Simple Summary
As the global burden of skin cancer rises, complex cases of locally advanced skin cancer are rising as well. Rare and aggressive dissemination mechanisms are often the cause for extensive skin cancer. Incohesive spreading patterns complicate correct diagnoses and may result in large tumors with multiple misguided treatment attempts. The aim of this study was to create awareness for cutaneous malignancies with rare, aggressive dissemination mechanisms to improve diagnostic and therapeutic strategies in such complex cases. We identified high-risk characteristics of locally advanced skin cancer warranting extended surgical treatment and a cohesive interdisciplinary approach.
Abstract
The globally increasing incidence of cutaneous malignancies leads, in parallel, to increasing numbers of locally advanced skin cancer resulting in reconstructive surgery. Reasons for locally advanced skin cancer may be a patient’s neglect or aggressive tumor growth, such as desmoplastic growth or perineural invasion. This study investigates characteristics of cutaneous malignancies requiring microsurgical reconstruction with the aim of identifying possible pitfalls and improving diagnostic and therapeutic processes. A retrospective data analysis from 2015 to 2020 was conducted. Seventeen patients (n = 17) were included. The mean age at reconstructive surgery was 68.5 (±13) years. The majority of patients (14/17, 82%) presented with recurrent skin cancer. The most common histological entity was squamous cell carcinoma (10/17, 59%). All neoplasms showed at least one of the following histopathological characteristics: desmoplastic growth (12/17, 71%), perineural invasion (6/17, 35%), or tumor thickness of at least 6 mm (9/17, 53%). The mean number of surgical resections until cancer-free resection margins (R0) were achieved was 2.4 (±0.7). The local recurrence rate and the rate of distant metastases were 36%. Identified high-risk neoplastic characteristics, such as desmoplastic growth, perineural invasion, and a tumor depth of at least 6 mm, require a more extensive surgical treatment without concerns about defect size.
1. Introduction
Cutaneous malignancies are the most common malignancies in humans and are generally found in sun-exposed areas such as the face, neck, and scalp due to chronic UV exposure [1]. The majority of skin cancers are treated by simple surgical excision; however, the globally increasing incidence of cutaneous malignancies leads, in parallel, to higher numbers of locally advanced skin neoplasms requiring reconstructive surgery [2]. Locally advanced skin cancer may be caused by a patient’s neglect or, rarely, by highly invasive entities [3]. A patient’s neglect usually results in tumor growth over a lengthy period of time and may, in certain cases, be associated with abnormal illness behavior, depression, somatic anxiety disorder, or schizophrenia [4,5,6]. Wax et al. have previously categorized two groups of patients with extensive skin cancer requiring microsurgical, free vascularized tissue transfer [7,8]. While the first group consisted of patients with neglected skin cancer, the second group represented patients with multiple previous treatment attempts. An in-depth analysis of neoplastic entities was not performed in this study [7,8]. In patients presenting with multiple previous treatment attempts, rare and insidious mechanisms of dissemination, such as desmoplastic growth and perineural invasion, might be the cause for misguided diagnosis and, thus, treatment [9]. Correct diagnosis and treatment are often delayed due to the clinically concealed character of certain neoplastic entities and due to the difficulty of histopathological diagnosis [10]. Meanwhile, aggressive growth can result in the invasion of deeper structures such as cartilage or bone and, thus, require extensive surgery followed by microsurgical, free vascularized tissue reconstruction as the last option at the top of the reconstructive ladder [11,12]. As the global burden of locally advanced skin cancer is rapidly increasing, examples of treatment strategies in such complex cases are warranted.
This study investigates characteristics of cutaneous malignancies in the head and neck region that ultimately result in microsurgical, free vascularized tissue reconstruction. Moreover, we aim to create awareness for aggressive skin cancer dissemination mechanisms and to indicate possible pitfalls in patients’ treatment pathways by sharing our personal experience and approach in this work.
2. Materials and Methods
A retrospective data analysis of all patients who underwent microsurgical reconstruction with free vascularized tissue transfer after skin cancer resection in the head and neck region was conducted between 1 January 2015 and 31 December 2020 within our department. Data acquisition and management were performed according to the Standards of Good Scientific Practice and the Declaration of Helsinki in its present version (64th WMA General Assembly, Fortaleza, Brazil, 2013). The institutional ethics committee granted ethical approval (No. 1310/2021). Written and oral informed consent for data and image processing was obtained from all patients. The medical chart review included patient demographics, Fitzpatrick skin type, and history of previous skin cancer, of previous malignant disease, and of immunosuppression. Characteristics of neoplasms including localization, entity, histological details regarding desmoplastic growth, perineural invasion, tumor thickness, and depth of tissue infiltration (e.g., bone infiltration) were recorded. Oncological staging and preoperative imaging were documented. Surgical characteristics including the number of resections needed to achieve histopathologically confirmed cancer-free resection margins, the reconstructive technique, and postoperative complications were recorded. Resections until histopathologically confirmed cancer-free resection margins are achieved, followed by staged reconstruction, is pursued as the standard of care within our department in cases of locally advanced skin cancer. Postoperative complications were classified according to the Clavien–Dindo classification [13]. If one patient had more than one complication, the complication with the highest Clavien–Dindo grade was used for statistical analysis in the individual patient. Adjuvant therapy, recurrent disease, and follow-up were also documented.
