Anti-Tumor Strategies by Harnessing the Phagocytosis of Macrophages
Abstract
Simple Summary
Abstract
1. Introduction
2. Mechanisms of Phagocytosis
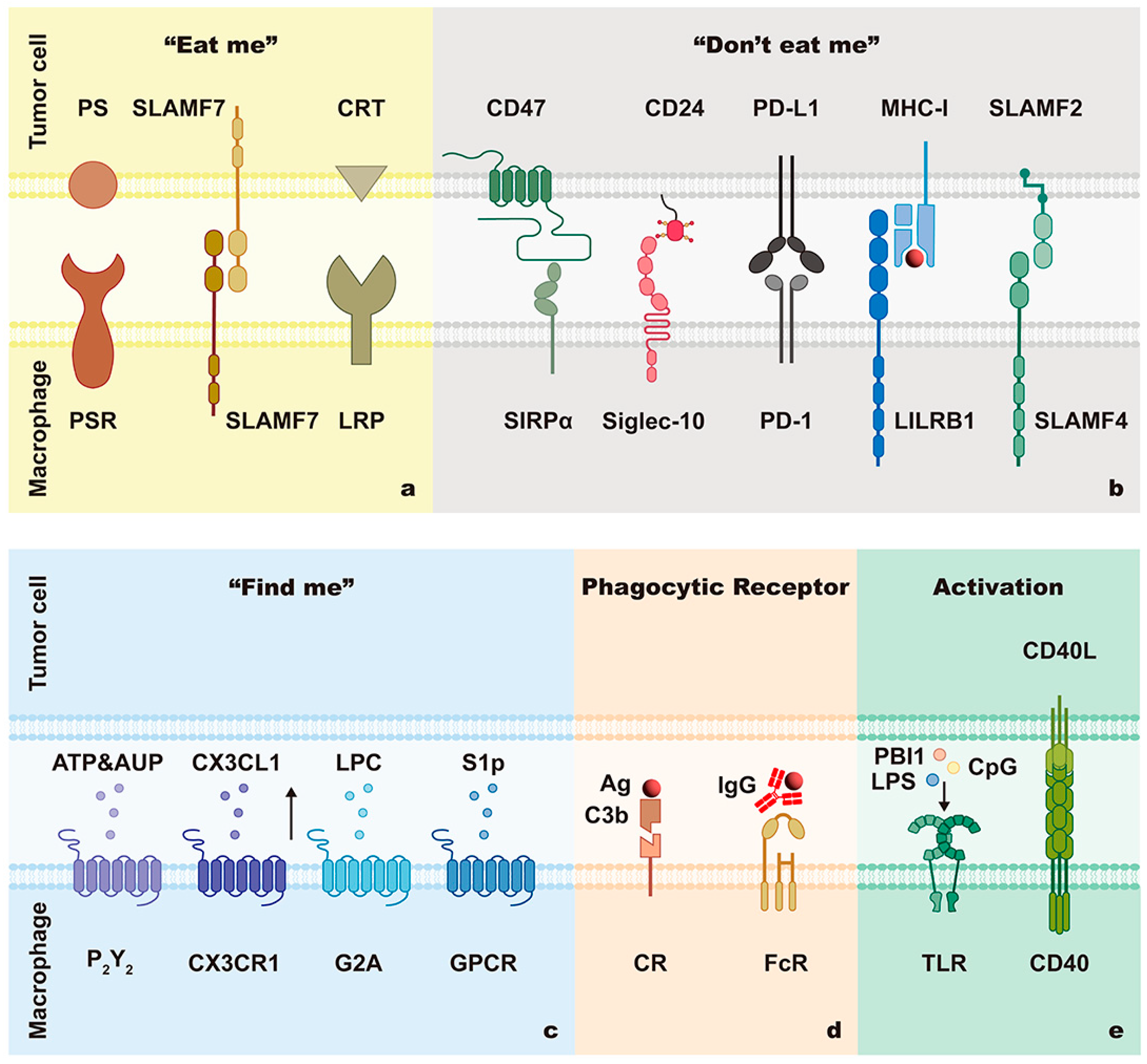
2.1. Phagocytic Signals
2.1.1. “Find Me” Signals
2.1.2. “Don’t Eat Me” Signals
CD47/SIRPα
CD24/Siglec-10
MHC-I/LILRB1
SLAMF3/SLAMF3 and SLAMF2/SLAMF4
2.1.3. “Eat Me” Signals
PS/PSR
CRT/LRP
SLAMF7
2.1.4. Specific Antigen-Mediated ADCP Signals
2.2. Phagocytic Ability of Macrophages
2.2.1. Phagocytic Receptors
2.2.2. Activation of Macrophages
2.2.3. Mitochondrial Fission
3. Clinical Translation of Therapeutic Strategies Targeting Macrophage Phagocytosis Pathways
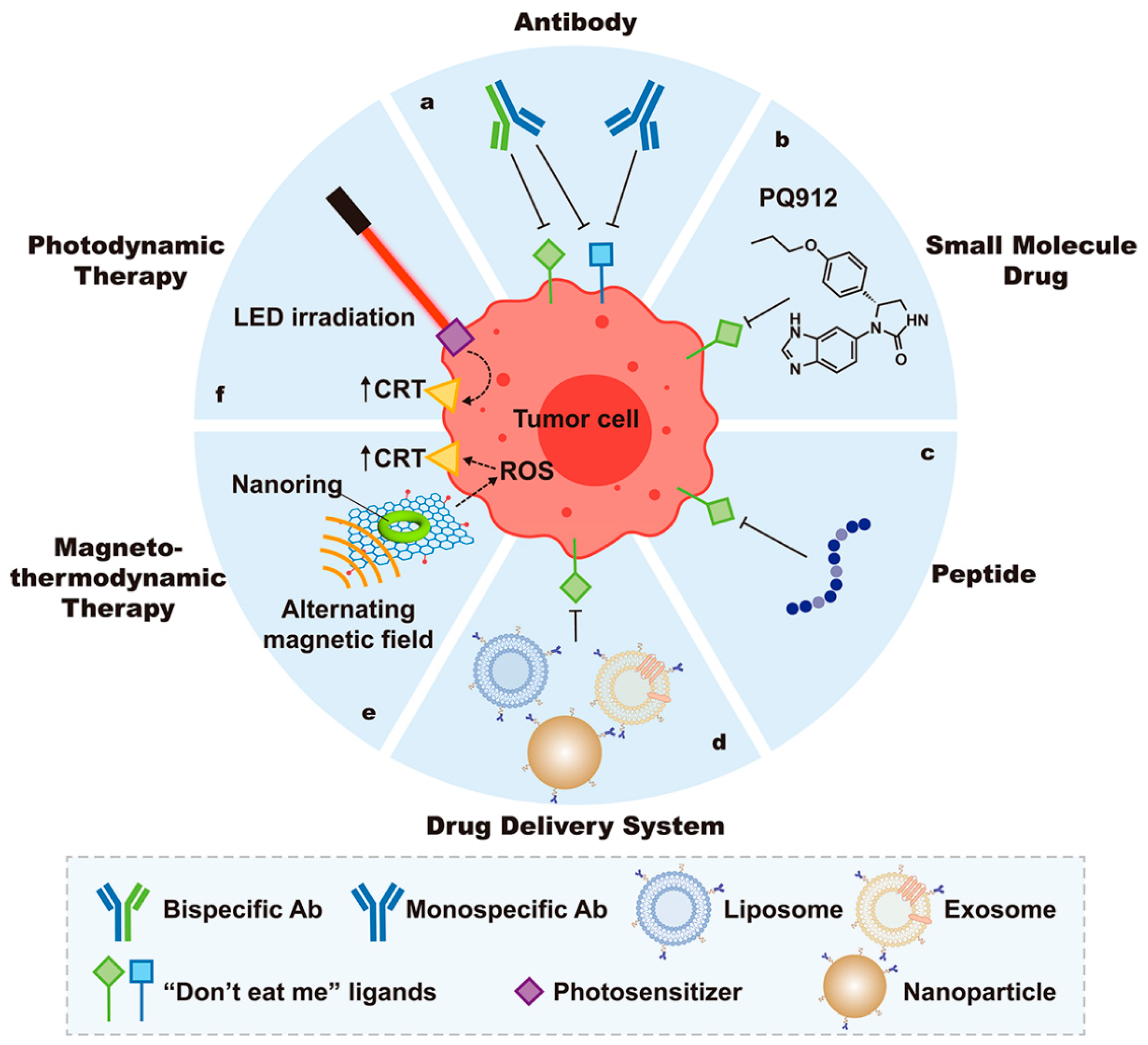
3.1. Therapeutic Applications Targeting Phagocytic Signals
3.1.1. Strategies Targeting “Find Me” Signals
3.1.2. “Don’t Eat Me” Signal Blockade
Monospecific Antibodies
Bispecific Antibodies
Small-Molecule Drugs
Peptides
Nanomedicine
3.1.3. Exposure of “Eat Me” Signals
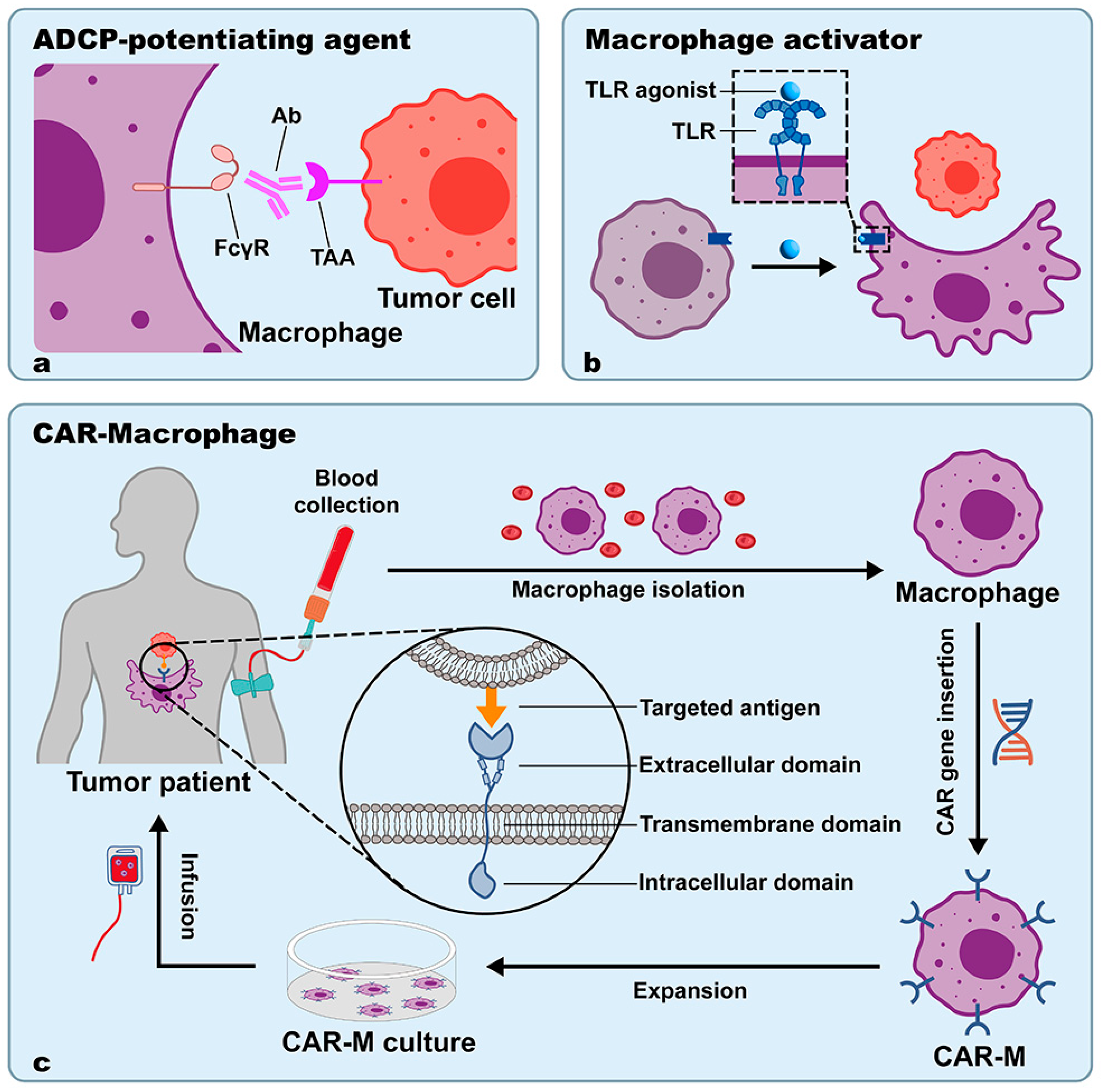
3.2. ADCP-Potentiating Agents
3.2.1. Application Status
3.2.2. Advantages
3.2.3. Limitations and Outlooks
3.3. Macrophage Activators
3.3.1. TLR Agonists
3.3.2. CSF-1 Inhibitors
3.3.3. HDAC Inhibitors
3.4. CAR-M
3.4.1. Application Status
3.4.2. Advantages
3.4.3. Limitations and Outlooks
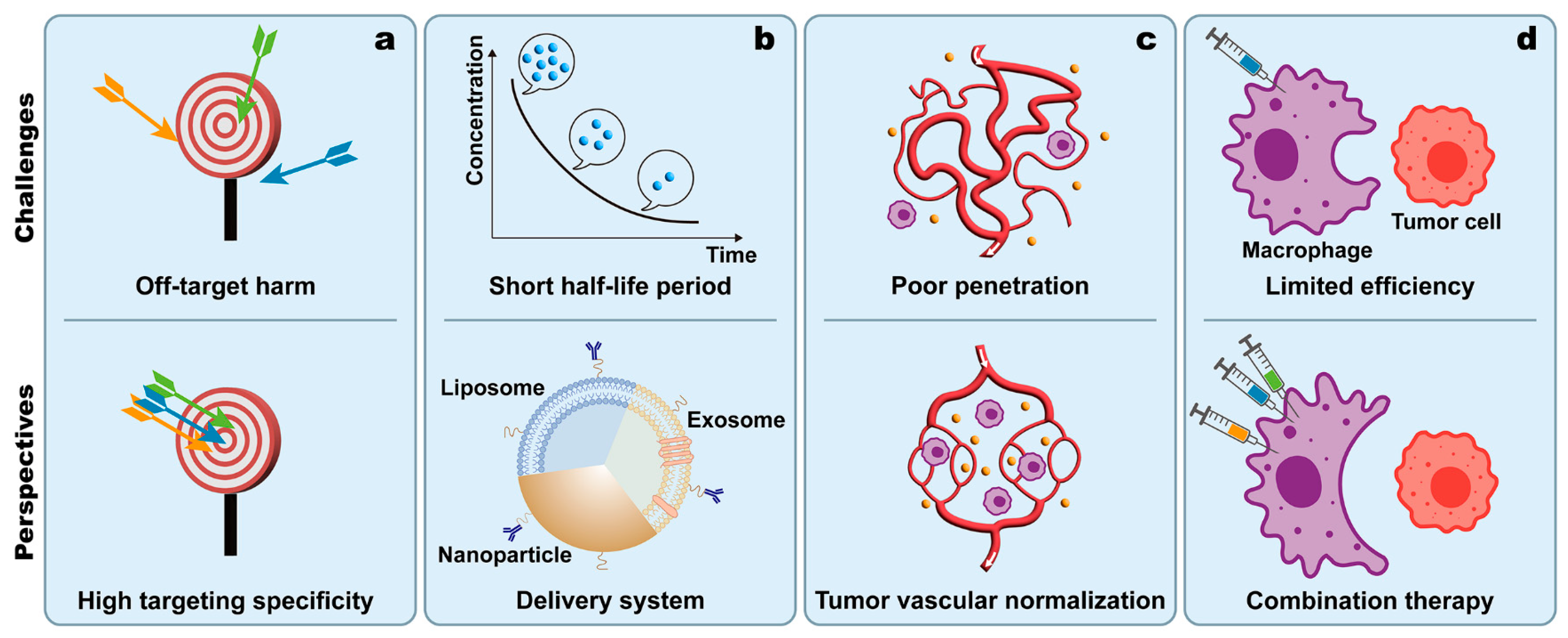
4. Perspectives and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Weiss, G.; Schaible, U.E. Macrophage defense mechanisms against intracellular bacteria. Immunol. Rev. 2015, 264, 182–203. [Google Scholar] [CrossRef]
- Chen, J.; Zhong, M.C.; Guo, H.; Davidson, D.; Mishel, S.; Lu, Y.; Rhee, I.; Perez-Quintero, L.A.; Zhang, S.; Cruz-Munoz, M.E.; et al. SLAMF7 is critical for phagocytosis of haematopoietic tumour cells via Mac-1 integrin. Nature 2017, 544, 493–497. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Li, W.; Zhang, Y.; Qi, L.; Zhao, Q.; Li, N.; Lu, Y.; Zhang, L.; Zhou, F.; Wu, Y.; et al. FLT4/VEGFR3 activates AMPK to coordinate glycometabolic reprogramming with autophagy and inflammasome activation for bacterial elimination. Autophagy 2022, 18, 1385–1400. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.J.; Wynn, T.A. Protective and pathogenic functions of macrophage subsets. Nat. Rev. Immunol. 2011, 11, 723–737. [Google Scholar] [CrossRef] [PubMed]
- Cavaillon, J.M. The historical milestones in the understanding of leukocyte biology initiated by Elie Metchnikoff. J. Leukoc. Biol. 2011, 90, 413–424. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Kemp, S.S.; Yang, X.; Wu, M.H.; Yuan, S.Y. Cellular mechanisms underlying the impairment of macrophage efferocytosis. Immunol. Lett. 2023, 254, 41–53. [Google Scholar] [CrossRef]
- Razi, S.; Yaghmoorian Khojini, J.; Kargarijam, F.; Panahi, S.; Tahershamsi, Z.; Tajbakhsh, A.; Gheibihayat, S.M. Macrophage efferocytosis in health and disease. Cell Biochem. Funct. 2023, 41, 152–165. [Google Scholar] [CrossRef]
- Weiskopf, K.; Weissman, I.L. Macrophages are critical effectors of antibody therapies for cancer. MAbs 2015, 7, 303–310. [Google Scholar] [CrossRef]
- Ruffell, B.; Affara, N.I.; Coussens, L.M. Differential macrophage programming in the tumor microenvironment. Trends Immunol. 2012, 33, 119–126. [Google Scholar] [CrossRef]
- Nesbit, M.; Schaider, H.; Miller, T.H.; Herlyn, M. Low-level monocyte chemoattractant protein-1 stimulation of monocytes leads to tumor formation in nontumorigenic melanoma cells. J. Immunol. 2001, 166, 6483–6490. [Google Scholar] [CrossRef]
- Imbert, P.R.C.; Saric, A.; Pedram, K.; Bertozzi, C.R.; Grinstein, S.; Freeman, S.A. An Acquired and Endogenous Glycocalyx Forms a Bidirectional “Don’t Eat” and “Don’t Eat Me” Barrier to Phagocytosis. Curr. Biol. 2021, 31, 77–89.e75. [Google Scholar] [CrossRef] [PubMed]
- Bingle, L.; Brown, N.J.; Lewis, C.E. The role of tumour-associated macrophages in tumour progression: Implications for new anticancer therapies. J. Pathol. 2002, 196, 254–265. [Google Scholar] [CrossRef] [PubMed]
- Cheng, N.; Bai, X.; Shu, Y.; Ahmad, O.; Shen, P. Targeting tumor-associated macrophages as an antitumor strategy. Biochem. Pharmacol. 2021, 183, 114354. [Google Scholar] [CrossRef] [PubMed]
- Park, J.B. Phagocytosis induces superoxide formation and apoptosis in macrophages. Exp. Mol. Med. 2003, 35, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Freeman, S.A.; Grinstein, S. Phagocytosis: Receptors, signal integration, and the cytoskeleton. Immunol. Rev. 2014, 262, 193–215. [Google Scholar] [CrossRef] [PubMed]
- Aderem, A.; Underhill, D.M. Mechanisms of phagocytosis in macrophages. Annu. Rev. Immunol. 1999, 17, 593–623. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.C.; Neher, J.J. Eaten alive! Cell death by primary phagocytosis: ‘phagoptosis’. Trends Biochem. Sci. 2012, 37, 325–332. [Google Scholar] [CrossRef]
- Kelley, S.M.; Ravichandran, K.S. Putting the brakes on phagocytosis: “don’t-eat-me” signaling in physiology and disease. EMBO Rep. 2021, 22, e52564. [Google Scholar] [CrossRef]
- Lauber, K.; Blumenthal, S.G.; Waibel, M.; Wesselborg, S. Clearance of apoptotic cells: Getting rid of the corpses. Mol. Cell. 2004, 14, 277–287. [Google Scholar] [CrossRef]
- Elliott, M.R.; Chekeni, F.B.; Trampont, P.C.; Lazarowski, E.R.; Kadl, A.; Walk, S.F.; Park, D.; Woodson, R.I.; Ostankovich, M.; Sharma, P.; et al. Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature 2009, 461, 282–286. [Google Scholar] [CrossRef]
- Niess, J.H.; Brand, S.; Gu, X.; Landsman, L.; Jung, S.; McCormick, B.A.; Vyas, J.M.; Boes, M.; Ploegh, H.L.; Fox, J.G.; et al. CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science 2005, 307, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.V.; Radu, C.G.; Wang, L.; Riedinger, M.; Witte, O.N. Gi-independent macrophage chemotaxis to lysophosphatidylcholine via the immunoregulatory GPCR G2A. Blood 2005, 105, 1127–1134. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.; Van Brocklyn, J.R.; Thangada, S.; Liu, C.H.; Hand, A.R.; Menzeleev, R.; Spiegel, S.; Hla, T. Sphingosine-1-phosphate as a ligand for the G protein-coupled receptor EDG-1. Science 1998, 279, 1552–1555. [Google Scholar] [CrossRef] [PubMed]
- Gude, D.R.; Alvarez, S.E.; Paugh, S.W.; Mitra, P.; Yu, J.; Griffiths, R.; Barbour, S.E.; Milstien, S.; Spiegel, S. Apoptosis induces expression of sphingosine kinase 1 to release sphingosine-1-phosphate as a “come-and-get-me” signal. FASEB J. 2008, 22, 2629–2638. [Google Scholar] [CrossRef]
- Nishiura, H.; Zhao, R.; Yamamoto, T. The role of the ribosomal protein S19 C-terminus in altering the chemotaxis of leucocytes by causing functional differences in the C5a receptor response. J. Biochem. 2011, 150, 271–277. [Google Scholar] [CrossRef]
- Hoffmann, P.R.; deCathelineau, A.M.; Ogden, C.A.; Leverrier, Y.; Bratton, D.L.; Daleke, D.L.; Ridley, A.J.; Fadok, V.A.; Henson, P.M. Phosphatidylserine (PS) induces PS receptor-mediated macropinocytosis and promotes clearance of apoptotic cells. J. Cell Biol. 2001, 155, 649–659. [Google Scholar] [CrossRef]
- Park, S.Y.; Jung, M.Y.; Kim, H.J.; Lee, S.J.; Kim, S.Y.; Lee, B.H.; Kwon, T.H.; Park, R.W.; Kim, I.S. Rapid cell corpse clearance by stabilin-2, a membrane phosphatidylserine receptor. Cell Death Differ. 2008, 15, 192–201. [Google Scholar] [CrossRef]
- Lillis, A.P.; Van Duyn, L.B.; Murphy-Ullrich, J.E.; Strickland, D.K. LDL receptor-related protein 1: Unique tissue-specific functions revealed by selective gene knockout studies. Physiol. Rev. 2008, 88, 887–918. [Google Scholar] [CrossRef]
- Kinchen, J.M.; Cabello, J.; Klingele, D.; Wong, K.; Feichtinger, R.; Schnabel, H.; Schnabel, R.; Hengartner, M.O. Two pathways converge at CED-10 to mediate actin rearrangement and corpse removal in C. elegans. Nature 2005, 434, 93–99. [Google Scholar] [CrossRef]
- Gardai, S.J.; McPhillips, K.A.; Frasch, S.C.; Janssen, W.J.; Starefeldt, A.; Murphy-Ullrich, J.E.; Bratton, D.L.; Oldenborg, P.A.; Michalak, M.; Henson, P.M. Cell-surface calreticulin initiates clearance of viable or apoptotic cells through trans-activation of LRP on the phagocyte. Cell 2005, 123, 321–334. [Google Scholar] [CrossRef]
- Oldenborg, P.A.; Gresham, H.D.; Lindberg, F.P. CD47-signal regulatory protein alpha (SIRPalpha) regulates Fcγ and complement receptor-mediated phagocytosis. J. Exp. Med. 2001, 193, 855–862. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Satoskar, A.; Faubion, W.; Howie, D.; Okamoto, S.; Feske, S.; Gullo, C.; Clarke, K.; Sosa, M.R.; Sharpe, A.H.; et al. The cell surface receptor SLAM controls T cell and macrophage functions. J. Exp. Med. 2004, 199, 1255–1264. [Google Scholar] [CrossRef]
