Perifocal Zone of Brain Gliomas: Application of Diffusion Kurtosis and Perfusion MRI Values for Tumor Invasion Border Determination
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Enrollment Criteria
2.2. Magnetic Resonance Imaging
2.3. Image Processing
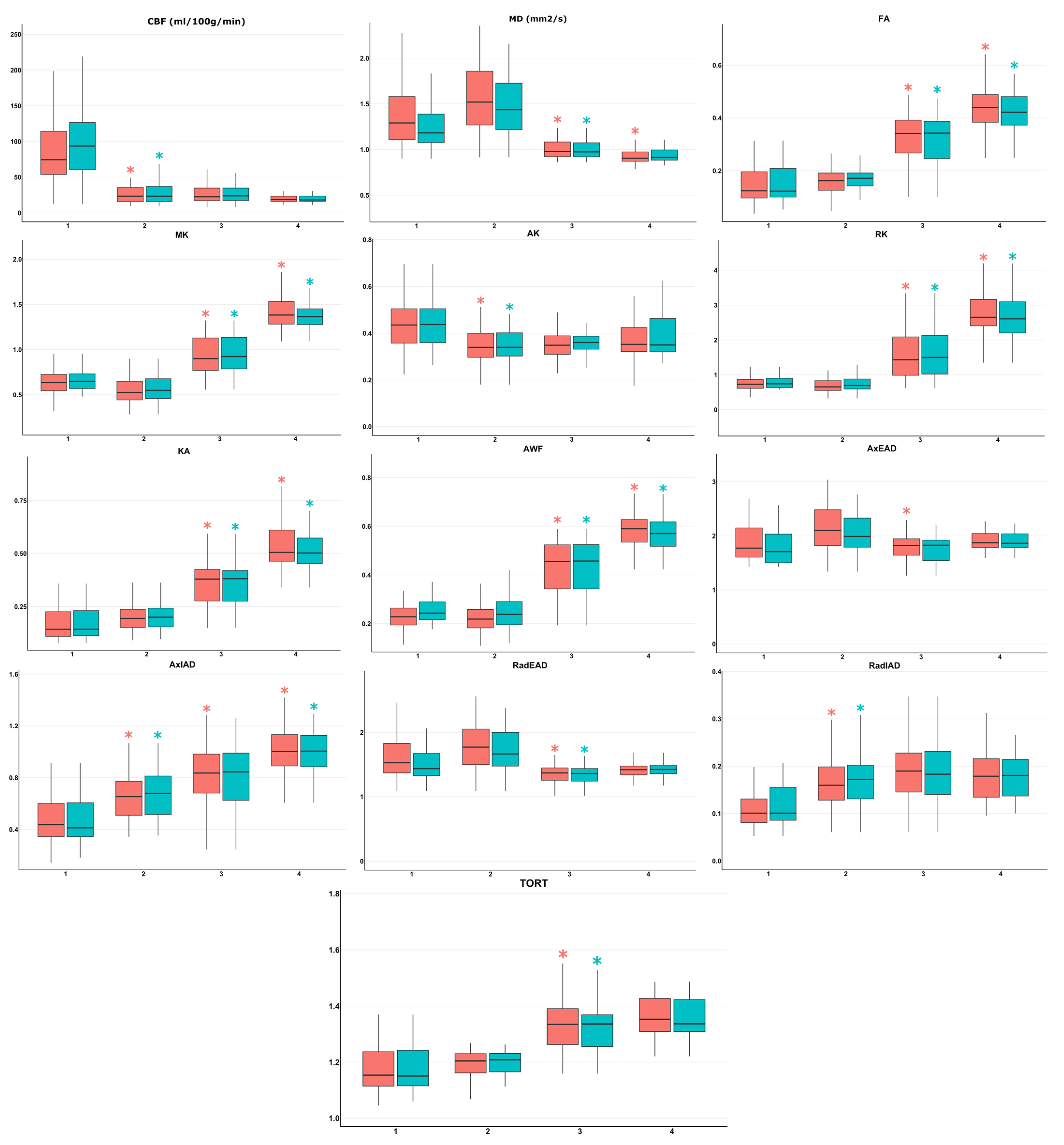
- ROI1—the highest perfusion focus in enhancing tumor part according to the fusion of perfusion maps and post-contrast T1 FSPGR. As CBF measured by ASL-perfusion has a correlation with tumor grade and the Ki-67 labeling index (LI), this site was regarded as the most malignant [45];
- ROI2—perifocal infiltrative edema zone according to the fusion of T2-FLAIR CUBE and post-contrast T1 FSPGR (T2-FLAIR CUBE hyperintense signal without signs of pathological contrast enhancement);
- ROI3—normal-appearing (intact on conventional MRI) white matter along surgical approach according to T2-FLAIR CUBE images (no pathological changes of MR signal);
- ROI4—contralateral (intact on conventional MRI) unaffected hemisphere normal-appearing white matter (centrum semiovale).
