Cardiovascular Complications of Pan-Cancer Therapies: The Need for Cardio-Oncology
Abstract
Simple Summary
Abstract
1. Introduction
2. Cardiovascular Complications of Different Types of Cancer Therapies
2.1. Conventional Chemotherapy
2.1.1. Mechanisms of Anthracycline-Induced Cardiotoxicity
2.1.2. Cardiotoxicity of Anthracyclines
2.1.3. Management and Follow-Up for Cardiotoxicity Induced by Anthracyclines
2.2. Radiation Therapy
2.2.1. Mechanism of Radiation Therapy-Induced Cardiotoxicity
2.2.2. Cardiotoxicity of Radiotherapy in Breast Cancer
2.2.3. Cardiotoxicity of Radiotherapy in Hodgkin’s Lymphoma
2.2.4. Cardiotoxicity of Radiotherapy in Non-Small-Cell Lung Cancer
2.2.5. Management and Follow-Up for Cardiotoxicity Induced by Radiotherapy
2.3. Targeted Therapy
2.3.1. Mechanism of Trastuzumab-Induced Cardiotoxicity
2.3.2. Cardiotoxicity of Trastuzumab
2.3.3. Cardiotoxicity of Other HER2-Targeted Drugs
2.3.4. Management and Follow-Up for Cardiotoxicity Induced by Targeted Therapies
3. Effects of Antiangiogenic Anticancer Drugs on the Cardiovascular System
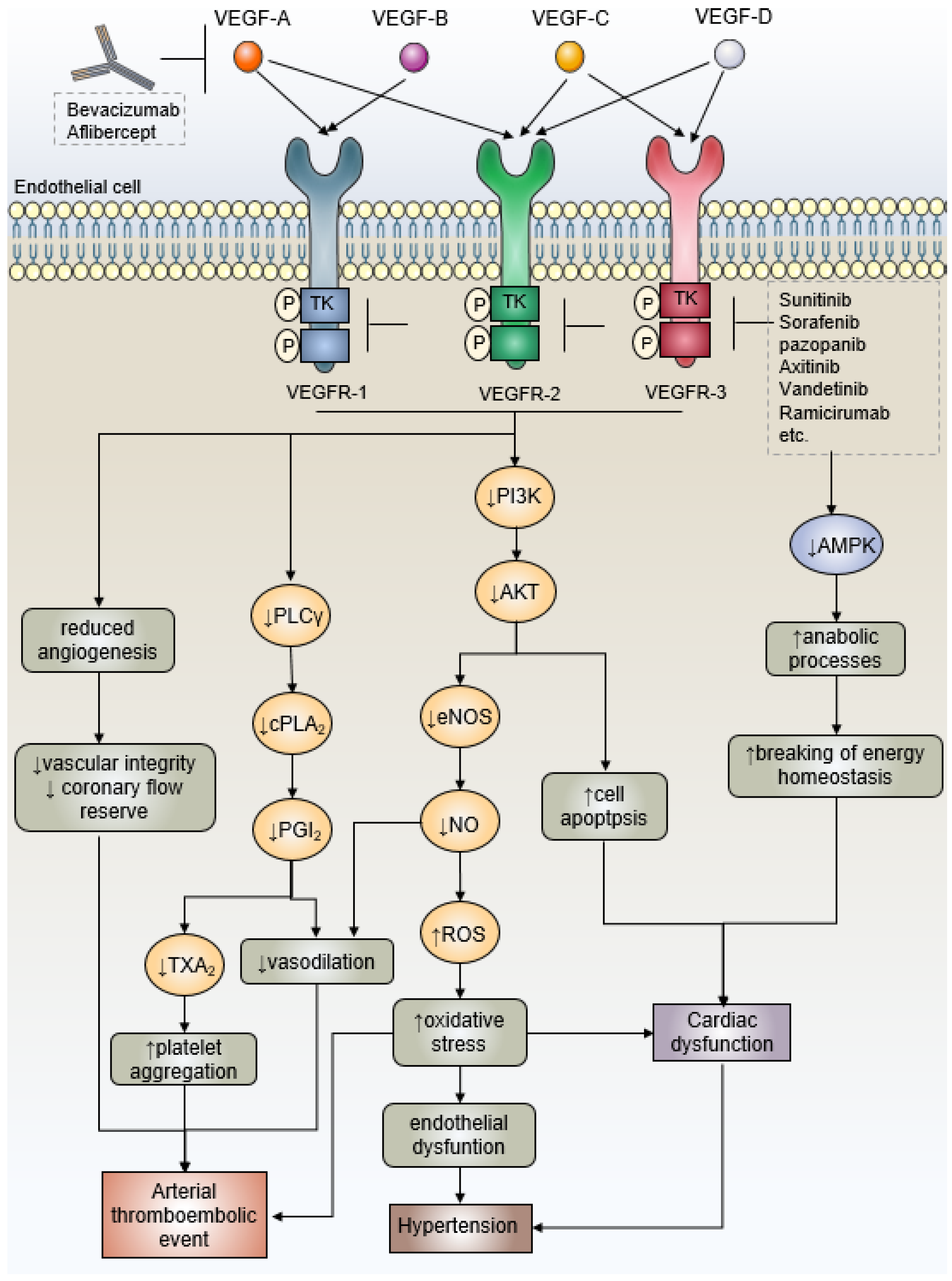
3.1. Mechanism of Antiangiogenic Drugs-Related Cardiac Side Effects—VEGF Signaling
3.2. Antiangiogenic Anticancer Drugs-Induced Hypertension
3.3. Antiangiogenic Anticancer Drugs-Induced Ventricular Dysfunction and Heart Failure
3.4. Antiangiogenic Anticancer Drugs-Induced Arterial Thromboembolic Events
4. Immune Checkpoint Inhibitors-Related Cardiovascular Diseases: Mechanisms, Identification and Management
4.1. ICI-Associated Myocarditis
4.1.1. Epidemiology of ICI-Associated Myocarditis
4.1.2. Immune Mechanisms Underlying Myocarditis
4.1.3. Management and Follow-Up in the Treatment of Cancer and Myocarditis
4.2. ICI-Associated Atherosclerosis
4.2.1. Epidemiology of ICI-Associated Atherosclerosis
4.2.2. Immune Mechanisms Underlying Atherosclerosis
4.2.3. Management and Follow-Up in the Treatment of Cancer and Atherosclerosis
4.3. Other Immuno-Oncology Agents
4.3.1. Mechanisms of Cardiotoxicity Induced by CAR-T Cell Therapy
4.3.2. Cardiotoxicity and Management of CAR-T Cell Therapy
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dobosz, P.; Dzieciatkowski, T. The Intriguing History of Cancer Immunotherapy. Front. Immunol. 2019, 10, 2965. [Google Scholar] [CrossRef]
- Bluethmann, S.M.; Mariotto, A.B.; Rowland, J.H. Anticipating the “Silver Tsunami”: Prevalence Trajectories and Comorbidity Burden among Older Cancer Survivors in the United States. Cancer Epidemiol. Biomark. Prev. 2016, 25, 1029–1036. [Google Scholar] [CrossRef] [PubMed]
- Farmakis, D.; Stafylas, P.; Giamouzis, G.; Maniadakis, N.; Parissis, J. The medical and socioeconomic burden of heart failure: A comparative delineation with cancer. Int. J. Cardiol. 2016, 203, 279–281. [Google Scholar] [CrossRef] [PubMed]
- Lyon, A.R.; Lopez-Fernandez, T.; Couch, L.S.; Asteggiano, R.; Aznar, M.C.; Bergler-Klein, J.; Boriani, G.; Cardinale, D.; Cordoba, R.; Cosyns, B.; et al. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur. Heart J. 2022, 43, 4229–4361. [Google Scholar] [CrossRef]
- Ewer, M.S.; Vooletich, M.T.; Durand, J.B.; Woods, M.L.; Davis, J.R.; Valero, V.; Lenihan, D.J. Reversibility of trastuzumab-related cardiotoxicity: New insights based on clinical course and response to medical treatment. J. Clin. Oncol. 2005, 23, 7820–7826. [Google Scholar] [CrossRef]
- Herrmann, J. Adverse cardiac effects of cancer therapies: Cardiotoxicity and arrhythmia. Nat. Rev. Cardiol. 2020, 17, 474–502. [Google Scholar] [CrossRef]
- Bhagat, A.; Kleinerman, E.S. Anthracycline-Induced Cardiotoxicity: Causes, Mechanisms, and Prevention. Adv. Exp. Med. Biol. 2020, 1257, 181–192. [Google Scholar] [CrossRef] [PubMed]
- McGowan, J.V.; Chung, R.; Maulik, A.; Piotrowska, I.; Walker, J.M.; Yellon, D.M. Anthracycline Chemotherapy and Cardiotoxicity. Cardiovasc. Drugs Ther. 2017, 31, 63–75. [Google Scholar] [CrossRef]
- Jirkovsky, E.; Jirkovska, A.; Bavlovic-Piskackova, H.; Skalicka, V.; Pokorna, Z.; Karabanovich, G.; Kollarova-Brazdova, P.; Kubes, J.; Lencova-Popelova, O.; Mazurova, Y.; et al. Clinically Translatable Prevention of Anthracycline Cardiotoxicity by Dexrazoxane Is Mediated by Topoisomerase II Beta and Not Metal Chelation. Circ. Heart Fail. 2021, 14, e008209. [Google Scholar] [CrossRef]
- Rajagopalan, S.; Politi, P.M.; Sinha, B.K.; Myers, C.E. Adriamycin-induced free radical formation in the perfused rat heart: Implications for cardiotoxicity. Cancer Res. 1988, 48, 4766–4769. [Google Scholar]
- Keizer, H.G.; Pinedo, H.M.; Schuurhuis, G.J.; Joenje, H. Doxorubicin (adriamycin): A critical review of free radical-dependent mechanisms of cytotoxicity. Pharmacol. Ther. 1990, 47, 219–231. [Google Scholar] [CrossRef] [PubMed]
- Jain, D.; Ahmad, T.; Cairo, M.; Aronow, W. Cardiotoxicity of cancer chemotherapy: Identification, prevention and treatment. Ann. Transl. Med. 2017, 5, 348. [Google Scholar] [CrossRef]
- Pai, V.B.; Nahata, M.C. Cardiotoxicity of chemotherapeutic agents: Incidence, treatment and prevention. Drug Saf. 2000, 22, 263–302. [Google Scholar] [CrossRef]