Statistical Analysis
Data were tabulated and stored in Microsoft Excel 16.16.21 (Microsoft, Redmond, DC, USA). Statistical analysis was performed using IBM SPSS Statistics 27 for Windows (IBM SPSS Software, Armond, NY, USA) and GraphPad Prism 8.4.3 (GraphPad Software LLC, La Jolla, CA, USA). Descriptive analysis data are presented as mean ± standard deviation (SD) unless stated otherwise.
3. Results
3.1. Patient Demographics
From 1 January 2015 to 31 December 2020, 17 patients (n = 17) fulfilled the inclusion criteria. All patients underwent microsurgical, free vascularized tissue reconstruction after a resection of locally advanced skin cancer in the head and neck region. All 17 patients were presented to an interdisciplinary tumor board and treatment was performed according to the tumor board’s decision. The mean age when reconstructive surgery occurred was 68.5 ± 13 years old. Eleven patients (11/17, 64.7%) were male and six patients (6/17, 35.3%) were female. The Fitzpatrick skin type was type 2 in all patients. The majority of patients (14/17, 82.4%) presented with a recurrent skin cancer and all of these patients (14/17, 82.4%) also had a history of prior skin malignancy at another localization. Three patients (3/17, 17.6%) had a history of previous malignant disease; one patient had carcinoma of the nasopharynx, another patient had non-Hodgkin’s lymphoma, and the third patient had lung cancer. Three other patients (3/17, 17.6%) received immunosuppressive medication; one patient for polycythemia vera and two patients after solid-organ transplantation.
3.2. Characteristics of Neoplasms
The most common localization was the scalp (6/17, 35.3%) and the most common histological entity was squamous cell carcinoma (SCC) (10/17, 58.8%). All neoplasms presented larger than 2 cm in diameter. Twelve neoplasms (12/17, 70.6%) were histopathologically diagnosed with desmoplastic growth and six neoplasms (6/17, 35.3%) with perineural invasion. Meanwhile, three neoplasms were histopathologically diagnosed with simultaneous desmoplastic growth and perineural invasion, and all neoplasms without these characteristics showed a tumor thickness of at least 6 mm (millimeters. The mean histological tumor thickness was 12.2 (±7.4) mm. Overall, clinical and histological bone infiltration was diagnosed in six cases (6/17, 35.3%), of which all but one showed desmoplastic growth. The clinical and histological characteristics are presented in Figure 1 and Table 1.
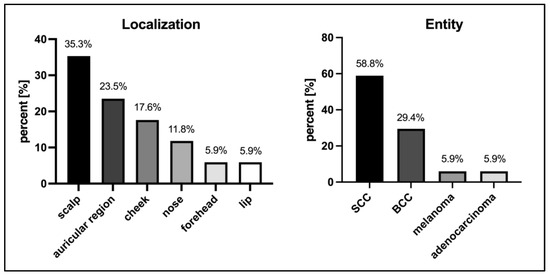
Figure 1.
Characteristics of neoplasms. SCC—squamous cell carcinoma; BCC—basal cell carcinoma.

Table 1.
Histological characteristics of neoplasms.
3.3. Oncological Staging and Imaging
Oncological staging was performed in 14 cases (14/17, 82.4%) during the current presentation and prior to microsurgical, free vascularized tissue reconstruction. Due to the low metastatic risk of basal cell carcinoma, staging was only performed in selected cases according to the most recent guidelines on basal cell carcinoma [14]; all cases without staging were cases diagnosed with basal cell carcinoma. Staging included radiological imaging, e.g., computed tomography (CT) (11/14, 78.6%), ultrasound of regional lymph nodes (11/14, 78.6%), positron emission tomography (PET) (2/14, 14.3%), and magnetic resonance imaging (MRI) (3/14, 21.4%). In more than half of the cases (8/14, 57.1%), staging imaging included ultrasound of regional lymph nodes alongside CT. During staging imaging, lymph node metastasis was suspected in five cases (5/17, 29.4%). Lymph node biopsy was performed in two of these cases and selective neck dissection was performed in the other three cases. Overall, lymph node biopsy was performed in four patients (4/17, 23.5%), of which two patients, as mentioned, were suspected of lymph node metastasis upon staging imaging and the other two patients each showed an enlarged lymph node intraoperatively, which was sent for histopathological diagnosis. In one patient, selective lymph node dissection was performed adjacent to a partial parotidectomy in a basal cell carcinoma of the auricular region. Overall, lymph node metastases were histologically confirmed in three patients (3/17, 17.6%), of which all showed previous suspicious imaging. One patient had a history of prior lymph node metastasis confirmed by histology. Distant metastasis of the lungs was suspected in two cases (2/17, 11.8%); one patient had scirrhous squamous cell carcinoma and one patient had adenocarcinoma.