2.4. Surgery and Biopsy Sampling
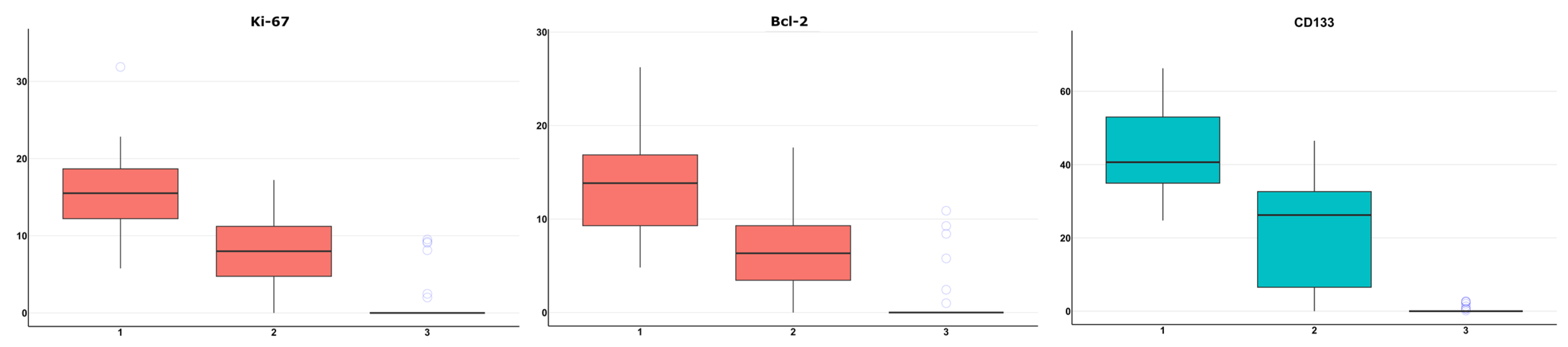
2.5. Morphological, Immunohistochemical, and Molecular Genetic Studies of Gliomas
2.6. Statistical Analysis
- ROI1 (enhancing tumor core with highest CBF) and ROI2 (perifocal infiltrative edema zone);
- ROI2 (perifocal infiltrative edema zone) and ROI3 (normal-appearing peritumoral white matter along surgical approach);
- ROI3 (normal-appearing peritumoral white matter along surgical approach) and ROI4 (contralateral unaffected hemisphere normal-appearing white matter).
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Claes, A.; Idema, A.J.; Wesseling, P. Diffuse glioma growth: A guerilla war. Acta Neuropathol. 2007, 114, 443–458. [Google Scholar] [CrossRef]
- Eisele, S.C.; Wen, P.Y.; Lee, E.Q. Assessment of Brain Tumor Response: RANO and Its Offspring. Curr. Treat. Options Oncol. 2016, 17, 35. [Google Scholar] [CrossRef] [PubMed]
- Blystad, I.; Warntjes, J.B.M.; Smedby, Ö.; Lundberg, P.; Larsson, E.-M.; Tisell, A. Quantitative MRI for analysis of peritumoral edema in malignant gliomas. PLoS ONE 2017, 12, e0177135. [Google Scholar] [CrossRef]
- Yamahara, T.; Numa, Y.; Oishi, T.; Kawaguchi, T.; Seno, T.; Asai, A.; Kawamoto, K. Morphological and flow cytometric analysis of cell infiltration in glioblastoma: A comparison of autopsy brain and neuroimaging. Brain Tumor Pathol. 2010, 27, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Sternberg, E.; Lipton, M.; Burns, J. Utility of Diffusion Tensor Imaging in Evaluation of the Peritumoral Region in Patients with Primary and Metastatic Brain Tumors. Am. J. Neuroradiol. 2013, 35, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Sanai, N.; Polley, M.Y.; McDermott, M.W.; Parsa, A.T.; Berger, M.S. An extent of resection threshold for newly diagnosed glioblastomas. J. Neurosurg. 2011, 115, 3–8. [Google Scholar] [CrossRef]
- Senft, C.; Bink, A.; Franz, K.; Vatter, H.; Gasser, T.; Seifert, V. Intraoperative MRI guidance and extent of resection in glioma surgery: A randomised, controlled trial. Lancet Oncol. 2011, 12, 997–1003. [Google Scholar] [CrossRef]
- Lemercier, P.; Maya, S.P.; Patrie, J.T.; Flors, L.; Leiva-Salinas, C. Gradient of Apparent Diffusion Coefficient Values in Peritumoral Edema Helps in Differentiation of Glioblastoma From Solitary Metastatic Lesions. Am. J. Roentgenol. 2014, 203, 163–169. [Google Scholar] [CrossRef]
- Batalov, A.I.; Zakharova, N.E.; Pogosbekyan, E.L.; Fadeeva, L.M.; Goryaynov, S.A.; Baev, A.A.; Shul’Ts, E.I.; Chelushkin, D.M.; Potapov, A.A.; Pronin, I.N. Non-contrast ASL perfusion in preoperative diagnosis of supratentorial gliomas. Vopr. neirokhirurgii Im. N.N. Burdenko 2018, 82, 15–22. [Google Scholar] [CrossRef]
- Batalov, A.I.; Zakharova, N.E.; Pronin, I.N.; Belyaev, A.Y.; Pogosbekyan, E.L.; Goryaynov, S.A.; Bykanov, A.E.; Tyurina, A.N.; Shevchenko, A.M.; Solozhentseva, K.D.; et al. 3D pCASL-perfusion in preoperative assessment of brain gliomas in large cohort of patients. Sci. Rep. 2022, 12, 1–7. [Google Scholar] [CrossRef]
