Graft-Versus-Host Disease Prophylaxis with Antithymocyte Globulin in Patients Receiving Stem Cell Transplantation from Unrelated Donors: An Observational Retrospective Single-Center Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Patients and Methods
2.1. Study Design and Procedures
2.2. Statistical Analysis
3. Results
3.1. Patients’ Characteristics
3.2. Cumulative Incidence of aGVHD and cGVHD
3.3. Cumulative Incidence of NRM and RI
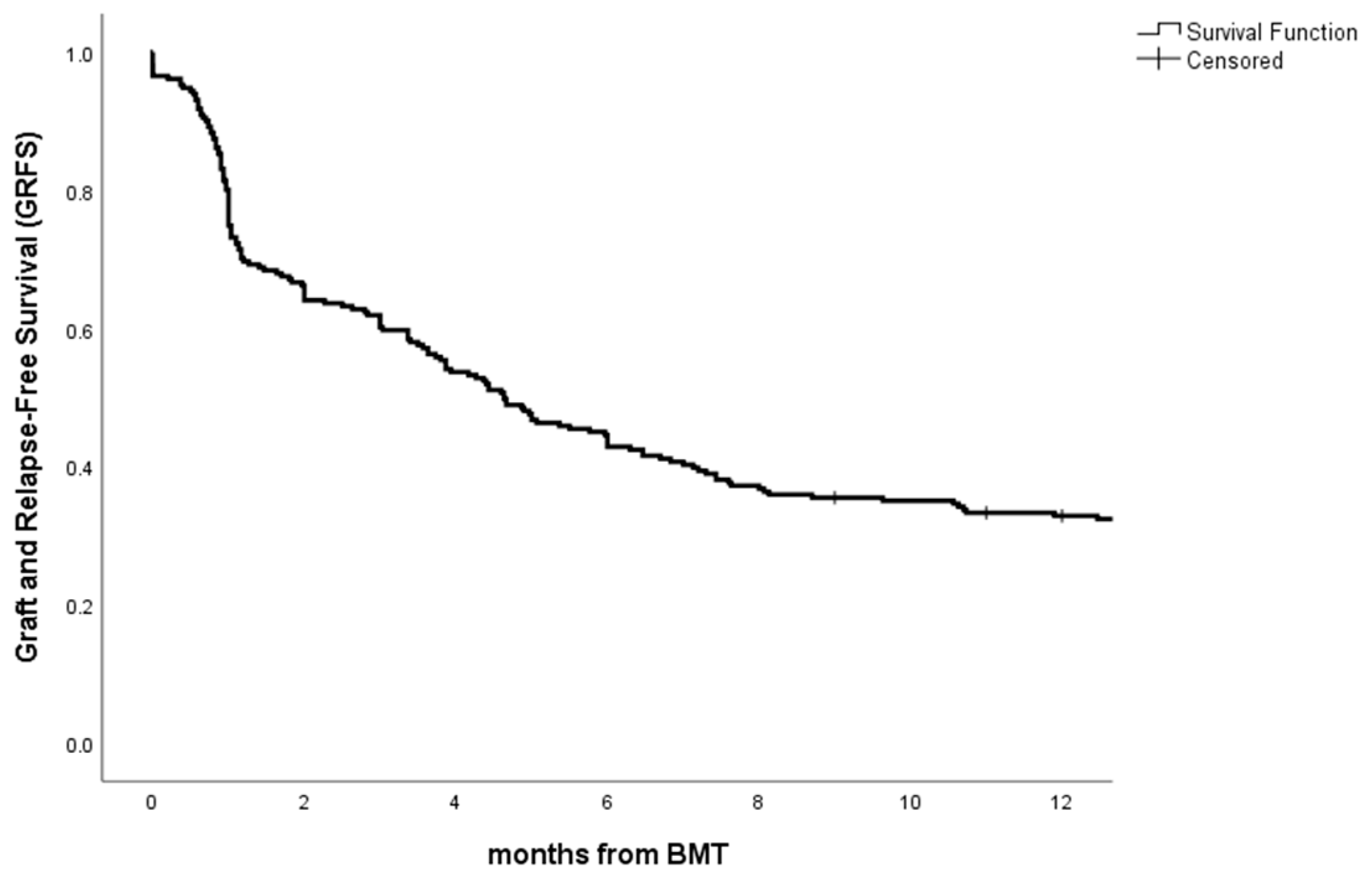
3.4. Overall Survival
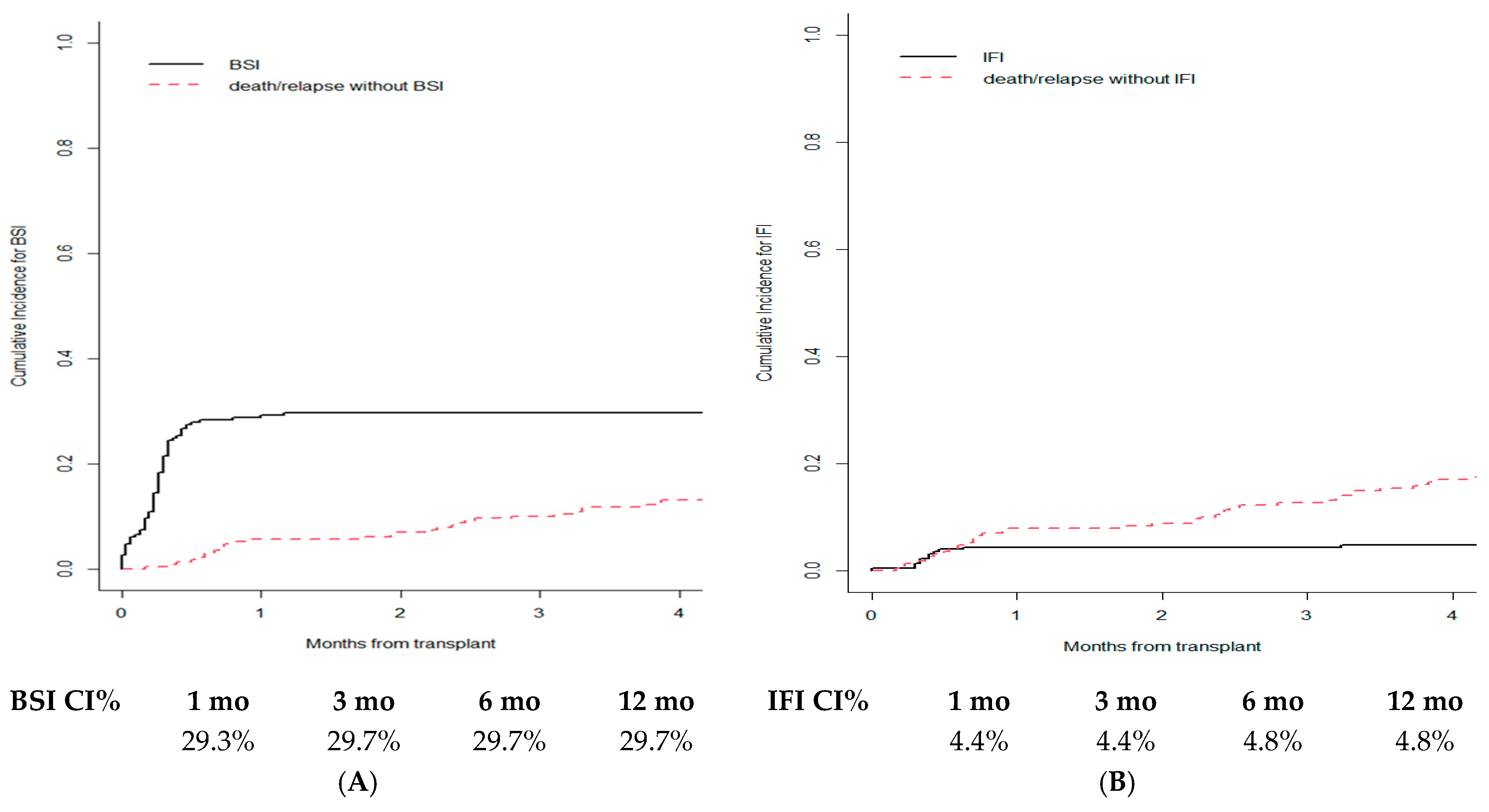
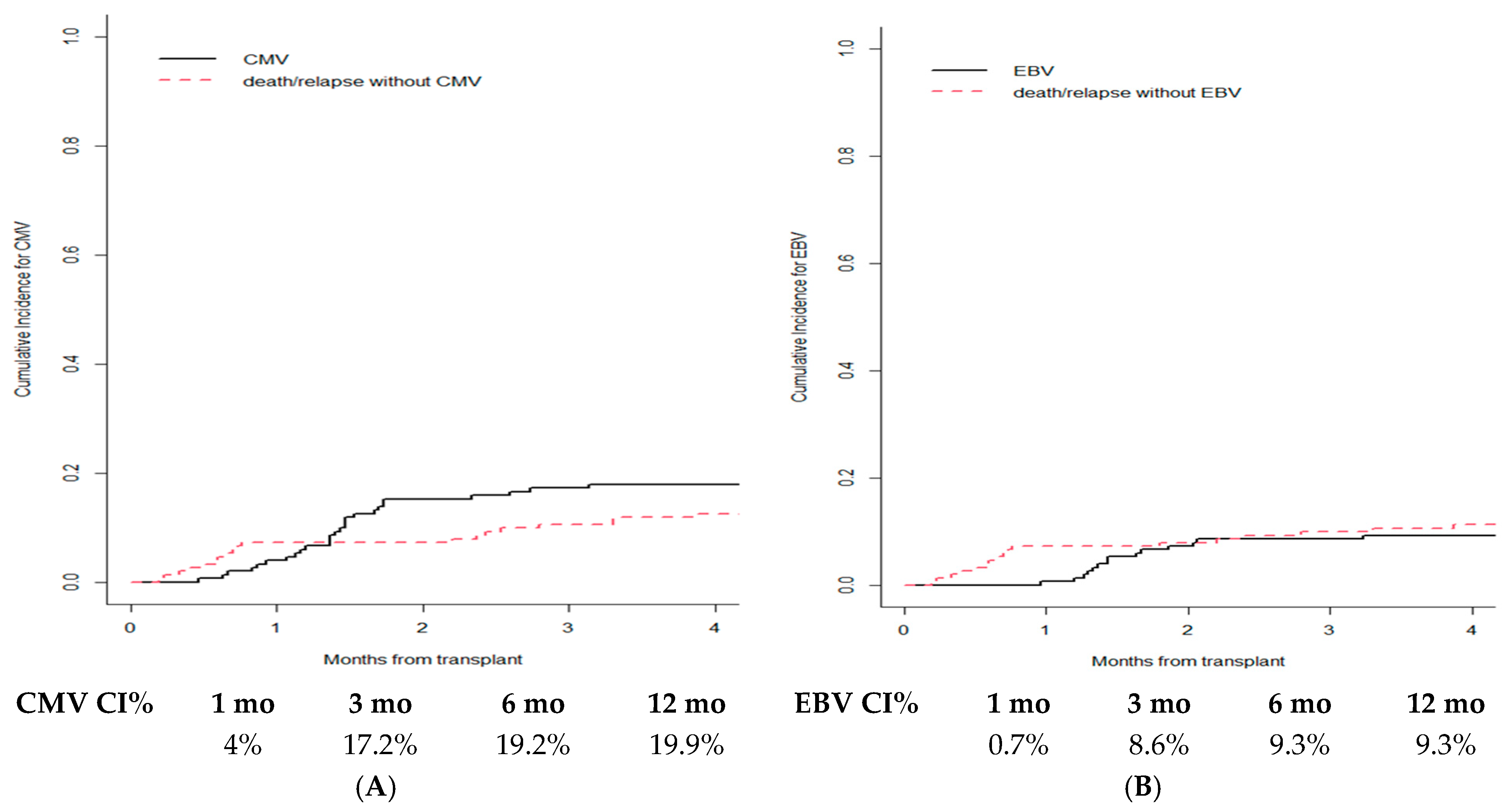
3.5. Infections
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Copelan, E.A.; Chojecki, A.; Lazarus, H.M.; Avalos, B.R. Allogeneic hematopoietic cell transplantation; the current renaissance. Blood Rev. 2019, 34, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Zeiser, R.; Blazar, B.R. Acute Graft-versus-Host Disease—Biologic Process, Prevention, and Therapy. N. Engl. J. Med. 2017, 377, 2167–2179. [Google Scholar] [CrossRef] [PubMed]
- Pidala, J.; Kurland, B.; Chai, X.; Majhail, N.; Weisdorf, D.J.; Pavletic, S.; Cutler, C.; Jacobsohn, D.; Palmer, J.; Arai, S.; et al. Patient-reported quality of life is associated with severity of chronic graft-versus-host disease as measured by NIH criteria: Report on baseline data from the chronic GVHD Consortium. Blood 2011, 117, 4851–4857. [Google Scholar] [CrossRef]
- Ruutu, T.; van Biezen, A.; Hertenstein, B.; Henseler, A.; Garderet, L.; Passweg, J.; Mohty, M.; Sureda, A.; Niederwieser, D.; Gratwohl, A.; et al. Prophylaxis and treatment of GVHD after allogeneic haematopoietic SCT: A survey of centre strategies by the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant. 2012, 47, 1459–1464. [Google Scholar] [CrossRef] [PubMed]
- Bonifazi, F.; Rubio, M.T.; Bacigalupo, A.; Boelens, J.J.; Finke, J.; Greinix, H.; Mohty, M.; Nagler, A.; Passweg, J.; Rambaldi, A.; et al. Rabbit ATG/ATLG in preventing graft-versus-host disease after allogeneic stem cell transplantation: Consensus-based recommendations by an international expert panel. Bone Marrow Transplant. 2020, 55, 1093–1102. [Google Scholar] [CrossRef]
