Noncoding RNAs Controlling Oxidative Stress in Cancer
Simple Summary
Abstract
1. Introduction
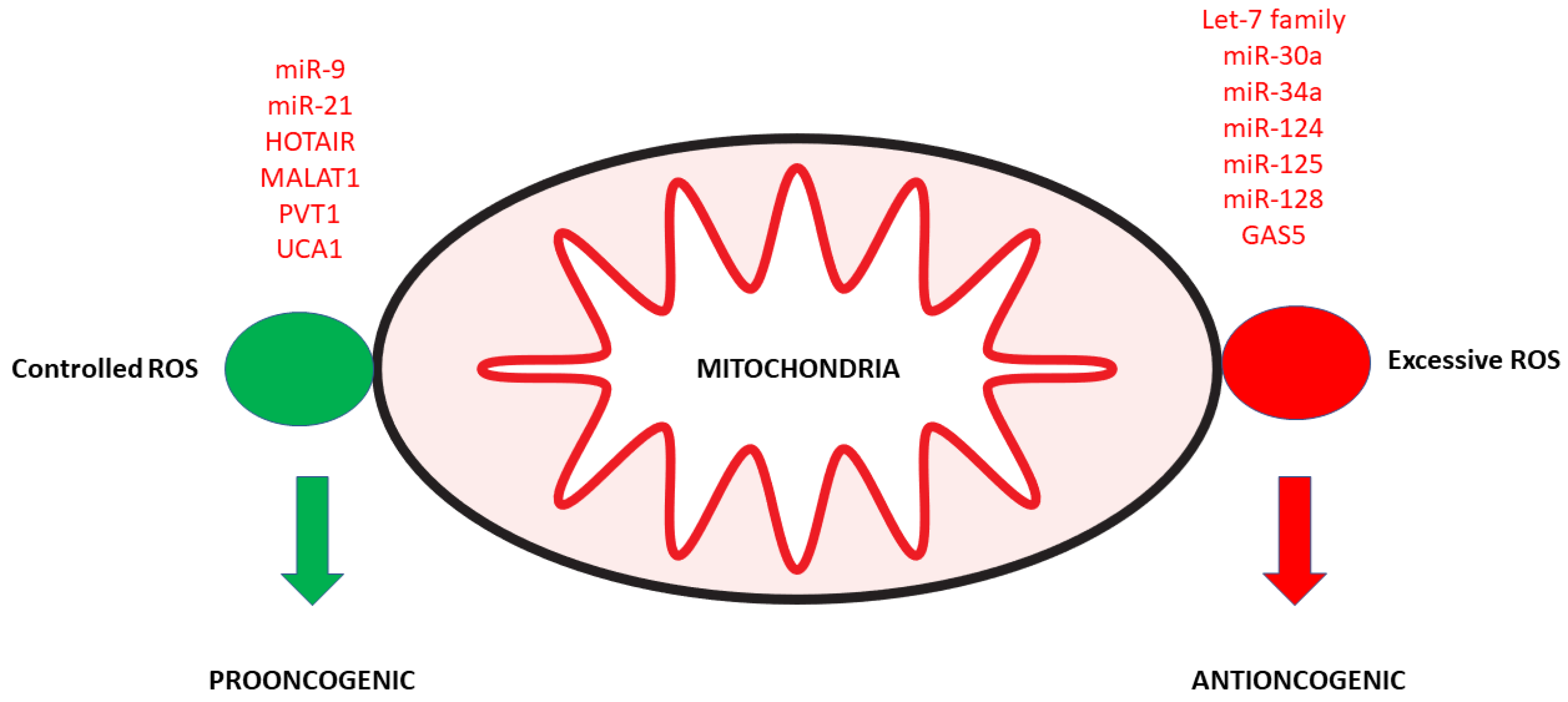
2. Noncoding RNAs and Mitochondrial Dysfunction
3. Noncoding RNAs and Antioxidant Activity
4. Immune Cells and ROS Generation
4.1. T Cells
4.2. Noncoding RNAs and T Cells
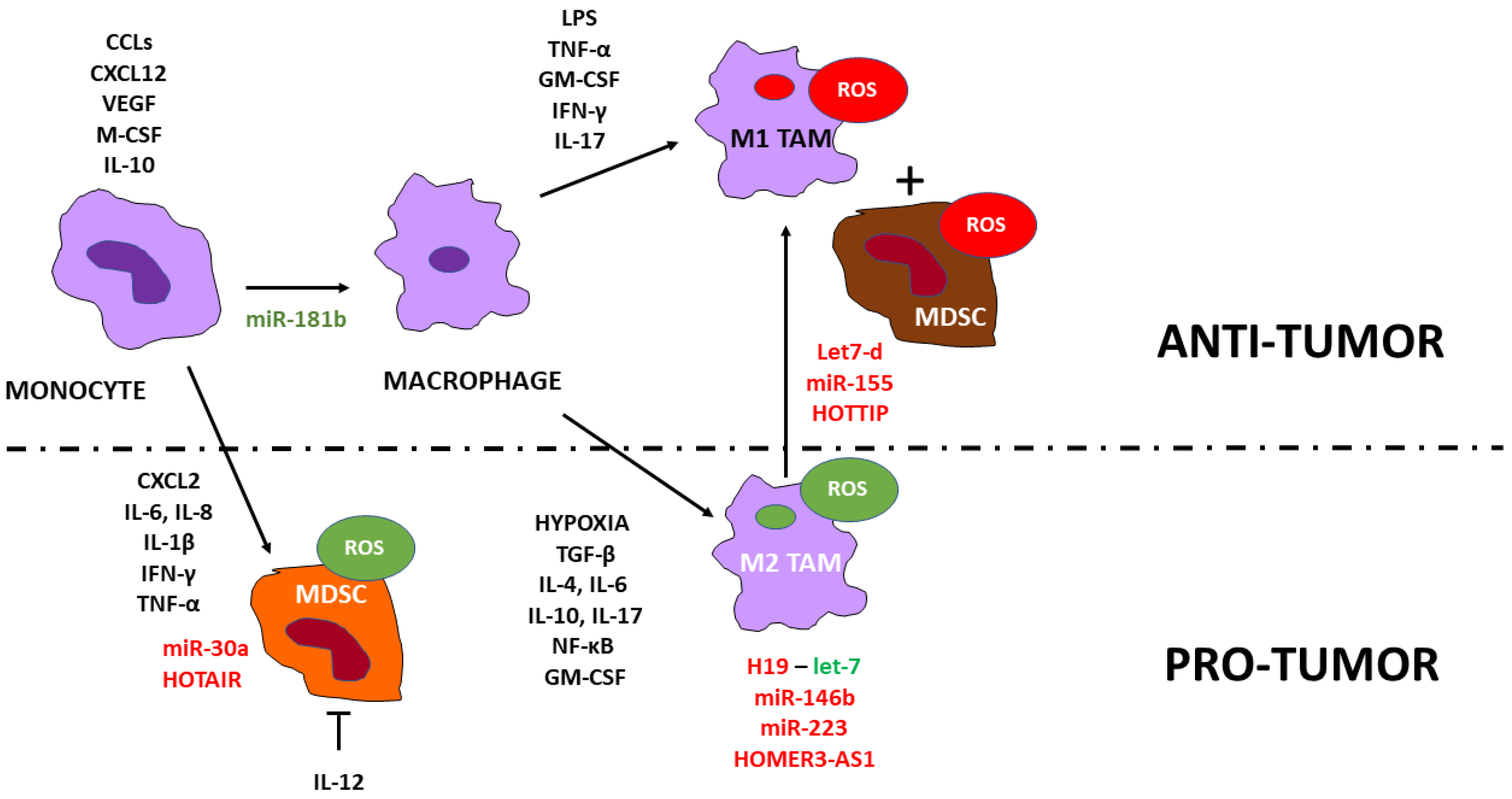
5. MDSCs and Macrophages
6. Noncoding RNAs and MDSCs, and Macrophages
7. Role of Noncoding RNA-Containing Microvesicles
8. Discussion
9. Conclusions
Funding
Conflicts of Interest
References
- Hanahan, D.; Weinberg, R.A. The Hallmarks of Cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef]
- Moloney, J.N.; Cotter, T.G. ROS signalling in the biology of cancer. Semin. Cell Dev. Biol. 2018, 80, 50–64. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Karakhanova, S.; Hartwig, W.; D’Haese, J.G.; Philippov, P.P.; Werner, J.; Bazhin, A.V. Mitochondria and Mitochondrial ROS in Cancer: Novel Targets for Anticancer Therapy. J. Cell. Physiol. 2016, 231, 2570–2581. [Google Scholar] [CrossRef]
- Sosa, V.; Moliné, T.; Somoza, R.; Paciucci, R.; Kondoh, H.; Lleonart, M.E. Oxidative stress and cancer: An overview. Ageing Res. Rev. 2013, 12, 376–390. [Google Scholar] [CrossRef] [PubMed]
- de la Cruz-Ojeda, P.; Flores-Campos, R.; Dios-Barbeito, S.; Navarro-Villarán, E.; Muntané, J. Role of Nitric Oxide in Gene Expression Regulation during Cancer: Epigenetic Modifications and Non-Coding RNAs. Int. J. Mol. Sci. 2021, 22, 6264. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Ye, S.; Chen, X.; Xu, P.; Li, K.; Zeng, S.; Huang, M.; Gao, W.; Chen, J.; Zhang, Q.; et al. Mitochondrial NOS1 suppresses apoptosis in colon cancer cells through increasing SIRT3 activity. Biochem. Biophys. Res. Commun. 2019, 515, 517–523. [Google Scholar] [CrossRef]
- Wang, R.; Geller, D.A.; Wink, D.A.; Cheng, B.; Billiar, T.R. NO and hepatocellular cancer. Br. J. Pharmacol. 2020, 177, 5459–5466. [Google Scholar] [CrossRef]
- DeNicola, G.M.; Karreth, F.A.; Humpton, T.J.; Gopinathan, A.; Wei, C.; Frese, K.; Mangal, D.; Yu, K.H.; Yeo, C.J.; Calhoun, E.S.; et al. Oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis. Nature 2011, 475, 106–109. [Google Scholar] [CrossRef]
- Jaramillo, M.C.; Zhang, D.D. The emerging role of the Nrf2–Keap1 signaling pathway in cancer. Genes Dev. 2013, 27, 2179–2191. [Google Scholar] [CrossRef]
- Tossetta, G.; Fantone, S.; Montanari, E.; Marzioni, D.; Goteri, G. Role of NRF2 in Ovarian Cancer. Antioxidants 2022, 11, 663. [Google Scholar] [CrossRef]
- Chen, X.; Cao, X.; Xiao, W.; Li, B.; Xue, Q. PRDX5 as a novel binding partner in Nrf2-mediated NSCLC progression under oxidative stress. Aging 2020, 12, 122–137. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Remark, R.; Becker, C.; Gomez, J.E.; Damotte, D.; Dieu-Nosjean, M.-C.; Sautès-Fridman, C.; Fridman, W.-H.; Powell, C.A.; Altorki, N.K.; Merad, M.; et al. The Non–Small Cell Lung Cancer Immune Contexture. A Major Determinant of Tumor Characteristics and Patient Outcome. Am. J. Respir. Crit. Care Med. 2015, 191, 377–390. [Google Scholar] [CrossRef]
- Van Acker, H.H.; Ma, S.; Scolaro, T.; Kaech, S.M.; Mazzone, M. How metabolism bridles cytotoxic CD8(+) T cells through epigenetic modifications. Trends Immunol. 2021, 42, 401–417. [Google Scholar] [CrossRef]
- Aydin, E.; Johansson, J.; Nazir, F.H.; Hellstrand, K.; Martner, A. Role of NOX2-Derived Reactive Oxygen Species in NK Cell–Mediated Control of Murine Melanoma Metastasis. Cancer Immunol. Res. 2017, 5, 804–811. [Google Scholar] [CrossRef]
- Wang, S.; Liu, G.; Li, Y.; Pan, Y. Metabolic Reprogramming Induces Macrophage Polarization in the Tumor Microenvironment. Front. Immunol. 2022, 13, 840029. [Google Scholar] [CrossRef]
- Chiossone, L.; Dumas, P.-Y.; Vienne, M.; Vivier, E. Natural killer cells and other innate lymphoid cells in cancer. Nat. Rev. Immunol. 2018, 18, 671–688. [Google Scholar] [CrossRef]
- Viola, A.; Munari, F.; Sánchez-Rodríguez, R.; Scolaro, T.; Castegna, A. The Metabolic Signature of Macrophage Responses. Front. Immunol. 2019, 10, 1462. [Google Scholar] [CrossRef]
- Kashfi, K.; Kannikal, J.; Nath, N. Macrophage Reprogramming and Cancer Therapeutics: Role of iNOS-Derived NO. Cells 2021, 10, 3194. [Google Scholar] [CrossRef]
- Jeannin, P.; Duluc, D.; Delneste, Y. IL-6 and leukemia-inhibitory factor are involved in the generation of tumor-associated macrophage: Regulation by IFN-γ. Immunotherapy 2011, 3 (Suppl 4), 23–26. [Google Scholar] [CrossRef]
- Centuori, S.M.; Trad, M.; LaCasse, C.J.; Alizadeh, D.; Larmonier, C.B.; Hanke, N.T.; Kartchner, J.; Janikashvili, N.; Bonnotte, B.; Larmonier, N.; et al. Myeloid-derived suppressor cells from tumor-bearing mice impair TGF-beta-induced differentiation of CD4+CD25+FoxP3+ Tregs from CD4+CD25-FoxP3- T cells. J. Leukoc. Biol. 2012, 92, 987–997. [Google Scholar] [CrossRef]
- Xiang, H.; Ramil, C.P.; Hai, J.; Zhang, C.; Wang, H.; Watkins, A.A.; Afshar, R.; Georgiev, P.; Sze, M.A.; Song, X.S.; et al. Cancer-Associated Fibroblasts Promote Immunosuppression by Inducing ROS-Generating Monocytic MDSCs in Lung Squamous Cell Carcinoma. Cancer Immunol. Res. 2020, 8, 436–450. [Google Scholar] [CrossRef]
- Aboelella, N.; Brandle, C.; Kim, T.; Ding, Z.-C.; Zhou, G. Oxidative Stress in the Tumor Microenvironment and Its Relevance to Cancer Immunotherapy. Cancers 2021, 13, 986. [Google Scholar] [CrossRef]
- Holvoet, P. Stress in Obesity and Associated Metabolic and Cardiovascular Disorders. Scientifica 2012, 2012, 205027. [Google Scholar] [CrossRef]
- Hulsmans, M.; Holvoet, P. MicroRNA-containing microvesicles regulating inflammation in association with atherosclerotic disease. Cardiovasc. Res. 2013, 100, 7–18. [Google Scholar] [CrossRef]
