Inhibitory Potential of Resveratrol in Cancer Metastasis: From Biology to Therapy
Abstract
Simple Summary
Abstract
1. Introduction
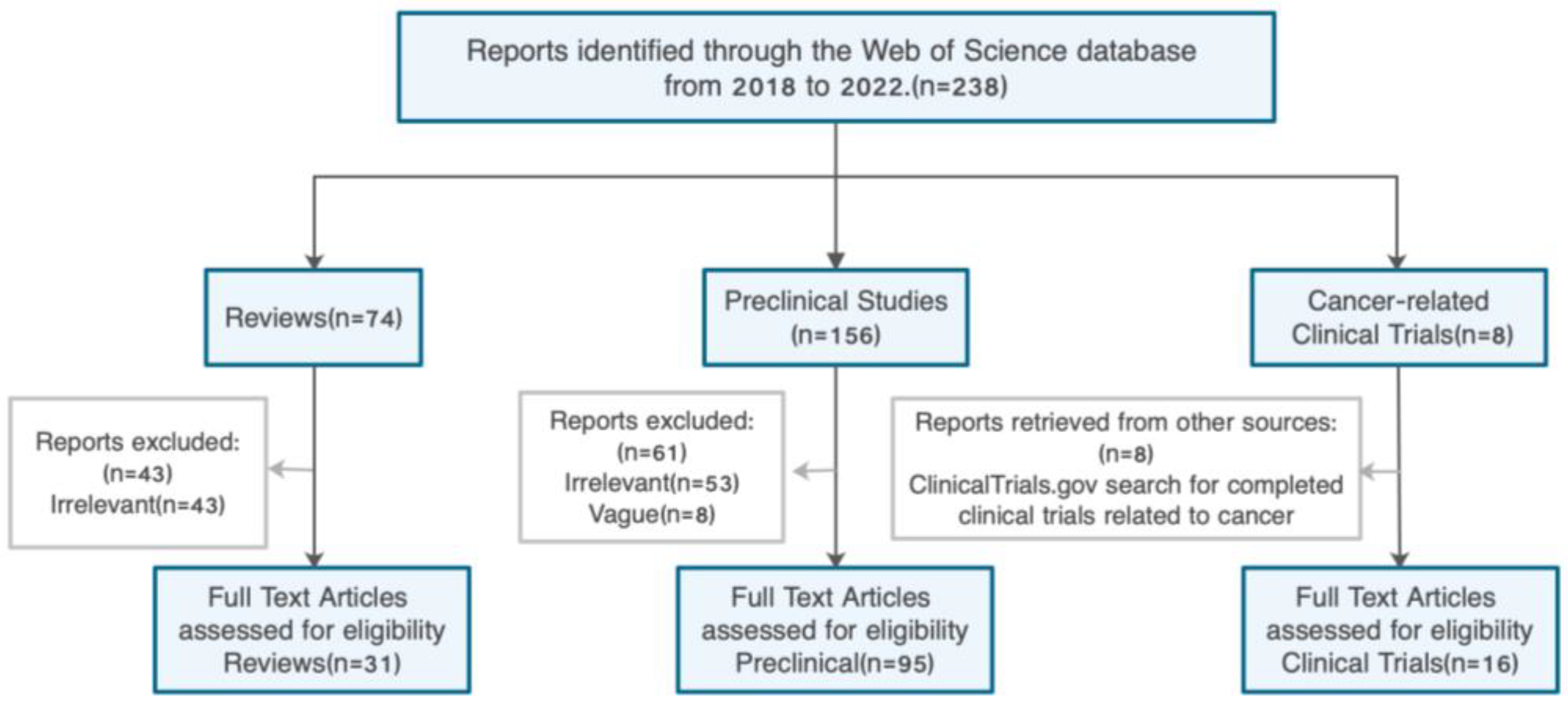
2. Literature Search
3. The Pathogenesis of Cancer Metastasis
4. Factors Associated with Cancer Metastasis
4.1. Angiogenesis
4.2. Epithelial–Mesenchymal Transition
4.3. Cancer Stem Cells
4.4. Tumor Microenvironment
4.5. Inflammation
4.6. Genetic and Epigenetic Factors
4.7. Extracellular Vesicles
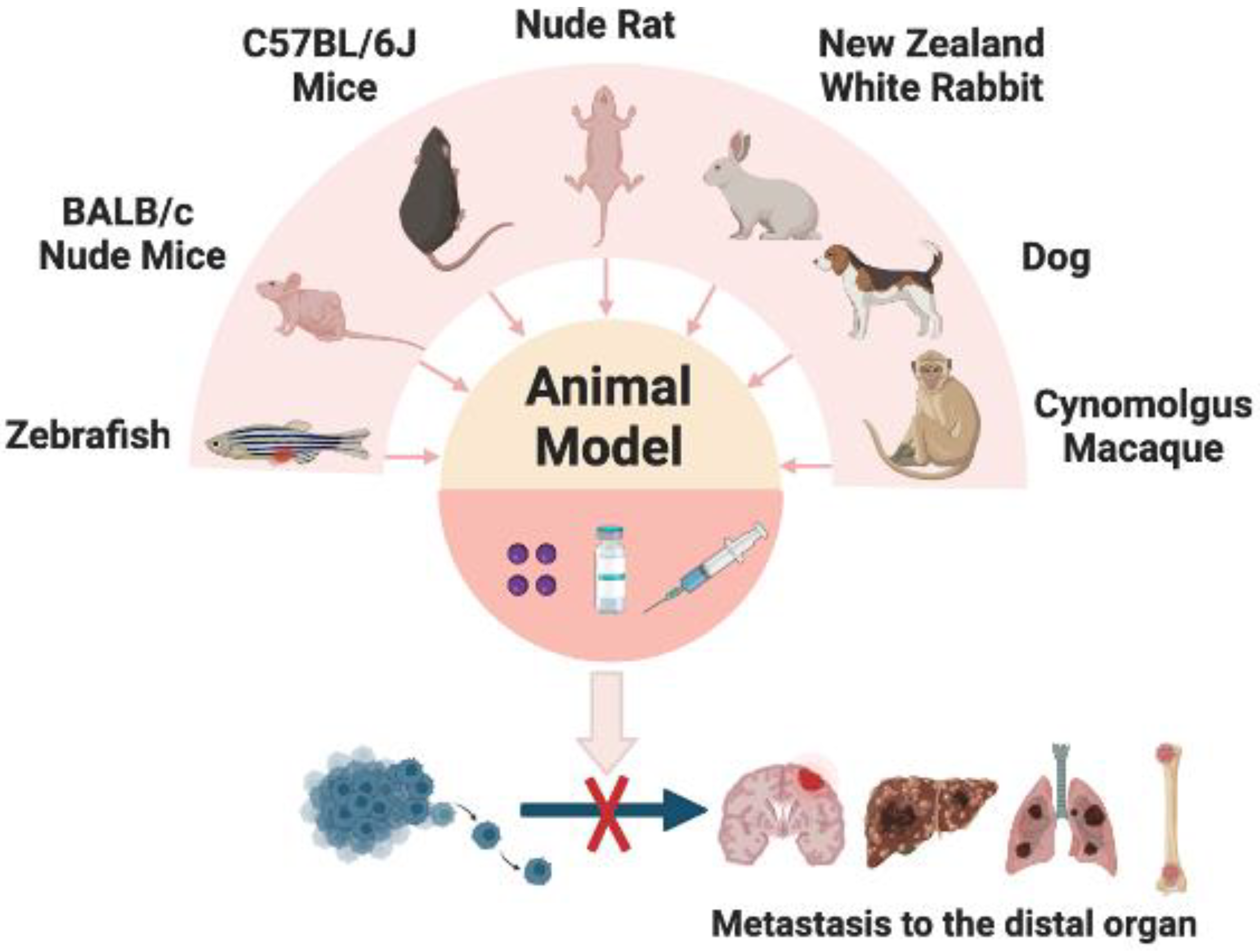
5. In Vivo Models of Metastatic Cancer
6. Resveratrol Inhibits Tumor Growth and Metastasis
6.1. In Vitro Studies of Resveratrol on Anticancer Activity
| Cancer Type | Biological Test | IC50 or Dose | Molecular Mechanisms | References |
|---|---|---|---|---|
| Colorectal cancer | In vitro (HCT116) | 5 µM | RES reduced the secretion of T-lymphocyte/fibroblast (TNF-β, TGF-β3) proteins, antagonized the T-lymphocyte/fibroblast-promoting NF-κB activation | [63] |
| Colon cancer | In vitro (HCT116, RKO, SW480) | 5 μM | RES inhibiting NF-κB pathway and focal adhesion kinase (FAK) regulation | [64] |
| Prostate cancer | In vitro (DU145 and PC3) | 50 μM | TRAF6/NF-kappa B/SLUG signaling pathway | [65] |
| Gastric cancer | In vitro (AGS and MKN45) | 5–25 μM | RES increases SOD activity but decreases NF-κB transcriptional activity | [66] |
| Colorectal cancer | In vitro (HCT116) | 5 μM | RES can block TNF-β/TNF-β-receptor-induced activation of NF-κB | [67] |
| Cervical cancer | In vitro (HeLa, SiHa); In vivo (BALB/C nude mice) | 10–40 μM | RES suppressed inactivating phosphorylation of STAT3 at Tyr705 | [69] |
| Ovarian cancer | In vitro (SKOV3, OVCAR3, OAW42, SKOV3-GFP-LC3) | 100 µM | PI3K-AKT, JAK-STAT and Hedgehog pathway | [70] |
| Gastric cancer | In vitro (SGC-7901) | 25, 50, or 100 μM | Hedgehog pathway | [71] |
| Gastric cancer | In vitro (SGC7901); In vivo (NOD/SCID mice) | 50 or 100 μM; 10 or 20 μM (intratumorally injection) | Raf/MAPK signaling pathway | [73] |
| Oral cancer | In vitro (CAL-27) | 10, 20, or 40 μM | ZNF750/RAC1 signaling pathway | [74] |
| Oral cancer | In vitro (CAR) | 50 μM | ERK/p-38 signaling pathway | [75] |
| Renal carcinoma | In vitro (ACHN and A498) | 132.9 ± 1.064 μM in ACHN, and 112.8 ± 1.191 μM in A498 | Akt and ERK1/2 signaling pathways | [76] |
| Colorectal cancer | In vitro (HT-29 and HCT 116) | 100 μM | RES binds and activates RKIP protein | [77] |
| Ovarian cancer | In vitro (A2780 and SKOV3); In vivo (BALB/c nude mice) | In A2780 and SKOV3 cells were 196.01 ± 33.09 μM and 56.99 ± 26.91 μM; 100 mg/kg/day, for 18 days (oral) | AMPK/mTOR signaling pathway | [79] |
| Lung cancer | In vitro (A549) | 50 μM | RES can protect mitochondria during EMT occurrence | [81] |
| Breast cancer | In vitro (4T1); In vivo (BALB/c nude mice) | 25 or 50 μM; 160 mg/kg (oral) | SIRT3/AMPK/autophagy signaling pathway | [82] |
| Gastric cancer | In vitro (SGC-790) | 5–20 μM | weakening the Hippo-YAP signaling pathway | [83] |
| Gastric cancer | In vitro (AGC, HGC-27); In vivo (BALB/c nude mice) | (20-CM, i.p. for 35 days) | Wnt/β-catenin signaling pathway | [85] |
| Ovarian cancer | In vitro (OVCAR3, OAW42, KURAMOCHI) | 10 µM | miR-1305 downregulation | [87] |
| Ovarian cancer | In vitro (OV-90 and SKOV-3) | 100 µM | miR-34a downregulation | [88] |
| Gastric cancer | In vitro (SGC7901, GES-1, MGC803, and AGS) | 50 or 75 µM | miR-155-5p downregulation | [89] |
| Gastric cancer | In vitro (SGC7901) | 1 or 5 µM | MALAT1/miR-383-5p/DDIT4 | [90] |
| Osteosarcoma | In vitro (U2OS and MG63) | 5, 10 or 20 µM | miR-139-5p/NOTCH1 | [91] |
| TNBC | In vitro (MDA-MB-231) | 16.37 ± 4.72 μM | Na+-dependent Pi transporter is inhibited by RES | [92] |
| Prostate cancer | In vitro (DU145 and PC3) | 123.90 ± 9.78 μM | Mg2+ influx via TRPM7 promotes cell migration by inducing EMT | [93] |
6.2. Research Progress of Resveratrol as Anticancer Agent in Clinical Trials
7. Pharmacokinetics and Toxicity Studies of Resveratrol
7.1. Issues of Pharmacokinetics
7.2. Toxicity Effects of Resveratrol
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Steeg, P.S. Tumor metastasis: Mechanistic insights and clinical challenges. Nat. Med. 2006, 12, 895–904. [Google Scholar] [CrossRef] [PubMed]
- Ren, B.X.; Kwah, M.X.Y.; Liu, C.L.; Ma, Z.W.; Shanmugam, M.K.; Ding, L.W.; Xiang, X.Q.; Ho, P.C.L.; Wang, L.Z.; Ong, P.S.; et al. Resveratrol for cancer therapy: Challenges and future perspectives. Cancer Lett. 2021, 515, 63–72. [Google Scholar] [CrossRef]
