Succinate-Directed Approaches for Warburg Effect-Targeted Cancer Management, an Alternative to Current Treatments?
Abstract
Simple Summary
Abstract
1. Introduction
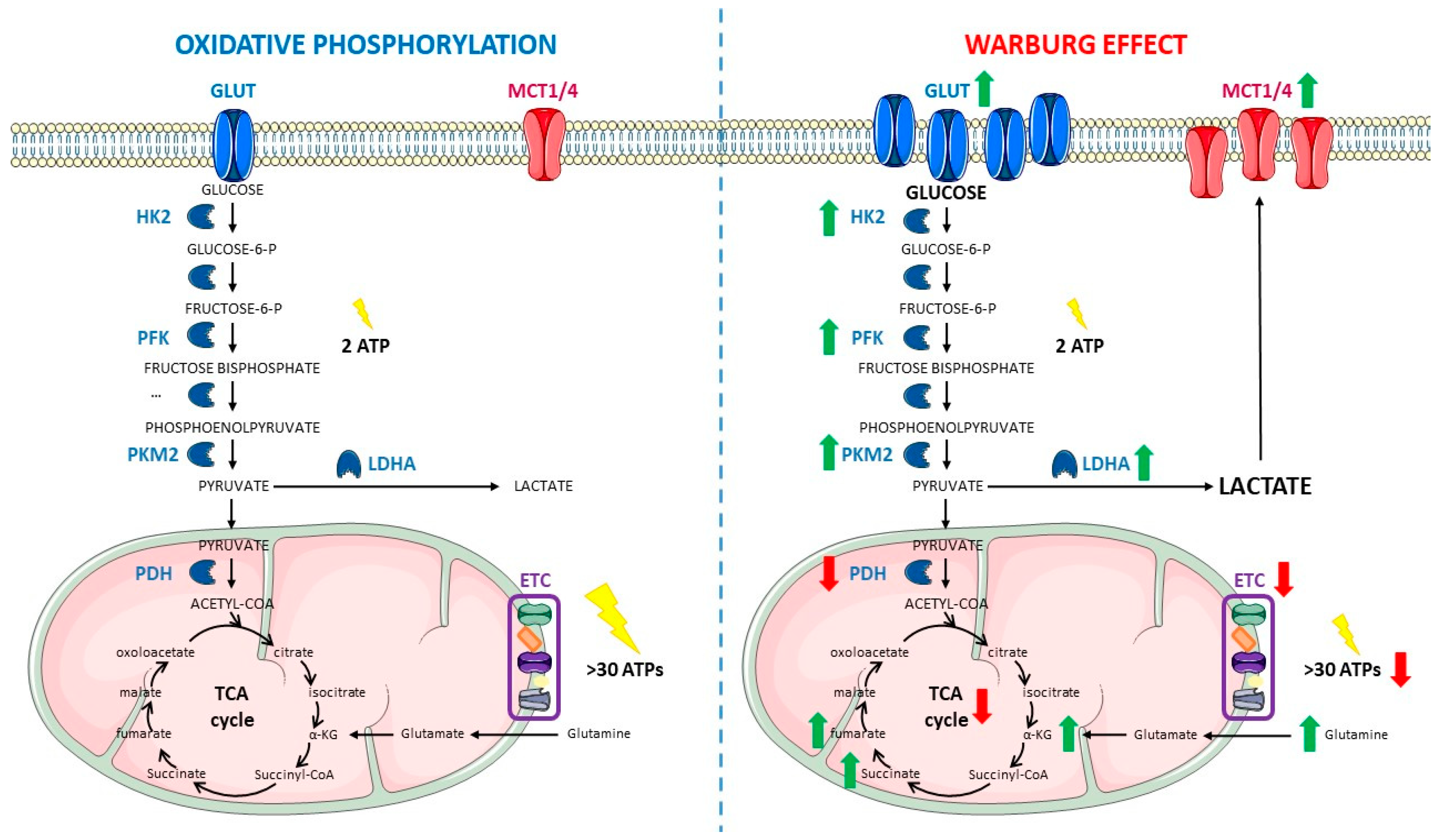
2. Warburg Effect
2.1. Cancer Cells Have a Different Metabolism
2.2. What Are the Causes of the Warburg Effect?
2.3. Main Features of the Warburg Effect
2.4. Classical Approaches Targeting the Warburg Effect to Fight Cancer
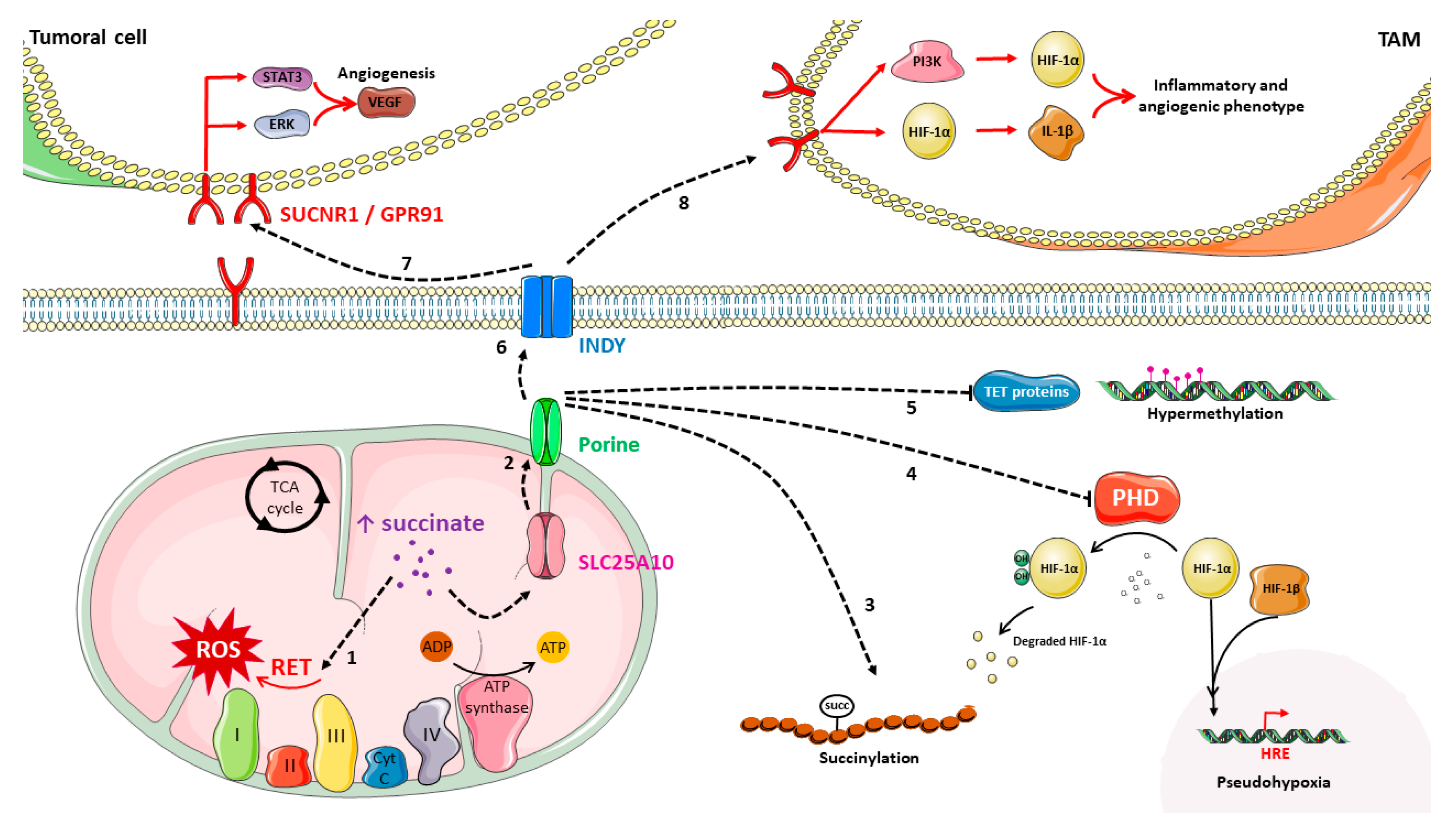
3. Succinate, the Forgotten Oncometabolite?
3.1. Succinate Metabolism and Accumulation
3.2. Succinate Role in Cancer
3.3. Succinate: A New Alternative to Target Cancer Metabolism?
3.3.1. SUCNR1 Could Be the Key to Suppress Succinate Extracellular Protumoral Actions
3.3.2. Sirtuin 3, a Booster to SDH Catalytic Activity
3.3.3. TRAP-1, an Inhibitor of SDH
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Global Cancer Observatory: Cancer Today; International Agency for Research on Cancer: Lyon, France, 2020. [Google Scholar]
- Cancer Facts Sheet—World Health Organization Website. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 16 February 2023).
