Impact of Postoperative Changes in Brain Anatomy on Target Volume Delineation for High-Grade Glioma
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
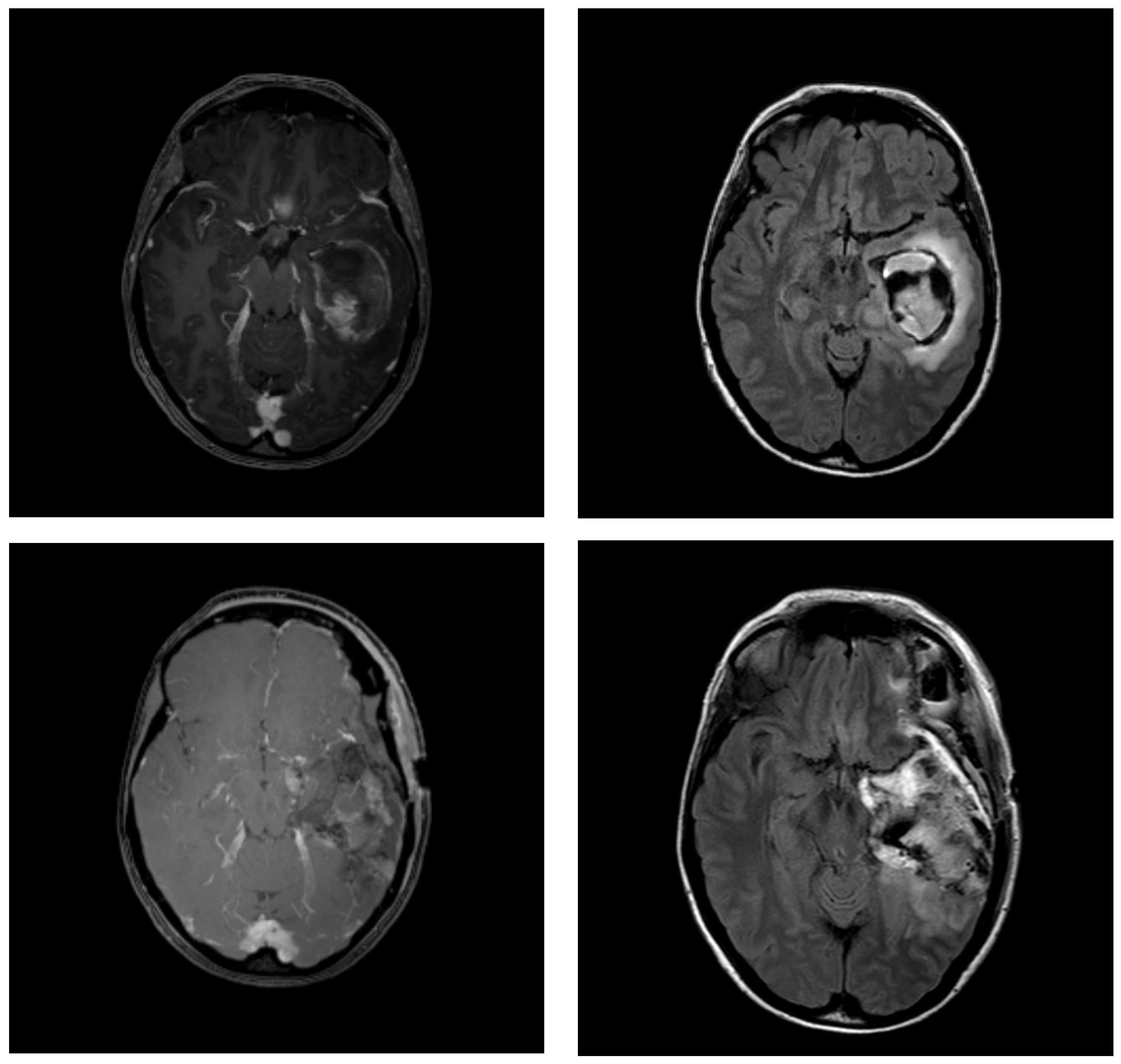
2.2. MRI Protocol
2.3. Contouring
2.4. Statistical Analysis
2.5. Literature Search
3. Results
3.1. Patient Characteristics
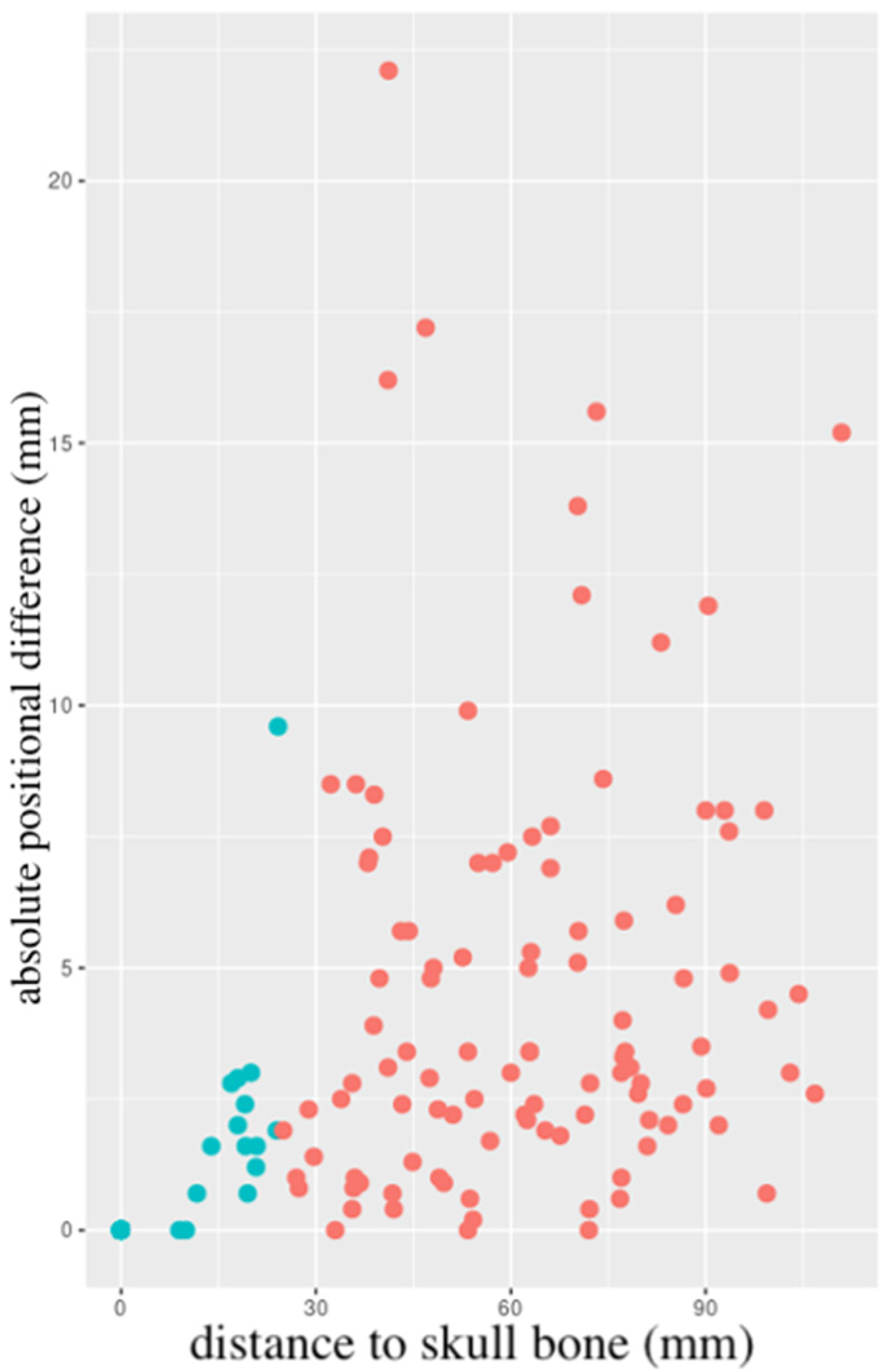
3.2. Changes in Resection Cavity Position
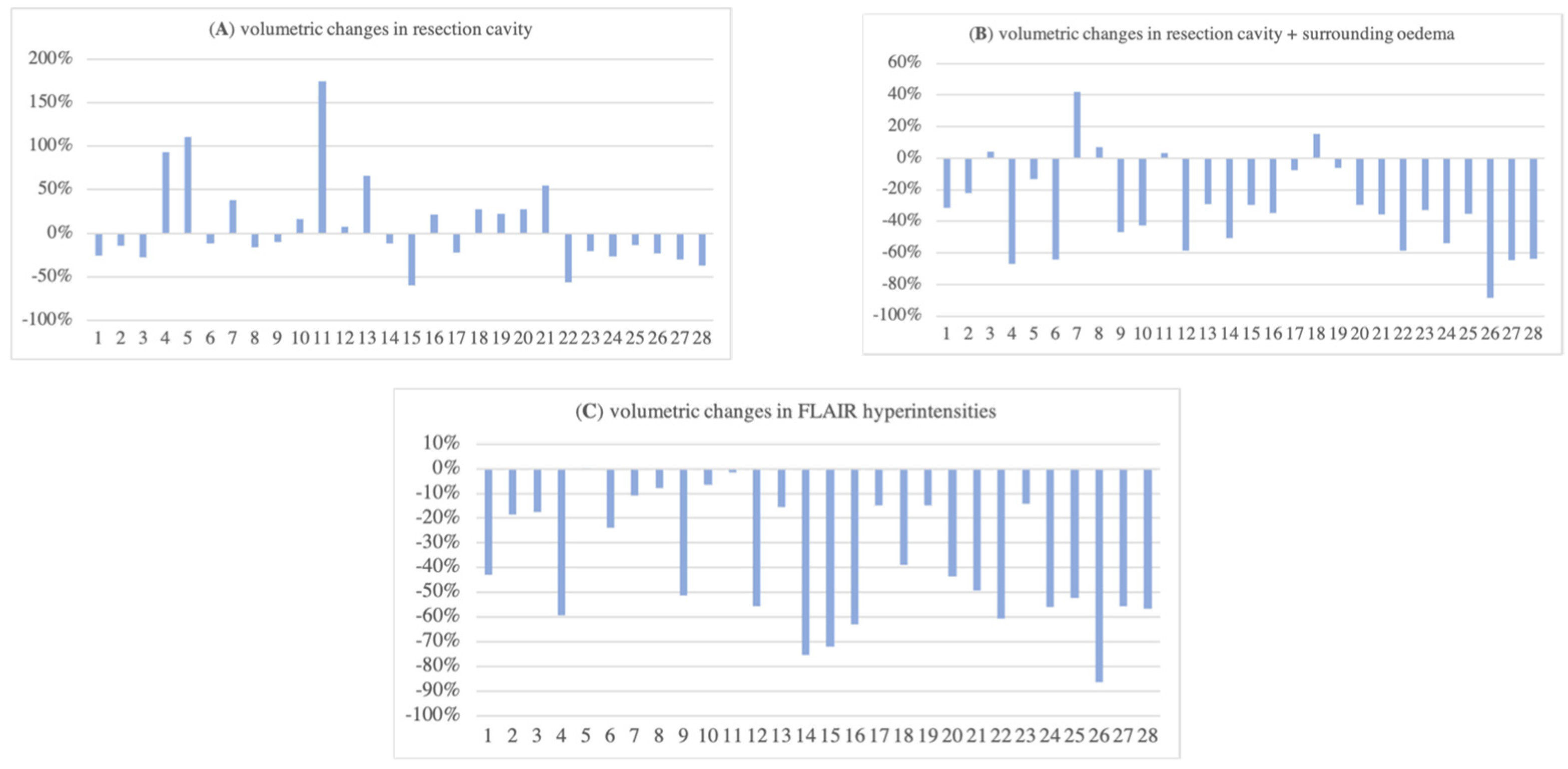
3.3. Volumetric Changes in Resection Cavity, Surrounding Oedema, and FLAIR Hyperintensities
3.4. Difference in Midline Shift and Subdural Haematoma
4. Discussion
| Author (Year) | n | WHO Grade | Timing MRI 1 | Timing MRI 2 | Contouring Guideline | Outcome |
|---|---|---|---|---|---|---|
| Manon et al. (2004) [26] | 15 | 4 (100%) | <24 h postoperative | <7 days prior to RT boost | RTOG | 80% had change in RC and GTV resulting in geographic miss |
| Shukla et al. (2005) [28] | 15 | 3 (47%) 4 (53%) | 1 day before RT | end of RT week 5 | RTOG | 80% had GTV decrease |
| Tsien et al. (2005) [29] | 21 | 3 (38%) 4 (62%) | 1–2 weeks before RT | RT week 1 and 3 | RTOG | 89% had GTV change |
| Champ et al. (2012) [30] | 24 | 3 (33%) 4 (67%) | <48 h postoperative | day of simulation median (range) 17 (7–32) days interval | RTOG | significant changes in GTV (22% volume decrease) and CTV (20% volume decrease) |
| Yang et al. (2016) [31] | 11 | 2 (36%) 3 (46%) 4 (18%) | before RT | end of RT | RTOG | decrease in RC, GTV, and PTV |
| Şenkesen et al. (2022) [32] | 24 | 4 (100%) | shortly before RT | shortly before RT boost median (range) 29 (25–38) days interval | RTOG | significant changes in GTV, CTV, and PTV |
| current series (2023) | 28 | 2 (7%) 4 (93%) | <24–48 h postoperative | before RT median (range) 19.5 (8–50) days interval | n/a | overall decrease in RC, surrounding oedema, and FLAIR |
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ostrom, Q.T.; Gittleman, H.; Farah, P.; Ondracek, A.; Chen, Y.; Wolinsky, Y.; Stroup, N.E.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2006–2010. Neuro-Oncology 2013, 15 (Suppl. S2), ii1–ii56. [Google Scholar] [CrossRef]