- Frishman, W.H.; Sung, H.M.; Yee, H.C.; Liu, L.L.; Keefe, D.; Einzig, A.I.; Dutcher, J. Cardiovascular toxicity with cancer chemotherapy. Curr. Probl. Cancer 1997, 21, 301–360. [Google Scholar] [CrossRef]
- Hu, G.F.; Fu, H.X.; Ma, J.F.; Hu, M.F.; Zhao, Z.N.; Hu, C.; Yang, J. [Clinical observation on the anthracyclines-induced cardiotoxicity in patients with early-stage breast cancer]. Zhonghua Xin Xue Guan Bing Za Zhi 2018, 46, 987–992. [Google Scholar] [CrossRef]
- Trudeau, M.; Charbonneau, F.; Gelmon, K.; Laing, K.; Latreille, J.; Mackey, J.; McLeod, D.; Pritchard, K.; Provencher, L.; Verma, S. Selection of adjuvant chemotherapy for treatment of node-positive breast cancer. Lancet Oncol. 2005, 6, 886–898. [Google Scholar] [CrossRef] [PubMed]
- Hershman, D.L.; McBride, R.B.; Eisenberger, A.; Tsai, W.Y.; Grann, V.R.; Jacobson, J.S. Doxorubicin, cardiac risk factors, and cardiac toxicity in elderly patients with diffuse B-cell non-Hodgkin’s lymphoma. J. Clin. Oncol. 2008, 26, 3159–3165. [Google Scholar] [CrossRef]
- Sawicki, K.T.; Sala, V.; Prever, L.; Hirsch, E.; Ardehali, H.; Ghigo, A. Preventing and Treating Anthracycline Cardiotoxicity: New Insights. Annu. Rev. Pharm. Toxicol. 2021, 61, 309–332. [Google Scholar] [CrossRef] [PubMed]
- Cardinale, D.; Iacopo, F.; Cipolla, C.M. Cardiotoxicity of Anthracyclines. Front. Cardiovasc. Med. 2020, 7, 26. [Google Scholar] [CrossRef] [PubMed]
- Stewart, F.A.; Heeneman, S.; Te Poele, J.; Kruse, J.; Russell, N.S.; Gijbels, M.; Daemen, M. Ionizing radiation accelerates the development of atherosclerotic lesions in ApoE-/- mice and predisposes to an inflammatory plaque phenotype prone to hemorrhage. Am. J. Pathol. 2006, 168, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Haubner, F.; Ohmann, E.; Pohl, F.; Prantl, L.; Strutz, J.; Gassner, H.G. Effects of radiation on the expression of adhesion molecules and cytokines in a static model of human dermal microvascular endothelial cells. Clin. Hemorheol. Microcirc. 2013, 54, 371–379. [Google Scholar] [CrossRef]
- Boerma, M.; Sridharan, V.; Mao, X.W.; Nelson, G.A.; Cheema, A.K.; Koturbash, I.; Singh, S.P.; Tackett, A.J.; Hauer-Jensen, M. Effects of ionizing radiation on the heart. Mutat. Res. Rev. Mutat. Res. 2016, 770, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Taunk, N.K.; Haffty, B.G.; Kostis, J.B.; Goyal, S. Radiation-induced heart disease: Pathologic abnormalities and putative mechanisms. Front. Oncol. 2015, 5, 39. [Google Scholar] [CrossRef]
- Lewis, G.D.; Farach, A. Cardiovascular Toxicities of Radiation Therapy. Methodist Debakey Cardiovasc. J. 2019, 15, 274–281. [Google Scholar] [CrossRef]
- Darby, S.C.; Ewertz, M.; McGale, P.; Bennet, A.M.; Blom-Goldman, U.; Bronnum, D.; Correa, C.; Cutter, D.; Gagliardi, G.; Gigante, B.; et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N. Engl. J. Med. 2013, 368, 987–998. [Google Scholar] [CrossRef]
- van den Bogaard, V.A.; Ta, B.D.; van der Schaaf, A.; Bouma, A.B.; Middag, A.M.; Bantema-Joppe, E.J.; van Dijk, L.V.; van Dijk-Peters, F.B.; Marteijn, L.A.; de Bock, G.H.; et al. Validation and Modification of a Prediction Model for Acute Cardiac Events in Patients With Breast Cancer Treated With Radiotherapy Based on Three-Dimensional Dose Distributions to Cardiac Substructures. J. Clin. Oncol. 2017, 35, 1171–1178. [Google Scholar] [CrossRef] [PubMed]
- Badiyan, S.N.; Puckett, L.L.; Vlacich, G.; Schiffer, W.; Pedersen, L.N.; Mitchell, J.D.; Bergom, C. Radiation-Induced Cardiovascular Toxicities. Curr. Treat. Options Oncol. 2022, 23, 1388–1404. [Google Scholar] [CrossRef]
- Zureick, A.H.; Grzywacz, V.P.; Almahariq, M.F.; Silverman, B.R.; Vayntraub, A.; Chen, P.Y.; Gustafson, G.S.; Jawad, M.S.; Dilworth, J.T. Dose to the Left Anterior Descending Artery Correlates With Cardiac Events After Irradiation for Breast Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2022, 114, 130–139. [Google Scholar] [CrossRef]
- van Nimwegen, F.A.; Schaapveld, M.; Cutter, D.J.; Janus, C.P.; Krol, A.D.; Hauptmann, M.; Kooijman, K.; Roesink, J.; van der Maazen, R.; Darby, S.C.; et al. Radiation Dose-Response Relationship for Risk of Coronary Heart Disease in Survivors of Hodgkin Lymphoma. J. Clin. Oncol. 2016, 34, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Schellong, G.; Riepenhausen, M.; Bruch, C.; Kotthoff, S.; Vogt, J.; Bolling, T.; Dieckmann, K.; Potter, R.; Heinecke, A.; Bramswig, J.; et al. Late valvular and other cardiac diseases after different doses of mediastinal radiotherapy for Hodgkin disease in children and adolescents: Report from the longitudinal GPOH follow-up project of the German-Austrian DAL-HD studies. Pediatr. Blood Cancer 2010, 55, 1145–1152. [Google Scholar] [CrossRef]
- Dess, R.T.; Sun, Y.; Matuszak, M.M.; Sun, G.; Soni, P.D.; Bazzi, L.; Murthy, V.L.; Hearn, J.W.D.; Kong, F.M.; Kalemkerian, G.P.; et al. Cardiac Events After Radiation Therapy: Combined Analysis of Prospective Multicenter Trials for Locally Advanced Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2017, 35, 1395–1402. [Google Scholar] [CrossRef] [PubMed]
- Atkins, K.M.; Rawal, B.; Chaunzwa, T.L.; Lamba, N.; Bitterman, D.S.; Williams, C.L.; Kozono, D.E.; Baldini, E.H.; Chen, A.B.; Nguyen, P.L.; et al. Cardiac Radiation Dose, Cardiac Disease, and Mortality in Patients With Lung Cancer. J. Am. Coll. Cardiol. 2019, 73, 2976–2987. [Google Scholar] [CrossRef] [PubMed]
