The Phosphorylation of Kv1.3: A Modulatory Mechanism for a Multifunctional Ion Channel
Abstract
Simple Summary
Abstract
1. Introduction
2. Phosphorylation
Kinases and Phosphatases
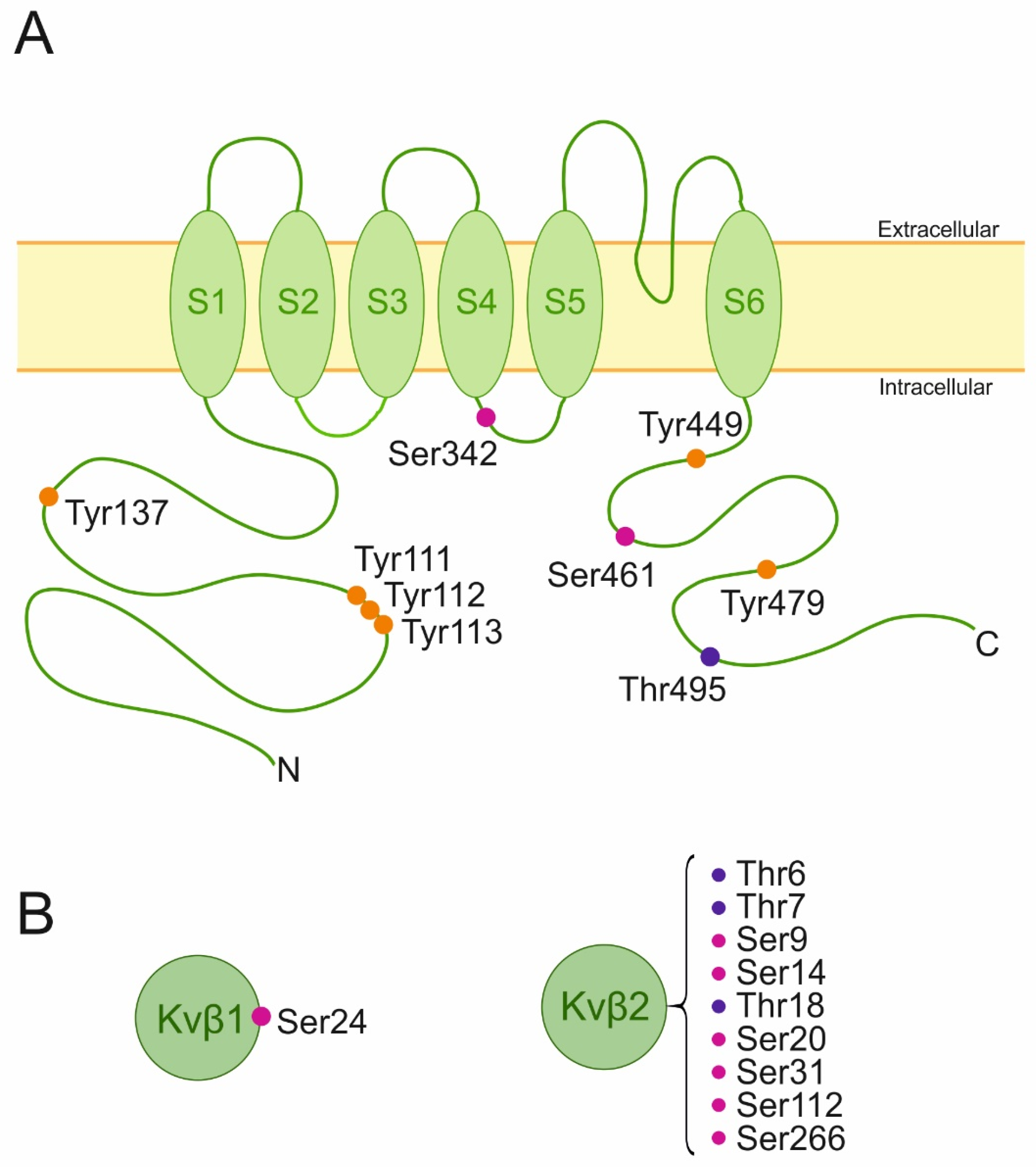
3. Kv1.3 Phosphorylation
3.1. Kv1.3 Ser/Thr Kinases
3.1.1. PKA
3.1.2. PKC
3.1.3. SGK1
3.1.4. ERK1/2
3.2. Kv1.3 Tyr Kinases
3.2.1. MAPK Family
3.2.2. Src Family
3.2.3. Receptor Tyrosine Kinases (RTKs)
EGFR
Insulin Receptor Kinase
Trk Family
4. Scaffolding Proteins
5. Phosphorylation of Regulatory Subunits
5.1. Kvβ
5.2. KCNEs
6. Functional Consequences of Kv1.3 Phosphorylation
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gouaux, E.; MacKinnon, R. Principles of Selective Ion Transport in Channels and Pumps. Science 2005, 310, 1461–1465. [Google Scholar] [CrossRef] [PubMed]
- Levitan, I.B. Modulation of ion channels by protein phosphorylation and dephosphorylation. Annu. Rev. Physiol. 1994, 56, 193–212. [Google Scholar] [CrossRef] [PubMed]
- Park, K.-S.; Yang, J.-W.; Seikel, E.; Trimmer, J.S. Potassium Channel Phosphorylation in Excitable Cells: Providing Dynamic Functional Variability to a Diverse Family of Ion Channels. Physiology 2008, 23, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Holmes, T.C.; Fadool, D.A.; Ren, R.; Levitan, I.B. Association of Src Tyrosine Kinase with a Human Potassium Channel Mediated by SH3 Domain. Science 1996, 274, 2089–2091. [Google Scholar] [CrossRef] [PubMed]
- Imbrici, P.; Tucker, S.J.; D’Adamo, M.C.; Pessia, M. Role of receptor protein tyrosine phosphatase alpha (RPTPalpha) and tyrosine phosphorylation in the sero-tonergic inhibition of voltage-dependent potassium channels. Pflug. Arch. 2000, 441, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Hattan, D.; Nesti, E.; Cachero, T.G.; Morielli, A.D. Tyrosine Phosphorylation of Kv1.2 Modulates Its Interaction with the Actin-binding Protein Cortactin. J. Biol. Chem. 2002, 277, 38596–38606. [Google Scholar] [CrossRef] [PubMed]
- Nesti, E.; Everill, B.; Morielli, A.D. Endocytosis as a Mechanism for Tyrosine Kinase-dependent Suppression of a Voltage-gated Potassium Channel. Mol. Biol. Cell 2004, 15, 4073–4088. [Google Scholar] [CrossRef]
- Johnson, R.P.; El-Yazbi, A.F.; Hughes, M.F.; Schriemer, D.C.; Walsh, E.J.; Walsh, M.P.; Cole, W.C. Identification and Functional Characterization of Protein Kinase A-catalyzed Phosphorylation of Potassium Channel Kv1.2 at Serine 449. J. Biol. Chem. 2009, 284, 16562–16574. [Google Scholar] [CrossRef]
- Roeper, J.; Lorra, C.; Pongs, O. Frequency-dependent inactivation of mammalian A-type K+ channel KV1.4 regulated by Ca2+/calmodulin-dependent protein kinase. J. Neurosci. 1997, 17, 3379–3391. [Google Scholar] [CrossRef]
- Jia, Q.; Jia, Z.; Zhao, Z.; Liu, B.; Liang, H.; Zhang, H. Activation of epidermal growth factor receptor inhibits KCNQ2/3 current through two distinct pathways: Membrane PtdIns(4,5)P2 hydrolysis and channel phosphorylation. J. Neurosci. 2007, 27, 2503–2512. [Google Scholar] [CrossRef]
- Anderson, A.E.; Adams, J.; Qian, Y.; Cook, R.G.; Pfaffinger, P.J.; Sweatt, J.D. Kv4.2 Phosphorylation by Cyclic AMP-dependent Protein Kinase. J. Biol. Chem. 2000, 275, 5337–5346. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.P.; Anderson, A.E.; Varga, A.W.; Dineley, K.T.; Cook, R.G.; Pfaffinger, P.J.; Sweatt, J.D. The A-Type Potassium Channel Kv4.2 Is a Substrate for the Mitogen-Activated Protein Kinase ERK. J. Neurochem. 2008, 75, 2277–2287. [Google Scholar] [CrossRef] [PubMed]
- Jerng, H.H.; Pfaffinger, P.J.; Covarrubias, M. Molecular physiology and modulation of somatodendritic A-type potassium channels. Mol. Cell. Neurosci. 2004, 27, 343–369. [Google Scholar] [CrossRef]
- Kanemasa, T.; Gan, L.; Perney, T.M.; Wang, L.Y.; Kaczmarek, L.K. Electrophysiological and pharmacological characterization of a mammalian Shaw channel expressed in NIH 3T3 fibroblasts. J. Neurophysiol. 1995, 74, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Critz, S.D.; Wible, B.A.; Lopez, H.S.; Brown, A.M. Stable Expression and Regulation of a Rat Brain K+ Channel. J. Neurochem. 1993, 60, 1175–1178. [Google Scholar] [CrossRef] [PubMed]
- Covarrubias, M.; Wei, A.; Salkoff, L.; Vyas, T.B. Elimination of rapid potassium channel inactivation by phosphorylation of the inactivation gate. Neuron 1994, 13, 1403–1412. [Google Scholar] [CrossRef]
- Park, K.-S.; Mohapatra, D.P.; Misonou, H.; Trimmer, J.S. Graded Regulation of the Kv2.1 Potassium Channel by Variable Phosphorylation. Science 2006, 313, 976–979. [Google Scholar] [CrossRef]
- Perez-Verdaguer, M.; Capera, J.; Serrano-Novillo, C.; Estadella, I.; Sastre, D.; Felipe, A. The voltage-gated potassium channel Kv1.3 is a promising multitherapeutic target against human pathologies. Expert Opin. Ther. Targets 2016, 20, 577–591. [Google Scholar] [CrossRef]
