Endoscopic Imaging for the Diagnosis of Neoplastic and Pre-Neoplastic Conditions of the Stomach
Abstract
Simple Summary
Abstract
1. Introduction
2. Indications for Endoscopic Screening of Gastric Cancer
3. White-Light Endoscopy (WLE)
3.1. Mucolytic and Defoaming Agents
3.2. Antispasmodic Agents
3.3. Inspection Time
3.4. High-Resolution Endoscopes
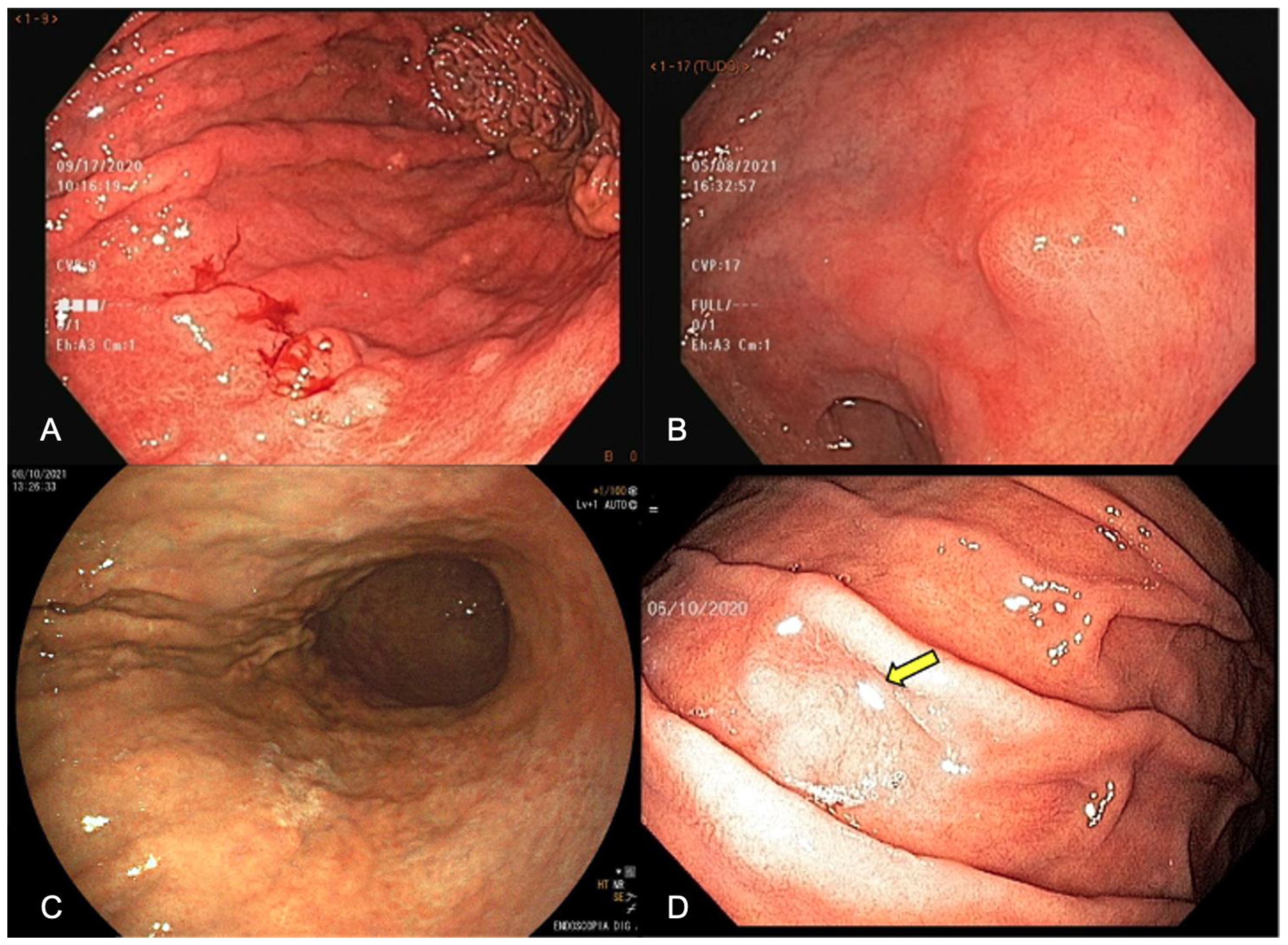
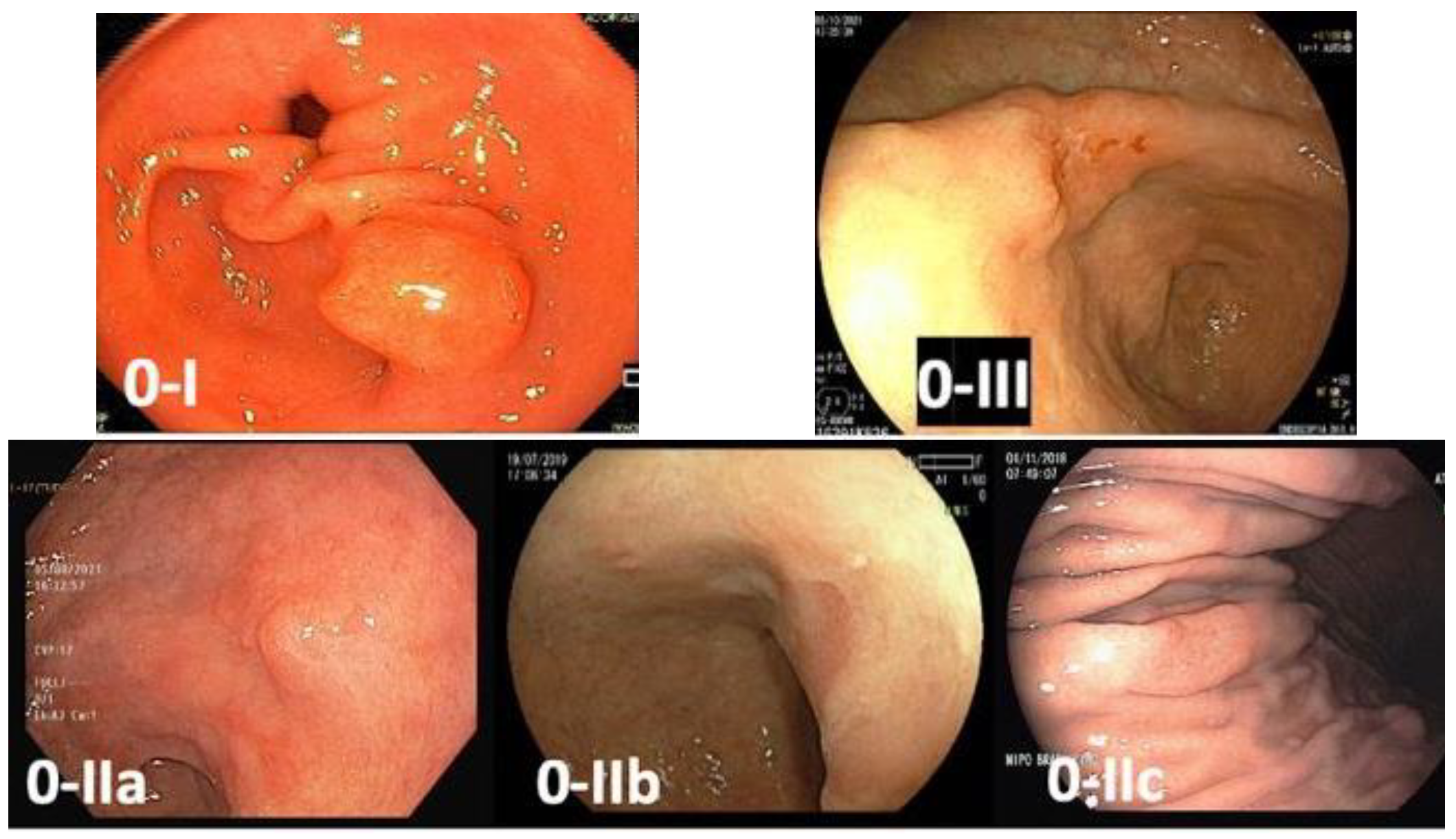
3.5. Obtain Index Images
3.6. Target Biopsies of Suspicious Lesions
4. Chromoendoscopy
4.1. Acetic Acid
4.2. Methylene Blue
4.3. Indigo Carmine
5. Virtual Chromoendoscopy
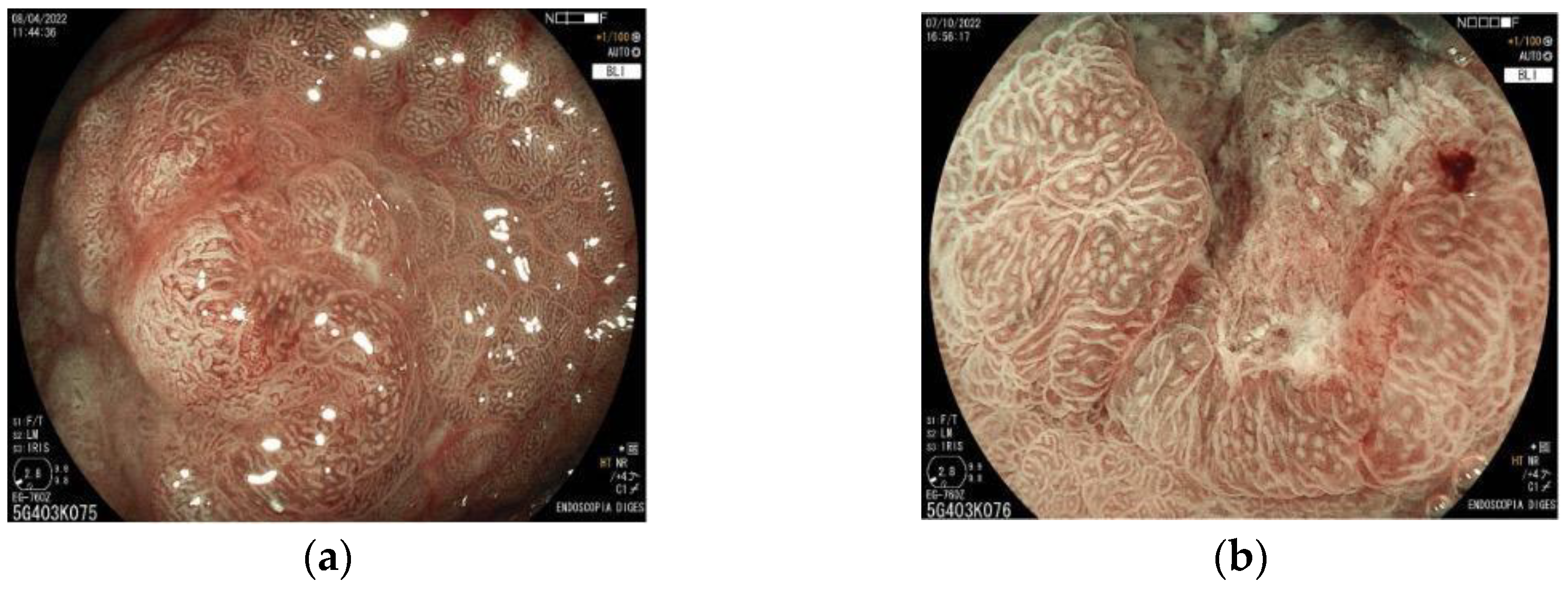
6. Magnifying Endoscopy
7. Artificial Intelligence
8. Other Methods
8.1. Confocal Laser Endomicroscopy
8.2. Endocytoscopy
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA. Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Crew, K.D.; Neugut, A.I. Epidemiology of Gastric Cancer. World J. Gastroenterol. 2006, 12, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Minicozzi, P.; Innos, K.; Sánchez, M.-J.; Trama, A.; Walsh, P.M.; Marcos-Gragera, R.; Dimitrova, N.; Botta, L.; Visser, O.; Rossi, S.; et al. Quality Analysis of Population-Based Information on Cancer Stage at Diagnosis across Europe, with Presentation of Stage-Specific Cancer Survival Estimates: A EUROCARE-5 Study. Eur. J. Cancer 2017, 84, 335–353. [Google Scholar] [CrossRef] [PubMed]
- Lauren, P. The two histological main types of gastric carcinoma: Diffuse and so-called intestinal-type carcinoma. an attempt at a histo-clinical classification. Acta Pathol. Microbiol. Scand. 1965, 64, 31–49. [Google Scholar] [CrossRef] [PubMed]
- Plummer, M.; de Martel, C.; Vignat, J.; Ferlay, J.; Bray, F.; Franceschi, S. Global Burden of Cancers Attributable to Infections in 2012: A Synthetic Analysis. Lancet Glob. Health 2016, 4, e609–e616. [Google Scholar] [CrossRef]
- Correa, P.; Houghton, J. Carcinogenesis of Helicobacter Pylori. Gastroenterology 2007, 133, 659–672. [Google Scholar] [CrossRef]
- Mabe, K.; Inoue, K.; Kamada, T.; Kato, K.; Kato, M.; Haruma, K. Endoscopic Screening for Gastric Cancer in Japan: Current Status and Future Perspectives. Dig. Endosc. Off. J. Jpn. Gastroenterol. Endosc. Soc. 2022, 34, 412–419. [Google Scholar] [CrossRef]
- Lee, Y.-C.; Chiang, T.-H.; Chou, C.-K.; Tu, Y.-K.; Liao, W.-C.; Wu, M.-S.; Graham, D.Y. Association Between Helicobacter Pylori Eradication and Gastric Cancer Incidence: A Systematic Review and Meta-Analysis. Gastroenterology 2016, 150, 1113–1124.e5. [Google Scholar] [CrossRef]
- Pimenta-Melo, A.R.; Monteiro-Soares, M.; Libânio, D.; Dinis-Ribeiro, M. Missing Rate for Gastric Cancer during Upper Gastrointestinal Endoscopy: A Systematic Review and Meta-Analysis. Eur. J. Gastroenterol. Hepatol. 2016, 28, 1041–1049. [Google Scholar] [CrossRef]
- Zhao, Z.; Yin, Z.; Wang, S.; Wang, J.; Bai, B.; Qiu, Z.; Zhao, Q. Meta-Analysis: The Diagnostic Efficacy of Chromoendoscopy for Early Gastric Cancer and Premalignant Gastric Lesions. J. Gastroenterol. Hepatol. 2016, 31, 1539–1545. [Google Scholar] [CrossRef]