- Li, D.; Xiong, W.; Wang, Y.; Feng, J.; He, Y.; Du, J.; Wang, J.; Yang, M.; Zeng, H.; Yang, Y.G.; et al. SLAMF3 and SLAMF4 are immune checkpoints that constrain macrophage phagocytosis of hematopoietic tumors. Sci. Immunol. 2022, 7, eabj5501. [Google Scholar] [PubMed]
- Barkal, A.A.; Brewer, R.E.; Markovic, M.; Kowarsky, M.; Barkal, S.A.; Zaro, B.W.; Krishnan, V.; Hatakeyama, J.; Dorigo, O.; Barkal, L.J.; et al. CD24 signalling through macrophage Siglec-10 is a target for cancer immunotherapy. Nature 2019, 572, 392–396. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S.R.; Maute, R.L.; Dulken, B.W.; Hutter, G.; George, B.M.; McCracken, M.N.; Gupta, R.; Tsai, J.M.; Sinha, R.; Corey, D.; et al. PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature 2017, 545, 495–499. [Google Scholar] [CrossRef]
- Patsoukis, N.; Duke-Cohan, J.S.; Chaudhri, A.; Aksoylar, H.I.; Wang, Q.; Council, A.; Berg, A.; Freeman, G.J.; Boussiotis, V.A. Interaction of SHP-2 SH2 domains with PD-1 ITSM induces PD-1 dimerization and SHP-2 activation. Commun. Biol. 2020, 3, 128. [Google Scholar] [CrossRef] [PubMed]
- Barkal, A.A.; Weiskopf, K.; Kao, K.S.; Gordon, S.R.; Rosental, B.; Yiu, Y.Y.; George, B.M.; Markovic, M.; Ring, N.G.; Tsai, J.M.; et al. Engagement of MHC class I by the inhibitory receptor LILRB1 suppresses macrophages and is a target of cancer immunotherapy. Nat. Immunol. 2018, 19, 76–84. [Google Scholar] [CrossRef]
- Ravichandran, K.S. Beginnings of a good apoptotic meal: The find-me and eat-me signaling pathways. Immunity 2011, 35, 445–455. [Google Scholar] [CrossRef]
- Veillette, A.; Chen, J. SIRPalpha-CD47 Immune Checkpoint Blockade in Anticancer Therapy. Trends Immunol. 2018, 39, 173–184. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Fan, J.; Chen, W.; Luan, J.; Mei, X.; Wang, S.; Li, Y.; Ye, L.; Li, S.; et al. Blocking CD47 efficiently potentiated therapeutic effects of anti-angiogenic therapy in non-small cell lung cancer. J. Immunother. Cancer 2019, 7, 346. [Google Scholar] [CrossRef]
- de Silva, S.; Fromm, G.; Shuptrine, C.W.; Johannes, K.; Patel, A.; Yoo, K.J.; Huang, K.; Schreiber, T.H. CD40 Enhances Type I Interferon Responses Downstream of CD47 Blockade, Bridging Innate and Adaptive Immunity. Cancer Immunol. Res. 2020, 8, 230–245. [Google Scholar] [CrossRef] [PubMed]
- Theruvath, J.; Menard, M.; Smith, B.A.H.; Linde, M.H.; Coles, G.L.; Dalton, G.N.; Wu, W.; Kiru, L.; Delaidelli, A.; Sotillo, E.; et al. Anti-GD2 synergizes with CD47 blockade to mediate tumor eradication. Nat. Med. 2022, 28, 333–344. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Bar, H.M.; Walters, A.A.; Lim, Y.; Rouatbi, N.; Qin, Y.; Gheidari, F.; Han, S.; Osman, R.; Wang, J.T.; Al-Jamal, K.T. An “eat me” combinatory nano-formulation for systemic immunotherapy of solid tumors. Theranostics 2021, 11, 8738–8754. [Google Scholar] [CrossRef] [PubMed]
- Lemke, G. How macrophages deal with death. Nat. Rev. Immunol. 2019, 19, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Utsugi, T.; Schroit, A.J.; Connor, J.; Bucana, C.D.; Fidler, I.J. Elevated expression of phosphatidylserine in the outer membrane leaflet of human tumor cells and recognition by activated human blood monocytes. Cancer Res. 1991, 51, 3062–3066. [Google Scholar]
- Sharma, B.; Kanwar, S.S. Phosphatidylserine: A cancer cell targeting biomarker. Semin. Cancer Biol. 2018, 52, 17–25. [Google Scholar] [CrossRef]
- Graham, D.K.; DeRyckere, D.; Davies, K.D.; Earp, H.S. The TAM family: Phosphatidylserine sensing receptor tyrosine kinases gone awry in cancer. Nat. Rev. Cancer 2014, 14, 769–785. [Google Scholar] [CrossRef]
- Lin, H.; Kryczek, I.; Li, S.; Green, M.D.; Ali, A.; Hamasha, R.; Wei, S.; Vatan, L.; Szeliga, W.; Grove, S.; et al. Stanniocalcin 1 is a phagocytosis checkpoint driving tumor immune resistance. Cancer Cell 2021, 39, 480–493.e486. [Google Scholar] [CrossRef]
- Wu, N.; Veillette, A. SLAM family receptors in normal immunity and immune pathologies. Curr. Opin. Immunol. 2016, 38, 45–51. [Google Scholar] [CrossRef]
- Su, S.; Zhao, J.; Xing, Y.; Zhang, X.; Liu, J.; Ouyang, Q.; Chen, J.; Su, F.; Liu, Q.; Song, E. Immune Checkpoint Inhibition Overcomes ADCP-Induced Immunosuppression by Macrophages. Cell 2018, 175, 442–457.e423. [Google Scholar] [CrossRef]
- Vachon, E.; Martin, R.; Plumb, J.; Kwok, V.; Vandivier, R.W.; Glogauer, M.; Kapus, A.; Wang, X.; Chow, C.W.; Grinstein, S.; et al. CD44 is a phagocytic receptor. Blood 2006, 107, 4149–4158. [Google Scholar] [CrossRef] [PubMed]
- May, R.C.; Caron, E.; Hall, A.; Machesky, L.M. Involvement of the Arp2/3 complex in phagocytosis mediated by FcγR or CR3. Nat. Cell Biol. 2000, 2, 246–248. [Google Scholar] [CrossRef] [PubMed]
- Olazabal, I.M.; Caron, E.; May, R.C.; Schilling, K.; Knecht, D.A.; Machesky, L.M. Rho-kinase and myosin-II control phagocytic cup formation during CR, but not FcγR, phagocytosis. Curr. Biol. 2002, 12, 1413–1418. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Guo, J.; Dzhagalov, I.; He, Y.W. An essential function for the calcium-promoted Ras inactivator in Fcγ receptor-mediated phagocytosis. Nat. Immunol. 2005, 6, 911–919. [Google Scholar] [CrossRef]
- Caron, E.; Hall, A. Identification of two distinct mechanisms of phagocytosis controlled by different Rho GTPases. Science 1998, 282, 1717–1721. [Google Scholar] [CrossRef]
- Lorenzi, R.; Brickell, P.M.; Katz, D.R.; Kinnon, C.; Thrasher, A.J. Wiskott-Aldrich syndrome protein is necessary for efficient IgG-mediated phagocytosis. Blood 2000, 95, 2943–2946. [Google Scholar] [CrossRef]
- D’Amico, A.E.; Wong, A.C.; Zajd, C.M.; Zhang, X.; Murali, A.; Trebak, M.; Lennartz, M.R. PKC-epsilon regulates vesicle delivery and focal exocytosis for efficient IgG-mediated phagocytosis. J. Cell Sci. 2021, 134, jcs258886. [Google Scholar] [CrossRef]
- Leverrier, Y.; Okkenhaug, K.; Sawyer, C.; Bilancio, A.; Vanhaesebroeck, B.; Ridley, A.J. Class I phosphoinositide 3-kinase p110beta is required for apoptotic cell and Fcγ receptor-mediated phagocytosis by macrophages. J. Biol. Chem. 2003, 278, 38437–38442. [Google Scholar] [CrossRef]
- Suzuki, T.; Kono, H.; Hirose, N.; Okada, M.; Yamamoto, T.; Yamamoto, K.; Honda, Z. Differential involvement of Src family kinases in Fcγ receptor-mediated phagocytosis. J. Immunol. 2000, 165, 473–482. [Google Scholar] [CrossRef]
- Swanson, J.A.; Hoppe, A.D. The coordination of signaling during Fc receptor-mediated phagocytosis. J. Leukoc. Biol. 2004, 76, 1093–1103. [Google Scholar] [CrossRef]
- Marion, S.; Mazzolini, J.; Herit, F.; Bourdoncle, P.; Kambou-Pene, N.; Hailfinger, S.; Sachse, M.; Ruland, J.; Benmerah, A.; Echard, A.; et al. The NF-kappaB signaling protein Bcl10 regulates actin dynamics by controlling AP1 and OCRL-bearing vesicles. Dev. Cell 2012, 23, 954–967. [Google Scholar] [CrossRef] [PubMed]
- Swanson, J.A. Shaping cups into phagosomes and macropinosomes. Nat. Rev. Mol. Cell Biol. 2008, 9, 639–649. [Google Scholar] [CrossRef] [PubMed]
- Braun, V.; Fraisier, V.; Raposo, G.; Hurbain, I.; Sibarita, J.B.; Chavrier, P.; Galli, T.; Niedergang, F. TI-VAMP/VAMP7 is required for optimal phagocytosis of opsonised particles in macrophages. EMBO J. 2004, 23, 4166–4176. [Google Scholar] [CrossRef] [PubMed]
- Niedergang, F.; Colucci-Guyon, E.; Dubois, T.; Raposo, G.; Chavrier, P. ADP ribosylation factor 6 is activated and controls membrane delivery during phagocytosis in macrophages. J. Cell Biol. 2003, 161, 1143–1150. [Google Scholar] [CrossRef] [PubMed]
- Nunes, P.; Demaurex, N. The role of calcium signaling in phagocytosis. J. Leukoc. Biol. 2010, 88, 57–68. [Google Scholar] [CrossRef]
- Peiser, L.; Mukhopadhyay, S.; Gordon, S. Scavenger receptors in innate immunity. Curr. Opin. Immunol. 2002, 14, 123–128. [Google Scholar] [CrossRef]
- Sulahian, T.H.; Imrich, A.; Deloid, G.; Winkler, A.R.; Kobzik, L. Signaling pathways required for macrophage scavenger receptor-mediated phagocytosis: Analysis by scanning cytometry. Respir. Res. 2008, 9, 59. [Google Scholar] [CrossRef]
- Strijbis, K.; Tafesse, F.G.; Fairn, G.D.; Witte, M.D.; Dougan, S.K.; Watson, N.; Spooner, E.; Esteban, A.; Vyas, V.K.; Fink, G.R.; et al. Bruton’s Tyrosine Kinase (BTK) and Vav1 contribute to Dectin1-dependent phagocytosis of Candida albicans in macrophages. PLoS Pathog. 2013, 9, e1003446. [Google Scholar] [CrossRef]
