18F-FDG PET-Derived Volume-Based Parameters to Predict Disease-Free Survival in Patients with Grade III Breast Cancer of Different Molecular Subtypes Candidates to Neoadjuvant Chemotherapy
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Patients
2.2. 18F-FDG-PET/CT Scanning and Analysis
2.3. Statistical Analysis
3. Results
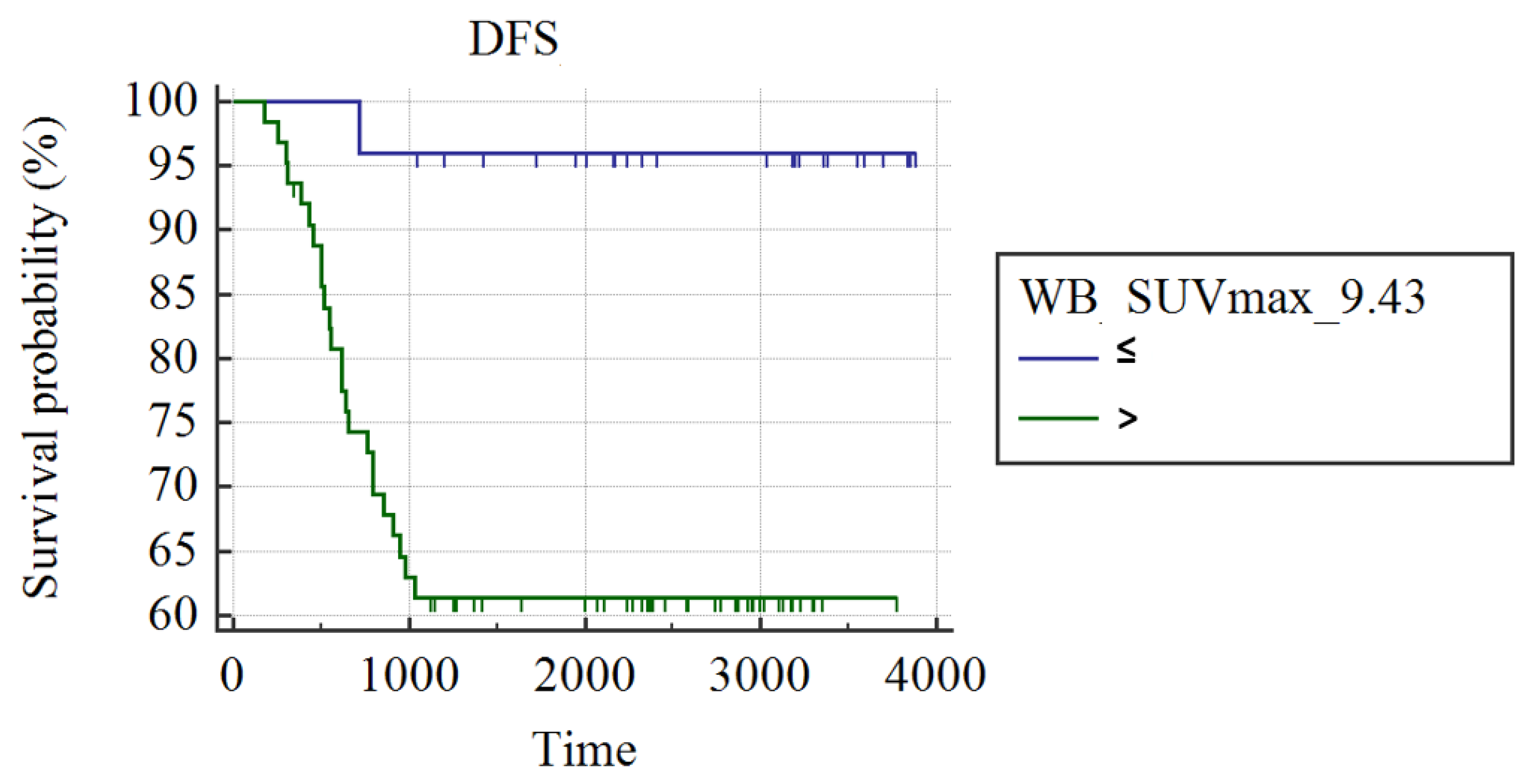
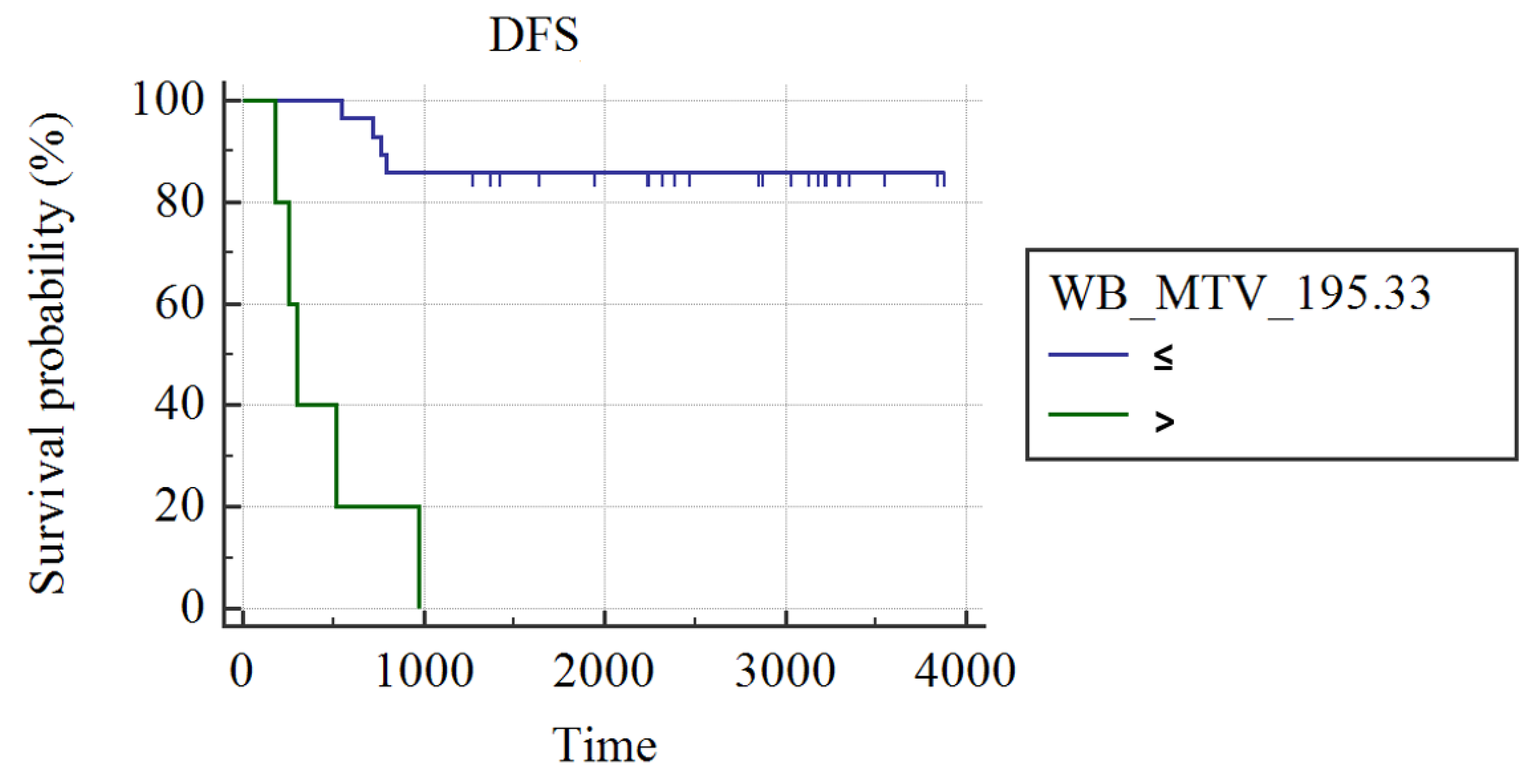
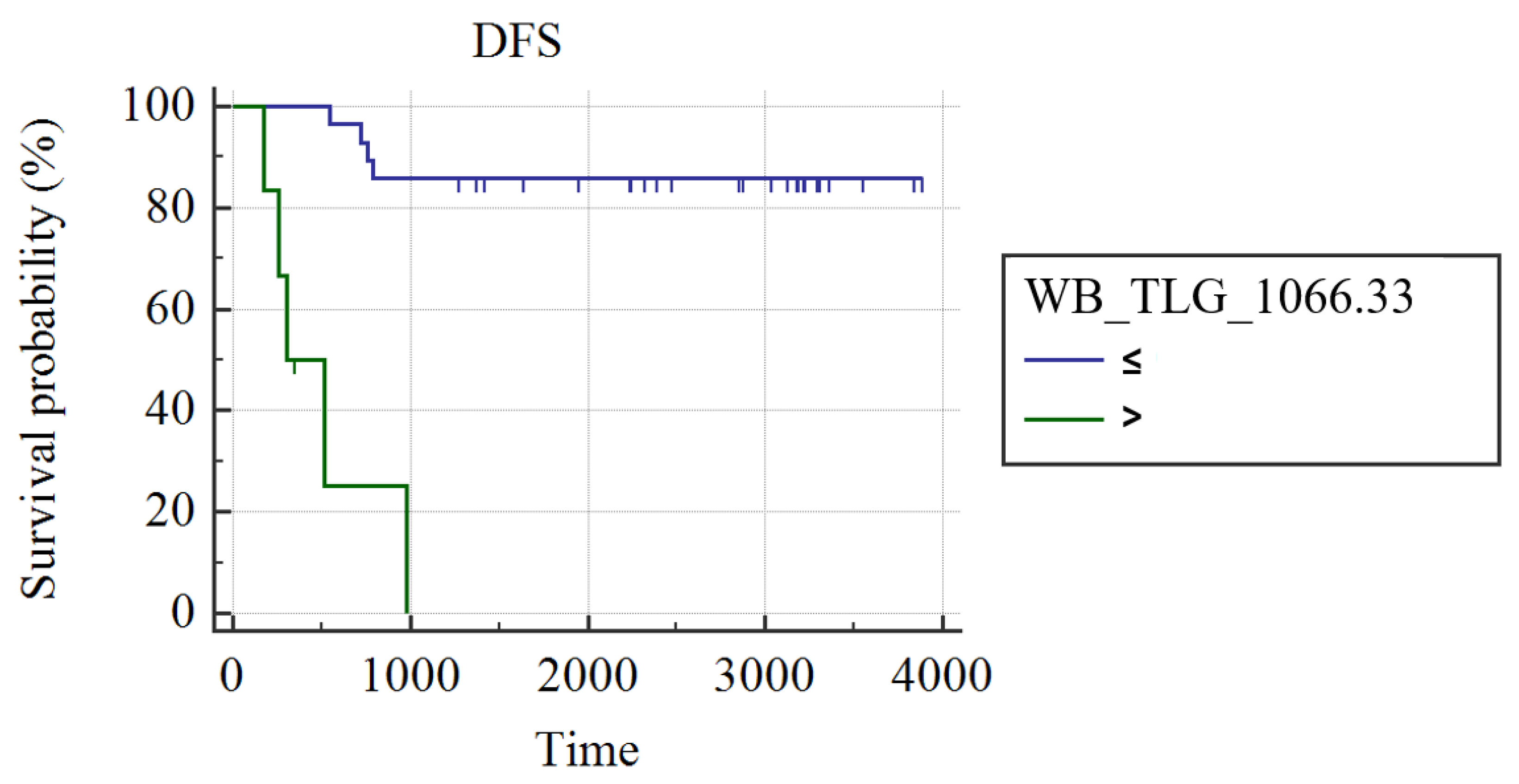
3.1. Whole Group Analysis
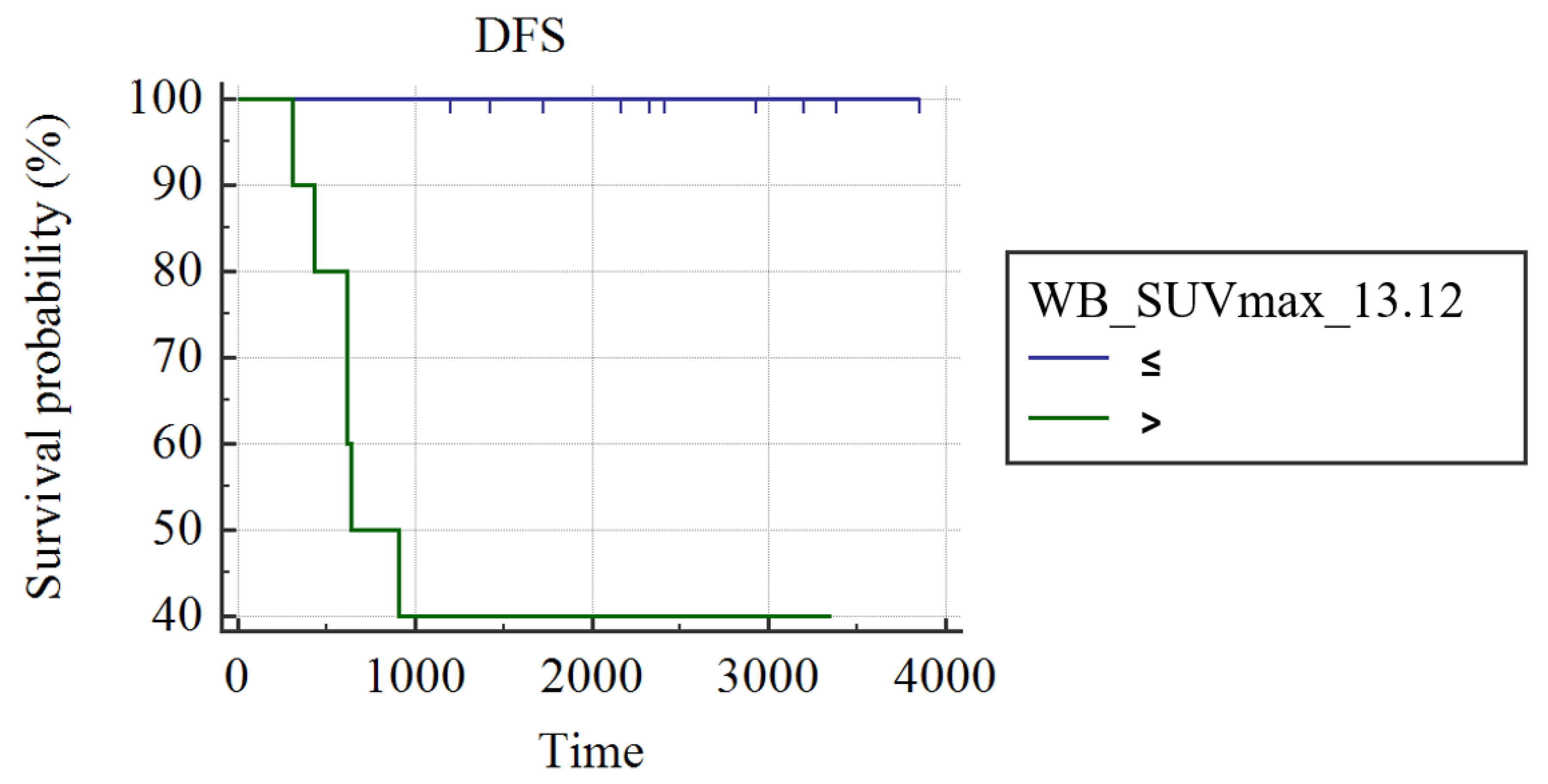
3.2. Subgroup Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Chen, K.; Zhang, J.; Beeraka, N.M.; Tang, C.; Babayeva, Y.V.; Sinelnikov, M.Y.; Zhang, X.; Zhang, J.; Liu, J.; Reshetov, I.V.; et al. Advances in the prevention and treatment of obesity-driven effects in breast cancers. Front. Oncol. 2022, 12, 820968. [Google Scholar] [CrossRef]
- Tryfonidis, K.; Senkus, E.; Cardoso, M.J.; Cardoso, F. Management of locally advanced breast cancer-perspectives and future directions. Nat. Rev. Clin. Oncol. 2015, 12, 147–162. [Google Scholar] [CrossRef]
- Perou, C.M.; Sørlie, T.; Eisen, M.B.; van de Rijn, M.; Jeffrey, S.S.; Rees, C.A.; Pollack, J.R.; Ross, D.T.; Johnsen, H.; Akslen, L.A.; et al. Molecular portraits of human breast tumours. Nature 2000, 406, 747–752. [Google Scholar] [CrossRef]
- Sreekumar, A.; Roarty, K.; Rosen, J.M. The mammary stem cell hierarchy: A looking glass into heterogeneous breast cancer landscapes. Endocr.-Relat. Cancer 2015, 22, T161–T176. [Google Scholar] [CrossRef]
- Schwartz, A.M.; Henson, D.E.; Chen, D.; Rajamarthandan, S. Histologic grade remains a prognostic factor for breast cancer regardless of the number of positive lymph nodes and tumor size: A study of 161,708 cases of breast cancer from the seer program. Arch. Pathol. Lab. Med. 2014, 138, 1048–1052. [Google Scholar] [CrossRef]
