Identification of Key Elements in Prostate Cancer for Ontology Building via a Multidisciplinary Consensus Agreement
Abstract
Simple Summary
Abstract
1. Introduction
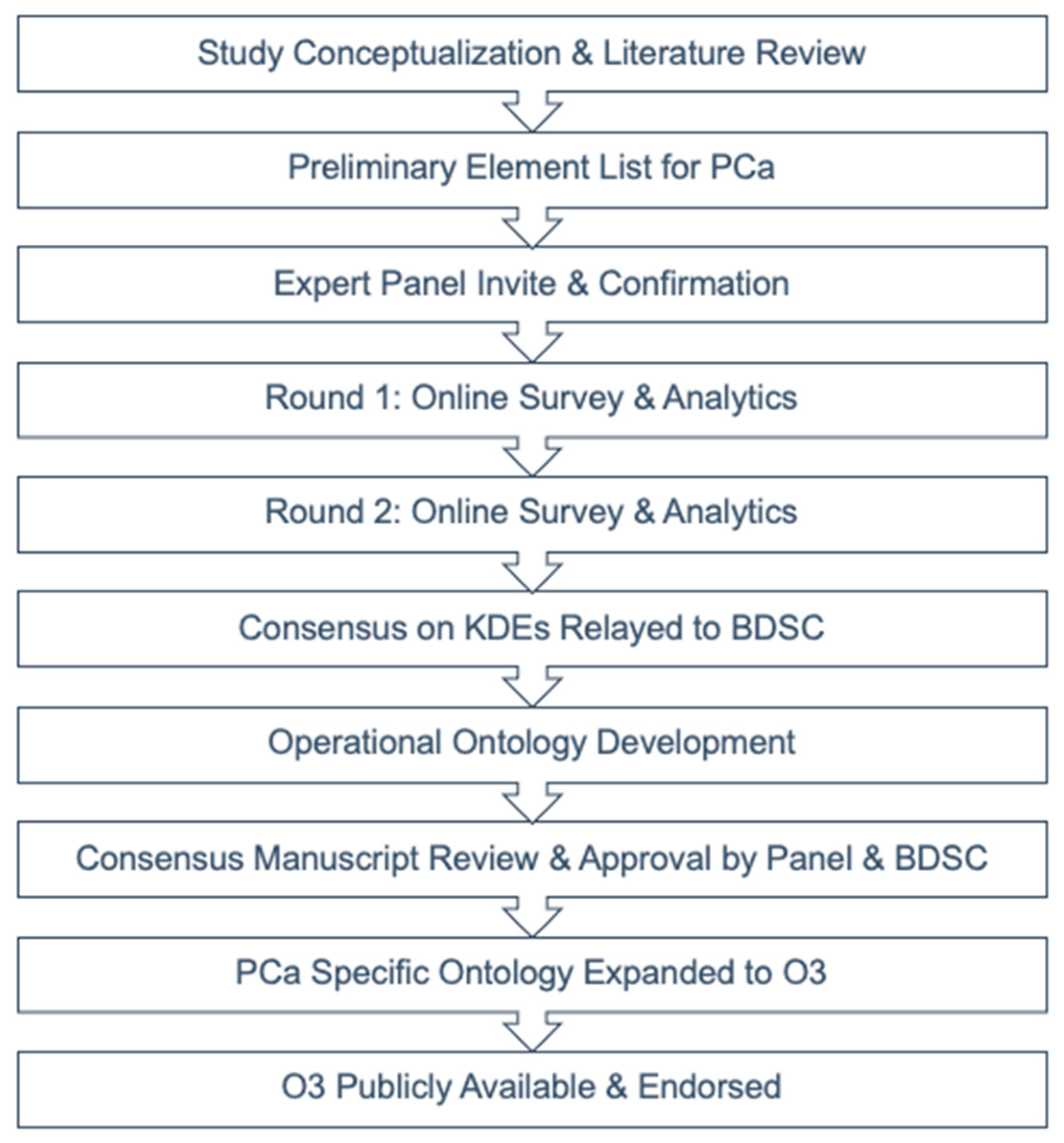
2. Materials and Methods
3. Results
3.1. Panel Characteristics and Participation
3.2. Treatment-Related Toxicity KDEs
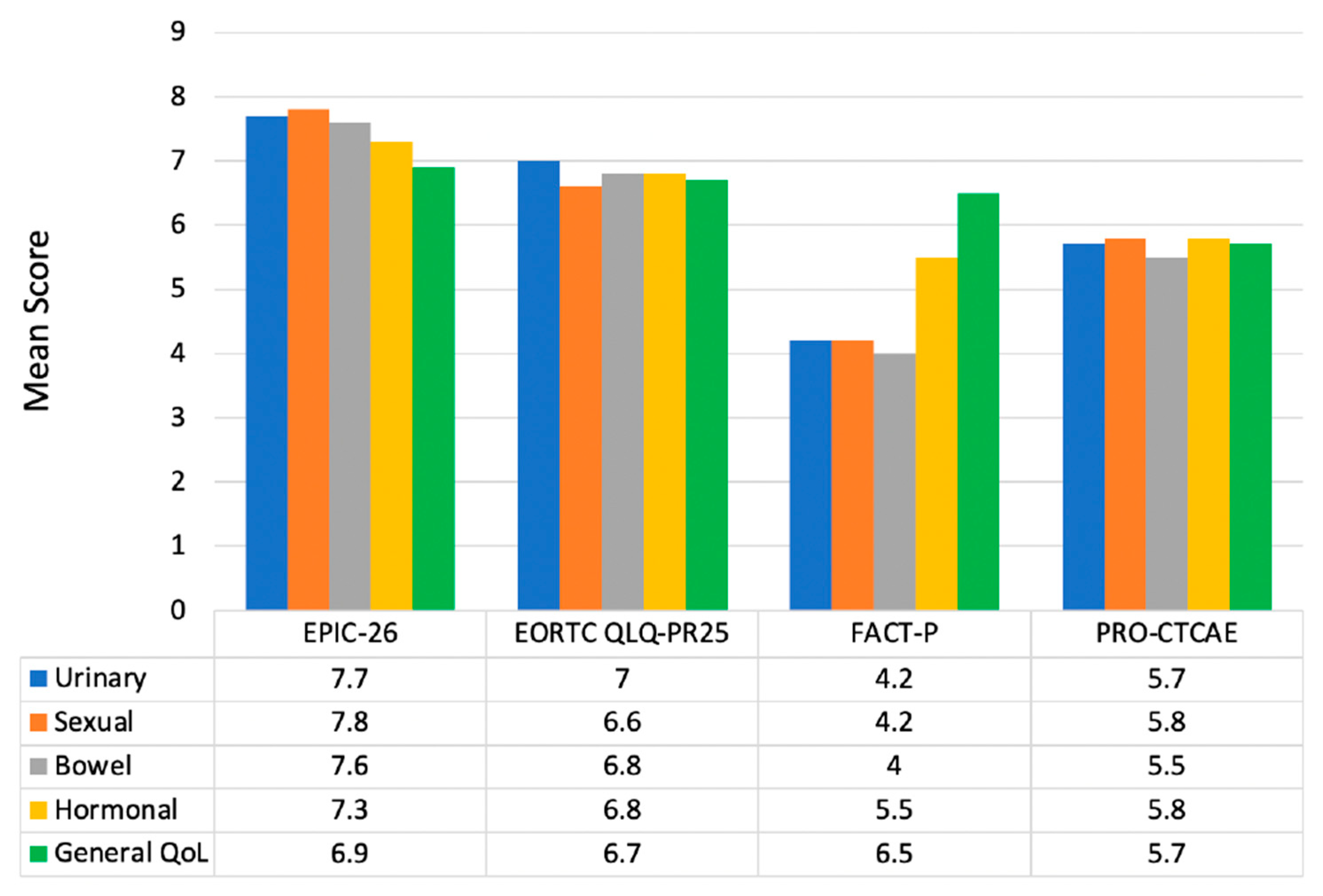
3.3. Patient-Reported Outcome Metrics KDEs
3.4. Disease Control Metrics KDEs
4. Discussion
4.1. Recommended Treatment-Related Toxicity Reporting
4.2. Recommended Patient-Reported Outcome/Symptom Surveying Tools
4.3. Recommended Reporting of Disease Control and Response Metrics
4.4. Study Limitations
4.5. Operational Ontology Build and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AAPM | American Association of Physicists in Medicine |
| AI | Agreement Index |
| BDSC | Big Data Sub Committee |
| DCM | Disease Control Metrics |
| EORTC QLQ-PR25 | European Organization for Research and Treatment of Cancer Quality of Life Questionnaire-Prostate Cancer |
| EPIC-26 | Expanded Prostate Index Composite-26 |
| FACT-P | Functional Assessment for Cancer Therapy- Prostate Cancer |
| GU | Genitourinary |
| ICC | Interclass Correlation Coefficient |
| KDE | Key Data Element |
| O3 | Operational Ontology for Oncology |
| PCa | Prostate Cancer |
| PRO-CTCAE | Patient-Reported Outcomes Common Terminology Criteria for Adverse Events |
| PROM | Patient-Reported Outcome Measure |
| TRT | Treatment-Related Toxicity |
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Henley, S.J.; Ward, E.M.; Scott, S.; Ma, J.; Anderson, R.N.; Firth, A.U.; Thomas, C.C.; Islami, F.; Weir, H.K.; Lewis, D.R.; et al. Annual Report to the Nation on the Status of Cancer, Part I: National Cancer Statistics. Cancer 2020, 126, 2225–2249. [Google Scholar] [CrossRef] [PubMed]
- Catton, C.N.; Lukka, H.; Gu, C.S.; Martin, J.M.; Supiot, S.; Chung, P.W.M.; Bauman, G.S.; Bahary, J.P.; Ahmed, S.; Cheung, P.; et al. Randomized Trial of a Hypofractionated Radiation Regimen for the Treatment of Localized Prostate Cancer. J. Clin. Oncol. 2017, 35, 1884–1890. [Google Scholar] [CrossRef] [PubMed]
