Risk of Second Primary Malignancies in Melanoma Survivors: A Population-Based Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Data Collection
2.3. Statistical Analysis
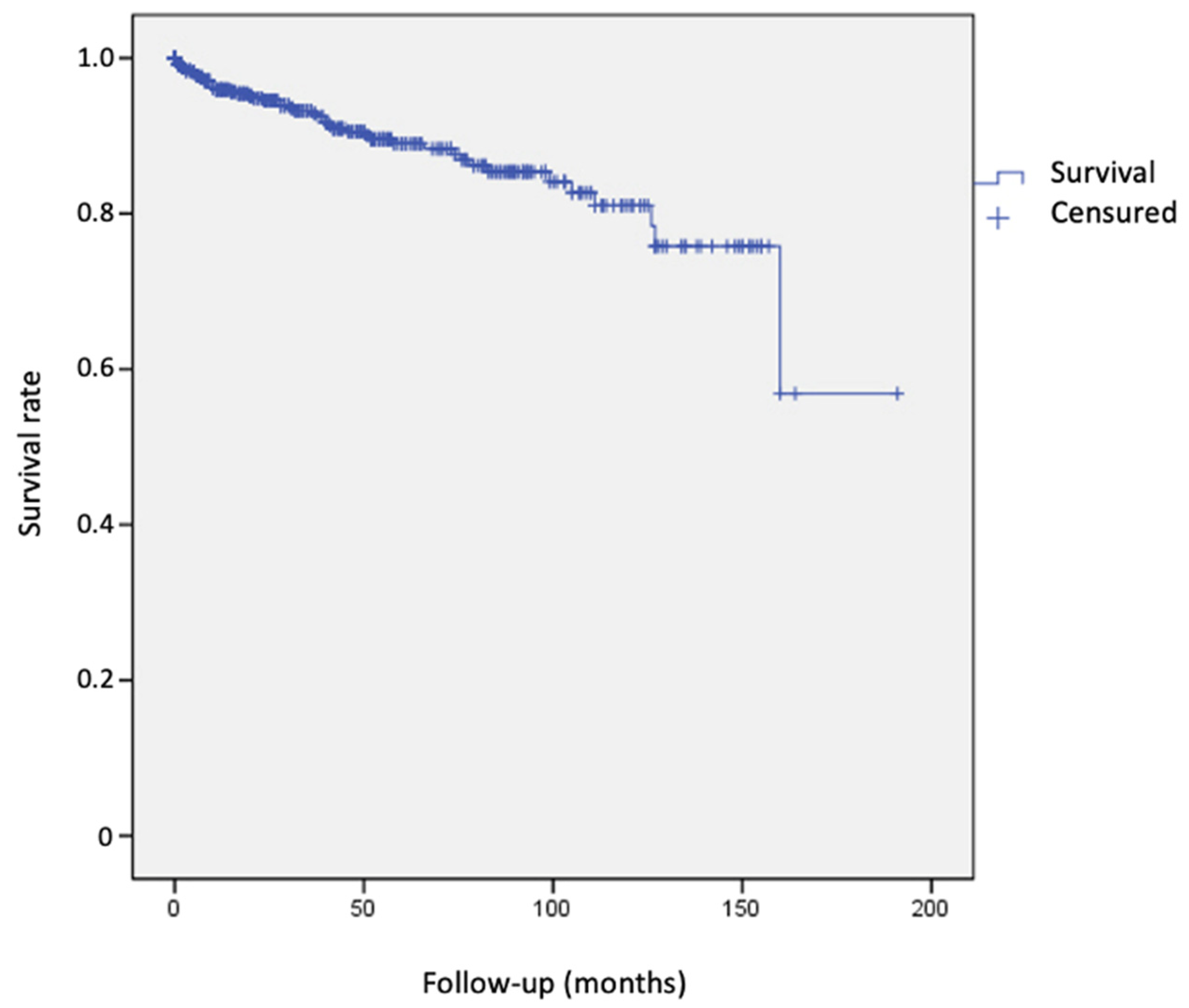
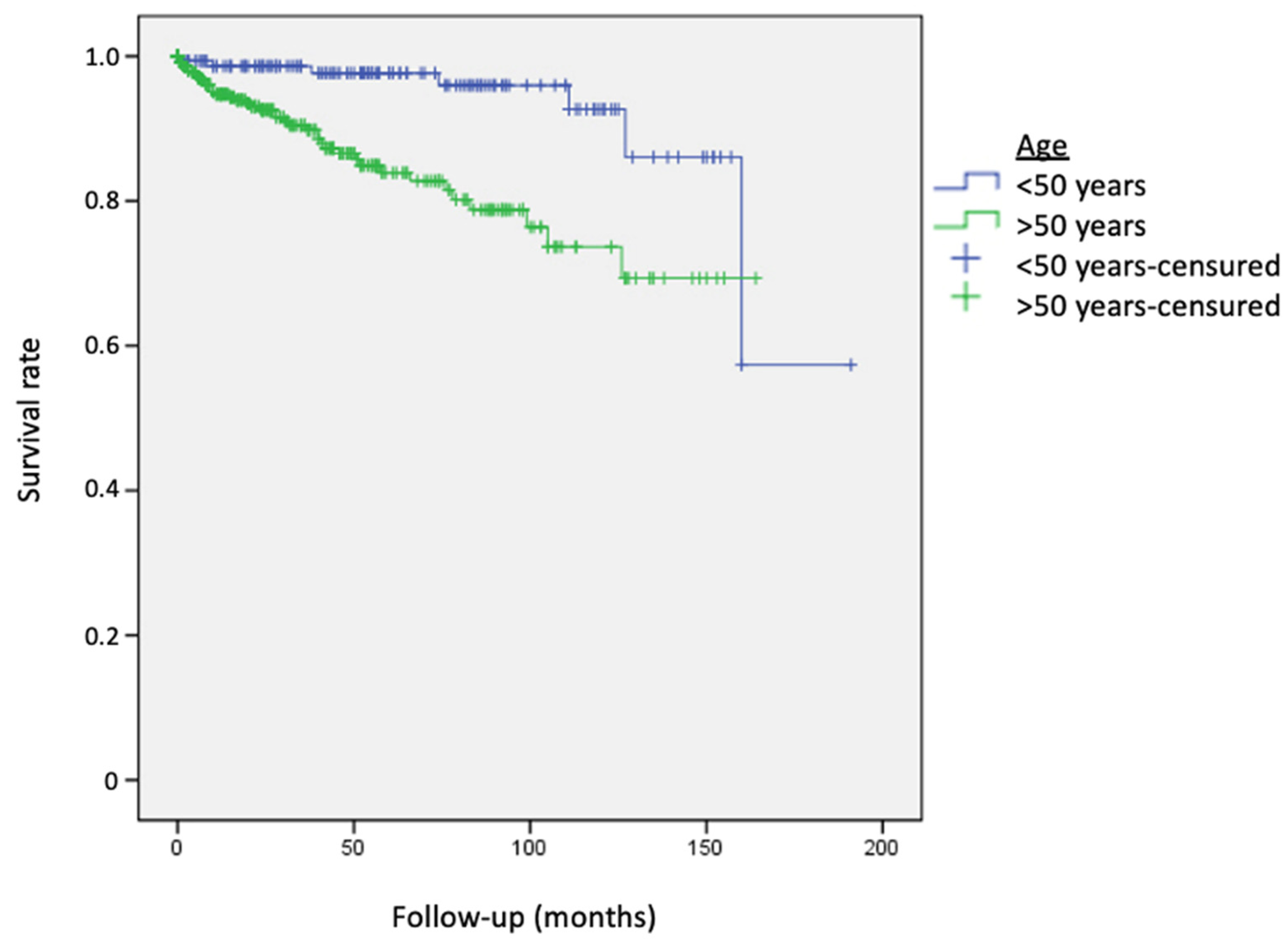
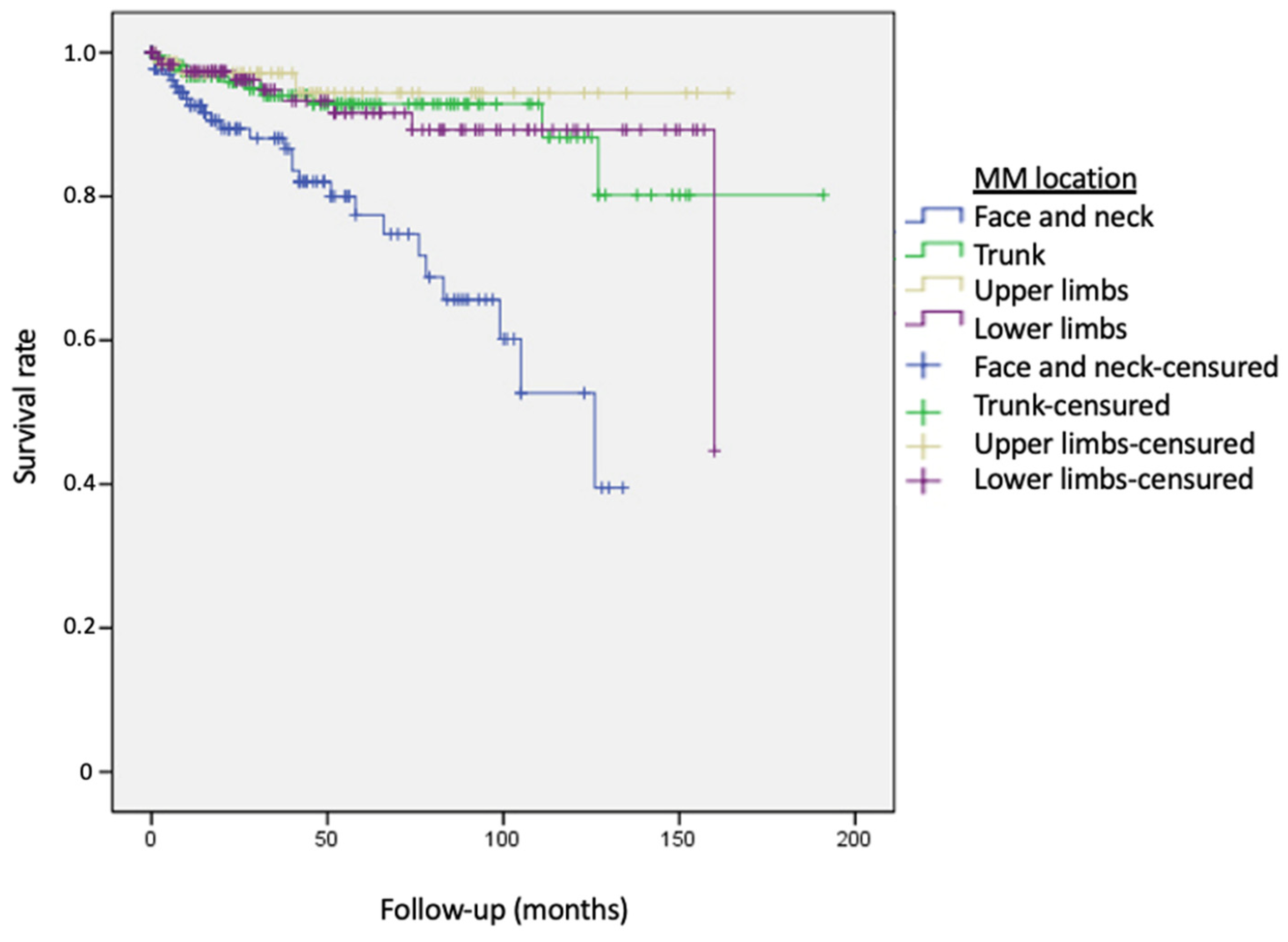
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Spanogle, J.P.; Clarke, C.A.; Aroner, S.; Swetter, S.M. Risk of second primary malignancies following cutaneous melanoma diagnosis: A population-based study. J. Am. Acad. Dermatol. 2010, 62, 757–767. [Google Scholar] [CrossRef]
- Sáenz, S.; Conejo-Mir, J.; Cayuela, A. Epidemiología del melanoma en España. Actas Dermo-Sifiliográficas 2005, 96, 411–418. [Google Scholar] [CrossRef]
- Pastor-Tomás, N.; Martínez-Franco, A.; Bañuls, J.; Peñalver, J.; Traves, V.; García-Casado, Z.; Requena, C.; Kumar, R.; Nagore, E. Risk factors for the development of a second melanoma in patients with cutaneous melanoma. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 2295–2302. [Google Scholar] [CrossRef]
- Goggins, W.B.; Finkelstein, D.M.; Tsao, H. Evidence for an association between cutaneous melanoma and non-Hodgkin lymphoma. Cancer 2001, 91, 874–880. [Google Scholar] [CrossRef] [PubMed]
- Yogi, T.; Hijioka, S.; Imaoka, H.; Mizuno, N.; Hara, K.; Tajika, M.; Tanaka, T.; Ishihara, M.; Shimizu, Y.; Hosoda, W.; et al. Risk factors for postoperative recurrence of intraductal papillary mucinous neoplasms of the pancreas based on a long-term follow-up study: Proposals for follow-up strategies. J. Hepato-Biliary-Pancreat. Sci. 2015, 22, 757–765. [Google Scholar] [CrossRef] [PubMed]
- Schmid-Wendtner, M.-H.; Baumert, J.; Wendtner, C.-M.; Plewig, G.; Volkenandt, M. Risk of second primary malignancies in patients with cutaneous melanoma. Br. J. Dermatol. 2001, 145, 981–985. [Google Scholar] [CrossRef] [PubMed]