- Ginckels, P.; Holvoet, P. Oxidative Stress and Inflammation in Cardiovascular Diseases and Cancer: Role of Non-coding RNAs. Yale. J. Biol. Med. 2022, 95, 129–152. [Google Scholar]
- Si, T.; Ning, X.; Zhao, H.; Zhang, M.; Huang, P.; Hu, Z.; Yang, L.; Lin, L. microRNA-9-5p regulates the mitochondrial function of hepatocellular carcinoma cells through suppressing PDK4. Cancer Gene Ther. 2021, 28, 706–718. [Google Scholar] [CrossRef]
- Wu, H.; Wang, J.; Ma, H.; Xiao, Z.; Dong, X. MicroRNA-21 inhibits mitochondria-mediated apoptosis in keloid. Oncotarget 2017, 8, 92914–92925. [Google Scholar] [CrossRef]
- Vallée, A.; Lecarpentier, Y. Crosstalk Between Peroxisome Proliferator-Activated Receptor Gamma and the Canonical WNT/β-Catenin Pathway in Chronic Inflammation and Oxidative Stress During Carcinogenesis. Front. Immunol. 2018, 9, 745. [Google Scholar] [CrossRef]
- Kong, L.; Zhou, X.; Wu, Y.; Wang, Y.; Chen, L.; Li, P.; Liu, S.; Sun, S.; Ren, Y.; Mei, M.; et al. Targeting HOTAIR Induces Mitochondria Related Apoptosis and Inhibits Tumor Growth in Head and Neck Squamous Cell Carcinoma in vitro and in vivo. Curr. Mol. Med. 2015, 15, 952–960. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhou, L.; Li, H.; Sun, T.; Wen, X.; Li, X.; Meng, Y.; Li, Y.; Liu, M.; Liu, S.; et al. Nuclear-Encoded lncRNA MALAT1 Epigenetically Controls Metabolic Reprogramming in HCC Cells through the Mitophagy Pathway. Mol. Ther. Nucleic Acids 2021, 23, 264–276. [Google Scholar] [CrossRef]
- Mishra, A.; Bonello, M.; Byron, A.; Langdon, S.P.; Sims, A.H. Evaluation of Gene Expression Data From Cybrids and Tumours Highlights Elevated NDRG1-Driven Proliferation in Triple-Negative Breast Cancer. Breast Cancer 2020, 14, 1178223420934447. [Google Scholar] [CrossRef]
- Li, H.-J.; Sun, X.-M.; Li, Z.-K.; Yin, Q.-W.; Pang, H.; Pan, J.-J.; Li, X.; Chen, W. LncRNA UCA1 Promotes Mitochondrial Function of Bladder Cancer via the MiR-195/ARL2 Signaling Pathway. Cell. Physiol. Biochem. 2017, 43, 2548–2561. [Google Scholar] [CrossRef]
- Zhang, X.; Mofers, A.; Hydbring, P.; Olofsson, M.H.; Guo, J.; Linder, S.; D’Arcy, P. MYC is downregulated by a mitochondrial checkpoint mechanism. Oncotarget 2017, 8, 90225–90237. [Google Scholar] [CrossRef]
- Yu, Y.; Yang, L.; Zhao, M.; Zhu, S.; Kang, R.; Vernon, P.; Tang, D.; Cao, L. Targeting microRNA-30a-mediated autophagy enhances imatinib activity against human chronic myeloid leukemia cells. Leukemia 2012, 26, 1752–1760. [Google Scholar] [CrossRef]
- Jin, L.; Miao, J.; Liu, Y.; Li, X.; Jie, Y.; Niu, Q.; Han, X. Icaritin induces mitochondrial apoptosis by up-regulating miR-124 in human oral squamous cell carcinoma cells. Biomed. Pharmacother. 2017, 85, 287–295. [Google Scholar] [CrossRef]
- Lo, Y.-L.; Wang, C.-S.; Chen, Y.-C.; Wang, T.-Y.; Chang, Y.-H.; Chen, C.-J.; Yang, C.-P. Mitochondrion-Directed Nanoparticles Loaded with a Natural Compound and a microRNA for Promoting Cancer Cell Death via the Modulation of Tumor Metabolism and Mitochondrial Dynamics. Pharmaceutics 2020, 12, 756. [Google Scholar] [CrossRef]
- Lian, B.; Yang, D.; Liu, Y.; Shi, G.; Li, J.; Yan, X.; Jin, K.; Liu, X.; Zhao, J.; Shang, W.; et al. miR-128 Targets the SIRT1/ROS/DR5 Pathway to Sensitize Colorectal Cancer to TRAIL-Induced Apoptosis. Cell. Physiol. Biochem. 2018, 49, 2151–2162. [Google Scholar] [CrossRef]
- Gao, J.; Liu, M.; Zou, Y.; Mao, M.; Shen, T.; Zhang, C.; Song, S.; Sun, M.; Zhang, S.; Wang, B.; et al. Long non-coding RNA growth arrest-specific transcript 5 is involved in ovarian cancer cell apoptosis through the mitochondria-mediated apoptosis pathway. Oncol. Rep. 2015, 34, 3212–3221. [Google Scholar] [CrossRef]
- Sang, L.; Ju, H.-Q.; Yang, Z.; Ge, Q.; Zhang, Z.; Liu, F.; Yang, L.; Gong, H.; Shi, C.; Qu, L.; et al. Mitochondrial long non-coding RNA GAS5 tunes TCA metabolism in response to nutrient stress. Nat. Metab. 2021, 3, 90–106. [Google Scholar] [CrossRef]
- Amodio, N.; Stamato, M.A.; Juli, G.; Morelli, E.; Fulciniti, M.; Manzoni, M.; Taiana, E.; Agnelli, L.; Cantafio, M.E.G.; Romeo, E.; et al. Drugging the lncRNA MALAT1 via LNA gapmeR ASO inhibits gene expression of proteasome subunits and triggers anti-multiple myeloma activity. Leukemia 2018, 32, 1948–1957. [Google Scholar] [CrossRef]
- Luo, Y.; Zhang, W.; Xu, L.; Chen, Y.; Xu, Y.; Yuan, L. Long Non-Coding RNA PVT1 Regulates the Resistance of the Breast Cancer Cell Line MDA-MB-231 to Doxorubicin via Nrf2. Technol. Cancer Res. Treat. 2020, 19, 1533033820980763. [Google Scholar] [CrossRef]
- Li, C.; Fan, K.; Qu, Y.; Zhai, W.; Huang, A.; Sun, X.; Xing, S. Deregulation of UCA1 expression may be involved in the development of chemoresistance to cisplatin in the treatment of non-small-cell lung cancer via regulating the signaling pathway of microRNA-495/NRF2. J. Cell. Physiol. 2020, 235, 3721–3730. [Google Scholar] [CrossRef]
- Sun, Z.; Huang, G.; Cheng, H. Transcription factor Nrf2 induces the up-regulation of lncRNA TUG1 to promote progression and adriamycin resistance in urothelial carcinoma of the bladder. Cancer Manag. Res. 2019, 11, 6079–6090. [Google Scholar] [CrossRef]
- Dong, H.; Wang, W.; Mo, S.; Liu, Q.; Chen, X.; Chen, R.; Zhang, Y.; Zou, K.; Ye, M.; He, X.; et al. Long non-coding RNA SNHG14 induces trastuzumab resistance of breast cancer via regulating PABPC1 expression through H3K27 acetylation. J. Cell. Mol. Med. 2018, 22, 4935–4947. [Google Scholar] [CrossRef]
- Nakashima, C.; Fujiwara-Tani, R.; Mori, S.; Kishi, S.; Ohmori, H.; Fujii, K.; Mori, T.; Miyagawa, Y.; Yamamoto, K.; Kirita, T.; et al. An Axis between the Long Non-Coding RNA HOXA11-AS and NQOs Enhances Metastatic Ability in Oral Squamous Cell Carcinoma. Int. J. Mol. Sci. 2022, 23, 10704. [Google Scholar] [CrossRef]
