Plasticity in Classical Hodgkin Composite Lymphomas: A Systematic Review
Abstract
Simple Summary
Abstract
1. Literature Review Section
Introduction
2. Materials and Methods
3. Results
3.1. Article Selection Process for Final Analysis
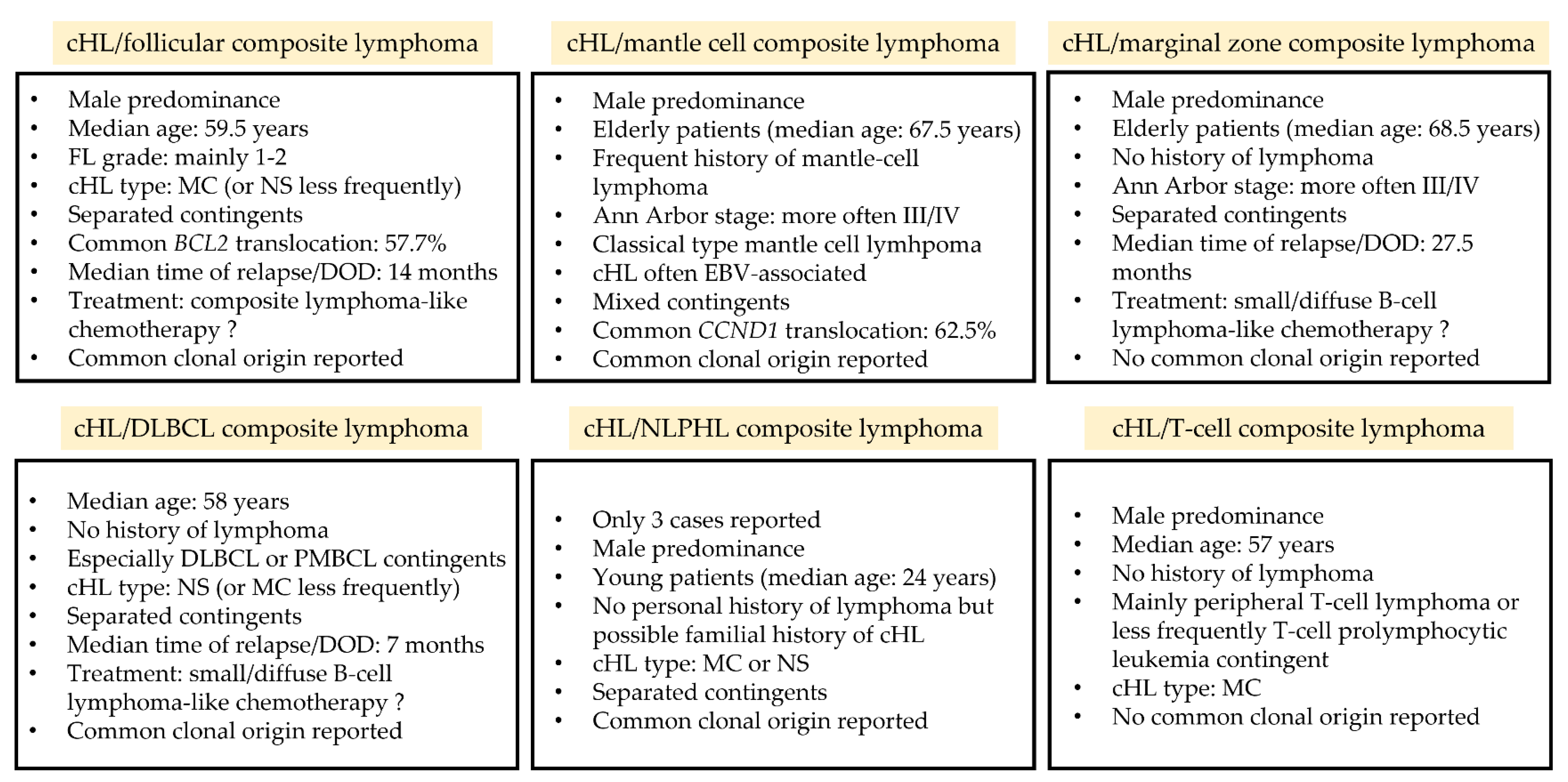
3.2. cHL and Follicular Composite Lymphomas
3.2.1. Clinical and Laboratory Data
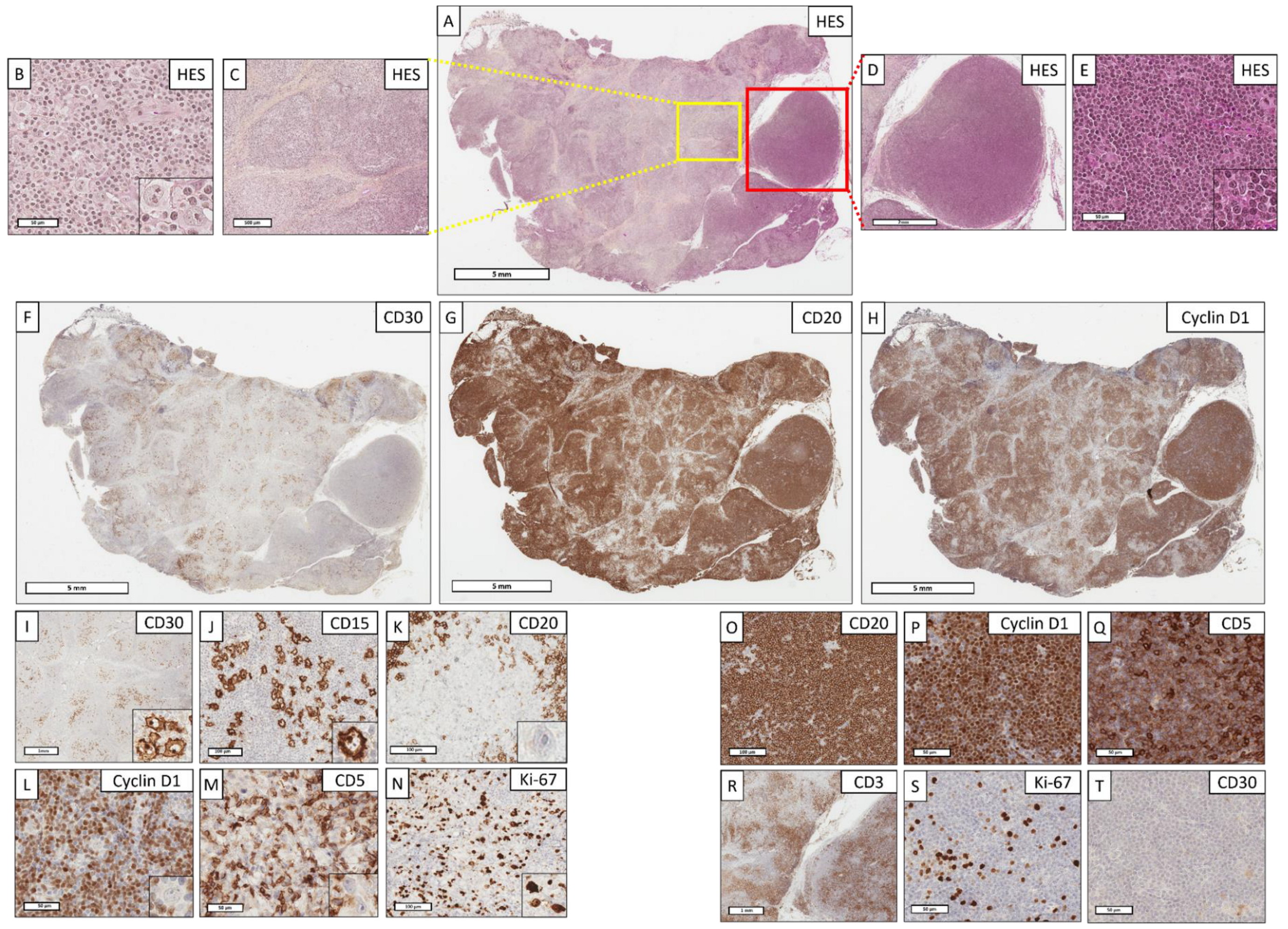
3.2.2. Pathological Data
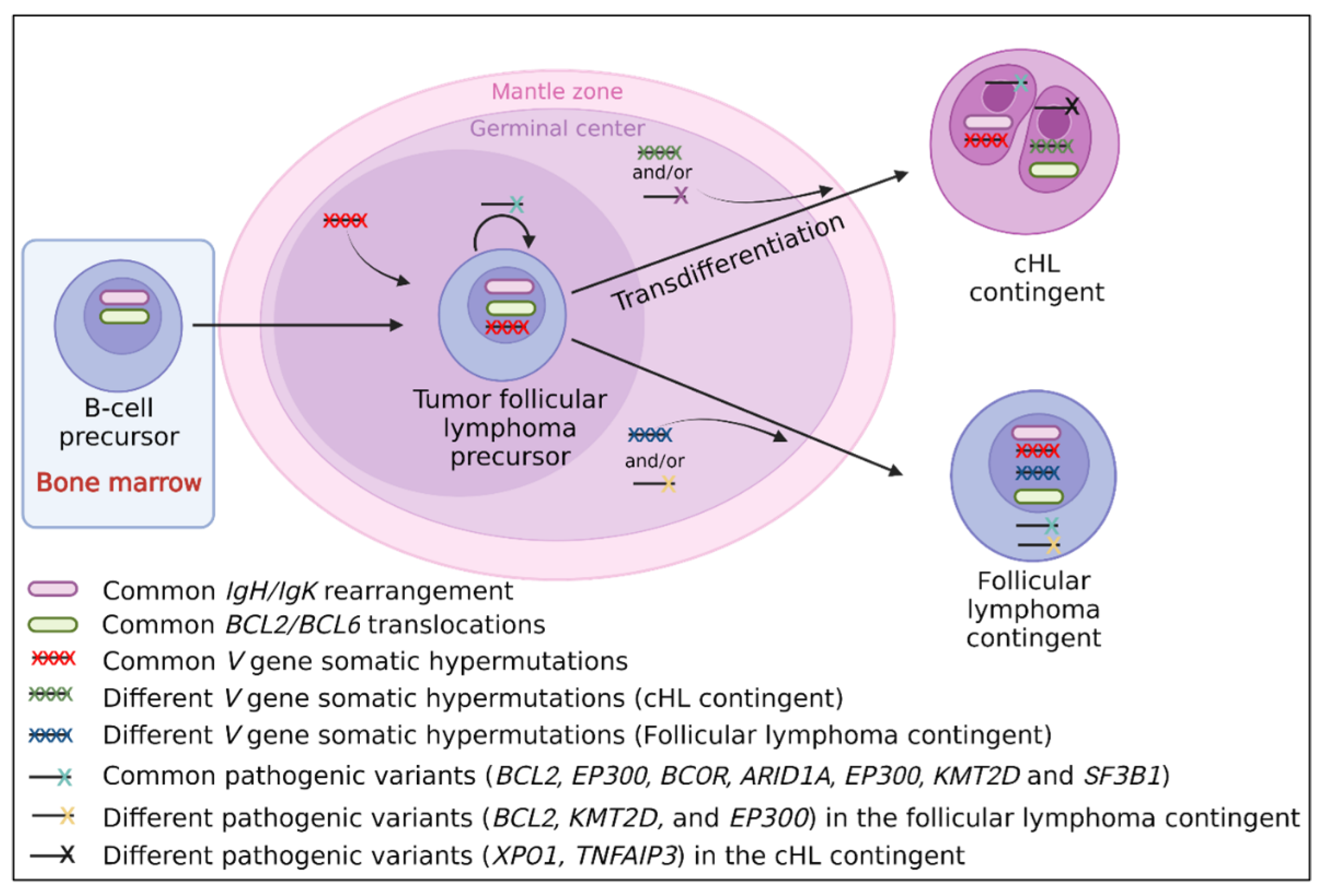
3.2.3. Molecular Data
3.3. cHL and Mantle Cell Composite Lymphomas
3.3.1. Clinical and Laboratory Data
3.3.2. Pathological Data
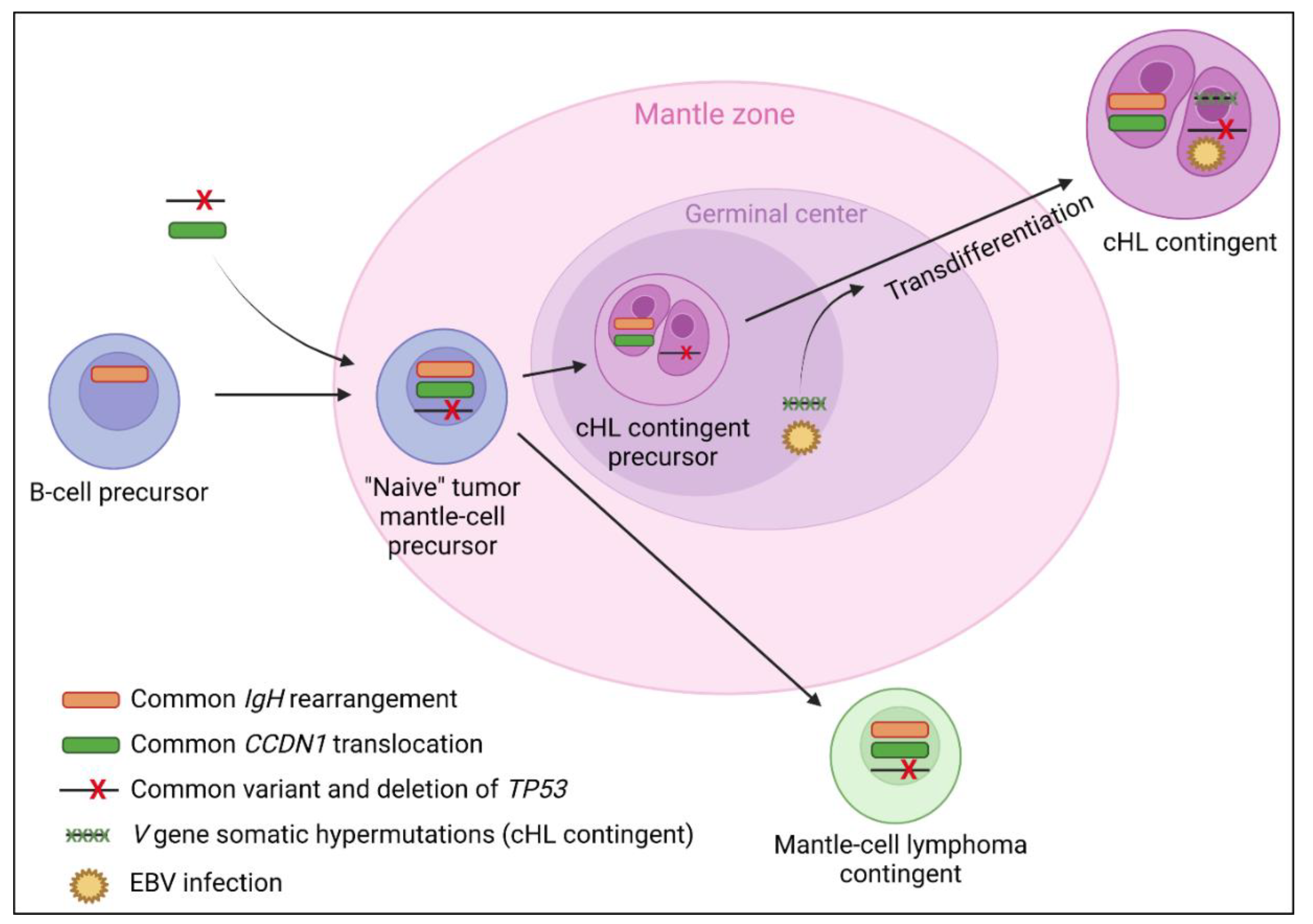
3.3.3. Molecular Data
3.4. cHL and Marginal Zone Composite Lymphomas
3.4.1. Clinical and Laboratory Data
3.4.2. Pathological Data
3.4.3. Molecular Data
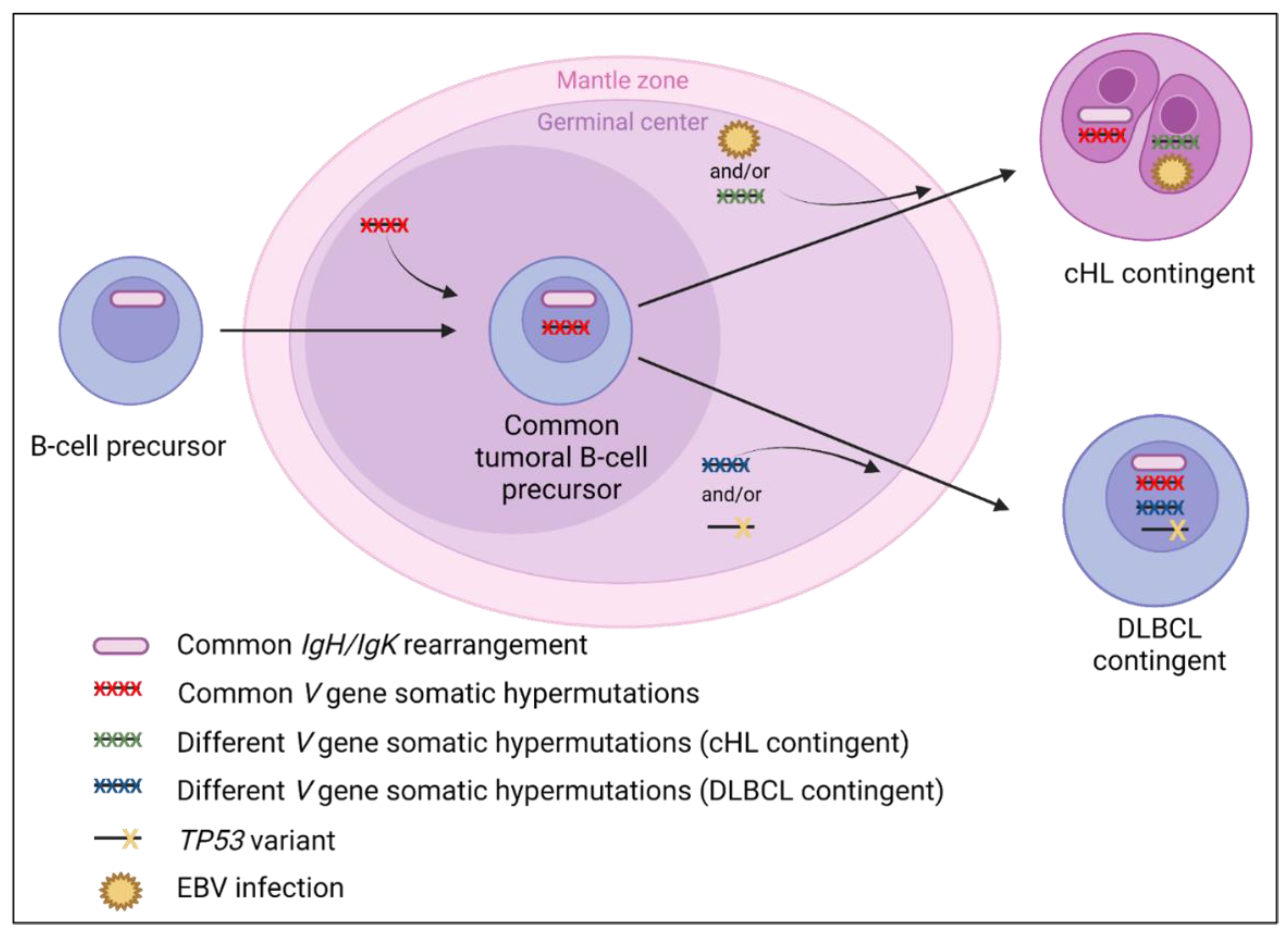
3.5. cHL and Diffuse Large B-Cell Composite Lymphomas
3.5.1. Clinical and Laboratory Data
3.5.2. Pathological Data
3.5.3. Molecular Data
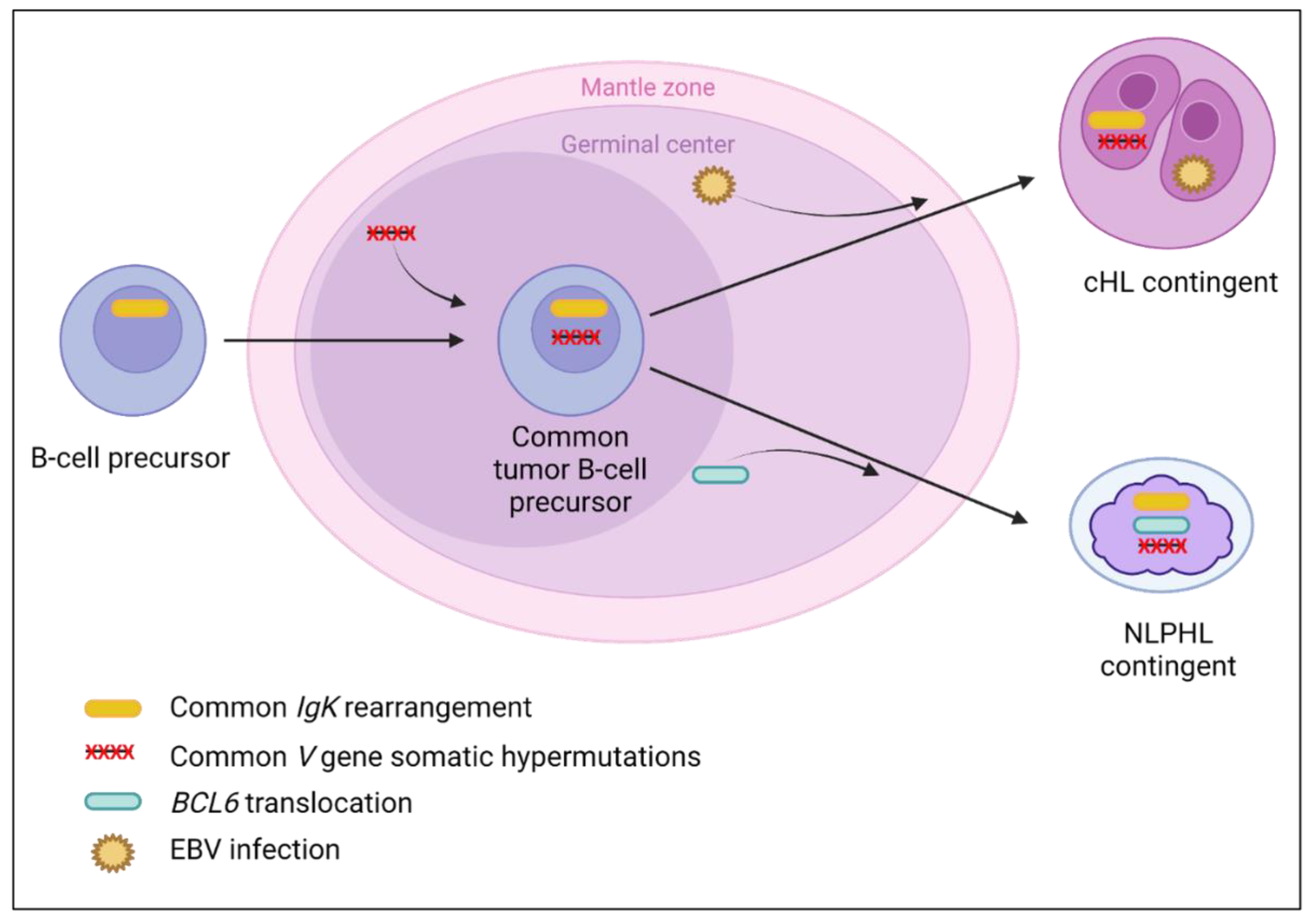
3.6. cHL and Nodular Lymphocyte-Predominant Hodgkin Composite Lymphomas
3.6.1. Clinical and Laboratory Data
3.6.2. Pathological Data
3.6.3. Molecular Data
3.7. cHL and T-cell Composite Lymphomas
3.7.1. Clinical and Laboratory Data
3.7.2. Pathological Data
3.7.3. Molecular Data
4. Discussion
4.1. cHL/Follicular Composite Lymphomas
4.2. cHL/Mantle Cell Composite Lymphomas
4.3. cHL/Marginal Zone Composite Lymphomas
4.4. cHL/NLPHL Composite Lymphomas
4.5. cHL/DLBCL Composite Lymphomas
4.6. Composite cHL/T-Cell Lymphomas
4.7. Limits and Conclusions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kim, H.; Hendrickson, R.; Dorfman, R.F. Composite lymphoma. Cancer 1977, 40, 959–976. [Google Scholar] [CrossRef]
- Jaffe, E.S.; Zarate-Osorno, A.; Kingma, D.W.; Raffeld, M.; Medeiros, L.J. The interrelationship between Hodgkin’s disease and non-Hodgkin’s lymphomas. Ann. Oncol. 1994, 5, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Maeshima, A.M.; Taniguchi, H.; Nomoto, J.; Makita, S.; Kitahara, H.; Fukuhara, S.; Munakata, W.; Suzuki, T.; Maruyama, D.; Kobayashi, Y.; et al. Clinicopathological features of classical Hodgkin lymphoma in patients ≥ 40 years old, with special reference to composite cases. Jpn. J. Clin. Oncol. 2015, 45, 921–928. [Google Scholar] [CrossRef]
- Custer, R. Pitfalls in the diagnosis of lymphoma and leukemia from the pathologist’s point of view. In Proceedings of the Second National Cancer Conference, Cincinnati, OH, USA, 3–5 March 1952; American Cancer Society: New York, NY, USA, 1954; pp. 554–557. [Google Scholar]
- Küppers, R.; Dührsen, U.; Hansmann, M.L. Pathogenesis, diagnosis, and treatment of composite lymphomas. Lancet. Oncol. 2014, 15, 435–446. [Google Scholar] [CrossRef]
- Schmitz, R.; Renné, C.; Rosenquist, R.; Tinguely, M.; Distler, V.; Menestrina, F.; Lestani, M.; Stankovic, T.; Austen, B.; Bräuninger, A.; et al. Insights into the multistep transformation process of lymphomas: IgH-associated translocations and tumor suppressor gene mutations in clonally related composite Hodgkin’s and non-Hodgkin’s lymphomas. Leukemia 2005, 19, 1452–1458. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, N.; Ohshima, K.; Abe, M.; Osamura, Y. Demonstration of chimeric DNA of bcl-2 and immunoglobulin heavy chain in follicular lymphoma and subsequent Hodgkin lymphoma from the same patient. J. Clin. Exp. Hematop. 2007, 47, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Schneider, S.; Crescenzi, B.; Schneider, M.; Ascani, S.; Hartmann, S.; Hansmann, M.L.; Falini, B.; Mecucci, C.; Tiacci, E.; Küppers, R. Subclonal evolution of a classical Hodgkin lymphoma from a germinal center B-cell-derived mantle cell lymphoma. Int. J. Cancer 2014, 134, 832–843. [Google Scholar] [CrossRef]
- Tinguely, M.; Rosenquist, R.; Sundström, C.; Amini, R.M.; Küppers, R.; Hansmann, M.L.; Bräuninger, A. Analysis of a clonally related mantle cell and Hodgkin lymphoma indicates Epstein-Barr virus infection of a Hodgkin/Reed-Sternberg cell precursor in a germinal center. Am. J. Surg. Pathol. 2003, 27, 1483–1488. [Google Scholar] [CrossRef]
- Trecourt, A.; Mauduit, C.; Szablewski, V.; Fontaine, J.; Balme, B.; Donzel, M.; Laurent, C.; Sesques, P.; Ghesquières, H.; Bachy, E.; et al. Plasticity of Mature B Cells Between Follicular and Classic Hodgkin Lymphomas: A Series of 22 Cases Expanding the Spectrum of Transdifferentiation. Am. J. Surg. Pathol. 2022, 46, 58–70. [Google Scholar] [CrossRef]
- Bräuninger, A.; Hansmann, M.L.; Strickler, J.G.; Dummer, R.; Burg, G.; Rajewsky, K.; Küppers, R. Identification of common germinal-center B-cell precursors in two patients with both Hodgkin’s disease and non-Hodgkin’s lymphoma. N. Engl. J. Med. 1999, 340, 1239–1247. [Google Scholar] [CrossRef]
- Küppers, R.; Sousa, A.B.; Baur, A.S.; Strickler, J.G.; Rajewsky, K.; Hansmann, M.L. Common germinal-center B-cell origin of the malignant cells in two composite lymphomas, involving classical Hodgkin’s disease and either follicular lymphoma or B-CLL. Mol. Med. 2001, 7, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Rosenquist, R.; Menestrina, F.; Lestani, M.; Küppers, R.; Hansmann, M.L.; Bräuninger, A. Indications for peripheral light-chain revision and somatic hypermutation without a functional B-cell receptor in precursors of a composite diffuse large B-cell and Hodgkin’s lymphoma. Lab. Invest. 2004, 84, 253–262. [Google Scholar] [CrossRef]
- Gonzalez, C.L.; Medeiros, L.J.; Jaffe, E.S. Composite lymphoma. A clinicopathologic analysis of nine patients with Hodgkin’s disease and B-cell non-Hodgkin’s lymphoma. Am. J. Clin. Pathol. 1991, 96, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Thirumala, S.; Esposito, M.; Fuchs, A. An unusual variant of composite lymphoma: A short case report and review of the literature. Arch. Pathol. Lab. Med. 2000, 124, 1376–1378. [Google Scholar] [CrossRef] [PubMed]
- Aussedat, G.; Traverse-Glehen, A.; Stamatoullas, A.; Molina, T.; Safar, V.; Laurent, C.; Michot, J.M.; Hirsch, P.; Nicolas-Virelizier, E.; Lamure, S.; et al. Composite and sequential lymphoma between classical Hodgkin lymphoma and primary mediastinal lymphoma/diffuse large B-cell lymphoma, a clinico-pathological series of 25 cases. Br. J. Haematol. 2020, 189, 244–256. [Google Scholar] [CrossRef] [PubMed]
- Weniger, M.A.; Küppers, R. Molecular biology of Hodgkin lymphoma. Leukemia 2021, 35, 968–981. [Google Scholar] [CrossRef] [PubMed]
