Influence of Sociodemographic Determinants on the Hodgkin Lymphoma Baseline Characteristics in Long Survivors Patients Enrolled in the Prospective Phase 3 Trial AHL2011
Abstract
Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Patients and Study Design
2.2. Socio-Demographical Data
2.3. PET/CT Acquisition and Analysis
2.4. Statistics
3. Results
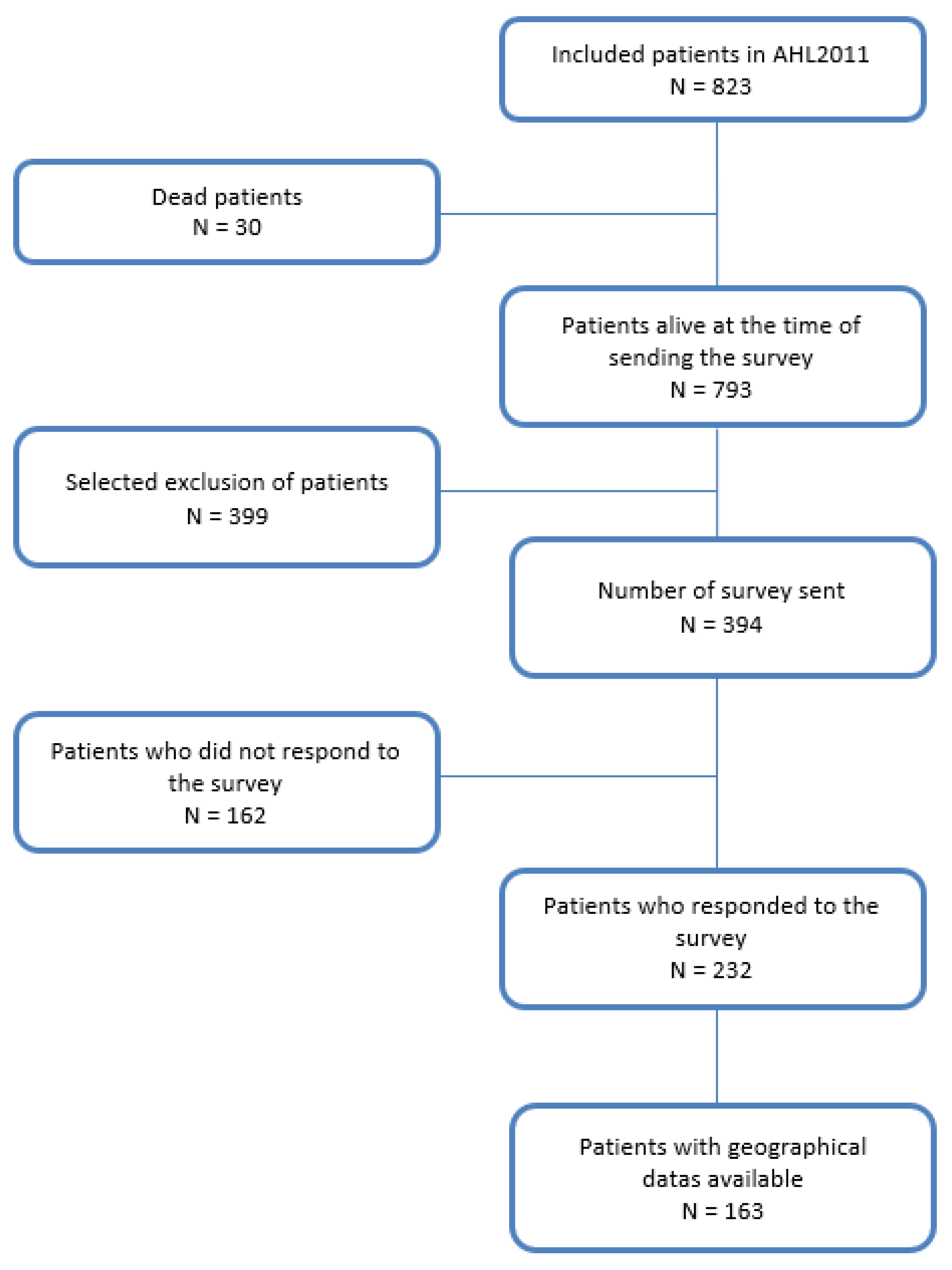
3.1. Patients
3.2. Analyses of Sociodemographic Characteristics
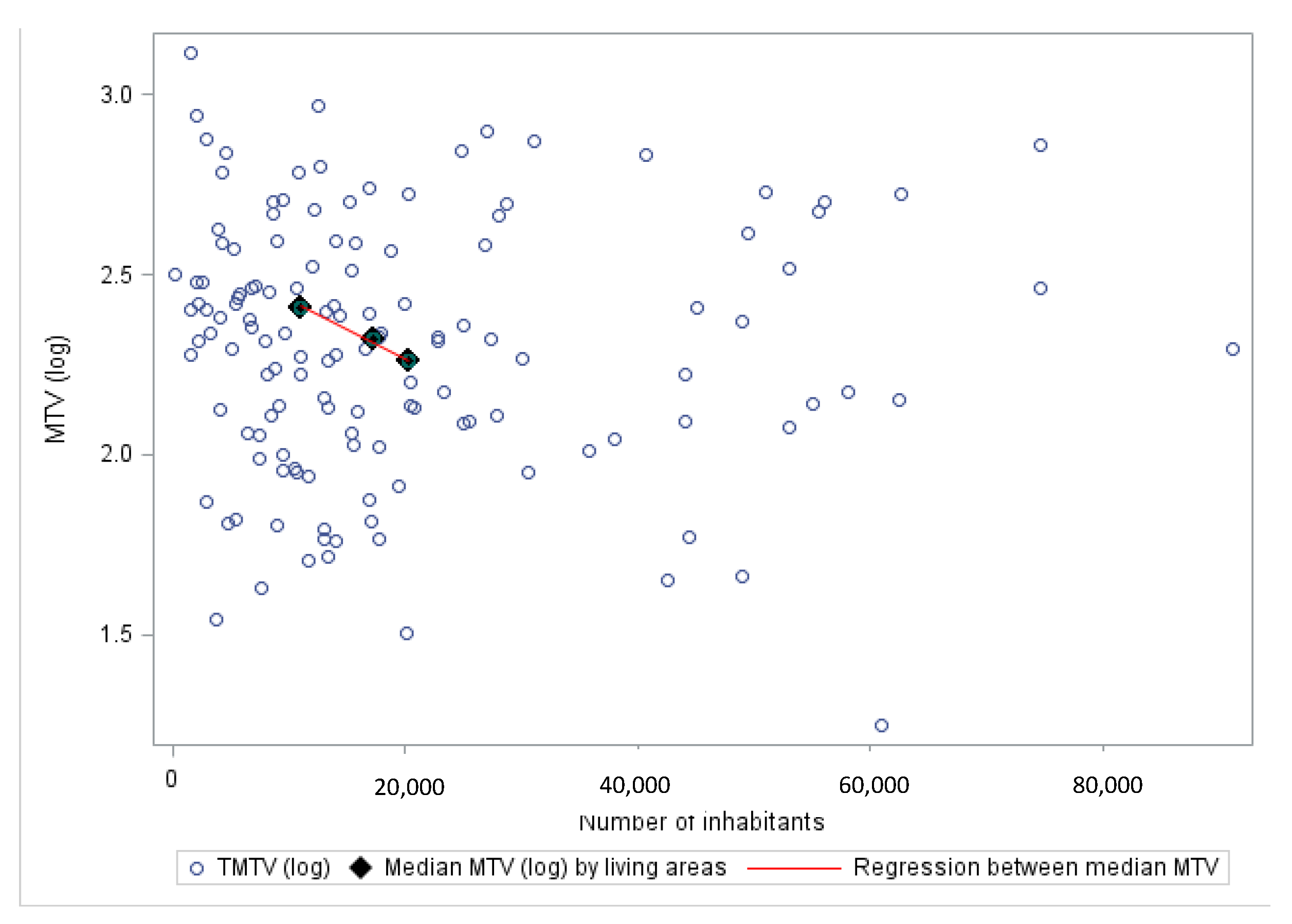
3.3. Relationships between Sociodemographic Features and Disease Characteristics
3.4. Relationships between Sociodemographic Features and PET Responses
3.5. Relationships of Sociodemographic Features and Outcome of These Long Survivor Patients
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
List of Presentations
References
- Le Guyader-Peyrou, S.; Delossez, G.; Dantony, E.; Mounier, M.; Cornet, E.; Uhry, Z.; Cowppli-Bony, A.; Maynadié, M.; Troussard, X.; Delafosse, P.; et al. Estimations Nationales de L’incidence et de la Mortalité par Cancer en France Métropolitaine Entre 1990 et 2018; Volume 2—Hémopathies malignes; Étude à partir des registres des cancers du réseau Francim; Santé Publique: Paris, France, 2019. [Google Scholar]
- SEER Cancer Statistics Review. 2018. Available online: https://seer.cancer.gov/archive/csr/1975_2015/results_merged/sect_09_hodgkins.pdf (accessed on 25 June 2019).
