Comparing Oncologic Outcomes and Toxicity for Combined Modality Therapy vs. Carbon-Ion Radiotherapy for Previously Irradiated Locally Recurrent Rectal Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. CMT Patients
3.2. CIRT Patients
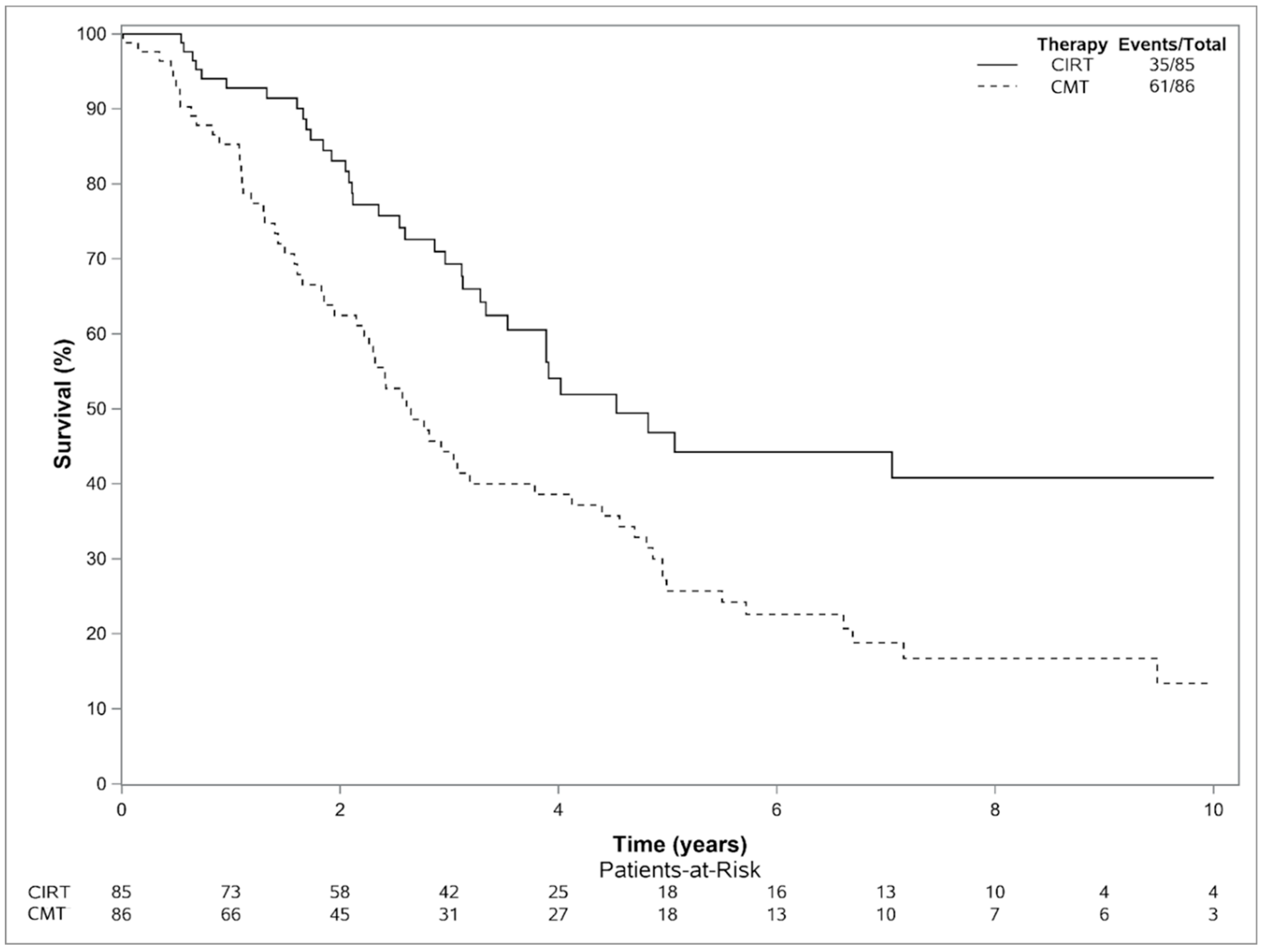
3.3. Outcomes
3.4. Toxicity
3.5. Cost Comparison
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mohiuddin, M.; Marks, G.; Marks, J. Long-term results of reirradiation for patients with recurrent rectal carcinoma. Cancer 2002, 95, 1144–1150. [Google Scholar] [CrossRef] [PubMed]
- Alektiar, K.M.; Zelefsky, M.J.; Paty, P.B.; Guillem, J.; Saltz, L.B.; Cohen, A.M.; Minsky, B.D. High-dose-rate intraoperative brachytherapy for recurrent colorectal cancer. Int. J. Radiat. Oncol. Biol. Phys. 2000, 48, 219–226. [Google Scholar] [CrossRef]
- Wiig, J.N.; Tveit, K.M.; Poulsen, J.P.; Olsen, D.R.; Giercksky, K.E. Preoperative irradiation and surgery for recurrent rectal cancer. Will intraoperative radiotherapy (IORT) be of additional benefit? A prospective study. Radiother. Oncol. 2002, 62, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Dresen, R.C.; Gosens, M.J.; Martijn, H.; Nieuwenhuijzen, G.A.; Creemers, G.J.; Daniels-Gooszen, A.W.; van den Brule, A.J.; van den Berg, H.A.; Rutten, H.J. Radical resection after IORT-containing multimodality treatment is the most important determinant for outcome in patients treated for locally recurrent rectal cancer. Ann. Surg. Oncol. 2008, 15, 1937–1947. [Google Scholar] [CrossRef] [PubMed]
- Haddock, M.G.; Miller, R.C.; Nelson, H.; Pemberton, J.H.; Dozois, E.J.; Alberts, S.R.; Gunderson, L.L. Combined modality therapy including intraoperative electron irradiation for locally recurrent colorectal cancer. Int. J. Radiat. Oncol. Biol. Phys. 2011, 79, 143–150. [Google Scholar] [CrossRef]
- Daly, M.E.; Kapp, D.S.; Maxim, P.G.; Welton, M.L.; Tran, P.T.; Koong, A.C.; Chang, D.T. Orthovoltage intraoperative radiotherapy for locally advanced and recurrent colorectal cancer. Dis. Colon. Rectum. 2012, 55, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Reddy, C.A.; Kolar, M.; Woody, N.; Mahadevan, A.; Deibel, F.C.; Dietz, D.W.; Remzi, F.H.; Suh, J.H. Intraoperative radiation therapy with the photon radiosurgery system in locally advanced and recurrent rectal cancer: Retrospective review of the Cleveland clinic experience. Radiat. Oncol. 2012, 7, 110. [Google Scholar] [CrossRef]