- Roger Stupp, M.D.; Warren, P.; Mason, M.D.; Martin, J.; van den Bent, M.D.; Michael Weller, M.D.; Barbara Fisher, M.D.; Martin, J.B.; Taphoorn, M.D.; Karl Belanger, M.D.; et al. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef]
- Wilson, T.A.; Karajannis, M.A.; Harter, D.H. Glioblastoma multiforme: State of the art and future therapeutics. Surg. Neurol. Int. 2014, 5, 64. [Google Scholar] [CrossRef]
- Davis, M.E. Glioblastoma: Overview of Disease and Treatment. Clin. J. Oncol. Nurs. 2016, 20, S2–S8. [Google Scholar] [CrossRef]
- Hanif, F.; Muzaffar, K.; Perveen, K.; Malhi, S.M.; Simjee, S.U. Glioblastoma Multiforme: A Review of its Epidemiology and Pathogenesis through Clinical Presentation and Treatment. Asian Pac. J. Cancer Prev. 2017, 18, 3–9. [Google Scholar] [CrossRef]
- Lorentini, S.; Amelio, D.; Giri, M.G.; Fellin, F.; Meliado, G.; Rizzotti, A.; Amichetti, M.; Schwarz, M. IMRT or 3D-CRT in glioblastoma? A dosimetric criterion for patient selection. Technol. Cancer Res. Treat. 2013, 12, 411–420. [Google Scholar] [CrossRef]
- Weller, M.; van den Bent, M.; Preusser, M.; Le Rhun, E.; Tonn, J.C.; Minniti, G.; Bendszus, M.; Balana, C.; Chinot, O.; Dirven, L.; et al. EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat. Rev. Clin. Oncol. 2021, 18, 170–186. [Google Scholar] [CrossRef]
- Niyazi, M.; Brada, M.; Chalmers, A.J.; Combs, S.E.; Erridge, S.C.; Fiorentino, A.; Grosu, A.L.; Lagerwaard, F.J.; Minniti, G.; Mirimanoff, R.-O.; et al. ESTRO-ACROP guideline “target delineation of glioblastomas”. Radiother. Oncol. 2016, 118, 35–42. [Google Scholar] [CrossRef]
- Niyazi, M.; Andratschke, N.; Bendszus, M.; Chalmers, A.J.; Erridge, S.C.; Galldiks, N.; Lagerwaard, F.J.; Navarria, P.; Munck Af Rosenschöld, P.; Ricardi, U.; et al. ESTRO-EANO guideline on target delineation and radiotherapy details for glioblastoma. Radiother. Oncol. 2023, 184, 109663. [Google Scholar] [CrossRef]
