The Potential Effect of General Anesthetics in Cancer Surgery: Meta-Analysis of Postoperative Metastasis and Inflammatory Cytokines
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Study Eligibility Criteria
- All randomized-controlled trials (RCT) and observational longitudinal studies (prospective and retrospective) comparing metastasis and recurrence after surgery with IHNA or TIVA were included.
- Studies reporting metastasis incidence, recurrence incidence, or recurrence rate were included.
- All randomized control trials in adult patients undergoing surgery under general anesthesia of IHNA or TIVA.
- Studies reporting at least one of the cytokines, IL-6, IL-10, or TNF-α.
- Studies including comparisons expressed as mean ± standard deviation or comparison values represented as median.
2.3. Data Extraction
2.4. Statistical Analysis
3. Results
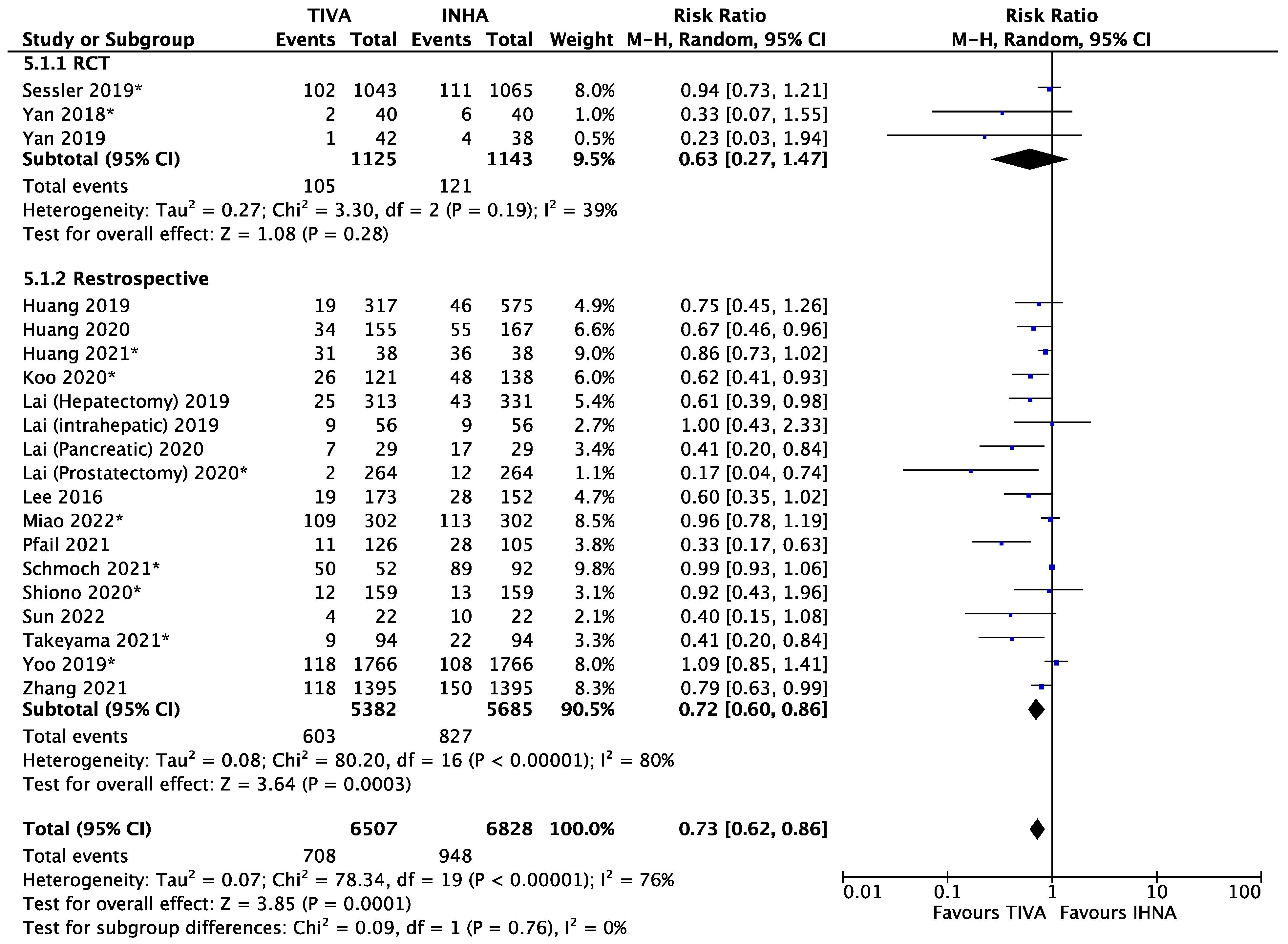
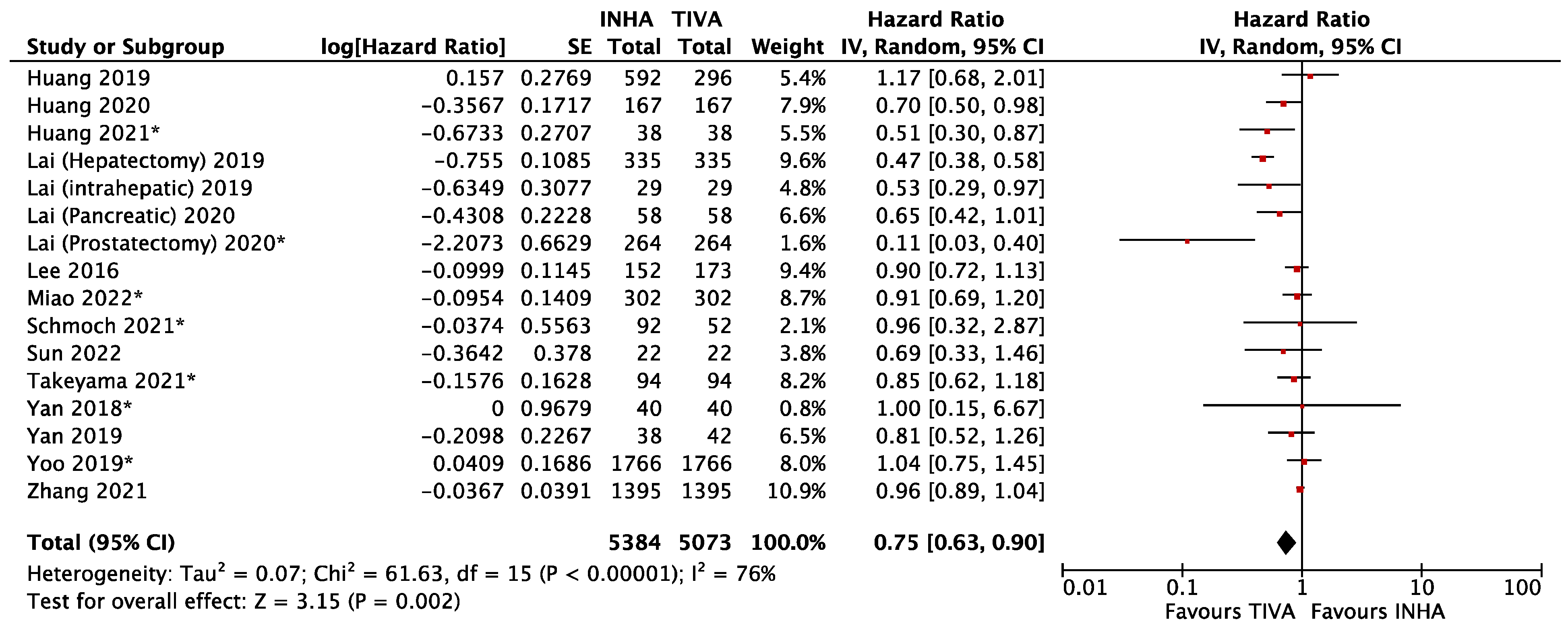
3.1. General Anesthetics and Cancer Metastasis
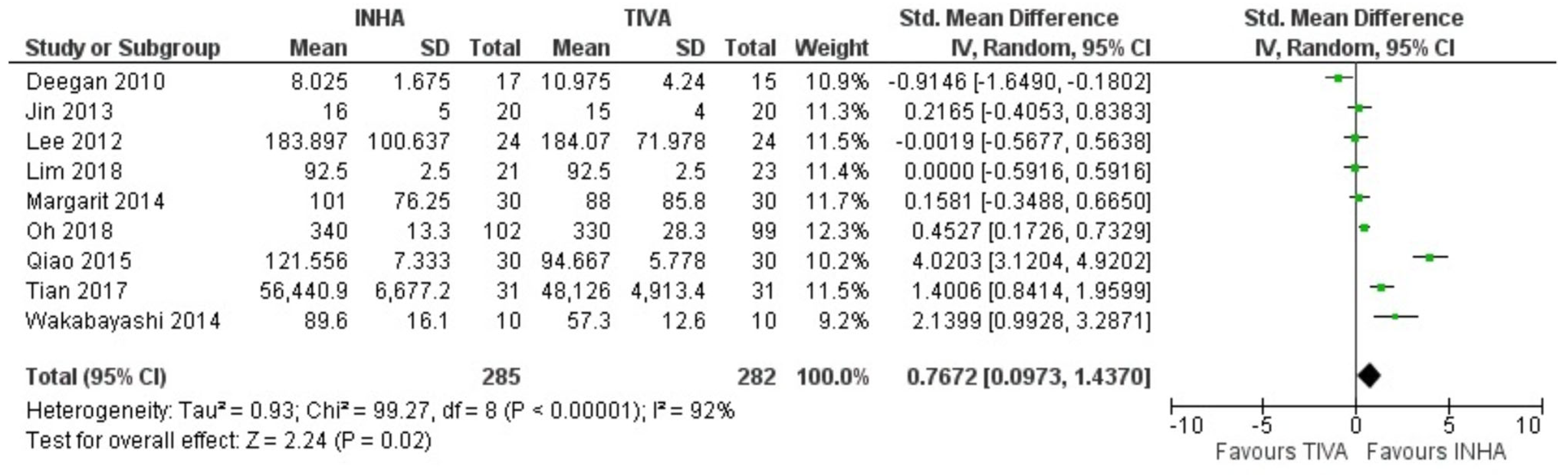
3.2. General Anesthetics and Inflammatory Cytokines
