Melatonin Modulates the SIRT1-Related Pathways via Transdermal Cryopass-Laser Administration in Prostate Tumor Xenograft
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Groups
2.2. Xenograft Morphological Evaluation
2.3. Immunofluorescence and Immunohistochemistry Assay
2.4. Statistical Analysis
3. Results
3.1. Prostate Tumor Xenograft
3.2. Sirtuin1 in Tumorigenesis
3.3. Sirtuin1 Signaling Pathway in Tumorigenesis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Cancer. World Health Organization: Geneva, Switzerland. Available online: https://www.who.int/health-topics/cancer#tab=tab_1 (accessed on 6 December 2022).
- Leslie, S.W.; Soon-Sutton, T.L.; Sajjad, H.; Siref, L.E. Prostate Cancer. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Kaiser, A.; Haskins, C.; Siddiqui, M.M.; Hussain, A.; D’Adamo, C. The evolving role of diet in prostate cancer risk and progression. Curr. Opin. Oncol. 2019, 31, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Jha, G.G.; Anand, V.; Soubra, A.; Konety, B.R. Challenges of managing elderly men with prostate cancer. Nat. Rev. Clin. Oncol. 2014, 11, 354–364. [Google Scholar] [CrossRef] [PubMed]
- Long, M.D.; Singh, P.K.; Russell, J.R.; Llimos, G.; Rosario, S.; Rizvi, A.; van den Berg, P.R.; Kirk, J.; Sucheston-Campbell, L.E.; Smiraglia, D.J.; et al. The miR-96 and RARγ signaling axis governs androgen signaling and prostate cancer progression. Oncogene 2019, 38, 421–444. [Google Scholar] [CrossRef] [PubMed]
- Cattley, R.C.; Radinsky, R.R. Cancer therapeutics: Understanding the mechanism of action. Toxicol. Pathol. 2004, 32, 116–121. [Google Scholar] [CrossRef]
- Davoodvandi, A.; Nikfar, B.; Reiter, R.J.; Asemi, Z. Melatonin and cancer suppression: Insights into its effects on DNA methylation. Cell. Mol. Biol. Lett. 2022, 27, 73. [Google Scholar] [CrossRef]