- Heil, J.; Kuerer, H.M.; Pfob, A.; Rauch, G.; Sinn, H.P.; Golatta, M.; Liefers, G.J.; Vrancken Peeters, M.J. Eliminating the breast cancer surgery paradigm after neoadjuvant systemic therapy: Current evidence and future challenges. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2020, 31, 61–71. [Google Scholar] [CrossRef]
- Chen, K.; Lu, P.; Beeraka, N.M.; Sukocheva, O.A.; Madhunapantula, S.V.; Liu, J.; Sinelnikov, M.Y.; Nikolenko, V.N.; Bulygin, K.V.; Mikhaleva, L.M.; et al. Mitochondrial mutations and mitoepigenetics: Focus on regulation of oxidative stress-induced responses in breast cancers. Semin. Cancer Biol. 2022, 83, 556–569. [Google Scholar] [CrossRef]
- Becker, J.; Schwarzenböck, S.M.; Krause, B.J. Fdg pet hybrid imaging. Recent Results Cancer Res. Fortschr. Der Krebsforschung. Prog. Dans Les Rech. Sur Le Cancer 2020, 216, 625–667. [Google Scholar]
- Boellaard, R.; Delgado-Bolton, R.; Oyen, W.J.; Giammarile, F.; Tatsch, K.; Eschner, W.; Verzijlbergen, F.J.; Barrington, S.F.; Pike, L.C.; Weber, W.A.; et al. Fdg pet/ct: Eanm procedure guidelines for tumour imaging: Version 2.0. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 328–354. [Google Scholar] [CrossRef]
- Cardoso, F.; Kyriakides, S.; Ohno, S.; Penault-Llorca, F.; Poortmans, P.; Rubio, I.T.; Zackrisson, S.; Senkus, E. Early breast cancer: Esmo clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2019, 30, 1194–1220. [Google Scholar] [CrossRef]
- Orsaria, P.; Chiaravalloti, A.; Caredda, E.; Marchese, P.V.; Titka, B.; Anemona, L.; Portarena, I.; Schillaci, O.; Petrella, G.; Palombi, L.; et al. Evaluation of the usefulness of fdg-pet/ct for nodal staging of breast cancer. Anticancer Res. 2018, 38, 6639–6652. [Google Scholar] [CrossRef]
