Oxidative Stress in Melanoma: Beneficial Antioxidant and Pro-Oxidant Therapeutic Strategies
Abstract
Simple Summary
Abstract
1. Introduction
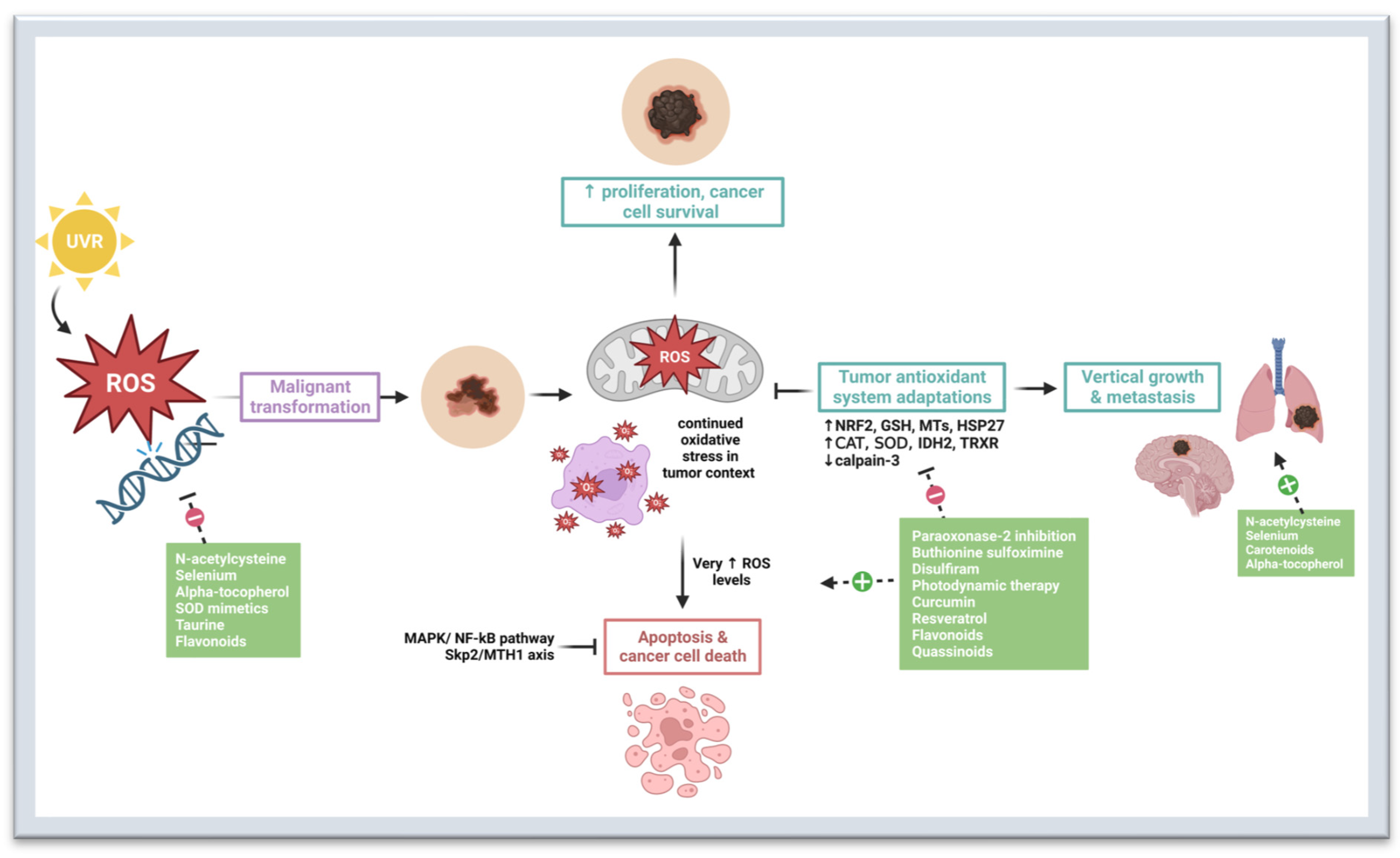
2. Oxidative Stress in Melanomagenesis
3. Preventive and Therapeutic Strategies That Target Oxidative Stress Pathways
3.1. N-Acetylcysteine
3.2. Selenium
3.3. Carotenoids
3.4. Alpha-Tocopherol
3.5. Superoxide Dismutase Mimetics
3.6. Taurine
3.7. Paraoxonase-2 Inhibitors
3.8. Buthionine Sulfoximine
3.9. Disulfiram
3.10. Photodynamic Therapy
3.11. Curcumin
3.12. Resveratrol
3.13. Flavonoids
3.14. Quassinoids
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- International Agency for Research on Cancer (IARC); WHO. Cancer Incidence in Five Continents, C15plus: IARC Cancer Base No.9. Available online: https://ci5.iarc.fr/Default.aspx (accessed on 28 March 2023).
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer Statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Melanoma of the Skin—Cancer Stat Facts. Available online: https://seer.cancer.gov/statfacts/html/melan.html (accessed on 17 July 2022).
- Gershenwald, J.E.; Scolyer, R.A.; Hess, K.R.; Sondak, V.K.; Long, G.V.; Ross, M.I.; Lazar, A.J.; Faries, M.B.; Kirkwood, J.M.; McArthur, G.A.; et al. Melanoma Staging: Evidence-Based Changes in the American Joint Committee on Cancer Eighth Edition Cancer Staging Manual. CA Cancer J. Clin. 2017, 67, 472–492. [Google Scholar] [CrossRef] [PubMed]
- Islami, F.; Ward, E.M.; Sung, H.; Cronin, K.A.; Tangka, F.K.L.; Sherman, R.L.; Zhao, J.; Anderson, R.N.; Henley, S.J.; Yabroff, K.R.; et al. Annual Report to the Nation on the Status of Cancer, Part 1: National Cancer Statistics. J. Natl. Cancer Inst. 2021, 113, 1648–1669. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.Y.; Fisher, D.E. Melanocyte Biology and Skin Pigmentation. Nature 2007, 445, 843–850. [Google Scholar] [CrossRef]
- Slominski, R.M.; Zmijewski, M.A.; Slominski, A.T. The Role of Melanin Pigment in Melanoma. Exp. Dermatol. 2015, 24, 258–259. [Google Scholar] [CrossRef]
- Davey, M.G.; Miller, N.; McInerney, N.M. A Review of Epidemiology and Cancer Biology of Malignant Melanoma. Cureus 2021, 13, e15087. [Google Scholar] [CrossRef]
- Flaherty, K.T.; Puzanov, I.; Kim, K.B.; Ribas, A.; McArthur, G.A.; Sosman, J.A.; O’Dwyer, P.J.; Lee, R.J.; Grippo, J.F.; Nolop, K.; et al. Inhibition of Mutated, Activated BRAF in Metastatic Melanoma. N. Engl. J. Med. 2010, 363, 809–819. [Google Scholar] [CrossRef]
- Arslanbaeva, L.R.; Santoro, M.M. Adaptive Redox Homeostasis in Cutaneous Melanoma. Redox Biol. 2020, 37, 101753. [Google Scholar] [CrossRef]
- Mullenders, L.H.F. Solar UV Damage to Cellular DNA: From Mechanisms to Biological Effects. Photochem. Photobiol. Sci. 2018, 17, 1842–1852. [Google Scholar] [CrossRef]
- Davis, E.J.; Johnson, D.B.; Sosman, J.A.; Chandra, S. Melanoma: What Do All the Mutations Mean? Cancer 2018, 124, 3490–3499. [Google Scholar] [CrossRef]
- Schadendorf, D.; van Akkooi, A.C.J.; Berking, C.; Griewank, K.G.; Gutzmer, R.; Hauschild, A.; Stang, A.; Roesch, A.; Ugurel, S. Melanoma. Lancet 2018, 392, 971–984. [Google Scholar] [CrossRef]
- Lim, S.Y.; Menzies, A.M.; Rizos, H. Mechanisms and Strategies to Overcome Resistance to Molecularly Targeted Therapy for Melanoma. Cancer 2017, 123, 2118–2129. [Google Scholar] [CrossRef]
- Leonardi, G.C.; Falzone, L.; Salemi, R.; Zanghì, A.; Spandidos, D.A.; Mccubrey, J.A.; Candido, S.; Libra, M. Cutaneous Melanoma: From Pathogenesis to Therapy (Review). Int. J. Oncol. 2018, 52, 1071–1080. [Google Scholar] [CrossRef]
- Ascierto, P.A.; Kirkwood, J.M.; Grob, J.-J.; Simeone, E.; Grimaldi, A.M.; Maio, M.; Palmieri, G.; Testori, A.; Marincola, F.M.; Mozzillo, N. The Role of BRAF V600 Mutation in Melanoma. J. Transl. Med. 2012, 10, 85. [Google Scholar] [CrossRef]
- Forbes, S.A.; Bhamra, G.; Bamford, S.; Dawson, E.; Kok, C.; Clements, J.; Menzies, A.; Teague, J.W.; Futreal, P.A.; Stratton, M.R. The Catalogue of Somatic Mutations in Cancer (COSMIC). In Current Protocols in Human Genetics; John Wiley & Sons: Hoboken, NJ, USA, 2008; Chapter 10: Unit 10.11. [Google Scholar] [CrossRef]
- Hayward, N.K.; Wilmott, J.S.; Waddell, N.; Johansson, P.A.; Field, M.A.; Nones, K.; Patch, A.-M.; Kakavand, H.; Alexandrov, L.B.; Burke, H.; et al. Whole-Genome Landscapes of Major Melanoma Subtypes. Nature 2017, 545, 175–180. [Google Scholar] [CrossRef]
- Domingues, B.; Lopes, J.M.; Soares, P.; Pópulo, H. Melanoma Treatment in Review. ImmunoTargets Ther. 2018, 7, 35–49. [Google Scholar] [CrossRef]
- Garbe, C.; Peris, K.; Hauschild, A.; Saiag, P.; Middleton, M.; Bastholt, L.; Grob, J.-J.; Malvehy, J.; Newton-Bishop, J.; Stratigos, A.J.; et al. Diagnosis and Treatment of Melanoma. European Consensus-Based Interdisciplinary Guideline—Update 2016. Eur. J. Cancer 2016, 63, 201–217. [Google Scholar] [CrossRef]
- Wilson, M.A.; Schuchter, L.M. Chemotherapy for Melanoma. Cancer Treat. Res. 2016, 167, 209–229. [Google Scholar] [CrossRef]