- Paroni, R.; Terraneo, L.; Bonomini, F.; Finati, E.; Virgili, E.; Bianciardi, P.; Favero, G.; Fraschini, F.; Reiter, R.J.; Rezzani, R.; et al. Antitumour activity of melatonin in a mouse model of human prostate cancer: Relationship with hypoxia signalling. J. Pineal Res. 2014, 57, 43–52. [Google Scholar] [CrossRef]
- Karasek, M. Does melatonin play a role in aging processes? J. Physiol. Pharmacol. 2007, 58, 105–113. [Google Scholar]
- Terraneo, L.; Bianciardi, P.; Virgili, E.; Finati, E.; Samaja, M.; Paroni, R. Transdermal administration of melatonin coupled to cryopass laser treatment as noninvasive therapy for prostate cancer. Drug Deliv. 2017, 24, 979–985. [Google Scholar] [CrossRef][Green Version]
- Haigis, M.C.; Guarente, L.P. Mammalian sirtuins: Emerging roles in physiology, aging, and calorie restriction. Genes. Dev. 2006, 21, 2913–2921. [Google Scholar] [CrossRef]
- Shi, Y.; Han, J.J.; Tennakoon, J.B.; Mehta, F.F.; Merchant, F.A.; Burns, A.R.; Howe, M.K.; McDonnell, D.P.; Frigo, D.E. Androgens promote prostate cancer cell growth through induction of autophagy. Mol. Endocrinol. 2013, 27, 280–295. [Google Scholar] [CrossRef]
- Pulla, V.K.; Alvala, M.; Sriram, D.S.; Viswanadha, S.; Sriram, D.; Yogeeswari, P. Structure-based drug design of small molecule SIRT1 modulators to treat cancer and metabolic disorders. J. Mol. Graph. Model. 2014, 52, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Luo, X.; Ye, X.; Li, H.; Liu, H.; Du, Q.; Zhai, Q. SIRT1/PGC-1α/PPAR-γ correlate with hypoxia-induced chemoresistance in non-small cell lung cancer. Front. Oncol. 2021, 11, 682762. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; He, H.; Zuo, Z.; Xu, Z.; Wei, Z.; Deng, J. The role of different SIRT1-mediated signaling pathways in toxic injury. Cell. Mol. Biol. Lett. 2019, 24, 36. [Google Scholar] [CrossRef]
- Onyiba, C.I.; Scarlett, C.J.; Weidenhofer, J. The Mechanistic Roles of Sirtuins in Breast and Prostate Cancer. Cancers 2022, 14, 5118. [Google Scholar] [CrossRef]