- Atkins, K.M.; Chaunzwa, T.L.; Lamba, N.; Bitterman, D.S.; Rawal, B.; Bredfeldt, J.; Williams, C.L.; Kozono, D.E.; Baldini, E.H.; Nohria, A.; et al. Association of Left Anterior Descending Coronary Artery Radiation Dose With Major Adverse Cardiac Events and Mortality in Patients With Non-Small Cell Lung Cancer. JAMA Oncol. 2021, 7, 206–219. [Google Scholar] [CrossRef] [PubMed]
- Banfill, K.; Giuliani, M.; Aznar, M.; Franks, K.; McWilliam, A.; Schmitt, M.; Sun, F.; Vozenin, M.C.; Faivre Finn, C.; IASLC Advanced Radiation Technology committee. Cardiac Toxicity of Thoracic Radiotherapy: Existing Evidence and Future Directions. J. Thorac. Oncol. 2021, 16, 216–227. [Google Scholar] [CrossRef] [PubMed]
- Lestuzzi, C.; Mascarin, M.; Coassin, E.; Canale, M.L.; Turazza, F. Cardiologic Long-Term Follow-Up of Patients Treated With Chest Radiotherapy: When and How? Front. Cardiovasc. Med. 2021, 8, 671001. [Google Scholar] [CrossRef]
- Nicolazzi, M.A.; Carnicelli, A.; Fuorlo, M.; Scaldaferri, A.; Masetti, R.; Landolfi, R.; Favuzzi, A.M.R. Anthracycline and trastuzumab-induced cardiotoxicity in breast cancer. Eur. Rev. Med. Pharm. Sci. 2018, 22, 2175–2185. [Google Scholar] [CrossRef]
- Barish, R.; Gates, E.; Barac, A. Trastuzumab-Induced Cardiomyopathy. Cardiol. Clin. 2019, 37, 407–418. [Google Scholar] [CrossRef]
- Tocchetti, C.G.; Ragone, G.; Coppola, C.; Rea, D.; Piscopo, G.; Scala, S.; De Lorenzo, C.; Iaffaioli, R.V.; Arra, C.; Maurea, N. Detection, monitoring, and management of trastuzumab-induced left ventricular dysfunction: An actual challenge. Eur. J. Heart Fail. 2012, 14, 130–137. [Google Scholar] [CrossRef]
- Riccio, G.; Coppola, C.; Piscopo, G.; Capasso, I.; Maurea, C.; Esposito, E.; De Lorenzo, C.; Maurea, N. Trastuzumab and target-therapy side effects: Is still valid to differentiate anthracycline Type I from Type II cardiomyopathies? Hum. Vaccin Immunother. 2016, 12, 1124–1131. [Google Scholar] [CrossRef] [PubMed]
- Maurea, N.; Coppola, C.; Ragone, G.; Frasci, G.; Bonelli, A.; Romano, C.; Iaffaioli, R.V. Women survive breast cancer but fall victim to heart failure: The shadows and lights of targeted therapy. J. Cardiovasc. Med. 2010, 11, 861–868. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Long, J.B.; Hurria, A.; Owusu, C.; Steingart, R.M.; Gross, C.P. Incidence of heart failure or cardiomyopathy after adjuvant trastuzumab therapy for breast cancer. J. Am. Coll. Cardiol. 2012, 60, 2504–2512. [Google Scholar] [CrossRef] [PubMed]
- Bowles, E.J.; Wellman, R.; Feigelson, H.S.; Onitilo, A.A.; Freedman, A.N.; Delate, T.; Allen, L.A.; Nekhlyudov, L.; Goddard, K.A.; Davis, R.L.; et al. Risk of heart failure in breast cancer patients after anthracycline and trastuzumab treatment: A retrospective cohort study. J. Natl. Cancer Inst. 2012, 104, 1293–1305. [Google Scholar] [CrossRef] [PubMed]
- De Keulenaer, G.W.; Doggen, K.; Lemmens, K. The vulnerability of the heart as a pluricellular paracrine organ: Lessons from unexpected triggers of heart failure in targeted ErbB2 anticancer therapy. Circ. Res. 2010, 106, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Dempsey, N.; Rosenthal, A.; Dabas, N.; Kropotova, Y.; Lippman, M.; Bishopric, N.H. Trastuzumab-induced cardiotoxicity: A review of clinical risk factors, pharmacologic prevention, and cardiotoxicity of other HER2-directed therapies. Breast Cancer Res. Treat. 2021, 188, 21–36. [Google Scholar] [CrossRef]
- Alhussein, M.M.; Mokbel, A.; Cosman, T.; Aghel, N.; Yang, E.H.; Mukherjee, S.D.; Dent, S.; Ellis, P.M.; Dhesy-Thind, S.; Leong, D.P. Pertuzumab Cardiotoxicity in Patients With HER2-Positive Cancer: A Systematic Review and Meta-analysis. CJC Open 2021, 3, 1372–1382. [Google Scholar] [CrossRef]
- Sendur, M.A.; Aksoy, S.; Altundag, K. Cardiotoxicity of novel HER2-targeted therapies. Curr. Med. Res. Opin. 2013, 29, 1015–1024. [Google Scholar] [CrossRef]
- Jerusalem, G.; Lancellotti, P.; Kim, S.B. HER2+ breast cancer treatment and cardiotoxicity: Monitoring and management. Breast Cancer Res. Treat. 2019, 177, 237–250. [Google Scholar] [CrossRef]
- Banke, A.; Fosbol, E.L.; Ewertz, M.; Videbaek, L.; Dahl, J.S.; Poulsen, M.K.; Cold, S.; Jensen, M.B.; Gislason, G.H.; Schou, M.; et al. Long-Term Risk of Heart Failure in Breast Cancer Patients After Adjuvant Chemotherapy With or Without Trastuzumab. JACC Heart Fail. 2019, 7, 217–224. [Google Scholar] [CrossRef]
- Khoury, K.; Lynce, F.; Barac, A.; Geng, X.; Dang, C.; Yu, A.F.; Smith, K.L.; Gallagher, C.; Pohlmann, P.R.; Nunes, R.; et al. Long-term follow-up assessment of cardiac safety in SAFE-HEaRt, a clinical trial evaluating the use of HER2-targeted therapies in patients with breast cancer and compromised heart function. Breast Cancer Res. Treat. 2021, 185, 863–868. [Google Scholar] [CrossRef]
- Potente, M.; Gerhardt, H.; Carmeliet, P. Basic and therapeutic aspects of angiogenesis. Cell 2011, 146, 873–887. [Google Scholar] [CrossRef]
- Folkman, J. Tumor angiogenesis: Therapeutic implications. N. Engl. J. Med. 1971, 285, 1182–1186. [Google Scholar] [CrossRef]