- Fiuza, F.; Maluf-Filho, F.; Ide, E.; Furuya, C.K.; Fylyk, S.N.; Ruas, J.N.; Stabach, L.; Araujo, G.A.; Matuguma, S.E.; Uemura, R.S.; et al. Association between Mucosal Surface Pattern under near Focus Technology and Helicobacter Pylori Infection. World J. Gastrointest. Endosc. 2021, 13, 518–528. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, F.; Chen, Z.-Y.; Wang, Z.; Zhi, F.-C.; Liu, S.-D.; Bai, Y. Comparison of the Diagnostic Efficacy of White Light Endoscopy and Magnifying Endoscopy with Narrow Band Imaging for Early Gastric Cancer: A Meta-Analysis. Gastric Cancer Off. J. Int. Gastric Cancer Assoc. Jpn. Gastric Cancer Assoc. 2016, 19, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Oshima, A.; Hirata, N.; Ubukata, T.; Umeda, K.; Fujimoto, I. Evaluation of a Mass Screening Program for Stomach Cancer with a Case-Control Study Design. Int. J. Cancer 1986, 38, 829–833. [Google Scholar] [CrossRef] [PubMed]
- Hamashima, C.; Shabana, M.; Okada, K.; Okamoto, M.; Osaki, Y. Mortality Reduction from Gastric Cancer by Endoscopic and Radiographic Screening. Cancer Sci. 2015, 106, 1744–1749. [Google Scholar] [CrossRef]
- Faria, L.; Silva, J.C.; Rodríguez-Carrasco, M.; Pimentel-Nunes, P.; Dinis-Ribeiro, M.; Libânio, D. Gastric Cancer Screening: A Systematic Review and Meta-Analysis. Scand. J. Gastroenterol. 2022, 57, 1178–1188. [Google Scholar] [CrossRef]
- Dinis-Ribeiro, M.; Areia, M.; de Vries, A.C.; Marcos-Pinto, R.; Monteiro-Soares, M.; O’Connor, A.; Pereira, C.; Pimentel-Nunes, P.; Correia, R.; Ensari, A.; et al. Management of Precancerous Conditions and Lesions in the Stomach (MAPS): Guideline from the European Society of Gastrointestinal Endoscopy (ESGE), European Helicobacter Study Group (EHSG), European Society of Pathology (ESP), and the Sociedade Portuguesa de Endoscopia Digestiva (SPED). Endoscopy 2012, 44, 74–94. [Google Scholar] [CrossRef] [PubMed]
- Pimentel-Nunes, P.; Libânio, D.; Marcos-Pinto, R.; Areia, M.; Leja, M.; Esposito, G.; Garrido, M.; Kikuste, I.; Megraud, F.; Matysiak-Budnik, T.; et al. Management of Epithelial Precancerous Conditions and Lesions in the Stomach (MAPS II): European Society of Gastrointestinal Endoscopy (ESGE), European Helicobacter and Microbiota Study Group (EHMSG), European Society of Pathology (ESP), and Sociedade Portuguesa de Endoscopia Digestiva (SPED) Guideline Update 2019. Endoscopy 2019, 51, 365–388. [Google Scholar] [CrossRef] [PubMed]
- Yue, H.; Shan, L.; Bin, L. The Significance of OLGA and OLGIM Staging Systems in the Risk Assessment of Gastric Cancer: A Systematic Review and Meta-Analysis. Gastric Cancer Off. J. Int. Gastric Cancer Assoc. Jpn. Gastric Cancer Assoc. 2018, 21, 579–587. [Google Scholar] [CrossRef]
- Gupta, S.; Li, D.; El Serag, H.B.; Davitkov, P.; Altayar, O.; Sultan, S.; Falck-Ytter, Y.; Mustafa, R.A. AGA Clinical Practice Guidelines on Management of Gastric Intestinal Metaplasia. Gastroenterology 2020, 158, 693–702. [Google Scholar] [CrossRef]
- Shah, S.C.; Piazuelo, M.B.; Kuipers, E.J.; Li, D. AGA Clinical Practice Update on the Diagnosis and Management of Atrophic Gastritis: Expert Review. Gastroenterology 2021, 161, 1325–1332.e7. [Google Scholar] [CrossRef]