- Lin, L.-C.; Lee, H.-T.; Chien, P.-J.; Huang, Y.-H.; Chang, M.-Y.; Lee, Y.-C.; Chang, W.-W. NAD(P)H:quinone oxidoreductase 1 determines radiosensitivity of triple negative breast cancer cells and is controlled by long non-coding RNA NEAT1. Int. J. Med. Sci. 2020, 17, 2214–2224. [Google Scholar] [CrossRef]
- Wang, M.; Cheng, H.; Wu, H.; Liu, C.; Li, S.; Li, B.; Su, J.; Luo, S.; Li, Q. Gambogenic acid antagonizes the expression and effects of long non-coding RNA NEAT1 and triggers autophagy and ferroptosis in melanoma. Biomed. Pharmacother. 2022, 154, 113636. [Google Scholar] [CrossRef]
- Liu, J.; Yao, L.; Zhang, M.; Jiang, J.; Yang, M.; Wang, Y. Downregulation of LncRNA-XIST inhibited development of non-small cell lung cancer by activating miR-335/SOD2/ROS signal pathway mediated pyroptotic cell death. Aging 2019, 11, 7830–7846. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, Y.; Ding, J.; Hu, W.; Tan, C.; Wang, M.; Tang, J.; Xu, Y. miR-17-3p Downregulates Mitochondrial Antioxidant Enzymes and Enhances the Radiosensitivity of Prostate Cancer Cells. Mol. Ther. Nucleic Acids 2018, 13, 64–77. [Google Scholar] [CrossRef]
- Wang, P.; Zhu, C.-F.; Ma, M.-Z.; Chen, G.; Song, M.; Zeng, Z.-L.; Lu, W.-H.; Yang, J.; Wen, S.; Chiao, P.J.; et al. Micro-RNA-155 is induced by K-Ras oncogenic signal and promotes ROS stress in pancreatic cancer. Oncotarget 2015, 6, 21148–21158. [Google Scholar] [CrossRef]
- Den Haan, J.M.; Lehar, S.M.; Bevan, M.J. CD8(+) but not CD8(-) dendritic cells cross-prime cytotoxic T cells in vivo. J. Exp. Med. 2000, 192, 1685–1696. [Google Scholar] [CrossRef]
- Manicassamy, S.; Reizis, B.; Ravindran, R.; Nakaya, H.; Salazar-Gonzalez, R.M.; Wang, Y.-C.; Pulendran, B. Activation of β-Catenin in Dendritic Cells Regulates Immunity Versus Tolerance in the Intestine. Science 2010, 329, 849–853. [Google Scholar] [CrossRef]
- Ou, Z.-L.; Luo, Z.; Wei, W.; Liang, S.; Gao, T.-L.; Lu, Y.-B. Hypoxia-induced shedding of MICA and HIF1A-mediated immune escape of pancreatic cancer cells from NK cells: Role of circ_0000977/miR-153 axis. RNA Biol. 2019, 16, 1592–1603. [Google Scholar] [CrossRef]
- Bream, J.H.; Curiel, R.E.; Yu, C.-R.; Egwuagu, C.E.; Grusby, M.J.; Aune, T.M.; Young, H.A. IL-4 synergistically enhances both IL-2– and IL-12–induced IFN-γ expression in murine NK cells. Blood 2003, 102, 207–214. [Google Scholar] [CrossRef]
- Barrow, A.D.; Edeling, M.A.; Trifonov, V.; Luo, J.; Goyal, P.; Bohl, B.; Bando, J.K.; Kim, A.H.; Walker, J.; Andahazy, M.; et al. Natural Killer Cells Control Tumor Growth by Sensing a Growth Factor. Cell 2018, 172, 534–548.e19. [Google Scholar] [CrossRef]
- Slattery, K.; Woods, E.; Zaiatz-Bittencourt, V.; Marks, S.; Chew, S.; Conroy, M.; Goggin, C.; MacEochagain, C.; Kennedy, J.; Lucas, S.; et al. TGFβ drives NK cell metabolic dysfunction in human metastatic breast cancer. J. Immunother. Cancer 2021, 9, e002044. [Google Scholar] [CrossRef]
- Trinchieri, G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat. Rev. Immunol. 2003, 3, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Abbas, A.K.; Trotta, E.; Simeonov, D.R.; Marson, A.; Bluestone, J.A. Revisiting IL-2: Biology and therapeutic prospects. Sci. Immunol. 2018, 3, eaat1482. [Google Scholar] [CrossRef]
- Kumar, A.; Khani, A.T.; Ortiz, A.S.; Swaminathan, S. GM-CSF: A Double-Edged Sword in Cancer Immunotherapy. Front. Immunol. 2022, 13, 901277. [Google Scholar] [CrossRef]
- Cenerenti, M.; Saillard, M.; Romero, P.; Jandus, C. The Era of Cytotoxic CD4 T Cells. Front. Immunol. 2022, 13, 867189. [Google Scholar] [CrossRef]
- Najafi, S.; Mirshafiey, A. The role of T helper 17 and regulatory T cells in tumor microenvironment. Immunopharmacol. Immunotoxicol. 2019, 41, 16–24. [Google Scholar] [CrossRef]
- Balkwill, F. Tumour necrosis factor and cancer. Nat. Rev. Cancer 2009, 9, 361–371. [Google Scholar] [CrossRef]
- Joshi, N.S.; Akama-Garren, E.H.; Lu, Y.; Lee, D.-Y.; Chang, G.P.; Li, A.; DuPage, M.; Tammela, T.; Kerper, N.R.; Farago, A.F.; et al. Regulatory T Cells in Tumor-Associated Tertiary Lymphoid Structures Suppress Anti-tumor T Cell Responses. Immunity 2015, 43, 579–590. [Google Scholar] [CrossRef]
- Togashi, Y.; Shitara, K.; Nishikawa, H. Regulatory T cells in cancer immunosuppression—implications for anticancer therapy. Nat. Rev. Clin. Oncol. 2019, 16, 356–371. [Google Scholar] [CrossRef]
- Huang, B.; Pan, P.-Y.; Li, Q.; Sato, A.I.; Levy, D.E.; Bromberg, J.; Divino, C.M.; Chen, S.-H. Gr-1+CD115+ Immature Myeloid Suppressor Cells Mediate the Development of Tumor-Induced T Regulatory Cells and T-Cell Anergy in Tumor-Bearing Host. Cancer Res. 2006, 66, 1123–1131. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Xu, L.; Wang, Y.; Jiang, Q.; Liu, Z.; Zhang, J.; Zhou, Q.; Zeng, H.; Tong, S.; Wang, T.; et al. Tumor-associated Macrophage-derived Interleukin-23 Interlinks Kidney Cancer Glutamine Addiction with Immune Evasion. Eur. Urol. 2019, 75, 752–763. [Google Scholar] [CrossRef]
- Fallah, Y.; Brundage, J.; Allegakoen, P.; Shajahan-Haq, A.N. MYC-Driven Pathways in Breast Cancer Subtypes. Biomolecules 2017, 7, 53. [Google Scholar] [CrossRef]
- Shachaf, C.M.; Kopelman, A.M.; Arvanitis, C.; Karlsson, A.; Beer, S.; Mandl, S.; Bachmann, M.; Borowsky, A.D.; Ruebner, B.; Cardiff, R.D.; et al. MYC inactivation uncovers pluripotent differentiation and tumour dormancy in hepatocellular cancer. Nature 2004, 431, 1112–1117. [Google Scholar] [CrossRef]
- Sipos, F.; Firneisz, G.; Műzes, G. Therapeutic aspects of c-MYC signaling in inflammatory and cancerous colonic diseases. World J. Gastroenterol. 2016, 22, 7938–7950. [Google Scholar] [CrossRef]