- Fan, E.; Zhang, K.; Jiang, S.; Yan, C.; Bai, Y. Analysis of trans-resveratrol in grapes by micro-high performance liquid chromatography. Anal. Sci. 2008, 24, 1019–1023. [Google Scholar] [CrossRef]
- Fan, E.G.; Lin, S.; Du, D.L.; Jia, Y.J.; Kang, L.; Zhang, K. Current separative strategies used for resveratrol determination from natural sources. Anal. Methods 2011, 3, 2454–2462. [Google Scholar] [CrossRef]
- Kiskova, T.; Kassayova, M. Resveratrol Action on Lipid Metabolism in Cancer. Int. J. Mol. Sci. 2019, 20, 2704. [Google Scholar] [CrossRef] [PubMed]
- Nonomura, S.; Kanagawa, H.; Makimoto, A. Chemical Constituents of Polygonaceous Plants. I. Studies on the Components of Ko-J O-Kon. (Polygonum Cuspidatum Sieb. Et Zucc.). Yakugaku Zasshi 1963, 83, 988–990. [Google Scholar] [CrossRef]
- Jang, M.; Cai, L.; Udeani, G.O.; Slowing, K.V.; Thomas, C.F.; Beecher, C.W.; Fong, H.H.; Farnsworth, N.R.; Kinghorn, A.D.; Mehta, R.G.; et al. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science 1997, 275, 218–220. [Google Scholar] [CrossRef]
- Kimura, Y.; Okuda, H. Resveratrol isolated from Polygonum cuspidatum root prevents tumor growth and metastasis to lung and tumor-induced neovascularization in Lewis lung carcinoma-bearing mice. J. Nutr. 2001, 131, 1844–1849. [Google Scholar] [CrossRef]
- Wenzel, E.; Somoza, V. Metabolism and bioavailability of trans-resveratrol. Mol. Nutr. Food Res. 2005, 49, 472–481. [Google Scholar] [CrossRef]
- Walle, T.; Hsieh, F.; DeLegge, M.H.; Oatis, J.E., Jr.; Walle, U.K. High absorption but very low bioavailability of oral resveratrol in humans. Drug Metab. Dispos. 2004, 32, 1377–1382. [Google Scholar] [CrossRef] [PubMed]
- Yao, Q.; Hou, S.X.; He, W.L.; Feng, J.L.; Wang, X.C.; Fei, H.X.; Chen, Z.H. Study on the preparation of resveratrol chitosan nanoparticles with free amino groups on the surface. Zhongguo Zhong Yao Za Zhi 2006, 31, 205–208. [Google Scholar]
- Gupta, G.P.; Massague, J. Cancer metastasis: Building a framework. Cell 2006, 127, 679–695. [Google Scholar] [CrossRef] [PubMed]
- Potente, M.; Gerhardt, H.; Carmeliet, P. Basic and Therapeutic Aspects of Angiogenesis. Cell 2011, 146, 873–887. [Google Scholar] [CrossRef] [PubMed]
- Prager, G.W.; Poettler, M. Angiogenesis in cancer. Basic mechanisms and therapeutic advances. Hamostaseologie 2012, 32, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Bielenberg, D.R.; Zetter, B.R. The Contribution of Angiogenesis to the Process of Metastasis. Cancer J. 2015, 21, 267–273. [Google Scholar] [CrossRef]
- Folkman, J. Tumor angiogenesis: Therapeutic implications. N. Engl. J. Med. 1971, 285, 1182–1186. [Google Scholar] [CrossRef]
- Rajabi, M.; Mousa, S.A. The Role of Angiogenesis in Cancer Treatment. Biomedicines 2017, 5, 34. [Google Scholar] [CrossRef]
- Pastushenko, I.; Blanpain, C. EMT Transition States during Tumor Progression and Metastasis. Trends Cell Biol. 2019, 29, 212–226. [Google Scholar] [CrossRef]
- Lambert, A.W.; Weinberg, R.A. Linking EMT programmes to normal and neoplastic epithelial stem cells. Nat. Rev. Cancer 2021, 21, 325–338. [Google Scholar] [CrossRef]
- Ma, Z.W.; Wang, L.Z.; Cheng, J.T.; Lam, W.S.T.; Ma, X.; Xiang, X.Q.; Wong, A.L.A.; Goh, B.C.; Gong, Q.; Sethi, G.; et al. Targeting Hypoxia-Inducible Factor-1-Mediated Metastasis for Cancer Therapy. Antioxid. Redox Signal. 2021, 34, 1484–1497. [Google Scholar] [CrossRef] [PubMed]
- Puisieux, A.; Brabletz, T.; Caramel, J. Oncogenic roles of EMT-inducing transcription factors. Nat. Cell Biol. 2014, 16, 488–494. [Google Scholar] [CrossRef]
- Das, V.; Bhattacharya, S.; Chikkaputtaiah, C.; Hazra, S.; Pal, M. The basics of epithelial-mesenchymal transition (EMT): A study from a structure, dynamics, and functional perspective. J. Cell. Physiol. 2019, 5, 14535–14555. [Google Scholar] [CrossRef] [PubMed]
- Mittal, V. Epithelial Mesenchymal Transition in Tumor Metastasis. Annu. Rev. Pathol. 2018, 13, 395–412. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Li, Q. Cancer stem cells and tumor metastasis (Review). Int. J. Oncol. 2014, 44, 1806–1812. [Google Scholar] [CrossRef]
- Sampieri, K.; Fodde, R. Cancer stem cells and metastasis. Semin. Cancer Biol. 2012, 22, 187–193. [Google Scholar] [CrossRef]
- Nandy, S.B.; Lakshmanaswamy, R. Cancer Stem Cells and Metastasis. Prog. Mol. Biol. Transl. Sci. 2017, 151, 137–176. [Google Scholar] [CrossRef]
- Arneth, B. Tumor Microenvironment. Medicina 2019, 56, 15. [Google Scholar] [CrossRef]
- Paget, S. The distribution of secondary growths in cancer of the breast. Cancer Metastasis Rev. 1989, 8, 98–101. [Google Scholar] [CrossRef]