- Leanza, L.; Zoratti, M.; Gulbins, E.; Szabo, I. Mitochondrial ion channels as oncological targets. Oncogene 2014, 33, 5569–5581. [Google Scholar] [CrossRef]
- Varanita, T.; Angi, B.; Scattolini, V.; Szabo, I. Kv1.3 K+ Channel Physiology Assessed by Genetic and Pharmacological Modulation. Physiology 2023, 38, 25–41. [Google Scholar] [CrossRef]
- Capera, J.; Pérez-Verdaguer, M.; Navarro-Pérez, M.; Felipe, A. Kv1.3 Controls Mitochondrial Dynamics during Cell Cycle Progression. Cancers 2021, 13, 4457. [Google Scholar] [CrossRef] [PubMed]
- Capera, J.; Pérez-Verdaguer, M.; Peruzzo, R.; Navarro-Pérez, M.; Martínez-Pinna, J.; Alberola-Die, A.; Morales, A.; Leanza, L.; Szabó, I.; Felipe, A. A novel mitochondrial Kv1.3-caveolin axis controls cell survival and apoptosis. Elife 2021, 10, e69099. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.C.; Douglass, J. In vivo and in vitro phosphorylation of the T lymphocyte type n (Kv1.3) potassium channel. J. Biol. Chem. 1993, 268, 23720–23727. [Google Scholar] [CrossRef] [PubMed]
- England, S.K.; Uebele, V.N.; Shear, H.; Kodali, J.; Bennett, P.B.; Tamkun, M.M. Characterization of a voltage-gated K+ channel beta subunit expressed in human heart. Proc. Natl. Acad. Sci. USA 1995, 92, 6309–6313. [Google Scholar] [CrossRef]
- Hu, Z.; Jepps, T.; Zhou, L.; Liu, J.; Li, M.; Abbott, G.W. Kcne4 deletion sex dependently inhibits the RISK pathway response and exacerbates hepatic ischemia-reperfusion injury in mice. Am. J. Physiol. Integr. Comp. Physiol. 2019, 316, R552–R562. [Google Scholar] [CrossRef]
- Liu, S.Q.; Yin, J.; Bhatnagar, A. Protein kinase C-dependent phosphorylation of the beta-subunit of the voltage-sensitive potassium channels (Kvbeta2). Chem. Biol. Interact. 2003, 143–144, 597–604. [Google Scholar] [CrossRef]
- Nelson, D.L.; Cox, M. Lehninger Principles of Biochemistry: International Edition 2021; Macmillan Learning: New York, NY, USA, 2021. [Google Scholar]
- Ardito, F.; Giuliani, M.; Perrone, D.; Troiano, G.; Lo Muzio, L. The crucial role of protein phosphorylation in cell signaling and its use as targeted therapy (Review). Int. J. Mol. Med. 2017, 40, 271–280. [Google Scholar] [CrossRef]
- Kemp, B.E.; Pearson, R.B. Protein kinase recognition sequence motifs. Trends Biochem. Sci. 1990, 15, 342–346. [Google Scholar] [CrossRef]
- Kennelly, P.J.; Krebs, E.G. Consensus sequences as substrate specificity determinants for protein kinases and protein phosphatases. J. Biol. Chem. 1991, 266, 15555–15558. [Google Scholar] [CrossRef]
- St-Denis, N.; Gabriel, M.; Turowec, J.P.; Gloor, G.B.; Li, S.S.-C.; Gingras, A.-C.; Litchfield, D.W. Systematic investigation of hierarchical phosphorylation by protein kinase CK2. J. Proteom. 2015, 118, 49–62. [Google Scholar] [CrossRef]
- Szebeni, A.; Hingorani, K.; Negi, S.; Olson, M.O.J. Role of Protein Kinase CK2 Phosphorylation in the Molecular Chaperone Activity of Nucleolar Protein B23. J. Biol. Chem. 2003, 278, 9107–9115. [Google Scholar] [CrossRef] [PubMed]
- Capera, J.; Navarro-Pérez, M.; Moen, A.S.; Szabó, I.; Felipe, A. The Mitochondrial Routing of the Kv1.3 Channel. Front. Oncol. 2022, 12, 865686. [Google Scholar] [CrossRef] [PubMed]
- Krebs, E.G.; A Beavo, J. Phosphorylation-Dephosphorylation of Enzymes. Annu. Rev. Biochem. 1979, 48, 923–959. [Google Scholar] [CrossRef] [PubMed]
- Cohen, P. The origins of protein phosphorylation. Nat. Cell Biol. 2002, 4, E127–E130. [Google Scholar] [CrossRef]
- Enjalbert, A.; Le Pechon-Vallee, C.; Kinases, P. Encyclopedia of Hormones; Henry, H.L., Norman, A.W., Eds.; Academic Press: New York, NY, USA, 2003; pp. 277–285. [Google Scholar]
- Barford, D. Protein phosphatases. Curr. Opin. Struct. Biol. 1995, 5, 728–734. [Google Scholar] [CrossRef]
- Cahalan, M.D.; Chandy, K.G.; E DeCoursey, T.; Gupta, S. A voltage-gated potassium channel in human T lymphocytes. J. Physiol. 1985, 358, 197–237. [Google Scholar] [CrossRef] [PubMed]
- Chandy, K.G.; Williams, C.B.; Spencer, R.H.; Aguilar, B.A.; Ghanshani, S.; Tempel, B.L.; Gutman, G.A. A Family of Three Mouse Potassium Channel Genes with Intronless Coding Regions. Science 1990, 247, 973–975. [Google Scholar] [CrossRef] [PubMed]
- Swanson, R.; Marshall, J.; Smith, J.S.; Williams, J.B.; Boyle, M.B.; Folander, K.; Luneau, C.J.; Antanavage, J.; Oliva, C.; Buhrow, S.A.; et al. Cloning and expression of cDNA and genomic clones encoding three delayed rectifier potassium channels in rat brain. Neuron 1990, 4, 929–939. [Google Scholar] [CrossRef]
- Attali, B.; Romey, G.; Honoré, E.; Schmid-Alliana, A.; Mattéi, M.G.; Lesage, F.; Ricard, P.; Barhanin, J.; Lazdunski, M. Cloning, functional expression, and regulation of two K+ channels in human T lymphocytes. J. Biol. Chem. 1992, 267, 8650–8657. [Google Scholar] [CrossRef]