- Das, D.K.; Sheikh, Z.A.; Al-Shama’a, M.H.; John, B.; Alawi, A.M.; Junaid, T.A. A case of composite classical and nodular lymphocyte predominant Hodgkin lymphoma with progression to diffuse large B-cell non-Hodgkin lymphoma: Diagnostic difficulty in fine-needle aspiration cytology. Diagn. Cytopathol. 2017, 45, 262–266. [Google Scholar] [CrossRef]
- Chan, W.C.; Griem, M.L.; Grozea, P.N.; Freel, R.J.; Variakojis, D. Mycosis fungoides and Hodgkin’s disease occurring in the same patient: Report of three cases. Cancer 1979, 44, 1408–1413. [Google Scholar] [CrossRef]
- Donald, D.; Green, J.A.; White, M. Mycosis fungoides associated with nodular sclerosing Hodgkin’s disease: A case report. Cancer 1980, 46, 2505–2508. [Google Scholar] [CrossRef]
- Hawkins, K.A.; Schinella, R.; Schwartz, M.; Ramsey, D.; Weintraub, A.H.; Silber, R.; Amorosi, E.L. Simultaneous occurrence of mycosis fungoides and Hodgkin disease: Clinical and histologic correlations in three cases with ultrastructural studies in two. Am. J. Hematol. 1983, 14, 355–362. [Google Scholar] [CrossRef]
- Park, C.S.; Chung, H.C.; Lim, H.Y.; Kim, D.L.; Koh, E.H.; Kim, J.H.; Roh, J.K.; Chun, S.I.; Yang, W.I.; Kim, G.E. Coexisting mycosis fungoides and Hodgkin’s disease as a composite lymphoma: A case report. Yonsei. Med. J. 1991, 32, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Bee, C.S.; Blaise, Y.P.; Dunphy, C.H. Composite lymphoma of Hodgkin lymphoma and mycosis fungoides: Previously undescribed in the same extracutaneous site. Leuk. Lymphoma 2001, 42, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Nong, L.; Li, X.; Wang, Y. Cutaneous composite lymphoma of mycosis fungoides and Hodgkin lymphoma: Response to sequential therapy. J. Cutan. Pathol. 2020, 47, 829–833. [Google Scholar] [CrossRef] [PubMed]
- Willenbrock, K.; Ichinohasama, R.; Kadin, M.E.; Miura, I.; Terui, T.; Meguro, K.; Fukuhara, O.; DeCoteau, J.F.; Hansmann, M.L. T-cell variant of classical Hodgkin’s lymphoma with nodal and cutaneous manifestations demonstrated by single-cell polymerase chain reaction. Lab. Invest. 2002, 82, 1103–1109. [Google Scholar] [CrossRef] [PubMed]
- Steinhoff, M.; Hummel, M.; Assaf, C.; Anagnostopoulos, I.; Treudler, R.; Geilen, C.C.; Stein, H.; Orfanos, C.E. Cutaneous T cell lymphoma and classic Hodgkin lymphoma of the B cell type within a single lymph node: Composite lymphoma. J. Clin. Pathol. 2004, 57, 329–331. [Google Scholar] [CrossRef] [PubMed]
- Delabie, J.; Greiner, T.C.; Chan, W.C.; Weisenburger, D.D. Concurrent lymphocyte predominance Hodgkin’s disease and T-cell lymphoma. A report of three cases. Am. J. Surg. Pathol. 1996, 20, 355–362. [Google Scholar] [CrossRef]
- Shimodaira, S.; Hidaka, E.; Katsuyama, T. Clonal identity of nodular lymphocyte-predominant Hodgkin’s disease and T-cell-rich B-cell lymphoma. N. Engl. J. Med. 2000, 343, 1124–1125. [Google Scholar] [CrossRef]
- Ohno, T.; Trenn, G.; Wu, G.; Abou-Elella, A.; Reis, H.E.; Chan, W.C. The clonal relationship between nodular sclerosis Hodgkin’s disease with a clonal Reed-Sternberg cell population and a subsequent B-cell small noncleaved cell lymphoma. Mod. Pathol. 1998, 11, 485–490. [Google Scholar]
- Dargent, J.L.; Lespagnard, L.; Meiers, I.; Bradstreet, C.; Heimann, P.; De Wolf-Peeters, C. Composite follicular lymphoma and nodular lymphocyte predominance Hodgkin’s disease. Virchows. Arch. 2005, 447, 778–780. [Google Scholar] [CrossRef]
- O’Neill, J.P.; Quinn, F.; Dowling, A.; Walker, J.; Hayes, T.; Bird, B.; Flavin, R. Composite t(14;18)-Negative Follicular Lymphoma and Nodular Lymphocyte-Predominant Hodgkin Lymphoma. Case. Rep. Hematol. 2018, 2018, 4312594. [Google Scholar] [CrossRef]
- Esper, A.; Alhoulaiby, S.; Zuhri-Yafi, R.; Alshehabi, Z. Composite lymphoma of T-cell rich, histiocyte-rich diffuse large B-cell lymphoma and nodular lymphocyte predominant Hodgkin lymphoma: A case report. J. Med. Case. Rep. 2021, 15, 163. [Google Scholar] [CrossRef] [PubMed]
- Ranganathan, S.; Webber, S.; Ahuja, S.; Jaffe, R. Hodgkin-like posttransplant lymphoproliferative disorder in children: Does it differ from posttransplant Hodgkin lymphoma? Pediat. Dev. Pathol. 2004, 7, 348–360. [Google Scholar]
- Karube, K.; Takatori, M.; Kohno, K.; Tomoyose, T.; Ohshiro, K.; Nakazato, I. Co-occurrence of EBV-positive classic Hodgkin lymphoma and B-cell lymphomas of different clonal origins: A case report and literature review. Pathol. Int. 2020, 70, 893–898. [Google Scholar] [CrossRef]
- Ashrafi, F.; Kowsari, F.; Darakhshandeh, A.; Adibi, P. Composite lymphoma in a patient with ulcerative colitis: A case report. Int. J. Hematol. Oncol. Stem. Cell. Res. 2014, 8, 45–48. [Google Scholar] [PubMed]
- Shin, S.S.; Ben-Ezra, J.; Burke, J.S.; Sheibani, K.; Rappaport, H. Reed-Sternberg-like cells in low-grade lymphomas are transformed neoplastic cells of B-cell lineage. Am. J. Clin. Pathol. 1993, 99, 658–662. [Google Scholar] [CrossRef]
- Bayerl, M.G.; Bentley, G.; Bellan, C.; Leoncini, L.; Ehmann, W.C.; Palutke, M. Lacunar and reed-sternberg-like cells in follicular lymphomas are clonally related to the centrocytic and centroblastic cells as demonstrated by laser capture microdissection. Am. J. Clin. Pathol. 2004, 122, 858–864. [Google Scholar] [CrossRef] [PubMed]
- Mori, D.; Matsuishi, E.; Akashi, M.; Shibaki, M.; Hirano, T.; Ide, M.; Tsutsumi, Y.; Tsukiji, H.; Gondo, H. Hodgkin-like peripheral T-cell lymphoma (PTCL) with preserved Hodgkin-like lesions at autopsy: A case report with an interesting clinical course. Pathol. Res. Pract. 2015, 211, 83–87. [Google Scholar] [CrossRef]
- Venkataraman, G.; Berkowitz, J.; Morris, J.C.; Janik, J.E.; Raffeld, M.A.; Pittaluga, S. Adult T-cell leukemia/lymphoma with Epstein-Barr virus-positive Hodgkin-like cells. Human. Pathol. 2011, 42, 1042–1046. [Google Scholar] [CrossRef]
- LeBrun, D.P.; Ngan, B.Y.; Weiss, L.M.; Huie, P.; Warnke, R.A.; Cleary, M.L. The bcl-2 oncogene in Hodgkin’s disease arising in the setting of follicular non-Hodgkin’s lymphoma. Blood 1994, 83, 223–230. [Google Scholar] [CrossRef]
- Niedobitek, G.; Baumann, I.; Brabletz, T.; Lisner, R.; Winkelmann, C.; Helm, G.; Kirchner, T. Hodgkin’s disease and peripheral T-cell lymphoma: Composite lymphoma with evidence of Epstein-Barr virus infection. J. Pathol. 2000, 191, 394–399. [Google Scholar] [CrossRef]
- Momose, H.; Jaffe, E.S.; Shin, S.S.; Chen, Y.Y.; Weiss, L.M. Chronic lymphocytic leukemia/small lymphocytic lymphoma with Reed-Sternberg-like cells and possible transformation to Hodgkin’s disease. Mediation by Epstein-Barr virus. Am. J. Surg. Pathol. 1992, 16, 859–867. [Google Scholar] [CrossRef] [PubMed]
- Cha, I.; Herndier, B.G.; Glassberg, A.B.; Hamill, T.R. A case of composite Hodgkin’s disease and chronic lymphocytic leukemia in bone marrow. Lack of Epstein-Barr virus. Arch. Pathol. Lab. Med. 1996, 120, 386–389. [Google Scholar] [PubMed]