- Wu, Q.; Bai, B.; Tian, C.; Li, D.; Yu, H.; Song, B.; Li, B.; Chu, X. The Molecular Mechanisms of Cardiotoxicity Induced by HER2, VEGF, and Tyrosine Kinase Inhibitors: An Updated Review. Cardiovasc. Drugs Ther. 2022, 36, 511–524. [Google Scholar] [CrossRef] [PubMed]
- Maurea, N.; Coppola, C.; Piscopo, G.; Galletta, F.; Riccio, G.; Esposito, E.; De Lorenzo, C.; De Laurentiis, M.; Spallarossa, P.; Mercuro, G. Pathophysiology of cardiotoxicity from target therapy and angiogenesis inhibitors. J. Cardiovasc. Med. 2016, 17, S19–S26. [Google Scholar] [CrossRef]
- Bejarano, L.; Jordao, M.J.C.; Joyce, J.A. Therapeutic Targeting of the Tumor Microenvironment. Cancer Discov. 2021, 11, 933–959. [Google Scholar] [CrossRef]
- Albini, A.; Pennesi, G.; Donatelli, F.; Cammarota, R.; De Flora, S.; Noonan, D.M. Cardiotoxicity of anticancer drugs: The need for cardio-oncology and cardio-oncological prevention. J. Natl. Cancer Inst. 2010, 102, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Tocchetti, C.G.; Gallucci, G.; Coppola, C.; Piscopo, G.; Cipresso, C.; Maurea, C.; Giudice, A.; Iaffaioli, R.V.; Arra, C.; Maurea, N. The emerging issue of cardiac dysfunction induced by antineoplastic angiogenesis inhibitors. Eur. J. Heart Fail. 2013, 15, 482–489. [Google Scholar] [CrossRef] [PubMed]
- Di Lisi, D.; Madonna, R.; Zito, C.; Bronte, E.; Badalamenti, G.; Parrella, P.; Monte, I.; Tocchetti, C.G.; Russo, A.; Novo, G. Anticancer therapy-induced vascular toxicity: VEGF inhibition and beyond. Int. J. Cardiol. 2017, 227, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Qadir, H.; Ethier, J.L.; Lee, D.S.; Thavendiranathan, P.; Amir, E. Cardiovascular toxicity of angiogenesis inhibitors in treatment of malignancy: A systematic review and meta-analysis. Cancer Treat. Rev. 2017, 53, 120–127. [Google Scholar] [CrossRef]
- Morelli, M.B.; Bongiovanni, C.; Da Pra, S.; Miano, C.; Sacchi, F.; Lauriola, M.; D’Uva, G. Cardiotoxicity of Anticancer Drugs: Molecular Mechanisms and Strategies for Cardioprotection. Front. Cardiovasc. Med. 2022, 9, 847012. [Google Scholar] [CrossRef]
- Kikuchi, S.; Yoshioka, Y.; Prieto-Vila, M.; Ochiya, T. Involvement of Extracellular Vesicles in Vascular-Related Functions in Cancer Progression and Metastasis. Int. J. Mol. Sci. 2019, 20, 2584. [Google Scholar] [CrossRef]
- Ciuffetti, G.; Schillaci, G.; Innocente, S.; Lombardini, R.; Pasqualini, L.; Notaristefano, S.; Mannarino, E. Capillary rarefaction and abnormal cardiovascular reactivity in hypertension. J. Hypertens. 2003, 21, 2297–2303. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.K.; Singhi, E.K.; Arroyo, J.P.; Ikizler, T.A.; Gould, E.R.; Brown, J.; Beckman, J.A.; Harrison, D.G.; Moslehi, J. Mechanisms of VEGF (Vascular Endothelial Growth Factor) Inhibitor-Associated Hypertension and Vascular Disease. Hypertension 2018, 71, e1–e8. [Google Scholar] [CrossRef] [PubMed]
- Camarda, N.; Travers, R.; Yang, V.K.; London, C.; Jaffe, I.Z. VEGF Receptor Inhibitor-Induced Hypertension: Emerging Mechanisms and Clinical Implications. Curr. Oncol. Rep. 2022, 24, 463–474. [Google Scholar] [CrossRef] [PubMed]
- Klahr, S. The role of nitric oxide in hypertension and renal disease progression. Nephrol. Dial. Transpl. 2001, 16 (Suppl. 1), 60–62. [Google Scholar] [CrossRef]
- Hurwitz, H.; Fehrenbacher, L.; Novotny, W.; Cartwright, T.; Hainsworth, J.; Heim, W.; Berlin, J.; Baron, A.; Griffing, S.; Holmgren, E.; et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N. Engl. J. Med. 2004, 350, 2335–2342. [Google Scholar] [CrossRef]
- Fuchs, C.S.; Tomasek, J.; Yong, C.J.; Dumitru, F.; Passalacqua, R.; Goswami, C.; Safran, H.; Dos Santos, L.V.; Aprile, G.; Ferry, D.R.; et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): An international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 2014, 383, 31–39. [Google Scholar] [CrossRef]
- Crino, L.; Dansin, E.; Garrido, P.; Griesinger, F.; Laskin, J.; Pavlakis, N.; Stroiakovski, D.; Thatcher, N.; Tsai, C.M.; Wu, Y.L.; et al. Safety and efficacy of first-line bevacizumab-based therapy in advanced non-squamous non-small-cell lung cancer (SAiL, MO19390): A phase 4 study. Lancet Oncol. 2010, 11, 733–740. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Tabernero, J.; Lakomy, R.; Prenen, H.; Prausova, J.; Macarulla, T.; Ruff, P.; van Hazel, G.A.; Moiseyenko, V.; Ferry, D.; et al. Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin-based regimen. J. Clin. Oncol. 2012, 30, 3499–3506. [Google Scholar] [CrossRef]
- Chen, H.X.; Cleck, J.N. Adverse effects of anticancer agents that target the VEGF pathway. Nat. Rev. Clin. Oncol. 2009, 6, 465–477. [Google Scholar] [CrossRef]
- Yang, J.C.; Haworth, L.; Sherry, R.M.; Hwu, P.; Schwartzentruber, D.J.; Topalian, S.L.; Steinberg, S.M.; Chen, H.X.; Rosenberg, S.A. A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N. Engl. J. Med. 2003, 349, 427–434. [Google Scholar] [CrossRef]
- Girardi, F.; Franceschi, E.; Brandes, A.A. Cardiovascular safety of VEGF-targeting therapies: Current evidence and handling strategies. Oncologist 2010, 15, 683–694. [Google Scholar] [CrossRef]