- Paydary, K.; Seraj, S.M.; Zadeh, M.Z.; Emamzadehfard, S.; Shamchi, S.P.; Gholami, S.; Werner, T.J.; Alavi, A. The evolving role of fdg-pet/ct in the diagnosis, staging, and treatment of breast cancer. Mol. Imaging Biol. MIB Off. Publ. Acad. Mol. Imaging 2019, 21, 1–10. [Google Scholar] [CrossRef]
- Evangelista, L.; Cervino, A.R.; Michieletto, S.; Saibene, T.; Ghiotto, C.; Guarneri, V.; Conte, P.; Reccia, P.; Saladini, G. Diagnostic and prognostic impact of fluorine-18-fluorodeoxyglucose pet/ct in preoperative and postoperative setting of breast cancer patients. Nucl. Med. Commun. 2017, 38, 537–545. [Google Scholar] [CrossRef]
- Li, X.; Dai, D.; Chen, B.; Tang, H.; Xie, X.; Wei, W. Clinicopathological and prognostic significance of cancer antigen 15-3 and carcinoembryonic antigen in breast cancer: A meta-analysis including 12,993 patients. Dis. Mrk. 2018, 2018, 9863092. [Google Scholar] [CrossRef]
- Negrão, E.M.S.; Bitencourt, A.G.V.; de Souza, J.A.; Marques, E.F. Accuracy of breast magnetic resonance imaging in evaluating the response to neoadjuvant chemotherapy: A study of 310 cases at a cancer center. Radiol. Bras. 2019, 52, 299–304. [Google Scholar] [CrossRef]
- Asaoka, M.; Narui, K.; Suganuma, N.; Chishima, T.; Yamada, A.; Sugae, S.; Kawai, S.; Uenaka, N.; Teraoka, S.; Miyahara, K.; et al. Clinical and pathological predictors of recurrence in breast cancer patients achieving pathological complete response to neoadjuvant chemotherapy. Eur. J. Surg. Oncol. J. Eur. Soc. Surg. Oncol. Br. Assoc. Surg. Oncol. 2019, 45, 2289–2294. [Google Scholar] [CrossRef]
- Evangelista, L.; Urso, L.; Caracciolo, M.; Stracuzzi, F.; Panareo, S.; Cistaro, A.; Catalano, O. Fdg pet/ct volume-based quantitative data and survival analysis in breast cancer patients: A systematic review of the literature. Curr. Med. Imaging 2022. [Google Scholar] [CrossRef]