- Servais, S.; Menten-Dedoyart, C.; Beguin, Y.; Seidel, L.; Gothot, A.; Daulne, C.; Willems, E.; Delens, L.; Humblet-Baron, S.; Hannon, M.; et al. Impact of pre-transplant anti-T cell globulin (ATG) on immune recovery after myeloablative allogeneic peripheral blood stem cell transplantation. PLoS ONE 2015, 10, e0130026. [Google Scholar] [CrossRef] [PubMed]
- Bosch, M.; Dhadda, M.; Hoegh-Petersen, M.; Liu, Y.; Hagel, L.M.; Podgorny, P.; Ugarte-Torres, A.; Khan, F.M.; Luider, J.; Auer-Grzesiak, I.; et al. Immune reconstitution after antithymocyte globulin-conditioned hematopoietic cell transplantation. Cytotherapy 2012, 14, 1258–1275. [Google Scholar] [CrossRef]
- Soiffer, R.J.; Kim, H.T.; McGuirk, J.; Horwitz, M.E.; Johnston, L.; Patnaik, M.M.; Rybka, W.; Artz, A.; Porter, D.L.; Shea, T.C.; et al. Prospective, randomized, double-blind, phase III clinical trial of anti-T-lymphocyte globulin to assess impact on chronic graft-versus-host disease-free survival in patients undergoing HLA-matched unrelated myeloablative hematopoietic cell transplantation. J. Clin. Oncol. 2017, 35, 4003–4011. [Google Scholar] [CrossRef]
- Admiraal, R.; Nierkens, S.; de Witte, M.A.; Petersen, E.J.; Fleurke, G.J.; Verrest, L.; Belitser, S.V.; Bredius, R.G.M.; Raymakers, R.A.P.; Knibbe, C.A.J.; et al. Association between anti-thymocyte globulin exposure and survival outcomes in adult unrelated haemopoietic cell transplantation: A multicentre, retrospective, pharmacodynamic cohort analysis. Lancet Haematol. 2017, 4, e183–e191. [Google Scholar] [CrossRef]
- Harris, A.C.; Young, R.; Devine, S.; Hogan, W.J.; Ayuk, F.; Bunworasate, U.; Chanswangphuwana, C.; Efebera, Y.A.; Holler, E.; Litzow, M.; et al. International, Multicenter Standardization of Acute Graft-versus-Host Disease Clinical Data Collection: A Report from the Mount Sinai Acute GVHD International Consortium. Biol. Blood Marrow Transplant. 2016, 22, 4–10. [Google Scholar] [CrossRef]