- Kanzler, H.; Küppers, R.; Helmes, S.; Wacker, H.H.; Chott, A.; Hansmann, M.L.; Rajewsky, K. Hodgkin and Reed-Sternberg-like cells in B-cell chronic lymphocytic leukemia represent the outgrowth of single germinal-center B-cell-derived clones: Potential precursors of Hodgkin and Reed-Sternberg cells in Hodgkin’s disease. Blood 2000, 95, 1023–1031. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, M.J.; Kaleem, Z.; Bolger, M.J.; Swanson, P.E.; Zutter, M.M. Composite prolymphocytoid and hodgkin transformation of chronic lymphocytic leukemia. Arch. Pathol. Lab. Med. 2000, 124, 907–909. [Google Scholar] [CrossRef] [PubMed]
- van den Berg, A.; Maggio, E.; Rust, R.; Kooistra, K.; Diepstra, A.; Poppema, S. Clonal relation in a case of CLL, ALCL, and Hodgkin composite lymphoma. Blood 2002, 100, 1425–1429. [Google Scholar] [CrossRef]
- Copur, M.S.; Ledakis, P.; Novinski, D.; Fu, K.; Hutchins, M.; Frankforter, S.; Mleczko, K.; Sanger, W.G.; Chan, W.C. An unusual case of composite lymphoma involving chronic lymphocytic leukemia follicular lymphoma and Hodgkin disease. Leuk. Lymphoma. 2004, 45, 1071–1076. [Google Scholar] [CrossRef]
- de Leval, L.; Vivario, M.; De Prijck, B.; Zhou, Y.; Boniver, J.; Harris, N.L.; Isaacson, P.; Du, M.Q. Distinct clonal origin in two cases of Hodgkin’s lymphoma variant of Richter’s syndrome associated With EBV infection. Am. J. Surg. Pathol. 2004, 28, 679–686. [Google Scholar] [CrossRef]
- Badea, M.; Dobrea, C.; Badea, D.; Genunche-Dumitrescu, A.; Mitruţ, P.; Duţă, D. The composite lymphoma: Chronic lymphocytic leukemia-classic Hodgkin’s lymphoma. Rom. J. Morphol. Embryol. 2010, 51, 353–358. [Google Scholar]
- Rathnam, K.; Karpurmath, S.; Cyriac, S.; Gnana, S.T.; Sundersingh, S. Composite Hodgkin lymphoma and chronic lymphocytic leukemia: A rare case. J. Cancer. Res. Ther. 2011, 7, 484–485. [Google Scholar] [CrossRef]
- Michelis, F.V.; Kourti, G.; Skertsou, M.; Karmiris, T.; Rontogianni, D.P.; Harhalakis, N. Richter transformation of chronic lymphocytic leukemia into composite diffuse large B-cell and Hodgkin lymphoma. Leuk. Lymphoma. 2012, 53, 2302–2303. [Google Scholar] [CrossRef]
- Saba, M.; Ehsani, M.; Moosavian, M.; Khooeei, A. Primary composite lymphoma of the lung: A case report. Tanaffos 2014, 13, 47–49. [Google Scholar] [PubMed]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 372, 160. [Google Scholar] [CrossRef] [PubMed]
- Urano, M.; Mizoguchi, Y.; Nishio, T.; Abe, M.; Kuroda, M.; Saito, S.; Sakurai, K. Composite lymphoma arising in the parotid gland: A case report. Auris. Nasus. Larynx. 2004, 31, 89–93. [Google Scholar] [CrossRef]
- Vasudevan, J.A.; Nair, R.A.; Sukumaran, R.; Nair, S.G. Composite lymphomas: Experience from a tertiary cancer center in Kerala, South India. Indian. J. Cancer 2017, 54, 358–361. [Google Scholar] [PubMed]
- Huang, Y.; Hu, S.; Larson, D.P.; Shi, M.; He, R.; Dave, B.J.; Greiner, T.C.; Fu, K.; McPhail, E.D.; Ketterling, R.P.; et al. Composite Classic Hodgkin Lymphoma and Follicular Lymphoma: A Clinicopathologic Study of 22 Cases With Review of 27 Additional Cases in the Literature. Am. J. Surg. Path. 2022, 46, 793–800. [Google Scholar] [CrossRef]
- Linck, D.; Lentini, G.; Tiemann, M.; Fauser, A.A.; Parwaresch, R.; Basara, N. Sequential application of chemotherapy and monoclonal CD 20 antibody: Successful treatment of advanced composite-lymphoma. Leuk. Lymphoma. 2005, 46, 285–288. [Google Scholar] [CrossRef]
- Jaffe, E.S.; Zarate-Osorno, A.; Medeiros, L.J. The interrelationship of Hodgkin’s disease and non-Hodgkin’s lymphomas-lessons learned from composite and sequential malignancies. Semin. Diagn. Pathol. 1992, 9, 297–303. [Google Scholar]
- Hansmann, M.L.; Fellbaum, C.; Hui, P.K.; Lennert, K. Morphological and immunohistochemical investigation of non-Hodgkin’s lymphoma combined with Hodgkin’s disease. Histopathology 1989, 15, 35–48. [Google Scholar] [CrossRef]
- Demurtas, A.; Aliberti, S.; Bonello, L.; Di Celle, P.F.; Cavaliere, C.; Barreca, A.; Novero, D.; Stacchini, A. Usefulness of multiparametric flow cytometry in detecting composite lymphoma: Study of 17 cases in a 12-year period. Am. J. Clin. Pathol. 2011, 135, 541–555. [Google Scholar] [CrossRef]
- Yoshida, M.; Ichikawa, A.; Miyoshi, H.; Takeuchi, M.; Kimura, Y.; Nino, D.; Ohshima, K. High frequency of t(14;18) in Hodgkin’s lymphoma associated with follicular lymphoma. Pathol. Int. 2012, 62, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.J.; Griffin, G.K.; Yenamandra, A.; Wheeler, F.C.; Ligon, A.H.; Nandedka, M.A.; Shaheen, S.P.; Mosse, C.A.; Kim, A.S. Transformation of follicular lymphoma into classical Hodgkin lymphoma showing t(14;18). Hematopathology 2016, 1, 23–33. [Google Scholar]
- Kim, H.N.; Jeon, M.J.; Yu, E.S.; Kim, D.S.; Choi, C.W.; Ko, Y.H. Composite follicular lymphoma and classic Hodgkin lymphoma. J. Pathol. Transl. Med. 2022, 56, 57–60. [Google Scholar] [CrossRef]
- Nishioka, A.; Ureshino, H.; Ando, T.; Kizuka, H.; Kusaba, K.; Sano, H.; Itamura, H.; Kubota, Y.; Kojima, K.; Ohshima, K.; et al. Three coexisting lymphomas in a single patient: Composite lymphoma derived from a common germinal center B-cell precursor and unrelated discordant lymphoma. Int. J. Hematol. 2018, 107, 703–708. [Google Scholar] [CrossRef]
- Pezzella, M.; Brogna, B.; Romano, A.; Torelli, F.; Esposito, G.; Petrillo, M.; Romano, F.M.; Di Martino, N.; Reginelli, A.; Grassi, R. Detecting a rare composite small bowel lymphoma by Magnetic Resonance Imaging coincidentally: A case report with radiological, surgical and histopathological features. Int. J. Surg. Case. Rep. 2018, 46, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Kingma, D.W.; Medeiros, L.J.; Barletta, J.; Raffeld, M.; Mann, R.B.; Ambinder, R.F.; Jaffe, E.S. Epstein-Barr virus is infrequently identified in non-Hodgkin’s lymphomas associated with Hodgkin’s disease. Am. J. Surg. Pathol. 1994, 18, 48–61. [Google Scholar] [CrossRef] [PubMed]
- Caleo, A.; Sánchez-Aguilera, A.; Rodríguez, S.; Dotor, A.M.; Beltrán, L.; de Larrinoa, A.F.; Menárguez, F.J.; Piris, M.A.; García, J.F. Composite Hodgkin lymphoma and mantle cell lymphoma: Two clonally unrelated tumors. Am. J. Surg. Pathol. 2003, 27, 1577–1580. [Google Scholar] [CrossRef]
- Giua, R.; Fontana, D.; Deda, G.; Bianchi, A.; Rabitti, C.; Antonelli Incalzi, R. Composite mantle-cell lymphoma and classical Hodgkin lymphoma in a very old adult. J. Am. Geriatr. Soc. 2015, 63, 824–826. [Google Scholar] [CrossRef]