- Burns-Cox, N.; Avery, N.C.; Gingell, J.C.; Bailey, A.J. Changes in collagen metabolism in prostate cancer: A host response that may alter progression. J. Urol. 2001, 166, 1698–1701. [Google Scholar] [CrossRef]
- Szadvari, I.; Krizanova, O.; Babula, P. Athymic nude mice as an experimental model for cancer treatment. Physiol. Res. 2016, 65, S441–S453. [Google Scholar] [CrossRef] [PubMed]
- Favero, G.; Lonati, C.; Giugno, L.; Castrezzati, S.; Rodella, L.F.; Rezzani, R. Obesity-related dysfunction of the aorta and prevention by melatonin treatment in ob/ob mice. Acta Histochem. 2013, 115, 783–788. [Google Scholar] [CrossRef]
- Favero, G.; Paini, A.; De Ciuceis, C.; Rodella, L.F.; Moretti, E.; Porteri, E.; Rossini, C.; Ministrini, S.; Solaini, L.; Stefano, C.; et al. Changes in extracellular matrix in subcutaneous small resistance arteries of patients with essential hypertension. Blood Press. 2018, 27, 231–239. [Google Scholar] [CrossRef]
- Bonomini, F.; Favero, G.; Rodella, L.F.; Moghadasian, M.H.; Rezzani, R. Melatonin Modulation of Sirtuin-1 Attenuates Liver Injury in a Hypercholesterolemic Mouse Model. BioMed Res. Int. 2018, 2018, 7968452. [Google Scholar] [CrossRef]
- Stacchiotti, A.; Favero, G.; Giugno, L.; Golic, I.; Korac, A.; Rezzani, R. Melatonin Efficacy in Obese Leptin-Deficient Mice Heart. Nutrients 2017, 9, 1323. [Google Scholar] [CrossRef]
- Favero, G.; Franco, C.; Stacchiotti, A.; Rodella, L.F.; Rezzani, R. Sirtuin1 Role in the Melatonin Protective Effects Against Obesity-Related Heart Injury. Front. Physiol. 2020, 11, 103. [Google Scholar] [CrossRef] [PubMed]
- Rodella, L.F.; Rossini, C.; Favero, G.; Foglio, E.; Loreto, C.; Rezzani, R. Nicotine-induced morphological changes in rat aorta: The protective role of melatonin. Cells Tissues Organs 2012, 195, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, R.; Maekawa, M.; Kiyoi, T.; Kurata, M.; Miura, N.; Kikugawa, T.; Higashiyama, S.; Saika, T. PSMA-positive membranes secreted from prostate cancer cells have potency to transform vascular endothelial cells into an angiogenic state. Prostate 2021, 81, 1390–1401. [Google Scholar] [CrossRef] [PubMed]