- Sahm, F.; Capper, D.; Jeibmann, A.; Habel, A.; Paulus, W.; Troost, D.; von Deimling, A. Addressing diffuse glioma as a systemic brain disease with single-cell analysis. Arch. Neurol. 2012, 69, 523–526. [Google Scholar] [CrossRef]
- Kelly, P.J.; Daumas-Duport, C.; Kispert, D.B.; Kall, B.A.; Scheithauer, B.W.; Illig, J.J. Imaging-based stereotaxic serial biopsies in untreated intracranial glial neoplasms. J. Neurosurg. 1987, 66, 865–874. [Google Scholar] [CrossRef]
- Campos, B.; Olsen, L.R.; Urup, T.; Poulsen, H.S. A comprehensive profile of recurrent glioblastoma. Oncogene 2016, 35, 5819–5825. [Google Scholar] [CrossRef]
- Da Cunha, M.L.V.; Maldaun, M.V.C. Metastasis from glioblastoma multiforme: A meta-analysis. Rev. Assoc. Med. Bras. 2019, 65, 424–433. [Google Scholar] [CrossRef]
- Press, R.H.; Shafer, S.L.; Jiang, R.; Buchwald, Z.S.; Abugideiri, M.; Tian, S.; Morgan, T.M.; Behera, M.; Sengupta, S.; Voloschin, A.D.; et al. Optimal timing of chemoradiotherapy after surgical resection of glioblastoma: Stratification by validated prognostic classification. Cancer 2020, 126, 3255–3264. [Google Scholar] [CrossRef]
- Bagley, S.J.; Kothari, S.; Rahman, R.; Lee, E.Q.; Dunn, G.P.; Galanis, E.; Chang, S.M.; Nabors, L.B.; Ahluwalia, M.S.; Stupp, R.; et al. Glioblastoma Clinical Trials: Current Landscape and Opportunities for Improvement. Clin. Cancer Res. 2022, 28, 594–602. [Google Scholar] [CrossRef]
- Ramírez-Guerrero, S.; Vargas-Cuellar, M.P.; Charry-Sánchez, J.D.; Talero-Gutiérrez, C. Cognitive sequelae of radiotherapy in primary brain tumors. Interdiscip. Neurosurg. 2021, 26, 101305. [Google Scholar] [CrossRef]
- Zikou, A.; Sioka, C.; Alexiou, G.A.; Fotopoulos, A.; Voulgaris, S.; Argyropoulou, M.I. Radiation Necrosis, Pseudoprogression, Pseudoresponse, and Tumor Recurrence: Imaging Challenges for the Evaluation of Treated Gliomas. Contrast Media Mol. Imaging 2018, 2018, 6828396. [Google Scholar] [CrossRef]