- Schreiber, R.D.; Old, L.J.; Smyth, M.J. Cancer Immunoediting: Integrating Immunity’s Roles in Cancer Suppression and Promotion. Science 2011, 331, 1565–1570. [Google Scholar] [CrossRef]
- Gurzu, S.; Beleaua, M.A.; Jung, I. The Role of Tumor Microenvironment in Development and Progression of Malignant Melanomas—A Systematic Review. Rom. J. Morphol. Embryol. 2018, 59, 23–28. [Google Scholar]
- Khair, D.O.; Bax, H.J.; Mele, S.; Crescioli, S.; Pellizzari, G.; Khiabany, A.; Nakamura, M.; Harris, R.J.; French, E.; Hoffmann, R.M.; et al. Combining Immune Checkpoint Inhibitors: Established and Emerging Targets and Strategies to Improve Outcomes in Melanoma. Front. Immunol. 2019, 10, 453. [Google Scholar] [CrossRef] [PubMed]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.-J.; Rutkowski, P.; Lao, C.D.; Cowey, C.L.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Five-Year Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2019, 381, 1535–1546. [Google Scholar] [CrossRef] [PubMed]
- Hughes, T.; Klairmont, M.; Sharfman, W.H.; Kaufman, H.L. Interleukin-2, Ipilimumab, and Anti-PD-1: Clinical Management and the Evolving Role of Immunotherapy for the Treatment of Patients with Metastatic Melanoma. Cancer Biol. Ther. 2021, 22, 513–526. [Google Scholar] [CrossRef] [PubMed]
- Mistry, H.B.; Orrell, D.; Eftimie, R. Model Based Analysis of the Heterogeneity in the Tumour Size Dynamics Differentiates Vemurafenib, Dabrafenib and Trametinib in Metastatic Melanoma. Cancer Chemother. Pharmacol. 2018, 81, 325–332. [Google Scholar] [CrossRef]
- Gide, T.N.; Wilmott, J.S.; Scolyer, R.A.; Long, G.V. Primary and Acquired Resistance to Immune Checkpoint Inhibitors in Metastatic Melanoma. Clin. Cancer Res. 2018, 24, 1260–1270. [Google Scholar] [CrossRef]
- van Breeschoten, J.; Wouters, M.W.J.M.; Hilarius, D.L.; Haanen, J.B.; Blank, C.U.; Aarts, M.J.B.; van den Berkmortel, F.W.P.J.; de Groot, J.-W.B.; Hospers, G.A.P.; Kapiteijn, E.; et al. First-Line BRAF/MEK Inhibitors versus Anti-PD-1 Monotherapy in BRAFV600-Mutant Advanced Melanoma Patients: A Propensity-Matched Survival Analysis. Br. J. Cancer 2021, 124, 1222–1230. [Google Scholar] [CrossRef]
- Denat, L.; Kadekaro, A.L.; Marrot, L.; Leachman, S.A.; Abdel-Malek, Z.A. Melanocytes as Instigators and Victims of Oxidative Stress. J. Investig. Dermatol. 2014, 134, 1512–1518. [Google Scholar] [CrossRef]
- Davalli, P.; Mitic, T.; Caporali, A.; Lauriola, A.; D’Arca, D. ROS, Cell Senescence, and Novel Molecular Mechanisms in Aging and Age-Related Diseases. Oxidative Med. Cell. Longev. 2016, 2016, 3565127. [Google Scholar] [CrossRef]
- Venza, M.; Visalli, M.; Beninati, C.; De Gaetano, G.V.; Teti, D.; Venza, I. Cellular Mechanisms of Oxidative Stress and Action in Melanoma. Oxidative Med. Cell. Longev. 2015, 2015, 481782. [Google Scholar] [CrossRef]
- Cannavò, S.P.; Tonacci, A.; Bertino, L.; Casciaro, M.; Borgia, F.; Gangemi, S. The Role of Oxidative Stress in the Biology of Melanoma: A Systematic Review. Pathol.-Res. Pract. 2019, 215, 21–28. [Google Scholar] [CrossRef]
- Carvalho, L.A.C.; Queijo, R.G.; Baccaro, A.L.B.; Siena, Á.D.D.; Silva, W.A.; Rodrigues, T.; Maria-Engler, S.S. Redox-Related Proteins in Melanoma Progression. Antioxidants 2022, 11, 438. [Google Scholar] [CrossRef]
- Emanuelli, M.; Sartini, D.; Molinelli, E.; Campagna, R.; Pozzi, V.; Salvolini, E.; Simonetti, O.; Campanati, A.; Offidani, A. The Double-Edged Sword of Oxidative Stress in Skin Damage and Melanoma: From Physiopathology to Therapeutical Approaches. Antioxidants 2022, 11, 612. [Google Scholar] [CrossRef]
- Wheeler, M.D.; Smutney, O.M.; Samulski, R.J. Secretion of Extracellular Superoxide Dismutase from Muscle Transduced with Recombinant Adenovirus Inhibits the Growth of B16 Melanomas in Mice. Mol. Cancer Res. 2003, 1, 871–881. [Google Scholar]
- Aggarwal, V.; Tuli, H.S.; Varol, A.; Thakral, F.; Yerer, M.B.; Sak, K.; Varol, M.; Jain, A.; Khan, M.d.A.; Sethi, G. Role of Reactive Oxygen Species in Cancer Progression: Molecular Mechanisms and Recent Advancements. Biomolecules 2019, 9, 735. [Google Scholar] [CrossRef]
- Liu-Smith, F.; Dellinger, R.; Meyskens, F.L. Updates of Reactive Oxygen Species in Melanoma Etiology and Progression. Arch. Biochem. Biophys. 2014, 563, 51–55. [Google Scholar] [CrossRef]
- Moloney, J.N.; Cotter, T.G. ROS Signalling in the Biology of Cancer. Semin. Cell Dev. Biol. 2018, 80, 50–64. [Google Scholar] [CrossRef]
- Sander, C.S.; Hamm, F.; Elsner, P.; Thiele, J.J. Oxidative Stress in Malignant Melanoma and Non-Melanoma Skin Cancer. Br. J. Dermatol. 2003, 148, 913–922. [Google Scholar] [CrossRef]
- Ortega, A.L.; Carretero, J.; Obrador, E.; Gambini, J.; Asensi, M.; Rodilla, V.; Estrela, J.M. Tumor Cytotoxicity by Endothelial Cells. Impairment of the Mitochondrial System for Glutathione Uptake in Mouse B16 Melanoma Cells That Survive after in Vitro Interaction with the Hepatic Sinusoidal Endothelium. J. Biol. Chem. 2003, 278, 13888–13897. [Google Scholar] [CrossRef]