Imaging of the head and neck by CT and/or magnetic resonance imaging (MRI) to investigate tumor depth and infiltration prior to surgical tumor resection was carried out in 14 patients (14/17, 82.4%).
3.4. Surgical Characteristics
Overall, the mean number of surgical resections prior to microsurgical, free vascularized tissue reconstruction was 2.2 (±0.8, range from 1 to 3). The mean number of days between the last surgical resection and staged reconstruction was 10 (±12.3) days. Immediate reconstruction was performed in seven cases (7/17, 41.2%) due to the extent of the defect created. Intraoperative frozen sections were analyzed in three of these cases. In four of these cases (4/17, 23.5%), the bone was so extensively infiltrated by the neoplasm that free resection margins could not be achieved despite bone resection. Due to the high morbidity due to resection and/or the anatomical impossibility of further resection, the interdisciplinary tumor board decided on adjuvant therapy in these cases. Two patients received adjuvant radiotherapy, one received adjuvant radiochemotherapy, and one received immunotherapy. The third patient, however, was diagnosed with lung cancer and was no longer eligible for surgical therapy escalation. In the remaining cases (13/17, 76.5%), the mean number of surgical resections until cancer-free resection margins (R0) were achieved was 2.4 (±0.7, range 1 to 3), which included the resection of infiltrated bone in three cases. The most frequently used free flap for reconstruction was the gracilis muscle flap combined with the split-thickness skin graft (8/17, 47.1%). The facial artery served as the recipient artery in 52.9% (9/17) of cases. One venous anastomosis was performed in all cases except one that required two venous anastomoses. Postoperative complications occurred in nine cases (9/17, 52.9%). All recorded complications were classified as Clavien–Dindo grade 3b. The most common complication was secondary hemorrhage at the recipient site (4/9, 44.4%). Total flap loss was observed in one patient (1/17, 5.9%). Surgical characteristics are presented in Table 2. One patient example is displayed in Figure 2.

Table 2.
Surgical characteristics.
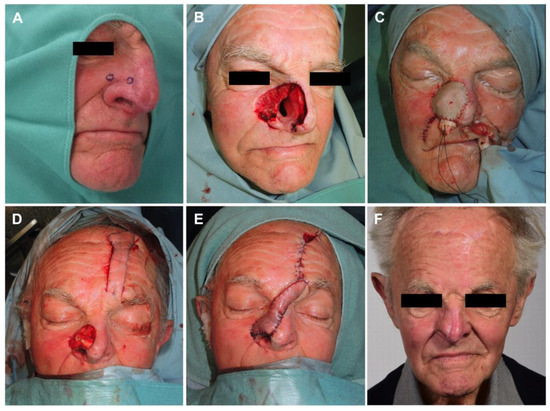
Figure 2.
(A) Biopsies taken upon suspicious skin changes; (B) resection defect after tumor-free resection margins reached; (C) first-step reconstruction of nasal inner lining with free radial forearm flap; (D) second-step reconstruction with nasal alar reconstruction by autologous cartilage graft and (E) pedicled paramedian forehead flap; (F) follow-up result 18 months postoperatively.
3.5. Follow-Up
The mean follow-up time after reconstructive surgery was 25.3 (±20.9, range 0–63) months. Only one patient was lost to follow-up. Three patients died and four patients refused further treatment or follow-up. In nine cases, follow-up is still ongoing.
Overall, seven patients received adjuvant therapy (7/17, 41.2%) after microsurgical, free vascularized tissue transfer. Five patients underwent radiation therapy, one patient underwent radiochemotherapy, and one patient received immunotherapy with pembrolizumab.
