Clinical Outcomes Depending on Sympathetic Innervation in Pancreatic Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. The Type of Study
2.2. Patients and Tissues
2.3. Immunohistochemistry
2.4. Quantitative Analysis of Neural Tissue
2.5. Statistical Analysis
3. Results
3.1. Immunoreactivity of Tyrosine Hydroxylase and Beta 2 Adrenergic Receptors in Pancreatic Cancer and Peritumoral Tissues
3.2. Baseline Clinicopathological Features
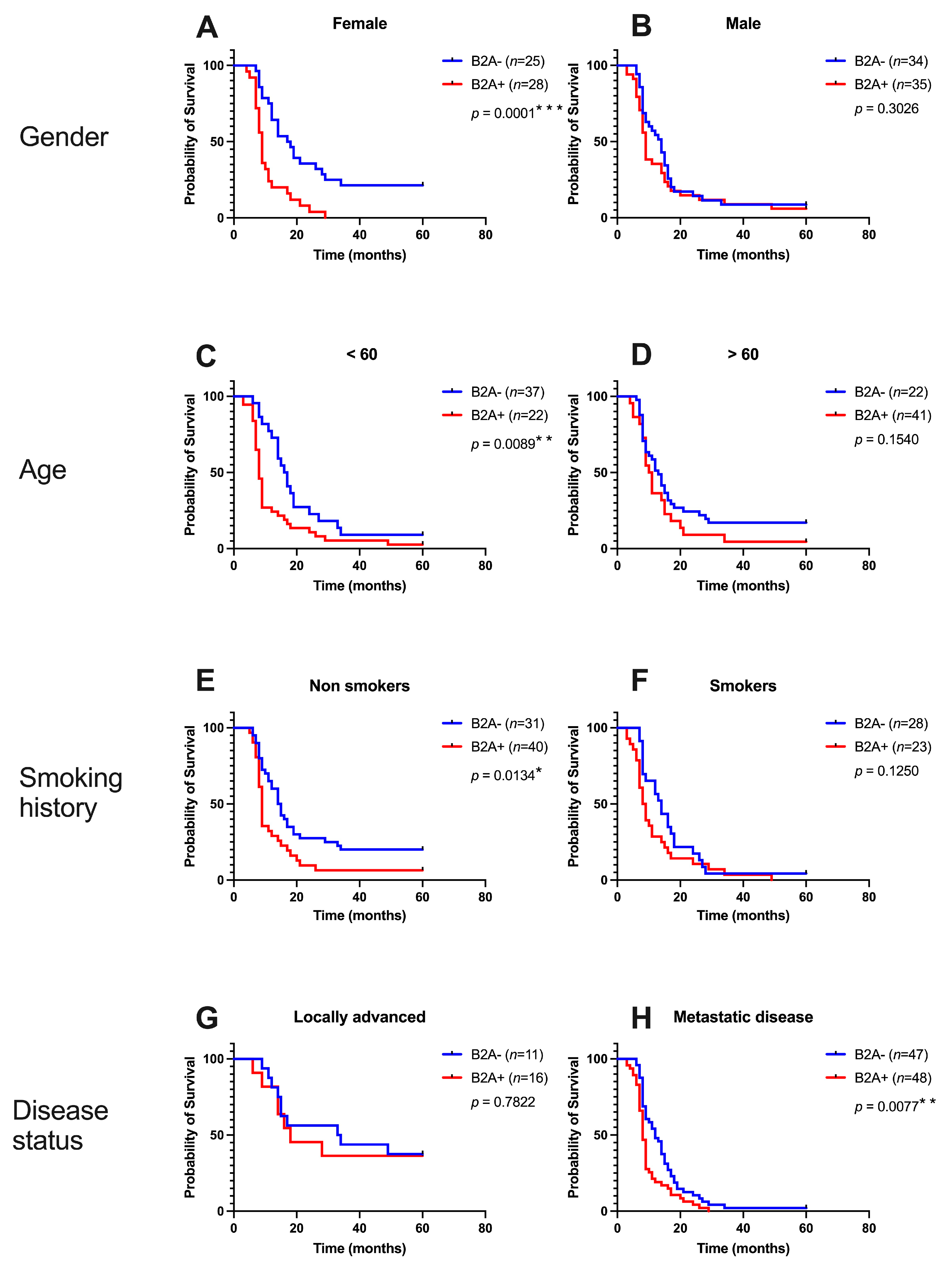
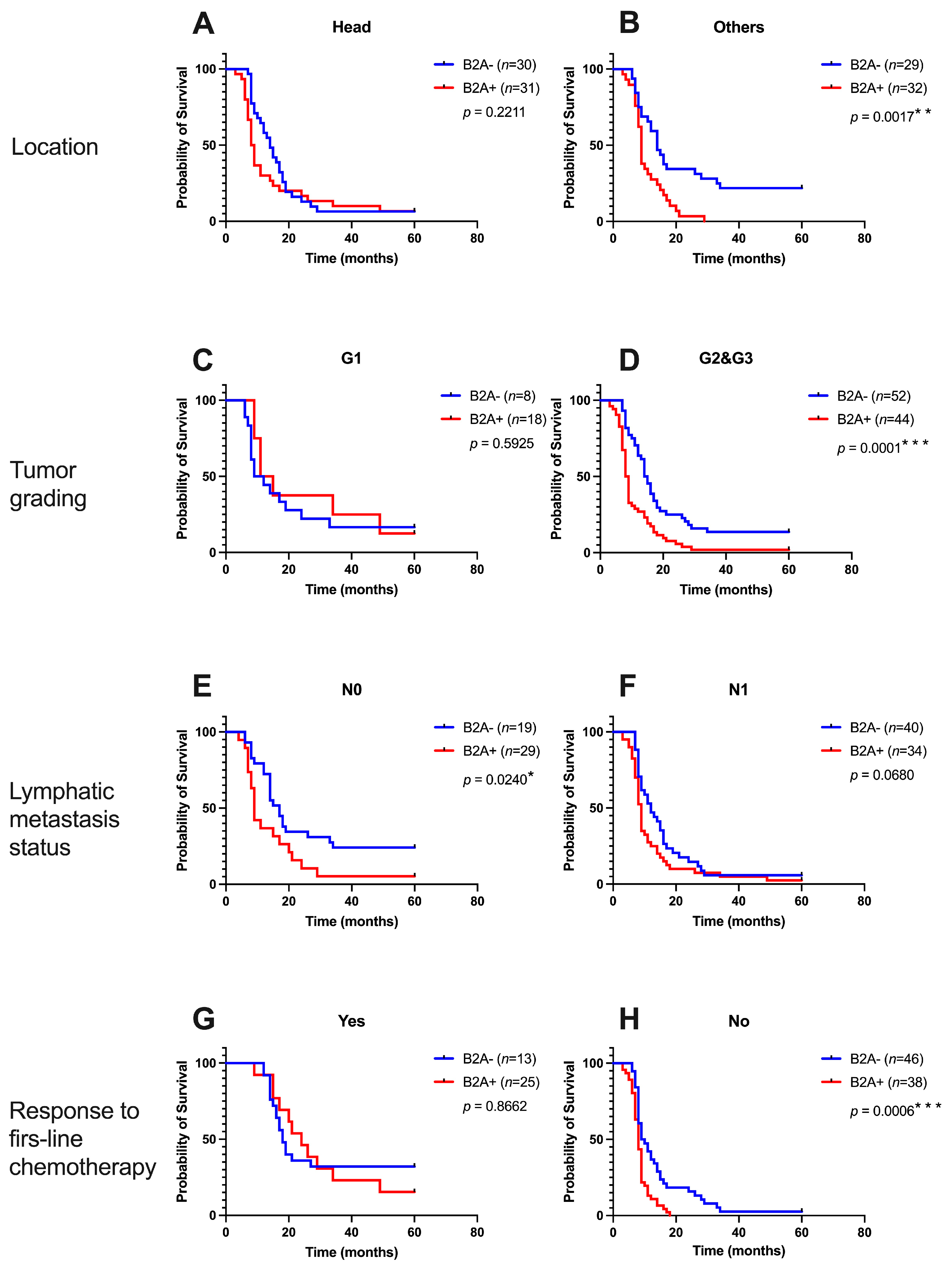
3.3. Impact of Beta 2 Adrenoreceptors from Peritumoral Tissues in Pancreatic Cancer
4. Discussion
4.1. Alterations of Sympathetic Nerve Fibers
4.2. Immunoreactivity of Beta 2 Adrenoreceptors
4.3. Limitations of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- National Cancer Institute. Available online: https://seer.cancer.gov/statfacts/html/pancreas.html (accessed on 10 May 2023).
- International Agency for Research on Cancer (World Health Organization). Available online: https://gco.iarc.fr (accessed on 10 May 2023).
