Peering through the PSMA PET Lens: The Role of the European Association of Urology Biochemical Recurrence Risk Groups after Radical Prostatectomy
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Population
2.2. Radiotracer Preparation
2.3. Imaging Procedure
2.4. Image Analysis
2.5. Variables and Outcomes
2.6. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Imaging Findings
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef] [PubMed]
- Paller, C.J.; Antonarakis, E.S. Management of biochemically recurrent prostate cancer after local therapy: Evolving standards of care and new directions. Clin. Adv. Hematol. Oncol. HO 2013, 11, 14–23. [Google Scholar]
- Toussi, A.; Stewart-Merrill, S.B.; Boorjian, S.A.; Psutka, S.P.; Thompson, R.H.; Frank, I.; Tollefson, M.K.; Gettman, M.T.; Carlson, R.E.; Rangel, L.J.; et al. Standardizing the Definition of Biochemical Recurrence after Radical Prostatectomy-What Prostate Specific Antigen Cut Point Best Predicts a Durable Increase and Subsequent Systemic Progression? J. Urol. 2016, 195, 1754–1759. [Google Scholar] [CrossRef] [PubMed]
- Boorjian, S.A.; Karnes, R.J.; Crispen, P.L.; Rangel, L.J.; Bergstralh, E.J.; Blute, M.L. Radiation therapy after radical prostatectomy: Impact on metastasis and survival. J. Urol. 2009, 182, 2708–2714. [Google Scholar] [CrossRef] [PubMed]
- Tilki, D.; Mandel, P.; Seeliger, F.; Kretschmer, A.; Karl, A.; Ergün, S.; Seitz, M.; Stief, C.G. Salvage lymph node dissection for nodal recurrence of prostate cancer after radical prostatectomy. J. Urol. 2015, 193, 484–490. [Google Scholar] [CrossRef]
- Phillips, R.; Shi, W.Y.; Deek, M.; Radwan, N.; Lim, S.J.; Antonarakis, E.S.; Rowe, S.P.; Ross, A.E.; Gorin, M.A.; Deville, C.; et al. Outcomes of Observation vs Stereotactic Ablative Radiation for Oligometastatic Prostate Cancer: The ORIOLE Phase 2 Randomized Clinical Trial. JAMA Oncol. 2020, 6, 650–659. [Google Scholar] [CrossRef]