- Dearnaley, D.; Syndikus, I.; Mossop, H.; Khoo, V.; Birtle, A.; Bloomfield, D.; Graham, J.; Kirkbride, P.; Logue, J.; Malik, Z.; et al. Conventional versus Hypofractionated High-Dose Intensity-Modulated Radiotherapy for Prostate Cancer: 5-Year Outcomes of the Randomised, Non-Inferiority, Phase 3 CHHiP Trial. Lancet Oncol. 2016, 17, 1047–1060. [Google Scholar] [CrossRef]
- Pollack, A.; Zagars, G.K.; Starkschall, G.; Antolak, J.A.; Lee, J.J.; Huang, E.; Von Eschenbach, A.C.; Kuban, D.A.; Rosen, I. Prostate Cancer Radiation Dose Response: Results of the M. D. Anderson Phase III Randomized Trial. Int. J. Radiat. Oncol. Biol. Phys. 2002, 53, 1097–1105. [Google Scholar] [CrossRef]
- Litwin, M.S.; Tan, H.J. The Diagnosis and Treatment of Prostate Cancer: A Review. JAMA J. Am. Med. Assoc. 2017, 317, 2532–2542. [Google Scholar] [CrossRef]
- Hamdy, F.C.; Donovan, J.L.; Lane, J.A.; Mason, M.; Metcalfe, C.; Holding, P.; Davis, M.; Peters, T.J.; Turner, E.L.; Martin, R.M.; et al. 10-Year Outcomes after Monitoring, Surgery, or Radiotherapy for Localized Prostate Cancer. N. Engl. J. Med. 2016, 375, 1415–1424. [Google Scholar] [CrossRef]
- Eastham, J.A.; Auffenberg, G.B.; Barocas, D.A.; Chou, R.; Crispino, T.; Davis, J.W.; Eggener, S.; Horwitz, E.M.; Kane, C.J.; Kirkby, E.; et al. Clinically Localized Prostate Cancer: AUA/ASTRO Guideline, Part I: Introduction, Risk Assessment, Staging, and Risk-Based Management. J. Urol. 2022, 208, 10–18. [Google Scholar] [CrossRef]
- Bruinsma, S.M.; Roobol, M.J.; Carroll, P.R.; Klotz, L.; Pickles, T.; Moore, C.M.; Gnanapragasam, V.J.; Villers, A.; Rannikko, A.; Valdagni, R.; et al. Semantics in Active Surveillance for Men with Localized Prostate Cancer—Results of a Modified Delphi Consensus Procedure. Nat. Rev. Urol. 2017, 14, 312–322. [Google Scholar] [CrossRef]
- Ramsey, I.; Eckert, M.; Hutchinson, A.D.; Marker, J.; Corsini, N. Core Outcome Sets in Cancer and Their Approaches to Identifying and Selecting Patient-Reported Outcome Measures: A Systematic Review. J. Patient Rep. Outcomes 2020, 4, 77. [Google Scholar] [CrossRef]
