Prognostic Values of Systemic Inflammatory Immunological Markers in Glioblastoma: A Systematic Review and Meta-Analysis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Selection Criteria
2.3. Eligibility Criteria
2.4. Data Extraction and Quality Assessment
2.5. Statistical Analysis
3. Results
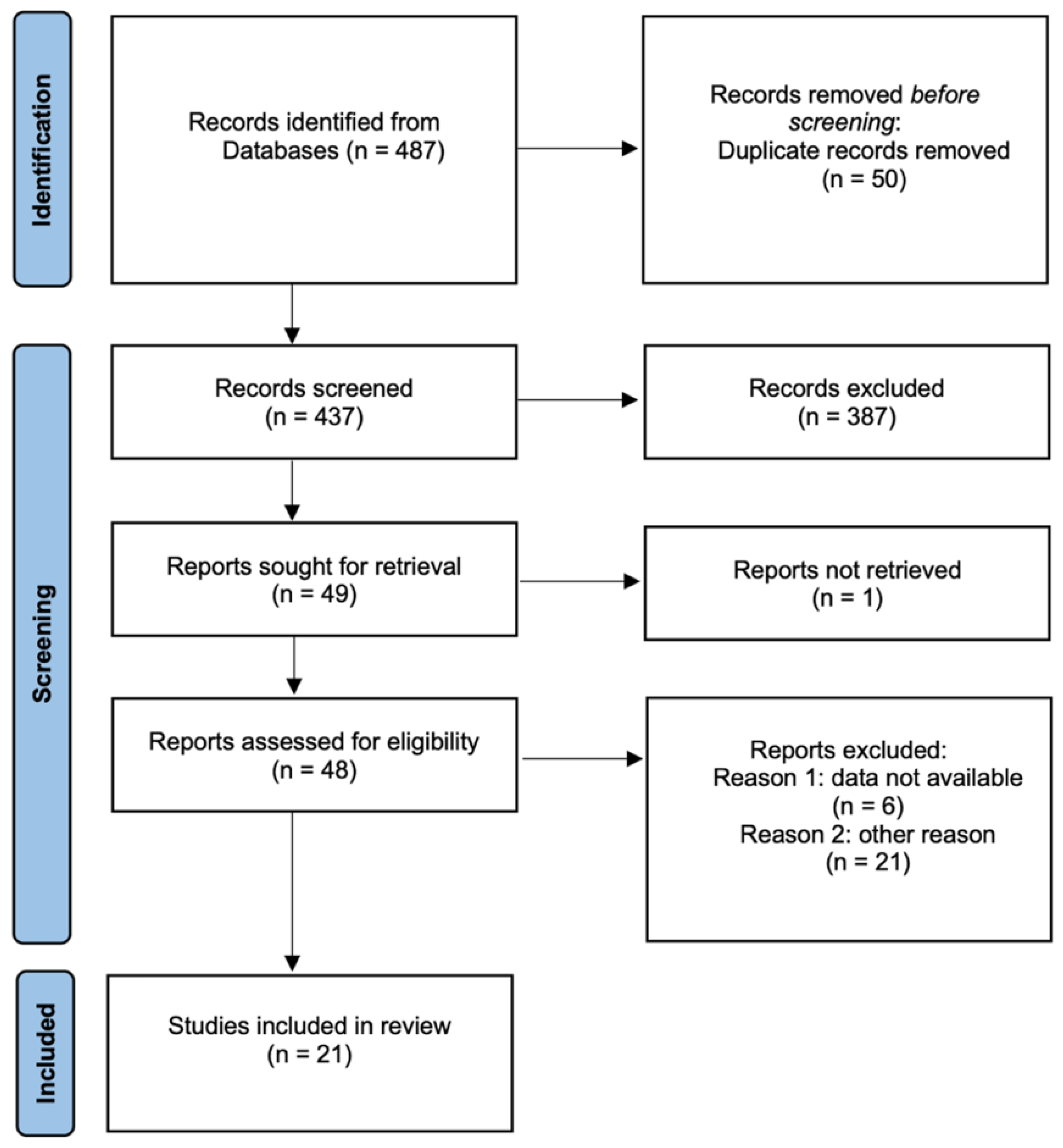
3.1. Study Search and Characteristics
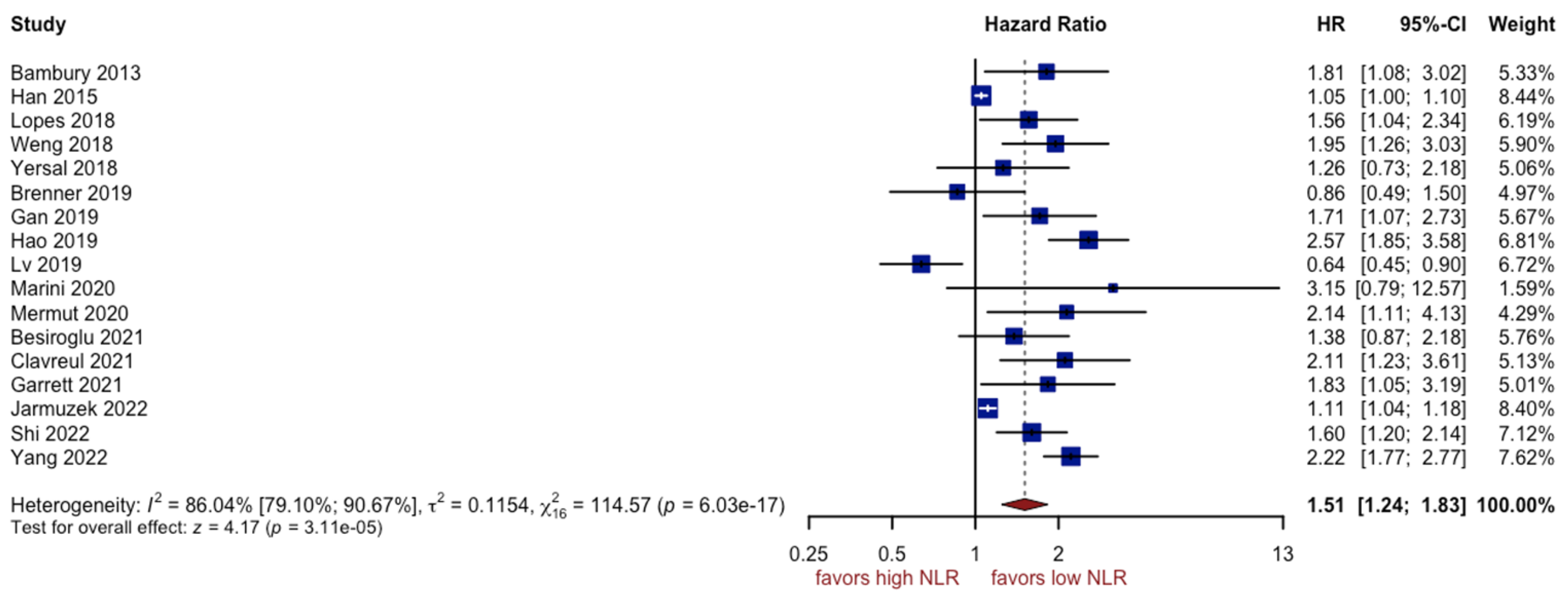
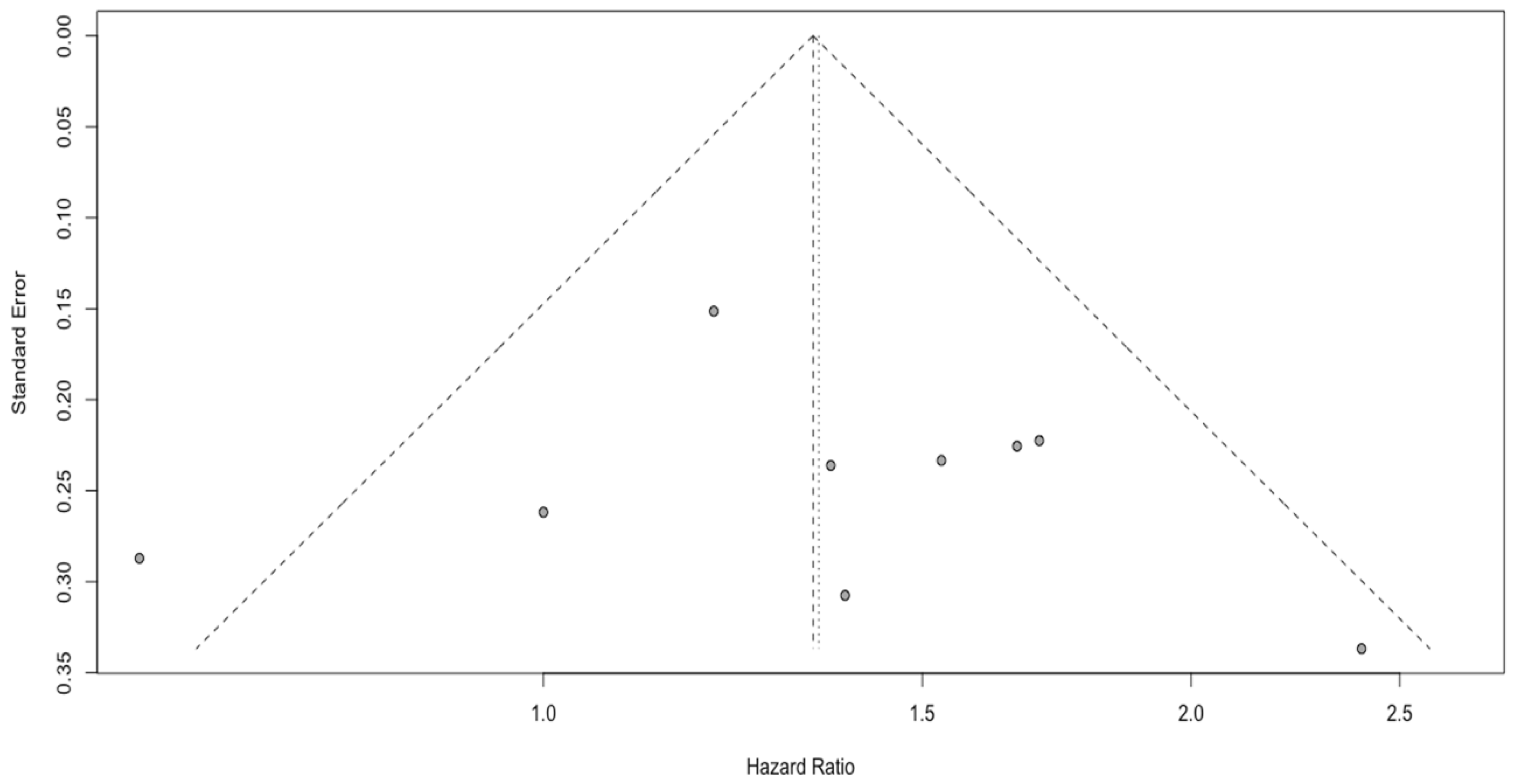
3.2. Analysis of NLR
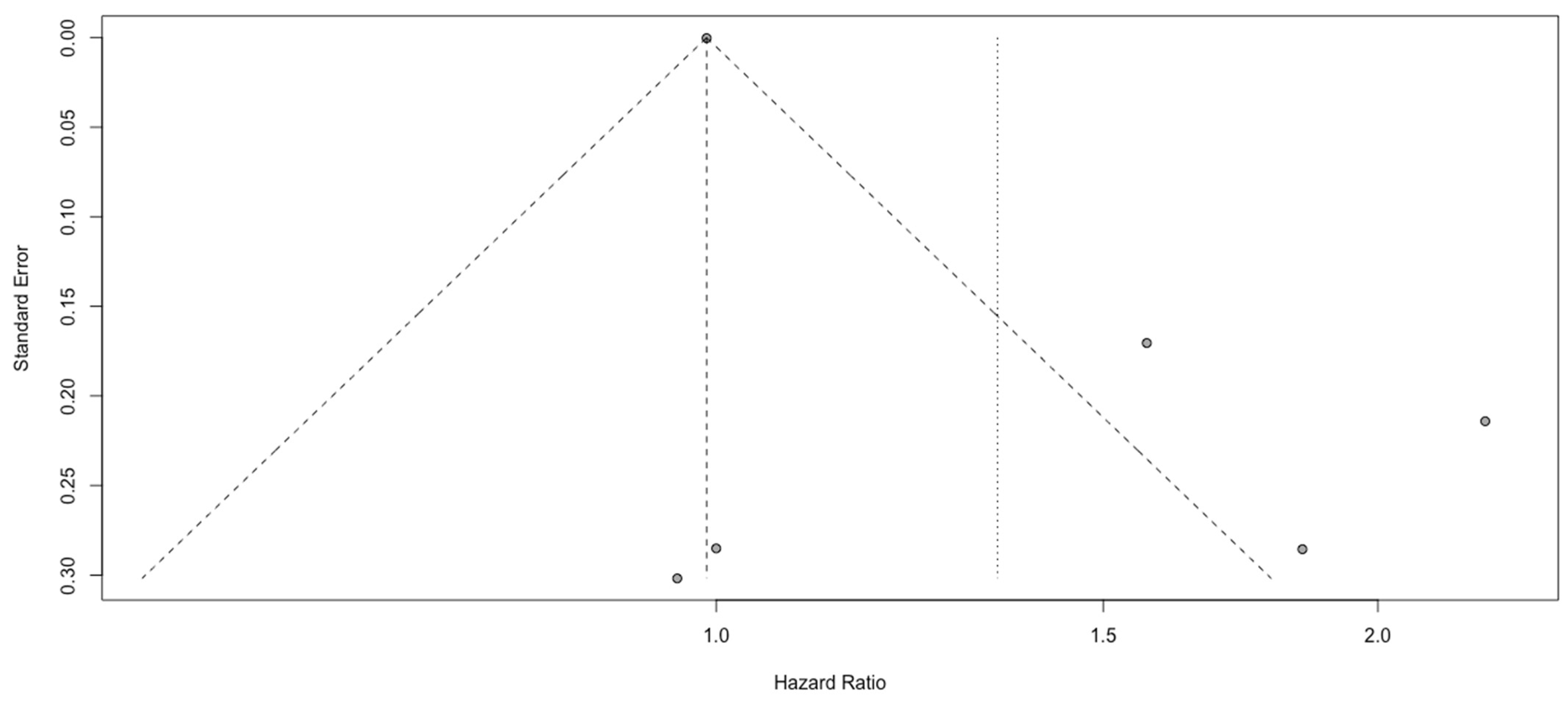
3.3. Analysis of PLR
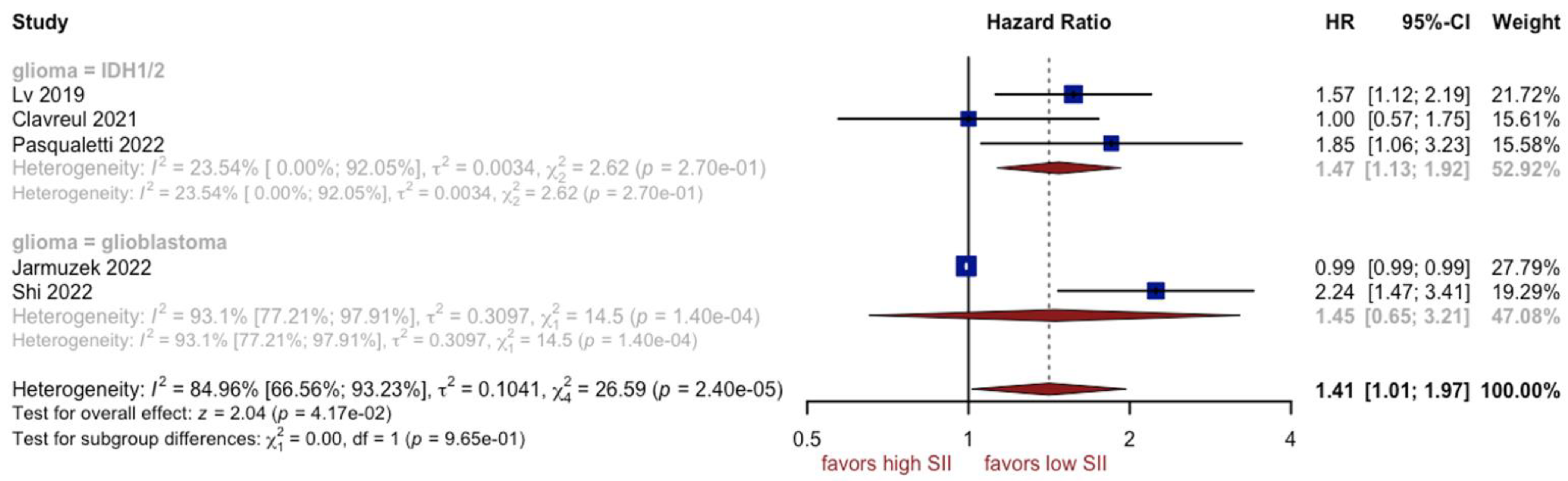
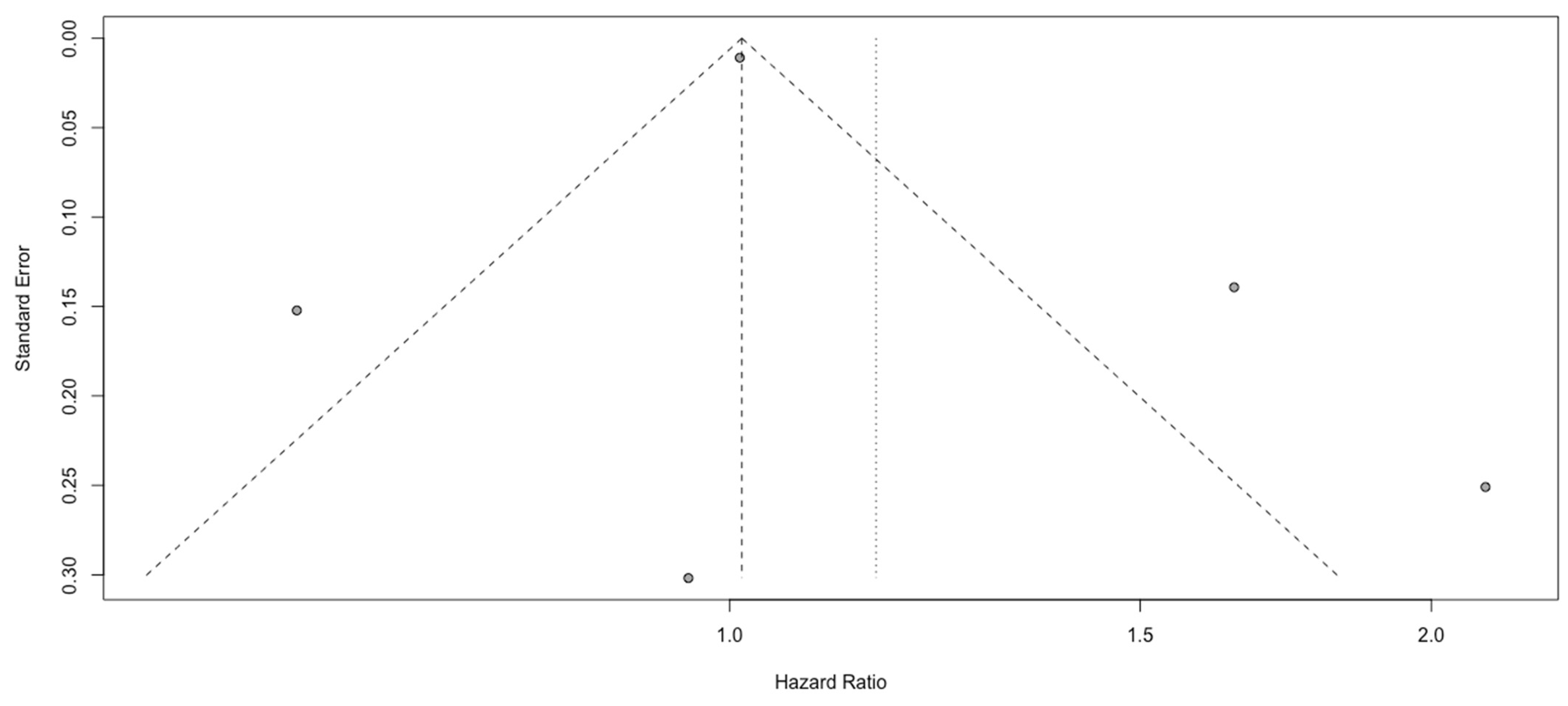
3.4. Analysis of SII
3.5. Analysis of SIRI
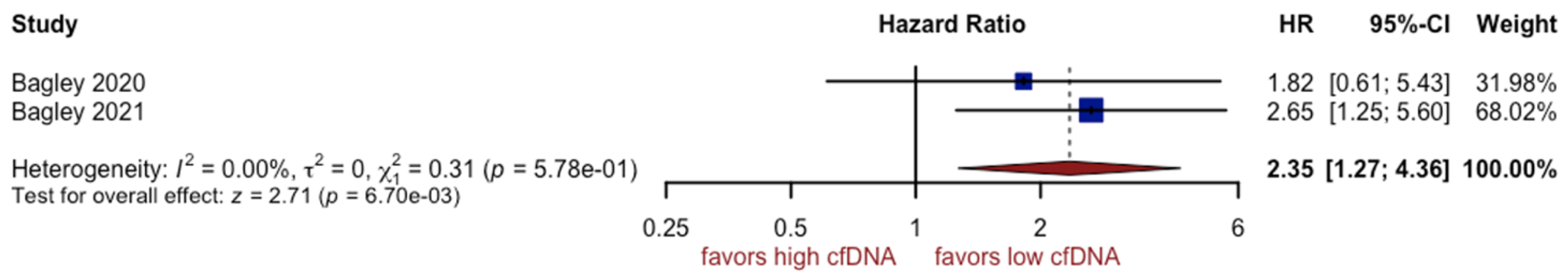
3.6. Analysis of cfDNA
4. Discussion
5. Conclusions
6. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Basheer, A.S.; Abas, F.; Othman, L.; Naidu, R. Role of Inflammatory Mediators, Macrophages, and Neutrophils in Glioma Maintenance and Progression: Mechanistic Understanding and Potential Therapeutic Applications. Cancers 2021, 13, 4226. [Google Scholar] [CrossRef]
- Chen, N.; Peng, C.; Li, D. Epigenetic Underpinnings of Inflammation: A Key to Unlock the Tumor Microenvironment in Glioblastoma. Front. Immunol. 2022, 13, 869307. [Google Scholar] [CrossRef]
- Wang, G.; Wang, J.; Niu, C.; Zhao, Y.; Wu, P. Neutrophils: New Critical Regulators of Glioma. Front. Immunol. 2022, 13, 927233. [Google Scholar] [CrossRef]
- Massara, M.; Persico, P.; Bonavita, O.; Poeta, V.M.; Locati, M.; Simonelli, M.; Bocecchi, R. Neutrophils in Glioma. Front. Immunol. 2017, 8, 1349. [Google Scholar] [CrossRef] [PubMed]
- Masucci, M.T.; Minopoli, M.; Carriero, M.V. Tumor Associated Neutrophils. Their Role in Tumorigenesis, Metastasis, Prognosis and Therapy. Front. Oncol. 2019, 9, 1146. [Google Scholar] [CrossRef]
- Jarmuzek, P.; Kot, M.; Defort, P.; Stawicki, J.; Komorzycka, J.; Nowak, K.; Tylutka, A.; Zembron-Lacny, A. Prognostic Values of Combined Ratios of White Blood Cells in Glioblastoma: A Retrospective Study. J. Clin. Med. 2022, 11, 3397. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.; Zhang, B.; Guo, X.; Zong, M.; Rahman, R.; Sanchez, D.; Winder, N.; Reardon, D.A.; Zhao, B.; Wen, P.Y.; et al. Multimodal imaging patterns predict survival in recurrent glioblastoma patients treated with bevacizumab. Neuro Oncol. 2016, 18, 1680–1687. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro Oncol. 2021, 23, 1231–1251. [Google Scholar] [CrossRef]