- Urso, L.; Quartuccio, N.; Caracciolo, M.; Evangelista, L.; Schirone, A.; Frassoldati, A.; Arnone, G.; Panareo, S.; Bartolomei, M. Impact on the long-term prognosis of fdg pet/ct in luminal-a and luminal-b breast cancer. Nucl. Med. Commun. 2022, 43, 212–219. [Google Scholar] [CrossRef]
- Goldhirsch, A.; Winer, E.P.; Coates, A.S.; Gelber, R.D.; Piccart-Gebhart, M.; Thürlimann, B.; Senn, H.J. Personalizing the treatment of women with early breast cancer: Highlights of the st gallen international expert consensus on the primary therapy of early breast cancer 2013. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2013, 24, 2206–2223. [Google Scholar] [CrossRef]
- Urso, L.; Evangelista, L.; Alongi, P.; Quartuccio, N.; Cittanti, C.; Rambaldi, I.; Ortolan, N.; Borgia, F.; Nieri, A.; Uccelli, L.; et al. The value of semiquantitative parameters derived from (18)f-fdg pet/ct for predicting response to neoadjuvant chemotherapy in a cohort of patients with different molecular subtypes of breast cancer. Cancers 2022, 14, 5869. [Google Scholar] [CrossRef]
- Sataloff, D.M.; Mason, B.A.; Prestipino, A.J.; Seinige, U.L.; Lieber, C.P.; Baloch, Z. Pathologic response to induction chemotherapy in locally advanced carcinoma of the breast: A determinant of outcome. J. Am. Coll. Surg. 1995, 180, 297–306. [Google Scholar]
- García Vicente, A.M.; Soriano Castrejón, Á.; León Martín, A.; Chacón López-Muñiz, I.; Muñoz Madero, V.; Muñoz Sánchez Mdel, M.; Palomar Muñoz, A.; Espinosa Aunión, R.; González Ageitos, A. Molecular subtypes of breast cancer: Metabolic correlation with 18f-fdg pet/ct. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 1304–1311. [Google Scholar] [CrossRef]