- Cohen, J.B.; Brown, N.J.; Brown, S.A.; Dent, S.; van Dorst, D.C.H.; Herrmann, S.M.; Lang, N.N.; Oudit, G.Y.; Touyz, R.M.; American Heart Association Council on Hypertension; et al. Cancer Therapy-Related Hypertension: A Scientific Statement From the American Heart Association. Hypertension 2023, 80, e46–e57. [Google Scholar] [CrossRef]
- de Jesus-Gonzalez, N.; Robinson, E.; Moslehi, J.; Humphreys, B.D. Management of antiangiogenic therapy-induced hypertension. Hypertension 2012, 60, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Xue, L.; Du, S.; Sun, M.; Hu, J.; Hao, L.; Gong, L.; Yeh, D.; Xiong, H.; Shao, S. Caveolin-1 knockdown is associated with the metastasis and proliferation of human lung cancer cell line NCI-H460. Biomed. Pharm. 2012, 66, 439–447. [Google Scholar] [CrossRef]
- Cheng, H.; Force, T. Molecular mechanisms of cardiovascular toxicity of targeted cancer therapeutics. Circ. Res. 2010, 106, 21–34. [Google Scholar] [CrossRef]
- Khakoo, A.Y.; Kassiotis, C.M.; Tannir, N.; Plana, J.C.; Halushka, M.; Bickford, C.; Trent, J., 2nd; Champion, J.C.; Durand, J.B.; Lenihan, D.J. Heart failure associated with sunitinib malate: A multitargeted receptor tyrosine kinase inhibitor. Cancer 2008, 112, 2500–2508. [Google Scholar] [CrossRef] [PubMed]
- Telli, M.L.; Witteles, R.M.; Fisher, G.A.; Srinivas, S. Cardiotoxicity associated with the cancer therapeutic agent sunitinib malate. Ann. Oncol. 2008, 19, 1613–1618. [Google Scholar] [CrossRef] [PubMed]
- Katoh, M. Therapeutics targeting angiogenesis: Genetics and epigenetics, extracellular miRNAs and signaling networks (Review). Int. J. Mol. Med. 2013, 32, 763–767. [Google Scholar] [CrossRef]
- Gao, X.; Wang, Y.C.; Liu, Y.; Yue, Q.; Liu, Z.; Ke, M.; Zhao, S.; Li, C. Nanoagonist-mediated endothelial tight junction opening: A strategy for safely increasing brain drug delivery in mice. J. Cereb. Blood Flow Metab. 2017, 37, 1410–1424. [Google Scholar] [CrossRef]
- Chu, T.F.; Rupnick, M.A.; Kerkela, R.; Dallabrida, S.M.; Zurakowski, D.; Nguyen, L.; Woulfe, K.; Pravda, E.; Cassiola, F.; Desai, J.; et al. Cardiotoxicity associated with tyrosine kinase inhibitor sunitinib. Lancet 2007, 370, 2011–2019. [Google Scholar] [CrossRef]
- Verma, N.; Swain, S.M. Bevacizumab and heart failure risk in patients with breast cancer: A thorn in the side? J. Clin. Oncol. 2011, 29, 603–606. [Google Scholar] [CrossRef]
- Motzer, R.J.; Hutson, T.E.; Tomczak, P.; Michaelson, M.D.; Bukowski, R.M.; Oudard, S.; Negrier, S.; Szczylik, C.; Pili, R.; Bjarnason, G.A.; et al. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J. Clin. Oncol. 2009, 27, 3584–3590. [Google Scholar] [CrossRef]
- Di Lorenzo, G.; Autorino, R.; Bruni, G.; Carteni, G.; Ricevuto, E.; Tudini, M.; Ficorella, C.; Romano, C.; Aieta, M.; Giordano, A.; et al. Cardiovascular toxicity following sunitinib therapy in metastatic renal cell carcinoma: A multicenter analysis. Ann. Oncol. 2009, 20, 1535–1542. [Google Scholar] [CrossRef] [PubMed]
- Richards, C.J.; Je, Y.; Schutz, F.A.; Heng, D.Y.; Dallabrida, S.M.; Moslehi, J.J.; Choueiri, T.K. Incidence and risk of congestive heart failure in patients with renal and nonrenal cell carcinoma treated with sunitinib. J. Clin. Oncol. 2011, 29, 3450–3456. [Google Scholar] [CrossRef]
- Glen, C.; Tan, Y.Y.; Waterston, A.; Evans, T.R.J.; Jones, R.J.; Petrie, M.C.; Lang, N.N. Mechanistic and Clinical Overview Cardiovascular Toxicity of BRAF and MEK Inhibitors: JACC: CardioOncology State-of-the-Art Review. JACC CardioOncol. 2022, 4, 1–18. [Google Scholar] [CrossRef]
- Loren, C.P.; Aslan, J.E.; Rigg, R.A.; Nowak, M.S.; Healy, L.D.; Gruber, A.; Druker, B.J.; McCarty, O.J. The BCR-ABL inhibitor ponatinib inhibits platelet immunoreceptor tyrosine-based activation motif (ITAM) signaling, platelet activation and aggregate formation under shear. Thromb. Res. 2015, 135, 155–160. [Google Scholar] [CrossRef]
- Gopal, S.; Miller, K.B.; Jaffe, I.Z. Molecular mechanisms for vascular complications of targeted cancer therapies. Clin. Sci. 2016, 130, 1763–1779. [Google Scholar] [CrossRef]
- Scappaticci, F.A.; Skillings, J.R.; Holden, S.N.; Gerber, H.P.; Miller, K.; Kabbinavar, F.; Bergsland, E.; Ngai, J.; Holmgren, E.; Wang, J.; et al. Arterial thromboembolic events in patients with metastatic carcinoma treated with chemotherapy and bevacizumab. J. Natl. Cancer Inst. 2007, 99, 1232–1239. [Google Scholar] [CrossRef] [PubMed]
- Qi, W.X.; Shen, Z.; Tang, L.N.; Yao, Y. Risk of arterial thromboembolic events with vascular endothelial growth factor receptor tyrosine kinase inhibitors: An up-to-date meta-analysis. Crit. Rev. Oncol. Hematol. 2014, 92, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Zaha, V.G.; Meijers, W.C.; Moslehi, J. Cardio-Immuno-Oncology. Circulation 2020, 141, 87–89. [Google Scholar] [CrossRef] [PubMed]
- Dall’Olio, F.G.; Marabelle, A.; Caramella, C.; Garcia, C.; Aldea, M.; Chaput, N.; Robert, C.; Besse, B. Tumour burden and efficacy of immune-checkpoint inhibitors. Nat. Rev. Clin. Oncol. 2022, 19, 75–90. [Google Scholar] [CrossRef] [PubMed]
- Postow, M.A.; Sidlow, R.; Hellmann, M.D. Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. N. Engl. J. Med. 2018, 378, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.W.; Razak, A.R.; Bedard, P.L.; Siu, L.L.; Hansen, A.R. A systematic review of immune-related adverse event reporting in clinical trials of immune checkpoint inhibitors. Ann. Oncol. 2015, 26, 1824–1829. [Google Scholar] [CrossRef]