- Ferdoushi, A.; Griffin, N.; Marsland, M.; Xu, X.; Faulkner, S.; Gao, F.; Liu, H.; King, S.J.; Denham, J.W.; van Helden, D.F.; et al. Tumor innervation and clinical outcome in pancreatic cancer. Sci. Rep. 2021, 11, 7390. [Google Scholar] [CrossRef] [PubMed]
- Ceyhan, G.O.; Demir, I.E.; Rauch, U.; Bergmann, F.; Müller, M.W.; Büchler, M.W.; Friess, H.; Schäfer, K.H. Pancreatic neuropathy results in “neural remodeling” and altered pancreatic innervation in chronic pancreatitis and pancreatic cancer. Am. J. Gastroenterol. 2009, 104, 2555–2565. [Google Scholar] [CrossRef]
- Renz, B.W.; Takahashi, R.; Tanaka, T.; Macchini, M.; Hayakawa, Y.; Dantes, Z.; Maurer, H.C.; Chen, X.; Jiang, Z.; Westphalen, C.B.; et al. β2 Adrenergic-Neurotrophin Feedforward Loop Promotes Pancreatic Cancer. Cancer Cell 2018, 33, 75–90.e7. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, T.; Hiraoka, N.; Ino, Y.; Nakajima, K.; Kishi, Y.; Nara, S.; Esaki, M.; Shimada, K.; Katai, H. Reduction of intrapancreatic neural density in cancer tissue predicts poorer outcome in pancreatic ductal carcinoma. Cancer Sci. 2019, 110, 1491–1502. [Google Scholar] [CrossRef] [PubMed]
- Schorn, S.; Demir, I.E.; Haller, B.; Scheufele, F.; Reyes, C.M.; Tieftrunk, E.; Sargut, M.; Goess, R.; Friess, H.; Ceyhan, G.O. The influence of neural invasion on survival and tumor recurrence in pancreatic ductal adenocarcinoma—A systematic review and meta-analysis. Surg. Oncol. 2017, 26, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Liebl, F.; Demir, I.E.; Mayer, K.; Schuster, T.; D’Haese, J.G.; Becker, K.; Langer, R.; Bergmann, F.; Wang, K.; Rosenberg, R.; et al. The impact of neural invasion severity in gastrointestinal malignancies: A clinicopathological study. Ann. Surg. 2014, 260, 900–908. [Google Scholar] [CrossRef]
- Bockman, D.E.; Büchler, M.; Beger, H.G. Interaction of pancreatic ductal carcinoma with nerves leads to nerve damage. Gastroenterology 1994, 107, 219–230. [Google Scholar] [CrossRef]
- Li, Z.J.; Cho, C.H. Neurotransmitters, more than meets the eye--neurotransmitters and their perspectives in cancer development and therapy. Eur. J. Pharmacol. 2011, 667, 17–22. [Google Scholar] [CrossRef]
- Jiang, S.H.; Hu, L.P.; Wang, X.; Li, J.; Zhang, Z.G. Neurotransmitters: Emerging targets in cancer. Oncogene 2020, 39, 503–515. [Google Scholar] [CrossRef]
- Kuol, N.; Stojanovska, L.; Apostolopoulos, V.; Nurgali, K. Crosstalk between cancer and the neuro-immune system. J. Neuroimmunol. 2018, 315, 15–23. [Google Scholar] [CrossRef]