3.3. General Anesthetics and Cancer Metastasis in Pre-Clinical Animal Studies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Horowitz, M.; Neeman, E.; Sharon, E.; Ben-Eliyahu, S. Exploiting the critical perioperative period to improve long-term cancer outcomes. Nat. Rev. Clin. Oncol. 2015, 12, 213–226. [Google Scholar] [CrossRef] [PubMed]
- Wigmore, T.; Gottumukkala, V.; Riedel, B. Making the Case for the Subspecialty of Onco-Anesthesia. Int. Anesthesiol. Clin. 2016, 54, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, J.; Jersky, J.; Katzav, S.; Feldman, M.; Segal, S. Anesthetic Drgs Accelerate the Progression of Postoperative Metastases of Mouse Tumors. J. Clin. Investig. 1981, 68, 678–685. [Google Scholar] [CrossRef] [PubMed]
- Melamed, R.; Bar-Yosef, S.; Shakhar, G.; Shakhar, K.; Ben-Eliyahu, S. Suppression of Natural Killer Cell Activity and Promotion of Tumor Metastasis by Ketamine, Thiopental, and Halothane, but Not by Propofol: Mediating Mechanisms and Prophylactic Measures. Anesth. Analg. 2003, 97, 1331–1339. [Google Scholar] [CrossRef]
- Buckley, A.; McQuaid, S.; Johnson, P.; Buggy, D.J. Effect of anaesthetic technique on the natural killer cell anti-tumour activity of serum from women undergoing breast cancer surgery: A pilot study. Br. J. Anaesth. 2014, 113, 56–62. [Google Scholar] [CrossRef]
- Jin, Z.; Li, R.; Liu, J.; Lin, J. Long-term prognosis after cancer surgery with inhalational anesthesia and total intravenous anesthesia: A systematic review and meta-analysis. Int. J. Physiol. Pathophysiol. Pharmacol. 2019, 11, 83–94. [Google Scholar]
- Desmond, F.; McCormack, J.; Mulligan, N.; Stokes, M.; Buggy, D.J. Effect of anaesthetic technique on immune cell infiltration in breast cancer: A follow-up pilot analysis of a prospective, randomised, investigator-masked study. Anticancer Res. 2015, 35, 1311–1319. [Google Scholar]
- Choi, H.; Hwang, W. Perioperative Inflammatory Response and Cancer Recurrence in Lung Cancer Surgery: A Narrative Review. Front. Surg. 2022, 9, 888630. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Hayden, J.A.; Van Der Windt, D.A.; Cartwright, J.L.; Côté, P.; Bombardier, C. Assessing bias in studies of prognostic factors. Ann. Intern. Med. 2013, 158, 280–286. [Google Scholar] [CrossRef]
- Rohatgi, A. WebPlotDigitizer. 2011. Available online: https://automeris.io/WebPlotDigitizer/ (accessed on 6 December 2022).