- Wang, S.-Q.; Yuan, Q.; Zhang, G.-T.; Qian, H.-P.; Liu, Z.-D.; Wang, J.-W.; Cai, H.-Q.; Wan, J.-H. Preoperative blood testing for glioblastoma, brain metastases, and primary central nervous system lymphoma differentiation. Transl. Cancer Res. 2022, 11, 63–71. [Google Scholar] [CrossRef]
- Connolly, I.D.; Li, Y.; Gephart, M.H.; Nagpal, S. The “Liquid Biopsy”: The Role of Circulating DNA and RNA in Central Nervous System Tumors. Curr. Neurol. Neurosci. Rep. 2016, 16, 25. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Li, H.; Xu, Y.; Nyalali, A.M.K.; Li, F. The prognostic value of the preoperative inflammatory index on the survival of glioblastoma patients. Neurol. Sci. 2022, 43, 5523–5531. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, S.; Zhang, S.; Liu, Y.; Ma, L.; Zhu, J.; Xin, Y.; Wang, Y.; Yang, C.; Cheng, Y. Systemic immune-inflammation index, neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio can predict clinical outcomes in patients with metastatic non-small-cell lung cancer treated with nivolumab. J. Clin. Lab. Anal. 2019, 33, e22964. [Google Scholar] [CrossRef]
- Lv, Y.; Zhang, S.; Liu, Z.; Tian, Y.; Liang, N.; Zhang, J. Prognostic value of preoperative neutrophil to lymphocyte ratio is superior to systemic immune inflammation index for survival in patients with Glioblastoma. Clin. Neurol. Neurosurg. 2019, 181, 24–27. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Guan, R.; Zou, Y.; Jian, Z.; Lin, Y.; Guo, R.; Jin, H. Nomogram Based on Inflammatory Biomarkers to Predict the Recurrence of Hepatocellular Carcinoma-A Multicentre Experience. J. Inflamm. Res. 2022, 15, 5089–5102. [Google Scholar] [CrossRef] [PubMed]
- Gawaz, M.; Langer, H.; May, A.E. Platelets in inflammation and atherogenesis. J. Clin. Investig. 2005, 115, 3378–3384. [Google Scholar] [CrossRef]