- Inoue, R.; Yasuma, T.; Fridman D’Alessandro, V.; Toda, M.; Ito, T.; Tomaru, A.; D’Alessandro-Gabazza, C.N.; Tsuruga, T.; Okano, T.; Takeshita, A.; et al. Amelioration of Pulmonary Fibrosis by Matrix Metalloproteinase-2 Overexpression. Int. J. Mol. Sci. 2023, 24, 6695. [Google Scholar] [CrossRef] [PubMed]
- Nicolas-Boluda, A.; Vaquero, J.; Vimeux, L.; Guilbert, T.; Barrin, S.; Kantari-Mimoun, C.; Ponzo, M.; Renault, G.; Deptula, P.; Pogoda, K.; et al. Tumor stiffening reversion through collagen crosslinking inhibition improves T cell migration and anti-PD-1 treatment. Elife 2021, 10, e58688. [Google Scholar] [CrossRef]
- Torzilli, P.A.; Bourne, J.W.; Cigler, T.; Vincent, C.T. A new paradigm for mechanobiological mechanisms in tumor metastasis. Semin. Cancer Biol. 2012, 22, 385–395. [Google Scholar] [CrossRef]
- Beck, A.H.; Espinosa, I.; Gilks, C.B.; van de Rijn, M.; West, R.B. The fibromatosis signature defines a robust stromal response in breast carcinoma. Lab. Investig. 2008, 88, 591–601. [Google Scholar] [CrossRef]
- Di Martino, J.S.; Nobre, A.R.; Mondal, C.; Taha, I.; Farias, E.F.; Fertig, E.J.; Naba, A.; Aguirre-Ghiso, J.A.; Bravo-Cordero, J.J. A tumor-derived type III collagen-rich ECM niche regulates tumor cell dormancy. Nat. Cancer 2022, 3, 90–107. [Google Scholar] [CrossRef]
- Ageeli, W.; Zhang, X.; Ogbonnaya, C.N.; Ling, Y.; Wilson, J.; Li, C.; Nabi, G. Characterisation of Collagen Re-Modelling in Localised Prostate Cancer Using Second-Generation Harmonic Imaging and Transrectal Ultrasound Shear Wave Elastography. Cancers 2021, 13, 5553. [Google Scholar] [CrossRef]