- Guilliams, M.; Bruhns, P.; Saeys, Y.; Hammad, H.; Lambrecht, B.N. The function of Fcγ receptors in dendritic cells and macrophages. Nat. Rev. Immunol. 2014, 14, 94–108. [Google Scholar] [CrossRef]
- Small, A.G.; Perveen, K.; Putty, T.; Patel, N.; Quinn, P.; Wechalekar, M.D.; Hii, C.S.; Quach, A.; Ferrante, A. Neutrophils Require Activation to Express Functional Cell-Surface Complement Receptor Immunoglobulin. Front. Immunol. 2022, 13, 840510. [Google Scholar] [CrossRef]
- Sica, A.; Schioppa, T.; Mantovani, A.; Allavena, P. Tumour-associated macrophages are a distinct M2 polarised population promoting tumour progression: Potential targets of anti-cancer therapy. Eur. J. Cancer 2006, 42, 717–727. [Google Scholar] [CrossRef] [PubMed]
- Musolino, A.; Gradishar, W.J.; Rugo, H.S.; Nordstrom, J.L.; Rock, E.P.; Arnaldez, F.; Pegram, M.D. Role of Fcγ receptors in HER2-targeted breast cancer therapy. J. Immunother. Cancer 2022, 10, e003171. [Google Scholar] [CrossRef] [PubMed]
- Shapouri-Moghaddam, A.; Mohammadian, S.; Vazini, H.; Taghadosi, M.; Esmaeili, S.A.; Mardani, F.; Seifi, B.; Mohammadi, A.; Afshari, J.T.; Sahebkar, A. Macrophage plasticity, polarization, and function in health and disease. J. Cell Physiol. 2018, 233, 6425–6440. [Google Scholar] [CrossRef] [PubMed]
- Kirschning, C.J.; Wesche, H.; Merrill Ayres, T.; Rothe, M. Human toll-like receptor 2 confers responsiveness to bacterial lipopolysaccharide. J. Exp. Med. 1998, 188, 2091–2097. [Google Scholar] [CrossRef]
- Toshchakov, V.; Jones, B.W.; Perera, P.Y.; Thomas, K.; Cody, M.J.; Zhang, S.; Williams, B.R.; Major, J.; Hamilton, T.A.; Fenton, M.J.; et al. TLR4, but not TLR2, mediates IFN-beta-induced STAT1alpha/beta-dependent gene expression in macrophages. Nat. Immunol. 2002, 3, 392–398. [Google Scholar] [CrossRef]
- Murakami, Y.; Tian, L.; Voss, O.H.; Margulies, D.H.; Krzewski, K.; Coligan, J.E. CD300b regulates the phagocytosis of apoptotic cells via phosphatidylserine recognition. Cell Death Differ. 2014, 21, 1746–1757. [Google Scholar] [CrossRef]
- Kong, X.N.; Yan, H.X.; Chen, L.; Dong, L.W.; Yang, W.; Liu, Q.; Yu, L.X.; Huang, D.D.; Liu, S.Q.; Liu, H.; et al. LPS-induced down-regulation of signal regulatory protein alpha contributes to innate immune activation in macrophages. J. Exp. Med. 2007, 204, 2719–2731. [Google Scholar] [CrossRef]
- Kong, L.; Ge, B.X. MyD88-independent activation of a novel actin-Cdc42/Rac pathway is required for Toll-like receptor-stimulated phagocytosis. Cell Res. 2008, 18, 745–755. [Google Scholar] [CrossRef]
- Pearson, L.L.; Castle, B.E.; Kehry, M.R. CD40-mediated signaling in monocytic cells: Up-regulation of tumor necrosis factor receptor-associated factor mRNAs and activation of mitogen-activated protein kinase signaling pathways. Int. Immunol. 2001, 13, 273–283. [Google Scholar] [CrossRef]
- Beatty, G.L.; Chiorean, E.G.; Fishman, M.P.; Saboury, B.; Teitelbaum, U.R.; Sun, W.; Huhn, R.D.; Song, W.; Li, D.; Sharp, L.L.; et al. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science 2011, 331, 1612–1616. [Google Scholar] [CrossRef]
- Liu, M.; O’Connor, R.S.; Trefely, S.; Graham, K.; Snyder, N.W.; Beatty, G.L. Metabolic rewiring of macrophages by CpG potentiates clearance of cancer cells and overcomes tumor-expressed CD47-mediated ‘don’t-eat-me’ signal. Nat. Immunol. 2019, 20, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Hardie, J.; Mas-Rosario, J.A.; Ha, S.; Rizzo, E.M.; Farkas, M.E. Macrophage activation by a substituted pyrimido[5,4-b]indole increases anti-cancer activity. Pharmacol. Res. 2019, 148, 104452. [Google Scholar] [CrossRef] [PubMed]
- Pyonteck, S.M.; Akkari, L.; Schuhmacher, A.J.; Bowman, R.L.; Sevenich, L.; Quail, D.F.; Olson, O.C.; Quick, M.L.; Huse, J.T.; Teijeiro, V.; et al. CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nat. Med. 2013, 19, 1264–1272. [Google Scholar] [CrossRef] [PubMed]
- Guerriero, J.L.; Sotayo, A.; Ponichtera, H.E.; Castrillon, J.A.; Pourzia, A.L.; Schad, S.; Johnson, S.F.; Carrasco, R.D.; Lazo, S.; Bronson, R.T.; et al. Class IIa HDAC inhibition reduces breast tumours and metastases through anti-tumour macrophages. Nature 2017, 543, 428–432. [Google Scholar] [CrossRef]
- Mitochondrial fission in macrophages fuels phagocytosis of tumor cells. Nat. Cancer 2022, 3, 384–385. [CrossRef] [PubMed]
- Li, J.; Ye, Y.; Liu, Z.; Zhang, G.; Dai, H.; Li, J.; Zhou, B.; Li, Y.; Zhao, Q.; Huang, J.; et al. Macrophage mitochondrial fission improves cancer cell phagocytosis induced by therapeutic antibodies and is impaired by glutamine competition. Nat. Cancer 2022, 3, 453–470. [Google Scholar] [CrossRef]
- Moradinasab, S.; Pourbagheri-Sigaroodi, A.; Ghaffari, S.H.; Bashash, D. Targeting macrophage-mediated tumor cell phagocytosis: An overview of phagocytosis checkpoints blockade, nanomedicine intervention, and engineered CAR-macrophage therapy. Int. Immunopharmacol. 2022, 103, 108499. [Google Scholar] [CrossRef]
- Hossain, M.; Shim, R.; Lee, W.Y.; Sharpe, A.H.; Kubes, P. Gata6+ resident peritoneal macrophages promote the growth of liver metastasis. Nat. Commun. 2022, 13, 4406. [Google Scholar] [CrossRef]
- Russ, A.; Hua, A.B.; Montfort, W.R.; Rahman, B.; Riaz, I.B.; Khalid, M.U.; Carew, J.S.; Nawrocki, S.T.; Persky, D.; Anwer, F. Blocking “don’t eat me” signal of CD47-SIRPalpha in hematological malignancies, an in-depth review. Blood Rev. 2018, 32, 480–489. [Google Scholar] [CrossRef]
- Hayat, S.M.G.; Bianconi, V.; Pirro, M.; Jaafari, M.R.; Hatamipour, M.; Sahebkar, A. CD47: Role in the immune system and application to cancer therapy. Cell. Oncol. 2020, 43, 19–30. [Google Scholar] [CrossRef]
- Brierley, C.K.; Staves, J.; Roberts, C.; Johnson, H.; Vyas, P.; Goodnough, L.T.; Murphy, M.F. The effects of monoclonal anti-CD47 on RBCs, compatibility testing, and transfusion requirements in refractory acute myeloid leukemia. Transfusion 2019, 59, 2248–2254. [Google Scholar] [CrossRef] [PubMed]
- Sikic, B.I.; Lakhani, N.; Patnaik, A.; Shah, S.A.; Chandana, S.R.; Rasco, D.; Colevas, A.D.; O’Rourke, T.; Narayanan, S.; Papadopoulos, K.; et al. First-in-Human, First-in-Class Phase I Trial of the Anti-CD47 Antibody Hu5F9-G4 in Patients With Advanced Cancers. J. Clin. Oncol. 2019, 37, 946–953. [Google Scholar] [CrossRef] [PubMed]
- Advani, R.; Flinn, I.; Popplewell, L.; Forero, A.; Bartlett, N.L.; Ghosh, N.; Kline, J.; Roschewski, M.; LaCasce, A.; Collins, G.P.; et al. CD47 Blockade by Hu5F9-G4 and Rituximab in Non-Hodgkin’s Lymphoma. N. Engl. J. Med. 2018, 379, 1711–1721. [Google Scholar] [CrossRef] [PubMed]
- Gholamin, S.; Mitra, S.S.; Feroze, A.H.; Liu, J.; Kahn, S.A.; Zhang, M.; Esparza, R.; Richard, C.; Ramaswamy, V.; Remke, M.; et al. Disrupting the CD47-SIRPalpha anti-phagocytic axis by a humanized anti-CD47 antibody is an efficacious treatment for malignant pediatric brain tumors. Sci. Transl. Med. 2017, 9, eaaf2968. [Google Scholar] [CrossRef] [PubMed]
- Zeidan, A.M.; DeAngelo, D.J.; Palmer, J.; Seet, C.S.; Tallman, M.S.; Wei, X.; Raymon, H.; Sriraman, P.; Kopytek, S.; Bewersdorf, J.P.; et al. Phase 1 study of anti-CD47 monoclonal antibody CC-90002 in patients with relapsed/refractory acute myeloid leukemia and high-risk myelodysplastic syndromes. Ann. Hematol. 2022, 101, 557–569. [Google Scholar] [CrossRef]
- Yu, X.Y.; Qiu, W.Y.; Long, F.; Yang, X.P.; Zhang, C.; Xu, L.; Chang, H.Y.; Du, P.; Hou, X.J.; Yu, Y.Z.; et al. A novel fully human anti-CD47 antibody as a potential therapy for human neoplasms with good safety. Biochimie 2018, 151, 54–66. [Google Scholar] [CrossRef]
- Ni, H.; Cao, L.; Wu, Z.; Wang, L.; Zhou, S.; Guo, X.; Gao, Y.; Jing, H.; Wu, M.; Liu, Y.; et al. Combined strategies for effective cancer immunotherapy with a novel anti-CD47 monoclonal antibody. Cancer Immunol. Immunother. 2022, 71, 353–363. [Google Scholar] [CrossRef]
- Peluso, M.O.; Adam, A.; Armet, C.M.; Zhang, L.; O’Connor, R.W.; Lee, B.H.; Lake, A.C.; Normant, E.; Chappel, S.C.; Hill, J.A.; et al. The Fully human anti-CD47 antibody SRF231 exerts dual-mechanism antitumor activity via engagement of the activating receptor CD32a. J. Immunother. Cancer 2020, 8, e000413. [Google Scholar] [CrossRef]