- Olsson Möller, U.; Beck, I.; Rydén, L.; Malmström, M. A Comprehensive Approach to Rehabilitation Interventions Following Breast Cancer Treatment—A Systematic Review of Systematic Reviews. BMC Cancer 2019, 19, 472. [Google Scholar] [CrossRef]
- Dilalla, V.; Chaput, G.; Williams, T.; Sultanem, K. Radiotherapy Side Effects: Integrating a Survivorship Clinical Lens to Better Serve Patients. Curr. Oncol. 2020, 27, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Johdi, N.A.; Sukor, N.F. Colorectal Cancer Immunotherapy: Options and Strategies. Front. Immunol. 2020, 11, 1624. [Google Scholar] [CrossRef]
- Riley, R.S.; June, C.H.; Langer, R.; Mitchell, M.J. Delivery Technologies for Cancer Immunotherapy. Nat. Rev. Drug Discov. 2019, 18, 175–196. [Google Scholar] [CrossRef] [PubMed]
- Schirrmacher, V. From Chemotherapy to Biological Therapy: A Review of Novel Concepts to Reduce the Side Effects of Systemic Cancer Treatment (Review). Int. J. Oncol. 2019, 54, 407–419. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.; Koktved, D.P.; Nielsen, L.L. Living with Side Effects from Cancer Treatment—A Challenge to Target Information. Scand. J. Caring Sci. 2013, 27, 715–723. [Google Scholar] [CrossRef]
- Bateman, A.C. Molecules in Cancer Immunotherapy: Benefits and Side Effects. J. Clin. Pathol. 2019, 72, 20–24. [Google Scholar] [CrossRef]
- Numico, G.; Longo, V.; Courthod, G.; Silvestris, N. Cancer Survivorship. Curr. Opin. Oncol. 2015, 27, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Bender, D.A.; Botham, K.M.; Weil, P.A.; Kenelly, P.; Murray, R.K.; Rodwell, V.W. Harper Bioquímica Ilustrada, 29th ed.; McGraw-Hill: New York, NY, USA, 2013. [Google Scholar]
- Warburg, O. The Metabolism of Carcinoma Cells. J. Cancer Res. 1925, 9, 148–163. [Google Scholar] [CrossRef]
- Warburg, O.; Posener, K.; Negelein, E. Ueber Den Stoffwechsel Der Tumoren. Biochem. Z. 1924, 9, 319–344. [Google Scholar]
- Warburg, O. On Respiratory Impairment in Cancer Cells. Science 1956, 124, 269–270. [Google Scholar] [CrossRef]
- Wallace, D.C. Mitochondria and Cancer. Nat. Rev. Cancer 2012, 12, 685–698. [Google Scholar] [CrossRef] [PubMed]
- Ju, Y.S.; Alexandrov, L.B.; Gerstung, M.; Martincorena, I.; Nik-Zainal, S.; Ramakrishna, M.; Davies, H.R.; Papaemmanuil, E.; Gundem, G.; Shlien, A.; et al. Origins and Functional Consequences of Somatic Mitochondrial DNA Mutations in Human Cancer. eLife 2014, 3, e02935. [Google Scholar] [CrossRef] [PubMed]
- Kernstine, K.H.; Faubert, B.; Do, Q.N.; Rogers, T.J.; Hensley, C.T.; Cai, L.; Torrealba, J.; Oliver, D.; Wachsmann, J.W.; Lenkinski, R.E.; et al. Does Tumor FDG-PET Avidity Represent Enhanced Glycolytic Metabolism in Non-Small Cell Lung Cancer? Ann. Thorac. Surg. 2020, 109, 1019–1025. [Google Scholar] [CrossRef] [PubMed]
- Kozal, K.; Jóźwiak, P.; Krześlak, A. Contemporary Perspectives on the Warburg Effect Inhibition in Cancer Therapy. Cancer Control 2021, 28, 107327482110412. [Google Scholar] [CrossRef]
- Bartman, C.R.; Weilandt, D.R.; Shen, Y.; Lee, W.D.; Han, Y.; TeSlaa, T.; Jankowski, C.S.R.; Samarah, L.; Park, N.R.; Silva-Diz, V.; et al. Slow TCA flux and ATP production in primary solid tumours but not metastases. Nature 2023, 614, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Fantin, V.R.; St-Pierre, J.; Leder, P. Attenuation of LDH-A Expression Uncovers a Link between Glycolysis, Mitochondrial Physiology, and Tumor Maintenance. Cancer Cell 2006, 9, 425–434. [Google Scholar] [CrossRef] [PubMed]
- DeBerardinis, R.J.; Chandel, N.S. We Need to Talk about the Warburg Effect. Nat. Metab. 2020, 2, 127–129. [Google Scholar] [CrossRef] [PubMed]
- Bouillaud, F.; Hammad, N.; Schwartz, L. Warburg Effect, Glutamine, Succinate, Alanine, When Oxygen Matters. Biology 2021, 10, 1000. [Google Scholar] [CrossRef]
- vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg Effect: The Metabolic Requirements of Cell Proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef]
- Le, A.; Cooper, C.R.; Gouw, A.M.; Dinavahi, R.; Maitra, A.; Deck, L.M.; Royer, R.E.; vander Jagt, D.L.; Semenza, G.L.; Dang, C.V. Inhibition of Lactate Dehydrogenase a Induces Oxidative Stress and Inhibits Tumor Progression. Proc. Natl. Acad. Sci. USA 2010, 107, 2037–2042. [Google Scholar] [CrossRef] [PubMed]
- Michelakis, E.D.; Sutendra, G.; Dromparis, P.; Webster, L.; Haromy, A.; Niven, E.; Maguire, C.; Gammer, T.-L.; Mackey, J.R.; Fulton, D.; et al. Metabolic Modulation of Glioblastoma with Dichloroacetate. Sci. Transl. Med. 2010, 2, 31ra34. [Google Scholar] [CrossRef] [PubMed]
- DeBerardinis, R.J.; Lum, J.J.; Hatzivassiliou, G.; Thompson, C.B. The Biology of Cancer: Metabolic Reprogramming Fuels Cell Growth and Proliferation. Cell Metab. 2008, 7, 11–20. [Google Scholar] [CrossRef]
- Christofk, H.R.; vander Heiden, M.G.; Harris, M.H.; Ramanathan, A.; Gerszten, R.E.; Wei, R.; Fleming, M.D.; Schreiber, S.L.; Cantley, L.C. The M2 Splice Isoform of Pyruvate Kinase Is Important for Cancer Metabolism and Tumour Growth. Nature 2008, 452, 230–233. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, T.; Schuster, S.; Bonhoeffer, S. Cooperation and Competition in the Evolution of ATP-Producing Pathways. Science 2001, 292, 504–507. [Google Scholar] [CrossRef]
- Shestov, A.A.; Liu, X.; Ser, Z.; Cluntun, A.A.; Hung, Y.P.; Huang, L.; Kim, D.; Le, A.; Yellen, G.; Albeck, J.G.; et al. Quantitative Determinants of Aerobic Glycolysis Identify Flux through the Enzyme GAPDH as a Limiting Step. eLife 2014, 3, e03342. [Google Scholar] [CrossRef]
- DeBerardinis, R.J.; Mancuso, A.; Daikhin, E.; Nissim, I.; Yudkoff, M.; Wehrli, S.; Thompson, C.B. Beyond Aerobic Glycolysis: Transformed Cells Can Engage in Glutamine Metabolism That Exceeds the Requirement for Protein and Nucleotide Synthesis. Proc. Natl. Acad. Sci. USA 2007, 104, 19345–19350. [Google Scholar] [CrossRef]
- Sonveaux, P.; Végran, F.; Schroeder, T.; Wergin, M.C.; Verrax, J.; Rabbani, Z.N.; de Saedeleer, C.J.; Kennedy, K.M.; Diepart, C.; Jordan, B.F.; et al. Targeting Lactate-Fueled Respiration Selectively Kills Hypoxic Tumor Cells in Mice. J. Clin. Investig. 2008, 118, 3930–3942. [Google Scholar] [CrossRef]
- Li, T.; Le, A. Glutamine Metabolism in Cancer. In The Heterogeneity of Cancer Metabolism; Springer: Cham, Switzerland, 2018; pp. 13–32. [Google Scholar] [CrossRef]
- Kao, T.-W.; Chuang, Y.-C.; Lee, H.-L.; Kuo, C.-C.; Shen, Y.-A. Therapeutic Targeting of Glutaminolysis as a Novel Strategy to Combat Cancer Stem Cells. Int. J. Mol. Sci. 2022, 23, 15296. [Google Scholar] [CrossRef]
- Altman, B.J.; Stine, Z.E.; Dang, C.V. From Krebs to Clinic: Glutamine Metabolism to Cancer Therapy. Nat. Rev. Cancer 2016, 16, 619–634. [Google Scholar] [CrossRef]
- Zong, W.-X.; Rabinowitz, J.D.; White, E. Mitochondria and Cancer. Mol. Cell 2016, 61, 667–676. [Google Scholar] [CrossRef]
- Hyroššová, P.; Milošević, M.; Škoda, J.; Vachtenheim, J., Jr.; Rohlena, J.; Rohlenová, K. Effects of Metabolic Cancer Therapy on Tumor Microenvironment. Front. Oncol. 2022, 12, 1046630. [Google Scholar] [CrossRef]
- Nagayama, Y.; Hamada, K. Reprogramming of Cellular Metabolism and Its Therapeutic Applications in Thyroid Cancer. Metabolites 2022, 12, 1214. [Google Scholar] [CrossRef] [PubMed]