- Kojima, K.; Ohhashi, R.; Fujita, Y.; Hamada, N.; Akao, Y.; Nozawa, Y.; Deguchi, T.; Ito, M. A role for SIRT1 in cell growth and chemoresistance in prostate cancer PC3 and DU145 cells. Biochem. Biophys. Res. Commun. 2008, 373, 423–428. [Google Scholar] [CrossRef]
- Jung-Hynes, B.; Nihal, M.; Zhong, W.; Ahmad, N. Role of sirtuin histone deacetylase SIRT1 in prostate cancer. A target for prostate cancer management via its inhibition? J. Biol. Chem. 2009, 284, 3823–3832. [Google Scholar] [CrossRef]
- Ruan, L.; Wang, L.; Wang, X.; He, M.; Yao, X. SIRT1 contributes to neuroendocrine differentiation of prostate cancer. Oncotarget 2018, 9, 2002–2016. [Google Scholar] [CrossRef]
- Byles, V.; Zhu, L.; Lovaas, J.D.; Chmilewski, L.K.; Wang, J.; Faller, D.V.; Dai, Y. SIRT1 induces EMT by cooperating with EMT transcription factors and enhances prostate cancer cell migration and metastasis. Oncogene 2012, 31, 4619–4629. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yang, B.; Ma, B. The UCA1/miR-204/Sirt1 axis modulates docetaxel sensitivity of prostate cancer cells. Cancer Chemother. Pharmacol. 2016, 78, 1025–1031. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Rivas, P.; Bedolla, R.; Thapa, D.; Reddick, R.L.; Ghosh, R.; Kumar, A.P. Dietary resveratrol prevents development of high-grade prostatic intraepithelial neoplasticlesions: Involvement of SIRT1/S6K axis. Cancer Prev. Res. 2013, 6, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Chalkiadaki, A.; Guarente, L. The multifaceted functions of sirtuins in cancer. Nat. Rev. Canc. 2015, 15, 608–624. [Google Scholar] [CrossRef]
- Wilking, M.J.; Ahmad, N. The role of SIRT1 in cancer: The saga continues. Am. J. Pathol. 2015, 185, 26–28. [Google Scholar] [CrossRef]
- Huang, S.B.; Thapa, D.; Munoz, A.R.; Hussain, S.S.; Yang, X.; Bedolla, R.G.; Osmulski, P.; Gaczynska, M.E.; Lai, Z.; Chiu, Y.C.; et al. Androgen deprivation-induced elevated nuclear SIRT1 promotes prostate tumor cell survival by reactivation of AR signaling. Cancer Lett. 2021, 505, 24–36. [Google Scholar] [CrossRef]