- Fidler, I.J. The pathogenesis of cancer metastasis: The ‘seed and soil’ hypothesis revisited. Nat. Rev. Cancer 2003, 3, 453–458. [Google Scholar] [CrossRef]
- Cox, T.R.; Rumney, R.M.H.; Schoof, E.M.; Perryman, L.; Hoye, A.M.; Agrawal, A.; Bird, D.; Ab Latif, N.; Forrest, H.; Evans, H.R.; et al. The hypoxic cancer secretome induces pre-metastatic bone lesions through lysyl oxidase. Nature 2015, 522, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Li, X.; Zhang, C.; Yang, Y.; Jiang, J.; Wu, C. Tumor-associated macrophages in cancers. Clin. Transl. Oncol. 2016, 18, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Pollard, J.W. Trophic macrophages in development and disease. Nat. Rev. Immunol. 2009, 9, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Neophytou, C.M.; Panagi, M.; Stylianopoulos, T.; Papageorgis, P. The Role of Tumor Microenvironment in Cancer Metastasis: Molecular Mechanisms and Therapeutic Opportunities. Cancers 2021, 13, 2053. [Google Scholar] [CrossRef]
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef]
- Qian, B.Z. Inflammation fires up cancer metastasis. Semin. Cancer Biol. 2017, 47, 170–176. [Google Scholar] [CrossRef]
- Gobel, A.; Dell’Endice, S.; Jaschke, N.; Pahlig, S.; Shahid, A.; Hofbauer, L.C.; Rachner, T.D. The Role of Inflammation in Breast and Prostate Cancer Metastasis to Bone. Int. J. Mol. Sci. 2021, 22, 5078. [Google Scholar] [CrossRef]
- Chatterjee, A.; Rodger, E.J.; Eccles, M.R. Epigenetic drivers of tumourigenesis and cancer metastasis. Semin. Cancer Biol. 2018, 51, 149–159. [Google Scholar] [CrossRef]
- Herman, J.G.; Baylin, S.B. Gene silencing in cancer in association with promoter hypermethylation. N. Engl. J. Med. 2003, 349, 2042–2054. [Google Scholar] [CrossRef]
- Baranwal, S.; Alahari, S.K. miRNA control of tumor cell invasion and metastasis. Int. J. Cancer 2010, 126, 1283–1290. [Google Scholar] [CrossRef]
- Weng, J.; Xiang, X.; Ding, L.; Wong, A.L.; Zeng, Q.; Sethi, G.; Wang, L.; Lee, S.C.; Goh, B.C. Extracellular vesicles, the cornerstone of next-generation cancer diagnosis? Semin. Cancer Biol. 2021, 74, 105–120. [Google Scholar] [CrossRef] [PubMed]
- Yokoi, A.; Ochiya, T. Exosomes and extracellular vesicles: Rethinking the essential values in cancer biology. Semin. Cancer Biol. 2021, 74, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Kosaka, N.; Yoshioka, Y.; Fujita, Y.; Ochiya, T. Versatile roles of extracellular vesicles in cancer. J. Clin. Investig. 2016, 126, 1163–1172. [Google Scholar] [CrossRef] [PubMed]
- Akoto, T.; Saini, S. Role of Exosomes in Prostate Cancer Metastasis. Int. J. Mol. Sci. 2021, 22, 3528. [Google Scholar] [CrossRef]
- Mo, Z.; Cheong, J.Y.A.; Xiang, L.; Le, M.T.N.; Grimson, A.; Zhang, D.X. Extracellular vesicle-associated organotropic metastasis. Cell Prolif. 2021, 54, e12948. [Google Scholar] [CrossRef]
- Cekanova, M.; Rathore, K. Animal models and therapeutic molecular targets of cancer: Utility and limitations. Drug Des. Dev. Ther. 2014, 8, 1911–1922. [Google Scholar] [CrossRef]
- Astell, K.R.; Sieger, D. Zebrafish In Vivo Models of Cancer and Metastasis. Cold Spring Harb. Perspect. Med. 2020, 10, a037077. [Google Scholar] [CrossRef]
- Tat, J.; Liu, M.; Wen, X.Y. Zebrafish cancer and metastasis models for in vivo drug discovery. Drug Discov. Today Technol. 2013, 10, e83–e89. [Google Scholar] [CrossRef]
- Chen, L.; Groenewoud, A.; Tulotta, C.; Zoni, E.; Kruithof-de Julio, M.; van der Horst, G.; van der Pluijm, G.; Ewa Snaar-Jagalska, B. A zebrafish xenograft model for studying human cancer stem cells in distant metastasis and therapy response. Methods Cell Biol. 2017, 138, 471–496. [Google Scholar] [CrossRef]
- Savio, M.; Ferraro, D.; Maccario, C.; Vaccarone, R.; Jensen, L.D.; Corana, F.; Mannucci, B.; Bianchi, L.; Cao, Y.H.; Stivala, L.A. Resveratrol analogue 4,4′-dihydroxy-trans-stilbene potently inhibits cancer invasion and metastasis. Sci. Rep. 2016, 6, 19973. [Google Scholar] [CrossRef]
- Paschall, A.V.; Liu, K.B. An Orthotopic Mouse Model of Spontaneous Breast Cancer Metastasis. JoVE J. Vis. Exp. 2016, 114, e54040. [Google Scholar] [CrossRef]
- Jin, X.; Demere, Z.; Nair, K.; Ali, A.; Ferraro, G.B.; Natoli, T.; Deik, A.; Petronio, L.; Tang, A.A.; Zhu, C.; et al. A metastasis map of human cancer cell lines. Nature 2020, 588, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, K.; Walker, S.M.; Jessup, J.M.; Fidler, I.J. In vivo selection of highly metastatic cells from surgical specimens of different primary human colon carcinomas implanted into nude mice. Cancer Res. 1988, 48, 1943–1948. [Google Scholar]