- Puro, R.J.; Bouchlaka, M.N.; Hiebsch, R.R.; Capoccia, B.J.; Donio, M.J.; Manning, P.T.; Frazier, W.A.; Karr, R.W.; Pereira, D.S. Development of AO-176, a Next-Generation Humanized Anti-CD47 Antibody with Novel Anticancer Properties and Negligible Red Blood Cell Binding. Mol. Cancer Ther. 2020, 19, 835–846. [Google Scholar] [CrossRef]
- Bouwstra, R.; van Meerten, T.; Bremer, E. CD47-SIRPalpha blocking-based immunotherapy: Current and prospective therapeutic strategies. Clin. Transl. Med. 2022, 12, e943. [Google Scholar] [CrossRef]
- Willingham, S.B.; Volkmer, J.P.; Gentles, A.J.; Sahoo, D.; Dalerba, P.; Mitra, S.S.; Wang, J.; Contreras-Trujillo, H.; Martin, R.; Cohen, J.D.; et al. The CD47-signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors. Proc. Natl. Acad. Sci. USA 2012, 109, 6662–6667. [Google Scholar] [CrossRef] [PubMed]
- Piccione, E.C.; Juarez, S.; Liu, J.; Tseng, S.; Ryan, C.E.; Narayanan, C.; Wang, L.; Weiskopf, K.; Majeti, R. A bispecific antibody targeting CD47 and CD20 selectively binds and eliminates dual antigen expressing lymphoma cells. MAbs 2015, 7, 946–956. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ni, H.; Zhou, S.; He, K.; Gao, Y.; Wu, W.; Wu, M.; Wu, Z.; Qiu, X.; Zhou, Y.; et al. Tumor-selective blockade of CD47 signaling with a CD47/PD-L1 bispecific antibody for enhanced anti-tumor activity and limited toxicity. Cancer Immunol. Immunother. 2021, 70, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Shi, W.; Shi, J.J.; Lu, J.J. Progress of CD47 immune checkpoint blockade agents in anticancer therapy: A hematotoxic perspective. J. Cancer Res. Clin. Oncol. 2022, 148, 1–14. [Google Scholar] [CrossRef]
- Voets, E.; Parade, M.; Lutje Hulsik, D.; Spijkers, S.; Janssen, W.; Rens, J.; Reinieren-Beeren, I.; van den Tillaart, G.; van Duijnhoven, S.; Driessen, L.; et al. Functional characterization of the selective pan-allele anti-SIRPalpha antibody ADU-1805 that blocks the SIRPalpha-CD47 innate immune checkpoint. J. Immunother. Cancer 2019, 7, 340. [Google Scholar] [CrossRef]
- Ansell, S.M.; Maris, M.B.; Lesokhin, A.M.; Chen, R.W.; Flinn, I.W.; Sawas, A.; Minden, M.D.; Villa, D.; Percival, M.M.; Advani, A.S.; et al. Phase I Study of the CD47 Blocker TTI-621 in Patients with Relapsed or Refractory Hematologic Malignancies. Clin. Cancer Res. 2021, 27, 2190–2199. [Google Scholar] [CrossRef]
- Querfeld, C.; Thompson, J.A.; Taylor, M.H.; DeSimone, J.A.; Zain, J.M.; Shustov, A.R.; Johns, C.; McCann, S.; Lin, G.H.Y.; Petrova, P.S.; et al. Intralesional TTI-621, a novel biologic targeting the innate immune checkpoint CD47, in patients with relapsed or refractory mycosis fungoides or Sezary syndrome: A multicentre, phase 1 study. Lancet Haematol. 2021, 8, e808–e817. [Google Scholar] [CrossRef]
- Lakhani, N.J.; Chow, L.Q.M.; Gainor, J.F.; LoRusso, P.; Lee, K.W.; Chung, H.C.; Lee, J.; Bang, Y.J.; Hodi, F.S.; Kim, W.S.; et al. Evorpacept alone and in combination with pembrolizumab or trastuzumab in patients with advanced solid tumours (ASPEN-01): A first-in-human, open-label, multicentre, phase 1 dose-escalation and dose-expansion study. Lancet Oncol. 2021, 22, 1740–1751. [Google Scholar] [CrossRef]
- He, H.; Tu, X.; Zhang, J.; Acheampong, D.O.; Ding, L.; Ma, Z.; Ren, X.; Luo, C.; Chen, Z.; Wang, T.; et al. A novel antibody targeting CD24 and hepatocellular carcinoma in vivo by near-infrared fluorescence imaging. Immunobiology 2015, 220, 1328–1336. [Google Scholar] [CrossRef]
- Sun, F.; Wang, Y.; Luo, X.; Ma, Z.; Xu, Y.; Zhang, X.; Lv, T.; Zhang, Y.; Wang, M.; Huang, Z.; et al. Anti-CD24 Antibody-Nitric Oxide Conjugate Selectively and Potently Suppresses Hepatic Carcinoma. Cancer Res. 2019, 79, 3395–3405. [Google Scholar] [CrossRef]
- Sousa, L.G.; Rajapakshe, K.; Rodriguez Canales, J.; Chin, R.L.; Feng, L.; Wang, Q.; Barrese, T.Z.; Massarelli, E.; William, W.; Johnson, F.M.; et al. ISA101 and nivolumab for HPV-16+ cancer: Updated clinical efficacy and immune correlates of response. J. Immunother. Cancer 2022, 10, e004232. [Google Scholar] [CrossRef] [PubMed]
- Armand, P.; Janssens, A.; Gritti, G.; Radford, J.; Timmerman, J.; Pinto, A.; Mercadal Vilchez, S.; Johnson, P.; Cunningham, D.; Leonard, J.P.; et al. Efficacy and safety results from CheckMate 140, a phase 2 study of nivolumab for relapsed/refractory follicular lymphoma. Blood 2021, 137, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Haag, G.M.; Springfeld, C.; Grun, B.; Apostolidis, L.; Zschabitz, S.; Dietrich, M.; Berger, A.K.; Weber, T.F.; Zoernig, I.; Schaaf, M.; et al. Pembrolizumab and maraviroc in refractory mismatch repair proficient/microsatellite-stable metastatic colorectal cancer—The PICCASSO phase I trial. Eur. J. Cancer 2022, 167, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Ni, J.; Zhou, Y.; Wu, L.; Ai, X.; Dong, X.; Chu, Q.; Han, C.; Wang, X.; Zhu, Z. Sintilimab, stereotactic body radiotherapy and granulocyte-macrophage colony stimulating factor as second-line therapy for advanced non-small cell lung cancer: Safety run-in results of a multicenter, single-arm, phase II trial. Radiat. Oncol. 2021, 16, 177. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Allavena, P.; Marchesi, F.; Garlanda, C. Macrophages as tools and targets in cancer therapy. Nat. Rev. Drug Discov. 2022, 21, 799–820. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; An, E.K.; Kim, S.J.; You, S.; Jin, J.O. Intranasal Administration of Codium fragile Polysaccharide Elicits Anti-Cancer Immunity against Lewis Lung Carcinoma. Int. J. Mol. Sci. 2021, 22, 10608. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Qi, Y.; Kong, X.; Wang, Z.; Fang, Y.; Wang, J. PD-1/PD-L1 Inhibitor-Associated Myocarditis: Epidemiology, Characteristics, Diagnosis, Treatment, and Potential Mechanism. Front. Pharmacol. 2022, 13, 835510. [Google Scholar] [CrossRef]
- Qin, W.; Hu, L.; Zhang, X.; Jiang, S.; Li, J.; Zhang, Z.; Wang, X. The Diverse Function of PD-1/PD-L Pathway Beyond Cancer. Front. Immunol. 2019, 10, 2298. [Google Scholar] [CrossRef]
- Su, C.; Wang, H.; Liu, Y.; Guo, Q.; Zhang, L.; Li, J.; Zhou, W.; Yan, Y.; Zhou, X.; Zhang, J. Adverse Effects of Anti-PD-1/PD-L1 Therapy in Non-small Cell Lung Cancer. Front. Oncol. 2020, 10, 554313. [Google Scholar] [CrossRef]
- Hahn, A.W.; Gill, D.M.; Agarwal, N.; Maughan, B.L. PD-1 checkpoint inhibition: Toxicities and management. Urol. Oncol. 2017, 35, 701–707. [Google Scholar] [CrossRef]
- Sato, R.; Imamura, K.; Sakata, S.; Ikeda, T.; Horio, Y.; Iyama, S.; Akaike, K.; Hamada, S.; Jodai, T.; Nakashima, K.; et al. Disorder of Coagulation-Fibrinolysis System: An Emerging Toxicity of Anti-PD-1/PD-L1 Monoclonal Antibodies. J. Clin. Med. 2019, 8, 762. [Google Scholar] [CrossRef] [PubMed]
- Salik, B.; Smyth, M.J.; Nakamura, K. Targeting immune checkpoints in hematological malignancies. J. Hematol. Oncol. 2020, 13, 111. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, F.; Guo, R.; Bian, Z.; Song, Y. Targeting macrophages in hematological malignancies: Recent advances and future directions. J. Hematol. Oncol. 2022, 15, 110. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, Z.; Yang, Y. Potential Role of CD47-Directed Bispecific Antibodies in Cancer Immunotherapy. Front. Immunol. 2021, 12, 686031. [Google Scholar] [CrossRef] [PubMed]
- Chauchet, X.; Cons, L.; Chatel, L.; Daubeuf, B.; Didelot, G.; Moine, V.; Chollet, D.; Malinge, P.; Pontini, G.; Masternak, K.; et al. CD47xCD19 bispecific antibody triggers recruitment and activation of innate immune effector cells in a B-cell lymphoma xenograft model. Exp. Hematol. Oncol. 2022, 11, 26. [Google Scholar] [CrossRef] [PubMed]
- Miller, T.W.; Amason, J.D.; Garcin, E.D.; Lamy, L.; Dranchak, P.K.; Macarthur, R.; Braisted, J.; Rubin, J.S.; Burgess, T.L.; Farrell, C.L.; et al. Quantitative high-throughput screening assays for the discovery and development of SIRPalpha-CD47 interaction inhibitors. PLoS ONE 2019, 14, e0218897. [Google Scholar] [CrossRef]
- Wu, Z.; Weng, L.; Zhang, T.; Tian, H.; Fang, L.; Teng, H.; Zhang, W.; Gao, J.; Hao, Y.; Li, Y.; et al. Identification of Glutaminyl Cyclase isoenzyme isoQC as a regulator of SIRPalpha-CD47 axis. Cell Res. 2019, 29, 502–505. [Google Scholar] [CrossRef]
- Logtenberg, M.E.W.; Jansen, J.H.M.; Raaben, M.; Toebes, M.; Franke, K.; Brandsma, A.M.; Matlung, H.L.; Fauster, A.; Gomez-Eerland, R.; Bakker, N.A.M.; et al. Glutaminyl cyclase is an enzymatic modifier of the CD47- SIRPalpha axis and a target for cancer immunotherapy. Nat. Med. 2019, 25, 612–619. [Google Scholar] [CrossRef]