- Hayes, S.J.; Banerjee, S.S.; Cook, Y.; Houghton, J.B.; Menasce, L.P. Composite mantle-cell lymphoma and classical Hodgkin lymphoma. Histopathology 2006, 48, 621–623. [Google Scholar] [CrossRef]
- Kanai, R.; Miyagawa-Hayashino, A.; Shishido-Hara, Y.; Nakamura, N.; Omatsu, I.; Morinaga, Y.; Shimura, Y.; Kuroda, J.; Imura, T.; Itoh, K.; et al. Mantle cell lymphoma with EBV-positive Hodgkin and Reed-Sternberg-like cells in a patient after autologous PBSCT: Phenotypically distinct but genetically related tumors. Pathol. Int. 2021, 71, 96–101. [Google Scholar] [CrossRef]
- Kramer, S.; Uppal, G.; Wang, Z.X.; Gong, J.Z. Mantle Cell Lymphoma With Hodgkin and Reed-Sternberg Cells: Review With Illustrative Case. Appl. Immunohistochem. Mol. Morphol. 2019, 27, 8–14. [Google Scholar] [CrossRef]
- Murray, C.; Quinn, F.; Illyes, G.; Walker, J.; Castriciano, G.; O’Sullivan, P.; Grant, C.; Vandenberghe, E.; Bird, B.; Flavin, R. Composite Blastoid Variant of Mantle Cell Lymphoma and Classical Hodgkin Lymphoma. Int. J. Surg. Pathol. 2017, 25, 281–286. [Google Scholar] [CrossRef]
- Sharma, S.; Singh, V.; Bisaria, D.; Tangri, R. Composite lymphoma comprising mantle cell lymphoma and Epstein-Barr virus positive classic Hodgkin lymphoma: A rare case. Indian. J. Pathol. Microbiol. 2019, 62, 488–490. [Google Scholar] [CrossRef] [PubMed]
- Oka, K.; Nagayama, R.; Yonekawa, N.; Nihei, T.; Sando, N.; Yatabe, Y.; Mori, N. Concurrent gastric MALT and Hodgkin lymphoma: A case report. Int. J. Surg. Pathol. 2012, 20, 201–204. [Google Scholar] [CrossRef] [PubMed]
- Harada, S.; Kalla, H.; Balasubramanian, M.; Brodsky, I.; Gladstone, D.; Hou, J.S. Classical Hodgkin lymphoma concurrently evolving in a patient with marginal zone B-cell lymphoma of the spleen. Ann. Diagn. Pathol. 2008, 12, 212–216. [Google Scholar] [CrossRef] [PubMed]
- Zettl, A.; Rüdiger, T.; Marx, A.; Müller-Hermelink, H.K.; Ott, G. Composite marginal zone B-cell lymphoma and classical Hodgkin’s lymphoma: A clinicopathological study of 12 cases. Histopathology 2005, 46, 217–228. [Google Scholar] [CrossRef]
- Elmahy, H.; Hawley, I.; Beard, J. Composite splenic marginal zone lymphoma and classic Hodgkin lymphoma—An unusual combination. Int. J. Lab. Hematol. 2007, 29, 461–463. [Google Scholar] [CrossRef]
- Oka, K.; Shinonaga, M.; Nagayama, R.; Kashimura, H.; Yonekawa, N.; Tatebe, S.; Kuraoka, S.; Yatabe, Y.; Mori, N. Coexistence of primary pulmonary Hodgkin lymphoma and gastric MALT lymphoma associated with Epstein-Barr virus infection: A case report. Pathol. Int. 2010, 60, 520–523. [Google Scholar] [CrossRef]
- Fung, E.K.; Neuhauser, T.S.; Thompson, L.D. Hodgkin-like transformation of a marginal zone B-cell lymphoma of the larynx. Ann. Diagn. Pathol. 2002, 6, 61–66. [Google Scholar] [CrossRef][Green Version]
- Aguilera, N.S.; Howard, L.N.; Brissette, M.D.; Abbondanzo, S.L. Hodgkin’s disease and an extranodal marginal zone B-cell lymphoma in the small intestine: An unusual composite lymphoma. Mod. Pathol. 1996, 9, 1020–1026. [Google Scholar]
- Auditeau, C.; Lambotte, O.; Feriel, J.; Lazure, T.; Turhan, A.; Aumont, C. A composite lymphoma combining a Hodgkin lymphoma and a marginal zone lymphoma transformed into a diffuse large B-cell lymphoma. Clin. Case. Rep. 2018, 6, 2341–2346. [Google Scholar] [CrossRef]
- Geladari, E.; Dimopoulou, G.; Margellou, E.; Paraskevas, A.; Kafetzis, G.; Rontogianni, D.; Vadiaka, M. Coexistence of Hodgkin and Non-Hodgkin Lymphoma; Composite Lymphoma [CL] in a Patient Presenting with Waxing and Waning Lymphadenopathy. Cardiovasc. Hematol. Disord. Drug Targets 2020, 20, 160–163. [Google Scholar] [CrossRef] [PubMed]
- Wilson, W.H.; Pittaluga, S.; Nicolae, A.; Camphausen, K.; Shovlin, M.; Steinberg, S.M.; Roschewski, M.; Staudt, L.M.; Jaffe, E.S.; Dunleavy, K. A prospective study of mediastinal gray-zone lymphoma. Blood 2014, 124, 1563–1569. [Google Scholar] [CrossRef]
- Bellan, C.; Lazzi, S.; Zazzi, M.; Lalinga, A.V.; Palummo, N.; Galieni, P.; Marafioti, T.; Tonini, T.; Cinti, C.; Leoncini, L.; et al. Immunoglobulin gene rearrangement analysis in composite hodgkin disease and large B-cell lymphoma: Evidence for receptor revision of immunoglobulin heavy chain variable region genes in Hodgkin-Reed-Sternberg cells? Diagn. Mol. Pathol. 2002, 11, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Wei, E.X.; Flamholz, R.B.; Lowery-Nordberg, M.; Veillon, D.M.; Behm, W.; Heldmann, M.; Cotelingam, J.D. Pathology case of the month. A mediastinal mass. Malignant lymphoma, composite (nodular sclerosis Hodgkin lymphoma and diffuse large B-cell lymphoma). J. La. State. Med. Soc. 2004, 156, 294–297. [Google Scholar] [PubMed]
- Traverse-Glehen, A.; Pittaluga, S.; Gaulard, P.; Sorbara, L.; Alonso, M.A.; Raffeld, M.; Jaffe, E.S. Mediastinal gray zone lymphoma: The missing link between classic Hodgkin’s lymphoma and mediastinal large B-cell lymphoma. Am. J. Surg. Pathol. 2005, 29, 1411–1421. [Google Scholar] [CrossRef]
- Quintanilla-Martinez, L.; de Jong, D.; de Mascarel, A.; Hsi, E.D.; Kluin, P.; Natkunam, Y.; Parrens, M.; Pileri, S.; Ott, G. Gray zones around diffuse large B cell lymphoma. Conclusions based on the workshop of the XIV meeting of the European Association for Hematopathology and the Society of Hematopathology in Bordeaux, France. J. Hematop. 2009, 2, 211–236. [Google Scholar] [CrossRef]
- Oschlies, I.; Burkhardt, B.; Salaverria, I.; Rosenwald, A.; d’Amore, E.S.; Szczepanowski, M.; Koch, K.; Hansmann, M.L.; Stein, H.; Möller, P.; et al. Clinical, pathological and genetic features of primary mediastinal large B-cell lymphomas and mediastinal gray zone lymphomas in children. Haematologica 2011, 96, 262–268. [Google Scholar] [CrossRef]
- Eberle, F.C.; Rodriguez-Canales, J.; Wei, L.; Hanson, J.C.; Killian, J.K.; Sun, H.W.; Adams, L.G.; Hewitt, S.M.; Wilson, W.H.; Pittaluga, S.; et al. Methylation profiling of mediastinal gray zone lymphoma reveals a distinctive signature with elements shared by classical Hodgkin’s lymphoma and primary mediastinal large B-cell lymphoma. Haematologica 2011, 96, 558–566. [Google Scholar] [CrossRef]
- Yu, G.; Kong, L.; Qu, G.; Zhang, Q.; Wang, W.; Jiang, L. Composite lymphoma in the anterior mediastinum: A case report and review of the literature. Diagn. Pathol. 2011, 6, 60. [Google Scholar] [CrossRef][Green Version]
- Wang, H.W.; Yang, W.; Wang, L.; Lu, Y.L.; Lu, J.Y. Composite diffuse large B-cell lymphoma and classical Hodgkin’s lymphoma of the stomach: Case report and literature review. World J. Gastroenterol. 2013, 19, 6304–6309. [Google Scholar] [CrossRef]