- Kurtul, A.; Yarlioglues, M.; Murat, S.N.; Ergun, G.; Duran, M.; Kasapkara, H.A.; Demircelik, M.B.; Cetin, M.; Ocek, A.H. Usefulness of the platelet-to-lymphocyte ratio in predicting angiographic reflow after primary percutaneous coronary intervention in patients with acute ST-segment elevation myocardial infarction. Am. J. Cardiol. 2014, 114, 342–347. [Google Scholar] [CrossRef]
- Furuncuoğlu, Y.; Tulgar, S.; Dogan, A.N.; Cakar, S.; Tulgar, Y.K.; Cakiroglu, B. How obesity affects the neutrophil/lymphocyte and platelet/lymphocyte ratio, systemic immune-inflammatory index and platelet indices: A retrospective study. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 1300–1306. [Google Scholar]
- Wang, W.; Chen, X.; Gong, S.; Guo, L.; Zhang, X. Preoperative neutrophil-lymphocyte ratio correlated with glioma grading and glioblastoma survival. Neurol. Res. 2018, 40, 917–922. [Google Scholar] [CrossRef]
- Schneider, D.J. Factors Contributing to Increased Platelet Reactivity in People with Diabetes. Diabetes Care 2009, 32, 525–527. [Google Scholar] [CrossRef]
- Tomás, T.C.; Eiriz, I.; Vitorino, M.; Vicente, R.; Gramaça, J.; Oliveira, A.G.; Luz, P.; Baleiras, M.; Spencer, M.S.; Costa, L.L.; et al. Neutrophile-to-lymphocyte, lymphocyte-to-monocyte, and platelet-to-lymphocyte ratios as prognostic and response biomarkers for resectable locally advanced gastric cancer. World J. Gastrointest. Oncol. 2022, 14, 1307–1323. [Google Scholar] [CrossRef]
- Xia, W.-K.; Liu, Z.-L.; Shen, D.; Lin, Q.-F.; Su, J.; Mao, W.-D. Prognostic performance of pre-treatment NLR and PLR in patients suffering from osteosarcoma. World J. Surg. Oncol. 2016, 14, 127. [Google Scholar] [CrossRef]
- Zheng, S.-H.; Huang, J.-L.; Chen, M.; Wang, B.-L.; Ou, Q.-S.; Huang, S.-Y. Diagnostic value of preoperative inflammatory markers in patients with glioma: A multicenter cohort study. J. Neurosurg. 2018, 129, 583–592. [Google Scholar] [CrossRef]
- Yang, R.; Chang, Q.; Meng, X.; Gao, N.; Wang, W. Prognostic value of Systemic immune-inflammation index in cancer: A meta-analysis. J. Cancer 2018, 9, 3295–3302. [Google Scholar] [CrossRef]
- Wang, Y.-T.; Kuo, L.-T.; Weng, H.-H.; Hsu, C.-M.; Tsai, M.-S.; Chang, G.-H.; Lee, Y.-C.; Huang, E.I.; Tsai, Y.-T. Systemic Immun e-Inflammation Index as a Predictor for Head and Neck Cancer Prognosis: A Meta-Analysis. Front. Oncol. 2022, 12, 899518. [Google Scholar] [CrossRef] [PubMed]