- Serrano-Albarrás, A.; Estadella, I.; Cirera-Rocosa, S.; Navarro-Pérez, M.; Felipe, A. Kv1.3: A multifunctional channel with many pathological implications. Expert Opin. Ther. Targets 2017, 22, 101–105. [Google Scholar] [CrossRef]
- Comes, N.; Bielanska, J.; Vallejo-Gracia, A.; Serrano-Albarrás, A.; Marruecos, L.; Gómez, D.; Soler, C.; Condom, E.; Cajal, S.R.Y.; Hernández-Losa, J.; et al. The voltage-dependent K+ channels Kv1.3 and Kv1.5 in human cancer. Front. Physiol. 2013, 4, 283. [Google Scholar] [CrossRef] [PubMed]
- Hou, P.; Zhang, R.; Liu, Y.; Feng, J.; Wang, W.; Wu, Y.; Ding, J. Physiological Role of Kv1.3 Channel in T Lymphocyte Cell Investigated Quantitatively by Kinetic Modeling. PLoS ONE 2014, 9, e89975. [Google Scholar] [CrossRef] [PubMed]
- Grissmer, S.; Nguyen, A.N.; Aiyar, J.; Hanson, D.C.; Mather, R.J.; A Gutman, G.; Karmilowicz, M.J.; Auperin, D.D.; Chandy, K.G. Pharmacological characterization of five cloned voltage-gated K+ channels, types Kv1.1, 1.2, 1.3, 1.5, and 3.1, stably expressed in mammalian cell lines. Mol. Pharmacol. 1994, 45, 1227–1234. [Google Scholar] [PubMed]
- Panyi, G. Biophysical and pharmacological aspects of K+ channels in T lymphocytes. Eur. Biophys. J. 2005, 34, 515–529. [Google Scholar] [PubMed]
- Payet, M.D.; Dupuis, G. Dual regulation of the n type K+ channel in Jurkat T lymphocytes by protein kinases A and C. J. Biol. Chem. 1992, 267, 18270–18273. [Google Scholar] [CrossRef]
- Holmes, T.; Fadool, D.; Levitan, I. Tyrosine phosphorylation of the Kv1.3 potassium channel. J. Neurosci. 1996, 16, 1581–1590. [Google Scholar] [CrossRef]
- Szabò, I.; Gulbins, E.; Apfel, H.; Zhang, X.; Barth, P.; Busch, A.E.; Schlottmann, K.; Pongs, O.; Lang, F. Tyrosine Phosphorylation-dependent Suppression of a Voltage-gated K+ Channel in T Lymphocytes upon Fas Stimulation. J. Biol. Chem. 1996, 271, 20465–20469. [Google Scholar] [CrossRef]
- Kupper, J.; Bowlby, M.R.; Marom, S.; Levitan, I.B. Intracellular and extracellular amino acids that influence C-type inactivation and its modulation in a volt-age-dependent potassium channel. Pflug. Arch. 1995, 430, 1–11. [Google Scholar] [CrossRef]
- Martel, J.; Dupuis, G.; Deschênes, P.; Payet, M.D. The sensitivity of the human Kv1.3 (hKv1.3) lymphocyte K+ channel to regulation by PKA and PKC is partially lost in HEK 293 host cells. J. Membr. Biol. 1998, 161, 183–196. [Google Scholar] [CrossRef]
- Chung, I.; Schlichter, L.C. Regulation of native Kv1.3 channels by cAMP-dependent protein phosphorylation. Am. J. Physiol. 1997, 273 Pt 1, C622–C633. [Google Scholar] [CrossRef]
- Chung, I.; Schlichter, L. Native Kv1.3 Channels are Upregulated by Protein Kinase C. J. Membr. Biol. 1997, 156, 73–85. [Google Scholar] [CrossRef]
- Isacoff, E.Y.; Jan, Y.N.; Jan, L.Y. Putative receptor for the cytoplasmic inactivation gate in the Shaker K+ channel. Nature 1991, 353, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Lucas, S.; Marais, R.; Graves, J.; Alexandere, D.; Parker, P.; Cantrell, D. Heterogeneity of protein kinase C expression and regulation in T lymphocytes. FEBS Lett. 1990, 260, 53–56. [Google Scholar] [CrossRef] [PubMed]
- Aiyar, J.; Grissmer, S.; Chandy, K.G. Full-length and truncated Kv1.3 K+ channels are modulated by 5-HT1c receptor activation and independently by PKC. Am. J. Physiol. Cell Physiol. 1993, 265, C1571–C1578. [Google Scholar] [CrossRef] [PubMed]
- Kotani, K.; Yonezawa, K.; Hara, K.; Ueda, H.; Kitamura, Y.; Sakaue, H.; Ando, A.; Chavanieu, A.; Calas, B.; Grigorescu, F. Involvement of phosphoinositide 3-kinase in insulin- or IGF-1-induced membrane ruffling. EMBO J. 1994, 13, 2313–2321. [Google Scholar] [CrossRef]
- Alessi, D.R.; Deak, M.; Casamayor, A.; Caudwell, F.B.; Morrice, N.; Norman, D.G.; Gaffney, P.; Reese, C.B.; MacDougall, C.N.; Harbison, D.; et al. 3-Phosphoinositide-dependent protein kinase-1 (PDK1): Structural and functional homology with the Drosophila DSTPK61 kinase. Curr. Biol. 1997, 7, 776–789. [Google Scholar] [CrossRef]
- Kobayashi, T.; Cohen, P. Activation of serum- and glucocorticoid-regulated protein kinase by agonists that activate phosphatidylinositide 3-kinase is mediated by 3-phosphoinositide-dependent protein kinase-1 (PDK1) and PDK2. Biochem. J. 1999, 339, 319–328. [Google Scholar] [CrossRef]
- Henke, G.; Maier, G.; Wallisch, S.; Boehmer, C.; Lang, F. Regulation of the voltage gated K+ channel Kv1.3 by the ubiquitin ligase Nedd4-2 and the serum and glu-cocorticoid inducible kinase SGK1. J. Cell. Physiol. 2004, 199, 194–199. [Google Scholar] [CrossRef]
- Altman, J.; Das, G.D. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J. Comp. Neurol. 1965, 124, 319–335. [Google Scholar] [CrossRef]
- Lois, C.; Alvarez-Buylla, A. Proliferating subventricular zone cells in the adult mammalian forebrain can differentiate into neurons and glia. Proc. Natl. Acad. Sci. USA 1993, 90, 2074–2077. [Google Scholar] [CrossRef]