- Wise, D.R.; Thompson, C.B. Glutamine Addiction: A New Therapeutic Target in Cancer. Trends Biochem. Sci. 2010, 35, 427–433. [Google Scholar] [CrossRef] [PubMed]
- van Geldermalsen, M.; Wang, Q.; Nagarajah, R.; Marshall, A.D.; Thoeng, A.; Gao, D.; Ritchie, W.; Feng, Y.; Bailey, C.G.; Deng, N.; et al. ASCT2/SLC1A5 Controls Glutamine Uptake and Tumour Growth in Triple-Negative Basal-like Breast Cancer. Oncogene 2016, 35, 3201–3208. [Google Scholar] [CrossRef]
- Vanhove, K.; Derveaux, E.; Graulus, G.-J.; Mesotten, L.; Thomeer, M.; Noben, J.-P.; Guedens, W.; Adriaensens, P. Glutamine Addiction and Therapeutic Strategies in Lung Cancer. Int. J. Mol. Sci. 2019, 20, 252. [Google Scholar] [CrossRef] [PubMed]
- Hensley, C.T.; Wasti, A.T.; DeBerardinis, R.J. Glutamine and Cancer: Cell Biology, Physiology, and Clinical Opportunities. J. Clin. Investig. 2013, 123, 3678–3684. [Google Scholar] [CrossRef]
- Yoo, H.C.; Yu, Y.C.; Sung, Y.; Han, J.M. Glutamine Reliance in Cell Metabolism. Exp. Mol. Med. 2020, 52, 1496–1516. [Google Scholar] [CrossRef]
- Vettore, L.; Westbrook, R.L.; Tennant, D.A. New Aspects of Amino Acid Metabolism in Cancer. Br. J. Cancer 2020, 122, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Prochownik, E.V.; Wang, H. The Metabolic Fates of Pyruvate in Normal and Neoplastic Cells. Cells 2021, 10, 762. [Google Scholar] [CrossRef]
- Linehan, W.M.; Schmidt, L.S.; Crooks, D.R.; Wei, D.; Srinivasan, R.; Lang, M.; Ricketts, C.J. The Metabolic Basis of Kidney Cancer. Cancer Discov. 2019, 9, 1006–1021. [Google Scholar] [CrossRef] [PubMed]
- Spencer, N.Y.; Stanton, R.C. The Warburg Effect, Lactate, and Nearly a Century of Trying to Cure Cancer. Semin. Nephrol. 2019, 39, 380–393. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-H.; Gundem, G.; Lee, W.; Chen, Y.-B.; Cross, J.R.; Dong, Y.; Redzematovic, A.; Mano, R.; Wei, E.Y.; Cheng, E.H.; et al. Persistent Severe Hyperlactatemia and Metabolic Derangement in Lethal SDHB-Mutated Metastatic Kidney Cancer: Clinical Challenges and Examples of Extreme Warburg Effect. JCO Precis. Oncol. 2017, 1, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Eijkelenkamp, K.; Osinga, T.E.; Links, T.P.; Horst-Schrivers, A.N.A. Clinical Implications of the Oncometabolite Succinate in SDHx -mutation Carriers. Clin. Genet. 2020, 97, 39–53. [Google Scholar] [CrossRef] [PubMed]
- Lussey-Lepoutre, C.; Buffet, A.; Gimenez-Roqueplo, A.-P.; Favier, J. Mitochondrial Deficiencies in the Predisposition to Paraganglioma. Metabolites 2017, 7, 17. [Google Scholar] [CrossRef] [PubMed]
- Selak, M.A.; Armour, S.M.; MacKenzie, E.D.; Boulahbel, H.; Watson, D.G.; Mansfield, K.D.; Pan, Y.; Simon, M.C.; Thompson, C.B.; Gottlieb, E. Succinate Links TCA Cycle Dysfunction to Oncogenesis by Inhibiting HIF-α Prolyl Hydroxylase. Cancer Cell 2005, 7, 77–85. [Google Scholar] [CrossRef]
- Teicher, B.A.; Linehan, W.M.; Helman, L.J. Targeting Cancer Metabolism. Clin. Cancer Res. 2012, 18, 5537–5545. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, Z.; Wang, Z.; Li, X.; Lu, G.; Tong, J. SDHA-mediated Warburg Effect in Malignantly Transformed Human Bronchial Epithelial Cells Following Long-term Exposure to Radon. Environ. Toxicol. 2020, 35, 861–866. [Google Scholar] [CrossRef]
- Seyfried, T.N.; Shelton, L.M. Cancer as a Metabolic Disease. Nutr. Metab. 2010, 7, 7. [Google Scholar] [CrossRef]
- Brandon, M.; Baldi, P.; Wallace, D.C. Mitochondrial Mutations in Cancer. Oncogene 2006, 25, 4647–4662. [Google Scholar] [CrossRef]
- Chinnery, P.F.; Samuels, D.C.; Elson, J.; Turnbull, D.M. Accumulation of Mitochondrial DNA Mutations in Ageing, Cancer, and Mitochondrial Disease: Is There a Common Mechanism? Lancet 2002, 360, 1323–1325. [Google Scholar] [CrossRef]
- Copeland, W.C.; Wachsman, J.T.; Johnson, F.M.; Penta, J.S. Mitochondrial DNA Alterations in Cancer. Cancer Investig. 2002, 20, 557–569. [Google Scholar] [CrossRef]
- Klein, K.; He, K.; Younes, A.I.; Barsoumian, H.B.; Chen, D.; Ozgen, T.; Mosaffa, S.; Patel, R.R.; Gu, M.; Novaes, J.; et al. Role of Mitochondria in Cancer Immune Evasion and Potential Therapeutic Approaches. Front. Immunol. 2020, 11, 573326. [Google Scholar] [CrossRef]
- Pelicano, H.; Martin, D.S.; Xu, R.-H.; Huang, P. Glycolysis Inhibition for Anticancer Treatment. Oncogene 2006, 25, 4633–4646. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Hong, X.; He, W.; Ou, L.; Chen, B.; Zhong, W.; Lin, Y.; Luo, X. The Construction and Analysis of Tricarboxylic Acid Cycle Related Prognostic Model for Cervical Cancer. Front. Genet. 2023, 14, 1092276. [Google Scholar] [CrossRef]
- Schiliro, C.; Firestein, B.L. Mechanisms of Metabolic Reprogramming in Cancer Cells Supporting Enhanced Growth and Proliferation. Cells 2021, 10, 1056. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Haleem, A.M.; Lewis, N.E.; Jamshidi, N.; Mineta, K.; Gao, X.; Gojobori, T. The Emerging Facets of Non-Cancerous Warburg Effect. Front. Endocrinol. 2017, 8, 279. [Google Scholar] [CrossRef] [PubMed]
- Gatenby, R.A.; Gillies, R.J. Why Do Cancers Have High Aerobic Glycolysis? Nat. Rev. Cancer 2004, 4, 891–899. [Google Scholar] [CrossRef]
- Lin, S.-C.; Liao, W.-L.; Lee, J.-C.; Tsai, S.-J. Hypoxia-Regulated Gene Network in Drug Resistance and Cancer Progression. Exp. Biol. Med. 2014, 239, 779–792. [Google Scholar] [CrossRef]
- Courtnay, R.; Ngo, D.C.; Malik, N.; Ververis, K.; Tortorella, S.M.; Karagiannis, T.C. Cancer Metabolism and the Warburg Effect: The Role of HIF-1 and PI3K. Mol. Biol. Rep. 2015, 42, 841–851. [Google Scholar] [CrossRef]
- Hayashi, Y.; Yokota, A.; Harada, H.; Huang, G. Hypoxia/Pseudohypoxia-mediated Activation of Hypoxia-inducible Factor-1α in Cancer. Cancer Sci. 2019, 110, 1510–1517. [Google Scholar] [CrossRef]
- Vaupel, P.; Multhoff, G. Revisiting the Warburg Effect: Historical Dogma versus Current Understanding. J. Physiol. 2021, 599, 1745–1757. [Google Scholar] [CrossRef] [PubMed]
- Huang, E.; Tiburcio, P.; Choi, H. Complex Role of HIF in Cancer: The Known, the Unknown, and the Unexpected. Hypoxia 2014, 2, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Qiu, Z.; Wang, Z.; Wang, Q.; Tan, N.; Chen, T.; Chen, Z.; Huang, S.; Gu, J.; Li, J.; et al. MiR-199a-5p Is Negatively Associated with Malignancies and Regulates Glycolysis and Lactate Production by Targeting Hexokinase 2 in Liver Cancer. Hepatology 2015, 62, 1132–1144. [Google Scholar] [CrossRef] [PubMed]
- Denko, N.C. Hypoxia, HIF1 and Glucose Metabolism in the Solid Tumour. Nat. Rev. Cancer 2008, 8, 705–713. [Google Scholar] [CrossRef]
- Stanton, R.C. Role of Glucose Metabolism and Mitochondrial Function in Diabetic Kidney Disease. Curr. Diab. Rep. 2021, 21, 6. [Google Scholar] [CrossRef]
- Altenberg, B.; Greulich, K.O. Genes of Glycolysis Are Ubiquitously Overexpressed in 24 Cancer Classes. Genomics 2004, 84, 1014–1020. [Google Scholar] [CrossRef]
- Marin-Hernandez, A.; Gallardo-Perez, J.; Ralph, S.; Rodriguez-Enriquez, S.; Moreno-Sanchez, R. HIF-1α Modulates Energy Metabolism in Cancer Cells by Inducing Over-Expression of Specific Glycolytic Isoforms. Mini-Rev. Med. Chem. 2009, 9, 1084–1101. [Google Scholar] [CrossRef]