- Cottereau, A.-S.; El-Galaly, T.C.; Becker, S.; Broussais, F.; Petersen, L.J.; Bonnet, C.; Prior, J.O.; Tilly, H.; Hutchings, M.; Casasnovas, O.; et al. Predictive Value of PET Response Combined with Baseline Metabolic Tumor Volume in Peripheral T-Cell Lymphoma Patients. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2018, 59, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Kanoun, S.; Rossi, C.; Berriolo-Riedinger, A.; Dygai-Cochet, I.; Cochet, A.; Humbert, O.; Toubeau, M.; Ferrant, E.; Brunotte, F.; Casasnovas, R.-O. Baseline metabolic tumour volume is an independent prognostic factor in Hodgkin lymphoma. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 1735–1743. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.; Strickland, K.; Easter, S.R.; Worley, M.; Feltmate, C.; Muto, M.; Horowitz, N.; Berkowitz, R.; Feldman, S. The impact of health insurance status on the stage of cervical cancer diagnosis at a tertiary care center in Massachusetts. Gynecol. Oncol. 2018, 150, 67–72. [Google Scholar] [CrossRef]
- Halpern, M.T.; Bian, J.; Ward, E.M.; Schrag, N.M.; Chen, A.Y. Insurance status and stage of cancer at diagnosis among women with breast cancer. Cancer 2007, 110, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Walker, G.V.; Grant, S.R.; Guadagnolo, B.A.; Hoffman, K.E.; Smith, B.D.; Koshy, M.; Allen, P.K.; Mahmood, U. Disparities in Stage at Diagnosis, Treatment, and Survival in Nonelderly Adult Patients With Cancer According to Insurance Status. J. Clin. Oncol. 2014, 32, 3118–3125. [Google Scholar] [CrossRef]
- Keegan, T.H.M.; DeRouen, M.C.; Parsons, H.M.; Clarke, C.A.; Goldberg, D.; Flowers, C.R.; Glaser, S.L. Impact of Treatment and Insurance on Socioeconomic Disparities in Survival after Adolescent and Young Adult Hodgkin Lymphoma: A Population-Based Study. Cancer Epidemiol. Biomarkers Prev. 2016, 25, 264–273. [Google Scholar] [CrossRef]
- Okoro, C.A.; Zhao, G.; Fox, J.B.; Eke, P.I.; Greenlund, K.J.; Town, M. Surveillance for Health Care Access and Health Services Use, Adults Aged 18–64 Years—Behavioral Risk Factor Surveillance System, United States, 2014. MMWR Surveill. Summ. 2017, 66, 1–42. [Google Scholar] [CrossRef]
- Available online: InstitutCurieDP_ObservatoireCancer_InstitutCurie_2019.pdf (accessed on 22 May 2019).