- Takenaka, Y.; Oya, R.; Kitamiura, T.; Ashida, N.; Shimizu, K.; Takemura, K.; Yamamoto, Y.; Uno, A. Prognostic role of neutrophil-to-lymphocyte ratio in head and neck cancer: A meta-analysis. Head Neck 2018, 40, 647–655. [Google Scholar] [CrossRef] [PubMed]
- Ying, H.-Q.; Deng, Q.-W.; He, B.-S.; Pan, Y.-Q.; Wang, F.; Sun, H.-L.; Chen, J.; Liu, X.; Wang, S.-K. The prognostic value of preoperative NLR, d-NLR, PLR and LMR for predicting clinical outcome in surgical colorectal cancer patients. Med. Oncol. 2014, 31, 305. [Google Scholar] [CrossRef]
- Auezova, R.; Ivanova, N.; Akshulakov, S.; Zhetpisbaev, B.; Kozhakhmetova, A.; Ryskeldiyev, N.; Mustafin, K.; Teltayev, D.; Auezova, L. Isocitrate dehydrogenase 1 mutation is associated with reduced levels of inflammation in glioma patients. Cancer Manag. Res. 2019, 11, 3227–3236. [Google Scholar] [CrossRef]
- Yang, C.; Wen, H.-B.; Zhao, Y.-H.; Huang, W.-H.; Wang, Z.-F.; Li, Z.-Q. Systemic Inflammatory Indicators as Prognosticators in Glioblastoma Patients: A Comprehensive Meta-Analysis. Front. Neurol. 2020, 11, 580101. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.-Y.; Li, Y.-T.; Hu, Q.-L.; Wang, J.; Sui, A.-X. Prognostic impact of neutrophil-to-lymphocyte ratio in gliomas: A systematic review and meta-analysis. World J. Surg. Oncol. 2019, 17, 152. [Google Scholar] [CrossRef]
- Guo, X.; Jiao, H.; Zhang, T.; Zhang, Y. Pre-Treatment and Preoperative Neutrophil-to-Lymphocyte Ratio Predicts Prognostic Value of Glioblastoma: A Meta-Analysis. Brain Sci. 2022, 12, 675. [Google Scholar] [CrossRef]
- Lu, X.; Xu, W.; Wei, Y.; Li, T.; Gao, L.; Fu, X.; Yao, Y.; Wang, L. Diagnostic performance of DWI for differentiating primary central nervous system lymphoma from glioblastoma: A systematic review and meta-analysis. Neurol. Sci. 2019, 40, 947–956. [Google Scholar] [CrossRef]
- Luo, H.; He, L.; Zhang, G.; Yu, J.; Chen, Y.; Yin, H.; Goyal, H.; Zhang, G.-M.; Xiao, Y.; Gu, C.; et al. Normal Reference Intervals of neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, lymphocyte-to-monocyte ratio, and systemic immune inflammation index in healthy adults: A large multi-center study from Western China. Clin. Lab. 2019, 65. [Google Scholar] [CrossRef] [PubMed]