- ASGE Standards of Practice Committee; Evans, J.A.; Chandrasekhara, V.; Chathadi, K.V.; Decker, G.A.; Early, D.S.; Fisher, D.A.; Foley, K.; Hwang, J.H.; Jue, T.L.; et al. The Role of Endoscopy in the Management of Premalignant and Malignant Conditions of the Stomach. Gastrointest. Endosc. 2015, 82, 1–8. [Google Scholar] [CrossRef]
- Banks, M.; Graham, D.; Jansen, M.; Gotoda, T.; Coda, S.; di Pietro, M.; Uedo, N.; Bhandari, P.; Pritchard, D.M.; Kuipers, E.J.; et al. British Society of Gastroenterology Guidelines on the Diagnosis and Management of Patients at Risk of Gastric Adenocarcinoma. Gut 2019, 68, 1545–1575. [Google Scholar] [CrossRef] [PubMed]
- Japanese Gastric Cancer Association. Japanese Classification of Gastric Carcinoma: 3rd English Edition. Gastric Cancer 2011, 14, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Park, J.M. Quality Indicators in Esophagogastroduodenoscopy. Clin. Endosc. 2022, 55, 319–331. [Google Scholar] [CrossRef]
- Bisschops, R.; Areia, M.; Coron, E.; Dobru, D.; Kaskas, B.; Kuvaev, R.; Pech, O.; Ragunath, K.; Weusten, B.; Familiari, P.; et al. Performance Measures for Upper Gastrointestinal Endoscopy: A European Society of Gastrointestinal Endoscopy (ESGE) Quality Improvement Initiative. Endoscopy 2016, 48, 843–864. [Google Scholar] [CrossRef]
- Chang, W.-K.; Yeh, M.-K.; Hsu, H.-C.; Chen, H.-W.; Hu, M.-K. Efficacy of Simethicone and N-Acetylcysteine as Premedication in Improving Visibility during Upper Endoscopy. J. Gastroenterol. Hepatol. 2014, 29, 769–774. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Park, J.M.; Cho, H.S.; Cho, Y.K.; Choi, M.-G. Assessment of Cimetropium Bromide Use for the Detection of Gastric Neoplasms During Esophagogastroduodenoscopy. JAMA Netw. Open 2022, 5, e223827. [Google Scholar] [CrossRef] [PubMed]
- Park, J.M.; Huo, S.M.; Lee, H.H.; Lee, B.-I.; Song, H.J.; Choi, M.-G. Longer Observation Time Increases Proportion of Neoplasms Detected by Esophagogastroduodenoscopy. Gastroenterology 2017, 153, 460–469.e1. [Google Scholar] [CrossRef]
- Kawamura, T.; Wada, H.; Sakiyama, N.; Ueda, Y.; Shirakawa, A.; Okada, Y.; Sanada, K.; Nakase, K.; Mandai, K.; Suzuki, A.; et al. Examination Time as a Quality Indicator of Screening Upper Gastrointestinal Endoscopy for Asymptomatic Examinees. Dig. Endosc. Off. J. Jpn. Gastroenterol. Endosc. Soc. 2017, 29, 569–575. [Google Scholar] [CrossRef]
- Jang, J.-Y. The Past, Present, and Future of Image-Enhanced Endoscopy. Clin. Endosc. 2015, 48, 466–475. [Google Scholar] [CrossRef]
- Bhat, Y.M.; Abu Dayyeh, B.K.; Chauhan, S.S.; Gottlieb, K.T.; Hwang, J.H.; Komanduri, S.; Konda, V.; Lo, S.K.; Manfredi, M.A.; Maple, J.T.; et al. High-Definition and High-Magnification Endoscopes. Gastrointest. Endosc. 2014, 80, 919–927. [Google Scholar] [CrossRef] [PubMed]
- Rey, J.F.; Lambert, R. ESGE Quality Assurance Committee ESGE Recommendations for Quality Control in Gastrointestinal Endoscopy: Guidelines for Image Documentation in Upper and Lower GI Endoscopy. Endoscopy 2001, 33, 901–903. [Google Scholar] [CrossRef] [PubMed]
- Yao, K. The Endoscopic Diagnosis of Early Gastric Cancer. Ann. Gastroenterol. 2013, 26, 11–22. [Google Scholar] [PubMed]