- Gillessen, S.; Attard, G.; Beer, T.M.; Beltran, H.; Bjartell, A.; Bossi, A.; Briganti, A.; Bristow, R.G.; Chi, K.N.; Clarke, N.; et al. Management of Patients with Advanced Prostate Cancer: Report of the Advanced Prostate Cancer Consensus Conference 2019. Eur. Urol. 2020, 77, 508–547. [Google Scholar] [CrossRef]
- Aluwini, S.S.; Mehra, N.; Lolkema, M.P.; Oprea-Lager, D.E.; Yakar, D.; Stoevelaar, H.; van der Poel, H.; Busstra, M.; de Jong, I.J.; de Reijke, T.; et al. Oligometastatic Prostate Cancer: Results of a Dutch Multidisciplinary Consensus Meeting. Eur. Urol. Oncol. 2019, 3, 231–238. [Google Scholar] [CrossRef]
- Ingrosso, G.; Becherini, C.; Lancia, A.; Caini, S.; Ost, P.; Francolini, G.; Høyer, M.; Bottero, M.; Bossi, A.; Zilli, T.; et al. Nonsurgical Salvage Local Therapies for Radiorecurrent Prostate Cancer: A Systematic Review and Meta-Analysis. Eur. Urol. Oncol. 2020, 3, 183–197. [Google Scholar] [CrossRef]
- Gómez-Caamaño, A.; González-San Segundo, C.; Henríquez, I.; Maldonado, X.; Zapatero, A.; Cabeza Rodríguez, M.A.; Celada Álvarez, F.; Ferrer Albiach, C.; Ferrer González, F.; Gómez-Iturriaga, A.; et al. Consensus on Management of Castration-Resistant Prostate Cancer on Behalf of the Urological Tumours Working Group (URONCOR) of the Spanish Society of Radiation Oncology. Clin. Transl. Oncol. 2019, 21, 420–432. [Google Scholar] [CrossRef]
- Dunn, J.; Green, A.; Ralph, N.; Newton, R.U.; Kneebone, A.; Frydenberg, M.; Chambers, S.K. Prostate Cancer Survivorship Essentials Framework: Guidelines for Practitioners. BJU Int. 2020, 128, 18–29. [Google Scholar] [CrossRef]
- Hogan, W.R.; Wagner, M.M. Free-Text Fields Change the Meaning of Coded Data. In Proceedings of the AMIA Annual Fall Symposium; American Medical Informatics Association: Bethesda, MA, USA, 1996; p. 517, PMCID: PMC2233180. [Google Scholar] [PubMed]