- Li, Z.; Gu, X.; Rao, D.; Lu, M.; Wen, J.; Chen, X.; Wang, H.; Cui, X.; Tang, W.; Xu, S.; et al. Luteolin promotes macrophage-mediated phagocytosis by inhibiting CD47 pyroglutamation. Transl. Oncol. 2021, 14, 101129. [Google Scholar] [CrossRef]
- Kanemitsu, N.; Kiyonaga, F.; Mizukami, K.; Maeno, K.; Nishikubo, T.; Yoshida, H.; Ito, H. Chronic treatment with the (iso-)glutaminyl cyclase inhibitor PQ529 is a novel and effective approach for glomerulonephritis in chronic kidney disease. Naunyn Schmiedeberg’s Arch. Pharmcol. 2021, 394, 751–761. [Google Scholar] [CrossRef]
- Li, C.; Liu, Y.; Li, D.; Wang, Q.; Zhou, S.; Zhang, H.; Wang, Y.; He, Z.; Liu, H.; Sun, J. Promising alternatives of CD47 monoclonal antibody: An injectable degradable hydrogel loaded with PQ912 for postoperative immunotherapy effectively blocks CD47-SIRPalpha signal. Theranostics 2022, 12, 4581–4598. [Google Scholar] [CrossRef] [PubMed]
- Lau, J.L.; Dunn, M.K. Therapeutic peptides: Historical perspectives, current development trends, and future directions. Bioorg. Med. Chem. 2018, 26, 2700–2707. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Sun, Y.; Zhou, X.; Chen, C.; Jiao, L.; Li, W.; Gou, S.; Li, Y.; Du, J.; Chen, G.; et al. CD47/SIRPalpha blocking peptide identification and synergistic effect with irradiation for cancer immunotherapy. J. Immunother. Cancer 2020, 8, e000905. [Google Scholar] [CrossRef] [PubMed]
- Reganon, E.; Vila, V.; Aznar, J.; Garrido, G.; Estelles, A.; Berenguer, J. Study of the formation of fibrin clot in cirrhotic patients. An approach to study of acquired dysfibrinogenemia. Thromb. Res. 1987, 46, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Moghimi, S.M.; Hunter, A.C.; Murray, J.C. Nanomedicine: Current status and future prospects. FASEB J. 2005, 19, 311–330. [Google Scholar] [CrossRef]
- Lian, S.; Xie, X.; Lu, Y.; Jia, L. Checkpoint CD47 Function On Tumor Metastasis And Immune Therapy. Onco. Targets Ther. 2019, 12, 9105–9114. [Google Scholar] [CrossRef]
- Steeland, S.; Vandenbroucke, R.E.; Libert, C. Nanobodies as therapeutics: Big opportunities for small antibodies. Drug Discov. Today 2016, 21, 1076–1113. [Google Scholar] [CrossRef]
- Kijanka, M.; Dorresteijn, B.; Oliveira, S.; van Bergen en Henegouwen, P.M. Nanobody-based cancer therapy of solid tumors. Nanomedicine 2015, 10, 161–174. [Google Scholar] [CrossRef]
- Ma, L.; Zhu, M.; Gai, J.; Li, G.; Chang, Q.; Qiao, P.; Cao, L.; Chen, W.; Zhang, S.; Wan, Y. Preclinical development of a novel CD47 nanobody with less toxicity and enhanced anti-cancer therapeutic potential. J. Nanobiotechnol. 2020, 18, 12. [Google Scholar] [CrossRef]
- Noble, G.T.; Stefanick, J.F.; Ashley, J.D.; Kiziltepe, T.; Bilgicer, B. Ligand-targeted liposome design: Challenges and fundamental considerations. Trends Biotechnol. 2014, 32, 32–45. [Google Scholar] [CrossRef]
- Cheng, L.; Zhang, X.; Tang, J.; Lv, Q.; Liu, J. Gene-engineered exosomes-thermosensitive liposomes hybrid nanovesicles by the blockade of CD47 signal for combined photothermal therapy and cancer immunotherapy. Biomaterials 2021, 275, 120964. [Google Scholar] [CrossRef] [PubMed]
- Ovais, M.; Guo, M.; Chen, C. Tailoring Nanomaterials for Targeting Tumor-Associated Macrophages. Adv. Mater. 2019, 31, e1808303. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, A.; Kumar, S.; Nguyen, A.; Brouillard, A.; Kulkarni, A. Lipid-based phagocytosis nanoenhancer for macrophage immunotherapy. Nanoscale 2020, 12, 1875–1885. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef]
- Nie, W.; Wu, G.; Zhang, J.; Huang, L.L.; Ding, J.; Jiang, A.; Zhang, Y.; Liu, Y.; Li, J.; Pu, K.; et al. Responsive Exosome Nano-bioconjugates for Synergistic Cancer Therapy. Angew. Chem. Int. Ed. Engl. 2020, 59, 2018–2022. [Google Scholar] [CrossRef]
- Kooijmans, S.A.A.; Vader, P.; Schiffelers, R.M. Tumour-bound RNA-laden exosomes. Nat. Biomed. Eng. 2017, 1, 634–636. [Google Scholar] [CrossRef]
- Zhang, K.L.; Wang, Y.J.; Sun, J.; Zhou, J.; Xing, C.; Huang, G.; Li, J.; Yang, H. Artificial chimeric exosomes for anti-phagocytosis and targeted cancer therapy. Chem. Sci. 2019, 10, 1555–1561. [Google Scholar] [CrossRef]
- Belhadj, Z.; He, B.; Deng, H.; Song, S.; Zhang, H.; Wang, X.; Dai, W.; Zhang, Q. A combined “eat me/don’t eat me” strategy based on extracellular vesicles for anticancer nanomedicine. J. Extracell. Vesicles 2020, 9, 1806444. [Google Scholar] [CrossRef]
- Pham, L.M.; Poudel, K.; Phung, C.D.; Nguyen, T.T.; Pandit, M.; Nguyen, H.T.; Chang, J.H.; Jin, S.G.; Jeong, J.H.; Ku, S.K.; et al. Preparation and evaluation of dabrafenib-loaded, CD47-conjugated human serum albumin-based nanoconstructs for chemoimmunomodulation. Colloids Surf. B Biointerfaces 2021, 208, 112093. [Google Scholar] [CrossRef]
- Chen, H.; Cong, X.; Wu, C.; Wu, X.; Wang, J.; Mao, K.; Li, J.; Zhu, G.; Liu, F.; Meng, X.; et al. Intratumoral delivery of CCL25 enhances immunotherapy against triple-negative breast cancer by recruiting CCR9+ T cells. Sci. Adv. 2020, 6, eaax4690. [Google Scholar] [CrossRef]
- Guo, Z.; Liu, Y.; Zhou, H.; Zheng, K.; Wang, D.; Jia, M.; Xu, P.; Ma, K.; Cui, C.; Wang, L. CD47-targeted bismuth selenide nanoparticles actualize improved photothermal therapy by increasing macrophage phagocytosis of cancer cells. Colloids Surf. B Biointerfaces 2019, 184, 110546. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Wang, C.; Zhang, X.; Chen, G.; Hu, Q.; Li, H.; Wang, J.; Wen, D.; Zhang, Y.; Lu, Y.; et al. In situ sprayed bioresponsive immunotherapeutic gel for post-surgical cancer treatment. Nat. Nanotechnol. 2019, 14, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.R.; Luo, J.Q.; Zhang, J.Y.; Miao, W.M.; Wu, J.S.; Huang, H.; Tong, Q.S.; Shen, S.; Leong, K.W.; Du, J.Z.; et al. Nanoparticle-Enabled Dual Modulation of Phagocytic Signals to Improve Macrophage-Mediated Cancer Immunotherapy. Small 2020, 16, e2004240. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zheng, J.; Sun, W.; Zhao, X.; Li, Y.; Gong, N.; Wang, Y.; Ma, X.; Zhang, T.; Zhao, L.Y.; et al. Ferrimagnetic Vortex Nanoring-Mediated Mild Magnetic Hyperthermia Imparts Potent Immunological Effect for Treating Cancer Metastasis. ACS Nano 2019, 13, 8811–8825. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yan, B.; Li, Y.; Ma, X.; Jiao, W.; Shi, K.; Zhang, T.; Chen, S.; He, Y.; Liang, X.J.; et al. Graphene Oxide-Grafted Magnetic Nanorings Mediated Magnetothermodynamic Therapy Favoring Reactive Oxygen Species-Related Immune Response for Enhanced Antitumor Efficacy. ACS Nano 2020, 14, 1936–1950. [Google Scholar] [CrossRef]
- Kimura, Y.; Aoki, H.; Soyama, T.; Sakuragi, A.; Otsuka, Y.; Nomoto, A.; Yano, S.; Nishie, H.; Kataoka, H.; Aoyama, M. Photodynamic therapy using mannose-conjugated chlorin e6 increases cell surface calreticulin in cancer cells and promotes macrophage phagocytosis. Med. Oncol. 2022, 39, 82. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J.; Zhang, J.; Marbach, S.; Xu, W.; Zhu, L. Targeting Tumor-Associated Macrophages by MMP2-Sensitive Apoptotic Body-Mimicking Nanoparticles. ACS Appl. Mater Interfaces 2020, 12, 52402–52414. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, X.; Huang, L. Macrophage-Mediated Tumor Cell Phagocytosis: Opportunity for Nanomedicine Intervention. Adv. Funct. Mater. 2021, 31, 2006220. [Google Scholar] [CrossRef]
- Chen, T.F.; Li, K.K.; Zhu, E.F.; Opel, C.F.; Kauke, M.J.; Kim, H.; Atolia, E.; Wittrup, K.D. Artificial Anti-Tumor Opsonizing Proteins with Fibronectin Scaffolds Engineered for Specificity to Each of the Murine FcγR Types. J. Mol. Biol. 2018, 430, 1786–1798. [Google Scholar] [CrossRef]
- Cao, X.; Chen, J.; Li, B.; Dang, J.; Zhang, W.; Zhong, X.; Wang, C.; Raoof, M.; Sun, Z.; Yu, J.; et al. Promoting antibody-dependent cellular phagocytosis for effective macrophage-based cancer immunotherapy. Sci. Adv. 2022, 8, eabl9171. [Google Scholar] [CrossRef]
- Kurdi, A.T.; Glavey, S.V.; Bezman, N.A.; Jhatakia, A.; Guerriero, J.L.; Manier, S.; Moschetta, M.; Mishima, Y.; Roccaro, A.; Detappe, A.; et al. Antibody-Dependent Cellular Phagocytosis by Macrophages is a Novel Mechanism of Action of Elotuzumab. Mol. Cancer Ther. 2018, 17, 1454–1463. [Google Scholar] [CrossRef]