- Pagliarulo, V.; Bracarda, S.; Eisenberger, M.A.; Mottet, N.; Schröder, F.H.; Sternberg, C.N.; Studer, U.E. Contemporary role of androgen deprivation therapy for prostate cancer. Eur. Urol. 2012, 61, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Ost, P.; Reynders, D.; Decaestecker, K.; Fonteyne, V.; Lumen, N.; De Bruycker, A.; Lambert, B.; Delrue, L.; Bultijnck, R.; Claeys, T.; et al. Surveillance or Metastasis-Directed Therapy for Oligometastatic Prostate Cancer Recurrence: A Prospective, Randomized, Multicenter Phase II Trial. J. Clin. Oncol. Off J. Am. Soc. Clin. Oncol. 2018, 36, 446–453. [Google Scholar] [CrossRef]
- Artigas, C.; Flamen, P.; Charlier, F.; Levillain, H.; Wimana, Z.; Diamand, R.; Albisinni, S.; Gil, T.; Van Velthoven, R.; Peltier, A.; et al. 68Ga-PSMA PET/CT-based metastasis-directed radiotherapy for oligometastatic prostate cancer recurrence after radical prostatectomy. World J. Urol. 2019, 37, 1535–1542. [Google Scholar] [CrossRef]
- Chang, S.S. Overview of prostate-specific membrane antigen. Rev. Urol. 2004, 6 (Suppl. S10), S13–S18. [Google Scholar]
- Eder, M.; Schäfer, M.; Bauder-Wüst, U.; Hull, W.-E.; Wängler, C.; Mier, W.; Haberkorn, U.; Eisenhut, M. 68Ga-complex lipophilicity and the targeting property of a urea-based PSMA inhibitor for PET imaging. Bioconjug. Chem. 2012, 23, 688–697. [Google Scholar] [CrossRef] [PubMed]
- Tulsyan, S.; Das, C.J.; Tripathi, M.; Seth, A.; Kumar, R.; Bal, C. Comparison of 68Ga-PSMA PET/CT and multiparametric MRI for staging of high-risk prostate. Nucl. Med. Commun. 2017, 38, 1094–1102. [Google Scholar] [CrossRef] [PubMed]
- Von Eyben, F.E.; Picchio, M.; von Eyben, R.; Rhee, H.; Bauman, G. 68Ga-Labeled Prostate-specific Membrane Antigen Ligand Positron Emission Tomography/Computed Tomography for Prostate Cancer: A Systematic Review and Meta-analysis. Eur. Urol. Focus 2018, 4, 686–693. [Google Scholar] [CrossRef] [PubMed]
- Morigi, J.J.; Stricker, P.D.; van Leeuwen, P.J.; Tang, R.; Ho, B.; Nguyen, Q.; Hruby, G.; Fogarty, G.; Jagavkar, R.; Kneebone, A.; et al. Prospective Comparison of 18F-Fluoromethylcholine Versus 68Ga-PSMA PET/CT in Prostate Cancer Patients Who Have Rising PSA After Curative Treatment and Are Being Considered for Targeted Therapy. J. Nucl. Med. Off Publ. Soc. Nucl. Med. 2015, 56, 1185–1190. [Google Scholar] [CrossRef] [PubMed]