- Cespedes, M.V.; Espina, C.; Garcia-Cabezas, M.A.; Trias, M.; Boluda, A.; Gomez del Pulgar, M.T.; Sancho, F.J.; Nistal, M.; Lacal, J.C.; Mangues, R. Orthotopic microinjection of human colon cancer cells in nude mice induces tumor foci in all clinically relevant metastatic sites. Am. J. Pathol. 2007, 170, 1077–1085. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, J.; Guan, Y.B.; Li, H.L.; Huang, L.Y.; Tang, H.L.; He, J.X. Establishment of an orthotopic lung cancer model in nude mice and its evaluation by spiral CT. J. Thorac. Dis. 2012, 4, 141–145. [Google Scholar] [CrossRef]
- De Meulenaere, V.; Descamps, B.; De Wever, O.; Vanhove, C.; Deblaere, K. In vivo selection of the MDA-MB-231br/eGFP cancer cell line to obtain a clinically relevant rat model for triple negative breast cancer brain metastasis. PLoS ONE 2020, 15, e0243156. [Google Scholar] [CrossRef]
- Leroy, B.E.; Northrup, N. Prostate cancer in dogs: Comparative and clinical aspects. Vet. J. 2009, 180, 149–162. [Google Scholar] [CrossRef]
- Kerboeuf, M.; Koppang, E.O.; Haaland, A.H.; Lingaas, F.; Bruland, O.S.; Teige, J.; Moe, L. Early immunohistochemical detection of pulmonary micrometastases in dogs with osteosarcoma. Acta Vet. Scand. 2021, 63, 41. [Google Scholar] [CrossRef]
- Knapp, D.W.; Ramos-Vara, J.A.; Moore, G.E.; Dhawan, D.; Bonney, P.L.; Young, K.E. Urinary bladder cancer in dogs, a naturally occurring model for cancer biology and drug development. ILAR J. 2014, 55, 100–118. [Google Scholar] [CrossRef]
- Huang, Y.W.; Guan, M.F.; Liu, J.H.; Lan, C.Y.; Wan, T.; Huang, X. Large animal model for retroperitoneal lymphatic and lung metastasis. Mol. Med. Rep. 2013, 8, 1617–1622. [Google Scholar] [CrossRef]
- Sun, H.; Zhang, T.; Gui, B.; Song, L.W.; Li, L.; Pan, Q.; Yang, R.F.; Shao, Y.; Liu, X.Y.; Sun, Z.Y. Establishment of prostate cancer in cynomolgus macaque animal model by orthotropic inoculation of PC-3 cancer cells in situ. Eur. J. Oncol. 2012, 17, 189–203. [Google Scholar]
- Suhail, M.; Tarique, M.; Muhammad, N.; Naz, H.; Hafeez, A.; Zughaibi, T.A.; Kamal, M.A.; Rehan, M. A Critical Transcription Factor NF-kappaB as a Cancer Therapeutic Target and its Inhibitors as Cancer Treatment Options. Curr. Med. Chem. 2021, 28, 4117–4132. [Google Scholar] [CrossRef] [PubMed]
- Buhrmann, C.; Shayan, P.; Brockmueller, A.; Shakibaei, M. Resveratrol Suppresses Cross-Talk between Colorectal Cancer Cells and Stromal Cells in Multicellular Tumor Microenvironment: A Bridge between In Vitro and In Vivo Tumor Microenvironment Study. Molecules 2020, 25, 4292. [Google Scholar] [CrossRef]
- Buhrmann, C.; Yazdi, M.; Popper, B.; Kunnumakkara, A.B.; Aggarwal, B.B.; Shakibaei, M. Induction of the Epithelial-to-Mesenchymal Transition of Human Colorectal Cancer by Human TNF-beta (Lymphotoxin) and its Reversal by Resveratrol. Nutrients 2019, 11, 704. [Google Scholar] [CrossRef] [PubMed]
- Khusbu, F.Y.; Zhou, X.; Roy, M.; Chen, F.Z.; Cao, Q.; Chen, H.C. Resveratrol induces depletion of TRAF6 and suppresses prostate cancer cell proliferation and migration. Int. J. Biochem. Cell B 2020, 118, 105644. [Google Scholar] [CrossRef] [PubMed]
- Rojo, D.; Madrid, A.; San Martin, S.; Parraga, M.; Pinhal, M.A.S.; Villena, J.; Valenzuela-Valderrama, M. Resveratrol Decreases the Invasion Potential of Gastric Cancer Cells. Molecules 2022, 27, 3047. [Google Scholar] [CrossRef]
- Buhrmann, C.; Yazdi, M.; Popper, B.; Shayan, P.; Goel, A.; Aggarwal, B.B.; Shakibaei, M. Evidence that TNF-beta induces proliferation in colorectal cancer cells and resveratrol can down-modulate it. Exp. Biol. Med. 2019, 244, 1–12. [Google Scholar] [CrossRef]
- Carpenter, R.L.; Lo, H.W. STAT3 Target Genes Relevant to Human Cancers. Cancers 2014, 6, 897–925. [Google Scholar] [CrossRef]
- Sun, X.D.; Xu, Q.Q.; Zeng, L.; Xie, L.X.; Zhao, Q.; Xu, H.X.; Wang, X.B.; Jiang, N.; Fu, P.; Sang, M. Resveratrol suppresses the growth and metastatic potential of cervical cancer by inhibiting STAT3(Tyr705)phosphorylation. Cancer Med. 2020, 9, 8685–8700. [Google Scholar] [CrossRef]
- Ferraresi, A.; Esposito, A.; Girone, C.; Vallino, L.; Salwa, A.; Ghezzi, I.; Thongchot, S.; Vidoni, C.; Dhanasekaran, D.N.; Isidoro, C. Resveratrol Contrasts LPA-Induced Ovarian Cancer Cell Migration and Platinum Resistance by Rescuing Hedgehog-Mediated Autophagy. Cells 2021, 10, 3213. [Google Scholar] [CrossRef]