- Shulman, H.M.; Cardona, D.M.; Greenson, J.K.; Hingorani, S.; Horn, T.; Huber, E.; Kreft, A.; Longerich, T.; Morton, T.; Myerson, D.; et al. NIH Consensus development project on criteria for clinical trials in chronic graft-versus-host disease: II. The 2014 Pathology Working Group Report. Biol. Blood Marrow Transplant. 2015, 21, 589–603. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, J.P.; Chen, S.C.; Kauffman, C.A.; Steinbach, W.J.; Baddley, J.W.; Verweij, P.E.; Clancy, C.J.; Wingard, J.R.; Lockhart, S.R.; Groll, A.H.; et al. Revision and Update of the Consensus Definitions of Invasive Fungal Disease from the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin. Infect. Dis. 2020, 71, 1367–1376. [Google Scholar] [CrossRef]
- Holtan, S.G.; DeFor, T.E.; Lazaryan, A.; Bejanyan, N.; Arora, M.; Brunstein, C.G.; Blazar, B.R.; MacMillan, M.L.; Weisdorf, D.J. Composite end point of graft-versus-host disease-free, relapse-free survival after allogeneic hematopoietic cell transplantation. Blood 2015, 125, 1333–1338. [Google Scholar] [CrossRef]
- Butera, S.; Cerrano, M.; Brunello, L.; Dellacasa, C.M.; Faraci, D.G.; Vassallo, S.; Mordini, N.; Sorasio, R.; Zallio, F.; Busca, A.; et al. Impact of anti-thymocyte globulin dose for graft-versus-host disease prophylaxis in allogeneic hematopoietic cell transplantation from matched unrelated donors: A multicenter experience. Ann. Hematol. 2021, 100, 1837–1847. [Google Scholar] [CrossRef]
- Bacigalupo, A.; Lamparelli, T.; Bruzzi, P.; Guidi, S.; Alessandrino, P.E.; di Bartolomeo, P.; Oneto, R.; Bruno, B.; Barbanti, M.; Sacchi, N.; et al. Antithymocyte globulin for graft-versus-host disease prophylaxis in transplants from unrelated donors: 2 randomized studies from Gruppo Italiano Trapianti Midollo Osseo (GITMO). Blood 2001, 98, 2942–2947. [Google Scholar] [CrossRef] [PubMed]