- Woźniak, A.; Drewa, G.; Woźniak, B.; Schachtschabel, D.O. Activity of Antioxidant Enzymes and Concentration of Lipid Peroxidation Products in Selected Tissues of Mice of Different Ages, Both Healthy and Melanoma-Bearing. Z. Gerontol. Geriatr. 2004, 37, 184–189. [Google Scholar] [CrossRef]
- Li, X.; Wu, J.; Zhang, X.; Chen, W. Glutathione Reductase-Mediated Thiol Oxidative Stress Suppresses Metastasis of Murine Melanoma Cells. Free Radic. Biol. Med. 2018, 129, 256–267. [Google Scholar] [CrossRef]
- Schott, M.; de Jel, M.M.; Engelmann, J.C.; Renner, P.; Geissler, E.K.; Bosserhoff, A.K.; Kuphal, S. Selenium-Binding Protein 1 Is down-Regulated in Malignant Melanoma. Oncotarget 2018, 9, 10445–10456. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zheng, Z.; Kim, K.-Y.; Jin, X.; Roh, M.R.; Jin, Z. Hypermethylation and Downregulation of Glutathione Peroxidase 3 Are Related to Pathogenesis of Melanoma. Oncol. Rep. 2016, 36, 2737–2744. [Google Scholar] [CrossRef] [PubMed]
- Yi, Z.; Jiang, L.; Zhao, L.; Zhou, M.; Ni, Y.; Yang, Y.; Yang, H.; Yang, L.; Zhang, Q.; Kuang, Y.; et al. Glutathione Peroxidase 3 (GPX3) Suppresses the Growth of Melanoma Cells through Reactive Oxygen Species (ROS)-Dependent Stabilization of Hypoxia-Inducible Factor 1-α and 2-α. J. Cell. Biochem. 2019, 120, 19124–19136. [Google Scholar] [CrossRef] [PubMed]
- Nogués, M.R.; Giralt, M.; Cervelló, I.; Del Castillo, D.; Espeso, O.; Argany, N.; Aliaga, A.; Mallol, J. Parameters Related to Oxygen Free Radicals in Human Skin: A Study Comparing Healthy Epidermis and Skin Cancer Tissue. J. Investig. Dermatol. 2002, 119, 645–652. [Google Scholar] [CrossRef]
- Schadendorf, D.; Jurgovsky, K.; Kohlmus, C.M.; Czarnetzki, B.M. Glutathione and Related Enzymes in Tumor Progression and Metastases of Human Melanoma. J. Investig. Dermatol. 1995, 105, 109–112. [Google Scholar] [CrossRef]
- Kanetsky, P.A.; Holmes, R.; Walker, A.; Najarian, D.; Swoyer, J.; Guerry, D.; Halpern, A.; Rebbeck, T.R. Interaction of Glutathione S-Transferase M1 and T1 Genotypes and Malignant Melanoma. Cancer Epidemiol. Biomark. Prev. 2001, 10, 509–513. [Google Scholar]
- Kwee, J.K.; Mitidieri, E.; Affonso, O.R. Lowered Superoxide Dismutase in Highly Metastatic B16 Melanoma Cells. Cancer Lett. 1991, 57, 199–202. [Google Scholar] [CrossRef]
- Schadendorf, D.; Zuberbier, T.; Diehl, S.; Schadendorf, C.; Czarnetzki, B.M. Serum Manganese Superoxide Dismutase Is a New Tumour Marker for Malignant Melanoma. Melanoma Res. 1995, 5, 351–353. [Google Scholar] [CrossRef]
- Radojičić, R.M.; Spasić, S.; Saičić, Z.S.; Jovanović, T.B.; Simić-Krstić, J.B. Superoxide Dismutase Activity as a Function of Culture Aging of B-16 Mouse Melanoma Cells. J. Serb. Chem. Soc. 2004, 69, 1005–1011. [Google Scholar] [CrossRef]
- Grammatico, P.; Maresca, V.; Roccella, F.; Roccella, M.; Biondo, L.; Catricalà, C.; Picardo, M. Increased Sensitivity to Peroxidizing Agents Is Correlated with an Imbalance of Antioxidants in Normal Melanocytes from Melanoma Patients. Exp. Dermatol. 1998, 7, 205–212. [Google Scholar] [CrossRef]
- Bisevac, J.P.; Djukic, M.; Stanojevic, I.; Stevanovic, I.; Mijuskovic, Z.; Djuric, A.; Gobeljic, B.; Banovic, T.; Vojvodic, D. Association Between Oxidative Stress and Melanoma Progression. J. Med. Biochem. 2018, 37, 12–20. [Google Scholar] [CrossRef]
- Meyskens, F.L.; McNulty, S.E.; Buckmeier, J.A.; Tohidian, N.B.; Spillane, T.J.; Kahlon, R.S.; Gonzalez, R.I. Aberrant Redox Regulation in Human Metastatic Melanoma Cells Compared to Normal Melanocytes. Free Radic. Biol. Med. 2001, 31, 799–808. [Google Scholar] [CrossRef]
- Wang, J.Y.; Liu, G.Z.; Wilmott, J.S.; La, T.; Feng, Y.C.; Yari, H.; Yan, X.G.; Thorne, R.F.; Scolyer, R.A.; Zhang, X.D.; et al. Skp2-Mediated Stabilization of MTH1 Promotes Survival of Melanoma Cells upon Oxidative Stress. Cancer Res. 2017, 77, 6226–6239. [Google Scholar] [CrossRef]
- Pieri, L.; Dominici, S.; Del Bello, B.; Maellaro, E.; Comporti, M.; Paolicchi, A.; Pompella, A. Redox Modulation of Protein Kinase/Phosphatase Balance in Melanoma Cells: The Role of Endogenous and Gamma-Glutamyltransferase-Dependent H2O2 Production. Biochim. Biophys. Acta 2003, 1621, 76–83. [Google Scholar] [CrossRef]
- Giommarelli, C.; Corti, A.; Supino, R.; Favini, E.; Paolicchi, A.; Pompella, A.; Zunino, F. Cellular Response to Oxidative Stress and Ascorbic Acid in Melanoma Cells Overexpressing Gamma-Glutamyltransferase. Eur. J. Cancer 2008, 44, 750–759. [Google Scholar] [CrossRef]
- Li, J.; Peng, L.; Wu, L.; Kuang, Y.; Su, J.; Yi, M.; Hu, X.; Li, D.; Xie, H.; Kanekura, T.; et al. Depletion of CD147 Sensitizes Human Malignant Melanoma Cells to Hydrogen Peroxide-Induced Oxidative Stress. J. Dermatol. Sci. 2010, 58, 204–210. [Google Scholar] [CrossRef]