- Kitajima, K.; Fukushima, K.; Miyoshi, Y.; Nishimukai, A.; Hirota, S.; Igarashi, Y.; Katsuura, T.; Maruyama, K.; Hirota, S. Association between 18f-fdg uptake and molecular subtype of breast cancer. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 1371–1377. [Google Scholar] [CrossRef]
- de Mooij, C.M.; Ploumen, R.A.W.; Nelemans, P.J.; Mottaghy, F.M.; Smidt, M.L.; van Nijnatten, T.J.A. The influence of receptor expression and clinical subtypes on baseline [18f]fdg uptake in breast cancer: Systematic review and meta-analysis. EJNMMI Res. 2023, 13, 5. [Google Scholar] [CrossRef]
- Groheux, D. Fdg-pet/ct for primary staging and detection of recurrence of breast cancer. Semin. Nucl. Med. 2022, 52, 508–519. [Google Scholar] [CrossRef]
- Evangelista, L.; Cervino, A.R.; Ghiotto, C.; Al-Nahhas, A.; Rubello, D.; Muzzio, P.C. Tumor marker-guided pet in breast cancer patients-a recipe for a perfect wedding: A systematic literature review and meta-analysis. Clin. Nucl. Med. 2012, 37, 467–474. [Google Scholar] [CrossRef]
- Panareo, S.; Urso, L.; Nieri, A.; Caracciolo, M.; Valpiani, G.; Torricelli, P.; Frassoldati, A.; Cittanti, C.; Rollo, M.; Bartolomei, M. Clinical-diagnostic relevance of breast “incidentaloma” detected during 18f-fluoro-2-deoxy-d-glucose positron emission tomography/computed tomography: Correlation with radiological imaging and histopathology. Indian J. Nucl. Med. 2021, 36, 385–390. [Google Scholar]
- Urso, L.; Manco, L.; Castello, A.; Evangelista, L.; Guidi, G.; Castellani, M.; Florimonte, L.; Cittanti, C.; Turra, A.; Panareo, S. Pet-derived radiomics and artificial intelligence in breast cancer: A systematic review. Int. J. Mol. Sci. 2022, 23, 13409. [Google Scholar] [CrossRef]
- Inic, Z.; Zegarac, M.; Inic, M.; Markovic, I.; Kozomara, Z.; Djurisic, I.; Inic, I.; Pupic, G.; Jancic, S. Difference between luminal a and luminal b subtypes according to ki-67, tumor size, and progesterone receptor negativity providing prognostic information. Clin. Med. Insights Oncol. 2014, 8, 107–111. [Google Scholar] [CrossRef]
