OSCC in Never-Smokers and Never-Drinkers Is Associated with Increased Expression of Tumor-Infiltrating Lymphocytes and Better Survival
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Data Collection
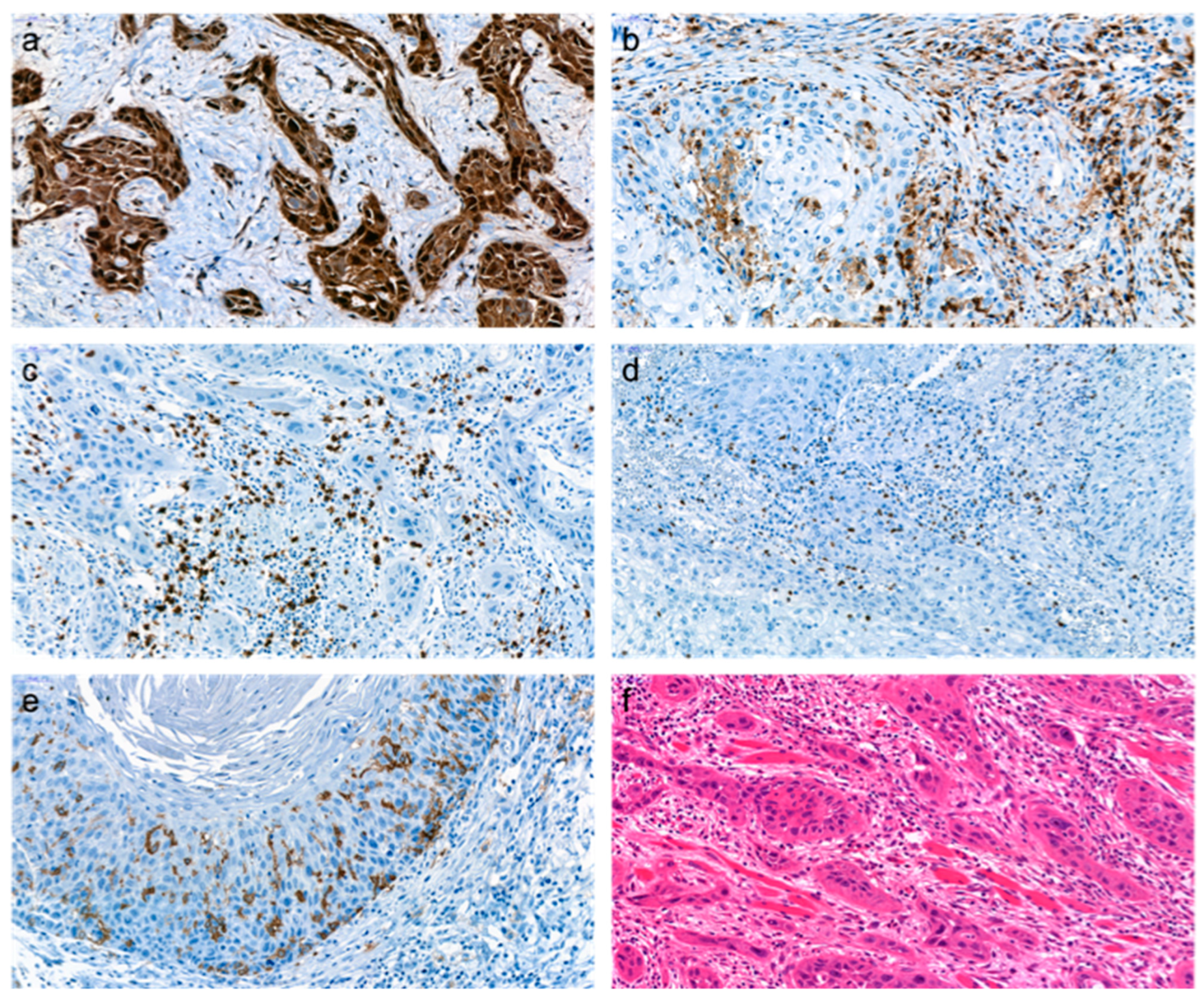
2.2. Immunohistochemistry (IHC)
2.2.1. Preparation and Staining of Samples
2.2.2. Assessment of Immunohistochemical Staining
2.3. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Associations between Clinicohistopathologic Characteristics and the Use of Smoking and Alcohol Consumption
3.3. Associations between NSND, Clinicohistopathologic Characteristics, and Survival
3.4. Immunohistochemical Expression and Associations with Clinicohistopathologic Characteristics
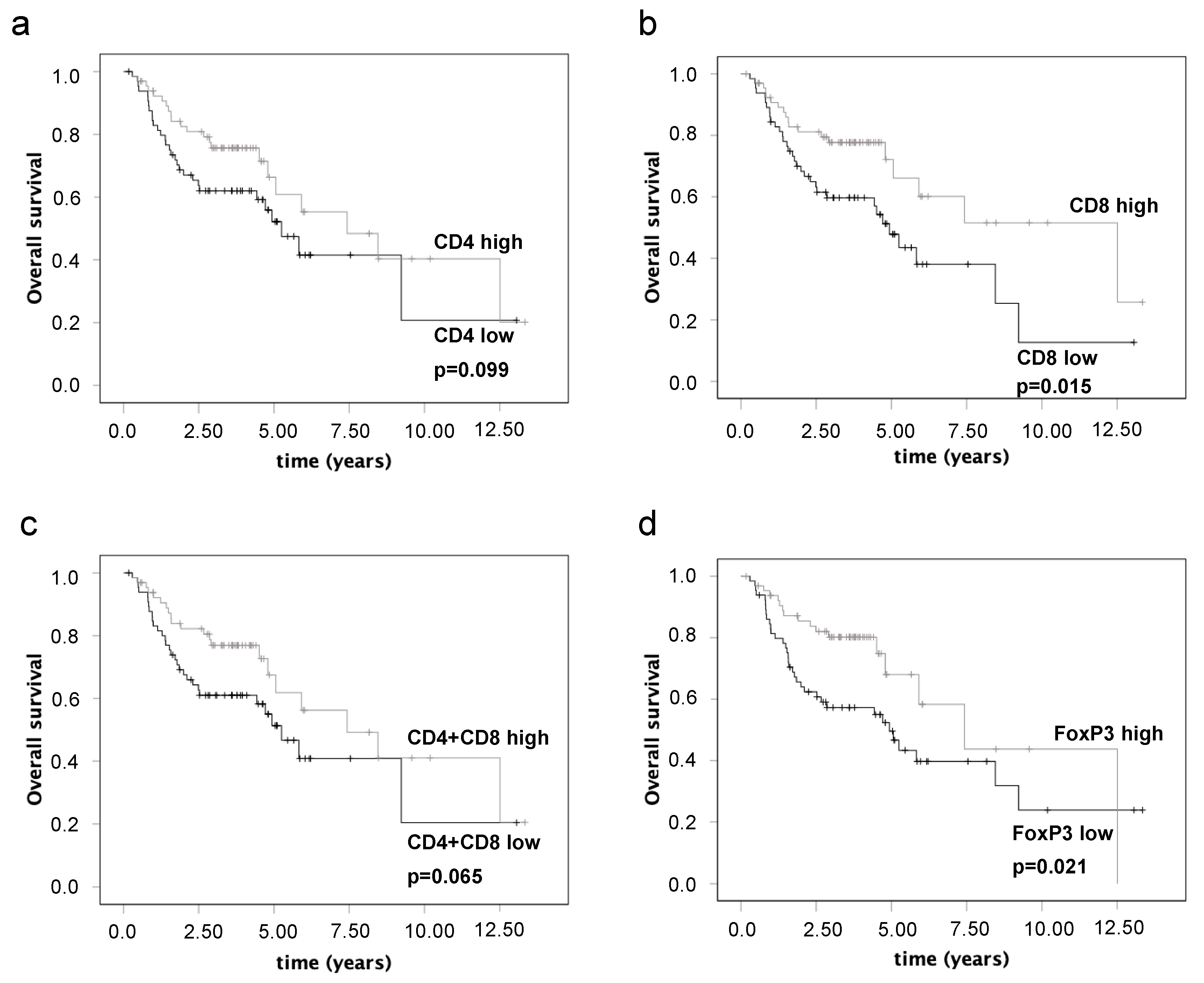
3.5. Associations of Immunohistochemical Parameters and Survival
3.6. Multivariate Survival Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gupta, B.; Johnson, N.; Kumar, N. Global Epidemiology of Head and Neck Cancers: A Continuing Challenge. Oncology 2016, 91, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Alexandrov, L.B.; Ju, Y.S.; Haase, K.; Van Loo, P.; Martincorena, I.; Nik-Zainal, S.; Totoki, Y.; Fujimoto, A.; Nakagawa, H.; Shibata, T.; et al. Mutational signatures associated with tobacco smoking in human cancer. Science 2016, 354, 618–622. [Google Scholar] [CrossRef] [PubMed]
- Boffetta, P.; Hashibe, M. Alcohol and cancer. Lancet Oncol. 2006, 7, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Blot, W.J.; McLaughlin, J.K.; Winn, D.M.; Austin, D.F.; Greenberg, R.S.; Preston-Martin, S.; Bernstein, L.; Schoenberg, J.B.; Stemhagen, A.; Fraumeni, J.F. Smoking and drinking in relation to oral and pharyngeal cancer. Cancer Res. 1988, 48, 3282–3287. [Google Scholar]
- Foy, J.P.; Bertolus, C.; Michallet, M.C.; Deneuve, S.; Incitti, R.; Bendriss-Vermare, N.; Albaret, M.-A.; Ortiz-Cuaran, S.; Thomas, E.; Colombe, A. The immune microenvironment of HPV-Negative oral squamous cell carcinoma from Never-Smokers and Never-Drinkers patients suggests higher clinical benefit of IDO1 and PD1/PD-L1 blockade. Ann. Oncol. 2017, 28, 1934–1941. [Google Scholar] [CrossRef]
- Koo, K.; Barrowman, R.; McCullough, M.; Iseli, T.; Wiesenfeld, D. Non-smoking non-drinking elderly females: A clinically distinct subgroup of oral squamous cell carcinoma patients. Int. J. Oral Maxillofac. Surg. 2013, 42, 929–933. [Google Scholar] [CrossRef]
- Lee, J.; Taneja, V.; Vassallo, R. Cigarette smoking and inflammation: Cellular and molecular mechanisms. J. Dent. Res. 2012, 91, 142–149. [Google Scholar] [CrossRef]