- Sharma, D.; Ulaganathan, S.P.; Sharma, V.; Piplani, S.; Niraj, R. Research Square. 2021. Available online: https://www.researchsquare.com/article/rs-828102/v1 (accessed on 6 December 2022).
- Bland, J.M.; Altman, D.G. Statistics notes. Logarithms. BMJ 1996, 312, 700. [Google Scholar] [CrossRef]
- Tierney, J.F.; Stewart, L.A.; Ghersi, D.; Burdett, S.; Sydes, M.R. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007, 8, 16. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Suurmond, R.; van Rhee, H.; Hak, T. Introduction, comparison, and validation of Meta-Essentials: A free and simple tool for meta-analysis. Res. Synth. Methods 2017, 8, 537–553. [Google Scholar] [CrossRef]
- Orwin, R.G. A Fail-Safe N for Effect Size in Meta-Analysis. J. Educ. Stat. 1983, 8, 157–159. [Google Scholar] [CrossRef]
- Huang, N.C.; Lee, M.S.; Lai, H.C.; Lin, H.T.; Huang, Y.H.; Lu, C.H.; Hsu, C.H.; Wu, Z.F. Propofol-based total intravenous anesthesia improves survival compared to desflurane anesthesia in gastric cancer surgery: A retrospective analysis. Medicine 2020, 99, e20714. [Google Scholar] [CrossRef]
- Huang, Y.H.; Lee, M.S.; Lou, Y.S.; Lai, H.C.; Yu, J.C.; Lu, C.H.; Wong, C.S.; Wu, Z.F. Propofol-based total intravenous anesthesia did not improve survival compared to desflurane anesthesia in breast cancer surgery. PLoS ONE 2019, 14, e0224728. [Google Scholar] [CrossRef]
- Lai, H.C.; Lee, M.S.; Lin, C.; Lin, K.T.; Huang, Y.H.; Wong, C.S.; Chan, S.M.; Wu, Z.F. Propofol-based total intravenous anaesthesia is associated with better survival than desflurane anaesthesia in hepatectomy for hepatocellular carcinoma: A retrospective cohort study. Br. J. Anaesth. 2019, 123, 151–160. [Google Scholar] [CrossRef]
- Lai, H.C.; Lee, M.S.; Lin, K.T.; Huang, Y.H.; Chen, J.Y.; Lin, Y.T.; Hung, K.C.; Wu, Z.F. Propofol-based total intravenous anesthesia is associated with better survival than desflurane anesthesia in robot-assisted radical prostatectomy. PLoS ONE 2020, 15, e0230290. [Google Scholar] [CrossRef]
- Lai, H.C.; Lee, M.S.; Liu, Y.T.; Lin, K.T.; Hung, K.C.; Chen, J.Y.; Wu, Z.F. Propofol-based intravenous anesthesia is associated with better survival than desflurane anesthesia in pancreatic cancer surgery. PLoS ONE 2020, 15, e0233598. [Google Scholar] [CrossRef]
- Schmoch, T.; Jungk, C.; Bruckner, T.; Haag, S.; Zweckberger, K.; von Deimling, A.; Brenner, T.; Unterberg, A.; Weigand, M.A.; Uhle, F.; et al. The anesthetist’s choice of inhalational vs. intravenous anesthetics has no impact on survival of glioblastoma patients. Neurosurg. Rev. 2021, 44, 2707–2715. [Google Scholar] [CrossRef] [PubMed]
- Takeyama, E.; Miyo, M.; Matsumoto, H.; Tatsumi, K.; Amano, E.; Hirao, M.; Shibuya, H. Long-term survival differences between sevoflurane and propofol use in general anesthesia for gynecologic cancer surgery. J. Anesth. 2021, 35, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.; Zhang, G.H.; Wang, B.N.; Sun, L.; Zheng, H. Effects of propofol/remifentanil-based total intravenous anesthesia versus sevoflurane-based inhalational anesthesia on the release of VEGF-C and TGF-beta and prognosis after breast cancer surgery: A prospective, randomized and controlled study. BMC Anesthesiol. 2018, 18, 131. [Google Scholar] [CrossRef]