- Courtney, D.; Davey, M.G.; Moloney, B.M.; Barry, M.K.; Sweeney, K.; McLaughlin, R.P.; Malone, C.M.; Lowery, A.J.; Kerin, M.J. Breast cancer recurrence: Factors impacting occurrence and survival. Ir. J. Med. Sci. 2022, 191, 2501–2510. [Google Scholar] [CrossRef]
- Pedersen, R.N.; Esen, B.Ö.; Mellemkjær, L.; Christiansen, P.; Ejlertsen, B.; Lash, T.L.; Nørgaard, M.; Cronin-Fenton, D. The incidence of breast cancer recurrence 10–32 years after primary diagnosis. JNCI J. Natl. Cancer Inst. 2021, 114, 391–399. [Google Scholar] [CrossRef]
- Liang, J.H.; Ding, C.Y.; Gale, R.P.; Wang, L.; Xu, J.; Qu, X.Y.; Fan, L.; Li, T.L.; Li, J.Y.; Xu, W. Prognostic value of whole-body suvmax of nodal and extra-nodal lesions detected by 18f-fdg pet/ct in extra-nodal nk/t-cell lymphoma. Oncotarget 2017, 8, 1737–1743. [Google Scholar] [CrossRef]
- Oliveira, F.R.A.; Santos, A.O.; de Lima, M.; Toro, I.F.C.; de Souza, T.F.; Amorim, B.J.; Barbeiro, A.S.; Etchebehere, E. The ratio between the whole-body and primary tumor burden, measured on (18)f-fdg pet/ct studies, as a prognostic indicator in advanced non-small cell lung cancer. Radiol. Bras. 2021, 54, 289–294. [Google Scholar] [CrossRef]
- Kurniawan, B.N.; Ferianto, D.; Pieter, J., Jr. Evaluation of breast cancer metastasis and mortality rates based on molecular subtype: A description study. Breast Dis. 2022, 41, 427–432. [Google Scholar] [CrossRef]
- Kwon, H.W.; Lee, J.H.; Pahk, K.; Park, K.H.; Kim, S. Clustering subtypes of breast cancer by combining immunohistochemistry profiles and metabolism characteristics measured using fdg pet/ct. Cancer Imaging 2021, 21, 55. [Google Scholar] [CrossRef]