- Qi, Q.; Zhuang, L.; Shen, Y.; Geng, Y.; Yu, S.; Chen, H.; Liu, L.; Meng, Z.; Wang, P.; Chen, Z. A novel systemic inflammation response index (SIRI) for predicting the survival of patients with pancreatic cancer after chemotherapy. Cancer 2016, 122, 2158–2167. [Google Scholar] [CrossRef] [PubMed]
- Palande, V.; Shay, D.R.; Frenkel-Morgenstern, M. Detection of Cell-Free DNA in Blood Plasma Samples of Cancer Patients. J. Vis. Exp. 2020, 163, e61449. [Google Scholar] [CrossRef]
- McMahon, J.T.; Studer, M.; Ulrich, B.; Barbero, J.M.R.; Pradilla, I.; Palacios-Ariza, M.A.; Pradilla, G. Circulating Tumor DNA in Adults with Glioma: A Systematic Review and Meta-Analysis of Biomarker Performance. Neurosurgery 2022, 91, 231–238. [Google Scholar] [CrossRef]
- Murao, A.; Aziz, M.; Wang, H.; Brenner, M.; Wang, P. Release mechanisms of major DAMPs. Apoptosis 2021, 26, 152–162. [Google Scholar] [CrossRef]
- Senhaji, N.; Houssaini, A.S.; Lamrabet, S.; Louati, S.; Bennis, S. Molecular and Circulating Biomarkers in Patients with Glioblastoma. Int. J. Mol. Sci. 2022, 23, 7474. [Google Scholar] [CrossRef]
- Cisneros-Villanueva, M.; Hidalgo-Pérez, L.; Rios-Romero, M.; Cedro-Tanda, A.; Ruiz-Villavicencio, C.A.; Page, K.; Hastings, R.; Fernandez-Garcia, D.; Allsopp, R.; Fonseca-Montaño, M.A.; et al. Cell-free DNA analysis in current cancer clinical trials: A review. Br. J. Cancer 2022, 126, 391–400. [Google Scholar] [CrossRef]
- Palande, V.; Siegal, T.; Detroja, R.; Gorohovski, A.; Glass, R.; Flueh, C.; Kanner, A.A.; Laviv, Y.; Har-Nof, S.; Levy-Barda, A.; et al. Detection of gene mutations and gene-gene fusions in circulating cell-free DNA of glioblastoma patients: An avenue for clinically relevant diagnostic analysis. Mol. Oncol. 2022, 16, 2098–2114. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Wells, G.; Shea, B.; O’Connell, D.; Robertson, J.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomized Studies in Meta-Analysis. 2000. Available online: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 20 December 2020).