- Min, H.; Manion, F.J.; Goralczyk, E.; Wong, Y.N.; Ross, E.; Beck, J.R. Integration of Prostate Cancer Clinical Data Using an Ontology. J. Biomed. Inform. 2009, 42, 1035–1045. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, C.; Liu, X.; Xi, T.; Xu, G.; Sun, Y.; Zhu, F.; Shen, B. PCLiON: An Ontology for Data Standardization and Sharing of Prostate Cancer Associated Lifestyles. Int. J. Med. Inform. 2021, 145, 104332. [Google Scholar] [CrossRef]
- Zhang, H.; Guo, Y.; Li, Q.; George, T.J.; Shenkman, E.; Modave, F.; Bian, J. An Ontology-Guided Semantic Data Integration Framework to Support Integrative Data Analysis of Cancer Survival. BMC Med. Inform. Decis. Mak. 2018, 18, 129–147. [Google Scholar] [CrossRef]
- Studer, R.; Benjamins, V.R.; Fensel, D. Knowledge Engineering: Principles and Methods. Data Knowl. Eng. 1998, 25, 161–197. [Google Scholar] [CrossRef]
- Jackson, R.; Matentzoglu, N.; Overton, J.A.; Vita, R.; Balhoff, J.P.; Buttigieg, P.L.; Carbon, S.; Courtot, M.; Diehl, A.D.; Dooley, D.M.; et al. OBO Foundry in 2021: Operationalizing Open Data Principles to Evaluate Ontologies. Database 2021, 2021, baab069. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, S.S.; Kobow, K.; Zhang, J.; Buchhalter, J.; Dayyani, M.; Upadhyaya, D.P.; Prantzalos, K.; Bhattacharjee, M.; Blumcke, I.; Wiebe, S.; et al. Ontology-Based Feature Engineering in Machine Learning Workflows for Heterogeneous Epilepsy Patient Records. Sci. Rep. 2022, 12, 19430. [Google Scholar] [CrossRef] [PubMed]
- Munir, K.; Sheraz Anjum, M. The Use of Ontologies for Effective Knowledge Modelling and Information Retrieval. Appl. Comput. Inform. 2018, 14, 116–126. [Google Scholar] [CrossRef]
- AAPM Task Group. Standardizing Nomenclatures in Radiation Oncology the Report of AAPM Task Group 263; AAPM: Alexandria, VA, USA, 2018. [Google Scholar]
- AAPM: The American Association of Physicists in Medicine. Available online: https://www.aapm.org/default.asp (accessed on 31 January 2023).
- AAPM Committee Tree—Big Data Subcommittee (BDS). Available online: https://www.aapm.org/org/structure/?committee_code=BDS (accessed on 31 January 2023).