Local recurrence occurred in 35.3% (6/17) of cases despite cancer-free resection margins (R0) being reached by histopathology. Local recurrence was present beneath the flap tissue; a tumor was found within the adjacent subcutaneous tissue in one case and skin lesions adjacent to the reconstructed tissue area were found in two cases. Progressive disease with the diagnosis of metastases was also observed in 35.3% of cases (6/17), of which two patients were diagnosed with novel distant metastases of the lung, two patients showed metastases after incomplete surgical resection (R1) (one patient showed an in-transit locoregional metastasis and the other patient showed bone metastasis), and two patients with known distant metastases of the lungs showed progression. Two of these patients were solid-organ transplant recipients under current immunosuppression. The mean time until the diagnosis of local recurrence was 7.7 (±13.9, range 0–36) months and the mean time until the diagnosis of metastases was 4.8 (±4.9, range 0–11) months.
Despite adjuvant therapy, local recurrence after adjuvant therapy occurred in 28.6% (2/7) of cases and progressive disease with metastases was observed in 57.1% (4/7) of cases. Of these six patients, one patient underwent surgical treatment again, two patients diagnosed with SCC and desmoplastic melanoma received immunotherapy with pembrolizumab, two patients diagnosed with BCC received immunotherapy with vismodegib, and one patient diagnosed with SCC after solid-organ transplantation received chemotherapy. Follow-up details are summarized in Table 3.

Table 3.
Follow-up.
4. Discussion
General risk factors for cutaneous malignancies are well studied and documented in the literature. They include advanced age, Fitzpatrick skin type 1 and 2, and male sex [15,16,17,18]. The most important risk factor for the development of cutaneous neoplasms remains chronic UV damage [16,17,18]. While our patient group fits well into this scheme, specific risk factors for the development of locally advanced skin cancer resulting in microsurgical, free vascularized tissue reconstruction are still to be defined.
Immunosuppression is one risk factor known to be associated with more aggressive growth and higher rates of recurrence and metastasis [19,20]. Higher rates of cutaneous malignancies have been observed in solid-organ transplant recipients [21], in patients with human immunodeficiency virus (HIV) [22], and in hematopoietic stem cell transplant recipients [23]. While the risk of developing cutaneous neoplasms increases with the duration of immunosuppression [24,25], patients under immunosuppression generally also present with more invasive types of skin cancer [26,27]. For example, aggressive dissemination characteristics such as perineural invasion have been reported in up to 39% of solid-organ transplant recipients compared to a range from 2.5% to 14% of immunocompetent patients [19,28]. Furthermore, immunosuppression bares a higher risk of local recurrence and a higher rate of metastasis [29,30,31]. In certain cases, immunosuppression after organ transplantation has even been associated with skin cancer mortality that is up to nine times higher compared to that in the general population [32]. Interestingly, our patient group only included 17.6% of patients taking active immunosuppressive medication, suggesting further risk factors for aggressive cancer growth expanding beyond the clinically visible tumor appearance.
Strikingly, 82.4% of our patients presented with a locally recurrent lesion and had a history of prior skin malignancies. As locally recurrent skin cancers may already hint at aggressive neoplastic characteristics [33,34], subclinical infiltration should be considered before therapy, potentially yielding for extended resection margins. Recurrent neoplasms show a higher risk of further relapse and are also known to be associated with irregular dissemination mechanisms such as desmoplasia [35,36,37]. Desmoplasia, also often referred to as “scirrhous” or “sclerodermiform” growth, was first described in 1997 and is histopathologically characterized by “Indian filing”, a strand-like growth pattern of neoplastic cells [38]. In melanoma as well as non-melanoma skin cancer, desmoplastic growth is a known prognostic factor for local recurrence and for metastasis [39,40,41]. While desmoplasia has also been referred to as a risk factor for perineural invasion, a recent study argues that, in squamous cell carcinoma, perineural invasion is exclusively attributed to desmoplastic growth [42,43]. Perineural invasion is defined as neoplastic cells “in close proximity to the nerve” involving at least one third of the nerve’s circumference [44] and is diagnosed by immunohistochemistry [10].
In our study population, 88.2% were diagnosed with a cutaneous malignancy showing desmoplastic growth and/or perineural invasion surpassing the number of patients presenting with recurrent skin cancer already. The remaining patients without histological findings of desmoplastic growth or perineural invasion were diagnosed with cutaneous malignancies showing a histological tumor depth of at least 6 mm; all tumors included in this study had a diameter larger than 2 cm. A tumor depth of over 6 mm has been described as an independent prognostic factor for metastasis and recurrence [33]. At the same time, an increased depth and diameter may be associated with desmoplastic growth and, hence, perineural invasion, although the dependence between desmoplasia and perineural invasion has still not been fully explained [43].