- Walker, I.; Panzarella, T.; Couban, S.; Couture, F.; Devins, G.; Elemary, M.; Gallagher, G.; Kerr, H.; Kuruvilla, J.; Lee, S.J.; et al. Addition of anti-thymocyte globulin to standard graft-versus-host disease prophylaxis versus standard treatment alone in patients with haematological malignancies undergoing transplantation from unrelated donors: Final analysis of a randomised, open-label, multicentre, phase 3 trial. Lancet Haematol. 2020, 7, e100–e111. [Google Scholar] [CrossRef] [PubMed]
- Finke, J.; Schmoor, C.; Bethge, W.A.; Ottinger, H.; Stelljes, M.; Volin, L.; Heim, D.; Bertz, H.; Grishina, O.; Socie, G. Long-term outcomes after standard graft-versus-host disease prophylaxis with or without anti-human-T-lymphocyte immunoglobulin in haemopoietic cell transplantation from matched unrelated donors: Final results of a randomised controlled trial. Lancet Haematol. 2017, 4, e293–e301. [Google Scholar] [CrossRef]
- Kuriyama, K.; Fuji, S.; Inamoto, Y.; Tajima, K.; Tanaka, T.; Inoue, Y.; Ito, R.; Hayashi, Y.; Ito, A.; Kurosawa, S.; et al. Impact of low-dose rabbit anti-thymocyte globulin in unrelated hematopoietic stem cell transplantation. Int. J. Hematol. 2016, 103, 453–460. [Google Scholar] [CrossRef]
- Othman, J.; Greenwood, M.; Moore, J.; Larsen, S.; Watson, A.M.; Arthur, C.; Bhattacharyya, A.; Bilmon, I.; Blyth, E.; Bryant, A.; et al. Unrelated Donor Transplant Recipients Given Thymoglobuline Have Superior GRFS When Compared to Matched Related Donor Recipients Undergoing Transplantation without ATG. Biol. Blood Marrow Transplant. 2020, 26, 1868–1875. [Google Scholar] [CrossRef]
- Battipaglia, G.; Labopin, M.; Kröger, N.; Vitek, A.; Afanasyev, B.; Hilgendorf, I.; Schetelig, J.; Ganser, A.; Blaise, D.; Itälä-Remes, M.; et al. Posttransplant cyclophosphamide vs antithymocyte globulin in HLA-mismatched unrelated donor transplantation. Blood 2019, 134, 892–899. [Google Scholar] [CrossRef]
- Brissot, E.; Labopin, M.; Moiseev, I.; Cornelissen, J.J.; Meijer, E.; Van Gorkom, G.; Rovira, M.; Ciceri, F.; Griskevicius, L.; Blaise, D.; et al. Post-transplant cyclophosphamide versus antithymocyte globulin in patients with acute myeloid leukemia in first complete remission undergoing allogeneic stem cell transplantation from 10/10 HLA-matched unrelated donors. J. Hematol. Oncol. 2020, 13, 87. [Google Scholar] [CrossRef] [PubMed]