- Roeder, F.; Goetz, J.M.; Habl, G.; Bischof, M.; Krempien, R.; Buechler, M.W.; Hensley, F.W.; Huber, P.E.; Weitz, J.; Debus, J. Intraoperative Electron Radiation Therapy (IOERT) in the management of locally recurrent rectal cancer. BMC Cancer 2012, 12, 592. [Google Scholar] [CrossRef]
- Calvo, F.A.; Sole, C.V.; de Sierra, P.A.; Gómez-Espí, M.; Blanco, J.; Lozano, M.A.; Del Valle, E.; Rodriguez, M.; Muñoz-Calero, A.; Turégano, F.; et al. Prognostic impact of external beam radiation therapy in patients treated with and without extended surgery and intraoperative electrons for locally recurrent rectal cancer: 16-year experience in a single institution. Int. J. Radiat. Oncol. Biol. Phys. 2013, 86, 892–900. [Google Scholar] [CrossRef]
- Alberda, W.J.; Verhoef, C.; Nuyttens, J.J.; Rothbarth, J.; van Meerten, E.; de Wilt, J.H.; Burger, J.W. Outcome in patients with resectable locally recurrent rectal cancer after total mesorectal excision with and without previous neoadjuvant radiotherapy for the primary rectal tumor. Ann. Surg. Oncol. 2014, 21, 520–526. [Google Scholar] [CrossRef]
- Holman, F.A.; Bosman, S.J.; Haddock, M.G.; Gunderson, L.L.; Kusters, M.; Nieuwenhuijzen, G.A.; van den Berg, H.; Nelson, H.; Rutten, H.J. Results of a pooled analysis of IOERT containing multimodality treatment for locally recurrent rectal cancer: Results of 565 patients of two major treatment centres. Eur. J. Surg. Oncol. 2017, 43, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Lindel, K.; Willett, C.G.; Shellito, P.C.; Ott, M.J.; Clark, J.; Grossbard, M.; Ryan, D.; Ancukiewicz, M. Intraoperative radiation therapy for locally advanced recurrent rectal or rectosigmoid cancer. Radiother. Oncol. 2001, 58, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Hyngstrom, J.R.; Tzeng, C.W.; Beddar, S.; Das, P.; Krishnan, S.; Delclos, M.E.; Crane, C.H.; Chang, G.J.; You, Y.N.; Feig, B.W.; et al. Intraoperative radiation therapy for locally advanced primary and recurrent colorectal cancer: Ten-year institutional experience. J. Surg. Oncol. 2014, 109, 652–658. [Google Scholar] [CrossRef] [PubMed]