- Meadows, G.G.; Zhang, H. Effects of Alcohol on Tumor Growth, Metastasis, Immune Response, and Host Survival. Alcohol Res. Curr. Rev. 2015, 37, 311–322. [Google Scholar]
- Gajewski, T.F.; Schreiber, H.; Fu, Y.-X. Innate and adaptive immune cells in the tumor microenvironment. Nat. Immunol. 2013, 14, 1014–1022. [Google Scholar] [CrossRef]
- De Meulenaere, A.; Vermassen, T.; Aspeslagh, S.; Vandecasteele, K.; Rottey, S.; Ferdinande, L. TILs in Head and Neck Cancer: Ready for Clinical Implementation and Why (Not)? Head Neck Pathol. 2017, 11, 354–363. [Google Scholar] [CrossRef]
- Xue, Y.; Song, X.; Fan, S.; Deng, R. The role of tumor-associated macrophages in oral squamous cell carcinoma. Front. Physiol. 2022, 13, 1779. [Google Scholar] [CrossRef]
- Mellman, I.; Coukos, G.; Dranoff, G. Cancer immunotherapy comes of age. Nature 2011, 480, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Milanes-Yearsley, M.; Hammond, M.E.H.; Pajak, T.F.; Cooper, J.S.; Chang, C.; Griffin, T.; Nelson, D.; Laramore, G.; Pilepich, M. Tissue Micro-Array: A Cost and Time-Effective Method for Correlative Studies by Regional and National Cancer Study Groups. Mod. Pathol. 2002, 15, 1366–1373. [Google Scholar] [CrossRef] [PubMed]
- Uddin, S.; Singh, A.; Mishra, V.; Agrawal, N.; Gooi, Z.; Izumchenko, E. Molecular drivers of oral cavity squamous cell carcinoma in non-smoking and non-drinking patients: What do we know so far? Oncol. Rev. 2022, 16, 549. [Google Scholar] [CrossRef] [PubMed]
- Myers, J.N.; Elkins, T.; Roberts, D.; Byers, R.M. Squamous cell carcinoma of the tongue in young adults: Increasing incidence and factors that predict treatment outcomes. Otolaryngol. Neck Surg. 2000, 122, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Farshadpour, F.; Hordijk, G.J.; Koole, R.; Slootweg, P.J. Non-smoking and non-drinking patients with head and neck squamous cell carcinoma: A distinct population. Oral Dis. 2007, 13, 239–243. [Google Scholar] [CrossRef]
- Li, R.; Faden, D.L.; Fakhry, C.; Langelier, C.; Jiao, Y.; Wang, Y.; Wilkerson, M.D.; Pedamallu, C.S.; Old, M.; Lang, J.; et al. Clinical, genomic, and metagenomic characterization of oral tongue squamous cell carcinoma in patients who do not smoke. Head Neck 2014, 37, 1642–1649. [Google Scholar] [CrossRef]
- Warnakulasuriya, S.; Kujan, O.; Aguirre-Urizar, J.M.; Bagan, J.V.; González-Moles, M.; Kerr, A.R.; Lodi, G.; Mello, F.W.; Monteiro, L.; Ogden, G.R.; et al. Oral potentially malignant disorders: A consensus report from an international seminar on nomenclature and classification, convened by the WHO Collaborating Centre for Oral Cancer. Oral Dis. 2020, 27, 1862–1880. [Google Scholar] [CrossRef]