- Tan, L.; Peng, D.; Cheng, Y. Significant position of C-myc in colorectal cancer: A promising therapeutic target. Clin. Transl. Oncol. 2022, 24, 2295–2304. [Google Scholar] [CrossRef]
- Martínez-Martín, S.; Soucek, L. MYC inhibitors in multiple myeloma. Cancer Drug Resist 2021, 4, 842–865. [Google Scholar] [CrossRef] [PubMed]
- Guo, A.; Huang, H.; Zhu, Z.; Chen, M.J.; Shi, H.; Yuan, S.; Sharma, P.; Connelly, J.P.; Liedmann, S.; Dhungana, Y.; et al. cBAF complex components and MYC cooperate early in CD8(+) T cell fate. Nature 2022, 607, 135–141. [Google Scholar] [CrossRef]
- Kikuchi, Y.; Tokita, S.; Hirama, T.; Kochin, V.; Nakatsugawa, M.; Shinkawa, T.; Hirohashi, Y.; Tsukahara, T.; Hata, F.; Takemasa, I.; et al. CD8(+) T–cell Immune Surveillance against a Tumor Antigen Encoded by the Oncogenic Long Noncoding RNA PVT1. Cancer Immunol. Res. 2021, 9, 1342–1353. [Google Scholar] [CrossRef]
- Kim, T.-D.; Jung, H.-R.; Seo, S.-H.; Oh, S.-C.; Ban, Y.; Tan, X.; Kim, J.M.; Lee, S.H.; Koh, D.-S.; Jung, H.; et al. MicroRNA-150 modulates intracellular Ca (2+) levels in naive CD8(+) T cells by targeting TMEM20. Sci. Rep. 2017, 7, 2623. [Google Scholar] [CrossRef]
- Bezman, N.A.; Chakraborty, T.; Bender, T.; Lanier, L.L. miR-150 regulates the development of NK and iNKT cells. J. Exp. Med. 2011, 208, 2717–2731. [Google Scholar] [CrossRef]
- Xu, Y.; Luo, X.; He, W.; Chen, G.; Li, Y.; Li, W.; Wang, X.; Lai, Y.; Ye, Y. Long Non-Coding RNA PVT1/miR-150/ HIG2 Axis Regulates the Proliferation, Invasion and the Balance of Iron Metabolism of Hepatocellular Carcinoma. Cell. Physiol. Biochem. 2018, 49, 1403–1419. [Google Scholar] [CrossRef]
- Obara, W.; Karashima, T.; Takeda, K.; Kato, R.; Kato, Y.; Kanehira, M.; Takata, R.; Inoue, K.; Katagiri, T.; Shuin, T.; et al. Effective induction of cytotoxic T cells recognizing an epitope peptide derived from hypoxia-inducible protein 2 (HIG2) in patients with metastatic renal cell carcinoma. Cancer Immunol. Immunother. 2017, 66, 17–24. [Google Scholar] [CrossRef]
- Dudda, J.C.; Salaun, B.; Ji, Y.; Palmer, D.C.; Monnot, G.C.; Merck, E.; Boudousquie, C.; Utzschneider, D.T.; Escobar, T.M.; Perret, R.; et al. MicroRNA-155 is required for effector CD8+ T cell responses to virus infection and cancer. Immunity 2013, 38, 742–753. [Google Scholar] [CrossRef]
- Xia, K.-G.; Wang, C.-M.; Shen, D.-Y.; Song, X.-Y.; Mu, X.-Y.; Zhou, J.-W.; Zhu, A.-Y.; Xuan, Q.; Tao, T. LncRNA NEAT1-associated aerobic glycolysis blunts tumor immunosurveillance by T cells in prostate cancer. Neoplasma 2022, 69, 594–602. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Zheng, J.; Yao, C.; Lu, X. LncRNA UCA1 attenuated the killing effect of cytotoxic CD8 + T cells on anaplastic thyroid carcinoma via miR-148a/PD-L1 pathway. Cancer Immunol. Immunother. 2021, 70, 2235–2245. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.F.; Gu, Z.S.; Zheng, L.L.; Zhao, M.X.; Wang, X.J. Long non-coding RNA GAS5 promotes natural killer cell cytotoxicity against gastric cancer by regulating miR-18a. Neoplasma 2020, 67, 1085–1093. [Google Scholar] [CrossRef] [PubMed]
- Fang, P.; Xiang, L.; Chen, W.; Li, S.; Huang, S.; Li, J.; Zhuge, L.; Jin, L.; Feng, W.; Chen, Y.; et al. LncRNA GAS5 enhanced the killing effect of NK cell on liver cancer through regulating miR-544/RUNX3. Innate Immun. 2019, 25, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Liu, M.; Li, Y.; Nie, Y.; Mi, Q.; Zhao, S. Ovarian tumor-associated microRNA-20a decreases natural killer cell cytotoxicity by downregulating MICA/B expression. Cell. Mol. Immunol. 2014, 11, 495–502. [Google Scholar] [CrossRef]
- Banerjee, A.; Schambach, F.; DeJong, C.S.; Hammond, S.M.; Reiner, S.L. Micro-RNA-155 inhibits IFN-γ signaling in CD4+ T cells. Eur. J. Immunol. 2010, 40, 225–231. [Google Scholar] [CrossRef]
- Jiang, S.; Li, C.; Olive, V.; Lykken, E.; Feng, F.; Sevilla, J.; Wan, Y.; He, L.; Li, Q.-J. Molecular dissection of the miR-17-92 cluster’s critical dual roles in promoting Th1 responses and preventing inducible Treg differentiation. Blood 2011, 118, 5487–5497. [Google Scholar] [CrossRef]
- Sun, J.; Wu, M.; Wang, L.; Wang, P.; Xiao, T.; Wang, S.; Liu, Q. miRNA-21, which disrupts metabolic reprogramming to facilitate CD4(+) T cell polarization toward the Th2 phenotype, accelerates arsenite-induced hepatic fibrosis. Ecotoxicol. Environ. Saf. 2022, 248, 114321. [Google Scholar] [CrossRef]
- Rossi, M.; Altomare, E.; Botta, C.; Cantafio, M.E.G.; Sarvide, S.; Caracciolo, D.; Riillo, C.; Gaspari, M.; Taverna, D.; Conforti, F.; et al. miR-21 antagonism abrogates Th17 tumor promoting functions in multiple myeloma. Leukemia 2021, 35, 823–834. [Google Scholar] [CrossRef]
- Sun, R.; Zhang, P.-P.; Weng, X.-Q.; Gao, X.-D.; Huang, C.-X.; Wang, L.; Hu, X.-X.; Xu, P.-P.; Cheng, L.; Jiang, L.; et al. Therapeutic targeting miR130b counteracts diffuse large B-cell lymphoma progression via OX40/OX40L-mediated interaction with Th17 cells. Signal Transduct. Target. Ther. 2022, 7, 80. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Z.-C.; Zhang, Z.-S.; Chen, F. MicroRNA-155 regulates cervical cancer via inducing Th17/Treg imbalance. Eur. Rev. Med Pharmacol. Sci. 2018, 22, 3719–3726. [Google Scholar]
- Yang, P.; Li, Q.-J.; Feng, Y.; Zhang, Y.; Markowitz, G.J.; Ning, S.; Deng, Y.; Zhao, J.; Jiang, S.; Yuan, Y.; et al. TGF-β-miR-34a-CCL22 Signaling-Induced Treg Cell Recruitment Promotes Venous Metastases of HBV-Positive Hepatocellular Carcinoma. Cancer Cell 2012, 22, 291–303. [Google Scholar] [CrossRef] [PubMed]
- El-Maadawy, E.A.; Elshal, M.F.; Bakry, R.M.; Moussa, M.M.; El-Naby, S.; Talaat, R.M. Regulation of CD4(+)CD25(+)FOXP3(+) cells in Pediatric Acute Lymphoblastic Leukemia (ALL): Implication of cytokines and miRNAs. Mol. Immunol. 2020, 124, 1–8. [Google Scholar] [CrossRef]