- Luo, Y.; Dallaglio, K.; Chen, Y.; Robinson, W.A.; Robinson, S.E.; McCarter, M.D.; Wang, J.; Gonzalez, R.; Thompson, D.C.; Norris, D.A.; et al. ALDH1A Isozymes Are Markers of Human Melanoma Stem Cells and Potential Therapeutic Targets. Stem Cells 2012, 30, 2100–2113. [Google Scholar] [CrossRef]
- Feng, Z.; Hom, M.E.; Bearrood, T.E.; Rosenthal, Z.C.; Fernández, D.; Ondrus, A.E.; Gu, Y.; McCormick, A.K.; Tomaske, M.G.; Marshall, C.R.; et al. Targeting Colorectal Cancer with Small-Molecule Inhibitors of ALDH1B1. Nat. Chem. Biol. 2022, 18, 1065–1075. [Google Scholar] [CrossRef]
- Mameishvili, E.; Serafimidis, I.; Iwaszkiewicz, S.; Lesche, M.; Reinhardt, S.; Bölicke, N.; Büttner, M.; Stellas, D.; Papadimitropoulou, A.; Szabolcs, M.; et al. Aldh1b1 Expression Defines Progenitor Cells in the Adult Pancreas and Is Required for Kras-Induced Pancreatic Cancer. Proc. Natl. Acad. Sci. USA 2019, 116, 20679–20688. [Google Scholar] [CrossRef]
- Wu, L.; Dong, B.; Zhang, F.; Li, Y.; Liu, L. Prediction of the Engendering Mechanism and Specific Genes of Primary Melanoma by Bioinformatics Analysis. Dermatol. Sin. 2016, 34, 14–19. [Google Scholar] [CrossRef]
- Yan, B.Y.; Garcet, S.; Gulati, N.; Kiecker, F.; Fuentes-Duculan, J.; Gilleaudeau, P.; Sullivan-Whalen, M.; Shemer, A.; Mitsui, H.; Krueger, J.G. Novel Immune Signatures Associated with Dysplastic Naevi and Primary Cutaneous Melanoma in Human Skin. Exp. Dermatol. 2019, 28, 35–44. [Google Scholar] [CrossRef]
- Zhai, Z.; Yamauchi, T.; Shangraw, S.; Hou, V.; Matsumoto, A.; Fujita, M. Ethanol Metabolism and Melanoma. Cancers 2023, 15, 1258. [Google Scholar] [CrossRef] [PubMed]
- Piskounova, E.; Agathocleous, M.; Murphy, M.M.; Hu, Z.; Huddlestun, S.E.; Zhao, Z.; Leitch, A.M.; Johnson, T.M.; DeBerardinis, R.J.; Morrison, S.J. Oxidative Stress Inhibits Distant Metastasis by Human Melanoma Cells. Nature 2015, 527, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Yoo, Y.H.; Lee, J.H.; Park, J.-W. Mitochondrial NADP(+)-Dependent Isocitrate Dehydrogenase Knockdown Inhibits Tumorigenicity of Melanoma Cells. Biochem. Biophys. Res. Commun. 2014, 451, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, E.L.; Becker, A.L.; Indra, A.K. NRF2 and Key Transcriptional Targets in Melanoma Redox Manipulation. Cancers 2022, 14, 1531. [Google Scholar] [CrossRef]
- Malakoutikhah, Z.; Mohajeri, Z.; Dana, N.; Haghjooy Javanmard, S. The Dual Role of Nrf2 in Melanoma: A Systematic Review. BMC Mol. Cell. Biol. 2023, 24, 5. [Google Scholar] [CrossRef]
- Jessen, C.; Kreß, J.K.C.; Baluapuri, A.; Hufnagel, A.; Schmitz, W.; Kneitz, S.; Roth, S.; Marquardt, A.; Appenzeller, S.; Ade, C.P.; et al. The Transcription Factor NRF2 Enhances Melanoma Malignancy by Blocking Differentiation and Inducing COX2 Expression. Oncogene 2020, 39, 6841–6855. [Google Scholar] [CrossRef]
- Davalos, V.; Lovell, C.D.; Von Itter, R.; Dolgalev, I.; Agrawal, P.; Baptiste, G.; Kahler, D.J.; Sokolova, E.; Moran, S.; Piqué, L.; et al. An Epigenetic Switch Controls an Alternative NR2F2 Isoform That Unleashes a Metastatic Program in Melanoma. Nat. Commun. 2023, 14, 1867. [Google Scholar] [CrossRef]
- Jasmer, K.J.; Hou, J.; Mannino, P.; Cheng, J.; Hannink, M. Heme Oxygenase Promotes B-Raf-Dependent Melanosphere Formation. Pigment Cell Melanoma Res. 2020, 33, 850–868. [Google Scholar] [CrossRef]
- Schmidlin, C.J.; Tian, W.; Dodson, M.; Chapman, E.; Zhang, D.D. FAM129B-Dependent Activation of NRF2 Promotes an Invasive Phenotype in BRAF Mutant Melanoma Cells. Mol. Carcinog. 2021, 60, 331–341. [Google Scholar] [CrossRef]
- Setaluri, V. Autophagy as a Melanocytic Self-Defense Mechanism. J. Investig. Dermatol. 2015, 135, 1215–1217. [Google Scholar] [CrossRef]
- Hintsala, H.-R.; Jokinen, E.; Haapasaari, K.-M.; Moza, M.; Ristimäki, A.; Soini, Y.; Koivunen, J.; Karihtala, P. Nrf2/Keap1 Pathway and Expression of Oxidative Stress Lesions 8-Hydroxy-2′-Deoxyguanosine and Nitrotyrosine in Melanoma. Anticancer Res. 2016, 36, 1497–1506. [Google Scholar]
- Ryšavá, A.; Vostálová, J.; Rajnochová Svobodová, A. Effect of Ultraviolet Radiation on the Nrf2 Signaling Pathway in Skin Cells. Int. J. Radiat. Biol. 2021, 97, 1383–1403. [Google Scholar] [CrossRef]
- Moretti, D.; Del Bello, B.; Allavena, G.; Corti, A.; Signorini, C.; Maellaro, E. Calpain-3 Impairs Cell Proliferation and Stimulates Oxidative Stress-Mediated Cell Death in Melanoma Cells. PLoS ONE 2015, 10, e0117258. [Google Scholar] [CrossRef]
- Coyle, P.; Philcox, J.C.; Carey, L.C.; Rofe, A.M. Metallothionein: The Multipurpose Protein. Cell. Mol. Life Sci. 2002, 59, 627–647. [Google Scholar] [CrossRef]
- Si, M.; Lang, J. The Roles of Metallothioneins in Carcinogenesis. J. Hematol. Oncol. 2018, 11, 107. [Google Scholar] [CrossRef]
- Kumari, M.V.; Hiramatsu, M.; Ebadi, M. Free Radical Scavenging Actions of Metallothionein Isoforms I and II. Free Radic. Res. 1998, 29, 93–101. [Google Scholar] [CrossRef]
- Richter, K.; Haslbeck, M.; Buchner, J. The Heat Shock Response: Life on the Verge of Death. Mol. Cell 2010, 40, 253–266. [Google Scholar] [CrossRef]