- Delphi Method|RAND. Available online: https://www.rand.org/topics/delphi-method.html (accessed on 1 August 2021).
- Ogbeifun, E.; Agwa-Ejon, J.; Mbohwa, C.; Pretorius, J.H. The Delphi Technique: A Credible Research Methodology. In Proceedings of the 2016 International Conference on Industrial Engineering and Operations Management, Kuala Lumpur, Malaysia, 8–10 March 2016. [Google Scholar]
- Avella, J.R. Delphi Panels: Research Design, Procedures, Advantages, and Challenges. Int. J. Dr. Stud. 2016, 11, 305–321. [Google Scholar] [CrossRef]
- Lange, T.; Kopkow, C.; Lützner, J.; Günther, K.P.; Gravius, S.; Scharf, H.P.; Stöve, J.; Wagner, R.; Schmitt, J. Comparison of Different Rating Scales for the Use in Delphi Studies: Different Scales Lead to Different Consensus and Show Different Test-Retest Reliability. BMC Med. Res. Methodol. 2020, 20, 28. [Google Scholar] [CrossRef]
- Li, X.; Fang, D.; Cooperberg, M.R.; Whitson, J.M.; Lue, T.F.; Zhou, L.; Shinohara, K. Long-Term Follow-up of International Prostate Symptom Score (IPSS) in Men Following Prostate Brachytherapy. World J. Urol. 2014, 32, 1061–1066. [Google Scholar] [CrossRef]
- Rosen, R.C.; Riley, A.; Wagner, G.; Osterloh, I.H.; Kirkpatrick, J.; Mishra, A. The International Index of Erectile Function (IIEF): A Multidimensional Scale for Assessment of Erectile Dysfunction. Urology 1997, 49, 822–830. [Google Scholar] [CrossRef]
- Cappelleri, J.C.; Rosen, R.C. The Sexual Health Inventory for Men (SHIM): A 5-Year Review of Research and Clinical Experience. Int. J. Impot. Res. 2005, 17, 307–319. [Google Scholar] [CrossRef]
- Mercieca-Bebber, R.; Stockler, M.R. Patient-Reported Outcomes and Localized Prostate Cancer Management. Nat. Rev. Urol. 2020, 17, 257–258. [Google Scholar] [CrossRef]
- Dueck, A.C.; Scher, H.I.; Bennett, A.V.; Mazza, G.L.; Thanarajasingam, G.; Schwab, G.; Weitzman, A.L.; Rogak, L.J.; Basch, E. Assessment of Adverse Events from the Patient Perspective in a Phase 3 Metastatic Castration-Resistant Prostate Cancer Clinical Trial. JAMA Oncol. 2020, 6, e193332. [Google Scholar] [CrossRef]
- Lee, J.; Koh, D.; Ong, C.N. Statistical Evaluation of Agreement between Two Methods for Measuring a Quantitative Variable. Comput. Biol. Med. 1989, 19, 61–70. [Google Scholar] [CrossRef]
- Liljequist, D.; Elfving, B.; Roaldsen, K.S. Intraclass Correlation—A Discussion and Demonstration of Basic Features. PLoS ONE 2019, 14, e0219854. [Google Scholar] [CrossRef]
- Beiderbeck, D.; Frevel, N.; von der Gracht, H.A.; Schmidt, S.L.; Schweitzer, V.M. Preparing, Conducting, and Analyzing Delphi Surveys: Cross-Disciplinary Practices, New Directions, and Advancements. MethodsX 2021, 8, 101401. [Google Scholar] [CrossRef]
- Hasson, F.; Keeney, S.; McKenna, H. Research Guidelines for the Delphi Survey Technique. J. Adv. Nurs. 2000, 32, 1008–1015. [Google Scholar] [CrossRef]
- Package “Lme4.” 2022. Available online: https://cran.r-project.org/web/packages/lme4/lme4.pdf (accessed on 30 May 2023).