- Kruser, T.J.; Bosch, W.R.; Badiyan, S.N.; Bovi, J.A.; Ghia, A.J.; Kim, M.M.; Solanki, A.A.; Sachdev, S.; Tsien, C.; Wang, T.J.C.; et al. NRG brain tumor specialists consensus guidelines for glioblastoma contouring. J. Neurooncol. 2019, 143, 157–166. [Google Scholar] [CrossRef]
- Chang, E.L.; Akyurek, S.; Avalos, T.; Rebueno, N.; Spicer, C.; Garcia, J.; Famiglietti, R.; Allen, P.K.; Chao, K.S.C.; Mahajan, A.; et al. Evaluation of peritumoral edema in the delineation of radiotherapy clinical target volumes for glioblastoma. Int. J. Radiat. Oncol. Biol. Phys. 2007, 68, 144–150. [Google Scholar] [CrossRef]
- Minniti, G.; Amelio, D.; Amichetti, M.; Salvati, M.; Muni, R.; Bozzao, A.; Lanzetta, G.; Scarpino, S.; Arcella, A.; Enrici, R.M. Patterns of failure and comparison of different target volume delineations in patients with glioblastoma treated with conformal radiotherapy plus concomitant and adjuvant temozolomide. Radiother. Oncol. 2010, 97, 377–381. [Google Scholar] [CrossRef]
- Dobelbower, M.C.; Burnett Iii, O.L.; Nordal, R.A.; Nabors, L.B.; Markert, J.M.; Hyatt, M.D.; Fiveash, J.B. Patterns of failure for glioblastoma multiforme following concurrent radiation and temozolomide. J. Med. Imaging Radiat. Oncol. 2011, 55, 77–81. [Google Scholar] [CrossRef]
- Petrecca, K.; Guiot, M.-C.; Panet-Raymond, V.; Souhami, L. Failure pattern following complete resection plus radiotherapy and temozolomide is at the resection margin in patients with glioblastoma. J. Neurooncol. 2013, 111, 19–23. [Google Scholar] [CrossRef]
- Zhao, F.; Li, M.; Kong, L.; Zhang, G.; Yu, J. Delineation of radiation therapy target volumes for patients with postoperative glioblastoma: A review. Onco. Targets Ther. 2016, 9, 3197–3204. [Google Scholar] [CrossRef]
- Chan, J.L.; Lee, S.W.; Fraass, B.A.; Normolle, D.P.; Greenberg, H.S.; Junck, L.R.; Gebarski, S.S.; Sandler, H.M. Survival and failure patterns of high-grade gliomas after three-dimensional conformal radiotherapy. J. Clin. Oncol. 2002, 20, 1635–1642. [Google Scholar] [CrossRef]