- Schwarzer, G. Meta: An R Package for Meta-Analysis. R News 2007, 7, 40–45. [Google Scholar]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Bambury, R.M.; Teo, M.Y.; Power, D.G.; Yusuf, A.; Murray, S.; Battley, J.E.; Drake, C.; O’Dea, P.; Bermingham, N.; Keohane, C.; et al. The association of pre-treatment neutrophil to lymphocyte ratio with overall survival in patients with glioblastoma multiforme. J. Neuro-Oncol. 2013, 114, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Liu, Y.; Li, Q.; Li, Z.; Hou, H.; Wu, A. Pre-treatment neutrophil-to-lymphocyte ratio is associated with neutrophil and T-cell infiltration and predicts clinical outcome in patients with glioblastoma. BMC Cancer 2015, 15, 617. [Google Scholar] [CrossRef]
- Lopes, M.; Carvalho, B.; Vaz, R.; Linhares, P. Influence of neutrophil–lymphocyte ratio in prognosis of glioblastoma multiforme. J. Neuro-Oncol. 2018, 136, 173–180. [Google Scholar] [CrossRef]
- Yersal, Ö.; Odabaşi, E.; Özdemir, Ö.; Kemal, Y. Prognostic significance of pre-treatment neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in patients with glioblastoma. Mol. Clin. Oncol. 2018, 9, 453–458. [Google Scholar] [CrossRef]
- Brenner, A.; Friger, M.; Geffen, D.B.; Kaisman-Elbaz, T.; Lavrenkov, K. The Prognostic Value of the Pretreatment Neutrophil/Lymphocyte Ratio in Patients with Glioblastoma Multiforme Brain Tumors: A Retrospective Cohort Study of Patients Treated with Combined Modality Surgery, Radiation Therapy, and Temozolomide Chemotherapy. Oncology 2019, 97, 255–263. [Google Scholar] [CrossRef]
- Hao, Y.; Li, X.; Chen, H.; Huo, H.; Liu, Z.; Tian, F.; Chai, E. A Cumulative Score Based on Preoperative Neutrophil-Lymphocyte Ratio and Fibrinogen in Predicting Overall Survival of Patients with Glioblastoma Multiforme. World Neurosurg. 2019, 128, e427–e433. [Google Scholar] [CrossRef]
- Gan, Y.; Zhou, X.; Niu, X.; Li, J.; Wang, T.; Zhang, H.; Yang, Y.; Liu, Y.; Mao, Q. Neutrophil/Lymphocyte Ratio Is an Independent Prognostic Factor in Elderly Patients with High-Grade Gliomas. World Neurosurg. 2019, 127, e261–e267. [Google Scholar] [CrossRef]
- Bagley, S.J.; Nabavizadeh, S.A.; Mays, J.J.; Till, J.E.; Ware, J.B.; Levy, S.; Sarchiapone, W.; Hussain, J.; Prior, T.; Guiry, S.; et al. Clinical Utility of Plasma Cell-Free DNA in Adult Patients with Newly Diagnosed Glioblastoma: A Pilot Prospective Study. Clin. Cancer Res. 2020, 26, 397–407. [Google Scholar] [CrossRef]
- Marini, A.; Dobran, M.; Aiudi, D.; Pesaresi, A.; Di Somma, L.G.M.; Iacoangeli, M. Pre-operative hematological markers as predictive factors for overall survival and progression free survival in glioblastomas. Clin. Neurol. Neurosurg. 2020, 197, 106162. [Google Scholar] [CrossRef] [PubMed]
- Mermut, O.; Inanc, B. The Effect of Indicators of Systemic Inflammatory Response on Survival in Glioblastoma Multiforme. Turk. Neurosurg. 2020, 30, 658–665. [Google Scholar] [CrossRef] [PubMed]
- Bagley, S.J.; Till, J.; Abdalla, A.; Sangha, H.K.; Yee, S.S.; Freedman, J.; Black, T.A.; Hussain, J.; Binder, Z.A.; Brem, S.; et al. Association of plasma cell-free DNA with survival in patients with IDH wild-type glioblastoma. Neuro-Oncol. Adv. 2021, 3, vdab011. [Google Scholar] [CrossRef]
- Besiroglu, M.; Shbair, A.T.; Yasin, A.I.; Topcu, A.; Turk, H.M.; Demir, T. Systemic Inflammatory Markers for Prediction of Bevacizumab Benefit in Glioblastoma Multiforme. J. Coll. Physicians Surg. Pak. 2021, 31, 39–44. [Google Scholar] [PubMed]
- Clavreul, A.; Lemée, J.-M.; Soulard, G.; Rousseau, A.; Menei, P. A Simple Preoperative Blood Count to Stratify Prognosis in Isocitrate Dehydrogenase-Wildtype Glioblastoma Patients Treated with Radiotherapy plus Concomitant and Adjuvant Temozolomide. Cancers 2021, 13, 5778. [Google Scholar] [CrossRef]
- Garrett, C.; Becker, T.M.; Lynch, D.; Po, J.; Xuan, W.; Scott, K.F.; De Souza, P. Comparison of neutrophil to lymphocyte ratio and prognostic nutritional index with other clinical and molecular biomarkers for prediction of glioblastoma multiforme outcome. PLoS ONE 2021, 16, e0252614. [Google Scholar] [CrossRef]
- Pasqualetti, F.; Giampietro, C.; Montemurro, N.; Giannini, N.; Gadducci, G.; Orlandi, P.; Natali, E.; Chiarugi, P.; Gonnelli, A.; Cantarella, M.; et al. Old and New Systemic Immune-Inflammation Indexes Are Associated with Overall Survival of Glioblastoma Patients Treated with Radio-Chemotherapy. Genes 2022, 13, 1054. [Google Scholar] [CrossRef]
- Wang, Z.; Li, J.; Yuan, Y.; Li, T.; Zuo, M.; Liu, Y. Prognostic significance of preoperative systemic inflammation response index in newly diagnosed glioblastoma patients underwent gross total resection: A propensity scores matching analysis. World J. Surg. Oncol. 2022, 20, 137. [Google Scholar] [CrossRef]
- Yang, C.; Lan, T.; Wang, Y.; Huang, W.-H.; Li, S.-M.; Li, J.; Li, F.-P.; Li, Y.-R.; Wang, Z.-F.; Li, Z.-Q. Cumulative Scoring Systems and Nomograms for Predicating Survival in Patients with Glioblastomas: A Study Based on Peripheral Inflammatory Markers. Front. Oncol. 2022, 12, 716295. [Google Scholar] [CrossRef]
- Vieira da Cunha, M.L.; Maldaun, M.V.C. Metastasis from glioblastoma multiforme: A meta-analysis. Rev. Da Assoc. Med. Bras. 2019, 65, 424–433. [Google Scholar] [CrossRef]