- Calais, J.; Ceci, F.; Eiber, M.; A Hope, T.; Hofman, M.S.; Rischpler, C.; Bach-Gansmo, T.; Nanni, C.; Savir-Baruch, B.; Elashoff, D.; et al. 18F-fluciclovine PET-CT and 68Ga-PSMA-11 PET-CT in patients with early biochemical recurrence after prostatectomy: A prospective, single-centre, single-arm, comparative imaging trial. Lancet Oncol. 2019, 20, 1286–1294. [Google Scholar] [CrossRef]
- Broeck, T.V.D.; Bergh, R.C.v.D.; Arfi, N.; Gross, T.; Moris, L.; Briers, E.; Cumberbatch, M.; De Santis, M.; Tilki, D.; Fanti, S.; et al. Prognostic Value of Biochemical Recurrence Following Treatment with Curative Intent for Prostate Cancer: A Systematic Review. Eur. Urol. 2019, 75, 967–987. [Google Scholar] [CrossRef]
- Van den Broeck, T.; van den Bergh, R.C.; Briers, E.; Cornford, P.; Cumberbatch, M.; Tilki, D.; De Santis, M.; Fanti, S.; Fossati, N.; Gillessen, S.; et al. Biochemical Recurrence in Prostate Cancer: The European Association of Urology Prostate Cancer Guidelines Panel Recommendations. Eur. Urol. Focus 2020, 6, 231–234. [Google Scholar] [CrossRef]
- Fanti, S.; Minozzi, S.; Morigi, J.J.; Giesel, F.; Ceci, F.; Uprimny, C.; Hofman, M.S.; Eiber, M.; Schwarzenbock, S.; Castellucci, P.; et al. Development of standardized image interpretation for 68Ga-PSMA PET/CT to detect prostate cancer recurrent lesions. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1622–1635. [Google Scholar] [CrossRef]
- Lievens, Y.; Guckenberger, M.; Gomez, D.; Hoyer, M.; Iyengar, P.; Kindts, I.; Romero, A.M.; Nevens, D.; Palma, D.; Park, C.; et al. Defining oligometastatic disease from a radiation oncology perspective: An ESTRO-ASTRO consensus document. Radiother Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2020, 148, 157–166. [Google Scholar] [CrossRef]
- Deek, M.P.; Van der Eecken, K.; Sutera, P.; Deek, R.A.; Fonteyne, V.; Mendes, A.A.; Decaestecker, K.; Kiess, A.P.; Lumen, N.; Phillips, R.; et al. Long-Term Outcomes and Genetic Predictors of Response to Metastasis-Directed Therapy Versus Observation in Oligometastatic Prostate Cancer: Analysis of STOMP and ORIOLE Trials. J. Clin. Oncol. 2022, 40, 3377–3382. [Google Scholar] [CrossRef]