- Groheux, D.; Giacchetti, S.; Moretti, J.-L.; Porcher, R.; Espié, M.; Lehmann-Che, J.; de Roquancourt, A.; Hamy, A.-S.; Cuvier, C.; Vercellino, L.; et al. Correlation of high 18f-fdg uptake to clinical, pathological and biological prognostic factors in breast cancer. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 426–435. [Google Scholar] [CrossRef]
- Groheux, D.; Martineau, A.; Teixeira, L.; Espié, M.; de Cremoux, P.; Bertheau, P.; Merlet, P.; Lemarignier, C. 18fdg-pet/ct for predicting the outcome in er+/her2− breast cancer patients: Comparison of clinicopathological parameters and pet image-derived indices including tumor texture analysis. Breast Cancer Res. 2017, 19, 3. [Google Scholar] [CrossRef]
| Molecular subtype | A | 5 |
| B | 34 | |
| B-He | 22 | |
| Her-2 enriched | 7 | |
| Triple-negative | 27 | |
| Histology | Invasive lobular | 8 |
| Invasive ductal | 87 | |
| Median age (range) at PET scan, years | 50 (26–76) |
| Luminal A | Luminal B | Luminal B-He | Her-2 Enriched | Triple-Negative | |
|---|---|---|---|---|---|
| Patients with recurrences | 0 | 13 | 5 | 1 | 10 |
| (38.2%) | (22.7%) | (14.3%) | (37%) | ||
| T | 0 | 13 | 5 | 1 | 9 |
| N | 0 | 8 | 3 | 1 | 6 |
| M | 0 | 4 | 1 | 1 | 3 |
| Semiquantitative Parameters | Molecular Subtypes | |||
|---|---|---|---|---|
| Luminal B | Luminal B-He | Triple Negative | ||
| T | SUVmax | 11.7 ± 7.5 | 9.1 ± 5.5 | 16.5 ± 10.2 |
| SUVmean | 5.5 ± 3.8 | 4.7 ± 5.5 | 16.6 ± 10.3 | |
| MTV | 70 ± 143.2 | 5.7 ± 3.9 | 25.9 ± 46.7 | |
| TLG | 340.7 ± 623.4 | 29 ± 23.6 | 16.6 ± 10.2 | |
| N | SUVmax | 10.7 ± 8.4 | 10.9 ± 8.8 | 7.1 ± 4.5 |
| SUVmean | 4.9 ± 3.8 | 5.6 ± 4.2 | 7.1 ± 4.5 | |
| MTV | 22.2 ± 50 | 39.9 ± 106 | 6.6 ± 11.5 | |
| TLG | 118.3 ± 273 | 24.3 ± 31.8 | 27.8 ± 53.9 | |
| M | SUVmax | 9.9 ± 7.3 | 6.2 ± 3 | 5 ± 2.5 |
| SUVmean | 4.6 ± 2.4 | 3.7 ± 0.9 | 3.1 ± 1.3 | |