Altogether, histological diagnosis referring to desmoplastic growth, perineural invasion, or a tumor depth of at least 6 mm should be alarming characteristics. Most of our patients necessitated at least two resections to achieve cancer-free resection margins and 35.3% developed local recurrence in only 7.7 (±13.9) months despite these cancer-free resection margins. Based on our findings, we suggest increased resection margins for cutaneous malignancies with these alarming characteristics that surpass the resection margins recommended by the guidelines. Interestingly, our study population included only one patient with desmoplastic melanoma, which might be attributed to the already larger resection margins applied in the therapy of melanoma [16,40]. Wider resection margins, complete histopathological margin analysis, and Moh’s surgery are recommended by the recent literature on complex skin cancer [45,46]. Nevertheless, Moh’s surgery and intraoperative frozen section control may be of limited value in lesions with desmoplastic growth or perineural invasion [47,48]. The incohesive spreading pattern of these aggressive dissemination mechanisms might not be displayed correctly on frozen sections and can, therefore, lead to a diagnostic discrepancy in a definitive histological diagnosis [49]. In these cases, immunohistochemical staining is the diagnostic tool of choice to detect desmoplastic growth and perineural invasion. Before performing microvascular, free vascularized tissue reconstruction, the histology ought to be definite, which demands staged reconstruction procedures with temporary defect coverage. Furthermore, in cases of suspected complex cutaneous malignancy, surgeons ought to prioritize cancer-free resection margins without concern for defect size. Additionally, imaging by MRI or CT early on should be considered to evaluate the infiltration of deeper structures prior to surgery.
Microvascular, free vascularized reconstruction in the head and neck represents a safe procedure and covers extended tissue defects. A good aesthetic and functional outcome is achieved provided that the defect analysis is carried out correctly and the adequate free flap is chosen for each individual application. The evaluation of complications in our patient collective showed only one total loss (5.9%) of a gracilis muscle flap, which was salvaged by secondary defect coverage using a deep inferior epigastric perforator (DIEP) flap. Increased morbidity in our patient collective originated from advanced age and the presence of underlying malignant diseases with a high prevalence of early local recurrence and distant metastases.
Regarding the local recurrence rate in this study, disease-free intervals before performing complex reconstructive surgery should be considered, and follow-up should be intensified and prolonged with a thorough evaluation of the surgical field in order to avoid the inconsiderate misinterpretation of recurrent lesions as a wound healing disorder. Additionally, adjuvant therapy with novel treatment options, e.g., biological agents, should be considered in these patients.
5. Conclusions
In conclusion, high-risk characteristics including desmoplastic growth, perineural invasion, or a tumor depth of at least 6 mm require a more aggressive surgical treatment. Resection margins should exceed those recommended for simple skin cancer excision, and resection ought to be carried out without concern for defect size. Although medical advancements offer a range of adjuvant therapy modalities, surgery still remains the best option for the curative treatment of primary skin cancer and microsurgical, free vascularized tissue reconstruction is a safe treatment option.
Thorough resection planning with the support of local imaging and an interdisciplinary approach with adequate follow-up regimens is necessary to achieve the best possible outcome for patients with locally advanced skin cancer and to diagnose aggressive dissemination mechanisms.
Author Contributions
T.R., data interpretation and manuscript writing; A.A., data acquisition and analysis; T.B.S., data acquisition, analysis and interpretation; B.W.Z., data analysis and manuscript editing; G.P., conceptualization and supervision; M.S., data analysis and manuscript editing; D.W., planning, supervision and data interpretation; E.M.M., conceptualization and manuscript writing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of the Medical University of Innsbruck (approval number: 1310/2021; approval date: 9 September 2021).
Informed Consent Statement
Written informed consent has been obtained from the patients for data and image processing.
Data Availability Statement
Data supporting the findings of this study are available from the authors upon request.