- Tang, C.; Sherry, A.D.; Haymaker, C.; Bathala, T.; Liu, S.; Fellman, B.; Cohen, L.; Aparicio, A.; Zurita, A.J.; Reuben, A.; et al. Addition of Metastasis-Directed Therapy to Intermittent Hormone Therapy for Oligometastatic Prostate Cancer: The EXTEND Phase 2 Randomized Clinical Trial. JAMA Oncol. 2023, e230161. [Google Scholar] [CrossRef] [PubMed]
- Pozdnyakov, A.; Kulanthaivelu, R.; Bauman, G.; Ortega, C.; Veit-Haibach, P.; Metser, U. The impact of PSMA PET on the treatment and outcomes of men with biochemical recurrence of prostate cancer: A systematic review and meta-analysis. Prostate Cancer Prostatic Dis. 2022. [Google Scholar] [CrossRef] [PubMed]
- Artigas, C.; Diamand, R.; Shagera, Q.A.; Plouznikoff, N.; Fokoue, F.; Otte, F.-X.; Gil, T.; Peltier, A.; Van Gestel, D.; Flamen, P. Oligometastatic Disease Detection with 68Ga-PSMA-11 PET/CT in Hormone-Sensitive Prostate Cancer Patients (HSPC) with Biochemical Recurrence after Radical Prostatectomy: Predictive Factors and Clinical Impact. Cancers 2021, 13, 4982. [Google Scholar] [CrossRef] [PubMed]
- Ferdinandus, J.; Fendler, W.P.; Farolfi, A.; Washington, S.; Mohammad, O.; Pampaloni, M.H.; Scott, P.J.; Rodnick, M.; Viglianti, B.L.; Eiber, M.; et al. PSMA PET Validates Higher Rates of Metastatic Disease for European Association of Urology Biochemical Recurrence Risk Groups: An International Multicenter Study. J. Nucl. Med. 2022, 63, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Su, Y.; Zhu, Y.; Markowski, M.C.; Xin, M.; Gorin, M.A.; Dong, B.; Pan, J.; Pomper, M.G.; Liu, J.; et al. The European Association of Urology Biochemical Recurrence Risk Groups Predict Findings on PSMA PET in Patients with Biochemically Recurrent Prostate Cancer After Radical Prostatectomy. J. Nucl. Med. 2022, 63, 248–252. [Google Scholar] [CrossRef]
- Roberts, M.J.; Chatfield, M.D.; Hruby, G.; Nandurkar, R.; Roach, P.; Watts, J.A.; Cusick, T.; Kneebone, A.; Eade, T.; Ho, B.; et al. Event-free survival after radical prostatectomy according to prostate-specific membrane antigen-positron emission tomography and European Association of Urology biochemical recurrence risk groups. BJU Int. 2022, 130, 32–39. [Google Scholar] [CrossRef]