- Xu, Q.H.; Xiao, Y.; Li, X.Q.; Fan, L.; Zhou, C.C.; Cheng, L.; Jiang, Z.D.; Wang, G.H. Resveratrol Counteracts Hypoxia-Induced Gastric Cancer Invasion and EMT through Hedgehog Pathway Suppression. Anti-Cancer Agents Med. Chem. 2020, 20, 1105–1114. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Dong, X.; Yap, J.; Hu, J. The MAPK and AMPK signalings: Interplay and implication in targeted cancer therapy. J. Hematol. Oncol. 2020, 13, 113. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Zhang, J.; Zhou, J.; Zhu, M.; Wang, L.; Yan, L. Resveratrol inhibits Interleukin-6 induced invasion of human gastric cancer cells. Biomed. Pharmacother. 2018, 99, 766–773. [Google Scholar] [CrossRef]
- Xiao, Y.; Duan, Y.; Wang, Y.; Yin, X. Resveratrol suppresses malignant progression of oral squamous cell carcinoma cells by inducing the ZNF750/RAC1 signaling pathway. Bioengineered 2021, 12, 2863–2873. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.S.; Tsai, C.W.; Yang, J.S.; Hsu, Y.M.; Shih, L.C.; Chiu, H.Y.; Bau, D.T.; Tsai, F.J. Resveratrol inhibited the metastatic behaviors of cisplatin-resistant human oral cancer cells via phosphorylation of ERK/p-38 and suppression of MMP-2/9. J. Food Biochem. 2021, 45, e13666. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Tang, H.; Zeng, X.; Ye, D.; Liu, J. Resveratrol inhibits proliferation, migration and invasion via Akt and ERK1/2 signaling pathways in renal cell carcinoma cells. Biomed. Pharmacother. 2018, 98, 36–44. [Google Scholar] [CrossRef]
- Dariya, B.; Behera, S.K.; Srivani, G.; Aliya, S.; Alam, A.; Nagaraju, G.P. Resveratrol binds and activates RKIP protein in colorectal cancer. Amino Acids 2020, 52, 1299–1306. [Google Scholar] [CrossRef] [PubMed]
- Yung, M.M.H.; Ngan, H.Y.S.; Chan, D.W. Targeting AMPK signaling in combating ovarian cancers: Opportunities and challenges. Acta Biochim. Biophys. Sin. 2016, 48, 301–317. [Google Scholar] [CrossRef]
- Liu, Y.; Tong, L.; Luo, Y.; Li, X.; Chen, G.W.; Wang, Y.F. Resveratrol inhibits the proliferation and induces the apoptosis in ovarian cancer cells via inhibiting glycolysis and targeting AMPK/mTOR signaling pathway. J. Cell. Biochem. 2018, 119, 6162–6172. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Najafi, M.; Orouei, S.; Zabolian, A.; Saleki, H.; Azami, N.; Sharifi, N.; Hushmandi, K.; Zarrabi, A.; Ahn, K.S. Resveratrol Modulates Transforming Growth Factor-Beta (TGF-beta) Signaling Pathway for Disease Therapy: A New Insight into Its Pharmacological Activities. Biomedicines 2020, 8, 261. [Google Scholar] [CrossRef]
- Zhang, J.X.; Zhang, W.; Zhang, T.; Zhou, Q.B.; Liu, J.Z.; Liu, Y.; Kong, D.Q.; Yu, W.H.; Liu, R.; Hai, C.X. TGF-beta 1 induces epithelial-to-mesenchymal transition via inhibiting mitochondrial functions in A549 cells. Free. Radic. Res. 2018, 52, 1432–1444. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Huang, P.; Pan, X.; Xia, C.; Zhang, H.; Zhao, H.; Yuan, Z.; Liu, J.; Meng, C.; Liu, F. Resveratrol reverses TGF-beta1-mediated invasion and metastasis of breast cancer cells via the SIRT3/AMPK/autophagy signal axis. Phytother. Res. 2023, 37, 211–230. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Zou, J.; Su, Y.F.; Wang, M.J.; Zhao, L.W. Resveratrol inhibits TGF-beta 1-induced EMT in gastric cancer cells through Hippo-YAP signaling pathway. Clin. Transl. Oncol. 2022, 24, 2210–2221. [Google Scholar] [CrossRef] [PubMed]
- Pai, S.G.; Carneiro, B.A.; Mota, J.M.; Costa, R.; Leite, C.A.; Barroso-Sousa, R.; Kaplan, J.B.; Chae, Y.K.; Giles, F.J. Wnt/beta-catenin pathway: Modulating anticancer immune response. J. Hematol. Oncol. 2017, 10, 101. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Zhang, R.; Hu, Y.; Li, W.; Wang, M.; Liang, Z.; Sun, Z.; Ji, R.; Xu, W.; Qian, H. Gastric-cancer-derived mesenchymal stem cells: A promising target for resveratrol in the suppression of gastric cancer metastasis. Hum. Cell 2020, 33, 652–662. [Google Scholar] [CrossRef] [PubMed]
- Oh-Hohenhorst, S.J.; Lange, T. Role of Metastasis-Related microRNAs in Prostate Cancer Progression and Treatment. Cancers 2021, 13, 4492. [Google Scholar] [CrossRef]
- Esposito, A.; Ferraresi, A.; Salwa, A.; Vidoni, C.; Dhanasekaran, D.N.; Isidoro, C. Resveratrol Contrasts IL-6 Pro-Growth Effects and Promotes Autophagy-Mediated Cancer Cell Dormancy in 3D Ovarian Cancer: Role of miR-1305 and of Its Target ARH-I. Cancers 2022, 14, 2142. [Google Scholar] [CrossRef]
- Yao, S.; Gao, M.; Wang, Z.; Wang, W.; Zhan, L.; Wei, B. Upregulation of MicroRNA-34a Sensitizes Ovarian Cancer Cells to Resveratrol by Targeting Bcl-2. Yonsei Med. J. 2021, 62, 691–701. [Google Scholar] [CrossRef]