- Schneider, B.J.; Naidoo, J.; Santomasso, B.D.; Lacchetti, C.; Adkins, S.; Anadkat, M.; Atkins, M.B.; Brassil, K.J.; Caterino, J.M.; Chau, I.; et al. Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: ASCO Guideline Update. J. Clin. Oncol. 2021, 39, 4073–4126. [Google Scholar] [CrossRef]
- Salem, J.E.; Manouchehri, A.; Moey, M.; Lebrun-Vignes, B.; Bastarache, L.; Pariente, A.; Gobert, A.; Spano, J.P.; Balko, J.M.; Bonaca, M.P.; et al. Cardiovascular toxicities associated with immune checkpoint inhibitors: An observational, retrospective, pharmacovigilance study. Lancet Oncol. 2018, 19, 1579–1589. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.B.; Balko, J.M.; Compton, M.L.; Chalkias, S.; Gorham, J.; Xu, Y.; Hicks, M.; Puzanov, I.; Alexander, M.R.; Bloomer, T.L.; et al. Fulminant Myocarditis with Combination Immune Checkpoint Blockade. N. Engl. J. Med. 2016, 375, 1749–1755. [Google Scholar] [CrossRef] [PubMed]
- Moslehi, J.J.; Salem, J.E.; Sosman, J.A.; Lebrun-Vignes, B.; Johnson, D.B. Increased reporting of fatal immune checkpoint inhibitor-associated myocarditis. Lancet 2018, 391, 933. [Google Scholar] [CrossRef]
- Moslehi, J.; Lichtman, A.H.; Sharpe, A.H.; Galluzzi, L.; Kitsis, R.N. Immune checkpoint inhibitor-associated myocarditis: Manifestations and mechanisms. J. Clin. Invest. 2021, 131, e145186. [Google Scholar] [CrossRef]
- Norwood, T.G.; Westbrook, B.C.; Johnson, D.B.; Litovsky, S.H.; Terry, N.L.; McKee, S.B.; Gertler, A.S.; Moslehi, J.J.; Conry, R.M. Smoldering myocarditis following immune checkpoint blockade. J. Immunother. Cancer 2017, 5, 91. [Google Scholar] [CrossRef]
- Tivol, E.A.; Borriello, F.; Schweitzer, A.N.; Lynch, W.P.; Bluestone, J.A.; Sharpe, A.H. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity 1995, 3, 541–547. [Google Scholar] [CrossRef]
- Tarrio, M.L.; Grabie, N.; Bu, D.X.; Sharpe, A.H.; Lichtman, A.H. PD-1 protects against inflammation and myocyte damage in T cell-mediated myocarditis. J. Immunol. 2012, 188, 4876–4884. [Google Scholar] [CrossRef] [PubMed]
- Gil-Cruz, C.; Perez-Shibayama, C.; De Martin, A.; Ronchi, F.; van der Borght, K.; Niederer, R.; Onder, L.; Lutge, M.; Novkovic, M.; Nindl, V.; et al. Microbiota-derived peptide mimics drive lethal inflammatory cardiomyopathy. Science 2019, 366, 881–886. [Google Scholar] [CrossRef] [PubMed]
- Myers, J.M.; Cooper, L.T.; Kem, D.C.; Stavrakis, S.; Kosanke, S.D.; Shevach, E.M.; Fairweather, D.; Stoner, J.A.; Cox, C.J.; Cunningham, M.W. Cardiac myosin-Th17 responses promote heart failure in human myocarditis. JCI Insight 2016, 1, e85851. [Google Scholar] [CrossRef]
- Moslehi, J.J. Cardiovascular Toxic Effects of Targeted Cancer Therapies. N. Engl. J. Med. 2016, 375, 1457–1467. [Google Scholar] [CrossRef]
- Mahmood, S.S.; Fradley, M.G.; Cohen, J.V.; Nohria, A.; Reynolds, K.L.; Heinzerling, L.M.; Sullivan, R.J.; Damrongwatanasuk, R.; Chen, C.L.; Gupta, D.; et al. Myocarditis in Patients Treated With Immune Checkpoint Inhibitors. J. Am. Coll. Cardiol. 2018, 71, 1755–1764. [Google Scholar] [CrossRef] [PubMed]
- Gilon, D.; Iakobishvili, Z.; Leibowitz, D. The Diagnosis and Management of Immune Checkpoint Inhibitor Cardiovascular Toxicity: Myocarditis and Beyond. Vaccines 2022, 10, 304. [Google Scholar] [CrossRef] [PubMed]
- Ganatra, S.; Neilan, T.G. Immune Checkpoint Inhibitor-Associated Myocarditis. Oncologist 2018, 23, 879–886. [Google Scholar] [CrossRef]
- Drobni, Z.D.; Alvi, R.M.; Taron, J.; Zafar, A.; Murphy, S.P.; Rambarat, P.K.; Mosarla, R.C.; Lee, C.; Zlotoff, D.A.; Raghu, V.K.; et al. Association Between Immune Checkpoint Inhibitors With Cardiovascular Events and Atherosclerotic Plaque. Circulation 2020, 142, 2299–2311. [Google Scholar] [CrossRef]
- Bar, J.; Markel, G.; Gottfried, T.; Percik, R.; Leibowitz-Amit, R.; Berger, R.; Golan, T.; Daher, S.; Taliansky, A.; Dudnik, E.; et al. Acute vascular events as a possibly related adverse event of immunotherapy: A single-institute retrospective study. Eur. J. Cancer 2019, 120, 122–131. [Google Scholar] [CrossRef]
- Gong, J.; Drobni, Z.D.; Alvi, R.M.; Murphy, S.P.; Sullivan, R.J.; Hartmann, S.E.; Gilman, H.K.; Lee, H.; Zubiri, L.; Raghu, V.K.; et al. Immune checkpoint inhibitors for cancer and venous thromboembolic events. Eur. J. Cancer 2021, 158, 99–110. [Google Scholar] [CrossRef]
- Oren, O.; Yang, E.H.; Molina, J.R.; Bailey, K.R.; Blumenthal, R.S.; Kopecky, S.L. Cardiovascular Health and Outcomes in Cancer Patients Receiving Immune Checkpoint Inhibitors. Am. J. Cardiol. 2020, 125, 1920–1926. [Google Scholar] [CrossRef] [PubMed]
- Solinas, C.; Saba, L.; Sganzerla, P.; Petrelli, F. Venous and arterial thromboembolic events with immune checkpoint inhibitors: A systematic review. Thromb. Res. 2020, 196, 444–453. [Google Scholar] [CrossRef] [PubMed]
- Brunner-Weinzierl, M.C.; Rudd, C.E. CTLA-4 and PD-1 Control of T-Cell Motility and Migration: Implications for Tumor Immunotherapy. Front. Immunol. 2018, 9, 2737. [Google Scholar] [CrossRef]