- Roberts, M.J.; Chatfield, M.D.; Hruby, G.; Nandurkar, R.; Roach, P.; Watts, J.A.; Cusick, T.; Kneebone, A.; Eade, T.; Ho, B.; et al. 3-Year Freedom from Progression After 68Ga-PSMA PET/CT-Triaged Management in Men with Biochemical Recurrence After Radical Prostatectomy: Results of a Prospective Multicenter Trial. J. Nucl. Med. Off Publ. Soc. Nucl. Med. 2020, 61, 866–872. [Google Scholar] [CrossRef]
- Rogowski, P.; Trapp, C.; von Bestenbostel, R.; Eze, C.; Ganswindt, U.; Li, M.; Unterrainer, M.; Zacherl, M.J.; Ilhan, H.; Beyer, L.; et al. Outcome after PSMA-PET/CT-based salvage radiotherapy for nodal recurrence after radical prostatectomy. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 1417–1428. [Google Scholar] [CrossRef]
- Murata, Y.; Tatsugami, K.; Yoshikawa, M.; Hamaguchi, M.; Yamada, S.; Hayakawa, Y.; Ueda, K.; Momosaki, S.; Sakamoto, N. Predictive factors of biochemical recurrence after radical prostatectomy for high-risk prostate cancer. Int. J. Urol. 2018, 25, 284–289. [Google Scholar] [CrossRef]
- Perera, M.; Papa, N.; Roberts, M.; Williams, M.; Udovicich, C.; Vela, I.; Christidis, D.; Bolton, D.; Hofman, M.S.; Lawrentschuk, N.; et al. Gallium-68 Prostate-specific Membrane Antigen Positron Emission Tomography in Advanced Prostate Cancer-Updated Diagnostic Utility, Sensitivity, Specificity, and Distribution of Prostate-specific Membrane Antigen-avid Lesions: A Systematic Review and Meta-analysis. Eur. Urol. 2020, 77, 403–417. [Google Scholar] [CrossRef]
| Risk Group | Characteristics |
|---|---|
| BCR after RP | |
| Low risk | PSAdt > 12 months and ISUP < 4 |
| High risk | PSAdt < 12 months and/or ISUP 4/5 |
| BCR after radiation therapy | |
| Low risk | TTR > 18 months and ISUP < 4 |
| High risk | TTR < 18 months and ISUP 4/5 |
| Characteristics | Values | |
|---|---|---|
| Age (years) | 71 (65–75) | |
| PSA at PET/CT (ng/mL), med (IQR) | 1.14 (0.5–3) | |
| PSAdt (months), med (IQR) | 6.7 (3.9–13) | |
| PSAvel (ng/mL/an), med (IQR) | 0.9 (0.3–2.8) | |
| pTstage, n (%) | ||
| T1c | 2 (0.5) | |
| T2a | 16 (3.7) | |
| T2b | 39 (9) | |
| T2c | 132 (30) | |
| T3a | 155 (36) | |
| T3b | 84 (19) | |
| Unknown | 7 (1.6) | |