- Su, N.; Li, L.; Zhou, E.; Li, H.; Wu, S.; Cao, Z. Resveratrol Downregulates miR-155-5p to Block the Malignant Behavior of Gastric Cancer Cells. BioMed Res. Int. 2022, 2022, 6968641. [Google Scholar] [CrossRef]
- Yang, Z.Y.; Xia, L. Resveratrol inhibits the proliferation, invasion, and migration, and induces the apoptosis of human gastric cancer cells through the MALAT1/miR-383-5p/DDIT4 signaling pathway. J. Gastrointest. Oncol. 2022, 13, 985–996. [Google Scholar] [CrossRef]
- Xiao, X.H.; Zhang, Y.Q.; Pan, W.N.; Chen, F. miR-139-mediated NOTCH1 regulation is crucial for the inhibition of osteosarcoma progression caused by resveratrol. Life Sci. 2020, 242, 117215. [Google Scholar] [CrossRef] [PubMed]
- Lacerda-Abreu, M.A.; Russo-Abrahao, T.; Meyer-Fernandes, J.R. Resveratrol is an inhibitor of sodium-dependent inorganic phosphate transport in triple-negative MDA-MB-231 breast cancer cells. Cell Biol. Int. 2021, 45, 1768–1775. [Google Scholar] [CrossRef]
- Sun, Y.; Schaar, A.; Sukumaran, P.; Dhasarathy, A.; Singh, B.B. TGFbeta-induced epithelial-to-mesenchymal transition in prostate cancer cells is mediated via TRPM7 expression. Mol. Carcinog. 2018, 57, 752–761. [Google Scholar] [CrossRef]
- Espinoza, J.L.; Trung, L.Q.; Inaoka, P.T.; Yamada, K.; An, D.T.; Mizuno, S.; Nakao, S.; Takami, A. The Repeated Administration of Resveratrol Has Measurable Effects on Circulating T-Cell Subsets in Humans. Oxidative Med. Cell. Longev. 2017, 2017, 6781872. [Google Scholar] [CrossRef] [PubMed]
- Holcombe, R.F.; Martinez, M.; Planutis, K.; Planutiene, M. Effects of a grape-supplemented diet on proliferation and Wnt signaling in the colonic mucosa are greatest for those over age 50 and with high arginine consumption. Nutr. J. 2015, 14, 62. [Google Scholar] [CrossRef] [PubMed]
- Boocock, D.J.; Faust, G.E.; Patel, K.R.; Schinas, A.M.; Brown, V.A.; Ducharme, M.P.; Booth, T.D.; Crowell, J.A.; Perloff, M.; Gescher, A.J.; et al. Phase I dose escalation pharmacokinetic study in healthy volunteers of resveratrol, a potential cancer chemopreventive agent. Cancer Epidemiol. Biomark. Prev. 2007, 16, 1246–1252. [Google Scholar] [CrossRef] [PubMed]
- Chow, H.H.; Garland, L.L.; Hsu, C.H.; Vining, D.R.; Chew, W.M.; Miller, J.A.; Perloff, M.; Crowell, J.A.; Alberts, D.S. Resveratrol modulates drug- and carcinogen-metabolizing enzymes in a healthy volunteer study. Cancer Prev. Res. 2010, 3, 1168–1175. [Google Scholar] [CrossRef]
- Brown, V.A.; Patel, K.R.; Viskaduraki, M.; Crowell, J.A.; Perloff, M.; Booth, T.D.; Vasilinin, G.; Sen, A.; Schinas, A.M.; Piccirilli, G.; et al. Repeat dose study of the cancer chemopreventive agent resveratrol in healthy volunteers: Safety, pharmacokinetics, and effect on the insulin-like growth factor axis. Cancer Res. 2010, 70, 9003–9011. [Google Scholar] [CrossRef]
- Howells, L.M.; Berry, D.P.; Elliott, P.J.; Jacobson, E.W.; Hoffmann, E.; Hegarty, B.; Brown, K.; Steward, W.P.; Gescher, A.J. Phase I Randomized, Double-Blind Pilot Study of Micronized Resveratrol (SRT501) in Patients with Hepatic Metastases-Safety, Pharmacokinetics, and Pharmacodynamics. Cancer Prev. Res. 2011, 4, 1419–1425. [Google Scholar] [CrossRef]
- Cai, H.; Scott, E.; Kholghi, A.; Andreadi, C.; Rufini, A.; Karmokar, A.; Britton, R.G.; Horner-Glister, E.; Greaves, P.; Jawad, D.; et al. Cancer chemoprevention: Evidence of a nonlinear dose response for the protective effects of resveratrol in humans and mice. Sci. Transl. Med. 2015, 7, 298ra117. [Google Scholar] [CrossRef]
- Nguyen, A.V.; Martinez, M.; Stamos, M.J.; Moyer, M.P.; Planutis, K.; Hope, C.; Holcombe, R.F. Results of a phase I pilot clinical trial examining the effect of plant-derived resveratrol and grape powder on Wnt pathway target gene expression in colonic mucosa and colon cancer. Cancer Manag. Res. 2009, 1, 25–37. [Google Scholar] [PubMed]
- Patel, K.R.; Brown, V.A.; Jones, D.J.; Britton, R.G.; Hemingway, D.; Miller, A.S.; West, K.P.; Booth, T.D.; Perloff, M.; Crowell, J.A.; et al. Clinical pharmacology of resveratrol and its metabolites in colorectal cancer patients. Cancer Res. 2010, 70, 7392–7399. [Google Scholar] [CrossRef] [PubMed]
- Van Die, M.D.; Williams, S.G.; Emery, J.; Bone, K.M.; Taylor, J.M.; Lusk, E.; Pirotta, M.V. A Placebo-Controlled Double-Blinded Randomized Pilot Study of Combination Phytotherapy in Biochemically Recurrent Prostate Cancer. Prostate 2017, 77, 765–775. [Google Scholar] [CrossRef]