- Ng, M.K.Y.; Leong, T.; Heriot, A.G.; Ngan, S.Y.K. Once-daily reirradiation for rectal cancer in patients who have received previous pelvic radiotherapy. J. Med. Imaging Radiat. Oncol. 2013, 57, 512–518. [Google Scholar] [CrossRef] [PubMed]
- Guren, M.G.; Undseth, C.; Rekstad, B.L.; Brændengen, M.; Dueland, S.; Spindler, K.L.; Glynne-Jones, R.; Tveit, K.M. Reirradiation of locally recurrent rectal cancer: A systematic review. Radiother. Oncol. 2014, 113, 151–157. [Google Scholar] [CrossRef]
- Jethwa, K.R.; Haddock, M.G.; Hallemeier, C.L. The use of intraoperative radiation therapy in the management of locally recurrent rectal cancer. Semin. Colon Rectal Surg. 2020, 31, 100763. [Google Scholar] [CrossRef]
- Malouff, T.D.; Mahajan, A.; Krishnan, S.; Beltran, C.; Seneviratne, D.S.; Trifiletti, D.M. Carbon Ion Therapy: A Modern Review of an Emerging Technology. Front. Oncol. 2020, 10, 82. [Google Scholar] [CrossRef]
- Kamada, T.; Tsujii, H.; Blakely, E.A.; Debus, J.; De Neve, W.; Durante, M.; Jäkel, O.; Mayer, R.; Orecchia, R.; Pötter, R.; et al. Carbon ion radiotherapy in Japan: An assessment of 20 years of clinical experience. Lancet Oncol. 2015, 16, e93–e100. [Google Scholar] [CrossRef]
- Shinoto, M.; Yamada, S.; Okamoto, M.; Shioyama, Y.; Ohno, T.; Nakano, T.; Nemoto, K.; Isozaki, Y.; Kawashiro, S.; Tsuji, H.; et al. Carbon-ion radiotherapy for locally recurrent rectal cancer: Japan carbon-ion radiation oncology study group (J-CROS) study 1404 rectum. Radiother. Oncol. 2019, 132, 236–240. [Google Scholar] [CrossRef]
- Tinganelli, W.; Durante, M. Carbon Ion Radiobiology. Cancers 2020, 12, 3022. [Google Scholar] [CrossRef]
- Yamada, S.; Kamada, T.; Ebner, D.K.; Shinoto, M.; Terashima, K.; Isozaki, Y.; Yasuda, S.; Makishima, H.; Tsuji, H.; Tsujii, H.; et al. Carbon-ion radiation therapy for pelvic recurrence of rectal cancer. Int. J. Radiat. Oncol. Biol. Phys. 2016, 96, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Haddock, M.G.; Gunderson, L.L.; Nelson, H.; Cha, S.S.; Devine, R.M.; Dozois, R.R.; Wolff, B.G. Intraoperative irradiation for locally recurrent colorectal cancer in previously irradiated patients. Int. J. Radiat. Oncol. Biol. Phys. 2001, 49, 1267–1274. [Google Scholar] [CrossRef] [PubMed]