- Minniti, G.; Tini, P.; Giraffa, M.; Capone, L.; Raza, G.; Russo, I.; Cinelli, E.; Gentile, P.; Bozzao, A.; Paolini, S.; et al. Feasibility of clinical target volume reduction for glioblastoma treated with standard chemoradiation based on patterns of failure analysis. Radiother. Oncol. 2023, 181, 109435. [Google Scholar] [CrossRef]
- Manon, R.; Hui, S.; Chinnaiyan, P.; Suh, J.; Chang, E.; Timmerman, R.; Phan, S.; Das, R.; Mehta, M. The impact of mid-treatment MRI on defining boost volumes in the radiation treatment of glioblastoma multiforme. Technol. Cancer Res. Treat. 2004, 3, 303–307. [Google Scholar] [CrossRef]
- Wach, J.; Hamed, M.; Schuss, P.; Güresir, E.; Herrlinger, U.; Vatter, H.; Schneider, M. Impact of initial midline shift in glioblastoma on survival. Neurosurg. Rev. 2021, 44, 1401–1409. [Google Scholar] [CrossRef]
- Shukla, D.; Huilgol, N.G.; Trivedi, N.; Mekala, C. T2 weighted MRI in assessment of volume changes during radiotherapy of high grade gliomas. J. Cancer Res. Ther. 2005, 1, 235–238. [Google Scholar] [CrossRef]
- Tsien, C.; Gomez-Hassan, D.; Ten Haken, R.K.; Tatro, D.; Junck, L.; Chenevert, T.L.; Lawrence, T. Evaluating changes in tumor volume using magnetic resonance imaging during the course of radiotherapy treatment of high-grade gliomas: Implications for conformal dose-escalation studies. Int. J. Radiat. Oncol. Biol. Phys. 2005, 62, 328–332. [Google Scholar] [CrossRef]
- Champ, C.E.; Siglin, J.; Mishra, M.V.; Shen, X.; Werner-Wasik, M.; Andrews, D.W.; Mayekar, S.U.; Liu, H.; Shi, W. Evaluating changes in radiation treatment volumes from post-operative to same-day planning MRI in High-grade gliomas. Radiat. Oncol. 2012, 7, 220. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, Z.; Wang, X.; Hu, Y.; Lyu, Z.; Huo, L.; Wei, R.; Fu, J.; Hong, J. Intensity-modulated radiotherapy for gliomas: Dosimetric effects of changes in gross tumor volume on organs at risk and healthy brain tissue. Onco. Targets Ther. 2016, 9, 3545–3554. [Google Scholar] [CrossRef] [PubMed]