- Parker, C.C.; James, N.D.; Brawley, C.D.; Clarke, N.W.; Hoyle, A.P.; Ali, A.; Ritchie, A.W.S.; Attard, G.; Chowdhury, S.; Cross, W.; et al. Radiotherapy to the Primary Tumour for Newly Diagnosed, Metastatic Prostate Cancer (STAMPEDE): A Randomised Controlled Phase 3 Trial. Lancet 2018, 392, 2353–2366. [Google Scholar] [CrossRef]
- Fizazi, K.; Tran, N.; Fein, L.; Matsubara, N.; Rodriguez-Antolin, A.; Alekseev, B.Y.; Özgüroğlu, M.; Ye, D.; Feyerabend, S.; Protheroe, A.; et al. Abiraterone plus Prednisone in Metastatic, Castration-Sensitive Prostate Cancer. N. Engl. J. Med. 2017, 377, 352–360. [Google Scholar] [CrossRef]
- Hoffman, K.E.; Penson, D.F.; Zhao, Z.; Huang, L.-C.; Conwill, R.; Laviana, A.A.; Joyce, D.D.; Luckenbaugh, A.N.; Goodman, M.; Hamilton, A.S.; et al. Patient-Reported Outcomes Through 5 Years for Active Surveillance, Surgery, Brachytherapy, or External Beam Radiation with or Without Androgen Deprivation Therapy for Localized Prostate Cancer. JAMA 2020, 323, 149–163. [Google Scholar] [CrossRef]
- Gulliford, S.L.; Foo, K.; Morgan, R.C.; Aird, E.G.; Bidmead, A.M.; Critchley, H.; Evans, P.M.; Gianolini, S.; Mayles, W.P.; Moore, A.R.; et al. Dose-Volume Constraints to Reduce Rectal Side Effects from Prostate Radiotherapy: Evidence from MRC RT01 Trial ISRCTN 47772397. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, 747–754. [Google Scholar] [CrossRef]
- Michalski, J.M.; Gay, H.; Jackson, A.; Tucker, S.L.; Deasy, J.O. Radiation Dose-Volume Effects in Radiation-Induced Rectal Injury. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, S123–S129. [Google Scholar] [CrossRef]
- Prada, P.J.; Gonzalez, H.; Menéndez, C.; Llaneza, A.; Fernández, J.; Santamarta, E.; Ricarte, P.P. Transperineal Injection of Hyaluronic Acid in the Anterior Perirectal Fat to Decrease Rectal Toxicity from Radiation Delivered with Low-Dose-Rate Brachytherapy for Prostate Cancer Patients. Brachytherapy 2009, 8, 210–217. [Google Scholar] [CrossRef]
- Smeenk, R.J.; van Lin, E.N.J.T.; van Kollenburg, P.; Kunze-Busch, M.; Kaanders, J.H.A.M. Anal Wall Sparing Effect of an Endorectal Balloon in 3D Conformal and Intensity-Modulated Prostate Radiotherapy. Radiother. Oncol. 2009, 93, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Van Lin, E.N.J.T.; Hoffmann, A.L.; Van Kollenburg, P.; Leer, J.W.; Visser, A.G. Rectal Wall Sparing Effect of Three Different Endorectal Balloons in 3D Conformal and IMRT Prostate Radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2005, 63, 565–576. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, K.E.; Voong, K.R.; Pugh, T.J.; Skinner, H.; Levy, L.B.; Takiar, V.; Choi, S.; Du, W.; Frank, S.J.; Johnson, J.; et al. Risk of Late Toxicity in Men Receiving Dose-Escalated Hypofractionated Intensity Modulated Prostate Radiation Therapy: Results from a Randomized Trial. Int. J. Radiat. Oncol. Biol. Phys. 2014, 88, 1074–1084. [Google Scholar] [CrossRef] [PubMed]
- Tree, A.C.; Ostler, P.; van der Voet, H.; Chu, W.; Loblaw, A.; Ford, D.; Tolan, S.; Jain, S.; Martin, A.; Staffurth, J.; et al. Intensity-Modulated Radiotherapy versus Stereotactic Body Radiotherapy for Prostate Cancer (PACE-B): 2-Year Toxicity Results from an Open-Label, Randomised, Phase 3, Non-Inferiority Trial. Lancet Oncol. 2022, 23, 1308–1320. [Google Scholar] [CrossRef]
- Mehta, R.; Radhakrishnan, N.S.; Warring, C.D.; Jain, A.; Fuentes, J.; Dolganiuc, A.; Lourdes, L.S.; Busigin, J.; Leverence, R.R. The Use of Evidence-Based, Problem-Oriented Templates as a Clinical Decision Support in an Inpatient Electronic Health Record System. Appl. Clin. Inform. 2016, 7, 790. [Google Scholar] [CrossRef]
- Martin, N.E.; Massey, L.; Stowell, C.; Bangma, C.; Briganti, A.; Bill-Axelson, A.; Blute, M.; Catto, J.; Chen, R.C.; D’Amico, A.V.; et al. Defining a Standard Set of Patient-Centered Outcomes for Men with Localized Prostate Cancer. Eur. Urol. 2015, 67, 460–467. [Google Scholar] [CrossRef]
- Lavallee, D.C.; Chenok, K.E.; Love, R.M.; Petersen, C.; Holve, E.; Segal, C.D.; Franklin, P.D. Incorporating Patient-Reported Outcomes into Health Care to Engage Patients and Enhance Care. Health Aff. 2016, 35, 575–582. [Google Scholar] [CrossRef]
- How REDCap Is Being Used in Response to COVID-19—REDCap. Available online: https://projectredcap.org/covid/ (accessed on 23 May 2023).