- Tran, Q.; Lee, H.; Park, J.; Kim, S.-H.; Park, J. Targeting Cancer Metabolism—Revisiting the Warburg Effects. Toxicol. Res. 2016, 32, 177–193. [Google Scholar] [CrossRef]
- O’Byrne, K.J.; Baird, A.-M.; Kilmartin, L.; Leonard, J.; Sacevich, C.; Gray, S.G. Epigenetic Regulation of Glucose Transporters in Non-Small Cell Lung Cancer. Cancers 2011, 3, 1550–1565. [Google Scholar] [CrossRef]
- Al-Azzam, N. Sirtuin 6 and Metabolic Genes Interplay in Warburg Effect in Cancers. J. Clin. Biochem. Nutr. 2020, 66, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Ansó, E.; Weinberg, S.E.; Diebold, L.P.; Thompson, B.J.; Malinge, S.; Schumacker, P.T.; Liu, X.; Zhang, Y.; Shao, Z.; Steadman, M.; et al. The Mitochondrial Respiratory Chain Is Essential for Haematopoietic Stem Cell Function. Nat. Cell Biol. 2017, 19, 614–625. [Google Scholar] [CrossRef] [PubMed]
- Vaupel, P.; Schmidberger, H.; Mayer, A. The Warburg Effect: Essential Part of Metabolic Reprogramming and Central Contributor to Cancer Progression. Int. J. Radiat. Biol. 2019, 95, 912–919. [Google Scholar] [CrossRef]
- Cairns, R.A.; Harris, I.S.; Mak, T.W. Regulation of Cancer Cell Metabolism. Nat. Rev. Cancer 2011, 11, 85–95. [Google Scholar] [CrossRef]
- Romero-Garcia, S.; Prado-Garcia, H.; Valencia-Camargo, A.D.; Alvarez-Pulido, A. Lactic Acidosis Promotes Mitochondrial Biogenesis in Lung Adenocarcinoma Cells, Supporting Proliferation under Normoxia or Survival under Hypoxia. Front. Oncol. 2019, 9, 1053. [Google Scholar] [CrossRef]
- Hoang, P.H.; Cornish, A.J.; Chubb, D.; Jackson, G.; Kaiser, M.; Houlston, R.S. Impact of Mitochondrial DNA Mutations in Multiple Myeloma. Blood Cancer J. 2020, 10, 46. [Google Scholar] [CrossRef]
- Wegiel, B.; Vuerich, M.; Daneshmandi, S.; Seth, P. Metabolic Switch in the Tumor Microenvironment Determines Immune Responses to Anti-Cancer Therapy. Front. Oncol. 2018, 8, 284. [Google Scholar] [CrossRef]
- Zhao, L.; Mao, Y.; Zhao, Y.; Cao, Y.; Chen, X. Role of Multifaceted Regulators in Cancer Glucose Metabolism and Their Clinical Significance. Oncotarget 2016, 7, 31572–31585. [Google Scholar] [CrossRef]
- Liu, C.; Jin, Y.; Fan, Z. The Mechanism of Warburg Effect-Induced Chemoresistance in Cancer. Front. Oncol. 2021, 11, 698023. [Google Scholar] [CrossRef] [PubMed]
- Cassim, S.; Vučetić, M.; Ždralević, M.; Pouyssegur, J. Warburg and Beyond: The Power of Mitochondrial Metabolism to Collaborate or Replace Fermentative Glycolysis in Cancer. Cancers 2020, 12, 1119. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.P.; Ganapathy, V. Lactate/GPR81 Signaling and Proton Motive Force in Cancer: Role in Angiogenesis, Immune Escape, Nutrition, and Warburg Phenomenon. Pharmacol. Ther. 2020, 206, 107451. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Shin, K.J.; Park, S.-A.; Park, K.S.; Park, S.; Heo, K.; Seo, Y.-K.; Noh, D.-Y.; Ryu, S.H.; Suh, P.-G. G-Protein-Coupled Receptor 81 Promotes a Malignant Phenotype in Breast Cancer through Angiogenic Factor Secretion. Oncotarget 2016, 7, 70898–70911. [Google Scholar] [CrossRef]
- Ochoa-Ruiz, E.; Diaz-Ruiz, R. Anaplerosis in Cancer: Another Step beyond the Warburg Effect. Am. J. Mol. Biol. 2012, 02, 291–303. [Google Scholar] [CrossRef]
- Dando, I.; Pozza, E.D.; Ambrosini, G.; Torrens-Mas, M.; Butera, G.; Mullappilly, N.; Pacchiana, R.; Palmieri, M.; Donadelli, M. Oncometabolites in Cancer Aggressiveness and Tumour Repopulation. Biol. Rev. 2019, 94, 1530–1546. [Google Scholar] [CrossRef]
- Eniafe, J.; Jiang, S. The Functional Roles of TCA Cycle Metabolites in Cancer. Oncogene 2021, 40, 3351–3363. [Google Scholar] [CrossRef]
- Akella, N.M.; Ciraku, L.; Reginato, M.J. Fueling the Fire: Emerging Role of the Hexosamine Biosynthetic Pathway in Cancer. BMC Biol. 2019, 17, 52. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Qian, Y.; Wu, S. The Warburg Effect: Evolving Interpretations of an Established Concept. Free Radic. Biol. Med. 2015, 79, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Miles, K.A. Warburg Revisited: Imaging Tumour Blood Flow and Metabolism. Cancer Imaging 2008, 8, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Di Cosimo, S.; Ferretti, G.; Papaldo, P.; Carlini, P.; Fabi, A.; Cognetti, F. Lonidamine: Efficacy and Safety in Clinical Trials for the Treatment of Solid Tumors. Drugs Today 2003, 39, 157. [Google Scholar] [CrossRef]
- Chelakkot, C.; Chelakkot, V.S.; Shin, Y.; Song, K. Modulating Glycolysis to Improve Cancer Therapy. Int. J. Mol. Sci. 2023, 24, 2606. [Google Scholar] [CrossRef]
- Ojelabi, O.A.; Lloyd, K.P.; Simon, A.H.; De Zutter, J.K.; Carruthers, A. WZB117 (2-Fluoro-6-(m-Hydroxybenzoyloxy) Phenyl m-Hydroxybenzoate) Inhibits GLUT1-Mediated Sugar Transport by Binding Reversibly at the Exofacial Sugar Binding Site. J. Biol. Chem. 2016, 291, 26762–26772. [Google Scholar] [CrossRef]
- Chan, D.A.; Sutphin, P.D.; Nguyen, P.; Turcotte, S.; Lai, E.W.; Banh, A.; Reynolds, G.E.; Chi, J.-T.; Wu, J.; Solow-Cordero, D.E.; et al. Targeting GLUT1 and the Warburg Effect in Renal Cell Carcinoma by Chemical Synthetic Lethality. Sci. Transl. Med. 2011, 3, 94ra70. [Google Scholar] [CrossRef]
- Zhong, X.; He, X.; Wang, Y.; Hu, Z.; Huang, H.; Zhao, S.; Wei, P.; Li, D. Warburg Effect in Colorectal Cancer: The Emerging Roles in Tumor Microenvironment and Therapeutic Implications. J. Hematol. Oncol. 2022, 15, 160. [Google Scholar] [CrossRef] [PubMed]
- Chae, H.-S.; Hong, S.-T. Overview of Cancer Metabolism and Signaling Transduction. Int. J. Mol. Sci. 2022, 24, 12. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Xiong, Y.; Qiao, T.; Li, X.; Jia, L.; Han, Y. Lactate Dehydrogenase A: A Key Player in Carcinogenesis and Potential Target in Cancer Therapy. Cancer Med. 2018, 7, 6124–6136. [Google Scholar] [CrossRef] [PubMed]
- Rupiani, S.; Guidotti, L.; Manerba, M.; Di Ianni, L.; Giacomini, E.; Falchi, F.; Di Stefano, G.; Roberti, M.; Recanatini, M. Synthesis of Natural Urolithin M6, a Galloflavin Mimetic, as a Potential Inhibitor of Lactate Dehydrogenase A. Org. Biomol. Chem. 2016, 14, 10981–10987. [Google Scholar] [CrossRef]
- Guan, X.; Bryniarski, M.A.; Morris, M.E. In Vitro and In Vivo Efficacy of the Monocarboxylate Transporter 1 Inhibitor AR-C155858 in the Murine 4T1 Breast Cancer Tumor Model. AAPS J. 2019, 21, 3. [Google Scholar] [CrossRef]
- Stine, Z.E.; Schug, Z.T.; Salvino, J.M.; Dang, C.V. Targeting Cancer Metabolism in the Era of Precision Oncology. Nat. Rev. Drug Discov. 2022, 21, 141–162. [Google Scholar] [CrossRef]
- Martínez-Sáez, O.; Gajate Borau, P.; Alonso-Gordoa, T.; Molina-Cerrillo, J.; Grande, E. Targeting HIF-2 α in Clear Cell Renal Cell Carcinoma: A Promising Therapeutic Strategy. Crit. Rev. Oncol. Hematol. 2017, 111, 117–123. [Google Scholar] [CrossRef]
- Zhou, Y.; Huang, Z.; Su, J.; Li, J.; Zhao, S.; Wu, L.; Zhang, J.; He, Y.; Zhang, G.; Tao, J.; et al. Benserazide Is a Novel Inhibitor Targeting PKM2 for Melanoma Treatment. Int. J. Cancer 2020, 147, 139–151. [Google Scholar] [CrossRef]
- Weber, D.D.; Aminzadeh-Gohari, S.; Tulipan, J.; Catalano, L.; Feichtinger, R.G.; Kofler, B. Ketogenic Diet in the Treatment of Cancer—Where Do We Stand? Mol. Metab. 2020, 33, 102–121. [Google Scholar] [CrossRef] [PubMed]