- DiFronzo, L.A.; Wanek, L.A.; Elashoff, R.; Morton, D.L. Increased Incidence of Second Primary Melanoma in Patients with a Previous Cutaneous Melanoma. Ann. Surg. Oncol. 1999, 6, 705–711. [Google Scholar] [CrossRef]
- Nashan, D.; Kocer, B.; Schiller, M.; Luger, T.; Grabbe, S. Significant Risk of a Second Melanoma in Patients with a History of Melanoma but No Further Predisposing Factors. Dermatology 2003, 206, 76–77. [Google Scholar] [CrossRef]
- Wassberg, C.; Thorn, M.; Yuen, J.; Ringborg, U.; Hakulinen, T. Second primary cancers in patients with cutaneous malignant melanoma: A population-based study in Sweden. Br. J. Cancer 1996, 73, 255–259. [Google Scholar] [CrossRef]
- Wolff, J.; Wollina, U. Second malignancies in melanoma patients in Thuringia. J. Eur. Acad. Dermatol. Venereol. 2000, 14, 479–483. [Google Scholar] [CrossRef]
- Jeyakumar, A.; Chua, T.C.; Lam, A.K.-Y.; Gopalan, V. The Melanoma and Breast Cancer Association: An Overview of their ‘Second Primary Cancers’ and the Epidemiological, Genetic and Biological correlations. Crit. Rev. Oncol. Hematol. 2020, 152, 102989. [Google Scholar] [CrossRef] [PubMed]
- Crocetti, E.; Guzzinati, S.; Paci, E.; Falcini, F.; Zanetti, R.; Vercelli, M.; Rashid, I.; De Lisi, V.; Russo, A.G.; Vitarelli, S.; et al. The risk of developing a second, different, cancer among 14 560 survivors of malignant cutaneous melanoma: A study by AIRTUM (the Italian Network of Cancer Registries). Melanoma Res. 2008, 18, 230–234. [Google Scholar] [CrossRef] [PubMed]
- Levi, F.; La Vecchia, C.; Randimbison, L.; Te, V.C.; Erler, G. Incidence of invasive cancers following cutaneous malignant melanoma. Int. J. Cancer 1997, 72, 776–779. [Google Scholar] [CrossRef]
- Doubrovsky, A.; Menzies, S.W. Enhanced Survival in Patients with Multiple Primary Melanoma. Arch. Dermatol. 2003, 139, 1013–1018. [Google Scholar] [CrossRef]