- Jain, R.; Poisson, L.M.; Gutman, D.; Scarpace, L.; Hwang, S.N.; Holder, C.A.; Wintermark, M.; Rao, A.; Colen, R.R.; Kirby, J.; et al. Outcome Prediction in Patients with Glioblastoma by Using Imaging, Clinical, and Genomic Biomarkers: Focus on the Nonenhancing Component of the Tumor. Radiology 2014, 272, 484–493. [Google Scholar] [CrossRef] [PubMed]
- Blasel, S.; Jurcoane, A.; Franz, K.; Morawe, G.; Pellikan, S.; Hattingen, E. Elevated peritumoural rCBV values as a mean to differentiate metastases from high-grade gliomas. Acta Neurochir. 2010, 152, 1893–1899. [Google Scholar] [CrossRef] [PubMed]
- Batalov, A.I.; Afandiev, R.M.; Zakharova, N.E.; Pogosbekyan, E.L.; Shulgina, A.A.; Kobyakov, G.L.; Potapov, A.A.; Pronin, I.N. 3D pseudo-continuous arterial spin labeling-MRI (3D PCASL-MRI) in the differential diagnosis between glioblastomas and primary central nervous system lymphomas. Neuroradiology 2022, 64, 1539–1545. [Google Scholar] [CrossRef] [PubMed]
- Solozhentseva, K.; Batalov, A.; Zakharova, N.; Goryaynov, S.; Pogosbekyan, E.; Pronin, I. The Role of 3D-pCASL MRI in the Differential Diagnosis of Glioblastoma and Brain Metastases. Front. Oncol. 2022, 12, 874924. [Google Scholar] [CrossRef] [PubMed]
- Provenzale, J.M.; McGraw, P.; Mhatre, P.; Guo, A.C.; Delong, D. Peritumoral Brain Regions in Gliomas and Meningiomas: Investigation with Isotropic Diffusion-weighted MR Imaging and Diffusion-Tensor MR Imaging1. Radiology 2004, 232, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Sundgren, P.C.; Fan, X.; Weybright, P.; Welsh, R.C.; Carlos, R.C.; Petrou, M.; McKeever, P.E.; Chenevert, T.L. Differentiation of recurrent brain tumor versus radiation injury using diffusion tensor imaging in patients with new contrast-enhancing lesions. Magn. Reson. Imaging 2006, 24, 1131–1142. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Yan, Y.; Zhong, D.; Yang, G.; Tang, W.; Lü, F.; Xie, B.; Liu, B. Quantitative analysis of glioma cell invasion by diffusion tensor imaging. J. Clin. Neurosci. 2010, 17, 1530–1536. [Google Scholar] [CrossRef] [PubMed]
- van Westen, D.; Lätt, J.; Englund, E.; Brockstedt, S.; Larsson, E.-M. Tumor extension in high-grade gliomas assessed with diffusion magnetic resonance imaging: Values and lesion-to-brain ratios of apparent diffusion coefficient and fractional anisotropy. Acta Radiol. 2006, 47, 311–319. [Google Scholar] [CrossRef]
- Kinoshita, M.; Hashimoto, N.; Goto, T.; Yanagisawa, T.; Okita, Y.; Kagawa, N.; Kishima, H.; Tanaka, H.; Fujita, N.; Shimosegawa, E.; et al. Use of fractional anisotropy for determination of the cut-off value in 11C-methionine positron emission tomography for glioma. Neuroimage 2009, 45, 312–318. [Google Scholar] [CrossRef]
- Jensen, J.H.; Helpern, J.A.; Ramani, A.; Lu, H.; Kaczynski, K. Diffusional kurtosis imaging: The quantification of non-gaussian water diffusion by means of magnetic resonance imaging. Magn. Reson. Med. 2005, 53, 1432–1440. [Google Scholar] [CrossRef]
- Jensen, J.H.; Helpern, J.A. MRI quantification of non-Gaussian water diffusion by kurtosis analysis. NMR Biomed. 2010, 23, 698–710. [Google Scholar] [CrossRef] [PubMed]
- Fieremans, E.; Jensen, J.H.; Helpern, J.A. White matter characterization with diffusional kurtosis imaging. Neuroimage 2011, 58, 177–188. [Google Scholar] [CrossRef]