- Wei, J.; Wang, F.; Kong, L.-Y.; Xu, S.; Doucette, T.; Ferguson, S.D.; Yang, Y.; McEnery, K.; Jethwa, K.; Gjyshi, O.; et al. miR-124 Inhibits STAT3 Signaling to Enhance T Cell–Mediated Immune Clearance of Glioma. Cancer Res. 2013, 73, 3913–3926. [Google Scholar] [CrossRef] [PubMed]
- Zhong, C.; Tao, B.; Li, X.; Xiang, W.; Peng, L.; Peng, T.; Chen, L.; Xia, X.; You, J.; Yang, X. HOXA-AS2 contributes to regulatory T cell proliferation and immune tolerance in glioma through the miR-302a/KDM2A/JAG1 axis. Cell Death Dis. 2022, 13, 160. [Google Scholar] [CrossRef] [PubMed]
- Veglia, F.; Sanseviero, E.; Gabrilovich, D.I. Myeloid-derived suppressor cells in the era of increasing myeloid cell diversity. Nat. Rev. Immunol. 2021, 21, 485–498. [Google Scholar] [CrossRef]
- Gabrilovich, D.I.; Nagaraj, S. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 2009, 9, 162–174. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Fang, D.; Liu, H.; Ou, X.; Zhang, C.; Zhao, Z.; Zhao, S.; Peng, J.; Cai, S.; He, Y.; et al. PMN-MDSCs accumulation induced by CXCL1 promotes CD8(+) T cells exhaustion in gastric cancer. Cancer Lett. 2022, 532, 215598. [Google Scholar] [CrossRef] [PubMed]
- Du-Rocher, B.; Binato, R.; De-Freitas-Junior, J.C.M.; Corrêa, S.; Mencalha, A.L.; Morgado-Díaz, J.A.; Abdelhay, E. IL-17 Triggers Invasive and Migratory Properties in Human MSCs, while IFNy Favors their Immunosuppressive Capabilities: Implications for the “Licensing” Process. Stem Cell Rev. Rep. 2020, 16, 1266–1279. [Google Scholar] [CrossRef] [PubMed]
- Kerkar, S.P.; Goldszmid, R.S.; Muranski, P.; Chinnasamy, D.; Yu, Z.; Reger, R.N.; Leonardi, A.J.; Morgan, R.A.; Wang, E.; Marincola, F.M.; et al. IL-12 triggers a programmatic change in dysfunctional myeloid-derived cells within mouse tumors. J. Clin. Investig. 2011, 121, 4746–4757. [Google Scholar] [CrossRef]
- Shan, T.; Chen, S.; Chen, X.; Wu, T.; Yang, Y.; Li, S.; Ma, J.; Zhao, J.; Lin, W.; Li, W.; et al. M2-TAM subsets altered by lactic acid promote T-cell apoptosis through the PD-L1/PD-1 pathway. Oncol. Rep. 2020, 44, 1885–1894. [Google Scholar] [CrossRef]
- Lopez, M.V.; Adris, S.K.; Bravo, A.I.; Chernajovsky, Y.; Podhajcer, O.L. IL-12 and IL-10 Expression Synergize to Induce the Immune-Mediated Eradication of Established Colon and Mammary Tumors and Lung Metastasis. J. Immunol. 2005, 175, 5885–5894. [Google Scholar] [CrossRef]
- Erin, N.; Grahovac, J.; Brozovic, A.; Efferth, T. Tumor microenvironment and epithelial mesenchymal transition as targets to overcome tumor multidrug resistance. Drug Resist. Updat. 2020, 53, 100715. [Google Scholar] [CrossRef]
- Guo, X.; Xue, H.; Shao, Q.; Wang, J.; Guo, X.; Chen, X.; Zhang, J.; Xu, S.; Li, T.; Zhang, P.; et al. Hypoxia promotes glioma-associated macrophage infiltration via periostin and subsequent M2 polarization by upregulating TGF-beta and M-CSFR. Oncotarget 2016, 7, 80521–80542. [Google Scholar] [CrossRef]
- Coosemans, A.; Decoene, J.; Baert, T.; Laenen, A.; Kasran, A.; Verschuere, T.; Seys, S.; Vergote, I. Immunosuppressive parameters in serum of ovarian cancer patients change during the disease course. Oncoimmunology 2016, 5, e1111505. [Google Scholar] [CrossRef]
- Hanazawa, A.; Ito, R.; Katano, I.; Kawai, K.; Goto, M.; Suemizu, H.; Kawakami, Y.; Ito, M.; Takahashi, T. Generation of Human Immunosuppressive Myeloid Cell Populations in Human Interleukin-6 Transgenic NOG Mice. Front. Immunol. 2018, 9, 152. [Google Scholar] [CrossRef]
- Groth, C.; Hu, X.; Weber, R.; Fleming, V.; Altevogt, P.; Utikal, J.; Umansky, V. Immunosuppression mediated by myeloid-derived suppressor cells (MDSCs) during tumour progression. Br. J. Cancer 2019, 120, 16–25. [Google Scholar] [CrossRef]
- Zhang, Q.; Atsuta, I.; Liu, S.; Chen, C.; Shi, S.; Shi, S.; Le, A.D. IL-17–Mediated M1/M2 Macrophage Alteration Contributes to Pathogenesis of Bisphosphonate-Related Osteonecrosis of the Jaws. Clin. Cancer Res. 2013, 19, 3176–3188. [Google Scholar] [CrossRef]
- Suarez-Carmona, M.; Lesage, J.; Cataldo, D.; Gilles, C. EMT and inflammation: Inseparable actors of cancer progression. Mol. Oncol. 2017, 11, 805–823. [Google Scholar] [CrossRef]
- Chen, Y.; Wen, H.; Zhou, C.; Su, Q.; Lin, Y.; Xie, Y.; Huang, Y.; Qiu, Q.; Lin, J.; Huang, X.; et al. TNF-α derived from M2 tumor-associated macrophages promotes epithelial-mesenchymal transition and cancer stemness through the Wnt/β-catenin pathway in SMMC-7721 hepatocellular carcinoma cells. Exp. Cell Res. 2019, 378, 41–50. [Google Scholar] [CrossRef]
- Meng, Z.; Zhang, R.; Wu, X.; Zhang, M.; Jin, T. PD-L1 mediates triple-negative breast cancer evolution via the regulation of TAM/M2 polarization. Int. J. Oncol. 2022, 61, 150. [Google Scholar] [CrossRef]
- Mazzieri, R.; Pucci, F.; Moi, D.; Zonari, E.; Ranghetti, A.; Berti, A.; Politi, L.S.; Gentner, B.; Brown, J.L.; Naldini, L.; et al. Targeting the ANG2/TIE2 Axis Inhibits Tumor Growth and Metastasis by Impairing Angiogenesis and Disabling Rebounds of Proangiogenic Myeloid Cells. Cancer Cell 2011, 19, 512–526. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Shan, K.; Yang, Q.; Qi, Y.; Qu, H.; Li, J.; Wang, R.; Jia, L.; Chen, W.; Feng, N.; et al. Prostaglandin E3 attenuates macrophage-associated inflammation and prostate tumour growth by modulating polarization. J. Cell. Mol. Med. 2021, 25, 5586–5601. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Han, W.; Ma, Y.; Cui, L.; Tian, Y.; Zhou, Z.; Wang, H. P53-dependent miRNAs mediate nitric oxide-induced apoptosis in colonic carcinogenesis. Free. Radic. Biol. Med. 2015, 85, 105–113. [Google Scholar] [CrossRef]