- Kroumpouzos, G.; Konstadoulakis, M.M.; Cabral, H.; Karakousis, C.P. Risk of Basal Cell and Squamous Cell Carcinoma in Persons with Prior Cutaneous Melanoma. Dermatol. Surg. 2000, 26, 547–550. [Google Scholar] [CrossRef] [PubMed]
- Hemminki, K.; Jiang, Y.; Steineck, G. Skin cancer and non-Hodgkin’s lymphoma as second malignancies: Markers of impaired immune function? Eur. J. Cancer 2003, 39, 223–229. [Google Scholar] [CrossRef]
- Lens, M.B.; Newton-Bishop, J.A. An association between cutaneous melanoma and non-Hodgkin’s lymphoma: Pooled analysis of published data with a review. Ann. Oncol. 2005, 16, 460–465. [Google Scholar] [CrossRef]
- Goggins, W.B.; Tsao, H. A population-based analysis of risk factors for a second primary cutaneous melanoma among melanoma survivors. Cancer 2003, 97, 639–643. [Google Scholar] [CrossRef]
- McKenna, D.B.; Stockton, D.; Brewster, D.H.; Doherty, V.R. Evidence for an association between cutaneous malignant melanoma and lymphoid malignancy: A population-based retrospective cohort study in Scotland. Br. J. Cancer 2003, 88, 74–78. [Google Scholar] [CrossRef]
- Wu, Y.-H.; Kim, G.H.; Wagner, J.D.; Hood, A.F.; Chuang, T.-Y. The association between malignant melanoma and noncutaneous malignancies. Int. J. Dermatol. 2006, 45, 529–534. [Google Scholar] [CrossRef]
- Zheng, G.; Chattopadhyay, S.; Sundquist, K.; Sundquist, J.; Försti, A.; Hemminki, A.; Hemminki, K. Types of second primary cancer influence overall survival in cutaneous melanoma. BMC Cancer 2021, 21, 1–8. [Google Scholar] [CrossRef] [PubMed]
- McMillan, M.T.; Lewis, R.S.; Drebin, J.A.; Teitelbaum, U.R.; Lee, M.K.; Roses, R.E.; Fraker, D.L.; Vollmer, C.M. The efficacy of adjuvant therapy for pancreatic invasive intraductal papillary mucinous neoplasm (IPMN). Cancer 2016, 122, 521–533. [Google Scholar] [CrossRef] [PubMed]