- Haddock, M.G. Irradiation of Very Locally Advanced and Recurrent Rectal Cancer. Semin. Radiat. Oncol. 2016, 26, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Tepper, J.E.; Gunderson, L.L.; Orlow, E.; Cohen, A.M.; Hedberg, S.E.; Shipley, W.U.; Blitzer, P.H.; Rich, T. Complications of intraoperative radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 1984, 10, 1831–1839. [Google Scholar] [CrossRef]
- Choi, K.; Molinelli, S.; Russo, S.; Mirandola, A.; Fiore, M.R.; Vischioni, B.; Fossati, P.; Petrucci, R.; Turturici, I.; Dale, J.E.; et al. Rectum Dose Constraints for Carbon Ion Therapy: Relative Biological Effectiveness Model Dependence in Relation to Clinical Outcomes. Cancers 2019, 12, 46. [Google Scholar] [CrossRef]
- Mohamad, O.; Tabuchi, T.; Nitta, Y.; Nomoto, A.; Sato, A.; Kasuya, G.; Makishima, H.; Choy, H.; Yamada, S.; Morishima, T.; et al. Risk of subsequent primary cancers after carbon ion radiotherapy, photon radiotherapy, or surgery for localised prostate cancer: A propensity score-weighted, retrospective, cohort study. Lancet Oncol. 2019, 20, 674–685. [Google Scholar] [CrossRef]
- Helm, A.; Tinganelli, W.; Simoniello, P.; Kurosawa, F.; Fournier, C.; Shimokawa, T.; Durante, M. Reduction of Lung Metastases in a Mouse Osteosarcoma Model Treated with Carbon Ions and Immune Checkpoint Inhibitors. Int. J. Radiat. Oncol. Biol. Phys. 2021 109, 594–602. [CrossRef]
- Shiba, S.; Okamoto, M.; Kiyohara, H.; Ohno, T.; Kaminuma, T.; Asao, T.; Ojima, H.; Shirabe, K.; Kuwano, H.; Nakano, T. Prospective Observational Study of High-Dose Carbon-Ion Radiotherapy for Pelvic Recurrence of Rectal Cancer (GUNMA 0801). Front. Oncol. 2019, 9, 702. [Google Scholar] [CrossRef]
- Habermehl, D.; Wagner, M.; Ellerbrock, M.; Büchler, M.W.; Jäkel, O.; Debus, J.; Combs, S.E. Reirradiation Using Carbon Ions in Patients with Locally Recurrent Rectal Cancer at HIT: First Results. Ann. Surg. Oncol. 2015, 22, 2068–2074. [Google Scholar] [CrossRef]
- Chung, S.Y.; Takiyama, H.; Kang, J.H.; Chang, J.S.; Min, B.S.; Tsuji, H.; Yamada, S.; Koom, W.S. Comparison of clinical outcomes between carbon ion radiotherapy and X-ray radiotherapy for reirradiation in locoregional recurrence of rectal cancer. Sci. Rep. 2022, 12, 1845. [Google Scholar] [CrossRef]