- Doetsch, F.; Petreanu, L.; Caille, I.; Garcia-Verdugo, J.-M.; Alvarez-Buylla, A. EGF Converts Transit-Amplifying Neurogenic Precursors in the Adult Brain into Multipotent Stem Cells. Neuron 2002, 36, 1021–1034. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Marmol, R.; Comes, N.; Styrczewska, K.; Pérez-Verdaguer, M.; Vicente, R.; Pujadas, L.; Soriano, E.; Sorkin, A.; Felipe, A. Unconventional EGF-induced ERK1/2-mediated Kv1.3 endocytosis. Cell. Mol. Life Sci. 2016, 73, 1515–1528. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Lee, M.-H.; Johnson, T.; Allie, R.; Hu, L.; Calabresi, P.A.; Nath, A. Activated T-Cells Inhibit Neurogenesis by Releasing Granzyme B: Rescue by Kv1.3 Blockers. J. Neurosci. 2010, 30, 5020–5027. [Google Scholar] [CrossRef] [PubMed]
- Liebau, S.; Pröpper, C.; Böckers, T.; Lehmann-Horn, F.; Storch, A.; Grissmer, S.; Wittekindt, O.H. Selective blockage of Kv1.3 and Kv3.1 channels increases neural progenitor cell proliferation. J. Neurochem. 2006, 99, 426–437. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.-Y.; Hou, G.-Q.; He, S.-W.; Xiao, Z.; Xu, H.-J.; Qiu, Y.-T.; Jiang, S.; Zheng, H.; Li, Z.-Y. Psora-4, a Kv1.3 Blocker, Enhances Differentiation and Maturation in Neural Progenitor Cells. CNS Neurosci. Ther. 2015, 21, 558–567. [Google Scholar] [CrossRef] [PubMed]
- Tucker, K.; Fadool, D.A. Neurotrophin modulation of voltage-gated potassium channels in rat through TrkB receptors is time and sensory experience dependent. J. Physiol. 2002, 542, 413–429. [Google Scholar] [CrossRef]
- Singh, V.; Ram, M.; Kumar, R.; Prasad, R.; Roy, B.K.; Singh, K.K. Phosphorylation: Implications in Cancer. Protein J. 2017, 36, 1–6. [Google Scholar] [CrossRef]
- Szabo, I.; Soddemann, M.; Leanza, L.; Zoratti, M.; Gulbins, E. Single-point mutations of a lysine residue change function of Bax and Bcl-xL expressed in Bax- and Bak-less mouse embryonic fibroblasts: Novel insights into the molecular mechanisms of Bax-induced apoptosis. Cell Death Differ. 2011, 18, 427–438. [Google Scholar] [CrossRef]
- Fadool, D.A.; Holmes, T.C.; Berman, K.; Dagan, D.; Levitan, I.B. Tyrosine Phosphorylation Modulates Current Amplitude and Kinetics of a Neuronal Voltage-Gated Potassium Channel. J. Neurophysiol. 1997, 78, 1563–1573. [Google Scholar] [CrossRef]
- Fadool, D.A.; Levitan, I.B. Modulation of Olfactory Bulb Neuron Potassium Current by Tyrosine Phosphorylation. J. Neurosci. 1998, 18, 6126–6137. [Google Scholar] [CrossRef]
- DeCoursey, T.E.; Chandy, K.G.; Gupta, S.; Cahalan, M.D. Voltage-gated K+ channels in human T lymphocytes: A role in mitogenesis? Nature 1984, 307, 465–468. [Google Scholar] [CrossRef] [PubMed]
- Cidad, P.; Jiménez-Pérez, L.; García-Arribas, D.; Miguel-Velado, E.; Tajada, S.; Ruiz-McDavitt, C.; López-López, J.R.; Pérez-García, M.T. Kv1.3 Channels Can Modulate Cell Proliferation During Phenotypic Switch by an Ion-Flux Independent Mechanism. Arter. Thromb. Vasc. Biol. 2012, 32, 1299–1307. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Pérez, L.; Cidad, P.; Álvarez-Miguel, I.; Santos-Hipólito, A.; Torres-Merino, R.; Alonso, E.; de la Fuente, M.Á.; López-López, J.R.; Pérez-García, M.T. Molecular Determinants of Kv1.3 Potassium Channels-induced Proliferation. J. Biol. Chem. 2016, 291, 3569–3580. [Google Scholar] [CrossRef] [PubMed]
- Kan, X.-H.; Gao, H.-Q.; Ma, Z.-Y.; Liu, L.; Ling, M.-Y.; Wang, Y.-Y. Kv1.3 potassium channel mediates macrophage migration in atherosclerosis by regulating ERK activity. Arch. Biochem. Biophys. 2016, 591, 150–156. [Google Scholar] [CrossRef]
- Szabo, I.; Nilius, B.; Zhang, X.; Busch, A.E.; Gulbins, E.; Suessbrich, H.; Lang, F. Inhibitory effects of oxidants on n-type K + channels in T lymphocytes and Xenopus oocytes. Pflug. Arch. 1997, 433, 626–632. [Google Scholar] [CrossRef] [PubMed]
- Cayabyab, F.S.; Khanna, R.; Jones, O.T.; Schlichter, L.C. Suppression of the rat microglia Kv1.3 current by src-family tyrosine kinases and oxygen/glucose dep-rivation. Eur. J. Neurosci. 2000, 12, 1949–1960. [Google Scholar] [CrossRef]
- Gulbins, E.; Szabo, I.; Baltzer, K.; Lang, F. Ceramide-induced inhibition of T lymphocyte voltage-gated potassium channel is mediated by tyrosine kinases. Proc. Natl. Acad. Sci. USA 1997, 94, 7661–7666. [Google Scholar] [CrossRef]
- Detre, C.; Kiss, E.; Varga, Z.; Ludányi, K.; Pászty, K.; Enyedi, A.; Kövesdi, D.; Panyi, G.; Rajnavölgyi, E.; Matkó, J. Death or survival: Membrane ceramide controls the fate and activation of antigen-specific T-cells depending on signal strength and duration. Cell. Signal. 2006, 18, 294–306. [Google Scholar] [CrossRef]