Acknowledgments
We would like to thank K. Langert for taking the photos.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Leiter, U.; Garbe, C. Epidemiology of melanoma and nonmelanoma skin cancer—The role of sunlight. Adv. Exp. Med. Biol. 2008, 624, 89–103. [Google Scholar] [CrossRef] [PubMed]
- Suk, S.; Shin, H.W.; Yoon, K.C.; Kim, J. Aggressive cutaneous squamous cell carcinoma of the scalp. Arch. Craniofacial Surg. 2020, 21, 363–367. [Google Scholar] [CrossRef] [PubMed]
- Purnell, J.C.; Gardner, J.M.; Brown, J.A.; Shalin, S.C. Conventional Versus Giant Basal Cell Carcinoma, a Review of 57 Cases: Histologic Differences Contributing to Excessive Growth. Indian J. Dermatol. 2018, 63, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Pavan, C.; Bassetto, F.; Vindigni, V. Psychological Aspects of a Patient with Neglected Skin Tumor of the Scalp. Plast. Reconstr. Surgery. Glob. Open 2017, 5, e1395. [Google Scholar] [CrossRef] [PubMed]
- Shah, H.A.; Lee, H.B.; Nunery, W.R. Neglected basal cell carcinoma in a schizophrenic patient. Ophthalmic Plast. Reconstr. Surg. 2008, 24, 495–497. [Google Scholar] [CrossRef]
- Andersen, R.M.; Lei, U. A massive neglected giant basal cell carcinoma in a schizophrenic patient treated successfully with vismodegib. J. Dermatol. Treat. 2015, 26, 575–576. [Google Scholar] [CrossRef]
- Wax, M.K. Free tissue transfer in the reconstruction of massive skin cancer. Facial Plast. Surg. Clin. North Am. 2009, 17, 279–286. [Google Scholar] [CrossRef]
- Wax, M.K.; Burkey, B.B.; Bascom, D.; Rosenthal, E.L. The role of free tissue transfer in the reconstruction of massive neglected skin cancers of the head and neck. Arch. Facial Plast. Surg. 2003, 5, 479–482. [Google Scholar] [CrossRef]
- Morandi, E.M.; Rauchenwald, T.; Puelzl, P.; Zelger, B.W.; Zelger, B.G.; Henninger, B.; Pierer, G.; Wolfram, D. Hide-and-seek: Neurotropic squamous cell carcinoma of the periorbital region—A series of five cases and review of the literature. J. Der Dtsch. Dermatol. Ges. J. Ger. Soc. Dermatol. JDDG 2021, 19, 1571–1580. [Google Scholar] [CrossRef]
- Kurtz, K.A.; Hoffman, H.T.; Zimmerman, M.B.; Robinson, R.A. Perineural and vascular invasion in oral cavity squamous carcinoma: Increased incidence on re-review of slides and by using immunohistochemical enhancement. Arch. Pathol. Lab. Med. 2005, 129, 354–359. [Google Scholar] [CrossRef]
- Ge, N.N.; McGuire, J.F.; Dyson, S.; Chark, D. Nonmelanoma skin cancer of the head and neck II: Surgical treatment and reconstruction. Am. J. Otolaryngol. 2009, 30, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Cannady, S.B.; Rosenthal, E.L.; Knott, P.D.; Fritz, M.; Wax, M.K. Free tissue transfer for head and neck reconstruction: A contemporary review. JAMA Facial Plast. Surg. 2014, 16, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Dindo, D.; Demartines, N.; Clavien, P.A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef]
- Lang, B.M.; Balermpas, P.; Bauer, A.; Blum, A.; Brölsch, G.F.; Dirschka, T.; Follmann, M.; Frank, J.; Frerich, B.; Fritz, K.; et al. S2k Guidelines for Cutaneous Basal Cell Carcinoma—Part 2: Treatment, Prevention and Follow-up. J. Dtsch. Dermatol. Ges 2019, 17, 214–230. [Google Scholar] [CrossRef]
- Fitzpatrick, T.B. The validity and practicality of sun-reactive skin types I through VI. Arch. Dermatol. 1988, 124, 869–871. [Google Scholar] [CrossRef]
- Swetter, S.M.; Tsao, H.; Bichakjian, C.K.; Curiel-Lewandrowski, C.; Elder, D.E.; Gershenwald, J.E.; Guild, V.; Grant-Kels, J.M.; Halpern, A.C.; Johnson, T.M.; et al. Guidelines of care for the management of primary cutaneous melanoma. J. Am. Acad. Dermatol. 2019, 80, 208–250. [Google Scholar] [CrossRef]
- Kim, J.Y.S.; Kozlow, J.H.; Mittal, B.; Moyer, J.; Olencki, T.; Rodgers, P. Guidelines of care for the management of basal cell carcinoma. J. Am. Acad. Dermatol. 2018, 78, 540–559. [Google Scholar] [CrossRef]
- Kim, J.Y.S.; Kozlow, J.H.; Mittal, B.; Moyer, J.; Olenecki, T.; Rodgers, P. Guidelines of care for the management of cutaneous squamous cell carcinoma. J. Am. Acad. Dermatol. 2018, 78, 560–578. [Google Scholar] [CrossRef] [PubMed]