- Wang, D.-P.; Kang, K.; Lin, Q.; Hai, J. Prognostic Significance of Preoperative Systemic Cellular Inflammatory Markers in Gliomas: A Systematic Review and Meta-Analysis. Clin. Transl. Sci. 2020, 13, 179–188. [Google Scholar] [CrossRef]
- Booth, T.C.; Grzeda, M.; Chelliah, A.; Roman, A.; Al Busaidi, A.; Dragos, C.; Shuaib, H.; Luis, A.; Mirchandani, A.; Alparslan, B.; et al. Imaging Biomarkers of Glioblastoma Treatment Response: A Systematic Review and Meta-Analysis of Recent Machine Learning Studies. Front. Oncol. 2022, 12, 799662. [Google Scholar] [CrossRef] [PubMed]
- Granot, Z.; Jablonska, J. Distinct Functions of Neutrophil in Cancer and Its Regulation. Mediat. Inflamm. 2015, 2015, 701067. [Google Scholar] [CrossRef] [PubMed]
- De Larco, J.E.; Wuertz, B.R.K.; Furcht, L.T. The potential role of neutrophils in promoting the metastatic phenotype of tumors releasing interleukin-8. Clin. Cancer Res. 2004, 10, 4895–4900. [Google Scholar] [CrossRef]
- Ardi, V.C.; Kupriyanova, T.A.; Deryugina, E.I.; Quigley, J.P. Human neutrophils uniquely release TIMP-free MMP-9 to provide a potent catalytic stimulator of angiogenesis. Proc. Natl. Acad. Sci. USA 2007, 104, 20262–20267. [Google Scholar] [CrossRef] [PubMed]
- Jablonska, E.; Puzewska, W.; Grabowska, Z.; Jablonski, J.; Talarek, Ł. VEGF, IL-18 and NO production by neutrophils and their serum levels in patients with oral cavity cancer. Cytokine 2005, 30, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Galon, J.; Costes, A.; Sanchez-Cabo, F.; Kirilovsky, A.; Mlecnik, B.; Lagorce-Pagès, C.; Tosolini, M.; Camus, M.; Berger, A.; Wind, P.; et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 2006, 313, 1960–1964. [Google Scholar] [CrossRef]
- Weng, Y.; Zhang, X.; Han, J.; Ouyang, L.; Liang, M.; Shi, Z.; Liu, A.; Cai, W. Do Selected Blood Inflammatory Markers Combined with Radiological Features Predict. Proliferation Index. in Glioma Patients? World Neurosurg. 2018, 118, e137–e146. [Google Scholar] [CrossRef]
- Cohen, J.T.; Miner, T.J.; Vezeridis, M.P. Is the neutrophil-to-lymphocyte ratio a useful prognostic indicator in melanoma patients? Melanoma Manag. 2020, 7, MMT47. [Google Scholar] [CrossRef]
- Ray-Coquard, I.; Cropet, C.; Van Glabbeke, M.; Sebban, C.; Le Cesne, A.; Judson, I.; Tredan, O.; Verweij, J.; Biron, P.; Labidi, I.; et al. European Organization for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group: Lymphopenia as a prognostic factor for overall survival in advanced carcinomas, sarcomas, and lymphomas. Cancer Res. 2009, 69, 5383–5391. [Google Scholar] [CrossRef]
- Cananzi, F.C.M.; Minerva, E.M.; Samà, L.; Ruspi, L.; Sicoli, F.; Conti, L.; Romario, U.F.; Quagliuolo, V.L. Preoperative monocyte-to-lymphocyte ratio predicts recurrence in gastrointestinal stromal tumors. J. Surg. Oncol. 2019, 119, 12–20. [Google Scholar] [CrossRef]
- Dupré, A.; Malik, H.Z. Inflammation and cancer: What a surgical oncologist should know. Eur. J. Surg. Oncol. 2018, 44, 566–570. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Yao, X.; Xie, X.; Wu, X.; Zheng, C.; Xia, W.; Ma, S. Prognostic value of preoperative NLR, dNLR, PLR and CRP in surgical renal cell carcinoma patients. World J. Urol. 2017, 35, 261–270. [Google Scholar] [CrossRef]
- Bao, Y.; Yang, M.; Jin, C.; Hou, S.; Shi, B.; Shi, J.; Lin, N. Preoperative Hematologic Inflammatory Markers as Prognostic Factors in Patients with Glioma. World Neurosurg. 2018, 119, e710–e716. [Google Scholar] [CrossRef]
- Di Vito, C.; Navone, S.E.; Marfia, G.; Hadi, L.A.; Mancuso, M.E.; Pecci, A.; Crisà, F.M.; Berno, V.; Rampini, P.; Campanella, R.; et al. Platelets from glioblastoma patients promote angiogenesis of tumor endothelial cells and exhibit increased VEGF content and release. Platelets 2017, 28, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Kayhan, A.; Korkmaz, T.S.; Baran, O.; Kemerdere, R.; Yeni, S.N.; Tanriverdi, T. Preoperative Systemic Inflammatory Markers in Different Brain Pathologies: An Analysis of 140 Patients. Turk. Neurosurg. 2019, 29, 799–803. [Google Scholar] [CrossRef]
- Topkan, E.; Kucuk, A.; Ozdemir, Y.; Mertsoylu, H.; Besen, A.A.; Sezen, D.; Bolukbasi, Y.; Pehlivan, B.; Selek, U. Systemic Inflammation Response Index Predicts Survival Outcomes in Glioblastoma Multiforme Patients Treated with Standard Stupp Protocol. J. Immunol. Res. 2020, 2020, 8628540. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.; Nguyen, H.; Drummond, K.; Morokoff, A. Circulating Biomarkers for Glioma: A Review. Neurosurgery 2021, 88, E221–E230. [Google Scholar] [CrossRef]
- Jelski, W.; Mroczko, B. Molecular and Circulating Biomarkers of Brain Tumors. Int. J. Mol. Sci. 2021, 22, 7039. [Google Scholar] [CrossRef] [PubMed]
- Kros, J.M.; Mustafa, D.M.; Dekker, L.J.M.; Smitt, P.E.A.S.; Luider, T.M.; Zheng, P.-P. Circulating glioma biomarkers. Neuro Oncol. 2015, 17, 343–360. [Google Scholar] [CrossRef] [PubMed]
- Tamai, S.; Ichinose, T.; Nakada, M. Liquid biomarkers in glioma. Brain Tumor Pathol. 2023, 40, 66–77. [Google Scholar] [CrossRef] [PubMed]

| Search # | Search Strategy | Items Found |
|---|---|---|
| 1 | “Glioblastoma” (Mesh) | 32,216 |
| 2 | “Glioblastoma/blood” (Mesh) | 326 |
| 3 | “Glioblastoma/immunology” (Mesh) | 1300 |
| 4 | “Glioblastoma/mortality” (Mesh) | 2900 |
| 5 | #1 AND (#2 OR #3 OR #4) | 4340 |