- Paller, C.J.; Rudek, M.A.; Zhou, X.C.; Wagner, W.D.; Hudson, T.S.; Anders, N.; Hammers, H.J.; Dowling, D.; King, S.; Antonarakis, E.S.; et al. A phase I study of muscadine grape skin extract in men with biochemically recurrent prostate cancer: Safety, tolerability, and dose determination. Prostate 2015, 75, 1518–1525. [Google Scholar] [CrossRef] [PubMed]
- Paller, C.J.; Zhou, X.C.; Heath, E.I.; Taplin, M.E.; Mayer, T.; Stein, M.N.; Bubley, G.J.; Pili, R.; Hudson, T.; Kakarla, R.; et al. Muscadine Grape Skin Extract (MPX) in Men with Biochemically Recurrent Prostate Cancer: A Randomized, Multicenter, Placebo-Controlled Clinical Trial. Clin. Cancer Res. 2018, 24, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Qin, W.; Zhang, K.; Rottinghaus, G.E.; Chen, Y.C.; Kliethermes, B.; Sauter, E.R. Trans-resveratrol alters mammary promoter hypermethylation in women at increased risk for breast cancer. Nutr. Cancer 2012, 64, 393–400. [Google Scholar] [CrossRef]
- Avila-Galvez, M.A.; Garcia-Villalba, R.; Martinez-Diaz, F.; Ocana-Castillo, B.; Monedero-Saiz, T.; Torrecillas-Sanchez, A.; Abellan, B.; Gonzalez-Sarrias, A.; Espin, J.C. Metabolic Profiling of Dietary Polyphenols and Methylxanthines in Normal and Malignant Mammary Tissues from Breast Cancer Patients. Mol. Nutr. Food Res. 2019, 63, e1801239. [Google Scholar] [CrossRef]
- Chow, H.H.; Garland, L.L.; Heckman-Stoddard, B.M.; Hsu, C.H.; Butler, V.D.; Cordova, C.A.; Chew, W.M.; Cornelison, T.L. A pilot clinical study of resveratrol in postmenopausal women with high body mass index: Effects on systemic sex steroid hormones. J. Transl. Med. 2014, 12, 223. [Google Scholar] [CrossRef]
- Popat, R.; Plesner, T.; Davies, F.; Cook, G.; Cook, M.; Elliott, P.; Jacobson, E.; Gumbleton, T.; Oakervee, H.; Cavenagh, J. A phase 2 study of SRT501 (resveratrol) with bortezomib for patients with relapsed and or refractory multiple myeloma. Br. J. Haematol. 2013, 160, 714–717. [Google Scholar] [CrossRef]
- Walle, T. Bioavailability of resveratrol. Ann. N. Y. Acad. Sci. 2011, 1215, 9–15. [Google Scholar] [CrossRef]
- Vitrac, X.; Desmouliere, A.; Brouillaud, B.; Krisa, S.; Deffieux, G.; Barthe, N.; Rosenbaum, J.; Merillon, J.M. Distribution of [14C]-trans-resveratrol, a cancer chemopreventive polyphenol, in mouse tissues after oral administration. Life Sci. 2003, 72, 2219–2233. [Google Scholar] [CrossRef] [PubMed]
- Almeida, L.; Vaz-da-Silva, M.; Falcao, A.; Soares, E.; Costa, R.; Loureiro, A.I.; Fernandes-Lopes, C.; Rocha, J.F.; Nunes, T.; Wright, L.; et al. Pharmacokinetic and safety profile of trans-resveratrol in a rising multiple-dose study in healthy volunteers. Mol. Nutr. Food Res. 2009, 53 (Suppl. S1), S7–S15. [Google Scholar] [CrossRef]
- Radko, Y.; Christensen, K.B.; Christensen, L.P. Semi-preparative isolation of dihydroresveratrol-3-O-beta-d-glucuronide and four resveratrol conjugates from human urine after oral intake of a resveratrol-containing dietary supplement. J. Chromatogr. B 2013, 930, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Scott, E.N.; Britton, R.G.; Parrott, E.; Ognibene, T.J.; Malfatti, M.; Khan, M.; Steward, W.P.; Brown, K. Distribution and metabolism of [14C]-resveratrol in human prostate tissue after oral administration of a “dietary-achievable” or “pharmacological” dose: What are the implications for anticancer activity? Am. J. Clin. Nutr. 2021, 113, 1115–1125. [Google Scholar] [CrossRef] [PubMed]
- Summerlin, N.; Soo, E.; Thakur, S.; Qu, Z.; Jambhrunkar, S.; Popat, A. Resveratrol nanoformulations: Challenges and opportunities. Int. J. Pharmaceut. 2015, 479, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Carletto, B.; Berton, J.; Ferreira, T.N.; Dalmolin, L.F.; Paludo, K.S.; Mainardes, R.M.; Farago, P.V.; Favero, G.M. Resveratrol-loaded nanocapsules inhibit murine melanoma tumor growth. Colloids Surf. B Biointerfaces 2016, 144, 65–72. [Google Scholar] [CrossRef]
- Pradhan, R.; Chatterjee, S.; Hembram, K.C.; Sethy, C.; Mandal, M.; Kundu, C.N. Nano formulated Resveratrol inhibits metastasis and angiogenesis by reducing inflammatory cytokines in oral cancer cells by targeting tumor associated macrophages. J. Nutr. Biochem. 2021, 92, 108624. [Google Scholar] [CrossRef]
- Baatout, S.; Derradji, H.; Jacquet, P.; Ooms, D.; Michaux, A.; Mergeay, M. Enhanced radiation-induced apoptosis of cancer cell lines after treatment with resveratrol. Int. J. Mol. Med. 2004, 13, 895–902. [Google Scholar] [CrossRef]
- Kapetanovic, I.M. Comment on ‘Resveratrol in human cancer chemoprevention--choosing the ‘right’ dose’. Mol. Nutr. Food Res. 2012, 56, 523. [Google Scholar] [CrossRef]