- Hanada, T.; Lin, L.; Chandy, K.G.; Oh, S.S.; Chishti, A.H. Human homologue of the Drosophila discs large tumor suppressor binds to p56lck tyrosine kinase and Shaker type Kv1.3 potassium channel in T lymphocytes. J. Biol. Chem. 1997, 272, 26899–26904. [Google Scholar] [CrossRef]
- Szigligeti, P.; Neumeier, L.; Duke, E.; Chougnet, C.; Takimoto, K.; Lee, S.M.; Filipovich, A.H.; Conforti, L. Signalling during hypoxia in human T lymphocytes-critical role of the src protein tyrosine kinase p56Lck in the O2sensitivity of Kv1.3 channels. J. Physiol. 2006, 573, 357–370. [Google Scholar] [CrossRef]
- Cahalan, M.D.; Chandy, K.G. The functional network of ion channels in T lymphocytes. Immunol. Rev. 2009, 231, 59–87. [Google Scholar] [CrossRef] [PubMed]
- Styles, F.L.; Al-Owais, M.M.; Scragg, J.L.; Chuntharpursat-Bon, E.; Hettiarachchi, N.T.; Lippiat, J.D.; Minard, A.; Bon, R.S.; Porter, K.; Sukumar, P.; et al. Kv1.3 voltage-gated potassium channels link cellular respiration to proliferation through a non-conducting mechanism. Cell Death Dis. 2021, 12, 372. [Google Scholar] [CrossRef] [PubMed]
- Bowlby, M.R.; Fadool, D.A.; Holmes, T.C.; Levitan, I.B. Modulation of the Kv1.3 Potassium Channel by Receptor Tyrosine Kinases. J. Gen. Physiol. 1997, 110, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Fadool, D.A. Tyrosine Phosphorylation Downregulates a Potassium Current in Rat Olfactory Bulb Neurons and a Cloned Kv1.3 Channela. Ann. N. Y. Acad. Sci. 1998, 855, 529–532. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Verdaguer, M.; Capera, J.; Ortego-Domínguez, M.; Bielanska, J.; Comes, N.; Montoro, R.J.; Camps, M.; Felipe, A. Caveolar targeting links Kv1.3 with the insulin-dependent adipocyte physiology. Cell. Mol. Life Sci. 2018, 75, 4059–4075. [Google Scholar] [CrossRef]
- Kues, W.A.; Wunder, F. Heterogeneous Expression Patterns of Mammalian Potassium Channel Genes in Developing and Adult Rat Brain. Eur. J. Neurosci. 1992, 4, 1296–1308. [Google Scholar] [CrossRef] [PubMed]
- Gupta, G.; Azam, M.; Baquer, N. Modulation of rat brain insulin receptor kinase activity in diabetes. Neurochem. Int. 1992, 20, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Fadool, D.A.; Tucker, K.; Phillips, J.J.; Simmen, J.A. Brain Insulin Receptor Causes Activity-Dependent Current Suppression in the Olfactory Bulb Through Multiple Phosphorylation of Kv1.3. J. Neurophysiol. 2000, 83, 2332–2348. [Google Scholar] [CrossRef]
- Das, P.; Parsons, A.; Scarborough, J.; Hoffman, J.; Wilson, J.; Thompson, R.; Overton, J.; Fadool, D. Electrophysiological and behavioral phenotype of insulin receptor defective mice. Physiol. Behav. 2005, 86, 287–296. [Google Scholar] [CrossRef]
- Marks, D.R.; Tucker, K.; Cavallin, M.A.; Mast, T.G.; Fadool, D.A. Awake Intranasal Insulin Delivery Modifies Protein Complexes and Alters Memory, Anxiety, and Olfactory Behaviors. J. Neurosci. 2009, 29, 6734–6751. [Google Scholar] [CrossRef]
- Brüning, J.C.; Gautam, D.; Burks, D.J.; Gillette, J.; Schubert, M.; Orban, P.C.; Klein, R.; Krone, W.; Müller-Wieland, D.; Kahn, C.R. Role of Brain Insulin Receptor in Control of Body Weight and Reproduction. Science 2000, 289, 2122–2125. [Google Scholar] [CrossRef] [PubMed]
- Hennige, A.M.; Sartorius, T.; Lutz, S.Z.; Tschritter, O.; Preissl, H.; Hopp, S.; Fritsche, A.; Rammensee, H.-G.; Ruth, P.; Häring, H.-U. Insulin-mediated cortical activity in the slow frequency range is diminished in obese mice and promotes physical inactivity. Diabetologia 2009, 52, 2416–2424. [Google Scholar] [CrossRef] [PubMed]
- Fadool, D.A.; Tucker, K.; Pedarzani, P. Mitral Cells of the Olfactory Bulb Perform Metabolic Sensing and Are Disrupted by Obesity at the Level of the Kv1.3 Ion Channel. PLoS ONE 2011, 6, e24921. [Google Scholar] [CrossRef] [PubMed]
- Bell, G.A.; Fadool, D.A. Awake, long-term intranasal insulin treatment does not affect object memory, odor discrimination, or reversal learning in mice. Physiol. Behav. 2017, 174, 104–113. [Google Scholar] [CrossRef]
- Colley, B.; Biju, K.; Visegrady, A.; Campbell, S.; Fadool, D. Neurotrophin B receptor kinase increases Kv subfamily member 1.3 (Kv1.3) ion channel half-life and surface expression. Neuroscience 2007, 144, 531–546. [Google Scholar] [CrossRef]
- Colley, B.; Tucker, K.; Fadool, D.A. Comparison of modulation of Kv1.3 channel by two receptor tyrosine kinases in ol-factory bulb neurons of rodents. Recept. Channels 2004, 10, 25–36. [Google Scholar] [CrossRef]
- Roig, S.R.E.; Estadella, I.; Cirera-Rocosa, S.; Navarro-Pérez, M.; Felipe, A. Kv1.3 In Microglia: Neuroinflammatory Determinant and Promising Pharmaceutical Target. J. Neurol. Neuromed. 2018, 3, 18–23. [Google Scholar]
- Holmes, T.C.; Berman, K.; Swartz, J.E.; Dagan, D.; Levitan, I.B. Expression of Voltage-Gated Potassium Channels Decreases Cellular Protein Tyrosine Phosphorylation. J. Neurosci. 1997, 17, 8964–8974. [Google Scholar] [CrossRef]