- Stopczynski, R.E.; Normolle, D.P.; Hartman, D.J.; Ying, H.; DeBerry, J.J.; Bielefeldt, K.; Rhim, A.D.; DePinho, R.A.; Albers, K.M.; Davis, B.M. Neuroplastic changes occur early in the development of pancreatic ductal adenocarcinoma. Cancer Res. 2014, 74, 1718–1727. [Google Scholar] [CrossRef] [PubMed]
- Biankin, A.V.; Waddell, N.; Kassahn, K.S.; Gingras, M.C.; Muthuswamy, L.B.; Johns, A.L.; Miller, D.K.; Wilson, P.J.; Patch, A.M.; Wu, J.; et al. Pancreatic cancer genomes reveal aberrations in axon guidance pathway genes. Nature 2012, 491, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Ceyhan, G.O.; Bergmann, F.; Kadihasanoglu, M.; Altintas, B.; Demir, I.E.; Hinz, U.; Müller, M.W.; Giese, T.; Büchler, M.W.; Giese, N.A.; et al. Pancreatic neuropathy and neuropathic pain--a comprehensive pathomorphological study of 546 cases. Gastroenterology 2009, 136, 177–186. [Google Scholar] [CrossRef]
- Ceyhan, G.O.; Schäfer, K.H.; Kerscher, A.G.; Rauch, U.; Demir, I.E.; Kadihasanoglu, M.; Böhm, C.; Müller, M.W.; Büchler, M.W.; Giese, N.A.; et al. Nerve growth factor and artemin are paracrine mediators of pancreatic neuropathy in pancreatic adenocarcinoma. Ann. Surg. 2010, 251, 923–931. [Google Scholar] [CrossRef]
- Haeberle, L.; Steiger, K.; Schlitter, A.M.; Safi, S.A.; Knoefel, W.T.; Erkan, M.; Esposito, I. Stromal heterogeneity in pancreatic cancer and chronic pancreatitis. Pancreatology 2018, 18, 536–549. [Google Scholar] [CrossRef] [PubMed]
- Helm, O.; Mennrich, R.; Petrick, D.; Goebel, L.; Freitag-Wolf, S.; Röder, C.; Kalthoff, H.; Röcken, C.; Sipos, B.; Kabelitz, D.; et al. Comparative characterization of stroma cells and ductal epithelium in chronic pancreatitis and pancreatic ductal adenocarcinoma. PLoS ONE 2014, 9, e94357. [Google Scholar] [CrossRef]
- Zhan, H.X.; Zhou, B.; Cheng, Y.G.; Xu, J.W.; Wang, L.; Zhang, G.Y.; Hu, S.Y. Crosstalk between stromal cells and cancer cells in pancreatic cancer: New insights into stromal biology. Cancer Lett. 2017, 392, 83–93. [Google Scholar] [CrossRef]
- Hori, S.; Shimada, K.; Ino, Y.; Oguro, S.; Esaki, M.; Nara, S.; Kishi, Y.; Kosuge, T.; Hattori, Y.; Sukeda, A.; et al. Macroscopic features predict outcome in patients with pancreatic ductal adenocarcinoma. Virchows Arch. 2016, 469, 621–634. [Google Scholar] [CrossRef]
- Nishida, T.; Yoshitomi, H.; Takano, S.; Kagawa, S.; Shimizu, H.; Ohtsuka, M.; Kato, A.; Furukawa, K.; Miyazaki, M. Low Stromal Area and High Stromal Microvessel Density Predict Poor Prognosis in Pancreatic Cancer. Pancreas 2016, 45, 593–600. [Google Scholar] [CrossRef]