- Aizer, A.A.; Chen, M.-H.; McCarthy, E.P.; Mendu, M.L.; Koo, S.; Wilhite, T.J.; Graham, P.L.; Choueiri, T.K.; Hoffman, K.E.; Martin, N.E.; et al. Marital status and survival in patients with cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2013, 31, 3869–3876. [Google Scholar] [CrossRef]
- Coughlin, S.S. Social determinants of breast cancer risk, stage, and survival. Breast Cancer Res. Treat. 2019, 177, 537–548. [Google Scholar] [CrossRef]
- Hung, P.; Deng, S.; Zahnd, W.E.; Adams, S.A.; Olatosi, B.; Crouch, E.L.; Eberth, J.M. Geographic disparities in residential proximity to colorectal and cervical cancer care providers. Cancer 2020, 126, 1068–1076. [Google Scholar] [CrossRef]
- Ambroggi, M.; Biasini, C.; Del Giovane, C.; Fornari, F.; Cavanna, L. Distance as a Barrier to Cancer Diagnosis and Treatment: Review of the Literature. The Oncologist 2015, 20, 1378–1385. [Google Scholar] [CrossRef] [PubMed]
- Le Guyader-Peyrou, S.; Orazio, S.; Dejardin, O.; Maynadié, M.; Troussard, X.; Monnereau, A. Factors related to the relative survival of patients with diffuse large B-cell lymphoma in a population-based study in France: Does socio-economic status have a role? Haematologica 2017, 102, 584–592. [Google Scholar] [CrossRef] [PubMed]
- Casasnovas, R.-O.; Bouabdallah, R.; Brice, P.; Lazarovici, J.; Ghesquieres, H.; Stamatoullas, A.; Dupuis, J.; Gac, A.-C.; Gastinne, T.; Joly, B.; et al. PET-adapted treatment for newly diagnosed advanced Hodgkin lymphoma (AHL2011): A randomised, multicentre, non-inferiority, phase 3 study. Lancet Oncol. 2019, 20, 202–215. [Google Scholar] [CrossRef] [PubMed]
- Casasnovas, R.-O.; Bouabdallah, R.; Brice, P.; Lazarovici, J.; Ghesquieres, H.; Stamatoullas, A.; Dupuis, J.; Gac, A.-C.; Gastinne, T.; Joly, B.; et al. Positron Emission Tomography-Driven Strategy in Advanced Hodgkin Lymphoma: Prolonged Follow-Up of the AHL2011 Phase III Lymphoma Study Association Study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2022, 40, 1091–1101. [Google Scholar] [CrossRef]
- Vijenthira, A.; Chan, K.; Cheung, M.C.; Prica, A. Cost-effectiveness of first-line treatment options for patients with advanced-stage Hodgkin lymphoma: A modelling study. Lancet Haematol. 2020, 7, e146–e156. [Google Scholar] [CrossRef]
- Available online: https://www.insee.fr/fr/information/2115018#:~:text=L’unit%C3%A9%20urbaine%20est%20une,population%20dans%20cette%20zone%20b%C3%A2tie (accessed on 1 March 2021).