- Emura, F.; Sharma, P.; Arantes, V.; Cerisoli, C.; Parra-Blanco, A.; Sumiyama, K.; Araya, R.; Sobrino, S.; Chiu, P.; Matsuda, K.; et al. Principles and Practice to Facilitate Complete Photodocumentation of the Upper Gastrointestinal Tract: World Endoscopy Organization Position Statement. Dig. Endosc. 2020, 32, 168–179. [Google Scholar] [CrossRef]
- Pouw, R.E.; Barret, M.; Biermann, K.; Bisschops, R.; Czakó, L.; Gecse, K.B.; de Hertogh, G.; Hucl, T.; Iacucci, M.; Jansen, M.; et al. Endoscopic Tissue Sampling—Part 1: Upper Gastrointestinal and Hepatopancreatobiliary Tracts. European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 2021, 53, 1174–1188. [Google Scholar] [CrossRef]
- Chiu, P.W.Y.; Uedo, N.; Singh, R.; Gotoda, T.; Ng, E.K.W.; Yao, K.; Ang, T.L.; Ho, S.H.; Kikuchi, D.; Yao, F.; et al. An Asian Consensus on Standards of Diagnostic Upper Endoscopy for Neoplasia. Gut 2019, 68, 186–197. [Google Scholar] [CrossRef] [PubMed]
- Dixon, M.F.; Genta, R.M.; Yardley, J.H.; Correa, P. Classification and Grading of Gastritis. The Updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am. J. Surg. Pathol. 1996, 20, 1161–1181. [Google Scholar] [CrossRef]
- Isajevs, S.; Liepniece-Karele, I.; Janciauskas, D.; Moisejevs, G.; Funka, K.; Kikuste, I.; Vanags, A.; Tolmanis, I.; Leja, M. The Effect of Incisura Angularis Biopsy Sampling on the Assessment of Gastritis Stage. Eur. J. Gastroenterol. Hepatol. 2014, 26, 510–513. [Google Scholar] [CrossRef]
- Varbanova, M.; Wex, T.; Jechorek, D.; Röhl, F.W.; Langner, C.; Selgrad, M.; Malfertheiner, P. Impact of the Angulus Biopsy for the Detection of Gastric Preneoplastic Conditions and Gastric Cancer Risk Assessment. J. Clin. Pathol. 2016, 69, 19–25. [Google Scholar] [CrossRef]
- Song, K.H.; Hwang, J.A.; Kim, S.M.; Ko, H.S.; Kang, M.K.; Ryu, K.H.; Koo, H.S.; Lee, T.H.; Huh, K.C.; Choi, Y.W.; et al. Acetic Acid Chromoendoscopy for Determining the Extent of Gastric Intestinal Metaplasia. Gastrointest. Endosc. 2017, 85, 349–356. [Google Scholar] [CrossRef]
- Ji, R.; Liu, J.; Zhang, M.-M.; Li, Y.-Y.; Zuo, X.-L.; Wang, X.; Li, Y.-Q. Optical Enhancement Imaging versus Acetic Acid for Detecting Gastric Intestinal Metaplasia: A Randomized, Comparative Trial. Dig. Liver Dis. 2020, 52, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Taghavi, S.A.; Membari, M.E.; Eshraghian, A.; Dehghani, S.M.; Hamidpour, L.; Khademalhoseini, F. Comparison of Chromoendoscopy and Conventional Endoscopy in the Detection of Premalignant Gastric Lesions. Can. J. Gastroenterol. J. Can. Gastroenterol. 2009, 23, 105–108. [Google Scholar] [CrossRef]
- Tada, M.; Katoh, S.; Kohli, Y.; Kawai, K. On the Dye Spraying Method in Colonofiberscopy. Endoscopy 1976, 8, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Le, H.; Wang, L.; Zhang, L.; Chen, P.; Xu, B.; Peng, D.; Yang, M.; Tan, Y.; Cai, C.; Li, H.; et al. Magnifying Endoscopy in Detecting Early Gastric Cancer: A Network Meta-Analysis of Prospective Studies. Medicine 2021, 100, e23934. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.-Y.; Lian, Q.-W.; Lin, Z.-H.; Zhong, J.; Xue, M.; Wang, L.-J. Diagnostic Performance of Magnifying Narrow-Band Imaging for Early Gastric Cancer: A Meta-Analysis. World J. Gastroenterol. 2015, 21, 7884–7894. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Chen, X.; Hendi, M.; Si, J.; Chen, S.; Deng, Y. Diagnostic Ability of Magnifying Narrow-Band Imaging for the Extent of Early Gastric Cancer: A Systematic Review and Meta-Analysis. Gastroenterol. Res. Pract. 2021, 2021, 5543556. [Google Scholar] [CrossRef]