- Lonial, S.; Dimopoulos, M.; Palumbo, A.; White, D.; Grosicki, S.; Spicka, I.; Walter-Croneck, A.; Moreau, P.; Mateos, M.V.; Magen, H.; et al. Elotuzumab Therapy for Relapsed or Refractory Multiple Myeloma. N. Engl. J. Med. 2015, 373, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Fan, X.; Deng, H.; Brezski, R.J.; Rycyzyn, M.; Jordan, R.E.; Strohl, W.R.; Zou, Q.; Zhang, N.; An, Z. Trastuzumab triggers phagocytic killing of high HER2 cancer cells in vitro and in vivo by interaction with Fcγ receptors on macrophages. J. Immunol. 2015, 194, 4379–4386. [Google Scholar] [CrossRef] [PubMed]
- Overdijk, M.B.; Verploegen, S.; Bogels, M.; van Egmond, M.; Lammerts van Bueren, J.J.; Mutis, T.; Groen, R.W.; Breij, E.; Martens, A.C.; Bleeker, W.K.; et al. Antibody-mediated phagocytosis contributes to the anti-tumor activity of the therapeutic antibody daratumumab in lymphoma and multiple myeloma. MAbs 2015, 7, 311–321. [Google Scholar] [CrossRef]
- Naicker, S.D.; Feerick, C.L.; Lynch, K.; Swan, D.; McEllistrim, C.; Henderson, R.; Leonard, N.A.; Treacy, O.; Natoni, A.; Rigalou, A.; et al. Cyclophosphamide alters the tumor cell secretome to potentiate the anti-myeloma activity of daratumumab through augmentation of macrophage-mediated antibody dependent cellular phagocytosis. Oncoimmunology 2021, 10, 1859263. [Google Scholar] [CrossRef] [PubMed]
- Roghanian, A.; Hu, G.; Fraser, C.; Singh, M.; Foxall, R.B.; Meyer, M.J.; Lees, E.; Huet, H.; Glennie, M.J.; Beers, S.A.; et al. Cyclophosphamide Enhances Cancer Antibody Immunotherapy in the Resistant Bone Marrow Niche by Modulating Macrophage FcγR Expression. Cancer Immunol. Res. 2019, 7, 1876–1890. [Google Scholar] [CrossRef]
- Upton, R.; Banuelos, A.; Feng, D.; Biswas, T.; Kao, K.; McKenna, K.; Willingham, S.; Ho, P.Y.; Rosental, B.; Tal, M.C.; et al. Combining CD47 blockade with trastuzumab eliminates HER2-positive breast cancer cells and overcomes trastuzumab tolerance. Proc. Natl. Acad. Sci. USA 2021, 118, e2026849118. [Google Scholar] [CrossRef]
- Gul, N.; van Egmond, M. Antibody-Dependent Phagocytosis of Tumor Cells by Macrophages: A Potent Effector Mechanism of Monoclonal Antibody Therapy of Cancer. Cancer Res. 2015, 75, 5008–5013. [Google Scholar] [CrossRef]
- Gogesch, P.; Dudek, S.; van Zandbergen, G.; Waibler, Z.; Anzaghe, M. The Role of Fc Receptors on the Effectiveness of Therapeutic Monoclonal Antibodies. Int. J. Mol. Sci. 2021, 22, 8947. [Google Scholar] [CrossRef]
- Bolli, E.; Scherger, M.; Arnouk, S.M.; Pombo Antunes, A.R.; Strassburger, D.; Urschbach, M.; Stickdorn, J.; De Vlaminck, K.; Movahedi, K.; Rader, H.J.; et al. Targeted Repolarization of Tumor-Associated Macrophages via Imidazoquinoline-Linked Nanobodies. Adv. Sci. 2021, 8, 2004574. [Google Scholar] [CrossRef]
- Ramesh, A.; Kumar, S.; Nandi, D.; Kulkarni, A. CSF1R- and SHP2-Inhibitor-Loaded Nanoparticles Enhance Cytotoxic Activity and Phagocytosis in Tumor-Associated Macrophages. Adv. Mater. 2019, 31, e1904364. [Google Scholar] [CrossRef] [PubMed]
- Yunna, C.; Mengru, H.; Lei, W.; Weidong, C. Macrophage M1/M2 polarization. Eur. J. Pharmacol. 2020, 877, 173090. [Google Scholar] [CrossRef] [PubMed]
- Akilesh, H.M.; Buechler, M.B.; Duggan, J.M.; Hahn, W.O.; Matta, B.; Sun, X.; Gessay, G.; Whalen, E.; Mason, M.; Presnell, S.R.; et al. Chronic TLR7 and TLR9 signaling drives anemia via differentiation of specialized hemophagocytes. Science 2019, 363, eaao5213. [Google Scholar] [CrossRef] [PubMed]
- Ni, K.; Luo, T.; Culbert, A.; Kaufmann, M.; Jiang, X.; Lin, W. Nanoscale Metal-Organic Framework Co-delivers TLR-7 Agonists and Anti-CD47 Antibodies to Modulate Macrophages and Orchestrate Cancer Immunotherapy. J. Am. Chem. Soc. 2020, 142, 12579–12584. [Google Scholar] [CrossRef]
- Li, F.; Zheng, X.; Wang, X.; Xu, J.; Zhang, Q. Macrophage polarization synergizes with oxaliplatin in lung cancer immunotherapy via enhanced tumor cell phagocytosis. Transl. Oncol. 2021, 14, 101202. [Google Scholar] [CrossRef]
- Li, H.; Somiya, M.; Kuroda, S. Enhancing antibody-dependent cellular phagocytosis by Re-education of tumor-associated macrophages with resiquimod-encapsulated liposomes. Biomaterials 2021, 268, 120601. [Google Scholar] [CrossRef]
- Kruit, W.H.; Suciu, S.; Dreno, B.; Mortier, L.; Robert, C.; Chiarion-Sileni, V.; Maio, M.; Testori, A.; Dorval, T.; Grob, J.J.; et al. Selection of immunostimulant AS15 for active immunization with MAGE-A3 protein: Results of a randomized phase II study of the European Organisation for Research and Treatment of Cancer Melanoma Group in Metastatic Melanoma. J. Clin. Oncol. 2013, 31, 2413–2420. [Google Scholar] [CrossRef]
- McQuade, J.L.; Homsi, J.; Torres-Cabala, C.A.; Bassett, R.; Popuri, R.M.; James, M.L.; Vence, L.M.; Hwu, W.J. A phase II trial of recombinant MAGE-A3 protein with immunostimulant AS15 in combination with high-dose Interleukin-2 (HDIL2) induction therapy in metastatic melanoma. BMC Cancer 2018, 18, 1274. [Google Scholar] [CrossRef]
- Sabado, R.L.; Pavlick, A.; Gnjatic, S.; Cruz, C.M.; Vengco, I.; Hasan, F.; Spadaccia, M.; Darvishian, F.; Chiriboga, L.; Holman, R.M.; et al. Resiquimod as an immunologic adjuvant for NY-ESO-1 protein vaccination in patients with high-risk melanoma. Cancer Immunol. Res. 2015, 3, 278–287. [Google Scholar] [CrossRef]
- Mehrotra, S.; Britten, C.D.; Chin, S.; Garrett-Mayer, E.; Cloud, C.A.; Li, M.; Scurti, G.; Salem, M.L.; Nelson, M.H.; Thomas, M.B.; et al. Vaccination with poly(IC:LC) and peptide-pulsed autologous dendritic cells in patients with pancreatic cancer. J. Hematol. Oncol. 2017, 10, 82. [Google Scholar] [CrossRef]
- Shen, X.; Burguillos, M.A.; Osman, A.M.; Frijhoff, J.; Carrillo-Jimenez, A.; Kanatani, S.; Augsten, M.; Saidi, D.; Rodhe, J.; Kavanagh, E.; et al. Glioma-induced inhibition of caspase-3 in microglia promotes a tumor-supportive phenotype. Nat. Immunol. 2016, 17, 1282–1290. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; He, Y.; Wu, C.; Zhang, M.; Gu, Z.; Zhang, J.; Liu, E.; Xu, Q.; Asrorov, A.M.; Huang, Y. Magnetism-mediated targeting hyperthermia-immunotherapy in “cold” tumor with CSF1R inhibitor. Theranostics 2021, 11, 6860–6872. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Sun, J.; Yang, Y.; Liu, Y.; Lou, J.; Pan, H.; Yao, J.; Han, W. TMP195 Exerts Antitumor Effects on Colorectal Cancer by Promoting M1 Macrophages Polarization. Int. J. Biol. Sci. 2022, 18, 5653–5666. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.; Li, F.; Li, Y.; Wang, Y.; Guo, X.; Cheng, Z.; Li, N.; Ma, X.; Nie, G.; Zhao, X. Biomimetic Nanoparticles Carrying a Repolarization Agent of Tumor-Associated Macrophages for Remodeling of the Inflammatory Microenvironment Following Photothermal Therapy. ACS Nano 2021, 15, 15166–15179. [Google Scholar] [CrossRef] [PubMed]
- Pan, K.; Farrukh, H.; Chittepu, V.; Xu, H.; Pan, C.X.; Zhu, Z. CAR race to cancer immunotherapy: From CAR T, CAR NK to CAR macrophage therapy. J. Exp. Clin. Cancer Res. 2022, 41, 119. [Google Scholar] [CrossRef] [PubMed]
- Keshavarz, A.; Salehi, A.; Khosravi, S.; Shariati, Y.; Nasrabadi, N.; Kahrizi, M.S.; Maghsoodi, S.; Mardi, A.; Azizi, R.; Jamali, S.; et al. Recent findings on chimeric antigen receptor (CAR)-engineered immune cell therapy in solid tumors and hematological malignancies. Stem. Cell Res. Ther. 2022, 13, 482. [Google Scholar] [CrossRef]
- Kang, M.; Lee, S.H.; Kwon, M.; Byun, J.; Kim, D.; Kim, C.; Koo, S.; Kwon, S.P.; Moon, S.; Jung, M.; et al. Nanocomplex-Mediated In Vivo Programming to Chimeric Antigen Receptor-M1 Macrophages for Cancer Therapy. Adv. Mater. 2021, 33, e2103258. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, Z.; Tan, X.; Jiang, H.; Xu, Z.; Fang, Y.; Han, D.; Hong, W.; Wei, W.; Tu, J. CAR-macrophage: A new immunotherapy candidate against solid tumors. Biomed. Pharmacother. 2021, 139, 111605. [Google Scholar] [CrossRef]
- Wang, S.; Yang, Y.; Ma, P.; Zha, Y.; Zhang, J.; Lei, A.; Li, N. CAR-macrophage: An extensive immune enhancer to fight cancer. EBioMedicine 2022, 76, 103873. [Google Scholar] [CrossRef]
- Morrissey, M.A.; Williamson, A.P.; Steinbach, A.M.; Roberts, E.W.; Kern, N.; Headley, M.B.; Vale, R.D. Chimeric antigen receptors that trigger phagocytosis. Elife 2018, 7, e36688. [Google Scholar] [CrossRef]
- Klichinsky, M.; Ruella, M.; Shestova, O.; Lu, X.M.; Best, A.; Zeeman, M.; Schmierer, M.; Gabrusiewicz, K.; Anderson, N.R.; Petty, N.E.; et al. Human chimeric antigen receptor macrophages for cancer immunotherapy. Nat. Biotechnol. 2020, 38, 947–953. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Tian, L.; Dai, X.; Yu, H.; Wang, J.; Lei, A.; Zhu, M.; Xu, J.; Zhao, W.; Zhu, Y.; et al. Pluripotent stem cell-derived CAR-macrophage cells with antigen-dependent anti-cancer cell functions. J. Hematol. Oncol. 2020, 13, 153. [Google Scholar] [CrossRef] [PubMed]