- Colley, B.S.; A Cavallin, M.; Biju, K.; Marks, D.R.; A Fadool, D. Brain-derived neurotrophic factor modulation of Kv1.3 channel is disregulated by adaptor proteins Grb10 and nShc. BMC Neurosci. 2009, 10, 8. [Google Scholar] [CrossRef]
- Cook, K.K.; Fadool, D.A. Two Adaptor Proteins Differentially Modulate the Phosphorylation and Biophysics of Kv1.3 Ion Channel by Src Kinase. J. Biol. Chem. 2002, 277, 13268–13280. [Google Scholar] [CrossRef]
- Marks, D.R.; Fadool, D.A. Post-synaptic density perturbs insulin-induced Kv1.3 channel modulation via a clustering mechanism involving the SH3 domain. J. Neurochem. 2007, 103, 1608–1627. [Google Scholar] [CrossRef] [PubMed]
- Morrione, A. Grb10 adapter protein as regulator of insulin-like growth factor receptor signaling. J. Cell. Physiol. 2003, 197, 307–311. [Google Scholar] [CrossRef]
- Liu, F.; A Roth, R. Grb-IR: A SH2-domain-containing protein that binds to the insulin receptor and inhibits its function. Proc. Natl. Acad. Sci. USA 1995, 92, 10287–10291. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Balas, B.; Christ-Roberts, C.Y.; Kim, R.Y.; Ramos, F.J.; Kikani, C.K.; Li, C.; Deng, C.; Reyna, S.; Musi, N.; et al. Peripheral Disruption of the Grb10 Gene Enhances Insulin Signaling and Sensitivity In Vivo. Mol. Cell. Biol. 2007, 27, 6497–6505. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.H.; Dickson, F.H.; Timmins, L.R.; Nabi, I.R. Tyrosine phosphorylation of tumor cell caveolin-1: Impact on cancer progression. Cancer Metastasis Rev. 2020, 39, 455–469. [Google Scholar] [CrossRef]
- Pérez-Verdaguer, M.; Capera, J.; Mármol, R.M.; Camps, M.; Comes, N.; Tamkun, M.M.; Felipe, A. Caveolin interaction governs Kv1.3 lipid raft targeting. Sci. Rep. 2016, 6, 22453. [Google Scholar] [CrossRef]
- Leanza, L.; Henry, B.; Sassi, N.; Zoratti, M.; Chandy, K.G.; Gulbins, E.; Szabò, I. Inhibitors of mitochondrial Kv1.3 channels induce Bax/Bak-independent death of cancer cells. EMBO Mol. Med. 2012, 4, 577–593. [Google Scholar] [CrossRef]
- Pongs, O.; Schwarz, J.R. Ancillary Subunits Associated With Voltage-Dependent K+Channels. Physiol. Rev. 2010, 90, 755–796. [Google Scholar] [CrossRef]
- Rettig, J.; Heinemann, S.H.; Wunder, F.; Lorra, C.; Parcej, D.N.; Dolly, J.O.; Pongs, O. Inactivation properties of voltage-gated K+ channels altered by presence of beta-subunit. Nature 1994, 369, 289–294. [Google Scholar] [CrossRef]
- Roig, S.R.; Cassinelli, S.; Navarro-Pérez, M.; Pérez-Verdaguer, M.; Estadella, I.; Capera, J.; Felipe, A. S-acylation-dependent membrane microdomain localization of the regulatory Kvbeta2.1 subunit. Cell. Mol. Life Sci. 2022, 79, 230. [Google Scholar] [CrossRef]
- Kwak, Y.G.; Hu, N.; Wei, J.; Jr, A.L.G.; Grobaski, T.D.; Tamkun, M.M.; Murray, K.T. Protein kinase A phosphorylation alters Kvbeta1.3 subunit-mediated inactivation of the Kv1.5 potassium channel. J. Biol. Chem. 1999, 274, 13928–13932. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, J.; Berkowski, S.M.; Knowleg, H.; Chandramouly, A.B.; Downens, M.; Prystowsky, M.B. Protein kinase C-mediated phosphorylation of Kv beta 2 in adult rat brain. Neurochem. Res. 2004, 29, 1879–1886. [Google Scholar] [CrossRef] [PubMed]
- Levin, G.; Chikvashvili, D.; Singer-Lahat, D.; Peretz, T.; Thornhill, W.B.; Lotan, I. Phosphorylation of a K+ channel alpha subunit modulates the inactivation conferred by a beta subunit. In-volvement of cytoskeleton. J. Biol. Chem. 1996, 271, 29321–29328. [Google Scholar] [CrossRef]
- Kim, S.J.; Ao, Z.; Warnock, G.; McIntosh, C.H.S. Incretin-stimulated interaction between β-cell Kv1.5 and Kvβ2 channel proteins involves acetyla-tion/deacetylation by CBP/SirT1. Biochem. J. 2013, 451, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Macias, A.; de la Cruz, A.; Peraza, D.A.; de Benito-Bueno, A.; Gonzalez, T.; Valenzuela, C. KV1.5–KVβ1.3 Recycling Is PKC-Dependent. Int. J. Mol. Sci. 2021, 22, 1336. [Google Scholar] [CrossRef] [PubMed]
- Macías, A.; de la Cruz, A.; Prieto, A.; Peraza, D.A.; Tamkun, M.M.; González, T.; Valenzuela, C. PKC inhibition results in a Kv 1.5 + Kv β1.3 pharmacology closer to Kv 1.5 channels. Br. J. Pharmacol. 2014, 171, 4914–4926. [Google Scholar] [CrossRef]
- Gong, J.; Xu, J.; Bezanilla, M.; van Huizen, R.; Derin, R.; Li, M. Differential Stimulation of PKC Phosphorylation of Potassium Channels by ZIP1 and ZIP2. Science 1999, 285, 1565–1569. [Google Scholar] [CrossRef]
- Abbott, G.W.; Butler, M.H.; Goldstein, S.A. Phosphorylation and protonation of neighboring MiRP2 sites: Function and pathophysiology of MiRP2-Kv3.4 potassium channels in periodic paralysis. FASEB J. 2006, 20, 293–301. [Google Scholar] [CrossRef]