- Zhou, F.; Wu, L.; Huang, M.; Jin, Q.; Qin, Y.; Chen, J. The Accuracy of Magnifying Narrow Band Imaging (ME-NBI) in Distinguishing between Cancerous and Noncancerous Gastric Lesions: A Meta-Analysis. Medicine 2018, 97, e9780. [Google Scholar] [CrossRef]
- Zhou, J.; Wu, H.; Fan, C.; Chen, S.; Liu, A. Comparison of the Diagnostic Efficacy of Blue Laser Imaging with Narrow Band Imaging for Gastric Cancer and Precancerous Lesions: A Meta-Analysis. Rev. Esp. Enferm. Dig. 2020, 112, 649–658. [Google Scholar] [CrossRef]
- Yashima, K.; Onoyama, T.; Kurumi, H.; Takeda, Y.; Yoshida, A.; Kawaguchi, K.; Yamaguchi, N.; Isomoto, H. Current Status and Future Perspective of Linked Color Imaging for Gastric Cancer Screening: A Literature Review. J. Gastroenterol. 2023, 58, 1–13. [Google Scholar] [CrossRef]
- Kanzaki, H.; Kawahara, Y.; Satomi, T.; Okanoue, S.; Hamada, K.; Kono, Y.; Iwamuro, M.; Kawano, S.; Okada, H. Differences in Color between Early Gastric Cancer and Cancer-Suspected Non-Cancerous Mucosa on Linked Color Imaging. Endosc. Int. Open 2023, 11, E90–E96. [Google Scholar] [CrossRef]
- Koh, M.; Lee, J.Y.; Han, S.-H.; Jeon, S.W.; Kim, S.J.; Cho, J.Y.; Kim, S.H.; Jang, J.Y.; Baik, G.H.; Jang, J.S. Comparison Trial between I-SCAN-Optical Enhancement and Chromoendoscopy for Evaluating the Horizontal Margins of Gastric Epithelial Neoplasms. Gut Liver 2022, 17, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Uedo, N.; Ishihara, R.; Iishi, H.; Yamamoto, S.; Yamamoto, S.; Yamada, T.; Imanaka, K.; Takeuchi, Y.; Higashino, K.; Ishiguro, S.; et al. A New Method of Diagnosing Gastric Intestinal Metaplasia: Narrow-Band Imaging with Magnifying Endoscopy. Endoscopy 2006, 38, 819–824. [Google Scholar] [CrossRef] [PubMed]
- Yao, K.; Oishi, T.; Matsui, T.; Yao, T.; Iwashita, A. Novel Magnified Endoscopic Findings of Microvascular Architecture in Intramucosal Gastric Cancer. Gastrointest. Endosc. 2002, 56, 279–284. [Google Scholar] [CrossRef]
- Muto, M.; Yao, K.; Kaise, M.; Kato, M.; Uedo, N.; Yagi, K.; Tajiri, H. Magnifying Endoscopy Simple Diagnostic Algorithm for Early Gastric Cancer (MESDA-G). Dig. Endosc. Off. J. Jpn. Gastroenterol. Endosc. Soc. 2016, 28, 379–393. [Google Scholar] [CrossRef]
- Yao, K.; Iwashita, A.; Tanabe, H.; Nishimata, N.; Nagahama, T.; Maki, S.; Takaki, Y.; Hirai, F.; Hisabe, T.; Nishimura, T.; et al. White Opaque Substance within Superficial Elevated Gastric Neoplasia as Visualized by Magnification Endoscopy with Narrow-Band Imaging: A New Optical Sign for Differentiating between Adenoma and Carcinoma. Gastrointest. Endosc. 2008, 68, 574–580. [Google Scholar] [CrossRef]
- Yao, K.; Doyama, H.; Gotoda, T.; Ishikawa, H.; Nagahama, T.; Yokoi, C.; Oda, I.; Machida, H.; Uchita, K.; Tabuchi, M. Diagnostic Performance and Limitations of Magnifying Narrow-Band Imaging in Screening Endoscopy of Early Gastric Cancer: A Prospective Multicenter Feasibility Study. Gastric Cancer Off. J. Int. Gastric Cancer Assoc. Jpn. Gastric Cancer Assoc. 2014, 17, 669–679. [Google Scholar] [CrossRef] [PubMed]