- Paoli, A.; Mancin, L.; Bianco, A.; Thomas, E.; Mota, J.F.; Piccini, F. Ketogenic Diet and Microbiota: Friends or Enemies? Genes 2019, 10, 534. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, B.; Raggi, P. The ketogenic diet: Pros and cons. Atherosclerosis 2020, 292, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Barry, D.; Ellul, S.; Watters, L.; Lee, D.; Haluska, R., Jr.; White, R. The ketogenic diet in disease and development. Int. J. Dev. Neurosci. 2018, 68, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Kao, K.C.; Vilbois, S.; Tsai, C.H.; Ho, P.C. Metabolic communication in the tumour-immune microenvironment. Nat. Cell Biol. 2022, 24, 1574–1583. [Google Scholar] [CrossRef]
- Hirschberger, S.; Strauß, G.; Effinger, D.; Marstaller, X.; Ferstl, A.; Müller, M.B.; Wu, T.; Hübner, M.; Rahmel, T.; Mascolo, H.; et al. Very-low-carbohydrate diet enhances human T-cell immunity through immunometabolic reprogramming. EMBO Mol. Med. 2021, 13, e14323. [Google Scholar] [CrossRef]
- Alden, R.S.; Kamran, M.Z.; Bashjawish, B.A.; Simone, B.A. Glutamine Metabolism and Radiosensitivity: Beyond the Warburg Effect. Front. Oncol. 2022, 12, 1070514. [Google Scholar] [CrossRef]
- Sainero-Alcolado, L.; Liaño-Pons, J.; Ruiz-Pérez, M.V.; Arsenian-Henriksson, M. Targeting Mitochondrial Metabolism for Precision Medicine in Cancer. Cell Death Differ. 2022, 29, 1304–1317. [Google Scholar] [CrossRef]
- Guarente, L. The Many Faces of Sirtuins: Sirtuins and the Warburg Effect. Nat. Med. 2014, 20, 24–25. [Google Scholar] [CrossRef]
- Faubert, B.; Solmonson, A.; DeBerardinis, R.J. Metabolic reprogramming and cancer progression. Science 2020, 368, eaaw5473. [Google Scholar] [CrossRef]
- Lapuente-Brun, E.; Moreno-Loshuertos, R.; Acín-Pérez, R.; Latorre-Pellicer, A.; Colás, C.; Balsa, E.; Perales-Clemente, E.; Quirós, P.M.; Calvo, E.; Rodríguez-Hernández, M.A.; et al. Supercomplex assembly determines electron flux in the mitochondrial electron transport chain. Science 2013, 340, 1567–1570. [Google Scholar] [CrossRef]
- da Veiga Moreira, J.; Schwartz, L.; Jolicoeur, M. Targeting Mitochondrial Singlet Oxygen Dynamics Offers New Perspectives for Effective Metabolic Therapies of Cancer. Front. Oncol. 2020, 18, 573399. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Lucas, A.; Lin, W.; Driscoll, P.C.; Legrave, N.; Novellasdemunt, L.; Xie, C.; Charles, M.; Wilson, Z.; Jones, N.P.; Rayport, S.; et al. Identifying Strategies to Target the Metabolic Flexibility of Tumours. Nat. Metab. 2020, 2, 335–350. [Google Scholar] [CrossRef] [PubMed]
- Cervantes-Madrid, D.; Dominguez-Gomez, G.; Gonzalez-Fierro, A.; Perez-Cardenas, E.; Taja-Chayeb, L.; Trejo-Becerril, C.; Duenas-Gonzalez, A. Feasibility and Antitumor Efficacy in Vivo, of Simultaneously Targeting Glycolysis, Glutaminolysis and Fatty Acid Synthesis Using Lonidamine, 6-Diazo-5-Oxo-L-Norleucine and Orlistat in Colon Cancer. Oncol. Lett. 2017, 13, 1905–1910. [Google Scholar] [CrossRef]
- Kouidhi, S.; ben Ayed, F.; Benammar Elgaaied, A. Targeting Tumor Metabolism: A New Challenge to Improve Immunotherapy. Front. Immunol. 2018, 9, 353. [Google Scholar] [CrossRef] [PubMed]
- Haas, R.; Cucchi, D.; Smith, J.; Pucino, V.; Macdougall, C.E.; Mauro, C. Intermediates of Metabolism: From Bystanders to Signalling Molecules. Trends Biochem. Sci. 2016, 41, 460–471. [Google Scholar] [CrossRef]
- Tretter, L.; Patocs, A.; Chinopoulos, C. Succinate, an Intermediate in Metabolism, Signal Transduction, ROS, Hypoxia, and Tumorigenesis. Biochim. Biophys. Acta (BBA)-Bioenerg. 2016, 1857, 1086–1101. [Google Scholar] [CrossRef]
- Kregiel, D. Succinate Dehydrogenase of Saccharomyces Cerevisiae—The Unique Enzyme of TCA Cycle—Current Knowledge and New Perspectives. In Dehydrogenases; InTech: Rang-Du-Fliers, France, 2012. [Google Scholar] [CrossRef]
- Wu, J.-Y.; Huang, T.-W.; Hsieh, Y.-T.; Wang, Y.-F.; Yen, C.-C.; Lee, G.-L.; Yeh, C.-C.; Peng, Y.-J.; Kuo, Y.-Y.; Wen, H.-T.; et al. Cancer-Derived Succinate Promotes Macrophage Polarization and Cancer Metastasis via Succinate Receptor. Mol. Cell 2020, 77, 213–227.e5. [Google Scholar] [CrossRef]
- Mu, X.; Zhao, T.; Xu, C.; Shi, W.; Geng, B.; Shen, J.; Zhang, C.; Pan, J.; Yang, J.; Hu, S.; et al. Oncometabolite Succinate Promotes Angiogenesis by Upregulating VEGF Expression through GPR91-Mediated STAT3 and ERK Activation. Oncotarget 2017, 8, 13174–13185. [Google Scholar] [CrossRef]
- Moreno, C.; Santos, R.M.; Burns, R.; Zhang, W.C. Succinate Dehydrogenase and Ribonucleic Acid Networks in Cancer and Other Diseases. Cancers 2020, 12, 3237. [Google Scholar] [CrossRef]
- Gimenez-Roqueplo, A.-P.; Favier, J.; Rustin, P.; Mourad, J.-J.; Plouin, P.-F.; Corvol, P.; Rötig, A.; Jeunemaitre, X. The R22X Mutation of the SDHD Gene in Hereditary Paraganglioma Abolishes the Enzymatic Activity of Complex II in the Mitochondrial Respiratory Chain and Activates the Hypoxia Pathway. Am. J. Hum. Genet. 2001, 69, 1186–1197. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.-C.; Wu, J.-Y.; Wu, K.K. Cancer-Derived Extracellular Succinate: A Driver of Cancer Metastasis. J. Biomed. Sci. 2022, 29, 93. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, R.K.; Luchtel, R.A.; Machha, V.; Tischer, A.; Zou, Y.; Pradhan, K.; Ashai, N.; Ramachandra, N.; Albanese, J.M.; Yang, J.; et al. Functional Succinate Dehydrogenase Deficiency Is a Common Adverse Feature of Clear Cell Renal Cancer. Proc. Natl. Acad. Sci. USA 2021, 118, e2106947118. [Google Scholar] [CrossRef]
- Chen, L.; Liu, T.; Zhang, S.; Zhou, J.; Wang, Y.; Di, W. Succinate Dehydrogenase Subunit B Inhibits the AMPK-HIF-1α Pathway in Human Ovarian Cancer in Vitro. J. Ovarian Res. 2014, 7, 115. [Google Scholar] [CrossRef]
- Zhao, T.; Mu, X.; You, Q. Succinate: An Initiator in Tumorigenesis and Progression. Oncotarget 2017, 8, 53819–53828. [Google Scholar] [CrossRef]
- Godel, M.; Ortone, G.; Anobile, D.P.; Pasino, M.; Randazzo, G.; Riganti, C.; Kopecka, J. Targeting Mitochondrial Oncometabolites: A New Approach to Overcome Drug Resistance in Cancer. Pharmaceutics 2021, 13, 762. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, J.M.; Venkatachalam, M.A.; Roeser, N.F.; Nissim, I. Mitochondrial Dysfunction during Hypoxia/Reoxygenation and Its Correction by Anaerobic Metabolism of Citric Acid Cycle Intermediates. Proc. Natl. Acad. Sci. USA 2000, 97, 2826–2831. [Google Scholar] [CrossRef]
- Hsu, P.P.; Sabatini, D.M. Cancer Cell Metabolism: Warburg and Beyond. Cell 2008, 134, 703–707. [Google Scholar] [CrossRef]
- Kes, M.M.G.; van den Bossche, J.; Griffioen, A.W.; Huijbers, E.J.M. Oncometabolites Lactate and Succinate Drive Pro-Angiogenic Macrophage Response in Tumors. Biochim. Biophys. Acta (BBA)-Rev. Cancer 2020, 1874, 188427. [Google Scholar] [CrossRef]
- Jha, A.K.; Huang, S.C.-C.; Sergushichev, A.; Lampropoulou, V.; Ivanova, Y.; Loginicheva, E.; Chmielewski, K.; Stewart, K.M.; Ashall, J.; Everts, B.; et al. Network Integration of Parallel Metabolic and Transcriptional Data Reveals Metabolic Modules That Regulate Macrophage Polarization. Immunity 2015, 42, 419–430. [Google Scholar] [CrossRef]
- Ryan, D.G.; O’Neill, L.A.J. Krebs Cycle Rewired for Macrophage and Dendritic Cell Effector Functions. FEBS Lett. 2017, 591, 2992–3006. [Google Scholar] [CrossRef]