- Miao, L.; Lv, X.; Huang, C.; Li, P.; Sun, Y.; Jiang, H. Long-term oncological outcomes after oral cancer surgery using propofol-based total intravenous anesthesia versus sevoflurane-based inhalation anesthesia: A retrospective cohort study. PLoS ONE 2022, 17, e0268473. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.-Y.; Hsu, C.-L.; Lee, M.-S.D.; Yeh, T.-T.M.; Lai, H.-C.; Wu, K.-L.; Wu, Z.-F.; Tseng, W.-C. Propofol-based total intravenous anesthesia is associated with better survival than desflurane anesthesia in limb-salvage surgery for osteosarcoma: A retrospective analysis. Medicine 2022, 101, e30840. [Google Scholar] [CrossRef]
- Huang, Y.H.; Wu, Z.F.; Lee, M.S.; Lou, Y.S.; Wu, K.L.; Cheng, K.I.; Lai, H.C. Propofol-based total intravenous anesthesia is associated with better survival than desflurane anesthesia in glioblastoma surgery. PLoS ONE 2021, 16, e0255627. [Google Scholar] [CrossRef]
- Zhang, J.; Chang, C.L.; Lu, C.Y.; Chen, H.M.; Wu, S.Y. Paravertebral block in regional anesthesia with propofol sedation reduces locoregional recurrence in patients with breast cancer receiving breast conservative surgery compared with volatile inhalational without propofol in general anesthesia. Biomed. Pharmacother. 2021, 142, 111991. [Google Scholar] [CrossRef]
- Yan, T.; Zhang, G.H.; Cheng, Y.Z.; Wu, L.X.; Liu, X.Y.; Sun, Y.L.; Zheng, H.; Sun, L. Effects of anesthetic technique and surgery on myeloid-derived suppressor cells and prognosis in women who underwent breast cancer surgery: A prospective study. Cancer Manag. Res. 2019, 11, 5513–5522. [Google Scholar] [CrossRef]
- Lai, H.C.; Lee, M.S.; Lin, K.T.; Chan, S.M.; Chen, J.Y.; Lin, Y.T.; Wu, Z.F. Propofol-based total intravenous anesthesia is associated with better survival than desflurane anesthesia in intrahepatic cholangiocarcinoma surgery. Medicine 2019, 98, e18472. [Google Scholar] [CrossRef]
- Yoo, S.; Lee, H.B.; Han, W.; Noh, D.Y.; Park, S.K.; Kim, W.H.; Kim, J.T. Total Intravenous Anesthesia versus Inhalation Anesthesia for Breast Cancer Surgery: A Retrospective Cohort Study. Anesthesiology 2019, 130, 31–40. [Google Scholar] [CrossRef]
- Pfail, J.L.; Katims, A.B.; Gul, Z.; Rosenzweig, S.J.; Razdan, S.; Nathaniel, S.; Martini, A.; Mehrazin, R.; Wiklund, P.N.; Loftus, K.; et al. Can anesthetics affect bladder cancer recurrence? Total intravenous versus volatile anesthesia in patients undergoing robot-assisted radical cystectomy: A single institution retrospective analysis. Urol. Oncol. 2021, 39, 233 e231–233 e238. [Google Scholar] [CrossRef]
- Sessler, D.I.; Pei, L.; Huang, Y.; Fleischmann, E.; Marhofer, P.; Kurz, A.; Mayers, D.B.; Meyer-Treschan, T.A.; Grady, M.; Tan, E.Y. Recurrence of breast cancer after regional or general anaesthesia: A randomised controlled trial. Lancet 2019, 394, 1807–1815. [Google Scholar] [CrossRef]
- Lee, J.H.; Kang, S.H.; Kim, Y.; Kim, H.-A.; Kim, B.S. Effects of propofol-based total intravenous anesthesia on recurrence and overall survival in patients after modified radical mastectomy: A retrospective study. Korean J. Anesthesiol. 2016, 69, 126–132. [Google Scholar] [CrossRef]
- Wu, Z.F.; Lee, M.S.; Wong, C.S.; Lu, C.H.; Huang, Y.S.; Lin, K.T.; Lou, Y.S.; Lin, C.; Chang, Y.C.; Lai, H.C. Propofol-based Total Intravenous Anesthesia Is Associated with Better Survival Than Desflurane Anesthesia in Colon Cancer Surgery. Anesthesiology 2018, 129, 932–941. [Google Scholar] [CrossRef]