- Tam, N.N.; Leav, I.; Ho, S.-M. Sex Hormones Induce Direct Epithelial and Inflammation-Mediated Oxidative/Nitrosative Stress That Favors Prostatic Carcinogenesis in the Noble Rat. Am. J. Pathol. 2007, 171, 1334–1341. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Ji, J.; Xu, J.; Li, D.; Shi, G.; Liu, F.; Ding, L.; Ren, J.; Dou, H.; Wang, T.; et al. MiR-30a increases MDSC differentiation and immunosuppressive function by targeting SOCS3 in mice with B-cell lymphoma. FEBS J. 2017, 284, 2410–2424. [Google Scholar] [CrossRef]
- Fujisaka, Y.; Iwata, T.; Tamai, K.; Nakamura, M.; Mochizuki, M.; Shibuya, R.; Yamaguchi, K.; Shimosegawa, T.; Satoh, K. Long non-coding RNA HOTAIR up-regulates chemokine (C-C motif) ligand 2 and promotes proliferation of macrophages and myeloid-derived suppressor cells in hepatocellular carcinoma cell lines. Oncol. Lett. 2017, 15, 509–514. [Google Scholar] [CrossRef]
- Liu, Y.-S.; Lin, H.-Y.; Lai, S.-W.; Huang, C.-Y.; Huang, B.-R.; Chen, P.-Y.; Wei, K.-C.; Lu, D.-Y. MiR-181b modulates EGFR-dependent VCAM-1 expression and monocyte adhesion in glioblastoma. Oncogene 2017, 36, 5006–5022. [Google Scholar] [CrossRef]
- Xiao, T.; Zou, Z.; Xue, J.; Syed, B.M.; Sun, J.; Dai, X.; Shi, M.; Li, J.; Wei, S.; Tang, H.; et al. LncRNA H19-mediated M2 polarization of macrophages promotes myofibroblast differentiation in pulmonary fibrosis induced by arsenic exposure. Environ. Pollut. 2021, 268, 115810. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, M.; Zhong, M.; Suo, Q.; Lv, K. Expression profiles of miRNAs in polarized macrophages. Int. J. Mol. Med. 2013, 31, 797–802. [Google Scholar] [CrossRef]
- Jeffries, J.; Zhou, W.; Hsu, A.Y.; Deng, Q. miRNA-223 at the crossroads of inflammation and cancer. Cancer Lett. 2019, 451, 136–141. [Google Scholar] [CrossRef]
- Pu, J.; Li, W.; Wang, A.; Zhang, Y.; Qin, Z.; Xu, Z.; Wang, J.; Lu, Y.; Tang, Q.; Wei, H. Long non-coding RNA HOMER3-AS1 drives hepatocellular carcinoma progression via modulating the behaviors of both tumor cells and macrophages. Cell Death Dis. 2021, 12, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Su, B.; Han, H.; Gong, Y.; Li, X.; Ji, C.; Yao, J.; Yang, J.; Hu, W.; Zhao, W.; Li, J.; et al. Let-7d inhibits intratumoral macrophage M2 polarization and subsequent tumor angiogenesis by targeting IL-13 and IL-10. Cancer Immunol. Immunother. 2021, 70, 1619–1634. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Xu, L.; Hu, Y.; Huang, Y.; Zhang, Y.; Zheng, X.; Wang, S.; Wang, Y.; Yu, Y.; Zhang, M.; et al. miRNA let-7b modulates macrophage polarization and enhances tumor-associated macrophages to promote angiogenesis and mobility in prostate cancer. Sci. Rep. 2016, 6, 25602. [Google Scholar] [CrossRef]
- Jia, X.; Li, X.; Shen, Y.; Miao, J.; Liu, H.; Li, G.; Wang, Z. MiR-16 regulates mouse peritoneal macrophage polarization and affects T-cell activation. J. Cell. Mol. Med. 2016, 20, 1898–1907. [Google Scholar] [CrossRef]
- Liang, X.; Shangguan, W.; Zhang, M.; Mei, S.; Wang, L.; Yang, R. miR-128 enhances dendritic cell-mediated anti-tumor immunity via targeting of p38. Mol. Med. Rep. 2017, 16, 1307–1313. [Google Scholar] [CrossRef]
- Zawislak, C.L.; Beaulieu, A.M.; Loeb, G.B.; Karo, J.; Canner, D.; Bezman, N.A.; Lanier, L.L.; Rudensky, A.Y.; Sun, J.C. Stage-specific regulation of natural killer cell homeostasis and response against viral infection by microRNA-155. Proc. Natl. Acad. Sci. USA 2013, 110, 6967–6972. [Google Scholar] [CrossRef]
- Cai, X.; Yin, Y.; Li, N.; Zhu, D.; Zhang, J.; Zhang, C.-Y.; Zen, K. Re-polarization of tumor-associated macrophages to pro-inflammatory M1 macrophages by microRNA-155. J. Mol. Cell Biol. 2012, 4, 341–343. [Google Scholar] [CrossRef]
- Liu, R.; Sun, X.; Hu, Z.; Peng, C.; Wu, T. Knockdown of long non-coding RNA MIR155HG suppresses melanoma cell proliferation, and deregulated MIR155HG in melanoma is associated with M1/M2 balance and macrophage infiltration. Cells Dev. 2022, 170, 203768. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Zhou, L.; Shen, N.; Ning, X.; Wu, D.; Jiang, K.; Huang, X. M1 macrophage-derived exosomes and their key molecule lncRNA HOTTIP suppress head and neck squamous cell carcinoma progression by upregulating the TLR5/NF-κB pathway. Cell Death Dis. 2022, 13, 183. [Google Scholar] [CrossRef]
- Baroni, S.; Romero-Cordoba, S.; Plantamura, I.; Dugo, M.; D’Ippolito, E.; Cataldo, A.; Cosentino, G.; Angeloni, V.; Rossini, A.; Daidone, M.G.; et al. Exosome-mediated delivery of miR-9 induces cancer-associated fibroblast-like properties in human breast fibroblasts. Cell Death Dis. 2016, 7, e2312. [Google Scholar] [CrossRef]
- Zhou, X.; Yan, T.; Huang, C.; Xu, Z.; Wang, L.; Jiang, E.; Wang, H.; Chen, Y.; Liu, K.; Shao, Z.; et al. Melanoma cell-secreted exosomal miR-155-5p induce proangiogenic switch of cancer-associated fibroblasts via SOCS1/JAK2/STAT3 signaling pathway. J. Exp. Clin. Cancer Res. 2018, 37, 242. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.-S.; Wang, C.-Y.; Chen, P.-S.; Hung, J.-H.; Yen, J.-H.; Wu, M.-J. 8-Hydroxydaidzein Downregulates JAK/STAT, MMP, Oxidative Phosphorylation, and PI3K/AKT Pathways in K562 Cells. Biomedicines 2021, 9, 1907. [Google Scholar] [CrossRef]
- Shu, S.; Yang, Y.; Allen, C.L.; Maguire, O.; Minderman, H.; Sen, A.; Ciesielski, M.J.; Collins, K.A.; Bush, P.J.; Singh, P.; et al. Metabolic reprogramming of stromal fibroblasts by melanoma exosome microRNA favours a pre-metastatic microenvironment. Sci. Rep. 2018, 8, 12905. [Google Scholar] [CrossRef]
- Jiang, M.; Zhang, W.; Zhang, R.; Liu, P.; Ye, Y.; Yu, W.; Guo, X.; Yu, J. Cancer exosome-derived miR-9 and miR-181a promote the development of early-stage MDSCs via interfering with SOCS3 and PIAS3 respectively in breast cancer. Oncogene 2020, 39, 4681–4694. [Google Scholar] [CrossRef]