- Yao, K.; Yao, T.; Matsui, T.; Iwashita, A.; Oishi, T. Hemoglobin Content in Intramucosal Gastric Carcinoma as a Marker of Histologic Differentiation: A Clinical Application of Quantitative Electronic Endoscopy. Gastrointest. Endosc. 2000, 52, 241–245. [Google Scholar] [CrossRef]
- Kanesaka, T.; Sekikawa, A.; Tsumura, T.; Maruo, T.; Osaki, Y.; Wakasa, T.; Shintaku, M.; Yao, K. Absent Microsurface Pattern Is Characteristic of Early Gastric Cancer of Undifferentiated Type: Magnifying Endoscopy with Narrow-Band Imaging. Gastrointest. Endosc. 2014, 80, 1194–1198.e1. [Google Scholar] [CrossRef]
- Ochiai, K.; Ozawa, T.; Shibata, J.; Ishihara, S.; Tada, T. Current Status of Artificial Intelligence-Based Computer-Assisted Diagnosis Systems for Gastric Cancer in Endoscopy. Diagnostics 2022, 12, 3153. [Google Scholar] [CrossRef]
- Khan, S.; Yong, S.-P. A Comparison of Deep Learning and Hand Crafted Features in Medical Image Modality Classification. In Proceedings of the 2016 3rd International Conference on Computer and Information Sciences (ICCOINS), Kuala Lumpur, Malaysia, 15–17 August 2016; pp. 633–638. [Google Scholar]
- Sharma, P.; Hassan, C. Artificial Intelligence and Deep Learning for Upper Gastrointestinal Neoplasia. Gastroenterology 2022, 162, 1056–1066. [Google Scholar] [CrossRef]
- Hirasawa, T.; Aoyama, K.; Tanimoto, T.; Ishihara, S.; Shichijo, S.; Ozawa, T.; Ohnishi, T.; Fujishiro, M.; Matsuo, K.; Fujisaki, J.; et al. Application of Artificial Intelligence Using a Convolutional Neural Network for Detecting Gastric Cancer in Endoscopic Images. Gastric Cancer Off. J. Int. Gastric Cancer Assoc. Jpn. Gastric Cancer Assoc. 2018, 21, 653–660. [Google Scholar] [CrossRef]
- Wu, L.; Shang, R.; Sharma, P.; Zhou, W.; Liu, J.; Yao, L.; Dong, Z.; Yuan, J.; Zeng, Z.; Yu, Y.; et al. Effect of a Deep Learning-Based System on the Miss Rate of Gastric Neoplasms during Upper Gastrointestinal Endoscopy: A Single-Centre, Tandem, Randomised Controlled Trial. Lancet Gastroenterol. Hepatol. 2021, 6, 700–708. [Google Scholar] [CrossRef]
- Xie, F.; Zhang, K.; Li, F.; Ma, G.; Ni, Y.; Zhang, W.; Wang, J.; Li, Y. Diagnostic Accuracy of Convolutional Neural Network-Based Endoscopic Image Analysis in Diagnosing Gastric Cancer and Predicting Its Invasion Depth: A Systematic Review and Meta-Analysis. Gastrointest. Endosc. 2022, 95, 599–609.e7. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, J.; Zhou, W.; An, P.; Shen, L.; Liu, J.; Jiang, X.; Huang, X.; Mu, G.; Wan, X.; et al. Randomised Controlled Trial of WISENSE, a Real-Time Quality Improving System for Monitoring Blind Spots during Esophagogastroduodenoscopy. Gut 2019, 68, 2161–2169. [Google Scholar] [CrossRef]
- Choi, S.J.; Khan, M.A.; Choi, H.S.; Choo, J.; Lee, J.M.; Kwon, S.; Keum, B.; Chun, H.J. Development of Artificial Intelligence System for Quality Control of Photo Documentation in Esophagogastroduodenoscopy. Surg. Endosc. 2022, 36, 57–65. [Google Scholar] [CrossRef]