- Steven, A.J.; Zhuo, J.; Melhem, E.R. Diffusion Kurtosis Imaging: An Emerging Technique for Evaluating the Microstructural Environment of the Brain. Am. J. Roentgenol. 2014, 202, W26–W33. [Google Scholar] [CrossRef] [PubMed]
- Grossman, E.J.; Kirov, I.I.; Gonen, O.; Novikov, D.S.; Davitz, M.S.; Lui, Y.W.; Grossman, R.I.; Inglese, M.; Fieremans, E. N -acetyl-aspartate levels correlate with intra-axonal compartment parameters from diffusion MRI. Neuroimage 2015, 118, 334–343. [Google Scholar] [CrossRef]
- Van Cauter, S.; Veraart, J.; Sijbers, J.; Peeters, R.R.; Himmelreich, U.; De Keyzer, F.; Van Gool, S.W.; Van Calenbergh, F.; De Vleeschouwer, S.; Van Hecke, W.; et al. Gliomas: Diffusion Kurtosis MR Imaging in Grading. Radiology 2012, 263, 492–501. [Google Scholar] [CrossRef] [PubMed]
- Van Cauter, S.; De Keyzer, F.; Sima, D.M.; Sava, A.C.; D’Arco, F.; Veraart, J.; Peeters, R.R.; Leemans, A.; Van Gool, S.; Wilms, G.; et al. Integrating diffusion kurtosis imaging, dynamic susceptibility-weighted contrast-enhanced MRI, and short echo time chemical shift imaging for grading gliomas. Neuro-Oncology 2014, 16, 1010–1021. [Google Scholar] [CrossRef]
- Maximov, I.I.; Tonoyan, A.S.; Pronin, I.N. Differentiation of glioma malignancy grade using diffusion MRI. Phys. Medica 2017, 40, 24–32. [Google Scholar] [CrossRef]
- Hempel, J.-M.; Bisdas, S.; Schittenhelm, J.; Brendle, C.; Bender, B.; Wassmann, H.; Skardelly, M.; Tabatabai, G.; Vega, S.C.; Ernemann, U.; et al. In vivo molecular profiling of human glioma using diffusion kurtosis imaging. J. Neuro-Oncology 2016, 131, 93–101. [Google Scholar] [CrossRef]
- Hempel, J.-M.; Schittenhelm, J.; Brendle, C.; Bender, B.; Bier, G.; Skardelly, M.; Tabatabai, G.; Vega, S.C.; Ernemann, U.; Klose, U. Effect of Perfusion on Diffusion Kurtosis Imaging Estimates for In Vivo Assessment of Integrated 2016 WHO Glioma Grades. Clin. Neuroradiol. 2017, 28, 481–491. [Google Scholar] [CrossRef]
- Hempel, J.-M.; Schittenhelm, J.; Bisdas, S.; Brendle, C.; Bender, B.; Bier, G.; Skardelly, M.; Tabatabai, G.; Vega, S.C.; Ernemann, U.; et al. In vivo assessment of tumor heterogeneity in WHO 2016 glioma grades using diffusion kurtosis imaging: Diagnostic performance and improvement of feasibility in routine clinical practice. J. Neuroradiol. 2018, 45, 32–40. [Google Scholar] [CrossRef]
- Abdalla, G.; Sanverdi, E.; Machado, P.; Kwong, J.S.W.; Panovska-Griffiths, J.; Rojas-Garcia, A.; Yoneoka, D.; Yousry, T.; Bisdas, S. Role of diffusional kurtosis imaging in grading of brain gliomas: A protocol for systematic review and meta-analysis. BMJ Open 2018, 8, e025123. [Google Scholar] [CrossRef] [PubMed]
- Abdalla, G.; Dixon, L.; Sanverdi, E.; Machado, P.; Kwong, J.S.W.; Panovska-Griffiths, J.; Rojas-Garcia, A.; Yoneoka, D.; Veraart, J.; Van Cauter, S.; et al. The diagnostic role of diffusional kurtosis imaging in glioma grading and differentiation of gliomas from other intra-axial brain tumours: A systematic review with critical appraisal and meta-analysis. Neuroradiology 2020, 62, 791–802. [Google Scholar] [CrossRef] [PubMed]