- Boellaard, R.; Delgado-Bolton, R.; Oyen, W.J.G.; Giammarile, F.; Tatsch, K.; Eschner, W.; Verzijlbergen, F.J.; Barrington, S.F.; Pike, L.C.; Weber, W.A.; et al. FDG PET/CT: EANM procedure guidelines for tumour imaging: Version 2.0. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 328–354. [Google Scholar] [CrossRef]
- Meignan, M.; Gallamini, A.; Meignan, M.; Gallamini, A.; Haioun, C. Report on the First International Workshop on interim-PET scan in lymphoma. Leuk. Lymphoma 2009, 50, 1257–1260. [Google Scholar] [CrossRef]
- Itti, E.; Juweid, M.E.; Haioun, C.; Yeddes, I.; Hamza-Maaloul, F.; El Bez, I.; Evangelista, E.; Lin, C.; Dupuis, J.; Meignan, M. Improvement of Early 18F-FDG PET Interpretation in Diffuse Large B-Cell Lymphoma: Importance of the Reference Background. J. Nucl. Med. 2010, 51, 1857–1862. [Google Scholar] [CrossRef]
- Patterson, P.; Allison, K.R.; Milley, K.M.; Chima, S.A.; Harrison, C. General practitioners’ management of cancers in Australian adolescents and young adults. Eur. J. Cancer Care 2018, 27, e12968. [Google Scholar] [CrossRef]
- Robards, J.; Evandrou, M.; Falkingham, J.; Vlachantoni, A. Marital status, health and mortality. Maturitas 2012, 73, 295–299. [Google Scholar] [CrossRef]
- De Roos, A.J.; Schinasi, L.H.; Miligi, L.; Cerhan, J.R.; Bhatti, P.; ‘t Mannetje, A.; Baris, D.; Benavente, Y.; Benke, G.; Clavel, J.; et al. Occupational insecticide exposure and risk of non-Hodgkin lymphoma: A pooled case-control study from the InterLymph Consortium. Int. J. Cancer 2021, 149, 1768–1786. [Google Scholar] [CrossRef] [PubMed]
- Hashim, D.; Boffetta, P. Occupational and Environmental Exposures and Cancers in Developing Countries. Ann. Glob. Health 2014, 80, 393. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Xie, X.; Yang, X.; Jiang, G.; Gu, J. The influence of marital status on the survival of patients with Hodgkin lymphoma. Oncotarget 2017, 8, 51016–51023. [Google Scholar] [CrossRef] [PubMed]
- Ghesquières, H.; Rossi, C.; Cherblanc, F.; Le Guyader-Peyrou, S.; Bijou, F.; Sujobert, P.; Fabbro-Peray, P.; Bernier, A.; Belot, A.; Chartier, L.; et al. A French multicentric prospective prognostic cohort with epidemiological, clinical, biological and treatment information to improve knowledge on lymphoma patients: Study protocol of the “REal world dAta in LYmphoma and survival in adults” (REALYSA) cohort. BMC Public Health 2021, 21, 432. [Google Scholar] [CrossRef] [PubMed]
| Patients’ Characteristics | AHL2011 Whole Cohort | Completed Survey | ZIP Code Available | ||
|---|---|---|---|---|---|
| N = 823 | N = 232 | Test | N = 163 | Test | |
| Median age. years (range) | 30 (16–60) | 32 (16–60) | Wilcoxon p = 0.002 | 32 (17–59) | Wilcoxon p = 0.018 |
| Male. No. (%) | 516 (62.7%) | 141 (60.8%) | Chi-2 p = 0.475 | 100 (61.3%) | Chi-2 p = 0.691 |
| ECOG. No. (%) | Chi-2 p = 0.766 | Chi-2 p = 0.839 | |||
| 0 | 396 (48.4%) | 112 (48.5%) | 76 (46.9%) | ||
| 1 | 365 (44.6%) | 105 (45.5%) | 73 (45.1%) | ||
| 2 | 58 (7.1%) | 14 (6.1%) | 13 (8.0%) | ||
| B symptoms. No. (%) | 263 (32.0%) | 66 (28.4%) | Chi-2 p = 0.176 | 48 (29.4%) | Chi-2 p = 0.443 |
| Ann Arbor stage. No. (%) | Chi-2 p = 0.139 | Chi-2 p = 0.092 | |||
| I or II | 98 (11.9%) | 36 (15.5%) | 28 (17.2%) | ||