- Ren, W.; Hou, J.; Yang, C.; Wang, H.; Wu, S.; Wu, Y.; Zhao, X.; Lu, C. Extracellular vesicles secreted by hypoxia pre-challenged mesenchymal stem cells promote non-small cell lung cancer cell growth and mobility as well as macrophage M2 polarization via miR-21-5p delivery. J. Exp. Clin. Cancer Res. 2019, 38, 62. [Google Scholar] [CrossRef]
- Fabbri, M. MicroRNAs and cancer: Towards a personalized medicine. Curr. Mol. Med. 2013, 13, 751–756. [Google Scholar] [CrossRef]
- Wang, B.; Wang, X.; Li, P.; Niu, X.; Liang, X.; Liu, G.; Liu, Z.; Ge, H. Osteosarcoma Cell-Derived Exosomal ELFN1-AS1 Mediates Macrophage M2 Polarization via Sponging miR-138-5p and miR-1291 to Promote the Tumorgenesis of Osteosarcoma. Front. Oncol. 2022, 12, 881022. [Google Scholar] [CrossRef]
- Moradi-Chaleshtori, M.; Bandehpour, M.; Heidari, N.; Mohammadi-Yeganeh, S.; Hashemi, S.M. Exosome-mediated miR-33 transfer induces M1 polarization in mouse macrophages and exerts antitumor effect in 4T1 breast cancer cell line. Int. Immunopharmacol. 2021, 90, 107198. [Google Scholar] [CrossRef]
- François, S.; Usunier, B.; Forgue-Lafitte, M.-E.; L’Homme, B.; Benderitter, M.; Douay, L.; Gorin, N.-C.; Larsen, A.K.; Chapel, A. Mesenchymal Stem Cell Administration Attenuates Colon Cancer Progression by Modulating the Immune Component within the Colorectal Tumor Microenvironment. Stem Cells Transl. Med. 2019, 8, 285–300. [Google Scholar] [CrossRef]
- Chang, W.-A.; Tsai, M.-J.; Hung, J.-Y.; Wu, K.-L.; Tsai, Y.-M.; Huang, Y.-C.; Chang, C.-Y.; Tsai, P.-H.; Hsu, Y.-L. miR-150-5p-Containing Extracellular Vesicles Are a New Immunoregulator That Favor the Progression of Lung Cancer in Hypoxic Microenvironments by Altering the Phenotype of NK Cells. Cancers 2021, 13, 6252. [Google Scholar] [CrossRef]
- Yang, M.; Chen, J.; Su, F.; Yu, B.; Su, F.; Lin, L.; Liu, Y.; Huang, J.-D.; Song, E. Microvesicles secreted by macrophages shuttle invasion-potentiating microRNAs into breast cancer cells. Mol. Cancer 2011, 10, 117. [Google Scholar] [CrossRef]
- Dong, D.; Zhang, Y.; Reece, E.A.; Wang, L.; Harman, C.R.; Yang, P. microRNA expression profiling and functional annotation analysis of their targets modulated by oxidative stress during embryonic heart development in diabetic mice. Reprod. Toxicol. 2016, 65, 365–374. [Google Scholar] [CrossRef]
- Zhu, X.; Shen, H.; Yin, X.; Yang, M.; Wei, H.; Chen, Q.; Feng, F.; Liu, Y.; Xu, W.; Li, Y. Macrophages derived exosomes deliver miR-223 to epithelial ovarian cancer cells to elicit a chemoresistant phenotype. J. Exp. Clin. Cancer Res. 2019, 38, 81. [Google Scholar] [CrossRef] [PubMed]
- Ismail, N.; Wang, Y.; Dakhlallah, D.; Moldovan, L.; Agarwal, K.; Batte, K.; Shah, P.; Wisler, J.; Eubank, T.D.; Tridandapani, S.; et al. Macrophage microvesicles induce macrophage differentiation and miR-223 transfer. Blood 2013, 121, 984–995. [Google Scholar] [CrossRef]
- Montecalvo, A.; Larregina, A.T.; Shufesky, W.J.; Beer Stolz, D.; Sullivan, M.L.G.; Karlsson, J.M.; Baty, C.J.; Gibson, G.A.; Erdos, G.; Wang, Z.; et al. Mechanism of transfer of functional microRNAs between mouse dendritic cells via exosomes. Blood 2012, 119, 756–766. [Google Scholar] [CrossRef]
- Bardi, G.T.; Smith, M.A.; Hood, J.L. Melanoma exosomes promote mixed M1 and M2 macrophage polarization. Cytokine 2018, 105, 63–72. [Google Scholar] [CrossRef]
- Cheng, C.-W.; Yu, J.-C.; Hsieh, Y.-H.; Liao, W.-L.; Shieh, J.-C.; Yao, C.-C.; Lee, H.-J.; Chen, P.-M.; Wu, P.-E.; Shen, C.-Y. Increased Cellular Levels of MicroRNA-9 and MicroRNA-221 Correlate with Cancer Stemness and Predict Poor Outcome in Human Breast Cancer. Cell. Physiol. Biochem. 2018, 48, 2205–2218. [Google Scholar] [CrossRef]
- Sui, X.; Jiao, Y.-N.; Yang, L.-H.; Liu, J. MiR-9 accelerates epithelial-mesenchymal transition of ovarian cancer cells via inhibiting e-cadherin. Eur. Rev. Med. Pharmacol. Sci. 2019, 23 (Suppl. 3), 209–216. [Google Scholar]
- Wang, H.; Zhang, W.; Zuo, Y.; Ding, M.; Ke, C.; Yan, R.; Zhan, H.; Liu, J.; Wang, J. miR-9 promotes cell proliferation and inhibits apoptosis by targeting LASS2 in bladder cancer. Tumor Biol. 2015, 36, 9631–9640. [Google Scholar] [CrossRef]
- Yao, X.; Xie, L.; Zeng, Y. MiR-9 Promotes Angiogenesis via Targeting on Sphingosine-1- Phosphate Receptor 1. Front. Cell Dev. Biol. 2020, 8, 755. [Google Scholar] [CrossRef]
- Ni, S.-J.; Zhao, L.-Q.; Wang, X.-F.; Wu, Z.-H.; Hua, R.-X.; Wan, C.-H.; Zhang, J.-Y.; Zhang, X.-W.; Huang, M.-Z.; Gan, L.; et al. CBX7 regulates stem cell-like properties of gastric cancer cells via p16 and AKT-NF-κB-miR-21 pathways. J. Hematol. Oncol. 2018, 11, 17. [Google Scholar] [CrossRef]
- Cretoiu, D.; Dai, L.; Chen, F.; Zheng, Y.; Zhang, D.; Qian, B.; Ji, H.; Long, F. miR-21 regulates growth and EMT in lung cancer cells via PTEN Akt GSK3 beta signaling. Front. Biosci. (Landmark Ed) 2019, 24, 1426–1439. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.-F.; Wang, M.-X.; Meng, L.-N.; Zhang, R.; Wang, W. MiR-21 regulates proliferation and apoptosis of oral cancer cells through TNF-α. Eur. Rev. Med Pharmacol. Sci. 2018, 22, 7735–7741. [Google Scholar]
- Johansson, J.; Berg, T.; Kurzejamska, E.; Pang, M.-F.; Tabor, V.; Jansson, M.; Roswall, P.; Pietras, K.; Sund, M.; Religa, P.; et al. MiR-155-mediated loss of C/EBPβ shifts the TGF-β response from growth inhibition to epithelial-mesenchymal transition, invasion and metastasis in breast cancer. Oncogene 2013, 32, 5614–5624. [Google Scholar] [CrossRef]
- Huang, P.; Ye, B.; Yang, Y.; Shi, J.; Zhao, H. MicroRNA-181 functions as a tumor suppressor in non-small cell lung cancer (NSCLC) by targeting Bcl-2. Tumor Biol. 2015, 36, 3381–3387. [Google Scholar] [CrossRef]