- Kimlin, M.G.; Youlden, D.R.; Brodie, A.M.; DiSipio, T.; Youl, P.; Nair-Shalliker, V.; Baade, P.D. Risk of Second Primary Cancer in Survivors of In Situ Melanoma. J. Investig. Dermatol. 2019, 139, 842–847. [Google Scholar] [CrossRef] [PubMed]
- Wiener, A.A.; Schumacher, J.R.; Racz, J.M.; Weber, S.M.; Xu, Y.G.; Neuman, H.B. Incidence of Second Primary Melanoma in Cutaneous Melanoma Survivors. Ann. Surg. Oncol. 2022, 29, 5925–5932. [Google Scholar] [CrossRef] [PubMed]
- Caini, S.; Boniol, M.; Botteri, E.; Tosti, G.; Bazolli, B.; Russell-Edu, W.; Giusti, F.; Testori, A.; Gandini, S. The risk of developing a second primary cancer in melanoma patients: A comprehensive review of the literature and meta-analysis. J. Dermatol. Sci. 2014, 75, 3–9. [Google Scholar] [CrossRef]
- Borg, Å.; Sandberg, T.; Johannsson, O.; Måsba, A.; Olsson, H. High frequency of multiple melanomas and breast and pancreas carcinomas in CDKN2A mutation-positive melanoma families. J. Natl. Cancer Inst. 2000, 92, 1260–1266. [Google Scholar] [CrossRef]
- Deng, W.; Wang, Y.; Liu, X.; Liu, J.; Wang, L.; Yang, Z.; Yang, M.; An, Y.; Tang, C.; Sanford, N.N.; et al. Assessment of Trends in Second Primary Cancers in Patients with Metastatic Melanoma from 2005 to 2016. JAMA Netw. Open 2020, 3, e2028627. [Google Scholar] [CrossRef]
- Lalla, S.C.; Kumar, A.B.; Lehman, J.S.; Lohse, C.M.; Brewer, J.D. Association of BRAF V600E Status of Incident Melanoma and Risk for a Second Primary Malignancy: A Population-Based Study. Cutis 2022, 110, 150–154. [Google Scholar] [CrossRef]
- Beroukhim, K.; Pourang, A.; Eisen, D.B. Risk of second primary cutaneous and noncutaneous melanoma after cutaneous melanoma diagnosis: A population-based study. J. Am. Acad. Dermatol. 2020, 82, 683–689. [Google Scholar] [CrossRef]
- Thomas, N.E.; Edmiston, S.N.; Alexander, A.; Millikan, R.C.; Groben, P.A.; Hao, H.; Tolbert, D.; Berwick, M.; Busam, K.; Begg, C.B.; et al. Number of Nevi and Early-Life Ambient UV Exposure Are Associated with BRAF-Mutant Melanoma. Cancer Epidemiol. Biomark. Prev. 2007, 16, 991–997. [Google Scholar] [CrossRef]