- Khan, U.; Hadid, T.; Ibrar, W.; Sano, D.; Al-Katib, A. Composite Lymphoma: Opposite Ends of Spectrum Meet. J. Clin. Med. Res. 2017, 9, 213–215. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Goyal, G.; Nguyen, A.H.; Kendric, K.; Caponetti, G.C. Composite lymphoma with diffuse large B-cell lymphoma and classical Hodgkin lymphoma components: A case report and review of the literature. Pathol. Res. Pract. 2016, 212, 1179–1190. [Google Scholar] [CrossRef] [PubMed]
- Khanna, A.D.; Burkhart, H.M.; Manduch, M.; Feldman, A.L.; Inwards, D.J.; Connolly, H.M. Composite hodgkin and non-hodgkin lymphoma of the mitral and aortic valves. J. Am. Soc. Echocardiogr. 2010, 23, 1113. [Google Scholar] [CrossRef]
- Casey, T.T.; Cousar, J.B.; Mangum, M.; Williams, M.E.; Lee, J.T.; Greer, J.P.; Collins, R.D. Monomorphic lymphomas arising in patients with Hodgkin’s disease. Correlation of morphologic, immunophenotypic, and molecular genetic findings in 12 cases. Am. J. Pathol. 1990, 136, 81–94. [Google Scholar] [PubMed]
- Guarner, J.; del Rio, C.; Hendrix, L.; Unger, E.R. Composite Hodgkin’s and non-Hodgkin’s lymphoma in a patient with acquired immune deficiency syndrome. In-situ demonstration of Epstein-Barr virus. Cancer 1990, 66, 796–800. [Google Scholar] [CrossRef]
- Huang, Q.; Wilczynski, S.P.; Chang, K.L.; Weiss, L.M. Composite recurrent hodgkin lymphoma and diffuse large B-cell lymphoma: One clone, two faces. Am. J. Clin. Pathol. 2006, 126, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, R. Composite lymphoma of cervical lymph nodes with classical Hodgkin lymphoma and diffuse large B cell lymphoma: A case report and literature review. Ann. Palliat. Med. 2020, 9, 3651–3662. [Google Scholar] [CrossRef] [PubMed]
- Kerl, K.; Girardet, C.; Borisch, B. A common B-cell precursor in composite lymphomas. N. Engl. J. Med. 1999, 341, 764–765. [Google Scholar] [CrossRef]
- Perwein, T.; Lackner, H.; Ebetsberger-Dachs, G.; Beham-Schmid, C.; Zach, K.; Tamesberger, M.; Simonitsch-Klupp, I.; Lüftinger, R.; Dworzak, M.; Mann, G.; et al. Management of children and adolescents with gray zone lymphoma: A case series. Pediatr. Blood Cancer 2020, 67, 28206. [Google Scholar] [CrossRef]
- Miyagaki, T.; Sugaya, M.; Minatani, Y.; Fujita, H.; Hangaishi, A.; Kurokawa, M.; Takazawa, Y.; Tamaki, K. Mycosis fungoides with recurrent Hodgkin’s lymphoma and diffuse large B-cell lymphoma. Acta Derm. Venereol. 2009, 89, 421–422. [Google Scholar] [CrossRef]
- Paulli, M.; Rosso, R.; Kindl, S.; Boveri, E.; Sirchi, M.; De Medici, A.; Invernizzi, R.; Magrini, U. Nodular sclerosing Hodgkin’s disease and large cell lymphoma. Immunophenotypic characterization of a composite case. Virchows Arch. A Pathol. Anat. Histopathol. 1992, 421, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.Y.; Leung, A.Y.; Lau, W.H.; Loong, F.; So, J.C.; Tse, E.; Kwong, Y.L. Synchronous Epstein-Barr virus-positive diffuse large B-cell lymphoma of the elderly and Epstein-Barr virus-positive classical Hodgkin lymphoma. Histopathology 2011, 59, 352–355. [Google Scholar] [CrossRef] [PubMed]
- Hell, K.; Hansmann, M.L.; Pringle, J.H.; Lauder, I.; Fischer, R. Combination of Hodgkin’s disease and diffuse large cell lymphoma: An in situ hybridization study for immunoglobulin light chain messenger RNA. Histopathology 1995, 27, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Soliman, D.S.; Fareed, S.; Alkuwari, E.; El-Omri, H.; Al-Sabbagh, A.; Gameel, A.; Yassin, M. Concomitant Classic Hodgkin Lymphoma of Lymph Node and cMYC-Positive Burkitt Leukemia/Lymphoma of the Bone Marrow Presented Concurrently at the Time of Presentation: A Rare Combination of Discordant Lymphomas. Clin. Med. Insights Blood Disord. 2016, 9, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Song, J.Y.; Eberle, F.C.; Xi, L.; Raffeld, M.; Rahma, O.; Wilson, W.H.; Dunleavy, K.; Pittaluga, S.; Jaffe, E.S. Coexisting and clonally identical classic hodgkin lymphoma and nodular lymphocyte predominant hodgkin lymphoma. Am. J. Surg. Pathol. 2011, 35, 767–772. [Google Scholar] [CrossRef] [PubMed]
- Szczepanowski, M.; Masqué-Soler, N.; Oschlies, I.; Schmidt, W.; Lück, A.; Klapper, W. Composite lymphoma of nodular lymphocyte-predominant and classical Hodgkin lymphoma-Epstein-Barr virus association suggests divergent pathogenesis despite clonal relatedness. Human. Pathol. 2013, 44, 1434–1439. [Google Scholar] [CrossRef]
- Gelb, A.B.; Dorfman, R.F.; Warnke, R.A. Coexistence of nodular lymphocyte predominance Hodgkin’s disease and Hodgkin’s disease of the usual type. Am. J. Surg. Pathol. 1993, 17, 364–374. [Google Scholar] [CrossRef]
- Ichikawa, A.; Miyoshi, H.; Yamauchi, T.; Arakawa, F.; Kawano, R.; Muta, H.; Sugita, Y.; Akashi, K.; Ohshima, K. Composite lymphoma of peripheral T-cell lymphoma and Hodgkin lymphoma, mixed cellularity type; pathological and molecular analysis. Pathol. Int. 2017, 67, 194–201. [Google Scholar] [CrossRef]
- Gualco, G.; Chioato, L.; Van Den Berg, A.; Weiss, L.M.; Bacchi, C.E. Composite lymphoma: EBV-positive classic Hodgkin lymphoma and peripheral T-cell lymphoma: A case report. App. Immunohistochem. Mol. Morphol. 2009, 17, 72–76. [Google Scholar] [CrossRef]
- Sanchez, S.; Holmes, H.; Katabi, N.; Newman, J.; Domiatti-Saad, R.; Stone, M.; Netto, G. Composite lymphocyte-rich Hodgkin lymphoma and peripheral T-cell lymphoma associated with Epstein-Barr virus: A case report and review of the literature. Arch. Pathol. Lab. Med. 2006, 130, 107–112. [Google Scholar] [CrossRef]
- Brown, J.R.; Weng, A.P.; Freedman, A.S. Hodgkin disease associated with T-cell non-Hodgkin lymphomas: Case reports and review of the literature. Am. J. Clin. Pathol. 2004, 121, 701–708. [Google Scholar] [CrossRef] [PubMed]
- Wlodarska, I.; Delabie, J.; De Wolf-Peeters, C.; Mecucci, C.; Stul, M.; Verhoef, G.; Cassiman, J.J.; Van den Berghe, H. T-cell lymphoma developing in Hodgkin’s disease: Evidence for two clones. J. Pathol. 1993, 170, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Gui, W.; Wang, J.; Ma, L.; Wang, Y.; Su, L. Clinicopathological analysis of composite lymphoma: A two-case report and literature review. Open Med. 2020, 15, 654–658. [Google Scholar] [CrossRef] [PubMed]
- Niino, D.; My Hanh, L.T.; Miura, S.; Nakashima, M.; Iwanaga, M. Incidence Patterns of Sequential or Composite Lymphoma: A Population-Based Cancer Registry Study. Tohoku. J. Exp. Med. 2021, 254, 123–127. [Google Scholar] [CrossRef]