- Saloman, J.L.; Albers, K.M.; Li, D.; Hartman, D.J.; Crawford, H.C.; Muha, E.A.; Rhim, A.D.; Davis, B.M. Ablation of sensory neurons in a genetic model of pancreatic ductal adenocarcinoma slows initiation and progression of cancer. Proc. Natl. Acad. Sci. USA 2016, 113, 3078–3083. [Google Scholar] [CrossRef]
- Magnon, C.; Hall, S.J.; Lin, J.; Xue, X.; Gerber, L.; Freedland, S.J.; Frenette, P.S. Autonomic nerve development contributes to prostate cancer progression. Science 2013, 341, 1236361. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.M.; Hayakawa, Y.; Kodama, Y.; Muthupalani, S.; Westphalen, C.B.; Andersen, G.T.; Flatberg, A.; Johannessen, H.; Friedman, R.A.; Renz, B.W.; et al. Denervation suppresses gastric tumorigenesis. Sci. Transl. Med. 2014, 6, 250ra115. [Google Scholar] [CrossRef] [PubMed]
- Ciurea, R.N.; Rogoveanu, I.; Pirici, D.; Târtea, G.C.; Streba, C.T.; Florescu, C.; Cătălin, B.; Puiu, I.; Târtea, E.A.; Vere, C.C. B2 adrenergic receptors and morphological changes of the enteric nervous system in colorectal adenocarcinoma. World J. Gastroenterol. 2017, 23, 1250–1261. [Google Scholar] [CrossRef]
- Ciurea, A.M.; Gheonea, D.I.; Schenker, M.; Mehedințeanu, A.M.; Târtea, G.C.; Vere, C.C. The Prognostic Correlation of Heart Rate Variability at Diagnosis with Survival of Patients with Hepatocellular Carcinoma. Diagnostics 2021, 11, 890. [Google Scholar] [CrossRef]
- Mehedințeanu, A.M.; Sfredel, V.; Stovicek, P.O.; Schenker, M.; Târtea, G.C.; Istrătoaie, O.; Ciurea, A.M.; Vere, C.C. Assessment of Epinephrine and Norepinephrine in Gastric Carcinoma. Int. J. Mol. Sci. 2021, 22, 2042. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Ma, Q.; Wang, Z.; Zhang, M.; Guo, K.; Wang, F.; Wu, E. β2-adrenoceptor blockage induces G1/S phase arrest and apoptosis in pancreatic cancer cells via Ras/Akt/NFκB pathway. Mol. Cancer 2011, 10, 146. [Google Scholar] [CrossRef]
- McGraw, D.W.; Liggett, S.B. Molecular mechanisms of beta2-adrenergic receptor function and regulation. Proc. Am. Thorac. Soc. 2005, 2, 292–312. [Google Scholar] [CrossRef]
- Luttrell, L.M.; Ferguson, S.S.; Daaka, Y.; Miller, W.E.; Maudsley, S.; Della Rocca, G.J.; Lin, F.; Kawakatsu, H.; Owada, K.; Luttrell, D.K.; et al. Beta-arrestin-dependent formation of beta2 adrenergic receptor-Src protein kinase complexes. Science 1999, 283, 655–661. [Google Scholar] [CrossRef]
- Vargiu, P.; De Abajo, R.; Garcia-Ranea, J.A.; Valencia, A.; Santisteban, P.; Crespo, P.; Bernal, J. The small GTP-binding protein, Rhes, regulates signal transduction from G protein-coupled receptors. Oncogene 2004, 23, 559–568. [Google Scholar] [CrossRef]
