Immunotherapy as a Promising Option for the Treatment of Advanced Chordoma: A Systemic Review
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
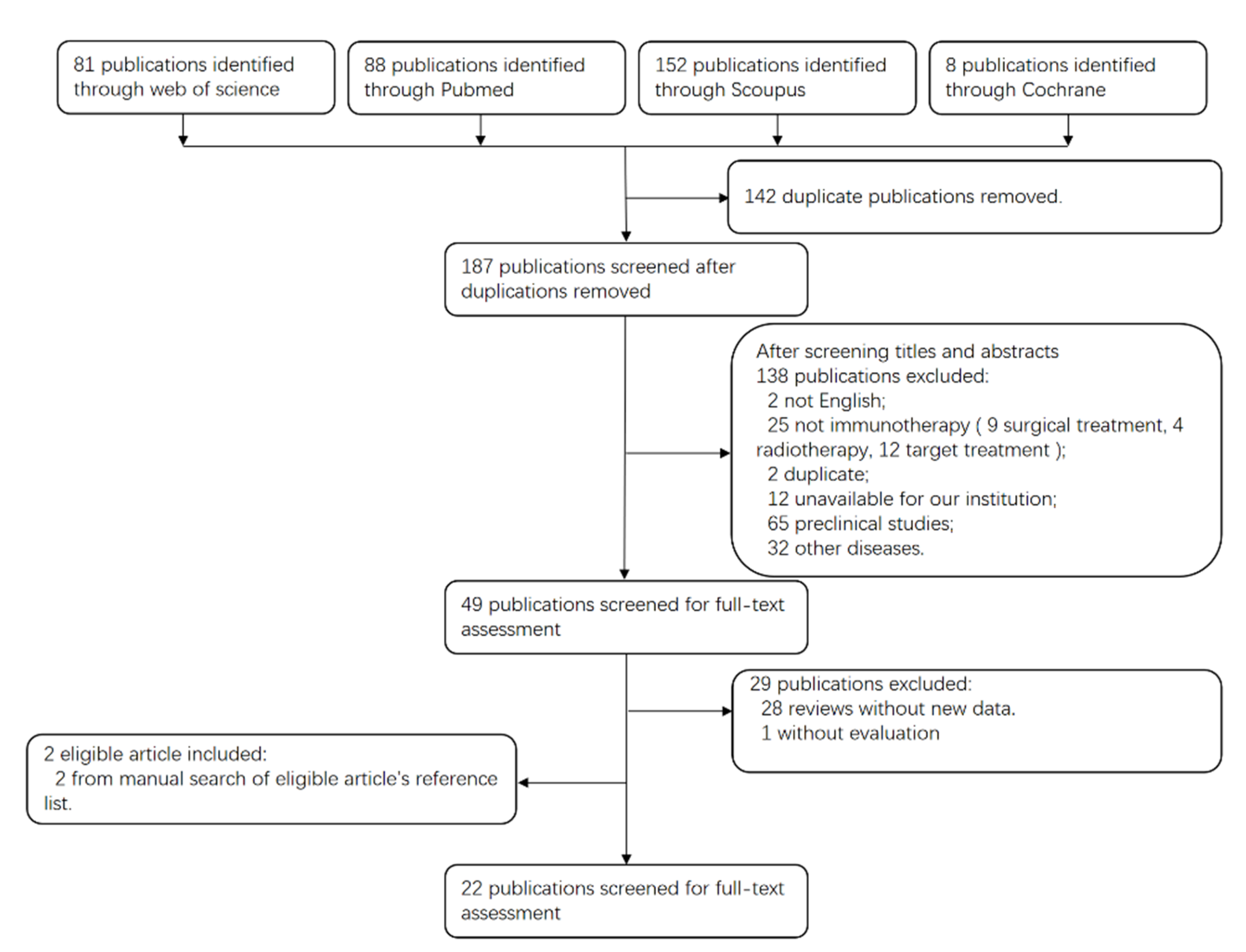
3.1. Search Results
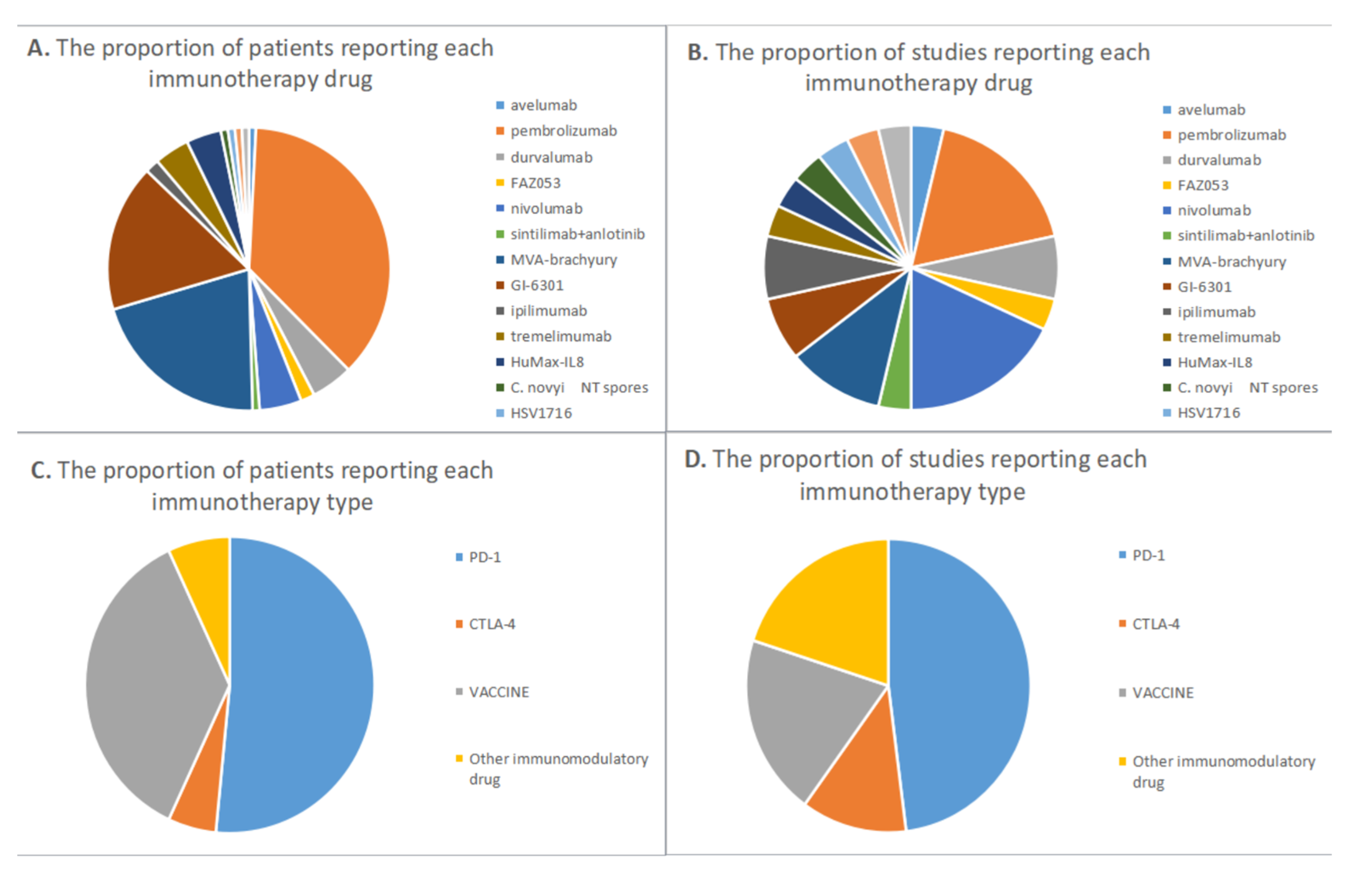
3.2. Study Characteristics
3.3. Efficacy and Safety of Immunotherapy in Chordoma Patients
3.4. PD-1/PD-L1 Immune Checkpoint Inhibitors
3.5. CTLA-4 Immune Checkpoint Inhibitor
3.6. Cancer Vaccine
3.7. Other Immunomodulatory Drugs
4. Discussion
4.1. Indications and Evaluation Criteria for Immunotherapy
4.2. Cancer Vaccines
4.3. Immune Checkpoint Inhibitor
4.3.1. PD-1/PD-L1
4.3.2. CTLA-4
4.3.3. Other Targets
4.3.4. Prediction of ICIs Response
4.4. Cell Therapy
5. Limitations
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Boriani, S.; Bandiera, S.; Biagini, R.; Bacchini, P.; Boriani, L.; Cappuccio, M.; Chevalley, F.; Gasbarrini, A.; Picci, P.; Weinstein, J.N. Chordoma of the mobile spine: Fifty years of experience. Spine 2006, 31, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Stiller, C.A.; Trama, A.; Serraino, D.; Rossi, S.; Navarro, C.; Chirlaque, M.D.; Casali, P.G.; Group, R.W. Descriptive epidemiology of sarcomas in Europe: Report from the RARECARE project. Eur. J. Cancer 2013, 49, 684–695. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Classification of Tumours of Soft Tissue and Bone: WHO Classification of Tumours; World Health Organization: Geneva, Switzerland, 2013; Volume 5.
- McMaster, M.L.; Goldstein, A.M.; Bromley, C.M.; Ishibe, N.; Parry, D.M. Chordoma: Incidence and survival patterns in the United States, 1973–1995. Cancer Causes Control 2001, 12, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Walcott, B.P.; Nahed, B.V.; Mohyeldin, A.; Coumans, J.V.; Kahle, K.T.; Ferreira, M.J. Chordoma: Current concepts, management, and future directions. Lancet Oncol. 2012, 13, e69–e76. [Google Scholar] [CrossRef] [PubMed]
- Stacchiotti, S.; Sommer, J.; Chordoma Global Consensus, G. Building a global consensus approach to chordoma: A position paper from the medical and patient community. Lancet Oncol. 2015, 16, e71-83. [Google Scholar] [CrossRef]
- Varga, P.P.; Szövérfi, Z.; Fisher, C.G.; Boriani, S.; Gokaslan, Z.L.; Dekutoski, M.B.; Chou, D.; Quraishi, N.A.; Reynolds, J.J.; Luzzati, A.J.E.S.J. Surgical treatment of sacral chordoma: Prognostic variables for local recurrence and overall survival. Eur. Spine J. 2015, 24, 1092–1101. [Google Scholar] [CrossRef]