- Gilligan, G.; Piemonte, E.; Lazos, J.; Simancas, M.C.; Panico, R.; Warnakulasuriya, S. Oral squamous cell carcinoma arising from chronic traumatic ulcers. Clin. Oral Investig. 2022, 27, 193–201. [Google Scholar] [CrossRef]
- Carrozzo, M.; Porter, S.; Mercadante, V.; Fedele, S. Oral lichen planus: A disease or a spectrum of tissue reactions? Types, causes, diagnostic algorhythms, prognosis, management strategies. Periodontology 2000 2019, 80, 105–125. [Google Scholar] [CrossRef]
- Best, D.L.; Herzog, C.; Powell, C.; Braun, T.; Ward, B.B.; Moe, J. Oral Lichen Planus-Associated Oral Cavity Squamous Cell Carcinoma Is Associated With Improved Survival and Increased Risk of Recurrence. J. Oral Maxillofac. Surg. 2020, 78, 1193–1202. [Google Scholar] [CrossRef] [PubMed]
- Baumeister, P.; Welz, C.; Jacobi, C.; Reiter, M. Is Perineural Invasion of Head and Neck Squamous Cell Carcinomas Linked to Tobacco Consumption? Otolaryngol.—Head Neck Surg. 2018, 158, 878–881. [Google Scholar] [CrossRef] [PubMed]
- Al Feghali, K.A.; Ghanem, A.I.; Burmeister, C.; Chang, S.S.; Ghanem, T.; Keller, C.; Siddiqui, F. Impact of smoking on pathological features in oral cavity squamous cell carcinoma. J. Cancer Res. Ther. 2019, 15, 582–588. [Google Scholar] [PubMed]
- Baochang, H.; Liu, F.; Chen, Q.; Chen, L.; Lin, J.; Chen, F.; Wang, J.; Qiu, Y.; Shi, B.; Pan, L.; et al. Propensity score analysis exploring the impact of smoking and drinking on the prognosis of patients with oral cancer. Head Neck 2020, 42, 1837–1847. [Google Scholar] [CrossRef]
- Descamps, G.; Karaca, Y.; Lechien, J.R.; Kindt, N.; Decaestecker, C.; Remmelink, M.; Larsimont, D.; Andry, G.; Hassid, S.; Rodriguez, A. Classical risk factors, but not HPV status, predict survival after chemoradiotherapy in advanced head and neck cancer patients. J. Cancer Res. Clin. Oncol. 2016, 142, 2185–2196. [Google Scholar] [CrossRef]
- Fan, Y.; Zheng, L.; Mao, M.-H.; Huang, M.-W.; Liu, S.-M.; Zhang, J.; Li, S.-L.; Zhang, J.-G. Survival Analysis of Oral Squamous Cell Carcinoma in a Subgroup of Young Patients. Asian Pac. J. Cancer Prev. 2014, 15, 8887–8891. [Google Scholar] [CrossRef]
- Bachar, G.; Hod, R.; Goldstein, D.; Irish, J.; Gullane, P.; Brown, D.; Gilbert, R.; Hadar, T.; Feinmesser, R.; Shpitzer, T. Outcome of oral tongue squamous cell carcinoma in patients with and without known risk factors. Oral Oncol. 2011, 47, 45–50. [Google Scholar] [CrossRef]
- Koo, K.; Mouradov, D.; Angel, C.M.; Iseli, T.A.; Wiesenfeld, D.; McCullough, M.J.; Burgess, A.W.; Sieber, O.M. Genomic Signature of Oral Squamous Cell Carcinomas from Non-Smoking Non-Drinking Patients. Cancers 2021, 13, 1029. [Google Scholar] [CrossRef]