| Histological Subtype | |
|---|---|
| Lentigo maligna MM | 63 (11.9%) |
| Superficial spreading MM | 213 (40.3%) |
| Nodular MM | 192 (36.3%) |
| Acral lentiginous MM | 26 (4.9%) |
| Non-cutaneous MM | 35 (6.6%) |
| MM location | |
| Face and neck | 142 (27.5%) |
| Trunk | 172 (33.3%) |
| Upper limbs | 76 (14.7%) |
| Lower limbs | 126 (24%) |
| Breslow index (mm) | |
| Mean (SD) | 2.67 (3.9) |
| Range | 0–44 |
| Clark level | |
| I (Intraepidermic) | 63 (12.8%) |
| II (Papillary dermis) | 106 (21.5%) |
| III (Medium dermis) | 159 (32.3%) |
| IV (Reticular dermis) | 116 (23.5%) |
| V (Hipodermis) | 49 (9.9%) |
| Second Primary Neoplasms | |||
|---|---|---|---|
| Total | Skin Tumors | Solid Organ Tumors * | |
| No. of patients | 529 | 34 (6.4%) | 20 (3.8%) |
| Gender | |||
| Male | 240 (45.4%) | 18 (52.9%) | 12 (60%) |
| Females | 289 (54.6%) | 16 (47.1%) | 8 (40%) |
| Age of diagnosis (years) | |||
| Mean (range) | 60 (11–94) | 66.4 (35–94) | 63.6 (30–82) |
| <50 years (n) | 157 (29.7%) | 6 (17.6%) | 2 (10%) |
| >50 years (n) | 372 (70.3%) | 28 (82.4%) | 18 (90%) |
| Age of diagnosis of SPN (years) | |||
| Mean (range) | 67.5 (35–94) | 69.1 (35–94) | 68.4 (44–89) |
| Follow-up (months) | |||
| Mean (range) | 45.4 (0–191) | 32.8 (1–127) | 55.8 (3–160) |
| 1 Year | 5 Years | 10 Years | |
|---|---|---|---|
| Global | 4.1% | 11% | 19% |
| Age ≤ 50 years | 1.4% | 2.3% | 7.3% |
| Age > 50 years | 5.3% | 16.1% | 26.3% |
| Solid organ tumors | 3.2% | 8.1% | 12.1% |
| Age ≤ 50 years | 1.4% | 2.3% | 7.3% |
| Age > 50 years | 4% | 11.5% | 14.3% |
| Solid organ tumors | 1.1% | 3.4% | 9% |
| Age ≤ 50 years | 0 | 0 | 0 |
| Age > 50 years | 1.7% | 7.3% | 15.9% |
| Variables | Univariant Analysis Rr (Ic 95%) | p Value | Multivariant Analysis Rr (Ic 95%) | p Value |