| pN stage, n (%) | ||
| N1 | 40 (9.2) | |
| N0 | 222 (51) | |
| Nx | 173 (40) | |
| ISUP grade group, n (%) | ||
| 1 | 69 (16) | |
| 2 | 160 (37) | |
| 3 | 117 (27) | |
| 4 | 66 (15) | |
| 5 | 23 (5.3) | |
| Surgical margins, n (%) | ||
| R1 | 142 (33) | |
| R0 | 178 (41) | |
| Rx | 115 (26) | |
| Pelvic lymph node dissection, n (%) | ||
| Performed | 243 (56) | |
| Not performed | 126 (29) | |
| Unknown | 66 (15) | |
| Adjuvant RT, n (%) | 122 (28) | |
| Salvage radiotherapy, n (%) | 200 (46) | |
| BCR status, n (%) | ||
| First BCR | 235 (54) | |
| Salvage RT | 200 (46) | |
| EAU BCR risk group, n (%) | ||
| Low risk | 97 (22) | |
| High risk | 338 (78) | |
| BCR Low Risk n = 97 | BCR High Risk n = 338 | Total n = 435 | p-Value | ||
|---|---|---|---|---|---|
| Positivity rate n (%) | 35 (36.08%) | 201 (59%) | 236 (54%) | <0.001 | |
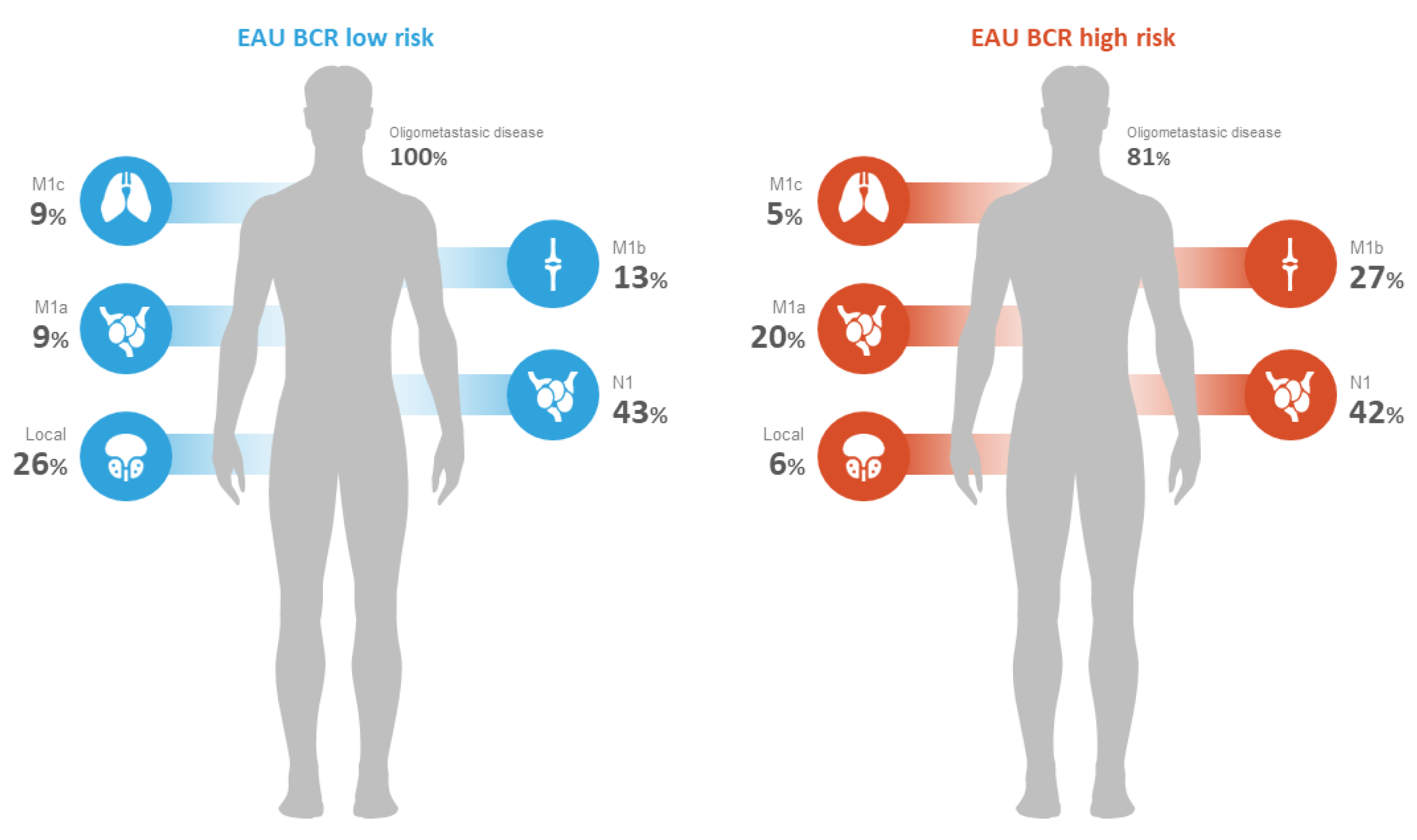
| Number of distant metastatic lesions, n (%) * | 1–5 | 11 (100%) | 85 (81%) | 96 (83%) | <0.001 |
| >5 | 0 (0%) | 20 (19%) | 20 (17%) | <0.001 | |
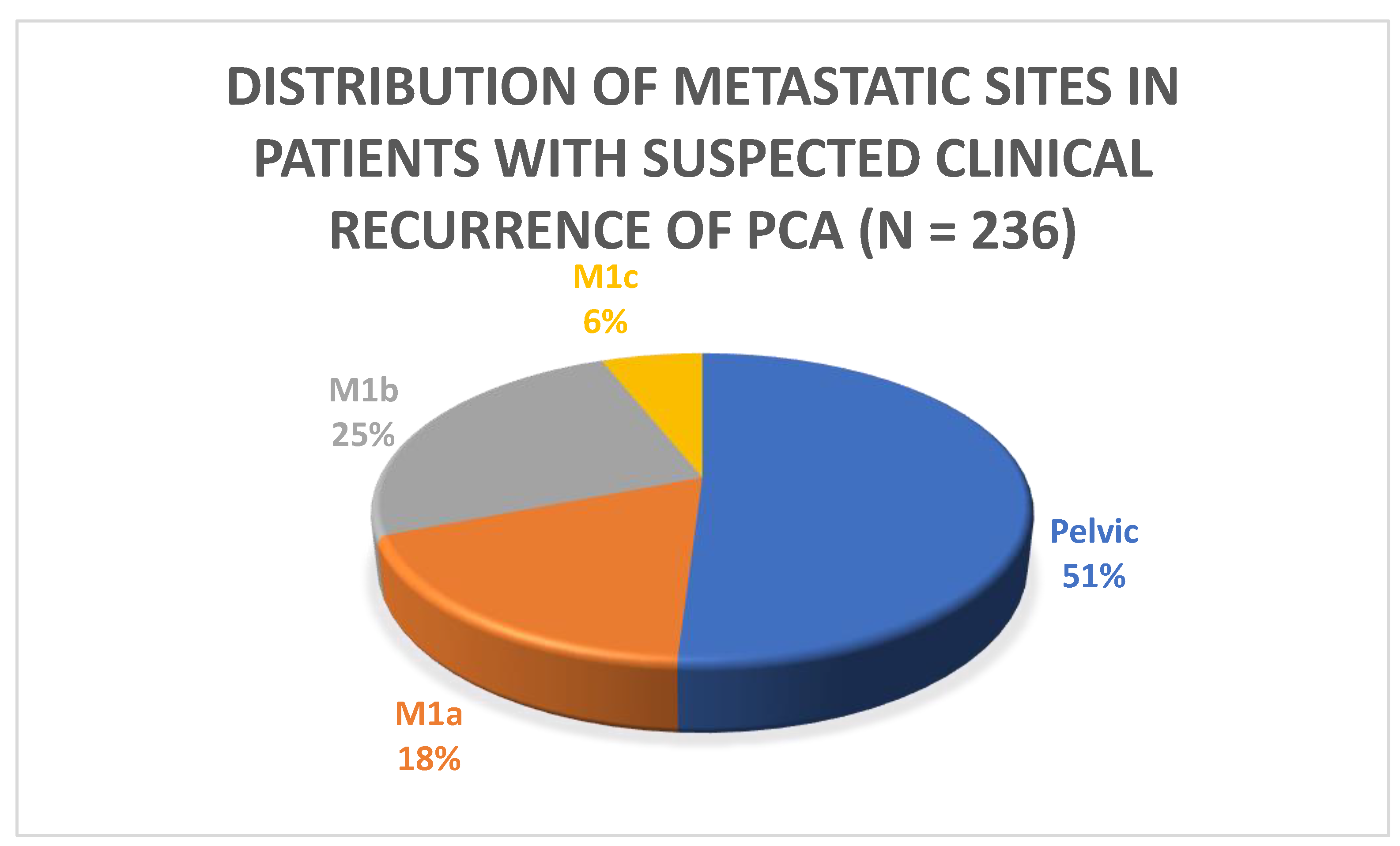
| Pattern of recurrence, n (%) ** | Local N1 | 9 (26%) 15 (43%) | 12 (6%) 84 (42%) | 21 (8.9%) 99 (42%) | <0.001 0.5 |
| M1a | 3 (9%) | 40 (20%) | 43 (18%) | 0.008 | |
| M1b | 5 (13%) | 54 (27%) | 59 (25%) | 0.007 | |
| M1c | 3 (9%) | 11 (5%) | 14 (6%) | 0.96 | |
| Univariable Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | |
| Positive predictive factors of PSMA PET/CT | ||||
| EAU BCR risk group (low vs. high) | 2.5 (1.6–4) | <0.001 | 2.2 (1.3–3.8) | 0.004 |
| Tumor stage (≥T3a vs. <T3a) | 1.7 (1.1–2.5) | 0.009 | 1.4 (0.9–2.2) | 0.109 |
| Lymph node staging (N0 vs. N1) | 1.4 (0.7–3) | 0.248 | - | - |
| Surgical Margins | 1.3 (0.8–2.1) | 0.207 | - | - |
| Lymph node dissection (yes/no) | 1.3 (0.8–2) | 0.224 | - | - |
| Adjuvant Radiotherapy (yes/no) | 2 (1.3–3.2) | 0.002 | 1.5 (0.9–2.5) | 0.094 |
| sRT (yes/no) | 1.2 (0.8–1.8) | 0.289 | - | - |
| PSA level before PET/CT (≥0.5 vs. <0.5 ng/mL) | 2.7 (1.7–4.2) | <0.001 | 2.7 (1.7–4.4) | <0.001 |
| PSAvel (≥0.4 vs. <0.4 ng/mL/year) | 2.6 (1.7–4) | <0.001 * | - | - |
| PSAdt (≥4 vs. <4 months) | 0.5 (0.3–0.8) | 0.002 | 0.8 (0.5–1.3) | 0.354 |