- Tumeh, P.; Harview, C.; Yearly, J.; Shintaku, I.; Taylor, E.; Robert, L.; Chmielowski, B.; Spasic, M.; Henry, G.; Ciobanu, V.; et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2018, 515, 568–571. [Google Scholar] [CrossRef]
- Gavrielatou, N.; Doumas, S.; Economopoulou, P.; Foukas, P.G.; Psyrri, A. Biomarkers for immunotherapy response in head and neck cancer. Cancer Treat. Rev. 2020, 84, 101977. [Google Scholar] [CrossRef]
- Kinoshita, T.; Muramatsu, R.; Fujita, T.; Nagumo, H.; Sakurai, T.; Noji, S.; Takahata, E.; Yaguchi, T.; Tsukamoto, N.; Kudo-Saito, C.; et al. Prognostic value of tumor-infiltrating lymphocytes differs depending on histological type and smoking habit in completely resected non-small-cell lung cancer. Ann. Oncol. 2016, 27, 2117–2123. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, S.; Yamaguchi, T.; Nomura, T.; Ono, M. Regulatory T Cells and Immune Tolerance. Cell 2008, 133, 775–787. [Google Scholar] [CrossRef] [PubMed]
- De Ruiter, E.J.; Ooft, M.L.; Devriese, L.A.; Willems, S.M. The prognostic role of tumor infiltrating T-lymphocytes in squamous cell carcinoma of the head and neck: A systematic review and meta-analysis. Oncoimmunology 2017, 6, e1356148. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Cho, K.J.; Lee, M.; Yoon, D.H.; Kim, S.-B. Importance of FOXP3 in prognosis and its relationship with p16 in tonsillar squamous cell carcinoma. Anticancer Res. 2013, 33, 5667–5673. [Google Scholar]
- Rochefort, J.; Karagiannidis, I.; Baillou, C.; Belin, L.; Guillot-Delost, M.; Macedo, R.; Le Moignic, A.; Mateo, V.; Soussan, P.; Brocheriou, I.; et al. Defining biomarkers in oral cancer according to smoking and drinking status. Front. Oncol. 2023, 12, 1068979. [Google Scholar] [CrossRef]
- Stähelin, H.; Francisco, A.L.N.; Mariano, F.V.; Kowalski, L.P.; Gondak, R. Impact of smoking on dendritic cells in patients with oral squamous cell carcinoma. Braz. Oral Res. 2021, 35, e075. [Google Scholar] [CrossRef]
- Sato, K.; Fujita, S. Dendritic cells: Nature and classification. Allergol. Int. 2007, 56, 183–191. [Google Scholar]
- Rizvi, N.A.; Hellmann, M.D.; Snyder, A.; Kvistborg, P.; Makarov, V.; Havel, J.J.; Lee, W.; Yuan, J.; Wong, P.; Ho, T.S.; et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non–small cell lung cancer. Science 2015, 348, 124–128. [Google Scholar] [CrossRef]
- Paijens, S.T.; Vledder, A.; de Bruyn, M.; Nijman, H.W. Tumor-infiltrating lymphocytes in the immunotherapy era. Cell. Mol. Immunol. 2020, 18, 842–859. [Google Scholar] [CrossRef]

| Antibody | Dilution | Heat Retrieval | Cell Conditioner | Incubation Time [min] |
|---|---|---|---|---|
| p16 | 1:4 | yes | CC1 | 60 |
| CD4 | 1:4 | yes | CC1 | 44 |
| CD8 | 1:4 | yes | CC1 | 32 |