- Chouchani, E.T.; Pell, V.R.; Gaude, E.; Aksentijević, D.; Sundier, S.Y.; Robb, E.L.; Logan, A.; Nadtochiy, S.M.; Ord, E.N.J.; Smith, A.C.; et al. Ischaemic Accumulation of Succinate Controls Reperfusion Injury through Mitochondrial ROS. Nature 2014, 515, 431–435. [Google Scholar] [CrossRef]
- Benzi, G.; Arrigoni, E.; Marzatico, F.; Villa, R.F. Influence of Some Biological Pyrimidines on the Succinate Cycle during and after Cerebral Ischemia. Biochem. Pharmacol. 1979, 28, 2545–2550. [Google Scholar] [CrossRef] [PubMed]
- Folbergrova´, J.; Ljunggren, B.; Norberg, K.; Siesjo¨, B.K. Influence of Complete Ischemia on Glycolytic Metabolites, Citric Acid Cycle Intermediates, and Associated Amino Acids in the Rat Cerebral Cortex. Brain Res. 1974, 80, 265–279. [Google Scholar] [CrossRef]
- Hui, S.; Ghergurovich, J.M.; Morscher, R.J.; Jang, C.; Teng, X.; Lu, W.; Esparza, L.A.; Reya, T.; Zhan, L.; Guo, J.Y.; et al. Glucose Feeds the TCA Cycle via Circulating Lactate. Nature 2017, 551, 115–118. [Google Scholar] [CrossRef] [PubMed]
- de Castro Fonseca, M.; Aguiar, C.J.; da Rocha Franco, J.A.; Gingold, R.N.; Leite, M.F. GPR91: Expanding the Frontiers of Krebs Cycle Intermediates. Cell Commun. Signal. 2016, 14, 3. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Yan, W. Succinate in the Cancer–Immune Cycle. Cancer Lett. 2017, 390, 45–47. [Google Scholar] [CrossRef]
- Najm, R.; Hachim, M.Y.; Kandasamy, R.K. Divulging a Pleiotropic Role of Succinate Receptor SUCNR1 in Renal Cell Carcinoma Microenvironment. Cancers 2022, 14, 6064. [Google Scholar] [CrossRef]
- Dalla Pozza, E.; Dando, I.; Pacchiana, R.; Liboi, E.; Scupoli, M.T.; Donadelli, M.; Palmieri, M. Regulation of Succinate Dehydrogenase and Role of Succinate in Cancer. Semin. Cell Dev. Biol. 2020, 98, 4–14. [Google Scholar] [CrossRef]
- Laukka, T.; Mariani, C.J.; Ihantola, T.; Cao, J.Z.; Hokkanen, J.; Kaelin, W.G.; Godley, L.A.; Koivunen, P. Fumarate and Succinate Regulate Expression of Hypoxia-Inducible Genes via TET Enzymes. J. Biol. Chem. 2016, 291, 4256–4265. [Google Scholar] [CrossRef]
- Aspuria, P.-J.P.; Lunt, S.Y.; Väremo, L.; Vergnes, L.; Gozo, M.; Beach, J.A.; Salumbides, B.; Reue, K.; Wiedemeyer, W.R.; Nielsen, J.; et al. Succinate Dehydrogenase Inhibition Leads to Epithelial-Mesenchymal Transition and Reprogrammed Carbon Metabolism. Cancer Metab. 2014, 2, 21. [Google Scholar] [CrossRef] [PubMed]
- Tannahill, G.M.; Curtis, A.M.; Adamik, J.; Palsson-McDermott, E.M.; McGettrick, A.F.; Goel, G.; Frezza, C.; Bernard, N.J.; Kelly, B.; Foley, N.H.; et al. Succinate Is an Inflammatory Signal That Induces IL-1β through HIF-1α. Nature 2013, 496, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Bauset, C.; Lis-Lopez, L.; Coll, S.; Gisbert-Ferrándiz, L.; Macias-Ceja, D.C.; Seco-Cervera, M.; Navarro, F.; Esplugues, J.V.; Calatayud, S.; Ortiz-Masia, D.; et al. SUCNR1 Mediates the Priming Step of the Inflammasome in Intestinal Epithelial Cells: Relevance in Ulcerative Colitis. Biomedicines 2022, 10, 532. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Prasad, H.K.; Kumar, M. The Double-Edged Sword Role of ROS in Cancer. In Handbook of Oxidative Stress in Cancer: Mechanistic Aspects; Springer: Singapore, 2021; pp. 1–17. [Google Scholar] [CrossRef]
- Liou, G.-Y.; Storz, P. Reactive Oxygen Species in Cancer. Free Radic. Res. 2010, 44, 479–496. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xie, L.; Qu, X.; Zhao, B.; Fu, W.; Wu, B.; Wu, J. GPR91, a Critical Signaling Mechanism in Modulating Pathophysiologic Processes in Chronic Illnesses. FASEB J. 2020, 34, 13091–13105. [Google Scholar] [CrossRef]
- Wu, B.; Luo, H.; Zhou, X.; Cheng, C.; Lin, L.; Liu, B.; Liu, K.; Li, P.; Yang, H. Succinate-Induced Neuronal Mitochondrial Fission and Hexokinase II Malfunction in Ischemic Stroke: Therapeutical Effects of Kaempferol. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2017, 1863, 2307–2318. [Google Scholar] [CrossRef]
- Lei, W.; Ren, W.; Ohmoto, M.; Urban, J.F.; Matsumoto, I.; Margolskee, R.F.; Jiang, P. Activation of Intestinal Tuft Cell-Expressed Sucnr1 Triggers Type 2 Immunity in the Mouse Small Intestine. Proc. Natl. Acad. Sci. USA 2018, 115, 5552–5557. [Google Scholar] [CrossRef] [PubMed]
- Mills, E.L.; Pierce, K.A.; Jedrychowski, M.P.; Garrity, R.; Winther, S.; Vidoni, S.; Yoneshiro, T.; Spinelli, J.B.; Lu, G.Z.; Kazak, L.; et al. Accumulation of Succinate Controls Activation of Adipose Tissue Thermogenesis. Nature 2018, 560, 102–106. [Google Scholar] [CrossRef]
- Reddy, A.; Bozi, L.H.M.; Yaghi, O.K.; Mills, E.L.; Xiao, H.; Nicholson, H.E.; Paschini, M.; Paulo, J.A.; Garrity, R.; Laznik-Bogoslavski, D.; et al. PH-Gated Succinate Secretion Regulates Muscle Remodeling in Response to Exercise. Cell 2020, 183, 62–75.e17. [Google Scholar] [CrossRef]
- Littlewood-Evans, A.; Sarret, S.; Apfel, V.; Loesle, P.; Dawson, J.; Zhang, J.; Muller, A.; Tigani, B.; Kneuer, R.; Patel, S.; et al. GPR91 Senses Extracellular Succinate Released from Inflammatory Macrophages and Exacerbates Rheumatoid Arthritis. J. Exp. Med. 2016, 213, 1655–1662. [Google Scholar] [CrossRef]
- Aguiar, C.J.; Rocha-Franco, J.A.; Sousa, P.A.; Santos, A.K.; Ladeira, M.; Rocha-Resende, C.; Ladeira, L.O.; Resende, R.R.; Botoni, F.A.; Barrouin Melo, M.; et al. Succinate Causes Pathological Cardiomyocyte Hypertrophy through GPR91 Activation. Cell Commun. Signal. 2014, 12, 78. [Google Scholar] [CrossRef]
- Trauelsen, M.; Hiron, T.K.; Lin, D.; Petersen, J.E.; Breton, B.; Husted, A.S.; Hjorth, S.A.; Inoue, A.; Frimurer, T.M.; Bouvier, M.; et al. Extracellular Succinate Hyperpolarizes M2 Macrophages through SUCNR1/GPR91-Mediated Gq Signaling. Cell Rep. 2021, 35, 109246. [Google Scholar] [CrossRef]
- Bhuniya, D.; Umrani, D.; Dave, B.; Salunke, D.; Kukreja, G.; Gundu, J.; Naykodi, M.; Shaikh, N.S.; Shitole, P.; Kurhade, S.; et al. Discovery of a Potent and Selective Small Molecule HGPR91 Antagonist. Bioorg. Med. Chem. Lett. 2011, 21, 3596–3602. [Google Scholar] [CrossRef] [PubMed]
- Rabe, P.; Liebing, A.-D.; Krumbholz, P.; Kraft, R.; Stäubert, C. Succinate Receptor 1 Inhibits Mitochondrial Respiration in Cancer Cells Addicted to Glutamine. Cancer Lett. 2022, 526, 91–102. [Google Scholar] [CrossRef]
- Zhang, J.; Xiang, H.; Liu, J.; Chen, Y.; He, R.-R.; Liu, B. Mitochondrial Sirtuin 3: New Emerging Biological Function and Therapeutic Target. Theranostics 2020, 10, 8315–8342. [Google Scholar] [CrossRef] [PubMed]
- Verdin, E.; Hirschey, M.D.; Finley, L.W.S.; Haigis, M.C. Sirtuin Regulation of Mitochondria: Energy Production, Apoptosis, and Signaling. Trends Biochem. Sci. 2010, 35, 669–675. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Fu, L.L.; Wen, X.; Wang, X.Y.; Liu, J.; Cheng, Y.; Huang, J. Sirtuin-3 (SIRT3), a Therapeutic Target with Oncogenic and Tumor-Suppressive Function in Cancer. Cell Death Dis. 2014, 5, e1047. [Google Scholar] [CrossRef]
- Jo, H.; Park, Y.; Kim, T.; Kim, J.; Lee, J.S.; Kim, S.Y.; Chung, J.; Ko, H.Y.; Pyun, J.-C.; Kim, K.S.; et al. Modulation of SIRT3 Expression through CDK4/6 Enhances the Anti-Cancer Effect of Sorafenib in Hepatocellular Carcinoma Cells. BMC Cancer 2020, 20, 332. [Google Scholar] [CrossRef]