- Hirsch, E.; Ciraolo, E.; Ghigo, A.; Costa, C. Taming the PI3K team to hold inflammation and cancer at bay. Pharmacol. Ther. 2008, 118, 192–205. [Google Scholar] [CrossRef]

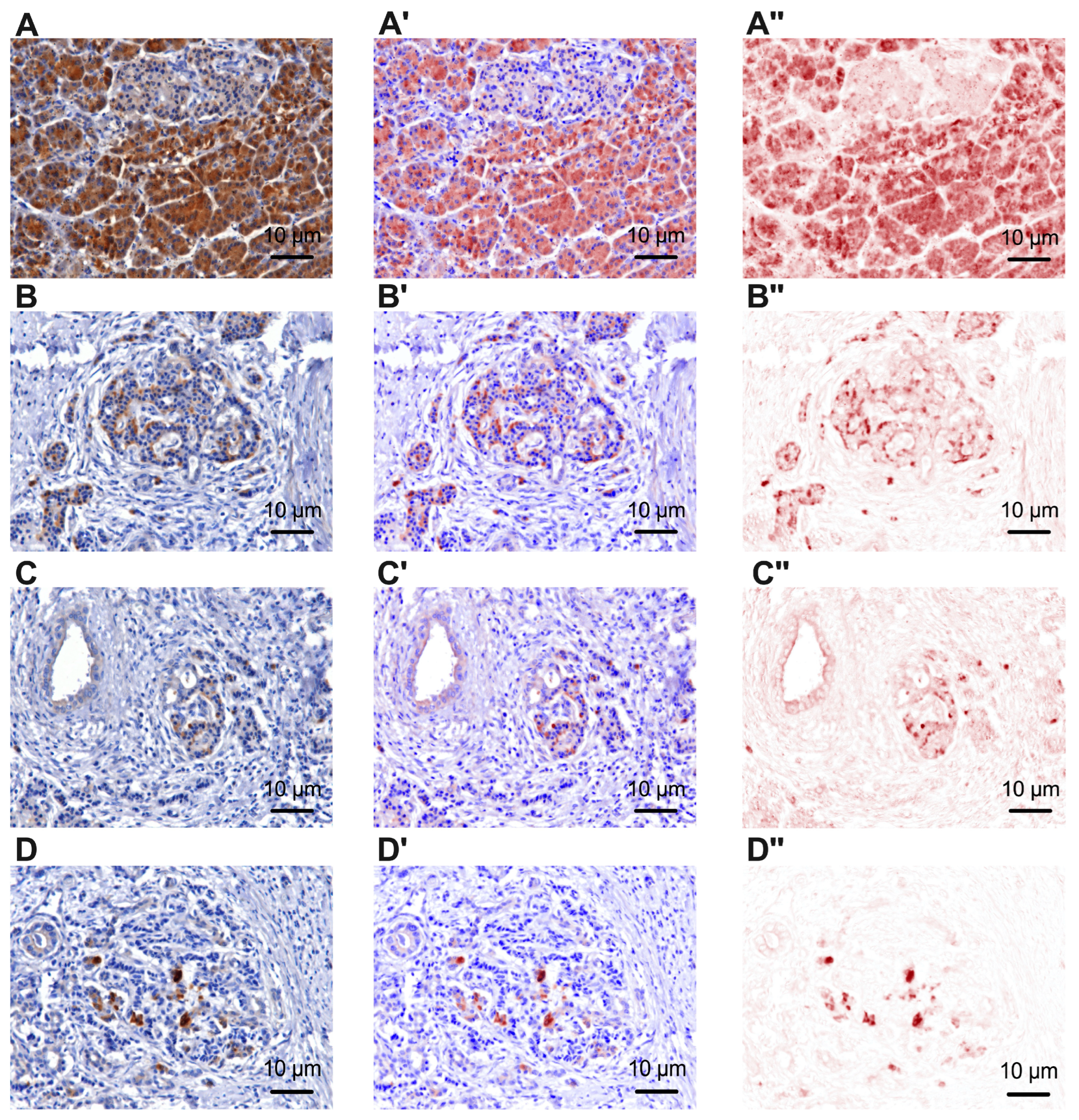


| B2A(−) | B2A(+) | |||
|---|---|---|---|---|
| Variable | Category | Value | Value | p |
| Age (years) | <60 (n = 59) | 22 (37.29%) | 37 (62.71%) | 0.0021 * |
| >60 (n = 63) | 41 (65.08%) | 22 (34.92%) | ||
| Gender | Female (n = 53) | 28 (52.83%) | 25 (47.17%) | 0.8564 |
| Male (n = 69) | 35 (50.72%) | 34 (49.28%) | ||
| Smoking history | No (n = 71) | 40 (56.34%) | 31 (43.66%) | 0.2713 |
| Yes (n = 51) | 23 (45.10%) | 28 (54.90%) | ||
| Disease status | Locally advanced (n = 27) | 16 (59.26%) | 11 (40.74%) | 0.5142 |
| Metastatic disease (n = 95) | 48 (50.53%) | 47 (49.47%) | ||
| Location | Head (n = 61) | 32 (52.46%) | 29 (47.54%) | 0.9999 |
| Others (n = 61) | 31 (50.82%) | 30 (49.18%) | ||
| Tumor grading | G1 (n = 26) | 19 (73.08%) | 7 (26.92%) | 0.0157 * |
| G2&G3 (n= 96) | 44 (45.83%) | 52 (54.17%) | ||
| Lymphatic metastasis status | N0 (n = 48) | 29 (60.42%) | 19 (39.58%) | 0.1398 |
| N1 (n = 74) | 34 (45.95%) | 40 (54.05%%) | ||
| Response to first-line chemotherapy | Yes (n = 38) | 25 (65.79%) | 13 (34.21%) | 0.0450 * |
| No (n = 84) | 38 (45.24%) | 46 (54.76%) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Târtea, E.-A.; Petrescu, M.; Udriștoiu, I.; Gheorman, V.; Biciușcă, V.; Petrescu, A.-R.; Ciurea, A.-M.; Vere, C.C. Clinical Outcomes Depending on Sympathetic Innervation in Pancreatic Cancer. Cancers 2023, 15, 3040. https://doi.org/10.3390/cancers15113040
Târtea E-A, Petrescu M, Udriștoiu I, Gheorman V, Biciușcă V, Petrescu A-R, Ciurea A-M, Vere CC. Clinical Outcomes Depending on Sympathetic Innervation in Pancreatic Cancer. Cancers. 2023; 15(11):3040. https://doi.org/10.3390/cancers15113040
Chicago/Turabian StyleTârtea, Elena-Anca, Mihai Petrescu, Ion Udriștoiu, Victor Gheorman, Viorel Biciușcă, Alexandra-Roxana Petrescu, Ana-Maria Ciurea, and Cristin Constantin Vere. 2023. "Clinical Outcomes Depending on Sympathetic Innervation in Pancreatic Cancer" Cancers 15, no. 11: 3040. https://doi.org/10.3390/cancers15113040
APA StyleTârtea, E.-A., Petrescu, M., Udriștoiu, I., Gheorman, V., Biciușcă, V., Petrescu, A.-R., Ciurea, A.-M., & Vere, C. C. (2023). Clinical Outcomes Depending on Sympathetic Innervation in Pancreatic Cancer. Cancers, 15(11), 3040. https://doi.org/10.3390/cancers15113040