- NI-RADs|American College of Radiology. Available online: https://www.acr.org/Clinical-Resources/Reporting-and-Data-Systems/NI-RADs (accessed on 3 August 2021).
- Winkler, J.; Moser, R. Biases in Future-Oriented Delphi Studies: A Cognitive Perspective. Technol. Forecast. Soc. Change 2016, 105, 63–76. [Google Scholar] [CrossRef]
- Keegan, N.M.; Vasselman, S.E.; Barnett, E.S.; Nweji, B.; Carbone, E.A.; Blum, A.; Morris, M.J.; Rathkopf, D.E.; Slovin, S.F.; Danila, D.C.; et al. Clinical Annotations for Prostate Cancer Research: Defining Data Elements, Creating a Reproducible Analytical Pipeline, and Assessing Data Quality. Prostate 2022, 82, 1107–1116. [Google Scholar] [CrossRef]
- AAPM Big Data Subcommittee (BDSC). Available online: https://aapmbdsc.azurewebsites.net/Home/About?ReturnUrl=%2F (accessed on 3 August 2021).
- Home|SNOMED International. Available online: https://www.snomed.org/ (accessed on 30 March 2023).
- Mayo, C.S.; Feng, M.U.; Brock, K.K.; Kudner, R.; Balter, P.; Buchsbaum, J.C.; Caissie, A.; Covington, E.; Daugherty, E.C.; Dekker, A.L.; et al. Operational Ontology for Oncology (O3)—A Professional Society Based, Multi-Stakeholder, Consensus Driven Informatics Standard Supporting Clinical and Research use of “Real -World” Data from Patients Treated for Cancer: Operational Ontology for Oncology, International Journal of Radiation Oncology Biology Physics. Available online: https://www.sciencedirect.com/science/article/pii/S0360301623005254?utm_campaign=STMJ_AUTH_SERV_PUBLISHED&utm_medium=email&utm_acid=28161953&SIS_ID=&dgcid=STMJ_AUTH_SERV_PUBLISHED&CMX_ID=&utm_in=DM376211&utm_source=AC_ (accessed on 30 May 2023).
- Dronkers, E.A.C.; Baatenburg de Jong, R.J.; van der Poel, E.F.; Sewnaik, A.; Offerman, M.P.J. Keys to Successful Implementation of Routine Symptom Monitoring in Head and Neck Oncology with “Healthcare Monitor” and Patients’ Perspectives of Quality of Care. Head Neck 2020, 42, 3590–3600. [Google Scholar] [CrossRef]
- Rocque, G.B.; Dent, D.N.; Ingram, S.A.; Caston, N.E.; Thigpen, H.B.; Lalor, F.R.; Jamy, O.H.; Giri, S.; Azuero, A.; Young Pierce, J.; et al. Adaptation of Remote Symptom Monitoring Using Electronic Patient-Reported Outcomes for Implementation in Real-World Settings. JCO Oncol. Pract. 2022, 18, e1943–e1952. [Google Scholar] [CrossRef]

| Panel Characteristics | Count (%) or Average |
|---|---|
| Age, mean (range), years | 49.5 (34–70) |
| Gender | |
| Male | 26 (67) |
| Female | 13 (33) |
| Specialty | |
| Radiation Oncology | 22 (56) |
| Radiation Physics | 7 (18) |
| Medical Oncology | 7 (18) |
| Urology | 3 (8) |
| Practice Setting | |
| Academic | 31 (79) |
| Private | 1 (3) |
| Not answered | 7 (18) |
| Approximate years in practice | 17.7 |
| Average patient caseload per week | 30 |
| Tier 1 TRTs: Minimum KDE | Selected Tier Percentage | AI |
|---|---|---|
| Rectal Hemorrhage | 96.4 | 0.93 |
| Urinary Incontinence | 92.9 | 0.86 |
| Urinary Retention | 92.6 | 0.86 |
| Erectile Dysfunction | 88.9 | 0.79 |
| Hematuria | 85.7 | 0.75 |
| Dysuria | 85.7 | 0.74 |
| Rectal Fistula | 77.8 | 0.64 |
| Urinary Urgency | 77.8 | 0.64 |
| Urinary Frequency | 77.8 | 0.63 |
| Urinary Fistula | 77.8 | 0.63 |
| Proctitis | 74.1 | 0.60 |
| Fecal Incontinence | 70.4 | 0.57 |