- Garrido, C.; Paul, C.; Seigneuric, R.; Kampinga, H.H. The Small Heat Shock Proteins Family: The Long Forgotten Chaperones. Int. J. Biochem. Cell Biol. 2012, 44, 1588–1592. [Google Scholar] [CrossRef]
- Schuster, C.; Akslen, L.A.; Straume, O. Expression of Heat Shock Protein 27 in Melanoma Metastases Is Associated with Overall Response to Bevacizumab Monotherapy: Analyses of Predictive Markers in a Clinical Phase II Study. PLoS ONE 2016, 11, e0155242. [Google Scholar] [CrossRef]
- Kaur, A.; Webster, M.R.; Marchbank, K.; Behera, R.; Ndoye, A.; Kugel, C.H.; Dang, V.M.; Appleton, J.; O’Connell, M.P.; Cheng, P.; et al. SFRP2 in the Aged Microenvironment Drives Melanoma Metastasis and Therapy Resistance. Nature 2016, 532, 250–254. [Google Scholar] [CrossRef]
- Rózanowska, M.; Sarna, T.; Land, E.J.; Truscott, T.G. Free Radical Scavenging Properties of Melanin Interaction of Eu- and Pheo-Melanin Models with Reducing and Oxidising Radicals. Free Radic. Biol. Med. 1999, 26, 518–525. [Google Scholar] [CrossRef] [PubMed]
- El-Naggar, N.E.-A.; El-Ewasy, S.M. Bioproduction, Characterization, Anticancer and Antioxidant Activities of Extracellular Melanin Pigment Produced by Newly Isolated Microbial Cell Factories Streptomyces Glaucescens NEAE-H. Sci. Rep. 2017, 7, 42129. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Kim, T.K.; Brozyna, A.A.; Janjetovic, Z.; Brooks, D.L.; Schwab, L.P.; Skobowiat, C.; Jozwicki, W.; Seagroves, T.N. The Role of Melanogenesis in Regulation of Melanoma Behavior: Melanogenesis Leads to Stimulation of HIF-1alpha Expression and HIF-Dependent Attendant Pathways. Arch. Biochem. Biophys. 2014, 563, 79–93. [Google Scholar] [CrossRef] [PubMed]
- Trachootham, D.; Alexandre, J.; Huang, P. Targeting Cancer Cells by ROS-Mediated Mechanisms: A Radical Therapeutic Approach? Nat. Rev. Drug Discov. 2009, 8, 579–591. [Google Scholar] [CrossRef]
- Goodson, A.G.; Cotter, M.A.; Cassidy, P.; Wade, M.; Florell, S.R.; Liu, T.; Boucher, K.M.; Grossman, D. Use of Oral N-Acetylcysteine for Protection of Melanocytic Nevi against UV-Induced Oxidative Stress: Towards a Novel Paradigm for Melanoma Chemoprevention. Clin. Cancer Res. 2009, 15, 7434–7440. [Google Scholar] [CrossRef]
- Cotter, M.A.; Thomas, J.; Cassidy, P.; Robinette, K.; Jenkins, N.; Florell, S.R.; Leachman, S.; Samlowski, W.E.; Grossman, D. N-Acetylcysteine Protects Melanocytes against Oxidative Stress/Damage and Delays Onset of Ultraviolet-Induced Melanoma in Mice. Clin. Cancer Res. 2007, 13, 5952–5958. [Google Scholar] [CrossRef]
- Cassidy, P.B.; Fain, H.D.; Cassidy, J.P.; Tran, S.M.; Moos, P.J.; Boucher, K.M.; Gerads, R.; Florell, S.R.; Grossman, D.; Leachman, S.A. Selenium for the Prevention of Cutaneous Melanoma. Nutrients 2013, 5, 725–749. [Google Scholar] [CrossRef]
- Gębka, N.; Gębka-Kępińska, B.; Adamczyk, J.; Mizgała-Izworska, E. The Use of Flavonoids in Skin Cancer Prevention and Treatment. J. Pre. Clin. Clin. Res. 2022, 16, 108–113. [Google Scholar] [CrossRef]
- Chung, J.H.; Seo, J.Y.; Lee, M.K.; Eun, H.C.; Lee, J.H.; Kang, S.; Fisher, G.J.; Voorhees, J.J. Ultraviolet Modulation of Human Macrophage Metalloelastase in Human Skin in Vivo. J. Investig. Dermatol. 2002, 119, 507–512. [Google Scholar] [CrossRef]
- Sfaxi, I.H.; Ferraro, D.; Fasano, E.; Pani, G.; Limam, F.; Marzouki, M.N. Inhibitory Effects of a Manganese Superoxide Dismutase Isolated from Garlic (Allium sativum L.) on in Vitro Tumoral Cell Growth. Biotechnol. Prog. 2009, 25, 257–264. [Google Scholar] [CrossRef]
- Yu, J.; Kim, A.K. Effect of Taurine on Antioxidant Enzyme System in B16F10 Melanoma Cells. Adv. Exp. Med. Biol. 2009, 643, 491–499. [Google Scholar] [CrossRef]
- Obrador, E.; Salvador-Palmer, R.; López-Blanch, R.; Oriol-Caballo, M.; Moreno-Murciano, P.; Estrela, J.M. N-Acetylcysteine Promotes Metastatic Spread of Melanoma in Mice. Cancers 2022, 14, 3614. [Google Scholar] [CrossRef]
- Kashif, M.; Yao, H.; Schmidt, S.; Chen, X.; Truong, M.; Tüksammel, E.; Liu, Y.; Bergo, M.O. ROS-Lowering Doses of Vitamins C and A Accelerate Malignant Melanoma Metastasis. Redox Biol. 2023, 60, 102619. [Google Scholar] [CrossRef]
- Campagna, R.; Bacchetti, T.; Salvolini, E.; Pozzi, V.; Molinelli, E.; Brisigotti, V.; Sartini, D.; Campanati, A.; Ferretti, G.; Offidani, A.; et al. Paraoxonase-2 Silencing Enhances Sensitivity of A375 Melanoma Cells to Treatment with Cisplatin. Antioxidants 2020, 9, 1238. [Google Scholar] [CrossRef]
- Ongaro, A.; Pellati, A.; De Mattei, M.; De Terlizzi, F.; Rossi, C.R.; Campana, L.G. Enhancement of Melphalan Activity by Buthionine Sulfoximine and Electroporation in Melanoma Cells. Anticancer Drugs 2015, 26, 284–292. [Google Scholar] [CrossRef]
- Calderon-Aparicio, A.; Cornejo, A.; Orue, A.; Rieber, M. Anticancer Response to Disulfiram May Be Enhanced by Co-Treatment with MEK Inhibitor or Oxaliplatin: Modulation by Tetrathiomolybdate, KRAS/BRAF Mutations and c-MYC/P53 Status. Ecancermedicalscience 2019, 13, 890. [Google Scholar] [CrossRef]