- Mehta, R.S.; Saliba, R.M.; Rondon, G.; Al-Atrash, G.; Bashir, Q.; Hosing, C.M.; Kebriaei, P.; Khouri, I.; Nieto, Y.; Oran, B.; et al. Post-Transplantation Cyclophosphamide versus Tacrolimus and Methotrexate Graft-Versus-Host Disease Prophylaxis for HLA-Matched Donor Transplantation. Transplant. Cell. Ther. 2022, 28, 695.e1–695.e10. [Google Scholar] [CrossRef] [PubMed]
| Characteristics. | All Patients |
|---|---|
| Number of patients/transplants | 226/231 |
| Age at transplant, median (IQR), years | 51 (43–60) |
| Gender Male Female | 126 (54.5%) 105 (45.5%) |
| Underlying disease AML/MDS ALL HL/NHL MPN/LMMC other | 123 (53.2%) 28 (12.1%) 42 (18.2%) 20 (8.7%) 18 (7.8%) |
| HCT-CI Low/intermediate (0–2) High (>3) | 119 (68.4%) 55 (31.6%) |
| Cytogenetics Low risk Intermediate/High risk | 53 (35.6%) 96 (64.4%) |
| Disease status at transplant CR1 CR2 PD | 117 (65.3%) 27 (15.1%) 35 (19.6%) |
| CMV Donor status negative positive | 128 (57.4%) 95 (42.6%) |
| CMV Recipient status negative positive | 67 (30.3%) 154 (69.7%) |
| Stem cell source PBSC BM | 218 (94.4%) 13 (5.6%) |
| Number of Ly at ATG administration, median (IQR), ×106/L | 200 (90–570) |
| Number of CD34+ cells infused, median (IQR), ×106/kg | 7.5 (5.8–10.6) |
| Number of CD3+ cells infused, median (IQR), ×108/kg | 2.7 (2.0–3.5) |
| Duration of neutropenia, median (IQR), days | 17 (15–21) |
| Conditioning regimen MAC RIC | 199 (86.1%) 32 (13.9%) |
| Recipient–donor HLA matched alleles 10/10 and 9/10 ≤8/10 | 179 (79.6%) 46 (20.4%) |
| Year of transplant 2008–2012 2013–2017 2018–2021 | 47 (20.3%) 98 (42.4%) 86 (37.3%) |
| Acute GvHD 0–I II–IV | 158 (68.4%) 73 (31.6%) |
| Chronic GvHD absent/mild moderate/severe | 131 (68.6%) 60 (31.4%) |
| Nonrelapse mortality and relapse incidence alive without relapse dead without relapse relapsed | 128 (55.5%) 50 (21.6%) 53 (22.9%) |
| Overall Survival alive dead | 133 (57.6%) 98 (42.4%) |