| Diarrhea | 70.4 | 0.53 |
| Rectal Perforation | 66.7 | 0.54 |
| Rectal Ulcer | 63.0 | 0.52 |
| Tier 2: Strongly Encouraged | ||
| Libido Decrease | 66.7 | 0.49 |
| Gynecomastia | 66.7 | 0.48 |
| Depression | 59.3 | 0.43 |
| Ejaculation Disorder | 59.3 | 0.42 |
| Rectal Fissure | 55.6 | 0.43 |
| Hemorrhoids | 53.9 | 0.45 |
| Rectal Mucositis | 51.9 | 0.37 |
| Rectal Stenosis | 51.9 | 0.37 |
| Fatigue | 51.9 | 0.36 |
| Hot Flashes | 51.9 | 0.40 |
| Rectal Pain | 48.2 | 0.48 |
| Cystitis (Non-infective) | 48.2 | 0.41 |
| Urinary Tract Pain | 48.2 | 0.35 |
| Bladder Spasms | 48.2 | 0.34 |
| Urinary Tract Obstruction | 40.7 | 0.42 |
| Tier 3: Not Required | ||
| Superficial Fibrosis | 96.2 | 0.92 |
| Anorexia | 88.5 | 0.79 |
| Nausea | 84.6 | 0.73 |
| Peripheral Neuropathy | 84.6 | 0.72 |
| Dehydration | 80.8 | 0.68 |
| Radiation Dermatitis | 80.8 | 0.66 |
| Vomiting | 80.8 | 0.66 |
| Pelvic Infection | 61.5 | 0.48 |
| Anal Mucositis | 57.7 | 0.49 |
| Constipation | 53.9 | 0.42 |
| Prostatic Hemorrhage | 50.0 | 0.39 |
| Urinary Tract Infection | 44.4 | 0.36 |
| Name | % Yes | AI * |
|---|---|---|
| Biochemical Recurrence | 100% | 1 |
| Recurrence at Primary, Pelvic Nodal, and Distant Sites | 92% | 0.85 |
| No evidence of disease (NED) or Complete response | 88% | 0.78 |
| Progressive Disease | 84% | 0.72 |
| Recurrence at Distant Site(s) Only | 80% | 0.67 |
| Recurrence at Primary and Pelvic Nodal Sites | 80% | 0.67 |
| Recurrence at Primary and Distant Sites | 80% | 0.67 |
| Recurrence at Pelvic Nodal and Distant Sites | 80% | 0.67 |
| Recurrence at Primary Site Only | 76% | 0.62 |
| Recurrence at Pelvic Nodal Site(s) Only | 76% | 0.62 |
| Under Treatment | 72% | 0.58 |
| Stable Disease | 52% | 0.48 |
| Indeterminate (possible pseudo-progression) | 46% | 0.48 |
| Partial Response | 40% | 0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreno, A.; Solanki, A.A.; Xu, T.; Lin, R.; Palta, J.; Daugherty, E.; Hong, D.; Hong, J.; Kamran, S.C.; Katsoulakis, E.; et al. Identification of Key Elements in Prostate Cancer for Ontology Building via a Multidisciplinary Consensus Agreement. Cancers 2023, 15, 3121. https://doi.org/10.3390/cancers15123121
Moreno A, Solanki AA, Xu T, Lin R, Palta J, Daugherty E, Hong D, Hong J, Kamran SC, Katsoulakis E, et al. Identification of Key Elements in Prostate Cancer for Ontology Building via a Multidisciplinary Consensus Agreement. Cancers. 2023; 15(12):3121. https://doi.org/10.3390/cancers15123121
Chicago/Turabian StyleMoreno, Amy, Abhishek A. Solanki, Tianlin Xu, Ruitao Lin, Jatinder Palta, Emily Daugherty, David Hong, Julian Hong, Sophia C. Kamran, Evangelia Katsoulakis, and et al. 2023. "Identification of Key Elements in Prostate Cancer for Ontology Building via a Multidisciplinary Consensus Agreement" Cancers 15, no. 12: 3121. https://doi.org/10.3390/cancers15123121
APA StyleMoreno, A., Solanki, A. A., Xu, T., Lin, R., Palta, J., Daugherty, E., Hong, D., Hong, J., Kamran, S. C., Katsoulakis, E., Brock, K., Feng, M., Fuller, C., Mayo, C., & BDSC Prostate Cancer. (2023). Identification of Key Elements in Prostate Cancer for Ontology Building via a Multidisciplinary Consensus Agreement. Cancers, 15(12), 3121. https://doi.org/10.3390/cancers15123121