- Şenkesen, Ö.; Tezcanlı, E.; Abacıoğlu, M.U.; Özen, Z.; Çöne, D.; Küçücük, H.; Göksel, E.O.; Arifoğlu, A.; Şengöz, M. Limited field adaptive radiotherapy for glioblastoma: Changes in target volume and organ at risk doses. Radiat. Oncol. J. 2022, 40, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Mehta, S.; Gajjar, S.R.; Padgett, K.R.; Asher, D.; Stoyanova, R.; Ford, J.C.; Mellon, E.A. Daily Tracking of Glioblastoma Resection Cavity, Cerebral Edema, and Tumor Volume with MRI-Guided Radiation Therapy. Cureus 2018, 10, e2346. [Google Scholar] [CrossRef] [PubMed]
- Dajani, S.; Hill, V.B.; Kalapurakal, J.A.; Horbinski, C.M.; Nesbit, E.G.; Sachdev, S.; Yalamanchili, A.; Thomas, T.O. Imaging of GBM in the Age of Molecular Markers and MRI Guided Adaptive Radiation Therapy. J. Clin. Med. 2022, 11, 5961. [Google Scholar] [CrossRef] [PubMed]
- Matsuyama, T.; Fukugawa, Y.; Kuroda, J.; Toya, R.; Watakabe, T.; Matsumoto, T.; Oya, N. A prospective comparison of adaptive and fixed boost plans in radiotherapy for glioblastoma. Radiat. Oncol. 2022, 17, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Stadlbauer, A.; Buchfelder, M.; Doelken, M.T.; Hammen, T.; Ganslandt, O. Magnetic resonance spectroscopic imaging for visualization of the infiltration zone of glioma. Cent. Eur. Neurosurg. 2011, 72, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Kis, D.; Szivos, L.; Rekecki, M.; Shukir, B.S.; Mate, A.; Hideghety, K.; Barzo, P. Predicting the true extent of glioblastoma based on probabilistic tractography. Front. Neurosci. 2022, 16, 886465. [Google Scholar] [CrossRef]
- Chambrelant, I.; Eber, J.; Antoni, D.; Burckel, H.; Noël, G.; Auvergne, R. Proton Therapy and Gliomas: A Systematic Review. Radiation 2021, 1, 218–233. [Google Scholar] [CrossRef]

| n (%) | |
|---|---|
| median age (range) in years | 61 (26–78) |
| sex | |
| male | 18 (64) |
| female | 10 (36) |
| diagnosis | |