- Franken, L.; Schiwon, M.; Kurts, C. Macrophages: Sentinels and regulators of the immune system. Cell Microbiol. 2016, 18, 475–487. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Bae, J.S. Tumor-Associated Macrophages and Neutrophils in Tumor Microenvironment. Mediators Inflamm. 2016, 2016, 6058147. [Google Scholar] [CrossRef]
- van der Heide, D.; Weiskirchen, R.; Bansal, R. Therapeutic Targeting of Hepatic Macrophages for the Treatment of Liver Diseases. Front. Immunol. 2019, 10, 2852. [Google Scholar] [CrossRef]
- Santoni, M.; Massari, F.; Montironi, R.; Battelli, N. Manipulating macrophage polarization in cancer patients: From nanoparticles to human chimeric antigen receptor macrophages. Biochim Biophys Acta Rev. Cancer 2021, 1876, 188547. [Google Scholar] [CrossRef]
- Kim, H.S.; Sun, X.; Lee, J.H.; Kim, H.W.; Fu, X.; Leong, K.W. Advanced drug delivery systems and artificial skin grafts for skin wound healing. Adv. Drug Deliv. Rev. 2019, 146, 209–239. [Google Scholar] [CrossRef]
- Stylianopoulos, T.; Munn, L.L.; Jain, R.K. Reengineering the Physical Microenvironment of Tumors to Improve Drug Delivery and Efficacy: From Mathematical Modeling to Bench to Bedside. Trends Cancer 2018, 4, 292–319. [Google Scholar] [CrossRef]
- Huang, Y.; Kim, B.Y.S.; Chan, C.K.; Hahn, S.M.; Weissman, I.L.; Jiang, W. Improving immune-vascular crosstalk for cancer immunotherapy. Nat. Rev. Immunol. 2018, 18, 195–203. [Google Scholar] [CrossRef]
- Huang, Y.; Yuan, J.; Righi, E.; Kamoun, W.S.; Ancukiewicz, M.; Nezivar, J.; Santosuosso, M.; Martin, J.D.; Martin, M.R.; Vianello, F.; et al. Vascular normalizing doses of antiangiogenic treatment reprogram the immunosuppressive tumor microenvironment and enhance immunotherapy. Proc. Natl. Acad. Sci. USA 2012, 109, 17561–17566. [Google Scholar] [CrossRef]
- Ye, Z.W.; Yu, Z.L.; Chen, G.; Jia, J. Extracellular vesicles in tumor angiogenesis and resistance to anti-angiogenic therapy. Cancer Sci. 2023; 1–11, ahead of print. [Google Scholar]
- Jing, H.; Yang, L.; Qi, J.; Qiu, L.; Fu, C.; Li, J.; Yang, M.; Qi, M.; Fan, N.; Ji, J.; et al. Safety and efficacy of daratumumab in Chinese patients with relapsed or refractory multiple myeloma: A phase 1, dose-escalation study (MMY1003). Ann. Hematol. 2022, 101, 2679–2690. [Google Scholar] [CrossRef] [PubMed]
- Mateos, M.V.; Nahi, H.; Legiec, W.; Grosicki, S.; Vorobyev, V.; Spicka, I.; Hungria, V.; Korenkova, S.; Bahlis, N.; Flogegard, M.; et al. Subcutaneous versus intravenous daratumumab in patients with relapsed or refractory multiple myeloma (COLUMBA): A multicentre, open-label, non-inferiority, randomised, phase 3 trial. Lancet Haematol. 2020, 7, e370–e380. [Google Scholar] [CrossRef] [PubMed]
- San-Miguel, J.; Usmani, S.Z.; Mateos, M.V.; van de Donk, N.; Kaufman, J.L.; Moreau, P.; Oriol, A.; Plesner, T.; Benboubker, L.; Liu, K.; et al. Subcutaneous daratumumab in patients with relapsed or refractory multiple myeloma: Part 2 of the open-label, multicenter, dose-escalation phase 1b study (PAVO). Haematologica 2021, 106, 1725–1732. [Google Scholar] [CrossRef] [PubMed]
- Facon, T.; Kumar, S.K.; Plesner, T.; Orlowski, R.Z.; Moreau, P.; Bahlis, N.; Basu, S.; Nahi, H.; Hulin, C.; Quach, H.; et al. Daratumumab, lenalidomide, and dexamethasone versus lenalidomide and dexamethasone alone in newly diagnosed multiple myeloma (MAIA): Overall survival results from a randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 1582–1596. [Google Scholar] [CrossRef]
- Chari, A.; Martinez-Lopez, J.; Mateos, M.V.; Blade, J.; Benboubker, L.; Oriol, A.; Arnulf, B.; Rodriguez-Otero, P.; Pineiro, L.; Jakubowiak, A.; et al. Daratumumab plus carfilzomib and dexamethasone in patients with relapsed or refractory multiple myeloma. Blood 2019, 134, 421–431. [Google Scholar] [CrossRef]
- Mateos, M.V.; Cavo, M.; Blade, J.; Dimopoulos, M.A.; Suzuki, K.; Jakubowiak, A.; Knop, S.; Doyen, C.; Lucio, P.; Nagy, Z.; et al. Overall survival with daratumumab, bortezomib, melphalan, and prednisone in newly diagnosed multiple myeloma (ALCYONE): A randomised, open-label, phase 3 trial. Lancet 2020, 395, 132–141. [Google Scholar] [CrossRef]
- Sonneveld, P.; Chanan-Khan, A.; Weisel, K.; Nooka, A.K.; Masszi, T.; Beksac, M.; Spicka, I.; Hungria, V.; Munder, M.; Mateos, M.V.; et al. Overall Survival With Daratumumab, Bortezomib, and Dexamethasone in Previously Treated Multiple Myeloma (CASTOR): A Randomized, Open-Label, Phase III Trial. J. Clin. Oncol. 2023, 41, 1600–1609. [Google Scholar] [CrossRef]
- Dimopoulos, M.A.; Oriol, A.; Nahi, H.; San-Miguel, J.; Bahlis, N.J.; Usmani, S.Z.; Rabin, N.; Orlowski, R.Z.; Suzuki, K.; Plesner, T.; et al. Overall Survival With Daratumumab, Lenalidomide, and Dexamethasone in Previously Treated Multiple Myeloma (POLLUX): A Randomized, Open-Label, Phase III Trial. J. Clin. Oncol. 2023, 41, 1590–1599. [Google Scholar] [CrossRef]
- Modi, S.; Jacot, W.; Yamashita, T.; Sohn, J.; Vidal, M.; Tokunaga, E.; Tsurutani, J.; Ueno, N.T.; Prat, A.; Chae, Y.S.; et al. Trastuzumab Deruxtecan in Previously Treated HER2-Low Advanced Breast Cancer. N. Engl. J. Med. 2022, 387, 9–20. [Google Scholar] [CrossRef]
- Li, B.T.; Smit, E.F.; Goto, Y.; Nakagawa, K.; Udagawa, H.; Mazieres, J.; Nagasaka, M.; Bazhenova, L.; Saltos, A.N.; Felip, E.; et al. Trastuzumab Deruxtecan in HER2-Mutant Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2022, 386, 241–251. [Google Scholar] [CrossRef]
- Siena, S.; Di Bartolomeo, M.; Raghav, K.; Masuishi, T.; Loupakis, F.; Kawakami, H.; Yamaguchi, K.; Nishina, T.; Fakih, M.; Elez, E.; et al. Trastuzumab deruxtecan (DS-8201) in patients with HER2-expressing metastatic colorectal cancer (DESTINY-CRC01): A multicentre, open-label, phase 2 trial. Lancet Oncol. 2021, 22, 779–789. [Google Scholar] [CrossRef] [PubMed]
- Salazar, L.G.; Lu, H.; Reichow, J.L.; Childs, J.S.; Coveler, A.L.; Higgins, D.M.; Waisman, J.; Allison, K.H.; Dang, Y.; Disis, M.L. Topical Imiquimod Plus Nab-paclitaxel for Breast Cancer Cutaneous Metastases: A Phase 2 Clinical Trial. JAMA Oncol. 2017, 3, 969–973. [Google Scholar] [CrossRef] [PubMed]
- Donin, N.M.; Chamie, K.; Lenis, A.T.; Pantuck, A.J.; Reddy, M.; Kivlin, D.; Holldack, J.; Pozzi, R.; Hakim, G.; Karsh, L.I.; et al. A phase 2 study of TMX-101, intravesical imiquimod, for the treatment of carcinoma in situ bladder cancer. Urol Oncol. 2017, 35, 39.e1–39.e7. [Google Scholar] [CrossRef] [PubMed]
- Shayan, G.; Kansy, B.A.; Gibson, S.P.; Srivastava, R.M.; Bryan, J.K.; Bauman, J.E.; Ohr, J.; Kim, S.; Duvvuri, U.; Clump, D.A.; et al. Phase Ib Study of Immune Biomarker Modulation with Neoadjuvant Cetuximab and TLR8 Stimulation in Head and Neck Cancer to Overcome Suppressive Myeloid Signals. Clin. Cancer Res. 2018, 24, 62–72. [Google Scholar] [CrossRef]
- Cassier, P.A.; Italiano, A.; Gomez-Roca, C.; Le Tourneau, C.; Toulmonde, M.; D’Angelo, S.P.; Weber, K.; Loirat, D.; Jacob, W.; Jegg, A.M.; et al. Long-term clinical activity, safety and patient-reported quality of life for emactuzumab-treated patients with diffuse-type tenosynovial giant-cell tumour. Eur. J. Cancer 2020, 141, 162–170. [Google Scholar] [CrossRef]
- Cassier, P.A.; Italiano, A.; Gomez-Roca, C.A.; Le Tourneau, C.; Toulmonde, M.; Cannarile, M.A.; Ries, C.; Brillouet, A.; Muller, C.; Jegg, A.M.; et al. CSF1R inhibition with emactuzumab in locally advanced diffuse-type tenosynovial giant cell tumours of the soft tissue: A dose-escalation and dose-expansion phase 1 study. Lancet Oncol. 2015, 16, 949–956. [Google Scholar] [CrossRef]
- Spierenburg, G.; Grimison, P.; Chevreau, C.; Stacchiotti, S.; Piperno-Neumann, S.; Le Cesne, A.; Ferraresi, V.; Italiano, A.; Duffaud, F.; Penel, N.; et al. Long-term follow-up of nilotinib in patients with advanced tenosynovial giant cell tumours: Long-term follow-up of nilotinib in TGCT. Eur. J. Cancer 2022, 173, 219–228. [Google Scholar] [CrossRef]
- Falchook, G.S.; Peeters, M.; Rottey, S.; Dirix, L.Y.; Obermannova, R.; Cohen, J.E.; Perets, R.; Frommer, R.S.; Bauer, T.M.; Wang, J.S.; et al. A phase 1a/1b trial of CSF-1R inhibitor LY3022855 in combination with durvalumab or tremelimumab in patients with advanced solid tumors. Investig. N. Drugs 2021, 39, 1284–1297. [Google Scholar] [CrossRef]