|---|---|---|---|---|
| Male gender | 0.6 (0.34–1.05) | NS | a | |
| Age (years) | 1.05 (1.03–1.07) | <0.0001 | 1.04 (1.02–1.07) | <0.0001 |
| Breslow index (mm) | 1.04 (0.97–1.11) | NS | a | |
| MM location: | ||||
| Reference group: Face and neck | ||||
| 0.26 (0.13–0.54) | <0.0001 | 0.24 (0.11–0.54) | <0.001 |
| 0.17 (0.05–0.55) | 0.003 | 0.13 (0.04–0.45) | <0.001 |
| 0.27 (0.12–0.58) | 0.001 | 0.20 (0.08–0.52) | <0.001 |
| MM histological subtype: | ||||
| Reference group: | ||||
| 0.13 (0.86–3.15) | NS | 0.94 (0.46–1.89) | NS |
| 0.27 (0.68–3.95) | NS | 0.30 (0.11–0.81) | 0.018 |
| 0.23 (0.62–7.32) | NS | 1.54 (0.37–6.43) | NS |
| 0.89 (0.26–4.84) | NS | 0.31 (0.07–1.45) | NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antoñanzas, J.; Morello-Vicente, A.; Garnacho-Saucedo, G.M.; Redondo, P.; Aguado-Gil, L.; Salido-Vallejo, R. Risk of Second Primary Malignancies in Melanoma Survivors: A Population-Based Study. Cancers 2023, 15, 3056. https://doi.org/10.3390/cancers15113056
Antoñanzas J, Morello-Vicente A, Garnacho-Saucedo GM, Redondo P, Aguado-Gil L, Salido-Vallejo R. Risk of Second Primary Malignancies in Melanoma Survivors: A Population-Based Study. Cancers. 2023; 15(11):3056. https://doi.org/10.3390/cancers15113056
Chicago/Turabian StyleAntoñanzas, Javier, Ana Morello-Vicente, Gloria Maria Garnacho-Saucedo, Pedro Redondo, Leyre Aguado-Gil, and Rafael Salido-Vallejo. 2023. "Risk of Second Primary Malignancies in Melanoma Survivors: A Population-Based Study" Cancers 15, no. 11: 3056. https://doi.org/10.3390/cancers15113056
APA StyleAntoñanzas, J., Morello-Vicente, A., Garnacho-Saucedo, G. M., Redondo, P., Aguado-Gil, L., & Salido-Vallejo, R. (2023). Risk of Second Primary Malignancies in Melanoma Survivors: A Population-Based Study. Cancers, 15(11), 3056. https://doi.org/10.3390/cancers15113056