- Koo, B.W.; Lim, D.J.; Oh, A.Y.; Na, H.S. Retrospective Comparison between the Effects of Propofol and Inhalation Anesthetics on Postoperative Recurrence of Early- and Intermediate-Stage Hepatocellular Carcinoma. Med. Princ. Pract. 2020, 29, 422–428. [Google Scholar] [CrossRef]
- Oh, C.S.; Lee, J.; Yoon, T.G.; Seo, E.H.; Park, H.J.; Piao, L.; Lee, S.H.; Kim, S.H. Effect of Equipotent Doses of Propofol versus Sevoflurane Anesthesia on Regulatory T Cells after Breast Cancer Surgery. Anesthesiology 2018, 129, 921–931. [Google Scholar] [CrossRef]
- Lim, J.A.; Oh, C.S.; Yoon, T.G.; Lee, J.Y.; Lee, S.H.; Yoo, Y.-B.; Yang, J.H.; Kim, S.H. The effect of propofol and sevoflurane on cancer cell, natural killer cell, and cytotoxic T lymphocyte function in patients undergoing breast cancer surgery: An in vitro analysis. BMC Cancer 2018, 18, 159. [Google Scholar] [CrossRef]
- Deegan, C.A.; Murray, D.; Doran, P.; Moriarty, D.C.; Sessler, D.I.; Mascha, E.; Kavanagh, B.P.; Buggy, D. Anesthetic technique and the cytokine and matrix metalloproteinase response to primary breast cancer surgery. Reg. Anesth. Pain. Med. 2010, 35, 490–495. [Google Scholar] [CrossRef]
- Wakabayashi, S.; Yamaguchi, K.; Kumakura, S.; Murakami, T.; Someya, A.; Kajiyama, Y.; Nagaoka, I.; Inada, E. Effects of anesthesia with sevoflurane and propofol on the cytokine/chemokine production at the airway epithelium during esophagectomy. Int. J. Mol. Med. 2014, 34, 137–144. [Google Scholar] [CrossRef]
- Qiao, Y.; Feng, H.; Zhao, T.; Yan, H.; Zhang, H.; Zhao, X. Postoperative cognitive dysfunction after inhalational anesthesia in elderly patients undergoing major surgery: The influence of anesthetic technique, cerebral injury and systemic inflammation. BMC Anesthesiol. 2015, 15, 154. [Google Scholar] [CrossRef]
- Margarit, S.C.; Vasian, H.N.; Balla, E.; Vesa, S.; Ionescu, D.C. The influence of total intravenous anaesthesia and isoflurane anaesthesia on plasma interleukin-6 and interleukin-10 concentrations after colorectal surgery for cancer: A randomised controlled trial. Eur. J. Anaesthesiol. 2014, 31, 678–684. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.J.; Kim, G.H.; Kim, J.A.; Yang, M.; Ahn, H.J.; Sim, W.S.; Park, K.J.; Jun, B.H. Comparison of pulmonary morbidity using sevoflurane or propofol-remifentanil anesthesia in an Ivor Lewis operation. J. Cardiothorac. Vasc. Anesth. 2012, 26, 857–862. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Zhao, X.; Li, H.; Wang, Z.; Wang, D. Effects of sevoflurane and propofol on the inflammatory response and pulmonary function of perioperative patients with one-lung ventilation. Exp. Ther. Med. 2013, 6, 781–785. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.T.; Duan, X.H.; Yang, Y.F.; Wang, Y.; Bai, Q.-L.; Zhang, X. Effects of propofol or sevoflurane anesthesia on the perioperative inflammatory response, pulmonary function and cognitive function in patients receiving lung cancer resection. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 5515–5522. [Google Scholar]
- Mosser, D.M.; Zhang, X. Interleukin-10: New perspectives on an old cytokine. Immunol. Rev. 2008, 226, 205–218. [Google Scholar] [CrossRef]
- King, A.; Balaji, S.; Le, L.D.; Crombleholme, T.M.; Keswani, S.G. Regenerative Wound Healing: The Role of Interleukin-10. Adv. Wound Care 2014, 3, 315–323. [Google Scholar] [CrossRef]
- Li, R.; Huang, Y.; Lin, J. Distinct effects of general anesthetics on lung metastasis mediated by IL-6/JAK/STAT3 pathway in mouse models. Nat. Commun. 2020, 11, 642. [Google Scholar] [CrossRef]