- Lee, J.H.; Chen, T.W.; Hsu, C.H.; Yen, Y.H.; Yang, J.C.; Cheng, A.L.; Sasaki, S.I.; Chiu, L.L.; Sugihara, M.; Ishizuka, T.; et al. A phase I study of pexidartinib, a colony-stimulating factor 1 receptor inhibitor, in Asian patients with advanced solid tumors. Investig. N. Drugs 2020, 38, 99–110. [Google Scholar] [CrossRef]
- Zhou, Y.B.; Zhang, Y.M.; Huang, H.H.; Shen, L.J.; Han, X.F.; Hu, X.B.; Yu, S.D.; Gao, A.H.; Sheng, L.; Su, M.B.; et al. Pharmacodynamic, pharmacokinetic, and phase 1a study of bisthianostat, a novel histone deacetylase inhibitor, for the treatment of relapsed or refractory multiple myeloma. Acta Pharmacol. Sin. 2022, 43, 1091–1099. [Google Scholar] [CrossRef]
- Heath, E.I.; Weise, A.; Vaishampayan, U.; Danforth, D.; Ungerleider, R.S.; Urata, Y. Phase Ia dose escalation study of OBP-801, a cyclic depsipeptide class I histone deacetylase inhibitor, in patients with advanced solid tumors. Investig. N. Drugs 2022, 40, 300–307. [Google Scholar] [CrossRef]
- Collier, K.A.; Valencia, H.; Newton, H.; Hade, E.M.; Sborov, D.W.; Cavaliere, R.; Poi, M.; Phelps, M.A.; Liva, S.G.; Coss, C.C.; et al. A phase 1 trial of the histone deacetylase inhibitor AR-42 in patients with neurofibromatosis type 2-associated tumors and advanced solid malignancies. Cancer Chemother. Pharmacol. 2021, 87, 599–611. [Google Scholar] [CrossRef] [PubMed]
- Sborov, D.W.; Canella, A.; Hade, E.M.; Mo, X.; Khountham, S.; Wang, J.; Ni, W.; Poi, M.; Coss, C.; Liu, Z.; et al. A phase 1 trial of the HDAC inhibitor AR-42 in patients with multiple myeloma and T- and B-cell lymphomas. Leuk. Lymphoma 2017, 58, 2310–2318. [Google Scholar] [CrossRef] [PubMed]
- Tsimberidou, A.M.; Beer, P.A.; Cartwright, C.A.; Haymaker, C.; Vo, H.H.; Kiany, S.; Cecil, A.R.L.; Dow, J.; Haque, K.; Silva, F.A.; et al. Preclinical Development and First-in-Human Study of KA2507, a Selective and Potent Inhibitor of Histone Deacetylase 6, for Patients with Refractory Solid Tumors. Clin. Cancer Res. 2021, 27, 3584–3594. [Google Scholar] [CrossRef]
- Tambo, Y.; Hosomi, Y.; Sakai, H.; Nogami, N.; Atagi, S.; Sasaki, Y.; Kato, T.; Takahashi, T.; Seto, T.; Maemondo, M.; et al. Phase I/II study of docetaxel combined with resminostat, an oral hydroxamic acid HDAC inhibitor, for advanced non-small cell lung cancer in patients previously treated with platinum-based chemotherapy. Investig. N. Drugs 2017, 35, 217–226. [Google Scholar] [CrossRef]
- Quinn, D.I.; Tsao-Wei, D.D.; Twardowski, P.; Aparicio, A.M.; Frankel, P.; Chatta, G.; Wright, J.J.; Groshen, S.G.; Khoo, S.; Lenz, H.J.; et al. Phase II study of the histone deacetylase inhibitor vorinostat (Suberoylanilide Hydroxamic Acid; SAHA) in recurrent or metastatic transitional cell carcinoma of the urothelium-an NCI-CTEP sponsored: California Cancer Consortium trial, NCI 6879. Investig. N. Drugs 2021, 39, 812–820. [Google Scholar] [CrossRef] [PubMed]
- Batlevi, C.L.; Crump, M.; Andreadis, C.; Rizzieri, D.; Assouline, S.E.; Fox, S.; van der Jagt, R.H.C.; Copeland, A.; Potvin, D.; Chao, R.; et al. A phase 2 study of mocetinostat, a histone deacetylase inhibitor, in relapsed or refractory lymphoma. Br. J. Haematol. 2017, 178, 434–441. [Google Scholar] [CrossRef]
- Jo, J.H.; Jung, D.E.; Lee, H.S.; Park, S.B.; Chung, M.J.; Park, J.Y.; Bang, S.; Park, S.W.; Cho, S.; Song, S.Y. A phase I/II study of ivaltinostat combined with gemcitabine and erlotinib in patients with untreated locally advanced or metastatic pancreatic adenocarcinoma. Int. J. Cancer 2022, 151, 1565–1577. [Google Scholar] [CrossRef]

| Phagocytic Signals | Ligands | Targets | Effects on Phagocytosis | Refs |
|---|---|---|---|---|
| Nucleotides (ATP, UTP) | P2Y2 | Apoptotic cells | Promote P2Y2-dependent recruitment of phagocytes | [20] |
| CX3CL1 | CX3CR1 | Bacteria | Control the clearance of entero-invasive pathogens by DCs | [21] |
| LPC | G2A | Apoptotic cells | Migrate macrophages toward LPC | [22] |
| S1P | GPCR | Apoptotic cells | Attract phagocytic cells | [23,24] |
| RP S19 | C5aR | Apoptotic cells | Migrate monocytes/macrophages | [25] |
| PS (PtdSer) | PSR (e.g., stabilin-2) | Apoptotic cells | Stimulate membrane ruffling, vesicle formation, “bystander” uptake of cells, promote clearance | [26,27] |
| CRT | LRP | Viable or apoptotic cells | Initiate clearance | [28,29,30] |
| CD47 | SIRPα | Opsonized RBCs, etc. | Regulate complement-mediated phagocytosis | [31] |
| SLAMF3 and SLAMF2 | Specific SFR members, mainly SLAMF3 and SLAMF4 | Hematopoietic cells | Inhibit “eat me” signals, mitigate macrophage phagocytosis, regulate signals transduced by TLR4 | [32,33] |
| CD24 | Siglec-10 | Tumor cells | Block cytoskeletal rearrangement | [34] |
| PD-1 | PD-L1 | Tumor cells | Inhibit phagocytosis | [35,36] |
| MHC-I | LILRB | Cancer cells | Inhibit phagocytosis | [37] |
| Receptors | Ligands | Downstream Signaling Molecules | Mechanisms | Refs |
|---|---|---|---|---|
| CD44 | / | Src family kinases, Syk, Rac1, PI-3K, Rho GTPases | Internalize large particle, induce mature phagosome formation | [51] |
| FcγR | IgG-opsonized particles | CAPRI, Cdc42, Rac, Rho | Internalize, recruit actin and Arp2/3 complex | [52,53,54,55] |
| Cdc42, WASp | Recruit P-Tyr proteins into the phagocytic cup, possibly assemble a regulated cytoskeletal complex at specialized sites of actin polymerization | [56] | ||
| PKC-ε | Regulate vesicle delivery and focal exocytosis | [57] | ||
| Lyn and Hck (Src family kinases), Syk kinase, PI3K, PI(3,4,5)P3 | Assemble a complex of proteins around the FcR | [58,59,60] | ||
| FcR | IgG-opsonized particles | Bcl10, vesicular OCRL phosphatase | Complete the phagosome closure, regulate PI(4,5)P2 and F-actin turnover | [61,62] |
| TI-VAMP /VAMP7 | Control exocytosis and membrane extension | [63] | ||
| ARF6 | Regulate membrane recycling | [64] | ||
| FcγR; CR | IgG-opsonized particles, complement-opsonized particles | PLC and PLD, Ca2+, InsP3 and S1P-SOCE channels, cytosolic Ca2+ elevation | Promote the actin meshwork solubilization, and phagosomes fusion with granules containing lytic enzymes, the assembly and activation of the superoxide-generating NADPH oxidase complex | [65] |
| CR3 | C3bi/complement-opsonized particles | Arp2/3 complex, Rho | Regulate actin assembly | [52,55] |
| SR | Effete components, such as apoptotic cells | Microtubules, PKC, tyrosine, MAPK, PI3K | / | [66,67] |
| Dectin-1 | Fungal β-glucan | BTK, Vav1, PLCγ2 | Ensue F-actin formation, participate in DAG production | [68] |
| Activators | Receptors | Targets | Critical Molecules or Pathways | Mechanisms | Refs |
|---|---|---|---|---|---|
| CD300b | PS | Apoptotic cells | Adaptor: DAP12 | Accumulate in phagocytic cups, facilitate engulfment | [76] |
| LPS | TLRs | Bacteria or their components | Actin-Cdc42/Rac (Rho family GTPase) pathway, MyD88-p38 signaling pathway | Regulate phagocytosis, help phagocytes sense bacteria | [77,78] |
| CD40 agonist | CD40 | Tumor cells | ERK1/2 pathway | Drive macrophage become tumoricidal, facilitate the depletion of tumor stroma | [79,80] |
| CpG | TLR9 | Tumor cells | FAO | Increase ECAR, basal OCR, and total mitochondria, change the central carbon metabolism, engulf CD47+ cancer cells | [81] |
| PBI1 | TLR4 | Tumor cells | / | Enhance macrophage phagocytic efficiency five-fold | [82] |
| GSF-1R inhibitor | CSF-1R | Tumor cells | / | Enhance phagocytosis | [83] |
| HDAC inhibitor | HDAC | Tumor cells | / | Modulate macrophage phenotypes | [84] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.-Y.; Guo, Y.-L.; Tian, J.-W.; Zhang, H.-J.; Li, R.-F.; Gong, P.; Yu, Z.-L. Anti-Tumor Strategies by Harnessing the Phagocytosis of Macrophages. Cancers 2023, 15, 2717. https://doi.org/10.3390/cancers15102717
Li S-Y, Guo Y-L, Tian J-W, Zhang H-J, Li R-F, Gong P, Yu Z-L. Anti-Tumor Strategies by Harnessing the Phagocytosis of Macrophages. Cancers. 2023; 15(10):2717. https://doi.org/10.3390/cancers15102717
Chicago/Turabian StyleLi, Si-Yuan, Yong-Lin Guo, Jia-Wen Tian, He-Jing Zhang, Rui-Fang Li, Ping Gong, and Zi-Li Yu. 2023. "Anti-Tumor Strategies by Harnessing the Phagocytosis of Macrophages" Cancers 15, no. 10: 2717. https://doi.org/10.3390/cancers15102717
APA StyleLi, S.-Y., Guo, Y.-L., Tian, J.-W., Zhang, H.-J., Li, R.-F., Gong, P., & Yu, Z.-L. (2023). Anti-Tumor Strategies by Harnessing the Phagocytosis of Macrophages. Cancers, 15(10), 2717. https://doi.org/10.3390/cancers15102717