- Mobaraki, A.; Ohno, T.; Yamada, S.; Sakurai, H.; Nakano, T. Cost-effectiveness of carbon ion radiation therapy for locally recurrent rectal cancer. Cancer Sci. 2010, 101, 1834–1839. [Google Scholar] [CrossRef] [PubMed]
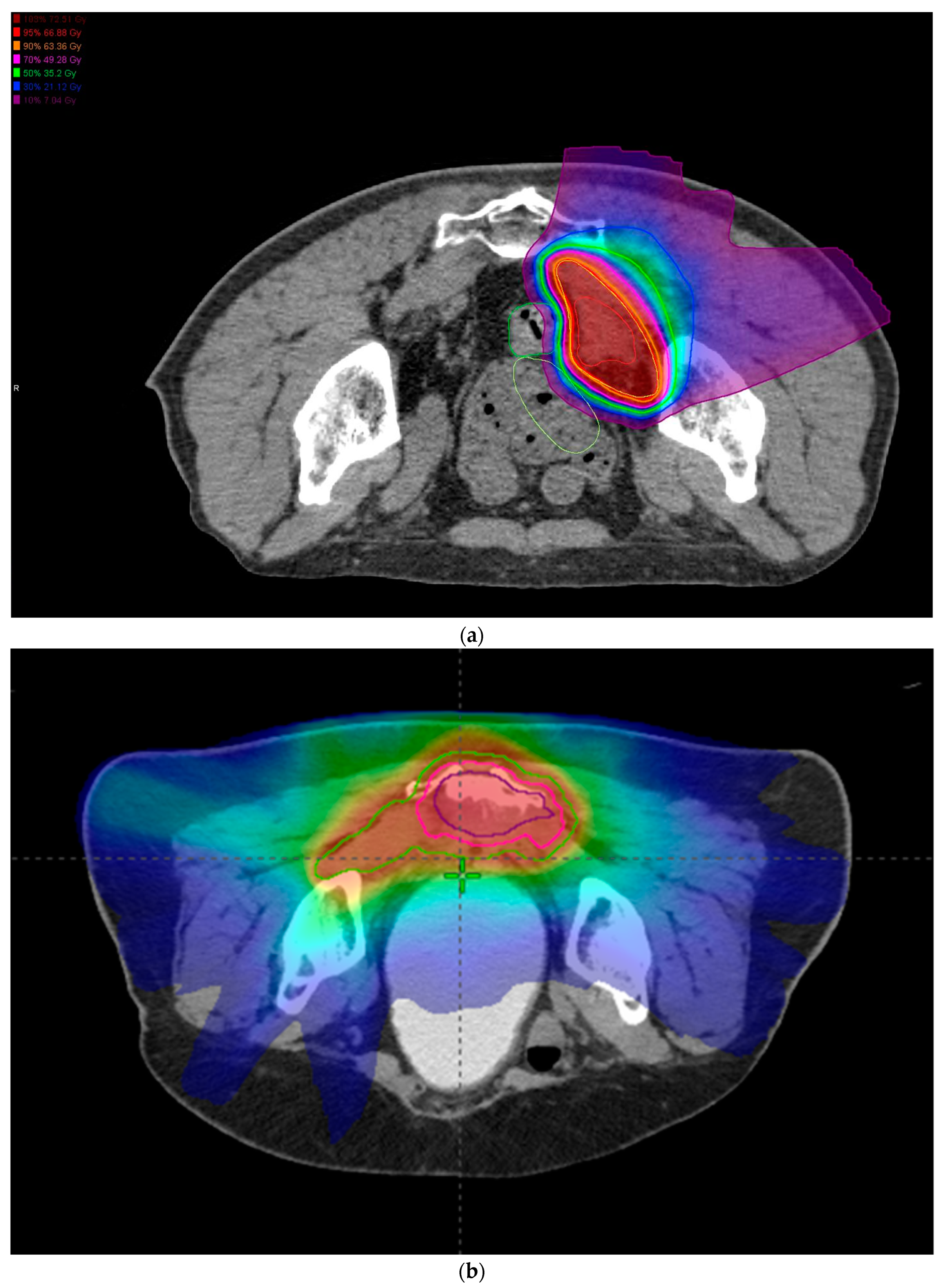

| CIRT (N = 85) | CMT (N = 86) | p-Value | |
|---|---|---|---|
| Sex (n, %) | NS | ||
| Female | 26 (30.6%) | 31 (36.0%) | |
| Male | 59 (69.4%) | 55 (64.0%) | |
| Age At Recurrence | NS | ||
| Median | 63.0 | 55.1 | |
| Q1, Q3 | 54.0, 68.0 | 48.6, 66.4 | |
| Lymph Node Status (n, %) | NS | ||
| Missing | 2 | 0 | |
| (−) | 54 (65.1%) | 70 (81.4%) | |
| (+) | 29 (34.9%) | 16 (18.6%) | |
| Concurrent Chemotherapy (n, %) | p < 0.01 | ||
| No | 85 (100.0%) | 4 (4.7%) | |
| Yes | 0 (0%) | 82 (95.3%) | |
| Year of RT Delivery, (n, %) | NS | ||
| 2006 | 3 (3.5%) | 6 (7.0%) | |
| 2007 | 2 (2.4%) | 6 (7.0%) | |
| 2008 | 6 (7.1%) | 6 (7.0%) | |
| 2009 | 5 (5.9%) | 6 (7.0%) | |
| 2010 | 4 (4.7%) | 16 (18.6%) | |
| 2011 | 9 (10.6%) | 9 (10.5%) | |
| 2012 | 7 (8.2%) | 6 (7.0%) | |
| 2013 | 7 (8.2%) | 13 (15.1%) | |
| 2014 | 6 (7.1%) | 8 (9.3%) | |
| 2015 | 7 (8.2%) | 6 (7.0%) | |
| 2016 | 9 (10.6%) | 1 (1.2%) | |
| 2017 | 4 (4.7%) | 3 (3.5%) | |
| 2018 | 8 (9.4%) | 0 (0.0%) | |
| 2019 | 8 (9.4%) | 0 (0.0%) | |
| Recurrence Size (cm) | NS | ||
| Median | 2.9 | 5.0 | |
| Q1, Q3 | 2.0, 4.5 | 3.5, 6.5 | |
| Chemotherapy Regimens (CMT Only) | NS | ||
| 5FU | 38 | ||
| Capecitabine | 33 | ||
| Capecitabine, Irinotecan, FOLFOX | 2 | ||
| Oxaliplatin, 5FU | 1 | ||
| Irinotecan, Oxaliplatin, Capecitabine | 1 | ||
| Capecitabine, Camptosar | 1 | ||
| 5FU, FOLFOX | 1 | ||
| Capecitabine, FOLFOX | 1 | ||
| Leucovorin, 5FU | 1 | ||
| None | 7 | ||
| Acute Toxicity Odds Ratio (95% Confidence Interval) | ||
|---|---|---|
| Technique | CMT vs. CIRT (Ref) | p-Value |
| Grade ≥ 2 GI | 2.23 (0.79–6.24) | 0.13 |
| Grade ≥ 3 GI | 21.81 (1.23–387.55) | 0.04 |
| Grade ≥ 2 GU | 2.74 (0.82–9.10) | 0.10 |
| Grade ≥ 3 GU | 12.76 (1.60–100.41) | 0.02 |
| Grade ≥ 2 Skin | 8.06 (3.59–18.12) | <0.01 |
| Grade ≥ 3 Skin | 3.07 (1.37–6.89) | <0.01 |
| Grade ≥ 2 Nerve | 0.79 (0.31–2.01) | 0.62 |
| Grade ≥ 3 Nerve | 3.00 (0.12–76.12) | 0.51 |
| Late Toxicity Cumulative Incidence (%) (95% Confidence Interval) | ||||
|---|---|---|---|---|
| Technique | Grade ≥ 2 GI | Grade ≥ 3 GI | Grade ≥ 2 GU | Grade ≥ 3 GU |