- Martín-Noguerol, T.; Mohan, S.; Santos-Armentia, E.; Cabrera-Zubizarreta, A.; Luna, A. Advanced MRI assessment of non-enhancing peritumoral signal abnormality in brain lesions. Eur. J. Radiol. 2021, 143, 109900. [Google Scholar] [CrossRef]
- Jiang, R.; Jiang, J.; Zhao, L.; Zhang, J.; Zhang, S.; Yao, Y.; Yang, S.; Shi, J.; Shen, N.; Su, C.; et al. Diffusion kurtosis imaging can efficiently assess the glioma grade and cellular proliferation. Oncotarget 2015, 6, 42380–42393. [Google Scholar] [CrossRef] [PubMed]
- Delgado, A.F.; Fahlström, M.; Nilsson, M.; Berntsson, S.G.; Zetterling, M.; Libard, S.; Alafuzoff, I.; van Westen, D.; Lätt, J.; Smits, A.; et al. Diffusion kurtosis imaging of gliomas grades II and III—A study of perilesional tumor infiltration, tumor grades and subtypes at clinical presentation. Radiol. Oncol. 2017, 51, 121–129. [Google Scholar] [CrossRef]
- Tan, Y.; Wang, X.-C.; Zhang, H.; Wang, J.; Qin, J.-B.; Wu, X.-F.; Zhang, L.; Wang, L. Differentiation of high-grade-astrocytomas from solitary-brain-metastases: Comparing diffusion kurtosis imaging and diffusion tensor imaging. Eur. J. Radiol. 2015, 84, 2618–2624. [Google Scholar] [CrossRef]
- Akbari, H.; Macyszyn, L.; Da, X.; Bilello, M.; Wolf, R.L.; Martinez-Lage, M.; Biros, G.; Alonso-Basanta, M.; O’Rourke, D.M.; Davatzikos, C. Imaging Surrogates of Infiltration Obtained Via Multiparametric Imaging Pattern Analysis Predict Subsequent Location of Recurrence of Glioblastoma. Neurosurgery 2016, 78, 572–580. [Google Scholar] [CrossRef]
- Turkin, A.M.; Pogosbekyan, E.L.; Tonoyan, A.C.; Shults, E.I.; Maximov, I.I.; Dolgushin, M.B.; Khachanova, N.V.; Fadeeva, L.M.; Melnikova-Pitskhelauri, T.V.; Pitskhelauri, D.I.; et al. Diffusion Kurtosis Imaging in the Assessment of Peritumoral Brain Edema in Glioblastomas and Brain Metastases. Med. Vis. 2017, 4, 97–112. [Google Scholar] [CrossRef]
- Kim, J.Y.; Yoon, M.J.; Park, J.E.; Choi, E.J.; Lee, J.; Kim, H.S. Radiomics in peritumoral non-enhancing regions: Fractional anisotropy and cerebral blood volume improve prediction of local progression and overall survival in patients with glioblastoma. Neuroradiology 2019, 61, 1261–1272. [Google Scholar] [CrossRef]
- Guo, J.; Yao, C.; Chen, H.; Zhuang, D.; Tang, W.; Ren, G.; Wang, Y.; Wu, J.; Huang, F.; Zhou, L. The relationship between Cho/NAA and glioma metabolism: Implementation for margin delineation of cerebral gliomas. Acta Neurochir. 2012, 154, 1361–1370. [Google Scholar] [CrossRef]
- Zetterling, M.; Roodakker, K.R.; Berntsson, S.G.; Edqvist, P.-H.; Latini, F.; Landtblom, A.-M.; Pontén, F.; Alafuzoff, I.; Larsson, E.-M.; Smits, A. Extension of diffuse low-grade gliomas beyond radiological borders as shown by the coregistration of histopathological and magnetic resonance imaging data. J. Neurosurg. 2016, 125, 1155–1166. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Reifenberger, G.; Von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro-Oncology 2021, 23, 1231–1251. [Google Scholar] [CrossRef]
- Veraart, J.; Rajan, J.; Peeters, R.R.; Leemans, A.; Sunaert, S.; Sijbers, J. Comprehensive framework for accurate diffusion MRI parameter estimation. Magn. Reson. Med. 2012, 70, 972–984. [Google Scholar] [CrossRef]