| MTV | 4.4 ± 5.3 | 1.4 ± 1.2 | 1.6 ± 1.9 | |
| TLG | 26.9 ± 38 | 6.1 ± 6.2 | 4.9 ± 5 | |
| WB | WB_SUVmax | 27.5 ± 42.6 | 63.8 ± 221.8 | 24.8 ± 15.4 |
| WB_MTV | 88.9 ± 150.1 | 8.6 ± 5.8 | 34.7 ± 49.7 | |
| WB_TLG | 427.6 ± 684 | 46.4 ± 42.1 | 348.8 ± 802 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quartuccio, N.; Alongi, P.; Urso, L.; Ortolan, N.; Borgia, F.; Bartolomei, M.; Arnone, G.; Evangelista, L. 18F-FDG PET-Derived Volume-Based Parameters to Predict Disease-Free Survival in Patients with Grade III Breast Cancer of Different Molecular Subtypes Candidates to Neoadjuvant Chemotherapy. Cancers 2023, 15, 2715. https://doi.org/10.3390/cancers15102715
Quartuccio N, Alongi P, Urso L, Ortolan N, Borgia F, Bartolomei M, Arnone G, Evangelista L. 18F-FDG PET-Derived Volume-Based Parameters to Predict Disease-Free Survival in Patients with Grade III Breast Cancer of Different Molecular Subtypes Candidates to Neoadjuvant Chemotherapy. Cancers. 2023; 15(10):2715. https://doi.org/10.3390/cancers15102715
Chicago/Turabian StyleQuartuccio, Natale, Pierpaolo Alongi, Luca Urso, Naima Ortolan, Francesca Borgia, Mirco Bartolomei, Gaspare Arnone, and Laura Evangelista. 2023. "18F-FDG PET-Derived Volume-Based Parameters to Predict Disease-Free Survival in Patients with Grade III Breast Cancer of Different Molecular Subtypes Candidates to Neoadjuvant Chemotherapy" Cancers 15, no. 10: 2715. https://doi.org/10.3390/cancers15102715
APA StyleQuartuccio, N., Alongi, P., Urso, L., Ortolan, N., Borgia, F., Bartolomei, M., Arnone, G., & Evangelista, L. (2023). 18F-FDG PET-Derived Volume-Based Parameters to Predict Disease-Free Survival in Patients with Grade III Breast Cancer of Different Molecular Subtypes Candidates to Neoadjuvant Chemotherapy. Cancers, 15(10), 2715. https://doi.org/10.3390/cancers15102715