- Schäfer, S.; Zernecke, A. CD8(+) T Cells in Atherosclerosis. Cells 2020, 10, 37. [Google Scholar] [CrossRef] [PubMed]
- Nettersheim, F.S.; Picard, F.S.R.; Hoyer, F.F.; Winkels, H. Immunotherapeutic Strategies in Cancer and Atherosclerosis-Two Sides of the Same Coin. Front. Cardiovasc. Med. 2021, 8, 812702. [Google Scholar] [CrossRef]
- Matsumoto, T.; Sasaki, N.; Yamashita, T.; Emoto, T.; Kasahara, K.; Mizoguchi, T.; Hayashi, T.; Yodoi, K.; Kitano, N.; Saito, T.; et al. Overexpression of Cytotoxic T-Lymphocyte-Associated Antigen-4 Prevents Atherosclerosis in Mice. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 1141–1151. [Google Scholar] [CrossRef]
- Jain, N.; Nguyen, H.; Chambers, C.; Kang, J. Dual function of CTLA-4 in regulatory T cells and conventional T cells to prevent multiorgan autoimmunity. Proc. Natl. Acad. Sci. USA 2010, 107, 1524–1528. [Google Scholar] [CrossRef]
- Ruocco, M.G.; Pilones, K.A.; Kawashima, N.; Cammer, M.; Huang, J.; Babb, J.S.; Liu, M.; Formenti, S.C.; Dustin, M.L.; Demaria, S. Suppressing T cell motility induced by anti-CTLA-4 monotherapy improves antitumor effects. J. Clin. Investig. 2012, 122, 3718–3730. [Google Scholar] [CrossRef]
- Bu, D.X.; Tarrio, M.; Maganto-Garcia, E.; Stavrakis, G.; Tajima, G.; Lederer, J.; Jarolim, P.; Freeman, G.J.; Sharpe, A.H.; Lichtman, A.H. Impairment of the programmed cell death-1 pathway increases atherosclerotic lesion development and inflammation. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 1100–1107. [Google Scholar] [CrossRef]
- Cochain, C.; Chaudhari, S.M.; Koch, M.; Wiendl, H.; Eckstein, H.H.; Zernecke, A. Programmed cell death-1 deficiency exacerbates T cell activation and atherogenesis despite expansion of regulatory T cells in atherosclerosis-prone mice. PLoS ONE 2014, 9, e93280. [Google Scholar] [CrossRef]
- Gotsman, I.; Grabie, N.; Dacosta, R.; Sukhova, G.; Sharpe, A.; Lichtman, A.H. Proatherogenic immune responses are regulated by the PD-1/PD-L pathway in mice. J. Clin. Investig. 2007, 117, 2974–2982. [Google Scholar] [CrossRef] [PubMed]
- Poels, K.; van Leent, M.M.T.; Reiche, M.E.; Kusters, P.J.H.; Huveneers, S.; de Winther, M.P.J.; Mulder, W.J.M.; Lutgens, E.; Seijkens, T.T.P. Antibody-Mediated Inhibition of CTLA4 Aggravates Atherosclerotic Plaque Inflammation and Progression in Hyperlipidemic Mice. Cells 2020, 9, 1987. [Google Scholar] [CrossRef] [PubMed]
- Poels, K.; van Leent, M.M.T.; Boutros, C.; Tissot, H.; Roy, S.; Meerwaldt, A.E.; Toner, Y.C.A.; Reiche, M.E.; Kusters, P.J.H.; Malinova, T.; et al. Immune Checkpoint Inhibitor Therapy Aggravates T Cell-Driven Plaque Inflammation in Atherosclerosis. JACC. CardioOncol. 2020, 2, 599–610. [Google Scholar] [CrossRef]
- Furukawa, A.; Tamura, Y.; Taniguchi, H.; Kawamura, A.; Nagase, S.; Hayashi, A.; Tada, Y.; Sase, K.; Hatake, K. Prospective screening for myocarditis in cancer patients treated with immune checkpoint inhibitors. J. Cardiol. 2023, 81, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Stein-Merlob, A.F.; Rothberg, M.V.; Ribas, A.; Yang, E.H. Cardiotoxicities of novel cancer immunotherapies. Heart 2021, 107, 1694–1703. [Google Scholar] [CrossRef] [PubMed]
- Totzeck, M.; Michel, L.; Lin, Y.; Herrmann, J.; Rassaf, T. Cardiotoxicity from chimeric antigen receptor-T cell therapy for advanced malignancies. Eur. Heart J. 2022, 43, 1928–1940. [Google Scholar] [CrossRef]
- Jin, Y.; Dong, Y.; Zhang, J.; Sun, J.; Liu, Y.; Chen, Y. The toxicity of cell therapy: Mechanism, manifestations, and challenges. J. Appl. Toxicol. 2021, 41, 659–667. [Google Scholar] [CrossRef]
- Goldman, A.; Maor, E.; Bomze, D.; Liu, J.E.; Herrmann, J.; Fein, J.; Steingart, R.M.; Mahmood, S.S.; Schaffer, W.L.; Perales, M.A.; et al. Adverse Cardiovascular and Pulmonary Events Associated With Chimeric Antigen Receptor T-Cell Therapy. J. Am. Coll. Cardiol. 2021, 78, 1800–1813. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Chen, D.H.; Guha, A.; Mackenzie, S.; Walker, J.M.; Roddie, C. CAR T Cell Therapy-Related Cardiovascular Outcomes and Management: Systemic Disease or Direct Cardiotoxicity? JACC CardioOncol. 2020, 2, 97–109. [Google Scholar] [CrossRef]


| Anticancer Treatment | Classical Drugs | Main Cardiovascular Complications | Mechanism | |
|---|---|---|---|---|
| Chemotherapeutics | Anthracyclines | Arrhythmias, ECG changes, HF, pericarditis, myocarditis, acute MI | Free-radical-mediated myocyte damage, lipid peroxidation of cell membrane, the accumulation of drug metabolites, the damage of mitochondria and DNA, sarcoplasmic reticulum stress, the release of circulating pro-inflammatory cytokines, the activity on drug transporters, Top I and II inhibition, effluent loss of calcium in sarcoplasmic reticulum. | |
| 5-Fluorouracil | Angina and MI, hypotension, arrhythmias, ECG changes | ↑Coronary vasospasm, ↑Oxidative stress. | ||
| Cisplatin | Angina and MI, arrhythmias, chest pain, hypotension | Cytotoxicity in endothelial cells, increasing of free radicals, mitochondrial damage, calcium ion disorder. | ||