- Liu, Q.; Li, R.; Lin, J. No Difference Among Inhaled Anesthetics on the Growth and Metastasis of Murine 4T1 Breast Cancers in a Mouse Model of Spontaneous Metastasis. Front. Pharmacol. 2022, 13, 184. [Google Scholar] [CrossRef]
- Mammoto, T.; Mukai, M.; Mammoto, A.; Yamanaka, Y.; Hayashi, Y.; Mashimo, T.; Kishi, Y.; Nakamura, H. Intravenous anesthetic, propofol inhibits invasion of cancer cells. Cancer Lett. 2002, 184, 165–170. [Google Scholar] [CrossRef]
- Liu, Q.; Sheng, Z.; Cheng, C.; Zheng, H.; Lanuti, M.; Liu, R.; Wang, P.; Shen, Y.; Xie, Z. Anesthetic Propofol Promotes Tumor Metastasis in Lungs via GABA(A) R-Dependent TRIM21 Modulation of Src Expression. Adv. Sci. 2021, 8, e2102079. [Google Scholar] [CrossRef]
- Moudgil, G.C.; Singal, D.P. Halothane and isoflurane enhance melanoma tumour metastasis in mice. Can. J. Anaesth. 1997, 44, 90–94. [Google Scholar] [CrossRef]
- Lu, N.; Piao, M.H.; Feng, C.S.; Yuan, Y. Isoflurane promotes epithelial-to-mesenchymal transition and metastasis of bladder cancer cells through HIF-1α-β-catenin/Notch1 pathways. Life Sci. 2020, 258, 118154. [Google Scholar] [CrossRef]
- Maeda, Y.; Takeuchi, H.; Matsuda, S.; Okamura, A.; Fukuda, K.; Miyasho, T.; Nakamura, R.; Suda, K.; Wada, N.; Kawakubo, H.; et al. Clinical significance of preoperative serum concentrations of interleukin-6 as a prognostic marker in patients with esophageal cancer. Esophagus 2020, 17, 279–288. [Google Scholar] [CrossRef]
- Shimazaki, J.; Goto, Y.; Nishida, K.; Tabuchi, T.; Motohashi, G.; Ubukata, H.; Tabuchi, T. In patients with colorectal cancer, preoperative serum interleukin-6 level and granulocyte/lymphocyte ratio are clinically relevant biomarkers of long-term cancer progression. Oncology 2013, 84, 356–361. [Google Scholar] [CrossRef]
- Barea, J.C.; De la Gala, F.; Piñeiro, P.; Reyes, A.; Simón, C.; Rancan, L.; Vara, E.; Paredes, S.; Bellón, J.M.; Martinez, I.G. Influence of postoperative complications on long-term outcome after oncologic lung resection surgery. Substudy of a randomized control trial. J. Clin. Monit. Comput. 2021, 35, 1183–1192. [Google Scholar] [CrossRef]
- Zhang, X.; Claerhout, S.; Prat, A.; Dobrolecki, L.E.; Petrovic, I.; Lai, Q.; Landis, M.D.; Wiechmann, L.; Schiff, R.; Giuliano, M.; et al. A renewable tissue resource of phenotypically stable, biologically and ethnically diverse, patient-derived human breast cancer xenograft models. Cancer Res. 2013, 73, 4885–4897. [Google Scholar] [CrossRef]

| Outcome | Anesthesia Term | Cancer Term |
|---|---|---|
| Metastasis/recurrence | TIVA/total intravenous anesthesia/propofol/inhaled anesthesia/volatile anesthesia | Cancer/malignancy/tumor/neoplasm |
| Cytokine/interleukin | TIVA/total intravenous anesthesia/propofol/inhaled anesthesia/volatile anesthesia | Cancer/malignancy/tumor/neoplasm |
| STUDY | EXPERIMENTAL MODEL | CANCER TYPE | ANESTHESIA | SURGERY | OUTCOMES | RESULTS | MECHANISM |
|---|---|---|---|---|---|---|---|
| FREEMAN 2019 | 4T1 orthotopic breast cancer spontaneous metastasis mouse model | Murine 4T1 breast cancer | Sevoflurane vs. sevoflurane + propofol vs. sevoflurane + lidocaine | Y | Post-operative pulmonary and hepatic metastasis; serum VEGF and IL-6 level at final | Propofol and lidocaine reduced pulmonary metastasis; No difference for hepatic metastasis or serum IL-6, VEGF at the end of observation | |