| III | 229 (27.8%) | 60 (25.9%) | 43 (26.4%) | ||
| IV | 496 (60.3%) | 136 (58.6%) | 92 (56.4%) | ||
| Stade IIB | 87 (10.6%) | 32 (13.8%) | Chi-2 p = 0.060 | 26 (16%) | Chi-2 p = 0.013 |
| Bulky Mass. No. (%). cm | Chi-2 p = 0.695 | ||||
| ≤10 | 462 (62.5%) | 138 (63.9%) | Chi-2 p = 0.62 | 99 (63.9%) | |
| >10 | 277 (37.5%) | 78 (36.1%) | 56 (36.1%) | ||
| IPS group. No. (%) | Chi-2 p = 0.559 | Chi-2 p = 0.409 | |||
| 0–2 | 343 (41.9%) | 101 (43.5%) | 73 (44.8%) | ||
| ≥3 | 475 (58.1%) | 131 (56.5%) | 90 (55.2%) | ||
| PET2 central review. No. (%) | Chi-2 p = 0.121 | Chi-2 p = 0.162 | |||
| Positive | 100 (12.6%) | 22 (9.7%) | 15 (9.3%) | ||
| Negative | 695 (87.4%) | 205 (90.3%) | 146 (90.7%) | ||
| PET4 central review. No. (%) | Chi-2 p = 0.932 | ||||
| Positive | 43 (5.7%) | 11 (5.1%) | Chi-2 p = 0.653 | 9 (5.8%) | |
| Negative | 716 (94.3%) | 206 (94.9%) | 146 (94.2%) | ||
| TMTV0 class | Chi-2 p = 0.994 | ||||
| < 220 cm3 | 386 (52.2%) | 118 (54.6%) | Chi-2 p = 0.402 | 81 (52.3%) | |
| ≥ 220 cm3 | 353 (47.8%) | 98 (45.4%) | 74 (47.7%) | ||
| Frequence N(%) | PFS 5 Years | |
|---|---|---|
| Marital status | ||
| Isolated | 143 (61.9%) | 92 % |
| Non-isolated | 88 (38.1%) | 86 % |
| Living area | ||
| Rural | 71 (31.1%) | 90.1% |
| City | 157 (68.9%) | 87.3% |
| Diploma | ||
| High school graduation or below | 131 (58.0%) | 88.5% |
| Above high school graduation | 95 (42.0%) | 88.4% |
| Socio-professional categories | ||
| Farmers/artisan/workers | 75 (33.3%) | 90.7% |
| Executives/employees/student | 150 (66.7%) | 87.3% |
| Professional context | ||
| Active | 163 (71.2%) | 89.4% |
| Non active | 66 (28.8%) | 87.7% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chevreux, S.; de Barros, S.; Laurent, C.; Durand, A.; Delpierre, C.; Robert, P.; Joubert, C.; Griolet, S.; Kanoun, S.; Bastie, J.-N.; et al. Influence of Sociodemographic Determinants on the Hodgkin Lymphoma Baseline Characteristics in Long Survivors Patients Enrolled in the Prospective Phase 3 Trial AHL2011. Cancers 2023, 15, 53. https://doi.org/10.3390/cancers15010053
Chevreux S, de Barros S, Laurent C, Durand A, Delpierre C, Robert P, Joubert C, Griolet S, Kanoun S, Bastie J-N, et al. Influence of Sociodemographic Determinants on the Hodgkin Lymphoma Baseline Characteristics in Long Survivors Patients Enrolled in the Prospective Phase 3 Trial AHL2011. Cancers. 2023; 15(1):53. https://doi.org/10.3390/cancers15010053
Chicago/Turabian StyleChevreux, Steeve, Sandra de Barros, Camille Laurent, Amandine Durand, Cyrille Delpierre, Philippine Robert, Clémentine Joubert, Samuel Griolet, Salim Kanoun, Jean-Noël Bastie, and et al. 2023. "Influence of Sociodemographic Determinants on the Hodgkin Lymphoma Baseline Characteristics in Long Survivors Patients Enrolled in the Prospective Phase 3 Trial AHL2011" Cancers 15, no. 1: 53. https://doi.org/10.3390/cancers15010053
APA StyleChevreux, S., de Barros, S., Laurent, C., Durand, A., Delpierre, C., Robert, P., Joubert, C., Griolet, S., Kanoun, S., Bastie, J.-N., Casasnovas, R.-O., & Rossi, C. (2023). Influence of Sociodemographic Determinants on the Hodgkin Lymphoma Baseline Characteristics in Long Survivors Patients Enrolled in the Prospective Phase 3 Trial AHL2011. Cancers, 15(1), 53. https://doi.org/10.3390/cancers15010053