| CD1a | 1:20 | yes | CC1 | 28 |
| FoxP3 | 1:120 | yes | CC1 | 32 |
| Noxious | Smoking | Drinking | Smoking and Drinking | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No | Yes | N | p-Value | No | Yes | N | p-Value | No | Yes | N | p-Value | |
| sex | ||||||||||||
| male | 20 (40.0%) | 64 (79.0%) | 84 (64.1%) | 26 (41.9%) | 57 (85.1%) | 83 (64.3%) | 30 (44.8%) | 53(85.5%) | 83 (64.3%) | |||
| female | 30 (60.0%) | 17 (21.0%) | 47 (35.9%) | <0.001 | 36 (58.1%) | 10 (14.9%) | 46 (35.7%) | <0.001 | 37 (55.2%) | 9 (14.5%) | 46 (35.7%) | <0.001 |
| Age at diagnosis | ||||||||||||
| <71 years | 27 (54.0%) | 71 (87.7%) | 98 (74.8%) | 37 (59.7%) | 59 (88.1%) | 96 (74.4%) | 40 (59.7%) | 56 (90.3%) | 96 (74.4%) | |||
| ≥71 years | 23 (46.0%) | 10 (12.3%) | 33 (25.2%) | <0.001 | 25 (40.3%) | 8 (11.9%) | 33 (25.6%) | <0.001 | 27 (40.3%) | 6 (9.7%) | 33 (25.6%) | <0.001 |
| Localization | ||||||||||||
| tongue | 12 (24.0%) | 17 (21.0%) | 29 (22.1%) | 19 (30,6%) | 10 (14.9%) | 29 (22.5%) | 20 (29.9%) | 9 (14.5%) | 29 (22.5%) | |||
| mandible | 12 (24.0%) | 11 (13.6%) | 23 (17.6%) | 14 (22.6%) | 7 (10.4%) | 21 (16.3%) | 14 (20.9%) | 7 (11.3%) | 21 (16.3%) | |||
| maxilla | 9 (18.0%) | 0 (0.0%) | 9 (6.9%) | 9 (14.5%) | 0 (0.0%) | 9 (7.0%) | 9 (13.4%) | 0 (0.0%) | 9 (7.0%) | |||
| floor of mouth | 12 (24.0%) | 44 (54.3%) | 56 (42.7%) | 16 (25.8%) | 40 (59.7%) | 56 (43.4%) | 19 (28.4%) | 37 (59.7%) | 56 (43.4%) | |||
| vestibule | 3 (6.0%) | 4 (4.9%) | 7 (5.3%) | 2 (3.2%) | 5 (7.5%) | 7 (5.4%) | 3 (4.5%) | 4 (6.5%) | 7 (5.4%) | |||
| soft palate | 2 (4.0%) | 5 (6.2%) | 7 (5.3%) | <0.001 | 2 (3.2%) | 5 (7.5%) | 7 (5.4%) | <0.001 | 2 (3.0%) | 5 (8.1%) | 7 (5.4%) | <0.001 |
| T-stage | ||||||||||||
| T1-2 | 28 (56.0%) | 52 (64.2%) | 80 (61.1%) | 38 (61.3%) | 41 (61.2%) | 79 (61.2%) | 40 (59.7%) | 39 (62.9%) | 79 (61.2%) | |||
| T3-4 | 22 (44.0%) | 29 (35.8%) | 51 (38.9%) | 0.363 | 24 (38.7%) | 26 (38.8%) | 50 (38.8%) | 1.000 | 27 (40.3%) | 23 (37.1%) | 50 (38.8%) | 0.722 |
| N-Stage | ||||||||||||
| N0 | 30 (61.2%) | 51 (63.0%) | 81 (62.3%) | 37 (60.7%) | 42 (62.7%) | 79 (61.7%) | 39 (59.1%) | 40 (64.5%) | 79 (61.7%) | |||
| N+ | 19 (38.8%) | 30 (37.0%) | 49 (37.7%) | 0.854 | 24 (39.3%) | 25 (37.3%) | 49 (38.3%) | 0.857 | 27 (40.9%) | 22 (35.5%) | 49 (38.3%) | 0.587 |
| M-stage | ||||||||||||
| M0 | 50 (100.0%) | 80 (100.0%) | 130 (100.0%) | 62 (100.0%) | 66 (100.0%) | 128 (100.0%) | 67 (100.0%) | 61 (100.0%) | 128 (100.0%) | |||
| M1 | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |||
| G-Stage | ||||||||||||
| G1-2 | 43 (86.0%) | 63 (77.8%) | 106 0,809% | 52 (83.9%) | 52 (77.6%) | 104 (80.6%) | 56 (83.6%) | 48 (77.4%) | 104 (80.6%) | |||