- Hassan, C.; Spadaccini, M.; Iannone, A.; Maselli, R.; Jovani, M.; Chandrasekar, V.T.; Antonelli, G.; Yu, H.; Areia, M.; Dinis-Ribeiro, M.; et al. Performance of Artificial Intelligence in Colonoscopy for Adenoma and Polyp Detection: A Systematic Review and Meta-Analysis. Gastrointest. Endosc. 2021, 93, 77–85.e6. [Google Scholar] [CrossRef] [PubMed]
- Shaukat, A.; Lichtenstein, D.R.; Somers, S.C.; Chung, D.C.; Perdue, D.G.; Gopal, M.; Colucci, D.R.; Phillips, S.A.; Marka, N.A.; Church, T.R.; et al. Computer-Aided Detection Improves Adenomas per Colonoscopy for Screening and Surveillance Colonoscopy: A Randomized Trial. Gastroenterology 2022, 163, 732–741. [Google Scholar] [CrossRef]
- Areia, M.; Mori, Y.; Correale, L.; Repici, A.; Bretthauer, M.; Sharma, P.; Taveira, F.; Spadaccini, M.; Antonelli, G.; Ebigbo, A.; et al. Cost-Effectiveness of Artificial Intelligence for Screening Colonoscopy: A Modelling Study. Lancet Digit. Health 2022, 4, e436–e444. [Google Scholar] [CrossRef] [PubMed]
- Pilonis, N.D.; Januszewicz, W.; di Pietro, M. Confocal Laser Endomicroscopy in Gastro-Intestinal Endoscopy: Technical Aspects and Clinical Applications. Transl. Gastroenterol. Hepatol. 2022, 7, 7. [Google Scholar] [CrossRef]
- Park, C.H.; Kim, H.; Jo, J.H.; Hahn, K.Y.; Yoon, J.-H.; Kim, S.Y.; Lee, Y.C.; Noh, S.H.; Chung, H.C.; Lee, S.K. Role of Probe-Based Confocal Laser Endomicroscopy-Targeted Biopsy in the Molecular and Histopathological Study of Gastric Cancer. J. Gastroenterol. Hepatol. 2019, 34, 84–91. [Google Scholar] [CrossRef]
- Bai, T.; Zhang, L.; Sharma, S.; Jiang, Y.D.; Xia, J.; Wang, H.; Qian, W.; Song, J.; Hou, X.H. Diagnostic Performance of Confocal Laser Endomicroscopy for Atrophy and Gastric Intestinal Metaplasia: A Meta-Analysis. J. Dig. Dis. 2017, 18, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Canakis, A.; Deliwala, S.S.; Kadiyala, J.; Bomman, S.; Canakis, J.; Bilal, M. The Diagnostic Performance of Probe-Based Confocal Laser Endomicroscopy in the Detection of Gastric Cancer: A Systematic Review and Meta-Analysis. Ann. Gastroenterol. 2022, 35, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Safatle-Ribeiro, A.V.; Ryoka Baba, E.; Corsato Scomparin, R.; Friedrich Faraj, S.; Simas de Lima, M.; Lenz, L.; Costa Martins, B.; Gusmon, C.; Shiguehissa Kawaguti, F.; Pennacchi, C.; et al. Probe-Based Confocal Endomicroscopy Is Accurate for Differentiating Gastric Lesions in Patients in a Western Center. Chin. J. Cancer Res. Chung-Kuo Yen Cheng Yen Chiu 2018, 30, 546–552. [Google Scholar] [CrossRef]
- Li, Z.; Zuo, X.-L.; Li, C.-Q.; Liu, Z.-Y.; Ji, R.; Liu, J.; Guo, J.; Li, Y.-Q. New Classification of Gastric Pit Patterns and Vessel Architecture Using Probe-Based Confocal Laser Endomicroscopy. J. Clin. Gastroenterol. 2016, 50, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Kong, R.; Wang, N.; Bao, W.; Mao, X.; Lu, J. Confocal Laser Endomicroscopy for Detection of Early Upper Gastrointestinal Cancer. Cancers 2023, 15, 776. [Google Scholar] [CrossRef]
- Misawa, M.; Kudo, S.-E.; Takashina, Y.; Akimoto, Y.; Maeda, Y.; Mori, Y.; Kudo, T.; Wakamura, K.; Miyachi, H.; Ishida, F.; et al. Clinical Efficacy of Endocytoscopy for Gastrointestinal Endoscopy. Clin. Endosc. 2021, 54, 455–463. [Google Scholar] [CrossRef]