- Rassidakis, G.Z.; Medeiros, L.J.; Viviani, S.; Bonfante, V.; Nadali, G.P.; Vassilakopoulos, T.P.; Mesina, O.; Herling, M.; Angelopoulou, M.K.; Giardini, R.; et al. CD20 expression in Hodgkin and Reed-Sternberg cells of classical Hodgkin’s disease: Associations with presenting features and clinical outcome. J. Clin. Oncol. 2002, 20, 1278–1287. [Google Scholar] [PubMed]
- Qin, Y.; Kang, S.Y.; He, X.H.; Zhou, S.Y.; Liu, P.; Yang, J.L.; Zhang, C.G.; Yang, S.; Gui, L.; Shi, Y.K. Clinical features and prognosis of CD20-positive classical Hodgkin lymphoma. Zhonghua Yi Xue Za Zhi 2016, 96, 2224–2228. [Google Scholar]
- Benharroch, D.; Nalbandyan, K.; Lazarev, I. CD20 Over-Expression in Hodgkin-Reed-Sternberg Cells of Classical Hodgkin Lymphoma: The Neglected Quest. J. Cancer 2015, 6, 1155–1159. [Google Scholar] [CrossRef]
- Abuelgasim, K.A.; Shammari, R.A.; Alshieban, S.; Alahmari, B.; Alzahrani, M.; Alhejazi, A.; Alaskar, A.; Damlaj, M. Impact of cluster of differentiation 20 expression and rituximab therapy in classical Hodgkin lymphoma: Real world experience. Leuk. Res. Rep. 2021, 15, 100240. [Google Scholar] [CrossRef]
- Tan, L.H. A practical approach to the understanding and diagnosis of lymphoma: An assessment of the WHO classification based on immunoarchitecture and immuno-ontogenic principles. Pathology 2009, 41, 305–326. [Google Scholar]
- Kridel, R.; Mottok, A.; Farinha, P.; Ben-Neriah, S.; Ennishi, D.; Zheng, Y.; Chavez, E.A.; Shulha, H.P.; Tan, K.; Chan, F.C.; et al. Cell of origin of transformed follicular lymphoma. Blood 2015, 126, 2118–2127. [Google Scholar] [CrossRef]
- Magnano, L.; Balagué, O.; Dlouhy, I.; Rovira, J.; Karube, K.; Pinyol, M.; Rivas-Delgado, A.; Costa, D.; Martínez-Trillos, A.; González-Farre, B.; et al. Clinicobiological features and prognostic impact of diffuse large B-cell lymphoma component in the outcome of patients with previously untreated follicular lymphoma. Ann. Oncol. 2017, 28, 2799–2805. [Google Scholar] [CrossRef] [PubMed]
- Feldman, A.L.; Arber, D.A.; Pittaluga, S.; Martinez, A.; Burke, J.S.; Raffeld, M.; Camos, M.; Warnke, R.; Jaffe, E.S. Clonally related follicular lymphomas and histiocytic/dendritic cell sarcomas: Evidence for transdifferentiation of the follicular lymphoma clone. Blood 2008, 111, 5433–5439. [Google Scholar] [CrossRef]
- Ohshima, K.; Kikuchi, M.; Yoshida, T.; Masuda, Y.; Kimura, N. Lymph nodes in incipient adult T-cell leukemia-lymphoma with Hodgkin’s disease-like histologic features. Cancer 1991, 67, 1622–1628. [Google Scholar] [CrossRef]
- Quintanilla-Martinez, L.; Fend, F.; Moguel, L.R.; Spilove, L.; Beaty, M.W.; Kingma, D.W.; Raffeld, M.; Jaffe, E.S. Peripheral T-cell lymphoma with Reed-Sternberg-like cells of B-cell phenotype and genotype associated with Epstein-Barr virus infection. Am. J. Surg. Pathol. 1999, 23, 1233–1240. [Google Scholar] [CrossRef]
- Kang, M.S.; Kieff, E. Epstein-Barr virus latent genes. Exp. Mol. Med. 2015, 47, 131. [Google Scholar] [CrossRef] [PubMed]
- Küppers, R. Mechanisms of B-cell lymphoma pathogenesis. Nat. Rev. Cancer 2005, 5, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Farrell, K.; Jarrett, R.F. The molecular pathogenesis of Hodgkin lymphoma. Histopathology 2011, 58, 15–25. [Google Scholar] [CrossRef]
- Spina, V.; Rossi, D. Molecular pathogenesis of splenic and nodal marginal zone lymphoma. Best Pract. Res. Clin. Haematol. 2017, 30, 5–12. [Google Scholar] [CrossRef]
- Donzel, M.; Baseggio, L.; Fontaine, J.; Pesce, F.; Ghesquières, H.; Bachy, E.; Verney, A.; Traverse-Glehen, A. New Insights into the Biology and Diagnosis of Splenic Marginal Zone Lymphomas. Curr. Oncol. 2021, 28, 3430–3447. [Google Scholar] [CrossRef]
- Saarinen, S.; Pukkala, E.; Vahteristo, P.; Mäkinen, M.J.; Franssila, K.; Aaltonen, L.A. High familial risk in nodular lymphocyte-predominant Hodgkin lymphoma. J. Clin. Oncol. 2013, 31, 938–943. [Google Scholar] [CrossRef]
- Wiernik, P.H.; Wickramasinghe, D.; Dutcher, J.P. Families with both Hodgkin lymphoma and multiple myeloma in their pedigrees. Clin. Adv. Hematol. Oncol. 2015, 13, 257–260. [Google Scholar] [PubMed]
- Kharazmi, E.; Fallah, M.; Pukkala, E.; Olsen, J.H.; Tryggvadottir, L.; Sundquist, K.; Tretli, S.; Hemminki, K. Risk of familial classical Hodgkin lymphoma by relationship, histology, age, and sex: A joint study from five Nordic countries. Blood 2015, 126, 1990–1995. [Google Scholar] [CrossRef] [PubMed]
- Köse, D.; Güzelçiçek, A.; Öz, Ö.; Erdem, A.Y.; Haliloğlu, Y.; Witzel, M.; Klein, C.; Ünal, E. The Mutation of CD27 Deficiency Presented With Familial Hodgkin Lymphoma and a Review of the Literature. J. Pediatr. Hematol. Oncol. 2022, 44, 833–843. [Google Scholar] [CrossRef]
- Kuhlen, M.; Hönscheid, A.; Schemme, J.; Merz, H.; Mauz-Körholz, C.; Borkhardt, A.; Troeger, A. Hodgkin lymphoma as a novel presentation of familial DICER1 syndrome. Eur. J. Pediatr. 2016, 175, 593–597. [Google Scholar] [CrossRef]
- Bandapalli, O.R.; Paramasivam, N.; Giangiobbe, S.; Kumar, A.; Benisch, W.; Engert, A.; Witzens-Harig, M.; Schlesner, M.; Hemminki, K.; Försti, A. Whole genome sequencing reveals DICER1 as a candidate predisposing gene in familial Hodgkin lymphoma. Int. J. Cancer 2019, 143, 2076–2078. [Google Scholar] [CrossRef] [PubMed]
- Harty, L.C.; Lin, A.Y.; Goldstein, A.M.; Jaffe, E.S.; Carrington, M.; Tucker, M.A.; Modi, W.S. HLA-DR, HLA-DQ, and TAP genes in familial Hodgkin disease. Blood 2002, 99, 690–693. [Google Scholar] [CrossRef] [PubMed]
- Salipante, S.J.; Mealiffe, M.E.; Wechsler, J.; Krem, M.M.; Liu, Y.; Namkoong, S.; Bhagat, G.; Kirchhoff, T.; Offit, K.; Lynch, H.; et al. Mutations in a gene encoding a midbody kelch protein in familial and sporadic classical Hodgkin lymphoma lead to binucleated cells. Proc. Natl. Acad. Sci. USA 2009, 106, 14920–14925. [Google Scholar] [CrossRef]
- Saarinen, S.; Aavikko, M.; Aittomäki, K.; Launonen, V.; Lehtonen, R.; Franssila, K.; Lehtonen, H.J.; Kaasinen, E.; Broderick, P.; Tarkkanen, J.; et al. Exome sequencing reveals germline NPAT mutation as a candidate risk factor for Hodgkin lymphoma. Blood 2011, 118, 493–498. [Google Scholar] [CrossRef]
- Ristolainen, H.; Kilpivaara, O.; Kamper, P.; Taskinen, M.; Saarinen, S.; Leppä, S.; d’Amore, F.; Aaltonen, L.A. Identification of homozygous deletion in ACAN and other candidate variants in familial classical Hodgkin lymphoma by exome sequencing. Br. J. Haematol. 2015, 170, 428–431. [Google Scholar] [CrossRef]
- Rotunno, M.; McMaster, M.L.; Boland, J.; Bass, S.; Zhang, X.; Burdett, L.; Hicks, B.; Ravichandran, S.; Luke, B.T.; Yeager, M.; et al. Whole exome sequencing in families at high risk for Hodgkin lymphoma: Identification of a predisposing mutation in the KDR gene. Haematologica 2016, 101, 853–860. [Google Scholar] [CrossRef]