- Batalov, A.I.; Zakharova, N.E.; Chekhonin, I.V.; Pogosbekyan, E.L.; Sudarikova, A.V.; Goryainov, S.A.; Shulgina, A.A.; Belyaev, A.Y.; Usachev, D.Y.; Pronin, I.N. Arterial Spin Labeling Perfusion in Determining the IDH1 Status and Ki-67 Index in Brain Gliomas. Diagnostics 2022, 12, 1444. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Deng, K.X.; Wang, P.; Chen, C.Y.; Luo, Y.; Yuan, S.Y.; Wen, J. Application of diffusion kurtosis imaging to the study of edema in solid and peritumoral areas of glioma. Magn. Reson. Imaging 2021, 86, 10–16. [Google Scholar] [CrossRef]
- Chekhonin, I.; Batalov, A.; Zakharova, N.; Pogosbekyan, E.; Nikitin, P.; Bykanov, A.; Pitskhelauri, D.; Pronin, I. Magnetic resonance relaxometry in high-grade glioma subregion assessment—Neuroimaging and morphological correlates. Vopr. neirokhirurgii Im. N.N. Burdenko 2021, 85, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Holly, K.; Barker, B.J.; Murcia, D.; Bennett, R.; Kalakoti, P.; Ledbetter, C.; Gonzalez-Toledo, E.; Nanda, A.; Sun, H. High-grade Gliomas Exhibit Higher Peritumoral Fractional Anisotropy and Lower Mean Diffusivity than Intracranial Metastases. Front. Surg. 2017, 4, 18. [Google Scholar] [CrossRef]
- Glenn, G.R.; Helpern, J.A.; Tabesh, A.; Jensen, J.H. Quantitative assessment of diffusional kurtosis anisotropy. NMR Biomed. 2015, 28, 448–459. [Google Scholar] [CrossRef]
- Hansen, B.; Jespersen, S.N. Kurtosis fractional anisotropy, its contrast and estimation by proxy. Sci. Rep. 2016, 6, 23999. [Google Scholar] [CrossRef]
- Raab, P.; Hattingen, E.; Franz, K.; Zanella, F.E.; Lanfermann, H. Cerebral Gliomas: Diffusional Kurtosis Imaging Analysis of Microstructural Differences. Radiology 2010, 254, 876–881. [Google Scholar] [CrossRef] [PubMed]
- Pogosbekian, E.L.; Pronin, I.N.; Zakharova, N.E.; Batalov, A.I.; Turkin, A.M.; Konakova, T.A.; Maximov, I.I. Feasibility of generalised diffusion kurtosis imaging approach for brain glioma grading. Neuroradiology 2021, 63, 1241–1251. [Google Scholar] [CrossRef]
- Price, S.; Jena, R.; Burnet, N.; Hutchinson, P.; Dean, A.; Peña, A.; Pickard, J.; Carpenter, T.; Gillard, J. Improved Delineation of Glioma Margins and Regions of Infiltration with the Use of Diffusion Tensor Imaging: An Image-Guided Biopsy Study. Am. J. Neuroradiol. 2006, 27, 1969–1974. [Google Scholar]
- Pallud, J.; Varlet, P.; Devaux, B.; Geha, S.; Badoual, M.; Deroulers, C.; Page, P.; Dezamis, E.; Daumas-Duport, C.; Roux, F.X. Diffuse low-grade oligodendrogliomas extend beyond MRI-defined abnormalities. Neurology 2010, 74, 1724–1731. [Google Scholar] [CrossRef]
- Capper, D.; Sahm, F.; Jeibmann, A.; Habel, A.; Paulus, W.; Troost, D.; Von Deimling, A. Addressing Diffuse Glioma as a Systemic Brain Disease With Single-Cell Analysis. Arch. Neurol. 2012, 69, 523–526. [Google Scholar] [CrossRef]
- Singh, S.K.; Hawkins, C.; Clarke, I.D.; Squire, J.A.; Bayani, J.; Hide, T.; Henkelman, R.M.; Cusimano, M.D.; Dirks, P.B. Identification of human brain tumour initiating cells. Nature 2004, 432, 396–401. [Google Scholar] [CrossRef]