- Zhu, H.R.; Wang, Z.Y.; Zhu, X.L.; Wu, X.X.; Li, E.G.; Xu, Y. Icariin protects against brain injury by enhancing SIRT1-dependent PGC-1alpha expression in experimental stroke. Neuropharmacology 2010, 59, 70–76. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, W.; Xie, C.; Dai, X.; Ma, J.; Lu, J. The Role of PGC-1α in Digestive System Malignant Tumours. Anti-Cancer Agents Med. Chem. 2020, 20, 276–285. [Google Scholar] [CrossRef]
- Wu, N.; Jin, W.; Zhao, Y.; Wang, H.; He, S.; Zhang, W.; Zhou, J. Sulfated Fucogalactan from Laminaria Japonica Ameliorates β-Cell Failure by Attenuating Mitochondrial Dysfunction via SIRT1-PGC1-α Signaling Pathway Activation. Front. Endocrinol. 2022, 13, 881256. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Widlund, H.R.; Puigserver, P. PGC-1 Coactivators: Shepherding the Mitochondrial Biogenesis of Tumors. Trends Cancer 2016, 2, 619–631. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Chen, S.; Hu, X. Peroxisome Proliferator-activated Receptor Gamma Coactivator-1 Alpha: A Double-edged Sword in Prostate Cancer. Curr. Cancer Drug Targets 2022, 22, 541–559. [Google Scholar] [CrossRef] [PubMed]
- Yun, C.W.; Han, Y.-S.; Lee, S.H. PGC-1α Controls mitochondrial biogenesis in drug-resistant colorectal cancer cells by regulating endoplasmic reticulum stress. Int. J. Mol. Sci. 2019, 20, 1707. [Google Scholar] [CrossRef]
- Jiang, J.; Wang, K.; Chen, Y.; Chen, H.; Nice, E.C.; Huang, C. Redox regulation in tumor cell epithelial-mesenchymal transition: Molecular basis and therapeutic strategy. Signal Transduct. Target. Ther. 2017, 2, 17036. [Google Scholar] [CrossRef]
- Kim, B.; Jung, J.W.; Jung, J.; Han, Y.; Suh, D.H.; Kim, H.S.; Dhanasekaran, D.N.; Song, Y.S. PGC1alpha induced by reactive oxygen species contributes to chemoresistance of ovarian cancer cells. Oncotarget 2017, 8, 60299–60311. [Google Scholar] [CrossRef]