- Tao, Y.; Chen, H.; Liu, D.; Dai, X. Survival among patients with composite and sequential lymphoma between primary mediastinal lymphoma/diffuse large B-cell lymphoma and classical Hodgkin lymphoma: A population-based study. Leuk. Res. 2021, 111, 106669. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Lezama, L.S.; Kurzer, J.; Oak, J.; Schultz, L.M.; Walkush, A.; Cheng, T.C.; Chen, E.H.; May, W.A.; Chang, C.; et al. Targeted Mutational Profiling Reveals Clonal Relationships in Metachronous Occurrence of Classic Hodgkin and Mediastinal Large B-Cell Lymphomas. Am. J. Surg. Pathol. 2022; in press. [Google Scholar] [CrossRef] [PubMed]
- Seitz, V.; Hummel, M.; Marafioti, T.; Anagnostopoulos, I.; Assaf, C.; Stein, H. Detection of clonal T-cell receptor gamma-chain gene rearrangements in Reed-Sternberg cells of classic Hodgkin disease. Blood 2000, 95, 3020–3024. [Google Scholar] [CrossRef]
- Müschen, M.; Rajewsky, K.; Bräuninger, A.; Baur, A.S.; Oudejans, J.J.; Roers, A.; Hansmann, M.L.; Küppers, R. Rare occurrence of classical Hodgkin’s disease as a T cell lymphoma. J. Exp. Med. 2000, 191, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Davis, T.H.; Morton, C.C.; Miller-Cassman, R.; Balk, S.P.; Kadin, M.E. Hodgkin’s disease, lymphomatoid papulosis, and cutaneous T-cell lymphoma derived from a common T-cell clone. N. Engl. J. Med. 1992, 326, 1115–1122. [Google Scholar] [CrossRef]
- Moroch, J.; Copie-Bergman, C.; de Leval, L.; Plonquet, A.; Martin-Garcia, N.; Delfau-Larue, M.H.; Molinier-Frenkel, V.; Belhadj, K.; Haioun, C.; Audouin, J.; et al. Follicular peripheral T-cell lymphoma expands the spectrum of classical Hodgkin lymphoma mimics. Am. J. Surg. Pathol. 2012, 36, 1636–1646. [Google Scholar] [CrossRef]
- Nicolae, A.; Pittaluga, S.; Venkataraman, G.; Vijnovich-Baron, A.; Xi, L.; Raffeld, M.; Jaffe, E.S. Peripheral T-cell lymphomas of follicular T-helper cell derivation with Hodgkin/Reed-Sternberg cells of B-cell lineage: Both EBV-positive and EBV-negative variants exist. Am. J. Surg. Pathol. 2013, 37, 816–826. [Google Scholar] [CrossRef]
- Haefliger, S.; Bihl, M.; Krasniqi, F.; Tzankov, A. PET-positive bone lesion due to Langerhans cell histiocytosis after BEACOPP therapy for Hodgkin lymphoma: How anamnesis, histopathological accuracy, and molecular analysis could resolve a clinical dilemma. Ann. Hematol. 2018, 97, 355–357. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
| Clinical, Laboratory, and Pathological Data | cHL/Follicular Lymphoma | cHL/Mantle Cell Lymphoma | cHL/Marginal Zone Lymphoma | cHL/DLBCL | cHL/NLPHL | cHL/T-Cell Lymphoma |
|---|---|---|---|---|---|---|
| Number of cases included | 76 | 11 | 21 | 79 | 3 | 11 |
| Median age at presentation | 59.5 | 67.5 | 68.5 | 58 | 24 | 57 |
| M/F ratio | 2 | 4 | 4 | 1.5 | 3/0 | 2.7 |
| History of lymphoma, % | ||||||
| Yes | 7.9 (6/76) | 54.5 (6/11) | 14.3 (3/21) | 6.3 (5/79) | 0 (0/3) | 27.3 (3/11) |
| No | 59.2 (45/76) | 45.5 (5/11) | 76.2 (16/21) | 57 (45/79) | 100 (3/3) | 72.7 (8/11) |
| NA | 32.9 (25/76) | 0 (0/11) | 9.5 (2/21) | 36.7 (29/79) | 0 (0/3) | 0 (0/11) |
| Location, % | ||||||
| Unique | 6.6 (5/76) | 9.1 (1/11) | 9.5 (2/21) | 10.1 (8/79) | 0 (0/3) | 9.1 (1/11) |
| Multiple | 34.2 (26/76) | 81.8 (9/11) | 76.2 (16/21) | 48.1 (38/79) | 66.7 (2/3) | 45.5 (5/11) |
| NA | 59.2 (45/76) | 9.1 (1/11) | 14.3 (3/21) | 41.8 (33/79) | 33.3 (1/3) | 45.5 (5/11) |
| Median size of larger LN/tumor (cm) | 4.15 | 3 | 3 | 3.75 | 3 | 3.05 |
| B symptoms, % | ||||||
| Yes | 21 (16/76) | 9.1 (1/11) | 9.5 (2/21) | 11.4 (9/79) | 33.3 (1/3) | 27.3 (3/11) |
| No | 25 (19/76) | 72.7 (8/11) | 76.2 (16/21) | 26.6 (21/79) | 66.7 (2/3) | 18.2 (2/11) |
| NA | 53.9 (41/76) | 18.2 (2/11) | 14.3 (3/21) | 62 (49/79) | 0 (0/3) | 54.5 (6/11) |
| LDH dosage, % | ||||||
| increased | 3.9 (3/76) | 9.1 (1/11) | 0 (0/21) | 5.1 (4/79) | 0 (0/3) | 9.1 (1/11) |
| normal range | 26.3 (20/76) | 9.1 (1/11) | 4.8 (1/21) | 2.5 (2/79) | 0 (0/3) | 9.1 (1/11) |
| NA | 69.7 (53/76) | 81.8 (9/11) | 95.2 (20/21) | 92.4 (73/79) | 100 (3/3) | 81.8 (9/11) |
| Ann Arbor stage, % | ||||||
| I/II | 18.4 (14/76) | 27.3 (3/11) | 9.5 (2/21) | 13.9 (11/79) | 33.3 (1/3) | 27.3 (3/11) |
| III/IV | 36.8 (28/76) | 63.6 (7/11) | 66.7 (14/21) | 21.5 (17/79) | 66.7 (2/3) | 45.5 (5/11) |
| NA | 44.7 (34/76) | 9.1 (1/11) | 23.8 (5/21) | 64.6 (51/79) | 0 (0/3) | 27.3 (3/11) |
| BM involvement, % | ||||||
| Yes | 19.7 (15/76) | 27.3 (3/11) | 14.3 (3/21) | 10.1 (8/79) | 0 (0/3) | 18.2 (2/11) |
| No | 13.2 (10/76) | 0 (0/11) | 4.8 (1/21) | 2.5 (2/79) | 66.7 (2/3) | 9.1 (1/11) |
| NA | 67.1 (51/76) | 72.7 (8/11) | 80.9 (17/21) | 87.3 (69/79) | 33.3 (1/3) | 72.7 (8/11) |
| cHL type, % | ||||||
| cHL-NS | 23.7 (18/76) | 9.1 (1/11) | 4.8 (1/21) | 53.2 (42/79) | 33.3 (1/3) | 9.1 (1/11) |
| cHL-MC/granulomatous | 60.5 (46/76) | 9.1 (1/11) | 57.1 (12/21) | 21.5 (17/79) | 66.7 (2/3) | 45.5 (5/11) |
| cHL-LR | 0 (0/76) | 18.2 (2/11) | 0 (0/21) | 1.3 (1/79) | 0 (0/3) | 0 (0/11) |
| unclassified | 15.8 (12/76) | 63.6 (7/11) | 38.1 (8/21) | 24 (19/79) | 0 (0/3) | 45.5 (5/11) |
| Location of both contingents, % | ||||||
| separated | 46.1 (35/76) | 9.1 (1/11) | 42.9 (9/21) | 34.2 (27/79) | 100 (3/3) | 9.1 (1/11) |
| mixed | 19.7 (15/76) | 54.5 (6/11) | 14.3 (3/21) | 0 (0/69) | 0 (0/3) | 9.1 (1/11) |
| NA | 34.2 (26/76) | 36.4 (4/11) | 42.9 (9/21) | 65.8 (52/79) | 0 (0/3) | 81.8 (9/11) |
| Outcome, % | ||||||
| CR or PR but no relapse (alive) | 28.9 (22/76) | 18.2 (2/11) | 38.1 (8/21) | 20.3 (16/79) | 66.7 (2/3) | 36.4 (4/11) |
| Relapse and/or DOD | 25 (19/76) | 9.1 (1/11) | 23.8 (5/21) | 11.4 (9/79) | 0 (0/3) | 18.2 (2/11) |
| Death from other cause | 0 (0/76) | 18.2 (2/11) | 9.5 (2/21) | 2.5 (2/79) | 0 (0/3) | 0 (0/11) |
| NA | 46.1 (35/76) | 54.5 (6/11) | 28.6 (6/21) | 65.8 (52/79) | 33.3 (1/3) | 45.5 (5/11) |
| Median follow-up duration, months | 23.5 | NA | 12 | 12 | NA | 30 |
| Median time of relapse/DOD, months | 14 | NA | 27.5 | 7 | NA | NA |
| Treatment (First Line) | cHL/Follicular Composite Lymphoma | cHL/Mantle Cell Composite Lymphoma | cHL/Marginal Zone Composite Lymphoma | cHL/DLBCL | cHL/NLPHL | cHL/T-Cell Composite Lymphoma |
|---|---|---|---|---|---|---|
| Data available, % | 52.6 (40/76) | 54.5 (6/11) | 81 (17/21) | 50.6 (40/79) | 66.7 (2/3) | 63.6 (7/11) |
| cHL-like chemotherapy, % | 25 (10/40) | - | 17.6 (3/17) | 5 (2/40) | 50 (1/2) | 42.9 (3/7) |
| Relapse and/or DOD | 50 (5/10) * | - | 33.3 (1/3) | 50 (1/2) | - | 33.3 (1/3) |
| Alive in remission | 50 (5/10) | - | 66.7 (2/3) | - | 100 (1/1) | 66.7 (2/3) |
| Loss of follow-up/NA | - | - | - | 50 (1/2) | - | - |