- Panza, S.; Russo, U.; Giordano, F.; Leggio, A.; Barone, I.; Bonofiglio, D.; Gelsomino, L.; Malivindi, R.; Conforti, F.L.; Naimo, G.D.; et al. Leptin and Notch Signaling Cooperate in Sustaining Glioblastoma Multiforme Progression. Biomolecules 2020, 10, 886. [Google Scholar] [CrossRef] [PubMed]
- Pirzkall, A.; Li, X.; Oh, J.; Chang, S.; Berger, M.S.; A Larson, D.; Verhey, L.J.; Dillon, W.P.; Nelson, S.J. 3D MRSI for resected high-grade gliomas before RT: Tumor extent according to metabolic activity in relation to MRI. Int. J. Radiat. Oncol. 2004, 59, 126–137. [Google Scholar] [CrossRef] [PubMed]
- Schlüter, M.; Stieltjes, B.; Hahn, H.K.; Rexilius, J.; Konrad-Verse, O.; Peitgen, H.-O. Detection of tumour infiltration in axonal fibre bundles using diffusion tensor imaging. Int. J. Med. Robot. Comput. Assist. Surg. 2005, 1, 80–86. [Google Scholar] [CrossRef] [PubMed]

| Diagnosis, Grade WHO 2016 | Diagnosis, Grade WHO 2021 | MGMT Methylation | IDH1 Mutation | IDH2 Mutation | Number of Patients |
|---|---|---|---|---|---|
| Anaplastic astrocytoma III | Astrocytoma 3 | + | + | − | 4 |
| Anaplastic astrocytoma III | Astrocytoma 3 | − | + | − | 1 |
| Anaplastic astrocytoma III | Glioblastoma 4 | − | − | − | 1 |
| Anaplastic oligodendroglioma III | Oligodendroglioma, 3 | + | + | − | 4 |
| Anaplastic oligodendroglioma III | Oligodendroglioma, 3 | − | + | − | 3 |
| Glioblastoma IV | Astrocytoma 4 | − | + | − | 1 |
| Glioblastoma IV | Glioblastoma 4 | + | − | − | 10 |
| Glioblastoma IV | Glioblastoma 4 | − | − | − | 26 |
| Total | 50 |
| Criterion | Feature |
|---|---|
| Enhancement pattern | None: 1 patient—anaplastic astrocytoma |
Multinodular: 4 patients:
| |
Diffuse: 6 patients:
| |
Ring-like: 40 patients:
| |
| Necrosis | Of 50 patients, present in 42, absent in 8 |
| Hemorrhage | Of 50 patients, present in 44, absent in 6 |
| ROI1 vs. ROI2 (Combined) | ROI1 vs. ROI2 (Glioblastoma) | |||||||
|---|---|---|---|---|---|---|---|---|
| AUC | Cutoff | Specificity | Sensitivity | AUC | Cutoff | Specificity | Sensitivity | |
| CBF | 0.89 | 50.13 | 0.81 | 0.93 | 0.92 | 50.9 | 0.9 | 0.9 |
| RadIAD | 0.78 | 0.11 | 0.66 | 0.89 | 0.77 | 0.5 | 0.69 | 0.79 |
| AxIAD | 0.76 | 0.51 | 0.71 | 0.76 | 0.77 | 0.11 | 0.66 | 0.9 |
| AK | 0.74 | 0.41 | 0.63 | 0.76 | 0.77 | 0.41 | 0.66 | 0.76 |
| AxEAD | 0.69 | 1.92 | 0.63 | 0.66 | 0.71 | 1.78 | 0.66 | 0.76 |
| MK | 0.67 | 0.57 | 0.71 | 0.63 | 0.67 | 0.57 | 0.76 | 0.59 |
| KA | 0.66 | 0.15 | 0.55 | 0.82 | 0.67 | 1.3 | 0.66 | 0.66 |
| MD | 0.65 | 1.4 | 0.63 | 0.63 | 0.66 | 0.15 | 0.55 | 0.86 |
| RadEAD | 0.64 | 1.58 | 0.63 | 0.66 | 0.65 | 1.48 | 0.59 | 0.76 |
| TORT | 0.63 | 1.16 | 0.58 | 0.74 | 0.62 | 1.16 | 0.59 | 0.76 |
| FA | 0.61 | 0.13 | 0.55 | 0.74 | 0.61 | 0.14 | 0.55 | 0.76 |
| RK | 0.6 | 0.73 | 0.53 | 0.71 | 0.59 | 0.72 | 0.62 | 0.62 |
| AWF | 0.56 | 0.22 | 0.55 | 0.55 | 0.56 | 0.24 | 0.55 | 0.55 |
| ROI2 vs. ROI3 (Combined) | ROI2 vs. ROI3 (Glioblastoma) | |||||||
| AUC | Cutoff | Specificity | Sensitivity | AUC | Cutoff | Specificity | Sensitivity | |
| AWF | 0.93 | 0.31 | 0.92 | 0.82 | 0.91 | 0.31 | 0.9 | 0.8 |
| MK | 0.93 | 0.72 | 0.92 | 0.79 | 0.9 | 0.77 | 0.93 | 0.77 |
| RK | 0.92 | 0.9 | 0.84 | 0.82 | 0.9 | 1 | 0.86 | 0.77 |
| FA | 0.9 | 0.21 | 0.87 | 0.85 | 0.88 | 0.21 | 0.83 | 0.87 |