| G3-4 | 7 (14.0%) | 18 (22.2%) | 25 0(19.1%) | 0.360 | 10 (16.1%) | 15 (22.4%) | 25 (19.4%) | 0.384 | 11 (16.4%) | 14 (22.6%) | 25 (19.4%) | 0.504 |
| Pn-Stage | ||||||||||||
| Pn0 | 48 (96.0%) | 70 (86.4%) | 118 (90.1%) | 61 (98.4%) | 55 (82.1%) | 116 (89.9%) | 64 (95.5%) | 52 (83.9%) | 116 (89.9%) | |||
| Pn1 | 2 (4.0%) | 11 (13.6%) | 13 (9.9%) | 0.130 | 1 (1.6%) | 12 (17.9%) | 13 (10.1%) | 0.002 | 3 (4.5%) | 10 (16.1%) | 13 (10.1%) | 0.039 |
| L-Stage | ||||||||||||
| L0 | 46 (92.0%) | 68 (84.%) | 114 (87.0%) | 57 (91.9%) | 55 (82.1%) | 112 (86.8%) | 61 (91.%) | 51 (82.3%) | 112 (86.8%) | |||
| L1 | 4 (8.0%) | 13 (16.0%) | 17 (13.0%) | 0.284 | 5 (8.1%) | 12 (17.9%) | 17 (13.2%) | 0.122 | 6 (9.0%) | 11 (17.7%) | 17 (13.2%) | 0.193 |
| V-Stage | ||||||||||||
| V0 | 49 (98.0%) | 79 (97.5%) | 128 (97.7%) | 62 (100.0%) | 64 (95.5%) | 126 (97.7%) | 66 (98.5%) | 60 (96.8%) | 126 (97.7%) | |||
| V1 | 1 (2.0%) | 2 (2.5%) | 3 (2.3%) | 1.000 | 0 (0.0%) | 3 (4.5%) | 3 (2.3%) | 0.245 | 1 (1.5%) | 2 (3.2%) | 3 (2.3%) | 0.608 |
| Lymphoma | ||||||||||||
| no | 47 (94.0%) | 80 (98.8%) | 127 (96.9%) | 58 (93.5%) | 67 (100.0%) | 125 (96.9%) | 63 (94.0%) | 62 (100.0%) | 125 (96.9%) | |||
| yes | 3 (6.0%) | 1 (12.0%) | 4 (3.1%) | 0.155 | 4 (6.5%) | 0 (0.0%) | 4 (3.1%) | 0.051 | 4 (6.0%) | 0 (0.00%) | 4 (3.1%) | 0.120 |
| OPMD | ||||||||||||
| no | 33 (66.0%) | 80 (98.8%) | 113 (86.3%) | 47 (75.8%) | 64 (95.5%) | 111 (86.0%) | 50 (74.6%) | 61 (98.4%) | 111 (86.0%) | |||
| yes | 17 (34.0%) | 1 (1.2%) | 18 (13.7%) | <0.001 | 15 (24.2%) | 3 (4.5%) | 18 (1.4%) | <0.001 | 17 (25.4%) | 1 (1.6%) | 18 (1.4%) | <0.001 |
| Antibody | CD4 | CD8 | CD4+CD8 | FoxP3 | CD1a | CD8/FoxP3 | CD4/CD8 |
|---|---|---|---|---|---|---|---|
| N | 130 | 131 | 130 | 130 | 130 | 128 | 130 |
| Median | 201.83 | 72.33 | 285.00 | 41.50 | 14.33 | 1.59 | 2.80 |
| Std. Deviation | 155.37 | 85.21 | 224.56 | 33.65 | 15.60 | 2.95 | 4.64 |
| Minimum | 11.67 | 1.67 | 13.33 | 0.00 | 0.33 | 0.14 | 0.55 |
| Maximum | 737.67 | 423.00 | 1047.50 | 170.33 | 92.00 | 29.54 | 41.00 |
| Noxious | Smoking | Drinking | Smoking and Drinking | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No | Yes | N | p-Value | No | Yes | N | p-Value | No | Yes | N | p-Value | |
| p16 | ||||||||||||
| negative | 44 (89.8%) | 74 (93.7%) | 118 (92.2%) | 54 (91.5%) | 62 (92.5%) | 116 (92.1%) | 58 (90.6%) | 58 (93.5%) | 116 (92.1%) | |||
| positive | 5 (10.2%) | 5 (6.3%) | 10 (7.8%) | 0.505 | 5 (8.5%) | 5 (7.5%) | 10 (7.9%) | 1.000 | 6 (9.4%) | 4 (6.5%) | 10 (7.9%) | 0.744 |
| CD4 | ||||||||||||
| low | 18 (36.0%) | 47 (58.0%) | 65 (49.6%) | 23 (37.1%) | 42 (62.7%) | 65 (50.4%) | 26 (38.8%) | 39 (62.9%) | 65 (50.4%) | |||