- Montes-de-Oca-García, A.; Corral-Pérez, J.; Velázquez-Díaz, D.; Perez-Bey, A.; Rebollo-Ramos, M.; Marín-Galindo, A.; Gómez-Gallego, F.; Calderon-Dominguez, M.; Casals, C.; Ponce-González, J.G. Influence of Peroxisome Proliferator-Activated Receptor (PPAR)-gamma Coactivator (PGC)-1 alpha gene rs8192678 polymorphism by gender on different health-related parameters in healthy young adults. Front. Physiol. 2022, 13, 885185. [Google Scholar] [CrossRef]
- Elix, C.; Pal, S.K.; Jones, J.O. The role of peroxisome proliferator-activated receptor gamma in prostate cancer. Asian J. Androl. 2018, 20, 238–243. [Google Scholar]
- Bao, Z.; Malki, M.I.; Forootan, S.S.; Adamson, J.; Forootan, F.S.; Chen, D.; Foster, C.S.; Rudland, P.S.; Ke, Y. A novel cutaneous fatty acid-binding protein-related signaling pathway leading to malignant progression in prostate cancer cells. Genes Cancer 2013, 4, 297–314. [Google Scholar] [CrossRef]
- Ahmad, I.; Mui, E.; Galbraith, L.; Patel, R.; Tan, E.H.; Salji, M.; Rust, A.G.; Repiscak, P.; Hedley, A.; Markert, E.; et al. Sleeping Beauty screen reveals Pparg activation in metastatic prostate cancer. Proc. Natl. Acad. Sci. USA 2016, 113, 8290–8829. [Google Scholar] [CrossRef]
- Xi, S.C.; Siu, S.W.; Fong, S.W.; Shiu, S.Y. Inhibition of androgen-sensitive LNCaP prostate cancer growth in vivo by melatonin: Association of antiproliferative action of the pineal hormone with mt1 receptor protein expression. Prostate. 2001, 46, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, C.; Choi, W.S. Use of Melatonin in Cancer Treatment: Where Are We? Int. J. Mol. Sci. 2022, 23, 3779. [Google Scholar] [CrossRef] [PubMed]
- Biava, P.M.; Bonizzoni, E.; Zafiropoulou, S.; Laudani, A.; Burigana, F.; Burian Lissoi, I.; Lotti, T. Stem cell growth and differentiation factors from Zebrafish embryo and their role as epigenetic regulators in hair regeneration: Results after transdermal administration using cryopass laser treatment. Aesthetic Med. 2020, 6, 11–19. [Google Scholar]