- Tsurudome, I.; Miyahara, R.; Funasaka, K.; Furukawa, K.; Matsushita, M.; Yamamura, T.; Ishikawa, T.; Ohno, E.; Nakamura, M.; Kawashima, H.; et al. In Vivo Histological Diagnosis for Gastric Cancer Using Endocytoscopy. World J. Gastroenterol. 2017, 23, 6894–6901. [Google Scholar] [CrossRef]
- Horiuchi, Y.; Hirasawa, T.; Ishizuka, N.; Tokura, J.; Ishioka, M.; Tokai, Y.; Namikawa, K.; Yoshimizu, S.; Ishiyama, A.; Yoshio, T.; et al. Evaluation of Microvascular Patterns Alone Using Endocytoscopy with Narrow-Band Imaging for Diagnosing Gastric Cancer. Digestion 2022, 103, 159–168. [Google Scholar] [CrossRef]
- Noda, H.; Kaise, M.; Higuchi, K.; Koizumi, E.; Yoshikata, K.; Habu, T.; Kirita, K.; Onda, T.; Omori, J.; Akimoto, T.; et al. Convolutional Neural Network-Based System for Endocytoscopic Diagnosis of Early Gastric Cancer. BMC Gastroenterol. 2022, 22, 237. [Google Scholar] [CrossRef]





| Technique | Advantages | Limitations |
|---|---|---|
| White-light endoscopy | Easy to perform Readily available | Low sensitivity and specificity |
| Dye-based chromoendoscopy | Low cost Widely available Useful for delineating lesions margins | Time-consuming |
| Virtual chromoendoscopy | Easy to perform Valuable tool for evaluation of microvessels | Low accuracy to predict tumor depth |
| Magnifying endoscopy | High accuracy to distinguish benign and malignant lesions More specific than WLE and dyes | Low accuracy to distinguish differentiated- from undifferentiated-type adenocarcinoma Limited field of view Low accuracy to predict tumor depth |
| Artificial intelligence | Real-time diagnosis | High cost Lack of validation from prospective studies |
| Confocal laser endomicroscopy | Real-time diagnosis | High cost Steep learning curve Limited field of view Need for intravenous or topical contrast |
| Endocytoscopy | Real-time diagnosis Technology integrated (dedicated endoscope) | High cost Steep learning curve Limited field of view |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martins, B.C.; Moura, R.N.; Kum, A.S.T.; Matsubayashi, C.O.; Marques, S.B.; Safatle-Ribeiro, A.V. Endoscopic Imaging for the Diagnosis of Neoplastic and Pre-Neoplastic Conditions of the Stomach. Cancers 2023, 15, 2445. https://doi.org/10.3390/cancers15092445
Martins BC, Moura RN, Kum AST, Matsubayashi CO, Marques SB, Safatle-Ribeiro AV. Endoscopic Imaging for the Diagnosis of Neoplastic and Pre-Neoplastic Conditions of the Stomach. Cancers. 2023; 15(9):2445. https://doi.org/10.3390/cancers15092445
Chicago/Turabian StyleMartins, Bruno Costa, Renata Nobre Moura, Angelo So Taa Kum, Carolina Ogawa Matsubayashi, Sergio Barbosa Marques, and Adriana Vaz Safatle-Ribeiro. 2023. "Endoscopic Imaging for the Diagnosis of Neoplastic and Pre-Neoplastic Conditions of the Stomach" Cancers 15, no. 9: 2445. https://doi.org/10.3390/cancers15092445
APA StyleMartins, B. C., Moura, R. N., Kum, A. S. T., Matsubayashi, C. O., Marques, S. B., & Safatle-Ribeiro, A. V. (2023). Endoscopic Imaging for the Diagnosis of Neoplastic and Pre-Neoplastic Conditions of the Stomach. Cancers, 15(9), 2445. https://doi.org/10.3390/cancers15092445