| high | 32 (64.0%) | 34 (42.0%) | 66 (50.4%) | 0.019 | 39 (62.9%) | 25 (37.3%) | 64 (49.6%) | 0.005 | 41 (61.2%) | 23 (37.1%) | 64 (49.6%) | 0.008 |
| CD8 | ||||||||||||
| low | 16 (32.0%) | 48 (59.3%) | 64 (48.9%) | 22 (35.5%) | 42 (62.7%) | 64 (49.6%) | 24 (35.8%) | 40 (64.5%) | 64 (49.6%) | |||
| high | 34 (68.0%) | 33 (40.7%) | 67 (51.1%) | 0.004 | 40 (64.5%) | 25 (37.3%) | 65 (50.4%) | 0.003 | 43 (64.2%) | 22 (35.5%) | 65 (50.4%) | 0.001 |
| CD4+CD8 | ||||||||||||
| low | 17 (34.0%) | 49 (61.3%) | 66 (50.8%) | 23 (37.7%) | 43 (64.2%) | 66 (51.6%) | 26 (39.4%) | 40 (64.5%) | 66 (51.6%) | |||
| high | 33 (66.0%) | 31 (38.8%) | 64 (49.2%) | 0.004 | 38 (62.3%) | 24 (35.8%) | 62 (48.4%) | 0.004 | 40 (60.6%) | 22 (35.5%) | 62 (48.4%) | 0.005 |
| FoxP3 | ||||||||||||
| low | 19 (38.0%) | 46 (57.5%) | 65 (50.0%) | 23 (37.7%) | 42 (62.7%) | 65 (50.8%) | 27 (41.5%) | 38 (58.5%) | 65 (50.8%) | |||
| high | 31 (62.0%) | 34 (42.5%) | 65 (50.0%) | 0.047 | 38 (62.3%) | 25 (37.3%) | 63 (49.2%) | 0.008 | 39 (59,1%) | 24 (38.7%) | 63 (49.2%) | 0.023 |
| CD1a | ||||||||||||
| low | 23 (46.0%) | 45 (56.3%) | 68 (52.3%) | 30 (49.2%) | 37 (55.2%) | 67 (52.3%) | 31 (47.0%) | 36 (58.1%) | 67 (52.3%) | |||
| high | 27 (54.0%) | 35 (43.8%) | 62 (47.7%) | 0.283 | 31 (50.8%) | 30 (44.8%) | 61 (47.7%) | 0.595 | 35 (53.0%) | 26 (41.9%) | 61 (47.7%) | 0.221 |
| CD4/CD8 | ||||||||||||
| low | 29 (58.0%) | 36 (45.0%) | 65 (50.0%) | 30 (49.2%) | 33 (49.3%) | 63 (49.2%) | 34 (51.5%) | 29 (46.8%) | 63 (49.2%) | |||
| high | 21 (42.0%) | 44 (55.0%) | 65 (50.0%) | 0.207 | 31 (50.8%) | 34 (50.7%) | 65 (50.8%) | 1.000 | 32 (48.5%) | 33 (53.2%) | 65 (50.8%) | 0.601 |
| CD8/FoxP3 | ||||||||||||
| low | 20 (40.8%) | 44 (55.7%) | 64 (50.0%) | 29 (49.2%) | 34 (50.7%) | 63 (50.0%) | 30 (46.9%) | 33 (53.2%) | 63 (50.0%) | |||
| high | 29 (59.2%) | 35 (44.3%) | 64 (50.0%) | 0.145 | 30 (50.8%) | 33 (49.3%) | 63 (50.0%) | 1.000 | 34 (53.1%) | 29 (46.8%) | 63 (50.0%) | 0.593 |
| Parameter (n = 131) | Univariate (Log-Rank) | Multivariate (Cox Regression) | |
|---|---|---|---|
| p-Value | p-Value (Step 11) | HR a (95% CI b) | |
| Noxious (NSND vs. SD) | 0.004 | 0.024 | 2.071 (1.099–3.903) |
| T-stage (T1/2 vs. T3/4) | 0.003 | 0.019 | 2.055 (1.127–3.749) |
| N-stage (N0 vs. N+) | 0.004 | 0.009 | 2.180 (1.217–3.903) |
| G-stage (G1 vs. G2 vs. G3 vs. G4) | <0.001 | ||
| L-stage (L0 vs. L1) | 0.014 | ||
| V-stage (V0 vs. V1) | 0.057 | 0.056 | 4.330 (0.962–19.482) |
| Pn-stage (Pn0 vs. Pn1) | 0.024 | ||
| Gender (female vs. male) | 0.022 | ||
| UICC-stage (I/II vs. III/IV) | 0.003 | ||