- La Porte, C.; Voduc, N.; Zhang, G.; Seguin, I.; Tardiff, D.; Singhal, N.; Cameron, D.W. Steady-State pharmacokinetics and tolerability of trans-resveratrol 2000 mg twice daily with food, quercetin and alcohol (ethanol) in healthy human subjects. Clin. Pharmacokinet. 2010, 49, 449–454. [Google Scholar] [CrossRef]
- Yiu, E.M.; Tai, G.; Peverill, R.E.; Lee, K.J.; Croft, K.D.; Mori, T.A.; Scheiber-Mojdehkar, B.; Sturm, B.; Praschberger, M.; Vogel, A.P.; et al. An open-label trial in Friedreich ataxia suggests clinical benefit with high-dose resveratrol, without effect on frataxin levels. J. Neurol. 2015, 262, 1344–1353. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Wang, J.; Xu, J.F.; Tang, F.; Chen, L.; Tan, Y.Z.; Rao, C.L.; Ao, H.; Peng, C. Panax ginseng and its ginsenosides: Potential candidates for the prevention and treatment of chemotherapy-induced side effects. J. Ginseng Res. 2021, 45, 617–630. [Google Scholar] [CrossRef] [PubMed]



| Phase | Tumor Type | Subjects | Administration | Dose/ Duration | Results | References |
|---|---|---|---|---|---|---|
| I | - | 9 (healthy) | Oral | 1 g, 28 days | Gastrointestinal side effects | [94] |
| I | - | 30 (healthy) | Oral (grapes) | 0.15/0.3/0.45 kg (~7.5/15/22.5 mg RES), 2 weeks | No side effects | [95] |
| I | - | 40 (healthy) | Oral | 0.5/1/2.5/5 g | Peak plasma levels of RES at the highest dose were 539 ± 384 ng/mL | [96] |
| I | - | 42 (healthy) | Oral | 1 g, 4 weeks | RES intervention inhibited the phenotypic indices of CYP3A4, CYP2D6, and CYP2C9 and induced the phenotypic indices of 1A2 | [97] |
| Ⅱ | - | 40 (healthy) | Oral | 0.5/1/2.5/5 g, 29 days | Only 2.5 and 5 g doses causing mild-to-moderate gastrointestinal symptoms | [98] |
| I | Colorectal cancer (hepatic metastasis) | 9 (patients) | Oral (SRT501) | 5 g, 14 days | Gastrointestinal side effects, cleaved caspase-3 was significantly increased by 39% | [99] |
| I | Colorectal cancer | 24 (patients) | Oral | 5 mg/1 g, 6 days | RSE ranged from 3.0 to 376.0 nmol/g in malignant tumor tissue | [100] |
| I | Colon cancer | 8 (patients) | Oral (Tablet/Grape Powder) | 20/80/160 mg (RES), 2 weeks | Not significantly inhibit the Wnt pathway in malignant colonic tissue | [101] |
| I | Colorectal cancer | 20 (patients) | Oral | 0.5/1 g, 8 days | 0.5 or 1.0 g of RES per day no side effects | [102] |
| I | Prostate cancer | 22 (patients) | Oral | ~30 mg, 12 weeks | RES (~30 mg) reported a non-significant prolongation of PSADT | [103] |
| I/Ⅱ | Prostate cancer | 14 (patients) | Oral (4.4 μg RES for per capsule) | 1/2/3/4 g (8.8/17.6/26.4/35.2 μg RES), 28 days | 4 patients developed gastrointestinal side effects in the high-dose group | [104] |
| Ⅱ | Prostate cancer | 125 (patients) | Oral (4.4 μg RES for per capsule) | 0.5/4 g (4.4/35.2 μg RES), 12 months | One patient developed gastrointestinal side effects in the high-dose group | [105] |
| I | Breast cancer | 39 (menopausal women) | Oral | 5/50 mg, 12 weeks | No side effects in the subjects, and trans-RES was detected in 20% of the subjects’ serum samples | [106] |
| I | Breast cancer | 19 (patients) | Oral | 161.55 mg, 6 ± 2 days | RES and its metabolites are more concentrated in malignant tumors compared to normal tissues | [107] |
| I | Breast cancer (revelation) | 40 (healthy) | Oral | 1 g, 12 weeks | 6 patients developed side effects leading to withdrawal | [108] |
| Ⅱ | Multiple myeloma | 24(patients) | Oral (SRT501) | 5 g, 21 days | Nephrotoxicity leads to study termination | [109] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, B.; Wang, W.; Tang, X.; Goh, R.M.W.-J.; Thuya, W.L.; Ho, P.C.L.; Chen, L.; Wang, L. Inhibitory Potential of Resveratrol in Cancer Metastasis: From Biology to Therapy. Cancers 2023, 15, 2758. https://doi.org/10.3390/cancers15102758
Song B, Wang W, Tang X, Goh RMW-J, Thuya WL, Ho PCL, Chen L, Wang L. Inhibitory Potential of Resveratrol in Cancer Metastasis: From Biology to Therapy. Cancers. 2023; 15(10):2758. https://doi.org/10.3390/cancers15102758
Chicago/Turabian StyleSong, Baohong, Wei Wang, Xuemei Tang, Robby Miguel Wen-Jing Goh, Win Lwin Thuya, Paul Chi Lui Ho, Lu Chen, and Lingzhi Wang. 2023. "Inhibitory Potential of Resveratrol in Cancer Metastasis: From Biology to Therapy" Cancers 15, no. 10: 2758. https://doi.org/10.3390/cancers15102758
APA StyleSong, B., Wang, W., Tang, X., Goh, R. M. W.-J., Thuya, W. L., Ho, P. C. L., Chen, L., & Wang, L. (2023). Inhibitory Potential of Resveratrol in Cancer Metastasis: From Biology to Therapy. Cancers, 15(10), 2758. https://doi.org/10.3390/cancers15102758