- Cen, D.; Brayton, D.; Shahandeh, B.; Meyskens, F.L.; Farmer, P.J. Disulfiram Facilitates Intracellular Cu Uptake and Induces Apoptosis in Human Melanoma Cells. J. Med. Chem. 2004, 47, 6914–6920. [Google Scholar] [CrossRef]
- Morrison, B.W.; Doudican, N.A.; Patel, K.R.; Orlow, S.J. Disulfiram Induces Copper-Dependent Stimulation of Reactive Oxygen Species and Activation of the Extrinsic Apoptotic Pathway in Melanoma. Melanoma Res. 2010, 20, 11–20. [Google Scholar] [CrossRef]
- Fontes, S.S.; Nogueira, M.L.; Dias, R.B.; Rocha, C.A.G.; Soares, M.B.P.; Vannier-Santos, M.A.; Bezerra, D.P. Combination Therapy of Curcumin and Disulfiram Synergistically Inhibits the Growth of B16-F10 Melanoma Cells by Inducing Oxidative Stress. Biomolecules 2022, 12, 1600. [Google Scholar] [CrossRef]
- Bush, J.A.; Cheung, K.J.; Li, G. Curcumin Induces Apoptosis in Human Melanoma Cells through a Fas Receptor/Caspase-8 Pathway Independent of P53. Exp. Cell Res. 2001, 271, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Kocyigit, A.; Guler, E.M. Curcumin Induce DNA Damage and Apoptosis through Generation of Reactive Oxygen Species and Reducing Mitochondrial Membrane Potential in Melanoma Cancer Cells. Cell. Mol. Biol. 2017, 63, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Darjatmoko, S.R.; Polans, A.S. Resveratrol Modulates the Malignant Properties of Cutaneous Melanoma through Changes in the Activation and Attenuation of the Antiapoptotic Protooncogenic Protein Akt/PKB. Melanoma Res. 2011, 21, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-J.; Chen, Y.-Y.; Lin, Y.-F.; Hu, H.-Y.; Liao, H.-F. Resveratrol Inhibits Alpha-Melanocyte-Stimulating Hormone Signaling, Viability, and Invasiveness in Melanoma Cells. Evid.-Based Complement. Alternat. Med. 2013, 2013, 632121. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.-R.; Kim, S.-M.; Hwang, K.-A.; Kang, J.-H.; Choi, K.-C. Resveratrol Induced Reactive Oxygen Species and Endoplasmic Reticulum Stress-mediated Apoptosis, and Cell Cycle Arrest in the A375SM Malignant Melanoma Cell Line. Int. J. Mol. Med. 2018, 42, 1427–1435. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Papp, L.V.; Fang, J.; Rodriguez-Nieto, S.; Zhivotovsky, B.; Holmgren, A. Inhibition of Mammalian Thioredoxin Reductase by Some Flavonoids: Implications for Myricetin and Quercetin Anticancer Activity. Cancer Res. 2006, 66, 4410–4418. [Google Scholar] [CrossRef]
- Netcharoensirisuk, P.; Abrahamian, C.; Tang, R.; Chen, C.-C.; Rosato, A.S.; Beyers, W.; Chao, Y.-K.; Filippini, A.; Di Pietro, S.; Bartel, K.; et al. Flavonoids Increase Melanin Production and Reduce Proliferation, Migration and Invasion of Melanoma Cells by Blocking Endolysosomal/Melanosomal TPC2. Sci. Rep. 2021, 11, 8515. [Google Scholar] [CrossRef]
- Liu-Smith, F.; Meyskens, F.L. Molecular Mechanisms of Flavonoids in Melanin Synthesis and the Potential for the Prevention and Treatment of Melanoma. Mol. Nutr. Food Res. 2016, 60, 1264–1274. [Google Scholar] [CrossRef]
- Balyan, R.; Kudugunti, S.K.; Hamad, H.A.; Yousef, M.S.; Moridani, M.Y. Bioactivation of Luteolin by Tyrosinase Selectively Inhibits Glutathione S-Transferase. Chem. Biol. Interact. 2015, 240, 208–218. [Google Scholar] [CrossRef]
- Cucci, M.A.; Grattarola, M.; Dianzani, C.; Damia, G.; Ricci, F.; Roetto, A.; Trotta, F.; Barrera, G.; Pizzimenti, S. Ailanthone Increases Oxidative Stress in CDDP-Resistant Ovarian and Bladder Cancer Cells by Inhibiting of Nrf2 and YAP Expression through a Post-Translational Mechanism. Free Radic. Biol. Med. 2020, 150, 125–135. [Google Scholar] [CrossRef]
- Daga, M.; Pizzimenti, S.; Dianzani, C.; Cucci, M.A.; Cavalli, R.; Grattarola, M.; Ferrara, B.; Scariot, V.; Trotta, F.; Barrera, G. Ailanthone Inhibits Cell Growth and Migration of Cisplatin Resistant Bladder Cancer Cells through Down-Regulation of Nrf2, YAP, and c-Myc Expression. Phytomedicine 2019, 56, 156–164. [Google Scholar] [CrossRef]
- Ding, H.; Yu, X.; Hang, C.; Gao, K.; Lao, X.; Jia, Y.; Yan, Z. Ailanthone: A Novel Potential Drug for Treating Human Cancer. Oncol. Lett. 2020, 20, 1489–1503. [Google Scholar] [CrossRef]
- Lara-Vega, I.; Vega-López, A. Combinational Photodynamic and Photothermal—Based Therapies for Melanoma in Mouse Models. Photodiagn. Photodyn. Ther. 2023, in press. [Google Scholar] [CrossRef]
- Le Gal, K.; Ibrahim, M.X.; Wiel, C.; Sayin, V.I.; Akula, M.K.; Karlsson, C.; Dalin, M.G.; Akyürek, L.M.; Lindahl, P.; Nilsson, J.; et al. Antioxidants Can Increase Melanoma Metastasis in Mice. Sci. Transl. Med. 2015, 7, 308re8. [Google Scholar] [CrossRef]
- Schrauzer, G.N.; Surai, P.F. Selenium in Human and Animal Nutrition: Resolved and Unresolved Issues. A Partly Historical Treatise in Commemoration of the Fiftieth Anniversary of the Discovery of the Biological Essentiality of Selenium, Dedicated to the Memory of Klaus Schwarz (1914–1978) on the Occasion of the Thirtieth Anniversary of His Death. Crit. Rev. Biotechnol. 2009, 29, 2–9. [Google Scholar] [CrossRef]