| CMT 1-year 2-year 3-year | 11.9 (6.2–22.9) 13.5 (7.4–24.9) 15.2 (8.6–26.9) | 8.9 (4.1–19.0) 8.9 (4.1–19.0) 10.5 (5.2–21.3) | 10.3 (5.1–20.7) 17.0 (9.9–29.1) 18.7 (11.2–31.2) | 8.8 (4.1–18.9) 13.8 (7.5–25.4) 15.5 (8.8–27.5) |
| CIRT 1-year 2-year 3-year | 10.7 (5.7–19.8) 13.5 (7.8–23.4) 16.7 (10.1–27.7) | 9.5 (4.9–18.4) 10.9 (5.9–20.2) 14.1 (8.1–24.6) | 1.2 (0.2–9.7) 4.1 (1.4–12.5) 4.1 (1.4–12.5) | - - - |
| Hazard Ratio (95% CI) CIRT (ref) p-value | 0.85 (0.38–1.89) 0.68 | 0.82 (0.32–2.11) 0.68 | 4.78 (1.56–14.68) <0.01 | 33.04 (1.71–638.40) 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeans, E.B.; Ebner, D.K.; Takiyama, H.; Qualls, K.; Cunningham, D.A.; Waddle, M.R.; Jethwa, K.R.; Harmsen, W.S.; Hubbard, J.M.; Dozois, E.J.; et al. Comparing Oncologic Outcomes and Toxicity for Combined Modality Therapy vs. Carbon-Ion Radiotherapy for Previously Irradiated Locally Recurrent Rectal Cancer. Cancers 2023, 15, 3057. https://doi.org/10.3390/cancers15113057
Jeans EB, Ebner DK, Takiyama H, Qualls K, Cunningham DA, Waddle MR, Jethwa KR, Harmsen WS, Hubbard JM, Dozois EJ, et al. Comparing Oncologic Outcomes and Toxicity for Combined Modality Therapy vs. Carbon-Ion Radiotherapy for Previously Irradiated Locally Recurrent Rectal Cancer. Cancers. 2023; 15(11):3057. https://doi.org/10.3390/cancers15113057
Chicago/Turabian StyleJeans, Elizabeth B., Daniel K. Ebner, Hirotoshi Takiyama, Kaitlin Qualls, Danielle A. Cunningham, Mark R. Waddle, Krishan R. Jethwa, William S. Harmsen, Joleen M. Hubbard, Eric J. Dozois, and et al. 2023. "Comparing Oncologic Outcomes and Toxicity for Combined Modality Therapy vs. Carbon-Ion Radiotherapy for Previously Irradiated Locally Recurrent Rectal Cancer" Cancers 15, no. 11: 3057. https://doi.org/10.3390/cancers15113057
APA StyleJeans, E. B., Ebner, D. K., Takiyama, H., Qualls, K., Cunningham, D. A., Waddle, M. R., Jethwa, K. R., Harmsen, W. S., Hubbard, J. M., Dozois, E. J., Mathis, K. L., Tsuji, H., Merrell, K. W., Hallemeier, C. L., Mahajan, A., Yamada, S., Foote, R. L., & Haddock, M. G. (2023). Comparing Oncologic Outcomes and Toxicity for Combined Modality Therapy vs. Carbon-Ion Radiotherapy for Previously Irradiated Locally Recurrent Rectal Cancer. Cancers, 15(11), 3057. https://doi.org/10.3390/cancers15113057