| Characteristics | aGVHD at d120 | p-Value * | cGVHD at 2 Years | p-Value * |
|---|---|---|---|---|
| Overall cumulative incidence | 29.9% | - | 29.8% | - |
| Age at transplant <40 years 40–60 years >60 years | 24.4% 27.1% 40.4% | 0.150 | 28.9% 27.3% 37.2% | 0.473 |
| Gender Male Female | 29.5% 30.2% | 0.719 | 30.1% 29.5% | 0.834 |
| Underlying disease AML/ALL other | 27.5% 31.1% | 0.799 | 29.5% 30.6% | 0.918 |
| HCT-CI 0–2 ≥3 | 31.9% 27.3% | 0.715 | 32.1% 22.0% | 0.177 |
| Cytogenetics Low risk Interm/high | 41.5% 24.0% | 0.018 | 29.8% 28.8% | 0.872 |
| Disease status at transplant CR1 other | 26.5% 38.7% | 0.071 | 29.4% 34.7% | 0.614 |
| CMV Donor status negative positive | 30.5% 28.4% | 0.395 | 27.5% 32.5% | 0.609 |
| CMV Recipient status negative positive | 26.9% 32.5% | 0.469 | 30.5% 30.2% | 0.920 |
| Stem cell source PBSC BM | 30.3% 23.1% | 0.444 | 18.2% 30.6% | 0.350 |
| Number of Ly at ATG administration, under median over median | 26.7% 33.6% | 0.172 | 28.3% 31.6% | 0.671 |
| Number of CD34+ cells infused under median over median | 33.0% 28.7% | 0.355 | 30.0% 28.6% | 0.926 |
| Number of CD3+ cells infused under median over median | 32.7% 31.9% | 0.974 | 25.0% 35.0% | 0.278 |
| Duration of neutropenia, under median over median | 34.8% 28.7% | 0.188 | 28.7% 30.7% | 0.606 |
| Conditioning regimen MAC RIC | 31.2% 21.9% | 0.570 | 30.4% 26.1% | 0.647 |
| Recipient–donor HLA matched alleles 10/10 & 9/10 ≤8/10 | 28.5% 37.0% | 0.230 | 25.0% 51.4% | 0.003 |
| Year of transplant 2008–2012 2013–2017 2018–2021 | 34.0% 30.6% 26.7% | 0.634 | 25.0% 37.2% 23.3% | 0.071 |
| Acute GvHD 0–I II–IV | - | - | 23.1% 41.4% | 0.007 |
| Characteristics | cGVHD SDHR (95%CI) | cGVHD p-Value * | NRM SDHR (95%CI) | NRM p-Value * | RI SDHR (95%CI) | RI p-Value * |
|---|---|---|---|---|---|---|
| Age at transplant (>60 vs. 40–60 vs. <40 years) | 3.52 (1.58–7.86) | 0.002 | ||||
| HCT-CI (≥3 vs. 0–2) | 1.32 (0.50–3.46) | 0.570 | 1.87 (0.90–3.89) | 0.092 | ||
| Cytogenetics (interm/high vs. low risk) | 3.00 (1.12–8.02) | 0.028 | ||||