- Finley, L.W.S.; Haas, W.; Desquiret-Dumas, V.; Wallace, D.C.; Procaccio, V.; Gygi, S.P.; Haigis, M.C. Succinate Dehydrogenase Is a Direct Target of Sirtuin 3 Deacetylase Activity. PLoS ONE 2011, 6, e23295. [Google Scholar] [CrossRef]
- Bell, E.L.; Emerling, B.M.; Ricoult, S.J.H.; Guarente, L. SirT3 Suppresses Hypoxia Inducible Factor 1α and Tumor Growth by Inhibiting Mitochondrial ROS Production. Oncogene 2011, 30, 2986–2996. [Google Scholar] [CrossRef]
- Alhazzazi, T.Y.; Kamarajan, P.; Verdin, E.; Kapila, Y.L. Sirtuin-3 (SIRT3) and the Hallmarks of Cancer. Genes Cancer 2013, 4, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Finley, L.W.S.; Carracedo, A.; Lee, J.; Souza, A.; Egia, A.; Zhang, J.; Teruya-Feldstein, J.; Moreira, P.I.; Cardoso, S.M.; Clish, C.B.; et al. SIRT3 Opposes Reprogramming of Cancer Cell Metabolism through HIF1α Destabilization. Cancer Cell 2011, 19, 416–428. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.A.; O’Callaghan, C.; Chang, E.D.; Jiang, H.; Vassilopoulos, A. Context-Dependent Roles for SIRT2 and SIRT3 in Tumor Development Upon Calorie Restriction or High Fat Diet. Front. Oncol. 2020, 9, 1462. [Google Scholar] [CrossRef] [PubMed]
- Baccelli, I.; Gareau, Y.; Lehnertz, B.; Gingras, S.; Spinella, J.-F.; Corneau, S.; Mayotte, N.; Girard, S.; Frechette, M.; Blouin-Chagnon, V.; et al. Mubritinib Targets the Electron Transport Chain Complex I and Reveals the Landscape of OXPHOS Dependency in Acute Myeloid Leukemia. Cancer Cell 2019, 36, 84–99.e8. [Google Scholar] [CrossRef]
- Ma, J.; Liu, B.; Yu, D.; Zuo, Y.; Cai, R.; Yang, J.; Cheng, J. SIRT3 Deacetylase Activity Confers Chemoresistance in AML via Regulation of Mitochondrial Oxidative Phosphorylation. Br. J. Haematol. 2019, 187, 49–64. [Google Scholar] [CrossRef]
- Lu, J.; Zhang, H.; Chen, X.; Zou, Y.; Li, J.; Wang, L.; Wu, M.; Zang, J.; Yu, Y.; Zhuang, W.; et al. A Small Molecule Activator of SIRT3 Promotes Deacetylation and Activation of Manganese Superoxide Dismutase. Free Radic. Biol. Med. 2017, 112, 287–297. [Google Scholar] [CrossRef]
- Pillai, V.B.; Samant, S.; Sundaresan, N.R.; Raghuraman, H.; Kim, G.; Bonner, M.Y.; Arbiser, J.L.; Walker, D.I.; Jones, D.P.; Gius, D.; et al. Honokiol Blocks and Reverses Cardiac Hypertrophy in Mice by Activating Mitochondrial Sirt3. Nat. Commun. 2015, 6, 6656. [Google Scholar] [CrossRef]
- Li, Y.; Ye, Z.; Lai, W.; Rao, J.; Huang, W.; Zhang, X.; Yao, Z.; Lou, T. Activation of Sirtuin 3 by Silybin Attenuates Mitochondrial Dysfunction in Cisplatin-Induced Acute Kidney Injury. Front. Pharmacol. 2017, 8, 178. [Google Scholar] [CrossRef]
- Xu, S.; Gao, Y.; Zhang, Q.; Wei, S.; Chen, Z.; Dai, X.; Zeng, Z.; Zhao, K. SIRT1/3 Activation by Resveratrol Attenuates Acute Kidney Injury in a Septic Rat Model. Oxid. Med. Cell Longev. 2016, 2016, 1–12. [Google Scholar] [CrossRef]
- Wang, J.; Wang, K.; Huang, C.; Lin, D.; Zhou, Y.; Wu, Y.; Tian, N.; Fan, P.; Pan, X.; Xu, D.; et al. SIRT3 Activation by Dihydromyricetin Suppresses Chondrocytes Degeneration via Maintaining Mitochondrial Homeostasis. Int. J. Biol. Sci. 2018, 14, 1873–1882. [Google Scholar] [CrossRef]
- Zhang, J.; Meruvu, S.; Bedi, Y.S.; Chau, J.; Arguelles, A.; Rucker, R.; Choudhury, M. Pyrroloquinoline Quinone Increases the Expression and Activity of Sirt1 and -3 Genes in HepG2 Cells. Nutr. Res. 2015, 35, 844–849. [Google Scholar] [CrossRef]
- Pillai, V.B.; Kanwal, A.; Fang, Y.H.; Sharp, W.W.; Samant, S.; Arbiser, J.; Gupta, M.P. Honokiol, an Activator of Sirtuin-3 (SIRT3) Preserves Mitochondria and Protects the Heart from Doxorubicin-Induced Cardiomyopathy in Mice. Oncotarget 2017, 8, 34082–34098. [Google Scholar] [CrossRef]
- Quan, Y.; Park, W.; Jin, J.; Kim, W.; Park, S.K.; Kang, K.P. Sirtuin 3 Activation by Honokiol Decreases Unilateral Ureteral Obstruction-Induced Renal Inflammation and Fibrosis via Regulation of Mitochondrial Dynamics and the Renal NF-ΚB-TGF-Β1/Smad Signaling Pathway. Int. J. Mol. Sci. 2020, 21, 402. [Google Scholar] [CrossRef]
- Yi, X.; Guo, W.; Shi, Q.; Yang, Y.; Zhang, W.; Chen, X.; Kang, P.; Chen, J.; Cui, T.; Ma, J.; et al. SIRT3-Dependent Mitochondrial Dynamics Remodeling Contributes to Oxidative Stress-Induced Melanocyte Degeneration in Vitiligo. Theranostics 2019, 9, 1614–1633. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Chen, L.; Lu, Y.; Lei, S.; Peng, M.; Xia, Z. SIRT3 Activator Honokiol Ameliorates Surgery/Anesthesia-induced Cognitive Decline in Mice through Anti-oxidative Stress and Anti-inflammatory in Hippocampus. CNS Neurosci. Ther. 2019, 25, 355–366. [Google Scholar] [CrossRef]
- Li, X.; Guan, S.; Li, H.; Li, D.; Liu, D.; Wang, J.; Zhu, W.; Xing, G.; Yue, L.; Cai, D.; et al. Polysialic Acid-Functionalized Liposomes for Efficient Honokiol Delivery to Inhibit Breast Cancer Growth and Metastasis. Drug Deliv. 2023, 30, 2181746. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Karoui, H.; Hardy, M.; Kalyanaraman, B. Polyphenolic Boronates Inhibit Tumor Cell Proliferation: Potential Mitigators of Oxidants in the Tumor Microenvironment. Cancers 2023, 15, 1089. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Shao, G.; Hong, X.; Shi, Y.; Zheng, Y.; Yu, Y.; Fu, C. Targeting Annexin A1 as a Druggable Player to Enhance the Anti-Tumor Role of Honokiol in Colon Cancer through Autophagic Pathway. Pharmaceuticals 2023, 16, 70. [Google Scholar] [CrossRef]
- Mao, R.; He, S.; Lan, J.; Zhu, W. Honokiol Ameliorates Cisplatin-induced Acute Kidney Injury via Inhibition of Mitochondrial Fission. Br. J. Pharmacol. 2022, 179, 3886–3904. [Google Scholar] [CrossRef]
- Bollard, J.; Miguela, V.; Ruiz de Galarreta, M.; Venkatesh, A.; Bian, C.B.; Roberto, M.P.; Tovar, V.; Sia, D.; Molina-Sánchez, P.; Nguyen, C.B.; et al. Palbociclib (PD-0332991), a Selective CDK4/6 Inhibitor, Restricts Tumour Growth in Preclinical Models of Hepatocellular Carcinoma. Gut 2017, 66, 1286–1296. [Google Scholar] [CrossRef]
- Hinshaw, D.C.; Shevde, L.A. The Tumor Microenvironment Innately Modulates Cancer Progression. Cancer Res. 2019, 79, 4557–4566. [Google Scholar] [CrossRef]
- Tao, N.-N.; Zhou, H.-Z.; Tang, H.; Cai, X.-F.; Zhang, W.-L.; Ren, J.-H.; Zhou, L.; Chen, X.; Chen, K.; Li, W.-Y.; et al. Sirtuin 3 Enhanced Drug Sensitivity of Human Hepatoma Cells through Glutathione S-Transferase Pi 1/JNK Signaling Pathway. Oncotarget 2016, 7, 50117–50130. [Google Scholar] [CrossRef]
- Cheng, R.; Liu, X.; Wang, Z.; Tang, K. ABT-737, a Bcl-2 Family Inhibitor, has a Synergistic Effect with Apoptosis by Inducing Urothelial Carcinoma Cell Necroptosis. Mol. Med. Rep. 2021, 23, 412. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.; Wang, R.; Wei, H.; Li, S.; Liu, L.; Lu, X.; Yu, H.; Liu, Z. ABT737 Enhances Ovarian Cancer Cells Sensitivity to Cisplatin through Regulation of Mitochondrial Fission via Sirt3 Activation. Life Sci. 2019, 232, 116561. [Google Scholar] [CrossRef]
- Sciacovelli, M.; Guzzo, G.; Morello, V.; Frezza, C.; Zheng, L.; Nannini, N.; Calabrese, F.; Laudiero, G.; Esposito, F.; Landriscina, M.; et al. The Mitochondrial Chaperone TRAP1 Promotes Neoplastic Growth by Inhibiting Succinate Dehydrogenase. Cell Metab. 2013, 17, 988–999. [Google Scholar] [CrossRef] [PubMed]