- Alzamora, R.; O’mahony, F.; Bustos, V.; Rapetti-Mauss, R.; Urbach, V.; Cid, L.P.; Sepúlveda, F.V.; Harvey, B.J. Sexual dimorphism and oestrogen regulation of KCNE3 expression modulates the functional properties of KCNQ1 K+channels. J. Physiol. 2011, 589, 5091–5107. [Google Scholar] [CrossRef]
- Kurakami, K.; Norota, I.; Nasu, F.; Ohshima, S.; Nagasawa, Y.; Konno, Y.; Obara, Y.; Ishii, K. KCNQ1 is internalized by activation of α1 adrenergic receptors. Biochem. Pharmacol. 2019, 169, 113628. [Google Scholar] [CrossRef]
- Xu, X.; Kanda, V.A.; Choi, E.; Panaghie, G.; Roepke, T.K.; Gaeta, S.A.; Christini, D.J.; Lerner, D.J.; Abbott, G.W. MinK-dependent internalization of the IKs potassium channel. Cardiovasc. Res. 2009, 82, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Kanda, V.A.; Purtell, K.; Abbott, G.W. Protein kinase C downregulates I(Ks) by stimulating KCNQ1-KCNE1 potassium channel endocytosis. Heart Rhythm 2011, 8, 1641–1647. [Google Scholar] [CrossRef] [PubMed]
- Varnum, M.D.; Busch, A.E.; Bond, C.T.; Maylie, J.; Adelman, J.P. The min K channel underlies the cardiac potassium current IKs and mediates species-specific responses to protein kinase C. Proc. Natl. Acad. Sci. USA 1993, 90, 11528–11532. [Google Scholar] [CrossRef] [PubMed]
- Kurokawa, J.; Bankston, J.R.; Kaihara, A.; Chen, L.; Furukawa, T.; Kass, R.S. KCNE variants reveal a critical role of the beta subunit carboxyl terminus in PKA-dependent regulation of the IKs potassium channel. Channels 2009, 3, 16–24. [Google Scholar] [CrossRef]
- Kurokawa, J.; Chen, L.; Kass, R.S. Requirement of subunit expression for cAMP-mediated regulation of a heart potassium channel. Proc. Natl. Acad. Sci. USA 2003, 100, 2122–2127. [Google Scholar] [CrossRef]
- Dvir, M.; Strulovich, R.; Sachyani, D.; Cohen, I.B.-T.; Haitin, Y.; Dessauer, C.; Pongs, O.; Kass, R.; Hirsch, J.A.; Attali, B. Long QT mutations at the interface between KCNQ1 helix C and KCNE1 disrupt I(KS) regulation by PKA and PIP2. J. Cell Sci. 2014, 127 Pt 18, 3943–3955. [Google Scholar]
- Zhou, L.; Köhncke, C.; Hu, Z.; Roepke, T.K.; Abbott, G.W. The KCNE2 potassium channel β subunit is required for normal lung function and resilience to ischemia and reperfusion injury. FASEB J. 2019, 33, 9762–9774. [Google Scholar] [CrossRef]
- Grunnet, M.; Rasmussen, H.B.; Hay-Schmidt, A.; Rosenstierne, M.; Klaerke, D.A.; Olesen, S.-P.; Jespersen, T. KCNE4 Is an Inhibitory Subunit to Kv1.1 and Kv1.3 Potassium Channels. Biophys. J. 2003, 85, 1525–1537. [Google Scholar] [CrossRef]
- Rauch, J.; Volinsky, N.; Romano, D.; Kolch, W. The secret life of kinases: Functions beyond catalysis. Cell Commun. Signal. 2011, 9, 23. [Google Scholar] [CrossRef]
- Miedema, F.; Petit, A.J.; Terpstra, F.G.; Schattenkerk, J.K.; De Wolf, F.; Al, B.J.; Roos, M.; Lange, J.M.; A Danner, S.; Goudsmit, J. Immunological abnormalities in human immunodeficiency virus (HIV)-infected asymptomatic homosexual men. HIV affects the immune system before CD4+ T helper cell depletion occurs. J. Clin. Investig. 1988, 82, 1908–1914. [Google Scholar] [CrossRef]
- Cayota, A.; Vuillier, F.; Siciliano, J.; Dighiero, G. Defective protein tyrosine phosphorylation and altered levels of p59fyn and p56lck in CD4 T cells from HIV-1 infected patients. Int. Immunol. 1994, 6, 611–621. [Google Scholar] [CrossRef] [PubMed]
- Morio, T.; Chatila, T.; Geha, R.S. HIV glycoprotein gp120 inhibits TCR-CD3-mediated activation of fyn and lck. Int. Immunol. 1997, 9, 53–64. [Google Scholar] [CrossRef] [PubMed]
- A Nokta, M.; I Hassan, M.; A Morgan, J.; A Loesch, K.; Pollard, R.B. Protein kinase C and intracellular free Ca++: Relationship to human immunodeficiency virus (HIV)-induced cellular hyporesponsiveness. Proc. Soc. Exp. Biol. Med. 1994, 207, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Dellis, O.; Bouteau, F.; Guenounou, M.; Rona, J.P. HIV-1 gp160 decreases the K+ voltage-gated current from Jurkat E6.1 T cells by up-phosphorylation. FEBS Lett. 1999, 443, 187–191. [Google Scholar] [CrossRef]
- Liu, H.; Liu, J.; Xu, E.; Tu, G.; Guo, M.; Liang, S.; Xiong, H. Human immunodeficiency virus protein Tat induces oligodendrocyte injury by enhancing outward K+ current conducted by K(V)1.3. Neurobiol. Dis. 2017, 97 Pt A, 1–10. [Google Scholar] [CrossRef]
- Choi, S.Y.; Kim, H.-R.; Ryu, P.D.; Lee, S.Y. Regulation of voltage-gated potassium channels attenuates resistance of side-population cells to gefitinib in the human lung cancer cell line NCI-H460. BMC Pharmacol. Toxicol. 2017, 18, 14. [Google Scholar] [CrossRef]
- Banerjee, S.; Li, Y.; Wang, Z.; Sarkar, F.H. Multi-targeted therapy of cancer by genistein. Cancer Lett. 2008, 269, 226–242. [Google Scholar] [CrossRef]
- Teisseyre, A.; Michalak, K. Genistein Inhibits the Activity of Kv1.3 Potassium Channels in Human T Lymphocytes. J. Membr. Biol. 2005, 205, 71–79. [Google Scholar] [CrossRef]