- Blot, W.J.; Li, J.Y.; Taylor, P.R.; Guo, W.; Dawsey, S.; Wang, G.Q.; Yang, C.S.; Zheng, S.F.; Gail, M.; Li, G.Y. Nutrition Intervention Trials in Linxian, China: Supplementation with Specific Vitamin/Mineral Combinations, Cancer Incidence, and Disease-Specific Mortality in the General Population. J. Natl. Cancer Inst. 1993, 85, 1483–1492. [Google Scholar] [CrossRef]
- Miura, K.; Green, A.C. Dietary Antioxidants and Melanoma: Evidence from Cohort and Intervention Studies. Nutr. Cancer 2015, 67, 867–876. [Google Scholar] [CrossRef]
- Maoka, T. Carotenoids as Natural Functional Pigments. J. Nat. Med. 2020, 74, 1–16. [Google Scholar] [CrossRef]
- Asgari, M.M.; Brasky, T.M.; White, E. Association of Vitamin A and Carotenoid Intake with Melanoma Risk in a Large Prospective Cohort. J. Investig. Dermatol. 2012, 132, 1573–1582. [Google Scholar] [CrossRef]
- Lee, I.M.; Cook, N.R.; Manson, J.E.; Buring, J.E.; Hennekens, C.H. Beta-Carotene Supplementation and Incidence of Cancer and Cardiovascular Disease: The Women’s Health Study. J. Natl. Cancer Inst. 1999, 91, 2102–2106. [Google Scholar] [CrossRef]
- Druesne-Pecollo, N.; Latino-Martel, P.; Norat, T.; Barrandon, E.; Bertrais, S.; Galan, P.; Hercberg, S. Beta-Carotene Supplementation and Cancer Risk: A Systematic Review and Metaanalysis of Randomized Controlled Trials. Int. J. Cancer 2010, 127, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Chhabra, G.; Ndiaye, M.A.; Garcia-Peterson, L.M.; Ahmad, N. Melanoma Chemoprevention: Current Status and Future Prospects. Photochem. Photobiol. 2017, 93, 975–989. [Google Scholar] [CrossRef] [PubMed]
- Hercberg, S.; Galan, P.; Preziosi, P.; Bertrais, S.; Mennen, L.; Malvy, D.; Roussel, A.-M.; Favier, A.; Briançon, S. The SU.VI.MAX Study: A Randomized, Placebo-Controlled Trial of the Health Effects of Antioxidant Vitamins and Minerals. Arch. Intern. Med. 2004, 164, 2335–2342. [Google Scholar] [CrossRef] [PubMed]
- Robbins, D.; Zhao, Y. Manganese Superoxide Dismutase in Cancer Prevention. Antioxid. Redox Signal. 2014, 20, 1628–1645. [Google Scholar] [CrossRef]
- Heer, C.D.; Davis, A.B.; Riffe, D.B.; Wagner, B.A.; Falls, K.C.; Allen, B.G.; Buettner, G.R.; Beardsley, R.A.; Riley, D.P.; Spitz, D.R. Superoxide Dismutase Mimetic GC4419 Enhances the Oxidation of Pharmacological Ascorbate and Its Anticancer Effects in an H2O2-Dependent Manner. Antioxidants 2018, 7, 18. [Google Scholar] [CrossRef]
- Mapuskar, K.A.; Anderson, C.M.; Spitz, D.R.; Batinic-Haberle, I.; Allen, B.G.; Oberley-Deegan, R. Utilizing Superoxide Dismutase Mimetics to Enhance Radiation Therapy Response While Protecting Normal Tissues. Semin. Radiat. Oncol. 2019, 29, 72–80. [Google Scholar] [CrossRef]
- Tu, S.; Zhang, X.-L.; Wan, H.-F.; Xia, Y.-Q.; Liu, Z.-Q.; Yang, X.-H.; Wan, F.-S. Effect of Taurine on Cell Proliferation and Apoptosis Human Lung Cancer A549 Cells. Oncol Lett 2018, 15, 5473–5480. [Google Scholar] [CrossRef]
- Mates, J.M.; Segura, J.A.; Alonso, F.J.; Marquez, J. Sulphur-Containing Non Enzymatic Antioxidants: Therapeutic Tools against Cancer. Front Biosci (Schol Ed) 2012, 4, 722–748. [Google Scholar] [CrossRef]
- Higuchi, K.; Sugiyama, K.; Tomabechi, R.; Kishimoto, H.; Inoue, K. Mammalian Monocarboxylate Transporter 7 (MCT7/Slc16a6) Is a Novel Facilitative Taurine Transporter. J. Biol. Chem. 2022, 298, 101800. [Google Scholar] [CrossRef]
- Dai, W.; Liu, H.; Chen, K.; Xu, X.; Qian, D.; Luo, S.; Amos, C.I.; Lee, J.E.; Li, X.; Nan, H.; et al. Genetic Variants in PDSS1 and SLC16A6 of the Ketone Body Metabolic Pathway Predict Cutaneous Melanoma-Specific Survival. Mol Carcinog 2020, 59, 640–650. [Google Scholar] [CrossRef]
- Altenhöfer, S.; Witte, I.; Teiber, J.F.; Wilgenbus, P.; Pautz, A.; Li, H.; Daiber, A.; Witan, H.; Clement, A.M.; Förstermann, U.; et al. One Enzyme, Two Functions: PON2 Prevents Mitochondrial Superoxide Formation and Apoptosis Independent from Its Lactonase Activity. J. Biol. Chem. 2010, 285, 24398–24403. [Google Scholar] [CrossRef]
- Devarajan, A.; Bourquard, N.; Hama, S.; Navab, M.; Grijalva, V.R.; Morvardi, S.; Clarke, C.F.; Vergnes, L.; Reue, K.; Teiber, J.F.; et al. Paraoxonase 2 Deficiency Alters Mitochondrial Function and Exacerbates the Development of Atherosclerosis. Antioxid. Redox Signal. 2011, 14, 341–351. [Google Scholar] [CrossRef]
- Bacchetti, T.; Salvolini, E.; Pompei, V.; Campagna, R.; Molinelli, E.; Brisigotti, V.; Togni, L.; Lucarini, G.; Sartini, D.; Campanati, A.; et al. Paraoxonase-2: A Potential Biomarker for Skin Cancer Aggressiveness. Eur. J. Clin. Investig. 2021, 51, e13452. [Google Scholar] [CrossRef]
- Meraz-Torres, F.; Plöger, S.; Garbe, C.; Niessner, H.; Sinnberg, T. Disulfiram as a Therapeutic Agent for Metastatic Malignant Melanoma-Old Myth or New Logos? Cancers 2020, 12, 3538. [Google Scholar] [CrossRef]