| Relapse (no relapse vs. relapse) | 0.005 | <0.001 | 2.999 (1.661–5.417) |
| OPMD (no OPMD vs. OPMD) | 0.020 | 0.112 | 0.380 (0.115–1.254) |
| Adj. RCT (no RCT vs. RCT) | 0.015 | ||
| CD4 (low expression vs. high expression) | 0.099 | ||
| CD8 (low expression vs. high expression) | 0.015 | 0.074 | 0.551 (0.287–1.058) |
| FoxP3 (low expression vs. high expression) | 0.021 |
| Parameter (n = 131) | Univariate (Log-Rank) | Multivariate (Cox Regression) | |
|---|---|---|---|
| p-Value | p-Value (Step 7) | HR a (95% CI b) | |
| Noxious (NSND vs. SD) | 0.029 | 0.027 | 2.326 (1.099–4.924) |
| T-stage (T1/2 vs. T3/4) | 0.062 | ||
| N-stage (N0 vs. N+) | 0.025 | ||
| G-stage (G1 vs. G2 vs. G3 vs. G4) | <0.001 | ||
| L-stage (L0 vs. L1) | 0.010 | ||
| V-stage (V0 vs. V1) | 0.016 | 0.035 | 5.994 (1.135–31.660) |
| Pn-stage (Pn0 vs. Pn1) | 0.031 | 0.056 | 2.884 (0.971–8.565) |
| Gender (female vs. male) | 0.020 | ||
| UICC-stage (I/II vs. III/IV) | 0.032 | 0.032 | 2.335 (1.074–5.075) |
| Relapse (no relapse vs. relapse) | <0.001 | <0.001 | 5.773 (2.587–12.881) |
| OPMD (no OPMD vs. OPMD) | 0.061 | ||
| CD8 (low expression vs. high expression) | 0.07 | 0.031 | 0.426 (0.196–0.924) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fiedler, M.; Off, A.; Eichberger, J.; Spoerl, S.; Schuderer, J.G.; Taxis, J.; Bauer, R.J.; Schreml, S.; Reichert, T.E.; Ettl, T.; et al. OSCC in Never-Smokers and Never-Drinkers Is Associated with Increased Expression of Tumor-Infiltrating Lymphocytes and Better Survival. Cancers 2023, 15, 2688. https://doi.org/10.3390/cancers15102688
Fiedler M, Off A, Eichberger J, Spoerl S, Schuderer JG, Taxis J, Bauer RJ, Schreml S, Reichert TE, Ettl T, et al. OSCC in Never-Smokers and Never-Drinkers Is Associated with Increased Expression of Tumor-Infiltrating Lymphocytes and Better Survival. Cancers. 2023; 15(10):2688. https://doi.org/10.3390/cancers15102688
Chicago/Turabian StyleFiedler, Mathias, Alisa Off, Jonas Eichberger, Steffen Spoerl, Johannes G. Schuderer, Juergen Taxis, Richard J. Bauer, Stephan Schreml, Torsten E. Reichert, Tobias Ettl, and et al. 2023. "OSCC in Never-Smokers and Never-Drinkers Is Associated with Increased Expression of Tumor-Infiltrating Lymphocytes and Better Survival" Cancers 15, no. 10: 2688. https://doi.org/10.3390/cancers15102688
APA StyleFiedler, M., Off, A., Eichberger, J., Spoerl, S., Schuderer, J. G., Taxis, J., Bauer, R. J., Schreml, S., Reichert, T. E., Ettl, T., & Weber, F. (2023). OSCC in Never-Smokers and Never-Drinkers Is Associated with Increased Expression of Tumor-Infiltrating Lymphocytes and Better Survival. Cancers, 15(10), 2688. https://doi.org/10.3390/cancers15102688