| MD | 0.9 | 1.2 | 0.82 | 0.87 | 0.88 | 1.11 | 0.86 | 0.83 |
| KA | 0.87 | 0.25 | 0.82 | 0.82 | 0.84 | 0.25 | 0.76 | 0.83 |
| TORT | 0.84 | 1.25 | 0.87 | 0.79 | 0.81 | 1.27 | 0.97 | 0.73 |
| RadEAD | 0.83 | 1.49 | 0.79 | 0.87 | 0.8 | 1.47 | 0.76 | 0.83 |
| AxEAD | 0.73 | 1.96 | 0.63 | 0.77 | 0.69 | 1.96 | 0.55 | 0.8 |
| AxIAD | 0.72 | 0.72 | 0.68 | 0.72 | 0.67 | 0.82 | 0.76 | 0.57 |
| RadIAD | 0.6 | 0.19 | 0.71 | 0.51 | 0.55 | 0.19 | 0.66 | 0.5 |
| AK | 0.52 | 0.35 | 0.58 | 0.54 | 0.53 | 0.34 | 0.52 | 0.67 |
| CBF | 0.5 | 23.92 | 0.5 | 0.56 | 0.51 | 17.65 | 0.4 | 0.72 |
| ROI3 vs. ROI4 (Combined) | ROI3 vs. ROI4 (Glioblastoma) | |||||||
| AUC | Cutoff | Specificity | Sensitivity | AUC | Cutoff | Specificity | Sensitivity | |
| MK | 0.92 | 1.21 | 0.9 | 0.82 | 0.89 | 1.25 | 0.9 | 0.77 |
| RK | 0.91 | 2.39 | 0.92 | 0.77 | 0.87 | 2.08 | 0.73 | 0.87 |
| AWF | 0.87 | 0.53 | 0.79 | 0.77 | 0.87 | 0.43 | 0.8 | 0.83 |
| KA | 0.87 | 0.44 | 0.79 | 0.85 | 0.83 | 0.53 | 0.77 | 0.7 |
| FA | 0.81 | 0.4 | 0.79 | 0.72 | 0.79 | 0.4 | 0.83 | 0.67 |
| MD | 0.75 | 0.94 | 0.69 | 0.69 | 0.75 | 0.93 | 0.7 | 0.67 |
| AxIAD | 0.74 | 0.93 | 0.69 | 0.69 | 0.68 | 0.91 | 0.87 | 0.5 |
| AxEAD | 0.64 | 1.84 | 0.59 | 0.67 | 0.67 | 1.42 | 0.73 | 0.6 |
| RadEAD | 0.62 | 1.42 | 0.72 | 0.51 | 0.64 | 1.84 | 0.6 | 0.63 |
| CBF | 0.6 | 22.3 | 0.54 | 0.71 | 0.64 | 22.34 | 0.55 | 0.73 |
| TORT | 0.57 | 1.37 | 0.72 | 0.49 | 0.56 | 1.37 | 0.73 | 0.43 |
| RadIAD | 0.56 | 0.19 | 0.51 | 0.62 | 0.53 | 0.19 | 0.5 | 0.6 |
| AK | 0.55 | 0.35 | 0.51 | 0.56 | 0.48 | 0.36 | 0.5 | 0.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zakharova, N.E.; Batalov, A.I.; Pogosbekian, E.L.; Chekhonin, I.V.; Goryaynov, S.A.; Bykanov, A.E.; Tyurina, A.N.; Galstyan, S.A.; Nikitin, P.V.; Fadeeva, L.M.; et al. Perifocal Zone of Brain Gliomas: Application of Diffusion Kurtosis and Perfusion MRI Values for Tumor Invasion Border Determination. Cancers 2023, 15, 2760. https://doi.org/10.3390/cancers15102760
Zakharova NE, Batalov AI, Pogosbekian EL, Chekhonin IV, Goryaynov SA, Bykanov AE, Tyurina AN, Galstyan SA, Nikitin PV, Fadeeva LM, et al. Perifocal Zone of Brain Gliomas: Application of Diffusion Kurtosis and Perfusion MRI Values for Tumor Invasion Border Determination. Cancers. 2023; 15(10):2760. https://doi.org/10.3390/cancers15102760
Chicago/Turabian StyleZakharova, Natalia E., Artem I. Batalov, Eduard L. Pogosbekian, Ivan V. Chekhonin, Sergey A. Goryaynov, Andrey E. Bykanov, Anastasia N. Tyurina, Suzanna A. Galstyan, Pavel V. Nikitin, Lyudmila M. Fadeeva, and et al. 2023. "Perifocal Zone of Brain Gliomas: Application of Diffusion Kurtosis and Perfusion MRI Values for Tumor Invasion Border Determination" Cancers 15, no. 10: 2760. https://doi.org/10.3390/cancers15102760
APA StyleZakharova, N. E., Batalov, A. I., Pogosbekian, E. L., Chekhonin, I. V., Goryaynov, S. A., Bykanov, A. E., Tyurina, A. N., Galstyan, S. A., Nikitin, P. V., Fadeeva, L. M., Usachev, D. Y., & Pronin, I. N. (2023). Perifocal Zone of Brain Gliomas: Application of Diffusion Kurtosis and Perfusion MRI Values for Tumor Invasion Border Determination. Cancers, 15(10), 2760. https://doi.org/10.3390/cancers15102760