- Lanz, J.; Bouwes Bavinck, J.N.; Westhuis, M.; Quint, K.D.; Harwood, C.A.; Nasir, S.; Van-de-Velde, V.; Proby, C.M.; Ferrándiz, C.; Genders, R.E.; et al. Aggressive Squamous Cell Carcinoma in Organ Transplant Recipients. JAMA Dermatol. 2019, 155, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Sargen, M.R.; Cahoon, E.K.; Yu, K.J.; Madeleine, M.M.; Zeng, Y.; Rees, J.R.; Lynch, C.F.; Engels, E.A. Spectrum of Nonkeratinocyte Skin Cancer Risk Among Solid Organ Transplant Recipients in the US. JAMA Dermatol. 2022, 158, 414. [Google Scholar] [CrossRef] [PubMed]
- Garrett, G.L.; Blanc, P.D.; Boscardin, J.; Lloyd, A.A.; Ahmed, R.L.; Anthony, T.; Bibee, K.; Breithaupt, A.; Cannon, J.; Chen, A.; et al. Incidence of and Risk Factors for Skin Cancer in Organ Transplant Recipients in the United States. JAMA Dermatol. 2017, 153, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Omland, S.H.; Ahlström, M.G.; Gerstoft, J.; Pedersen, G.; Mohey, R.; Pedersen, C.; Kronborg, G.; Larsen, C.S.; Kvinesdal, B.; Gniadecki, R.; et al. Risk of skin cancer in patients with HIV: A Danish nationwide cohort study. J. Am. Acad. Dermatol. 2018, 79, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Omland, S.H.; Gniadecki, R.; Hædersdal, M.; Helweg-Larsen, J.; Omland, L.H. Skin Cancer Risk in Hematopoietic Stem-Cell Transplant Recipients Compared With Background Population and Renal Transplant Recipients: A Population-Based Cohort Study. JAMA Dermatol. 2016, 152, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Boukamp, P. Non-melanoma skin cancer: What drives tumor development and progression? Carcinogenesis 2005, 26, 1657–1667. [Google Scholar] [CrossRef]
- Fania, L.; Abeni, D.; Esposito, I.; Spagnoletti, G.; Citterio, F.; Romagnoli, J.; Castriota, M.; Ricci, F.; Moro, F.; Perino, F.; et al. Behavioral and demographic factors associated with occurrence of non-melanoma skin cancer in organ transplant recipients. G. Ital. Di Dermatol. E Venereol. Organo Uff. Soc. Ital. Di Dermatol. E Sifilogr. 2020, 155, 669–675. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.J.; Hamza, S.; Skelton, H. Histologic features in primary cutaneous squamous cell carcinomas in immunocompromised patients focusing on organ transplant patients. Dermatol. Surg. Off. Publ. Am. Soc. Dermatol. Surg. 2004, 30, 634–641. [Google Scholar] [CrossRef]
- Harwood, C.A.; Proby, C.M.; McGregor, J.M.; Sheaff, M.T.; Leigh, I.M.; Cerio, R. Clinicopathologic features of skin cancer in organ transplant recipients: A retrospective case-control series. J. Am. Acad. Dermatol. 2006, 54, 290–300. [Google Scholar] [CrossRef]
- Karia, P.S.; Morgan, F.C.; Ruiz, E.S.; Schmults, C.D. Clinical and Incidental Perineural Invasion of Cutaneous Squamous Cell Carcinoma: A Systematic Review and Pooled Analysis of Outcomes Data. JAMA Dermatol. 2017, 153, 781–788. [Google Scholar] [CrossRef]
- Euvrard, S.; Kanitakis, J.; Claudy, A. Skin cancers after organ transplantation. New Engl. J. Med. 2003, 348, 1681–1691. [Google Scholar] [CrossRef]
- Berg, D.; Otley, C.C. Skin cancer in organ transplant recipients: Epidemiology, pathogenesis, and management. J. Am. Acad. Dermatol. 2002, 47, 1–17; quiz 18-20. [Google Scholar] [CrossRef]
- Thompson, A.K.; Kelley, B.F.; Prokop, L.J.; Murad, M.H.; Baum, C.L. Risk Factors for Cutaneous Squamous Cell Carcinoma Recurrence, Metastasis, and Disease-Specific Death: A Systematic Review and Meta-analysis. JAMA Dermatol. 2016, 152, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Garrett, G.L.; Lowenstein, S.E.; Singer, J.P.; He, S.Y.; Arron, S.T. Trends of skin cancer mortality after transplantation in the United States: 1987 to 2013. J. Am. Acad. Dermatol. 2016, 75, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Brantsch, K.D.; Meisner, C.; Schönfisch, B.; Trilling, B.; Wehner-Caroli, J.; Röcken, M.; Breuninger, H. Analysis of risk factors determining prognosis of cutaneous squamous-cell carcinoma: A prospective study. Lancet Oncol. 2008, 9, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Silverman, M.K.; Kopf, A.W.; Grin, C.M.; Bart, R.S.; Levenstein, M.J. Recurrence rates of treated basal cell carcinomas. Part 1: Overview. J. Dermatol. Surg. Oncol. 1991, 17, 713–718. [Google Scholar] [CrossRef] [PubMed]