- Cvek, B.; Dvorak, Z. The Value of Proteasome Inhibition in Cancer. Can the Old Drug, Disulfiram, Have a Bright New Future as a Novel Proteasome Inhibitor? Drug Discov. Today 2008, 13, 716–722. [Google Scholar] [CrossRef]
- Li, C.; Zhou, S.; Chen, C.; Zhu, L.; Li, S.; Song, Z.; Liang, J.; Tang, C.; Xu, N.; Liu, T.; et al. DDTC-Cu(I) Based Metal-Organic Framework (MOF) for Targeted Melanoma Therapy by Inducing SLC7A11/GPX4-Mediated Ferroptosis. Colloids Surf. B Biointerfaces 2023, 225, 113253. [Google Scholar] [CrossRef]
- Tang, S.-J.; Wang, M.-F.; Yang, R.; Liu, M.; Li, Q.-F.; Gao, F. More-Is-Better Strategy for Constructing Homoligand Polypyridyl Ruthenium Complexes as Photosensitizers for Infrared Two-Photon Photodynamic Therapy. Inorg. Chem. 2023, 62, 8210–8218. [Google Scholar] [CrossRef]
- Balas, M.; Nistorescu, S.; Badea, M.A.; Dinischiotu, A.; Boni, M.; Dinache, A.; Smarandache, A.; Udrea, A.-M.; Prepelita, P.; Staicu, A. Photodynamic Activity of TMPyP4/TiO2 Complex under Blue Light in Human Melanoma Cells: Potential for Cancer-Selective Therapy. Pharmaceutics 2023, 15, 1194. [Google Scholar] [CrossRef]
- Xin, J.; Wang, J.; Yao, Y.; Wang, S.; Zhang, Z.; Yao, C. Improved Simulated-Daylight Photodynamic Therapy and Possible Mechanism of Ag-Modified TiO2 on Melanoma. Int. J. Mol. Sci. 2023, 24, 7061. [Google Scholar] [CrossRef]
- Liao, W.; Xiang, W.; Wang, F.-F.; Wang, R.; Ding, Y. Curcumin Inhibited Growth of Human Melanoma A375 Cells via Inciting Oxidative Stress. Biomed. Pharmacother. 2017, 95, 1177–1186. [Google Scholar] [CrossRef]
- Szlasa, W.; Supplitt, S.; Drąg-Zalesińska, M.; Przystupski, D.; Kotowski, K.; Szewczyk, A.; Kasperkiewicz, P.; Saczko, J.; Kulbacka, J. Effects of Curcumin Based PDT on the Viability and the Organization of Actin in Melanotic (A375) and Amelanotic Melanoma (C32)—In Vitro Studies. Biomed. Pharmacother. 2020, 132, 110883. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Mishra, A.P.; Nigam, M.; Sener, B.; Kilic, M.; Sharifi-Rad, M.; Fokou, P.V.T.; Martins, N.; Sharifi-Rad, J. Resveratrol: A Double-Edged Sword in Health Benefits. Biomedicines 2018, 6, 91. [Google Scholar] [CrossRef] [PubMed]
- Ravikumar, P.; Katariya, M.; Patil, S.; Tatke, P.; Pillai, R. Skin Delivery of Resveratrol Encapsulated Lipidic Formulation for Melanoma Chemoprevention. J. Microencapsul. 2019, 36, 535–551. [Google Scholar] [CrossRef] [PubMed]
- Davoodvandi, A.; Darvish, M.; Borran, S.; Nejati, M.; Mazaheri, S.; Reza Tamtaji, O.; Hamblin, M.R.; Masoudian, N.; Mirzaei, H. The Therapeutic Potential of Resveratrol in a Mouse Model of Melanoma Lung Metastasis. Int. Immunopharmacol. 2020, 88, 106905. [Google Scholar] [CrossRef]
- Júnior, R.G.d.O.; Ferraz, C.A.A.; Silva, M.G.e.; Lavor, É.M.d.; Rolim, L.A.; Lima, J.T.; Fleury, A.; Picot, L.; Siqueira Quintans, J.; Júnior, L.; et al. Flavonoids: Promising Natural Products for Treatment of Skin Cancer (Melanoma). In Natural Products and Cancer Drug Discovery; Intech: London, UK, 2017. [Google Scholar] [CrossRef]
- Carpenter, E.L.; Wyant, M.B.; Indra, A.; Ito, S.; Wakamatsu, K.; Merrill, G.F.; Moos, P.J.; Cassidy, P.B.; Leachman, S.A.; Ganguli-Indra, G.; et al. Thioredoxin Reductase 1 Modulates Pigmentation and Photobiology of Murine Melanocytes in Vivo. J. Investig. Dermatol. 2022, 142, 1903–1911.e5. [Google Scholar] [CrossRef]
- Sak, K. Anticancer Effects of Flavonoids on Melanoma Cells: Are Murine Cells More Sensitive Compared to Humans? Int. J. Phytomed. 2013, 5, 441–445. [Google Scholar]
- Wang, M.; Shi, G.; Bian, C.; Nisar, M.F.; Guo, Y.; Wu, Y.; Li, W.; Huang, X.; Jiang, X.; Bartsch, J.W.; et al. UVA Irradiation Enhances Brusatol-Mediated Inhibition of Melanoma Growth by Downregulation of the Nrf2-Mediated Antioxidant Response. Oxidative Med. Cell. Longev. 2018, 2018, 9742154. [Google Scholar] [CrossRef]
| Intervention | Proposed Mechanism(s) of Action |
|---|---|
| N-acetylcysteine |
|
| Selenium | |
| Carotenoids |
|
| Alpha-tocopherol |
|
| SOD mimetics |
|
| Taurine |
|
| Paraoxonase -2 inhibitors |
|
| Buthioninesulfoximine |
|
| Disulfiram |
|
| Photodynamic therapy | |
| Curcumin | |
| Resveratrol |
|
| Flavanoids | |
| Quassinoids |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Becker, A.L.; Indra, A.K. Oxidative Stress in Melanoma: Beneficial Antioxidant and Pro-Oxidant Therapeutic Strategies. Cancers 2023, 15, 3038. https://doi.org/10.3390/cancers15113038
Becker AL, Indra AK. Oxidative Stress in Melanoma: Beneficial Antioxidant and Pro-Oxidant Therapeutic Strategies. Cancers. 2023; 15(11):3038. https://doi.org/10.3390/cancers15113038
Chicago/Turabian StyleBecker, Alyssa L., and Arup K. Indra. 2023. "Oxidative Stress in Melanoma: Beneficial Antioxidant and Pro-Oxidant Therapeutic Strategies" Cancers 15, no. 11: 3038. https://doi.org/10.3390/cancers15113038
APA StyleBecker, A. L., & Indra, A. K. (2023). Oxidative Stress in Melanoma: Beneficial Antioxidant and Pro-Oxidant Therapeutic Strategies. Cancers, 15(11), 3038. https://doi.org/10.3390/cancers15113038