| 6 | “NLR” (tiab) | 13,458 |
| 7 | “PLR” (tiab) | 5803 |
| 8 | “SII” (tiab) | 3635 |
| 9 | “SIRI” (tiab) | 591 |
| 10 | “Cell-free DNA” (tiab) | 6465 |
| 11 | #5 AND (#6 OR #7 OR #8 OR #9 OR #10) | 29 |
| Study | Duration | Sample Size Female/Male | NLR Cut-Off Value [103/µL] | PLR Cut-Off Value [103/µL] | SII Cut-Off Value [103/µL] | SIRI Cut-Off Value [103/µL] | cfDNA Cut-Off Value [ng/mL] | Outcome | Glioblastoma Type |
|---|---|---|---|---|---|---|---|---|---|
| Bambury 2013 [45] | 2004–2009 | 74 (19/65) | 4 | - | - | multivariate | GBM | ||
| Han 2015 [46] | 2010–2014 | 152 (57/95) | 4 | - | - | - | - | multivariate | grade IV |
| Lopes 2018 [47] | 2005–2013 | 140 (42/98) | 5 | - | - | - | - | multivariate | GBM |
| Weng 2018 [19] | 2011–2014 | 239 (108/131) | 4 | - | - | - | - | multivariate | 44% IV grade |
| Yersal 2018 [48] | 2012–2017 | 80 (41/39) | 4 | 135 | - | - | - | univariate | GBM |
| Brenner 2019 [49] | 2005–2016 | 89 (43/46) | 4 | - | - | - | - | multivariate | GBM |
| Hao 2019 [50] | 2012–2017 | 187 (71/116) | 4.1 | 228.6 | - | - | - | uni/multivariate | GBM |
| Gan 2019 [51] | 2014–2018 | 135 (48/89) | 3 | - | - | - | - | multivariate | 84% IV grade |
| Lv 2019 [14] | 2006–2018 | 192 (79/113) | 2.7 | 87 | 718 | - | - | uni/multivariate | IDH -1 mutant and wild-type |
| Bagley 2020 [52] | - | 42 (20/22) | - | - | - | - | 13.4 | multivariate | IDH 1 and 2 wild-type glioblastomas (83%) |
| Marini 2020 [53] | 2013–2019 | 124 (65/59) | 4 | 175 | - | - | - | multivariate | IDH -1 mutant (48%) and wild-type (52%) |
| Mermut 2020 [54] | 2011–2018 | 75 (28/47) | 4 | - | - | - | - | univariate | GBM |
| Bagley 2021 [55] | 2018–2020 | 62 (23/39) | - | - | - | - | 25.2 | multivariate | IDH wild-type glioblastomas |
| Besiroglu 2021 [56] | 2014–2019 | 107 (49/58) | 2.9 | 159 | 785 | - | - | multivariate | GBM |
| Clavreul 2021 [57] | 2012–2020 | 85 (20/65) | 2.42 | 180.9 | 502.39 | 2.55 | - | univariate | IDH-wildtype |
| Garrett 2021 [58] | 2013–2019 | 87 (33/54) | 5.07 | - | - | - | - | univariate | GBM |
| Jarmuzek 2022 [7] | 2004–2021 | 358 (195/163) | 4.56 | - | 2003 | 3.03 | - | multivariate | 66% IV grade |
| Pasqualetti 2022 [59] | 2010–2020 | 77 (34/43) | - | 250 | 1200 | - | - | univariate | IDH 1 and 2 wild-type glioblastomas |
| Shi 2022 [12] | 2014–2018 | 132 (105/127) | 2.54 | 158.56 | 659.1 | 1.78 | - | uni/multivariate | grade IV |
| Wang 2022 [60] | 2015–2019 | 291 (105/106) | 4.86 | - | - | 1.26 | - | multivariate | grade IV |
| Yang 2022 [61] | 2016–2019 | 187 (76/111) | 2 | 213 | - | - | - | uni/multivariate | 47% IV grade |
| Study | Marker | S1 | S2 | S3 | S4 | C1 | O1 | O2 | O3 | Total |
|---|---|---|---|---|---|---|---|---|---|---|
| Bambury 2013 [45] | NLR | * | * | * | 0 | * | * | * | * | 7 |
| Han 2015 [46] | NLR | * | * | * | 0 | ** | * | * | * | 8 |
| Lopes 2018 [47] | NLR | * | * | * | 0 | * | * | * | * | 7 |
| Weng 2018 [19] | NLR | * | * | * | 0 | * | * | * | * | 7 |
| Yersal 2018 [48] | NLR, PLR | * | * | * | 0 | ** | * | * | * | 8 |
| Brenner 2019 [49] | NLR | * | * | * | 0 | ** | * | * | * | 8 |
| Hao 2019 [50] | NLR, PLR | * | * | * | 0 | ** | * | * | * | 8 |
| Gan 2019 [51] | NLR | * | * | * | 0 | * | * | * | * | 7 |
| Lv 2019 [14] | NLR, PLR, SII | * | * | * | 0 | ** | * | * | * | 8 |
| Bagley 2020 [52] | cfDNA | * | * | * | 0 | * | * | * | * | 7 |
| Marini 2020 [53] | NLR, PLR | * | * | * | 0 | ** | * | * | * | 8 |
| Mermut 2020 [54] | NLR | * | * | * | 0 | ** | * | * | * | 8 |
| Bagley 2021 [55] | cfDNA | * | * | * | 0 | * | * | * | * | 7 |
| Besiroglu 2021 [56] | NLR, PLR, SII | * | * | * | 0 | * | * | * | * | 7 |
| Clavreul 2021 [57] | NLR, PLR, SII, SIRI | * | * | * | 0 | ** | * | * | * | 8 |
| Garrett 2021 [58] | NLR | * | * | * | 0 | * | * | * | * | 7 |
| Jarmuzek 2022 [7] | NLR, SII, SIRI | * | * | * | 0 | ** | * | * | * | 8 |
| Pasqualetti 2022 [59] | PLR, SII | * | * | * | 0 | ** | * | * | * | 8 |
| Shi 2022 [12] | NLR, PLR, SII, SIRI | * | * | * | 0 | ** | * | * | * | 8 |
| Wang 2022 [60] | NLR, SIRI | * | * | * | 0 | ** | * | * | * | 8 |
| Yang 2022 [61] | NLR, PLR | * | * | * | 0 | ** | * | * | * | 8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jarmuzek, P.; Kozlowska, K.; Defort, P.; Kot, M.; Zembron-Lacny, A. Prognostic Values of Systemic Inflammatory Immunological Markers in Glioblastoma: A Systematic Review and Meta-Analysis. Cancers 2023, 15, 3339. https://doi.org/10.3390/cancers15133339
Jarmuzek P, Kozlowska K, Defort P, Kot M, Zembron-Lacny A. Prognostic Values of Systemic Inflammatory Immunological Markers in Glioblastoma: A Systematic Review and Meta-Analysis. Cancers. 2023; 15(13):3339. https://doi.org/10.3390/cancers15133339
Chicago/Turabian StyleJarmuzek, Pawel, Klaudia Kozlowska, Piotr Defort, Marcin Kot, and Agnieszka Zembron-Lacny. 2023. "Prognostic Values of Systemic Inflammatory Immunological Markers in Glioblastoma: A Systematic Review and Meta-Analysis" Cancers 15, no. 13: 3339. https://doi.org/10.3390/cancers15133339
APA StyleJarmuzek, P., Kozlowska, K., Defort, P., Kot, M., & Zembron-Lacny, A. (2023). Prognostic Values of Systemic Inflammatory Immunological Markers in Glioblastoma: A Systematic Review and Meta-Analysis. Cancers, 15(13), 3339. https://doi.org/10.3390/cancers15133339