- Xu, G.; Wei, X.; Tu, Q.; Zhou, C. Up-regulated microRNA-33b inhibits epithelial–mesenchymal transition in gallbladder cancer through down-regulating CROCC. Biosci. Rep. 2020, 40, BSR20190108. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhou, X.; Shan, B.; Han, J.; Wang, F.; Fan, X.; Lv, Y.; Chang, L.; Liu, W. Downregulation of microRNA-33a promotes cyclin-dependent kinase 6, cyclin D1 and PIM1 expression and gastric cancer cell proliferation. Mol. Med. Rep. 2015, 12, 6491–6500. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, W.; Liu, J.; Li, W. hsa-miR-33-5p as a Therapeutic Target Promotes Apoptosis of Breast Cancer Cells via Selenoprotein T. Front. Med. 2021, 8, 651473. [Google Scholar] [CrossRef] [PubMed]
- Ito, M.; Teshima, K.; Ikeda, S.; Kitadate, A.; Watanabe, A.; Nara, M.; Yamashita, J.; Ohshima, K.; Sawada, K.; Tagawa, H. MicroRNA-150 inhibits tumor invasion and metastasis by targeting the chemokine receptor CCR6, in advanced cutaneous T-cell lymphoma. Blood 2014, 123, 1499–1511. [Google Scholar] [CrossRef]
- Yang, B.; Miao, S. lncRNA ELFN1-AS1 predicts poor prognosis and promotes tumor progression of non-small cell lung cancer by sponging miR-497. Cancer Biomark. 2022, 34, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Jing, X.; Du, L.; Shi, S.; Niu, A.; Wu, J.; Wang, Y.; Wang, C. Hypoxia-Induced Upregulation of lncRNA ELFN1-AS1 Promotes Colon Cancer Growth and Metastasis Through Targeting TRIM14 via Sponging miR-191-5p. Front. Pharmacol. 2022, 13, 806682. [Google Scholar] [CrossRef]



| Noncoding RNA | Observed Changes in Cancer | Possible Mechanism Supporting Tumor Growth | Possible Mechanism Inhibiting Tumor Growth |
|---|---|---|---|
| miR-9 | high | Retaining mitochondrial function and protecting against apoptosis; miR-9 in exosomes released by cancer cells induces differentiation of fibroblasts to CAFs | |
| miR-20a | high | Suppressing NK cell cytotoxicity | |
| miR-21 | high | Protecting mitochondrial function; inducing CD4+ T cells and differentiating them to Th2 cells; differentiating Th17 and Treg cells; miR-21 in microvesicles from cancer cells suppresses M1 macrophage polarization; miR-21 in microvesicles secreted by CAFs induces M2 macrophage polarization; miR-21 in microvesicles from macrophages and dendritic cells in tumor-adjacent tissues promotes M2 macrophage polarization in the tumor microenvironment | |
| miR-130b | high | Activating Th17 cells | |
| miR-146b | high | Activating M2 TAMs and controlling inflammation | |
| miR-150 | high | Reducing CD8+ cytotoxic and NK cells; miR-150 in microvesicles secreted by mesenchymal cells and cancer cells, and tumor-adjacent tissues inhibits NK cell action and improves IL-10 secretion by M2 macrophages | |
| miR-223 | high | Inhibiting and NLRP3 inflammasome, suppressing neutrophil and M1 macrophage polarization; miR-223 in exosomes derived from hypoxic macrophages induces PI3K/AKT signaling, crucial for controlling oxidative stress; miR-223 in microvesicles secreted by tumor-adjacent tissue suppresses the inflammatory tumor microenvironment | |
| HOTAIR | high | Protecting mitochondrial function; inducing MDSC differentiation; increasing the proportion of M2 macrophages | |
| MALAT1 | high | Protecting mitochondrial function by targeting COX2; increasing antioxidant defense | |
| UCA1 | high | Protecting mitochondrial function; increasing antioxidant defense; attenuated the killing effect of cytotoxic CD8+ T cells | |
| XIST | high | Increasing antioxidant protection | |
| Let-7b | high | Increasing M1 TAMs | |
| Let-7d | high | Increasing M1 TAMs | |
| miR-16 | high | Increasing M1 TAMs | |
| miR-34a | high | Perturbating mitochondrial function; inhibiting recruitment of Treg cells | |
| miR-125 | high | Impairing mitochondrial function | |
| miR-128 | high | Impairing mitochondrial function and increasing ROS; inducing M1 macrophage polarization | |
| miR-155 | high | Reducing antioxidant capacity; increasing CD8+ T cells and cytotoxicity of NK cells; promoting the differentiation of Th1 cells and inhibiting differentiation of Treg cells; reprogramming TAMs to pro-inflammatory M1 macrophages; miR-155 in melanoma cell-derived microvesicles induced differentiation of fibroblasts to CAFs; miR-155 in microvesicles secreted by tumor-adjacent tissues exerts anti-tumor effects | |
| GAS5 | high | Impairing mitochondrial function; promoting action of NK cells | |
| HOTTIP | high | Inducing polarization of M2 into M1 TAMs | |
| miR-17–19 | high | Suppressing the inhibitor of the AKT signaling pathway and activating the AKT pathway; inducing Th17 cell differentiation. However, HOTAIRM1 might downregulate miR-17-5p | Inducing Th1 and suppressing Treg cell differentiation |
| miR-30a | high | Inducing MDSC differentiation | Inducing mitochondria-dependent apoptosis |
| miR-124 | high | Inducing differentiation of Treg cells | Inducing mitochondria-dependent apoptosis; |
| NEAT1 | high | Increasing antioxidant defense | Promoting the secretion of CD8+ T-lymphocyte factors, including TNF-α and IFN-γ |
| PVT1 | high | Protecting mitochondrial function; increasing antioxidant | Enriching CD8+ cytotoxic T cell subsets |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Holvoet, P. Noncoding RNAs Controlling Oxidative Stress in Cancer. Cancers 2023, 15, 1155. https://doi.org/10.3390/cancers15041155
Holvoet P. Noncoding RNAs Controlling Oxidative Stress in Cancer. Cancers. 2023; 15(4):1155. https://doi.org/10.3390/cancers15041155
Chicago/Turabian StyleHolvoet, Paul. 2023. "Noncoding RNAs Controlling Oxidative Stress in Cancer" Cancers 15, no. 4: 1155. https://doi.org/10.3390/cancers15041155
APA StyleHolvoet, P. (2023). Noncoding RNAs Controlling Oxidative Stress in Cancer. Cancers, 15(4), 1155. https://doi.org/10.3390/cancers15041155