- Chae, Y.C.; Angelin, A.; Lisanti, S.; Kossenkov, A.V.; Speicher, K.D.; Wang, H.; Powers, J.F.; Tischler, A.S.; Pacak, K.; Fliedner, S.; et al. Landscape of the Mitochondrial Hsp90 Metabolome in Tumours. Nat. Commun. 2013, 4, 2139. [Google Scholar] [CrossRef] [PubMed]
- Dharaskar, S.P.; Amere Subbarao, S. The Mitochondrial Chaperone TRAP-1 Regulates the Glutamine Metabolism in Tumor Cells. Mitochondrion 2023, 69, 159–170. [Google Scholar] [CrossRef]
- Lisanti, S.; Tavecchio, M.; Chae, Y.C.; Liu, Q.; Brice, A.K.; Thakur, M.L.; Languino, L.R.; Altieri, D.C. Deletion of the Mitochondrial Chaperone TRAP-1 Uncovers Global Reprogramming of Metabolic Networks. Cell Rep. 2014, 8, 671–677. [Google Scholar] [CrossRef]
- Coller, H.A.; Grandori, C.; Tamayo, P.; Colbert, T.; Lander, E.S.; Eisenman, R.N.; Golub, T.R. Expression Analysis with Oligonucleotide Microarrays Reveals That MYC Regulates Genes Involved in Growth, Cell Cycle, Signaling, and Adhesion. Proc. Natl. Acad. Sci. USA 2000, 97, 3260–3265. [Google Scholar] [CrossRef]
- Costantino, E.; Maddalena, F.; Calise, S.; Piscazzi, A.; Tirino, V.; Fersini, A.; Ambrosi, A.; Neri, V.; Esposito, F.; Landriscina, M. TRAP1, a Novel Mitochondrial Chaperone Responsible for Multi-Drug Resistance and Protection from Apoptotis in Human Colorectal Carcinoma Cells. Cancer Lett. 2009, 279, 39–46. [Google Scholar] [CrossRef]
- Nakagawa, T.; Shimizu, S.; Watanabe, T.; Yamaguchi, O.; Otsu, K.; Yamagata, H.; Inohara, H.; Kubo, T.; Tsujimoto, Y. Cyclophilin D-Dependent Mitochondrial Permeability Transition Regulates Some Necrotic but Not Apoptotic Cell Death. Nature 2005, 434, 652–658. [Google Scholar] [CrossRef] [PubMed]
- Lisanti, S.; Garlick, D.S.; Bryant, K.G.; Tavecchio, M.; Mills, G.B.; Lu, Y.; Kossenkov, A.V.; Showe, L.C.; Languino, L.R.; Altieri, D.C. Transgenic Expression of the Mitochondrial Chaperone TNFR-Associated Protein 1 (TRAP1) Accelerates Prostate Cancer Development. J. Biol. Chem. 2016, 291, 25247–25254. [Google Scholar] [CrossRef] [PubMed]
- Vartholomaiou, E.; Madon-Simon, M.; Hagmann, S.; Mühlebach, G.; Wurst, W.; Floss, T.; Picard, D. Cytosolic Hsp90α and Its Mitochondrial Isoform Trap1 Are Differentially Required in a Breast Cancer Model. Oncotarget 2017, 8, 17428–17442. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Li, M.; Jiang, A.; Sun, R.; Jin, H.; Gui, H.; Xiao, F.; Ding, X.; Fu, Z.; Feng, J. Overexpression of the Mitochondrial Chaperone Tumor Necrosis Factor Receptor-associated Protein 1 Is Associated with the Poor Prognosis of Patients with Colorectal Cancer. Oncol. Lett. 2018, 15, 5451–5458. [Google Scholar] [CrossRef] [PubMed]
- Song, H.Y.; Dunbar, J.D.; Zhang, Y.X.; Guo, D.; Donner, D.B. Identification of a Protein with Homology to Hsp90 That Binds the Type 1 Tumor Necrosis Factor Receptor. J. Biol. Chem. 1995, 270, 3574–3581. [Google Scholar] [CrossRef]
- Kang, B.H.; Plescia, J.; Dohi, T.; Rosa, J.; Doxsey, S.J.; Altieri, D.C. Regulation of Tumor Cell Mitochondrial Homeostasis by an Organelle-Specific Hsp90 Chaperone Network. Cell 2007, 131, 257–270. [Google Scholar] [CrossRef] [PubMed]
- Altieri, D.C.; Stein, G.S.; Lian, J.B.; Languino, L.R. TRAP-1, the Mitochondrial Hsp90. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2012, 1823, 767–773. [Google Scholar] [CrossRef] [PubMed]
- Gesualdi, N.M.; Chirico, G.; Pirozzi, G.; Costantino, E.; Landriscina, M.; Esposito, F. Tumor Necrosis Factor-Associated Protein 1 (TRAP-1) Protects Cells from Oxidative Stress and Apoptosis. Stress 2007, 10, 342–350. [Google Scholar] [CrossRef]
- Hua, G.; Zhang, Q.; Fan, Z. Heat Shock Protein 75 (TRAP1) Antagonizes Reactive Oxygen Species Generation and Protects Cells from Granzyme M-Mediated Apoptosis. J. Biol. Chem. 2007, 282, 20553–20560. [Google Scholar] [CrossRef]
- Pridgeon, J.W.; Olzmann, J.A.; Chin, L.-S.; Li, L. PINK1 Protects against Oxidative Stress by Phosphorylating Mitochondrial Chaperone TRAP1. PLoS Biol. 2007, 5, e172. [Google Scholar] [CrossRef]
- Kang, B.H.; Plescia, J.; Song, H.Y.; Meli, M.; Colombo, G.; Beebe, K.; Scroggins, B.; Neckers, L.; Altieri, D.C. Combinatorial Drug Design Targeting Multiple Cancer Signaling Networks Controlled by Mitochondrial Hsp90. J. Clin. Investig. 2009, 119, 454–464. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.H.; Siegelin, M.D.; Plescia, J.; Raskett, C.M.; Garlick, D.S.; Dohi, T.; Lian, J.B.; Stein, G.S.; Languino, L.R.; Altieri, D.C. Preclinical Characterization of Mitochondria-Targeted Small Molecule Hsp90 Inhibitors, Gamitrinibs, in Advanced Prostate Cancer. Clin. Cancer Res. 2010, 16, 4779–4788. [Google Scholar] [CrossRef] [PubMed]
- Leav, I.; Plescia, J.; Goel, H.L.; Li, J.; Jiang, Z.; Cohen, R.J.; Languino, L.R.; Altieri, D.C. Cytoprotective Mitochondrial Chaperone TRAP-1 As a Novel Molecular Target in Localized and Metastatic Prostate Cancer. Am. J. Pathol. 2010, 176, 393–401. [Google Scholar] [CrossRef]
- Kang, B.H.; Tavecchio, M.; Goel, H.L.; Hsieh, C.-C.; Garlick, D.S.; Raskett, C.M.; Lian, J.B.; Stein, G.S.; Languino, L.R.; Altieri, D.C. Targeted Inhibition of Mitochondrial Hsp90 Suppresses Localised and Metastatic Prostate Cancer Growth in a Genetic Mouse Model of Disease. Br. J. Cancer 2011, 104, 629–634. [Google Scholar] [CrossRef]
- Amash, V.; Paithankar, K.; Dharaskar, S.P.; Arunachalam, A.; Amere Subbarao, S. Development of Nanocarrier-Based Mitochondrial Chaperone, TRAP-1 Inhibitor to Combat Cancer Metabolism. ACS Appl. Bio Mater. 2020, 3, 4188–4197. [Google Scholar] [CrossRef]
- Menezes, D.L.; Taverna, P.; Jensen, M.R.; Abrams, T.; Stuart, D.; Yu, G.K.; Duhl, D.; Machajewski, T.; Sellers, W.R.; Pryer, N.K.; et al. The Novel Oral Hsp90 Inhibitor NVP-HSP990 Exhibits Potent and Broad-Spectrum Antitumor Activities In Vitro and In Vivo. Mol. Cancer Ther. 2012, 11, 730–739. [Google Scholar] [CrossRef] [PubMed]
- Avendaño, C.; Menéndez, J.C. Drug Targeting in Anticancer Chemotherapy. In Medicinal Chemistry of Anticancer Drugs; Elsevier: Amsterdam, The Netherlands, 2008; pp. 351–385. [Google Scholar] [CrossRef]
- Zhang, X.; Li, X.; You, Q.; Zhang, X. Prodrug Strategy for Cancer Cell-Specific Targeting: A Recent Overview. Eur. J. Med. Chem. 2017, 139, 542–563. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casas-Benito, A.; Martínez-Herrero, S.; Martínez, A. Succinate-Directed Approaches for Warburg Effect-Targeted Cancer Management, an Alternative to Current Treatments? Cancers 2023, 15, 2862. https://doi.org/10.3390/cancers15102862
Casas-Benito A, Martínez-Herrero S, Martínez A. Succinate-Directed Approaches for Warburg Effect-Targeted Cancer Management, an Alternative to Current Treatments? Cancers. 2023; 15(10):2862. https://doi.org/10.3390/cancers15102862
Chicago/Turabian StyleCasas-Benito, Adrian, Sonia Martínez-Herrero, and Alfredo Martínez. 2023. "Succinate-Directed Approaches for Warburg Effect-Targeted Cancer Management, an Alternative to Current Treatments?" Cancers 15, no. 10: 2862. https://doi.org/10.3390/cancers15102862
APA StyleCasas-Benito, A., Martínez-Herrero, S., & Martínez, A. (2023). Succinate-Directed Approaches for Warburg Effect-Targeted Cancer Management, an Alternative to Current Treatments? Cancers, 15(10), 2862. https://doi.org/10.3390/cancers15102862