| glioblastoma | 26 (93) |
| oligodendroglioma | 2 (7) |
| tumour location | |
| frontal lobe | 13 (46) |
| parietal lobe | 7 (25) |
| temporal lobe | 7 (25) |
| occipital lobe | 1 (4) |
| interval MRI 1–2 (range) in days | 19.5 (8–50) |
| Total | Skull-Near (≤25 mm) | Skull-Distant (>25 mm) | |
|---|---|---|---|
| number of measurements (n) | 168 | 64 | 104 |
| mean difference ± SD (mm) | 3.04 ± 3.90 | 0.50 ± 1.40 | 4.61 ± 4.12 |
| minimum (mm) | 0 | 0 | 0 |
| maximum (mm) | 22.1 | 9.6 | 22.1 |
| Reference | Dose | GTV | CTV | PTV |
|---|---|---|---|---|
| ESTRO-ACROP [8] | 60 Gy | T1 + cavity | +2 cm | +3–5 mm |
| RTOG [18] | 46 Gy 16 Gy | GTV1 = T1 + cavity + T2 GTV2 = T1 + cavity | +2 cm | +3–5 mm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dejonckheere, C.S.; Thelen, A.; Simon, B.; Greschus, S.; Köksal, M.A.; Schmeel, L.C.; Wilhelm-Buchstab, T.; Leitzen, C. Impact of Postoperative Changes in Brain Anatomy on Target Volume Delineation for High-Grade Glioma. Cancers 2023, 15, 2840. https://doi.org/10.3390/cancers15102840
Dejonckheere CS, Thelen A, Simon B, Greschus S, Köksal MA, Schmeel LC, Wilhelm-Buchstab T, Leitzen C. Impact of Postoperative Changes in Brain Anatomy on Target Volume Delineation for High-Grade Glioma. Cancers. 2023; 15(10):2840. https://doi.org/10.3390/cancers15102840
Chicago/Turabian StyleDejonckheere, Cas Stefaan, Anja Thelen, Birgit Simon, Susanne Greschus, Mümtaz Ali Köksal, Leonard Christopher Schmeel, Timo Wilhelm-Buchstab, and Christina Leitzen. 2023. "Impact of Postoperative Changes in Brain Anatomy on Target Volume Delineation for High-Grade Glioma" Cancers 15, no. 10: 2840. https://doi.org/10.3390/cancers15102840
APA StyleDejonckheere, C. S., Thelen, A., Simon, B., Greschus, S., Köksal, M. A., Schmeel, L. C., Wilhelm-Buchstab, T., & Leitzen, C. (2023). Impact of Postoperative Changes in Brain Anatomy on Target Volume Delineation for High-Grade Glioma. Cancers, 15(10), 2840. https://doi.org/10.3390/cancers15102840