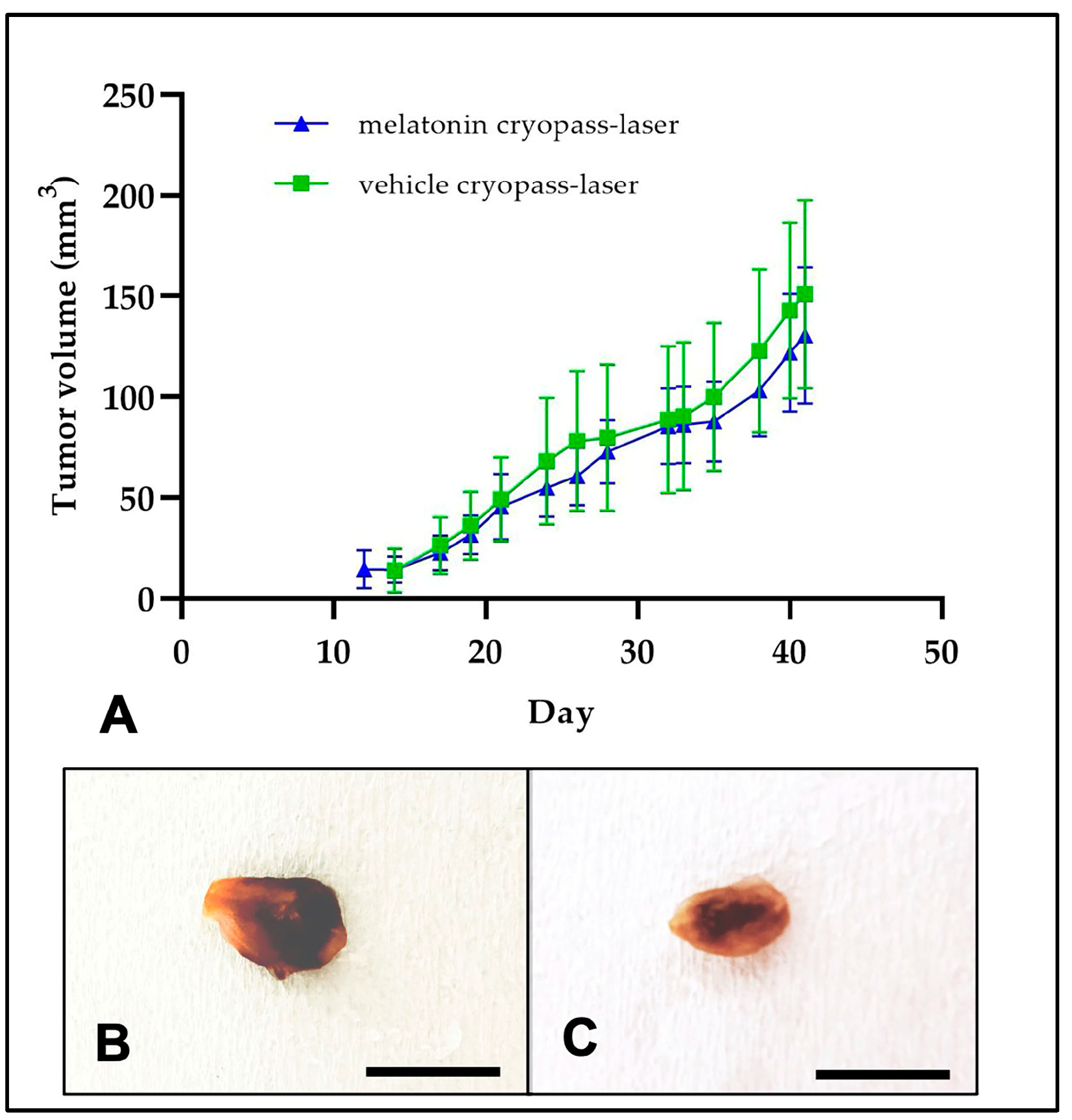
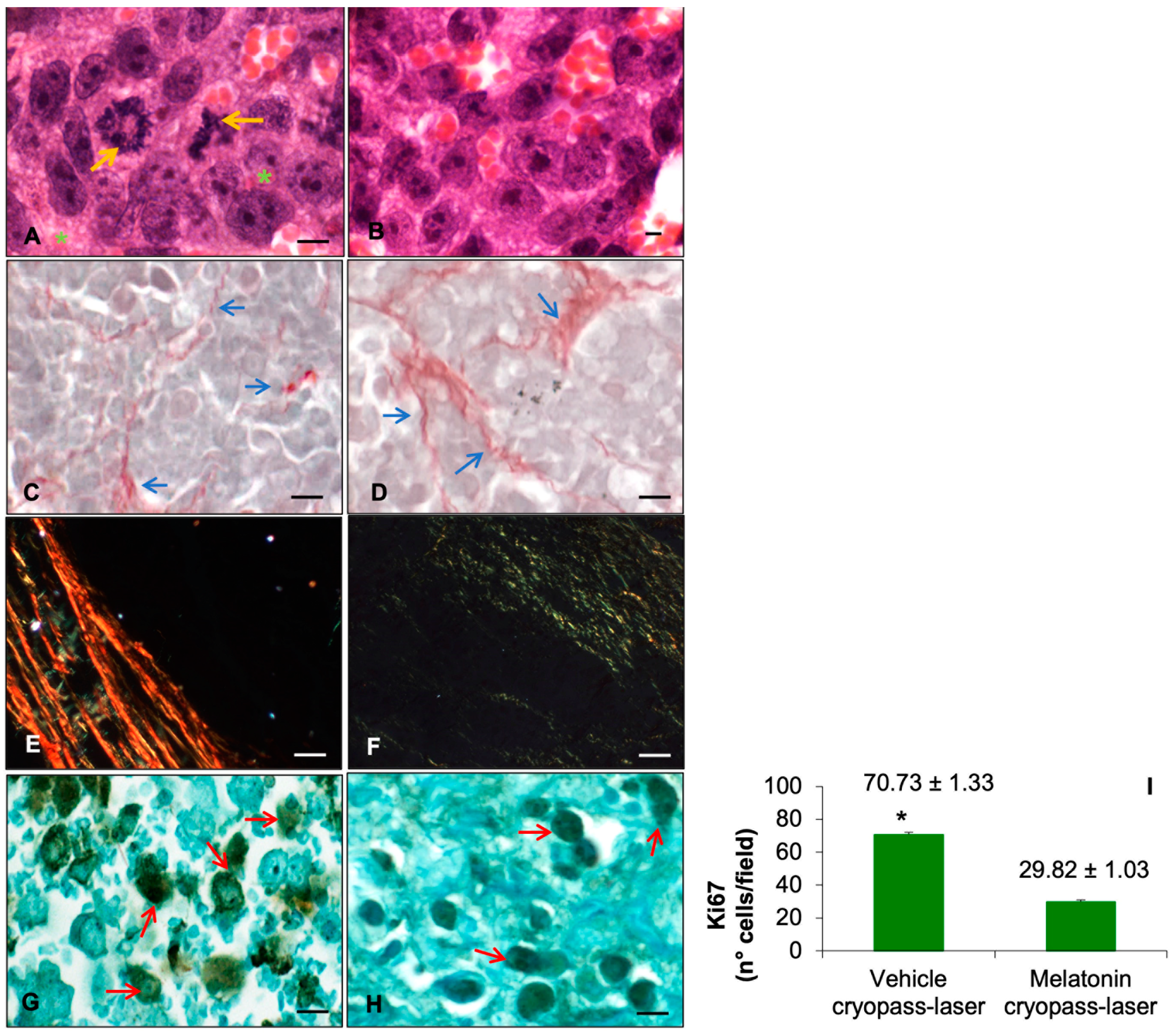
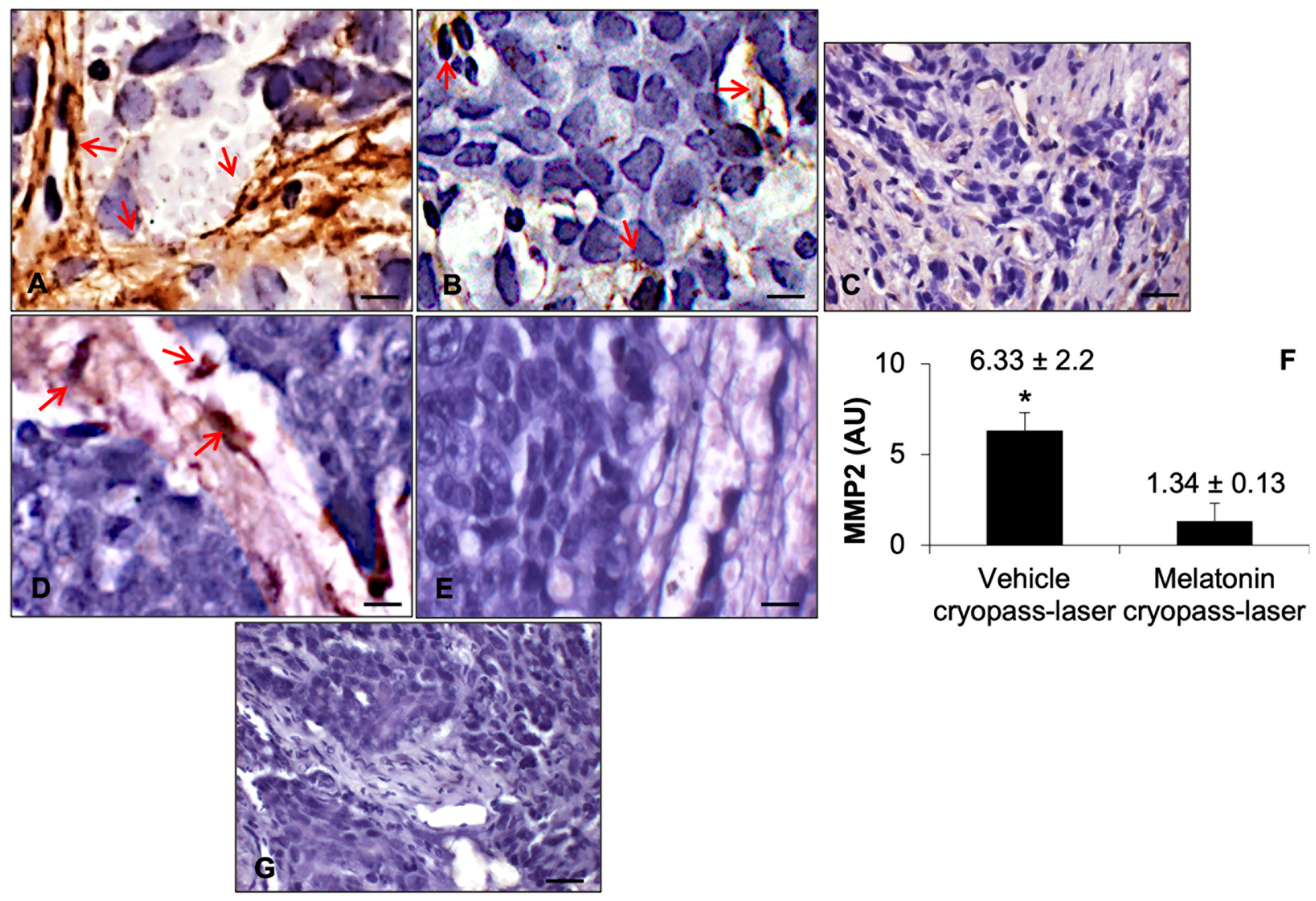



| Melatonin Cryopass-Laser | Vehicle Cryopass-Laser | ||||||
|---|---|---|---|---|---|---|---|
| Days | BW (g) | TV (mm3) | TV/BW (mm3/g) | BW (g) | TV (mm3) | TV/BW (mm3/g) | |
| Time after tumor cell inoculum | 2 | 27.4± 0.5 | 0 | 0 | 27.5 ± 0.5 | 0 | 0 |
| 12 | 29.5± 0.7 | 0 | 0 | 29.2 ± 0.8 | 0 | 0 | |
| 22 | 29.3 ± 0.9 | 45 ± 16 | 1.62 ± 0.6 | 29.0 ± 1.2 | 49 ± 21 | 1.81 ± 0.83 | |
| 32 | 28.3 ± 0.9 | 85 ± 19 | 3.14 ± 0.7 | 27.7 ± 1.3 | 89 ± 37 | 3.53 ± 1.5 | |
| 42 | 28.3 ± 1 | 131 ± 34 | 4.82 ± 1.3 | 27.5 ± 1.4 | 151 ± 47 | 5.94 ± 1.9 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonomini, F.; Favero, G.; Petroni, A.; Paroni, R.; Rezzani, R. Melatonin Modulates the SIRT1-Related Pathways via Transdermal Cryopass-Laser Administration in Prostate Tumor Xenograft. Cancers 2023, 15, 4908. https://doi.org/10.3390/cancers15204908
Bonomini F, Favero G, Petroni A, Paroni R, Rezzani R. Melatonin Modulates the SIRT1-Related Pathways via Transdermal Cryopass-Laser Administration in Prostate Tumor Xenograft. Cancers. 2023; 15(20):4908. https://doi.org/10.3390/cancers15204908
Chicago/Turabian StyleBonomini, Francesca, Gaia Favero, Anna Petroni, Rita Paroni, and Rita Rezzani. 2023. "Melatonin Modulates the SIRT1-Related Pathways via Transdermal Cryopass-Laser Administration in Prostate Tumor Xenograft" Cancers 15, no. 20: 4908. https://doi.org/10.3390/cancers15204908
APA StyleBonomini, F., Favero, G., Petroni, A., Paroni, R., & Rezzani, R. (2023). Melatonin Modulates the SIRT1-Related Pathways via Transdermal Cryopass-Laser Administration in Prostate Tumor Xenograft. Cancers, 15(20), 4908. https://doi.org/10.3390/cancers15204908