| Positive predictive factors of PSMA PET/CT in the BCR low-risk group | ||||
| Tumor stage (≥T3a vs. <T3a) | 0.8 (0.4–2) | 0.773 | ||
| Lymph node staging (N0 vs. N1) | 2 (0.1–35.3) | 0.616 | ||
| Surgical Margins | 0.6 (0.2–1.6) | 0.331 | ||
| Lymph node dissection (yes/no) | 0.7 (0.3–1.7) | 0.437 | ||
| Adjuvant Radiotherapy (yes/no) | 2.7 (0.9–8) | 0.079 | ||
| sRT (yes/no) | 1.3 (0.6–3) | 0.499 | ||
| PSA level before PET/CT (≥0.5 vs. <0.5 ng/mL) | 3.9 (1.5–10.2) | 0.006 | ||
| PSAvel (≥0.4 vs. <0.4 ng/mL/year) | 5.8 (2–17) | 0.001 * | ||
| PSAdt (≥4 vs. <4 months) | 1 (0.9–1) | 0.092 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leplat, C.; Jabbour, T.; Diamand, R.; Baudewyns, A.; Bourgeno, H.A.; Shagera, Q.A.; Flamen, P.; Roumeguere, T.; Peltier, A.; Artigas, C. Peering through the PSMA PET Lens: The Role of the European Association of Urology Biochemical Recurrence Risk Groups after Radical Prostatectomy. Cancers 2023, 15, 2926. https://doi.org/10.3390/cancers15112926
Leplat C, Jabbour T, Diamand R, Baudewyns A, Bourgeno HA, Shagera QA, Flamen P, Roumeguere T, Peltier A, Artigas C. Peering through the PSMA PET Lens: The Role of the European Association of Urology Biochemical Recurrence Risk Groups after Radical Prostatectomy. Cancers. 2023; 15(11):2926. https://doi.org/10.3390/cancers15112926
Chicago/Turabian StyleLeplat, Charles, Teddy Jabbour, Romain Diamand, Arthur Baudewyns, Henri Alexandre Bourgeno, Qaid Ahmed Shagera, Patrick Flamen, Thierry Roumeguere, Alexandre Peltier, and Carlos Artigas. 2023. "Peering through the PSMA PET Lens: The Role of the European Association of Urology Biochemical Recurrence Risk Groups after Radical Prostatectomy" Cancers 15, no. 11: 2926. https://doi.org/10.3390/cancers15112926
APA StyleLeplat, C., Jabbour, T., Diamand, R., Baudewyns, A., Bourgeno, H. A., Shagera, Q. A., Flamen, P., Roumeguere, T., Peltier, A., & Artigas, C. (2023). Peering through the PSMA PET Lens: The Role of the European Association of Urology Biochemical Recurrence Risk Groups after Radical Prostatectomy. Cancers, 15(11), 2926. https://doi.org/10.3390/cancers15112926