| CMV Recipient status (positive vs. negative) | 1.09 (0.37–3.22) | 0.880 | ||||
| Duration of neutropenia (over vs. under median) | 2.55 (1.01–6.40) | 0.047 | ||||
| Conditioning regimen (RIC vs. MAC) | 1.13 (0.34–3.75) | 0.850 | ||||
| Recipient-donor HLA matched alleles (≤8/10 vs. ≥9/10 vs) | 2.14 (1.22–3.77) | 0.008 | ||||
| Acute GvHD (II–IV vs. 0–I) | 1.86 (1.10–3.13) | 0.021 | 0.97 (0.40–2.37) | 0.950 | ||
| Chronic GvHD (moderate/severe vs. absent/mild) | 0.08 (0.01–0.59) | 0.013 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lo Schirico, M.; Passera, R.; Gill, J.; Dellacasa, C.; Dogliotti, I.; Giaccone, L.; Zompi, S.; Busca, A. Graft-Versus-Host Disease Prophylaxis with Antithymocyte Globulin in Patients Receiving Stem Cell Transplantation from Unrelated Donors: An Observational Retrospective Single-Center Study. Cancers 2023, 15, 2761. https://doi.org/10.3390/cancers15102761
Lo Schirico M, Passera R, Gill J, Dellacasa C, Dogliotti I, Giaccone L, Zompi S, Busca A. Graft-Versus-Host Disease Prophylaxis with Antithymocyte Globulin in Patients Receiving Stem Cell Transplantation from Unrelated Donors: An Observational Retrospective Single-Center Study. Cancers. 2023; 15(10):2761. https://doi.org/10.3390/cancers15102761
Chicago/Turabian StyleLo Schirico, Mariella, Roberto Passera, Jessica Gill, Chiara Dellacasa, Irene Dogliotti, Luisa Giaccone, Sofia Zompi, and Alessandro Busca. 2023. "Graft-Versus-Host Disease Prophylaxis with Antithymocyte Globulin in Patients Receiving Stem Cell Transplantation from Unrelated Donors: An Observational Retrospective Single-Center Study" Cancers 15, no. 10: 2761. https://doi.org/10.3390/cancers15102761
APA StyleLo Schirico, M., Passera, R., Gill, J., Dellacasa, C., Dogliotti, I., Giaccone, L., Zompi, S., & Busca, A. (2023). Graft-Versus-Host Disease Prophylaxis with Antithymocyte Globulin in Patients Receiving Stem Cell Transplantation from Unrelated Donors: An Observational Retrospective Single-Center Study. Cancers, 15(10), 2761. https://doi.org/10.3390/cancers15102761