| Small/diffuse B-cell lymphoma-like chemotherapy, % | 30 (12/40) | 66.7 (4/6) | 47.1 (8/17) | 60 (24/40) | - | - |
| Relapse and/or DOD | 41.7 (5/12) ** | 25 (1/4) | 25 (2/8) | 16.7 (4/24) | - | - |
| Alive in remission | 41.7 (5/12) | 50 (2/4) | 62.5 (5/8) | 41.7 (10/24) | - | - |
| Loss of follow-up/NA | 16.7 (2/12) | - | 12.5 (1/8) | 37.5 (9/24) | - | - |
| Death from another cause | - | 25 (1/4) | - | 4.2 (1/24) | - | - |
| T-cell lymphoma-like chemotherapy, % | - | - | - | - | - | 14.3 (1/7) |
| Loss of follow-up/NA | - | - | - | - | - | 100 (1/1) |
| Composite lymphoma-like chemotherapy, % | 35 (14/40) | 16.7 (1/6) | 11.8 (2/17) | 20 (8/40) | - | 42.9 (3/7) |
| Relapse and/or DOD | 35.7 (5/14) *** | - | 50 (1/2) | 37.5 (3/8) | - | - |
| Alive in remission | 57.1 (8/14) | - | - | 25 (2/8) | - | 66.7 (2/3) |
| Loss of follow-up/NA | 7.1 (1/14) | - | - | 37.5 (3/8) | - | - |
| Death from another cause | - | 100 (1/1) | 50 (1/2) | - | - | 33.3 (1/3) |
| Radiotherapy only, % | 2.5 (1/40) | - | 11.8 (2/17) | 2.5 (1/40) | 50 (1/2) | - |
| Relapse and/or DOD | - | - | - | - | - | - |
| Alive in remission | 100 (1/1) | - | 50 (1/2) | 100 (1/1) | 100 (1/1) | - |
| Loss of follow-up/NA | - | - | 50 (1/2) | - | - | - |
| No treatment, % | 7.5 (3/40) | - | - | - | - | - |
| Relapse and/or DOD | 66.7 (2/3) | - | - | - | - | - |
| Alive in remission | - | - | - | - | - | - |
| Loss of follow-up/NA | 33.3 (1/3) | - | - | - | - | - |
| Other treatment, % | - | 16.7 (1/6) ¥ | 11.8 (2/17) Ψ | 12.5 (5/40) £ | - | - |
| Markers | cHL | Follicular Lymphoma | cHL | Mantle Cell Lymphoma | cHL | Marginal Zone Lymphoma | cHL | DLBCL | cHL | NLPHL | cHL | T-Cell Lymphoma |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CD30 **, % | 100 (59/59) | 0 (0/21) | 100 (10/10) | 0 (0/4) | 100 (19/19) | 6.7 (1/15) * | 100 (46/46) | 29 (9/31) * | 100 (3/3) | 33.3 (1/3) * | 100 (10/10) | 0 (0/4) |
| CD15, % | 62.1 (41/66) | 0 (0/21) | 90 (9/10) | 0 (0/4) | 73.7 (14/19) | 0 (0/14) | 81.8 (45/55) | 2.4 (1/42) * | 100 (3/3) | 0 (0/3) | 80 (8/10) | 0 (0/3) |
| CD45/LCA, % | 10.7 (3/28) | 100 (24/24) | 25 (1/4) * | 100 (4/4) | 0 (0/16) | 92.9 (13/14) | 7.7 (2/26) | 100 (29/29) | NA | 50 (1/2) | 0 (0/3) | 100 (2/2) |
| CD20, % | 33.3 (20/60) * | 100 (56/56) | 33.3 (3/10) * | 100 (9/9) | 55.6 (10/18) * | 100 (19/19) | 27.7 (13/47) * | 96.7 (59/61) | 50 (1/2) * | 100 (2/2) | 22.2 (2/9) | NA |
| CD79a, % | 42.9 (6/14) * | 85.7 (6/7) | 33.3 (1/3) | 100 (4/4) | 7.7 (1/13) * | 100 (12/12) | 5.9 (1/17) | 93.8 (15/16) | NA | NA | NA | NA |
| PAX5, % | 97.8 (45/46) | 87.5 (7/8) | 100 (5/5) | 100 (4/4) | 66.7 (2/3) | NA | 84.6 (11/13) | 100 (11/11) | 100 (2/2) | 100 (2/2) | 100 (6/6) | NA |
| MUM1, % | 100 (13/13) | 22.2 (2/9) | NA | NA | 100 (7/7) | 57.1 (4/7) | 90 (9/10) | 75 (8/12) | NA | NA | NA | NA |
| OCT2, % | 46.1 (6/13) | 80 (4/5) | 75 (3/4) | 100 (2/2) | 0 (0/4) | NA | 37.5 (3/8) | 100 (5/5) | 50 (1/2) | 100 (2/2) | NA | NA |
| BOB1, % | 38.5 (5/13) | 60 (3/5) | 33.3 (1/3) | NA | 0 (0/4) | NA | 28.6 (2/7) | 100 (6/6) | NA | NA | NA | NA |
| EBER, % | 26.2 (11/42) | 0 (0/18) | 75 (6/8) | 0 (0/5) | 58.3 (7/12) | 7.1 (1/14) | 39.2 (20/51) | 29.2 (14/48) | 50 (1/2) | 0 (0/3) | 33.3 (3/9) | 0 (0/5) |
| LMP-1, % | NA | NA | 100 (4/4) | 0 (0/3) | 53.3 (8/15) | 7.7 (1/13) | 54.5 (6/11) | 10 (1/10) | NA | NA | NA | NA |
| BCL2, % | 48.5 (16/33) | 95.8 (46/48) | 25 (1/4) | 85.7 (6/7) | 88.9 (8/9) | 100 (11/11) | 87.5 (7/8) | 75 (9/12) | NA | NA | NA | NA |
| BCL6, % | 43.3 (13/30) | 100 (37/37) | 0 (0/3) | 0 (0/5) | NA | NA | 20 (1/5) | 33.3 (5/15) | 0 (0/2) | 100 (2/2) | NA | NA |
| CD10, % | 6.7 (2/30) | 92.7 (38/41) | 0 (0/2) | 0 (0/5) | NA | NA | 0 (0/6) | 17.6 (3/17) | NA | NA | NA | 0 (0/2) |
| Cyclin D1, % | NA | NA | 42.9 (3/7) | 100 (10/10) | NA | 0 (0/2) | 0 (0/2) | NA | NA | NA | NA | NA |
| SOX11, % | NA | NA | 50 (1/2) | 100 (2/2) | NA | NA | NA | NA | NA | NA | NA | NA |
| Kappa light chain, % | NA | NA | NA | NA | 0 (0/11) | 33.3 (4/12) | NA | NA | NA | NA | NA | NA |
| Lambda light chain, % | NA | NA | NA | NA | 0 (0/11) | 16.7 (2/12) | NA | NA | NA | NA | NA | NA |
| MAL, % | NA | NA | NA | NA | NA | NA | 0 (0/2) | 100 (7/7) *** | NA | NA | NA | NA |
| ALK-1, % | NA | NA | 0 (0/2) | NA | NA | 0 (0/2) | 0 (0/2) | NA | NA | NA | NA | NA |
| CD23, % | NA | NA | 0 (0/2) | 0 (0/8) | NA | NA | NA | NA | NA | NA | NA | NA |
| CD2, % | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | 100 (2/2) |
| CD3, % | NA | NA | 0 (0/3) | 0 (0/2) | 0 (0/2) | 0 (0/2) | 0 (0/11) | 0 (0/11) | NA | NA | 0 (0/3) | 100 (11/11) |
| CD4, % | NA | NA | NA | NA | NA | NA | 0 (0/2) | NA | NA | NA | NA | 50 (4/8) |
| CD5, % | NA | NA | 20 (1/5) * | 50 (4/8) | 0 (0/2) | 0 (0/3) | 0 (0/4) | 0 (0/4) | NA | NA | NA | 60 (3/5) |
| CD7, % | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | 33.3 (1/3) |
| CD8, % | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | 66.7 (6/9) |
| p53, % | NA | NA | 100 (2/2) | 50 (1/2) | 100 (7/7) | 0 (0/8) | 100 (6/6) | 16.7 (1/6) | NA | NA | NA | NA |
| MYC, % | NA | NA | NA | NA | NA | NA | NA | 100 (2/2) | NA | NA | NA | NA |
| TIA1, % | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | 83.3 (5/6) |
| Granzyme B, % | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | 0 (0/6) |
| FOXP3, % | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | 50 (1/2) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trecourt, A.; Donzel, M.; Fontaine, J.; Ghesquières, H.; Jallade, L.; Antherieu, G.; Laurent, C.; Mauduit, C.; Traverse-Glehen, A. Plasticity in Classical Hodgkin Composite Lymphomas: A Systematic Review. Cancers 2022, 14, 5695. https://doi.org/10.3390/cancers14225695
Trecourt A, Donzel M, Fontaine J, Ghesquières H, Jallade L, Antherieu G, Laurent C, Mauduit C, Traverse-Glehen A. Plasticity in Classical Hodgkin Composite Lymphomas: A Systematic Review. Cancers. 2022; 14(22):5695. https://doi.org/10.3390/cancers14225695
Chicago/Turabian StyleTrecourt, Alexis, Marie Donzel, Juliette Fontaine, Hervé Ghesquières, Laurent Jallade, Gabriel Antherieu, Camille Laurent, Claire Mauduit, and Alexsandra Traverse-Glehen. 2022. "Plasticity in Classical Hodgkin Composite Lymphomas: A Systematic Review" Cancers 14, no. 22: 5695. https://doi.org/10.3390/cancers14225695
APA StyleTrecourt, A., Donzel, M., Fontaine, J., Ghesquières, H., Jallade, L., Antherieu, G., Laurent, C., Mauduit, C., & Traverse-Glehen, A. (2022). Plasticity in Classical Hodgkin Composite Lymphomas: A Systematic Review. Cancers, 14(22), 5695. https://doi.org/10.3390/cancers14225695