| Radiotherapy | / | Lesions of vascular segments present (e.g., accelerated coronary artery disease, stenotic aortic lesion), MI, HF, arrhythmias, pericarditis, valvular disease | Radiation disruption of endothelial barrier integrity, oxidative stress, upregulation of inflammatory/pro-fibrotic factors, decline in microvascular density, affecting mitochondrial function through Nrf2 pathway. | |
| Targeted therapy | Anti-ERBB2 monoclonal antibody | Trastuzumab | LV dysfunction/HF, arrhythmias, hypertension, thromboembolism | Restraint of ERK-MAPK and PI3K-Akt pathways, oxidative stress, upregulation of the ratio of pro-apoptotic proteins BCL-XS disrupting mitochondrial membrane integrity and activating apoptosis pathways. |
| Pertuzumab | LV dysfunction/HF | Restraint of ERK-MAPK pathway, inhibition on AMPK, disorder of sarcomeres/myofibers. | ||
| Anti-VEGF/VEGFR inhibition | Sunitinib, sorafenib | Hypertension, LV dysfunction, thromboembolism, arrhythmias (e.g., QT prolongation), MI, bleeding | Mitochondrial damage, endothelial dysfunction, retention of sodium and water, capillary rarefaction, oxidative stress, coronary vasospasm, hypercoagulability. | |
| Immuno-oncology agents | CTLA-4 inhibitor, PD-1 and PDL-1 inhibitor | Myocarditis, vasculitis, pericarditis, arrhythmias (e.g., supraventricular and ventricular tachycardia, heart block), and atherosclerosis | ↑T lymphocyte proliferation targeting homologous antigens shared by both the tumor and myocardium, macrophage infiltration→infiltrating into cardiomyocytes by activated T lymphocytes→the inhibition of innate immune protective mechanisms in the heart→inflammation and injury. ↑Atherosclerotic inflammatory activity and progression of atherosclerotic plaque volume. | |
| Yescarta, Kymriah, Tecartus | LV systolic dysfunction, ECG changes, supraventricular arrhythmias (e.g., sinus tachycardia, atrial fibrillation), pericardial effusion, cardiogenic or vasodilatory shock, refractory hypotension, cardiomyopathy, and cardiac arrest | “On-target, on-tumor” effect (transferred T cells are activated and tumor cell contents are released after being attacked→multiple cytokines are released→cytokine release syndrome and cytotoxic effects on cardiomyocytes). “On-target, off-tumor” effect (T cells attack normal tissue that shares some similarities with tumor). “Off-target, off-tumor” effect (T cells attack normal tissue with certain antigens which are cross-creative with the tumor antigen, and it is related to molecular mimicry of antigens). | ||
| First Author (References) | Patients | Study Types | Follow-Up | Main Findings | Conclusions |
|---|---|---|---|---|---|
| Drobni et al. [104] | 2842 (patients)/2842 (controls, a 1:1 ratio matching based on respect age, CVDs history and cancer type) | Retrospective single-center matched cohort study and case-crossover analysis | a median of 5 cycles/2 years | In the matched cohort study, the incidence of CVDs was three times higher post-ICI when compared to controls (HR 3.3, 95% CI 2.0–5.5, p < 0.001). In the case-crossover analysis, CVDs rose from 1.37 to 6.55 per 100 person-years within 2 years (adjusted HR 4.8, 95% CI 3.5–6.5, p < 0.001). | Following the start of ICIs, CV events increased, maybe caused by accelerated atherosclerosis development. Optimal CV risk factors should be considered during the whole process of treatment. |
| Bar et al. [105] | 1215 | Retrospective single-center cohort study | 6 months | Approximately 2.6% (95% (CI): 1.8–3.6) of patients who did receive ICIs developed an arterial thrombotic accident (MI or ischaemic stroke). Survival of patients with acute vascular events had a worse prognosis than that of those without events. | ICIs initiation augmented the risk of acute vascular events, suggesting that caution should be exercised for ICI-related risk factors. |
| Gong et al. [106] | 2854 | Retrospective single-center cohort study | 2 years | The rate of VTE increased by more than 4-fold after ICI treatment set on (HR 4.98, 95% CI 3.65–8.59, p < 0.001) | Patients who have taken an ICI have a high rate of VTE. |
| Oren et al. [107] | 3326 | Retrospective single-center cohort study | a mean follow-up of 16 months | The incidence of MI and stroke was presented, respectively, in 213 (6.4%) and 227 (6.8%) patients. | In cancer patients receiving ICIs, CV factors are correlated with clinical outcomes and may be utilized to estimate mortality. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, M.; Xue, J.; Wang, M.; Yang, J.; Chen, T. Cardiovascular Complications of Pan-Cancer Therapies: The Need for Cardio-Oncology. Cancers 2023, 15, 3055. https://doi.org/10.3390/cancers15113055
Chen M, Xue J, Wang M, Yang J, Chen T. Cardiovascular Complications of Pan-Cancer Therapies: The Need for Cardio-Oncology. Cancers. 2023; 15(11):3055. https://doi.org/10.3390/cancers15113055
Chicago/Turabian StyleChen, Mengjia, Jianing Xue, Maoling Wang, Junyao Yang, and Ting Chen. 2023. "Cardiovascular Complications of Pan-Cancer Therapies: The Need for Cardio-Oncology" Cancers 15, no. 11: 3055. https://doi.org/10.3390/cancers15113055
APA StyleChen, M., Xue, J., Wang, M., Yang, J., & Chen, T. (2023). Cardiovascular Complications of Pan-Cancer Therapies: The Need for Cardio-Oncology. Cancers, 15(11), 3055. https://doi.org/10.3390/cancers15113055