| LI 2020 | orthotopic breast cancer spontaneous metastasis mouse model | Human MDA-MB-231 breast cancer and murine 4T1 breast cancer | Sevoflurane vs. Propofol | Y | Post-operative pulmonary metastasis; post-operative IL-6 | Surgery under sevoflurane significantly increased lung metastasis than with propofol; Sevoflurane increased serum IL-6 and infiltration of CD11b+ myeloid cells into lung | Sevoflurane induced pro-metastatic effects by activation of IL-6/STAT3 pathway and infiltrated CD11b+ cells. |
| LIU 2021 | Experimental metastasis model | Human colorectal carcinoma | vehicle (DMSO) vs. propofol | N | Pulmonary metastasis formation | Propofol promote tumor metastasis to the lungs as compared to control | Propofol enhanced adhesion and extension of tumor cells to endothelial cells by activation of GABAAR-dependent TRIM21 modulation of Src expression |
| LIU 2022 | 4T1 orthotopic breast cancer spontaneous metastasis mouse model | Murine 4T1 breast cancer | Isoflurane vs. sevoflurane vs. desflurane | Y | Post-operative pulmonary metastasis; serum level of IL-6, CCL-1, MCP-1, and VEGF at final | No difference in pulmonary metastasis or inflammatory cytokines under different inhalational anesthetics | |
| LU 2020 | Orthotopic tumor model and experimental metastasis model | Human T24 bladder cancer | control vs. isoflurane | N | Primary tumor growth; hepatic metastasis | Isoflurane exposure accelerated formation of primary tumor and hepatic metastases | Isoflurane promotes epithelial-mesenchymal transition and metastasis by HIF-1alpha-beta-catanin/Notch1 pathway |
| MAMMOTO 2002 | Subcutaneous inoculation | Murine osteosarcoma | vehicle (DMSO) vs. propofol | N | Primary tumor growth; pulmonary metastatic nodule | No difference in primary tumor volume; Continuous infusion of propofol inhibited pulmonary metastasis of LM 8 cells in mice | |
| MOUDGIL 1997 | Experimental metastasis model | Murine B16 melanoma | control vs. halothane vs. isoflurane | N | Pulmonary metastasis | More metastases were observed in animals’ exposure to halothane or isoflurane than in the control |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, R.; Mukherjee, M.B.; Jin, Z.; Liu, H.; Lin, K.; Liu, Q.; Dilger, J.P.; Lin, J. The Potential Effect of General Anesthetics in Cancer Surgery: Meta-Analysis of Postoperative Metastasis and Inflammatory Cytokines. Cancers 2023, 15, 2759. https://doi.org/10.3390/cancers15102759
Li R, Mukherjee MB, Jin Z, Liu H, Lin K, Liu Q, Dilger JP, Lin J. The Potential Effect of General Anesthetics in Cancer Surgery: Meta-Analysis of Postoperative Metastasis and Inflammatory Cytokines. Cancers. 2023; 15(10):2759. https://doi.org/10.3390/cancers15102759
Chicago/Turabian StyleLi, Ru, Mousumi Beto Mukherjee, Zhaosheng Jin, Hengrui Liu, Kevin Lin, Qiuyue Liu, James P. Dilger, and Jun Lin. 2023. "The Potential Effect of General Anesthetics in Cancer Surgery: Meta-Analysis of Postoperative Metastasis and Inflammatory Cytokines" Cancers 15, no. 10: 2759. https://doi.org/10.3390/cancers15102759
APA StyleLi, R., Mukherjee, M. B., Jin, Z., Liu, H., Lin, K., Liu, Q., Dilger, J. P., & Lin, J. (2023). The Potential Effect of General Anesthetics in Cancer Surgery: Meta-Analysis of Postoperative Metastasis and Inflammatory Cytokines. Cancers, 15(10), 2759. https://doi.org/10.3390/cancers15102759