- Hornbeck, P.V.; Zhang, B.; Murray, B.; Kornhauser, J.M.; Latham, V.; Skrzypek, E. PhosphoSitePlus, 2014: Mutations, PTMs and recalibrations. Nucleic Acids Res. 2014, 43, D512–D520. [Google Scholar] [CrossRef]
| Serine/Threonine Kinases | |||
|---|---|---|---|
| Group | Families | Principal Members | General Traits |
| AGC group | 14 | PKA, PKG, PKC, SGK, AKT/PKB, PDK1, PKN/PRK, RSK, NDR, MAST, YANK, DMPK, GRK, SGK494 | Activated by cyclic nucleotides (PKA by cAMP and PKG by cGMP), regulated by Ca2+, diacylglycerol, or phosphatidylserine (PKC), among others. The “PIF pocket” serves as kinase regulation site and therapeutic target. |
| Ca2+/calmodulin kinase (CaMK) | 17 | CaMK1, CaMK2, PSK, DAPK, MLCK, CASK, PHK, DCAMKL, MAPKAPK, CAMKL, TSSK, PIM, Trbl, PKD, RAD53, Trio, VACAMKL | Binding of Ca2+/CaM complex results in release of the auto-inhibitory domain from the N-terminal kinase domain. Classified into substrate-specific or multifunctional kinases. Key activators of transcription factors. |
| Cell Kinase 1 (CK1) | 3 | CK1, VRK, TTBK | Regulators of signal transduction pathways related to proliferation, cell differentiation, chromosome segregation, and circadian rhythms. |
| CMGC group | 8 | GSKs, MAPK, CDKs, CTD, DYRK, SRPK, CLK, RCK | Essential modulators of the cell cycle (CDKs), glycogen metabolism (GSKs), diverse signaling cascades involved in proliferation, differentiation, neural plasticity (MAPK), and the spliceosomal complex (CLK), among many others. |
| STE | 3 | Ste7/MAP2K, Ste11/MAP3K, Ste20/MAP4K | Also known as MAP2K, MEK, or MKKs, important activators of MAPK family. |
| Tyrosine kinase-like (TKL) | 7 | IRAK, MLK, RIPK, STKR, RAF, LRRK, LISK | Most diverse group with sequence similarity to tyrosine kinases but lacking specific motifs. |
| Other Protein Kinases (OPK) | 37 | PLK, Aur, CAMKK, TLK, ULK, IKK, NAK, NEK, TTK, MOS, TOPK, PEK, WEE, CDC7, NKF1, CK2, BUB, Bud32, Haspin, IRE, NKF4, NKF2, NKF3, NKF5, NRBP, Wnt, SLOB, SCY1, VPS15, TBCK… | Extremely diverse group of Ser/Thr and dual kinases. |
| Tyrosine Kinases | |||
| Group | Families | Principal Members | General Traits |
| Cytoplasmic tyrosine kinases | 32 | ABL, ACK, CSK, FAK, FES, FRK, JAK, SRC, SYK, TEC… | Critical regulators of the immune system by controlling cell growth, proliferation, differentiation, migration, and apoptosis. |
| Receptor tyrosine kinases (RTKs) | 20 | EGFR/ErbB, IR, PDGFR, VEGFR, FGFR, CCKR, NGFR, HGFR, EphR, AXLR, TIER, RYKR, DDRR, RETR, ROSR, LTKR, RORR, MuSKR, LMRR, other RTKs | They contain a transmembrane domain and a highly conserved intracellular C-terminal region with kinase domains. |
| Serine/Threonine Kinases | ||
|---|---|---|
| Kinase/Trigger | Residues | Outcome |
| PKC [23] | Ser 342 | Modulation of Kv1.3 ion conductivity |
| ERK1/2 [59,70] | Ser 461 Thr 495 | Kv1.3-induced cell proliferation EGF-dependent Kv1.3 endocytosis |
| Tyrosine Kinases | ||
| Kinase/Trigger | Residues | Outcome |
| v-Src [66] | Tyr 111-113 Tyr 137 Tyr 449 Tyr 479 | Current suppression and fast deactivation (Tyr 137, Tyr 449) Slow C-type inactivation (more than 3 Tyr) |
| EGFR [80] | Tyr 479 | Decreased peak current independent of inactivation |
| PDGFR-ERK1/2 [70] | Tyr 449 | Kv1.3 induced mitochondrial respiration and cell proliferation |
| IRK [67] | Tyr 111-113 Tyr 137 Tyr 479 | Insulin-mediated current suppression |
| TrkB [93] | Tyr 111-113 Tyr 137 Tyr449 | BDNF-induced current decrease (Tyr 111-113, Tyr 137, Tyr 449) Modulation of BDNF-dependent inactivation (Tyr 137) |
| Pervanadate [67] | Tyr 111-113 Tyr 449 | Pervanadate-dependent current suppression |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Navarro-Pérez, M.; Estadella, I.; Benavente-Garcia, A.; Orellana-Fernández, R.; Petit, A.; Ferreres, J.C.; Felipe, A. The Phosphorylation of Kv1.3: A Modulatory Mechanism for a Multifunctional Ion Channel. Cancers 2023, 15, 2716. https://doi.org/10.3390/cancers15102716
Navarro-Pérez M, Estadella I, Benavente-Garcia A, Orellana-Fernández R, Petit A, Ferreres JC, Felipe A. The Phosphorylation of Kv1.3: A Modulatory Mechanism for a Multifunctional Ion Channel. Cancers. 2023; 15(10):2716. https://doi.org/10.3390/cancers15102716
Chicago/Turabian StyleNavarro-Pérez, María, Irene Estadella, Anna Benavente-Garcia, Ruth Orellana-Fernández, Anna Petit, Joan Carles Ferreres, and Antonio Felipe. 2023. "The Phosphorylation of Kv1.3: A Modulatory Mechanism for a Multifunctional Ion Channel" Cancers 15, no. 10: 2716. https://doi.org/10.3390/cancers15102716
APA StyleNavarro-Pérez, M., Estadella, I., Benavente-Garcia, A., Orellana-Fernández, R., Petit, A., Ferreres, J. C., & Felipe, A. (2023). The Phosphorylation of Kv1.3: A Modulatory Mechanism for a Multifunctional Ion Channel. Cancers, 15(10), 2716. https://doi.org/10.3390/cancers15102716