- Skulsky, S.L.; O’Sullivan, B.; McArdle, O.; Leader, M.; Roche, M.; Conlon, P.J.; O’Neill, J.P. Review of high-risk features of cutaneous squamous cell carcinoma and discrepancies between the American Joint Committee on Cancer and NCCN Clinical Practice Guidelines In Oncology. Head Neck 2017, 39, 578–594. [Google Scholar] [CrossRef]
- Zeng, S.; Fu, L.; Zhou, P.; Ling, H. Identifying risk factors for the prognosis of head and neck cutaneous squamous cell carcinoma: A systematic review and meta-analysis. PloS ONE 2020, 15, e0239586. [Google Scholar] [CrossRef]
- Santos-Arroyo, A.; Carrasquillo, O.Y.; Cardona, R.; Sánchez, J.L.; Valentín-Nogueras, S. Non-Melanoma Skin Cancer Tumor’s Characteristics and Histologic Subtype as a Predictor for Subclinical Spread and Number of Mohs Stages required to Achieve Tumor-Free Margins. Puerto Rico Health Sci. J. 2019, 38, 40–45. [Google Scholar]
- Breuninger, H.; Schaumburg-Lever, G.; Holzschuh, J.; Horny, H.P. Desmoplastic squamous cell carcinoma of skin and vermilion surface: A highly malignant subtype of skin cancer. Cancer 1997, 79, 915–919. [Google Scholar] [CrossRef]
- Scrivener, Y.; Grosshans, E.; Cribier, B. Variations of basal cell carcinomas according to gender, age, location and histopathological subtype. Br. J. Dermatol. 2002, 147, 41–47. [Google Scholar] [CrossRef]
- Leitlinienprogramm Onkologie (Deutsche Krebsgesellschaft, Deutsche Krebshilfe, AWMF): Diagnostik Therapie und Nachsorge des Melanoms. Langverion 3.3, 2020, AWMF Registernummer: 032/024OL. Available online: http://www.leitlinienprogramm-onkologie.de/leitlinien/melanom (accessed on 1 May 2022).
- S3-Leitlinie Aktinische Keratose und Plattenepithelkarzinom der Haut. Langversion 1.1, 2020, AWMF Registernummer: 032/022OL. Available online: https://www.leitlinienprogramm-onkologie.de/leitlinien/aktinische-keratose-und-plattenepithelkarzinom-der-haut/ (accessed on 1 May 2022).
- Roscher, I.; Falk, R.S.; Vos, L.; Clausen, O.P.F.; Helsing, P.; Gjersvik, P.; Robsahm, T.E. Validating 4 Staging Systems for Cutaneous Squamous Cell Carcinoma Using Population-Based Data: A Nested Case-Control Study. JAMA Dermatol. 2018, 154, 428–434. [Google Scholar] [CrossRef]
- Haug, K.; Breuninger, H.; Metzler, G.; Eigentler, T.; Eichner, M.; Häfner, H.M.; Schnabl, S.M. Prognostic Impact of Perineural Invasion in Cutaneous Squamous Cell Carcinoma: Results of a Prospective Study of 1,399 Tumors. J. Invest. Dermatol. 2020, 140, 1968–1975. [Google Scholar] [CrossRef]
- Liebig, C.; Ayala, G.; Wilks, J.A.; Berger, D.H.; Albo, D. Perineural invasion in cancer: A review of the literature. Cancer 2009, 115, 3379–3391. [Google Scholar] [CrossRef] [PubMed]
- Zhou, A.E.; Hoegler, K.M.; Khachemoune, A. Review of Perineural Invasion in Keratinocyte Carcinomas. Am. J. Clin. Dermatol. 2021, 22, 653–666. [Google Scholar] [CrossRef] [PubMed]
- Fraga, S.D.; Besaw, R.J.; Murad, F.; Schmults, C.D.; Waldman, A. Complete Margin Assessment Versus Sectional Assessment in Surgically Excised High-Risk Keratinocyte Carcinomas: A Systematic Review and Meta-Analysis. Dermatol. Surg. 2022, 48, 704–710. [Google Scholar] [CrossRef]
- Chambers, K.J.; Kraft, S.; Emerick, K. Evaluation of frozen section margins in high-risk cutaneous squamous cell carcinomas of the head and neck. Laryngoscope 2015, 125, 636–639. [Google Scholar] [CrossRef]
- Brown, I.S. Pathology of Perineural Spread. J. Neurol. Surg. B Skull Base 2016, 77, 124–130. [Google Scholar] [CrossRef]
- Yanofsky, V.R.; Mercer, S.E.; Phelps, R.G. Histopathological variants of cutaneous squamous cell carcinoma: A review. J. Ski. Cancer 2011, 2011, 210813. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).