- Stacchiotti, S.; Gronchi, A.; Fossati, P.; Akiyama, T.; Alapetite, C.; Baumann, M.; Blay, J.Y.; Bolle, S.; Boriani, S.; Bruzzi, P.; et al. Best practices for the management of local-regional recurrent chordoma: A position paper by the Chordoma Global Consensus Group. Ann. Oncol. 2017, 28, 1230–1242. [Google Scholar] [CrossRef]
- MacCarty, C.S.; Waugh, J.M.; Coventry, M.B.; Cope, W.F., Jr. Surgical treatment of sacral and presacral tumors other than sacrococcygeal chordoma. J. Neurosurg. 1965, 22, 458–464. [Google Scholar] [CrossRef]
- Ozaki, T.; Hillmann, A.; Winkelmann, W. Surgical Treatment of sacrococcygeal chordoma. J. Surg. Oncol. 1997, 64, 274–279. [Google Scholar] [CrossRef]
- Magrini, S.M.; Papi, M.G.; Marletta, F.; Tomaselli, S.; Cellai, E.; Mungai, V.; Biti, G.J.A.O. Chordoma-natural history, treatment and prognosis the Florence radiotherapy department experience (1956–1990) and a critical review of the literature. Acta Oncol. 1992, 31, 847–851. [Google Scholar] [CrossRef]
- Radaelli, S.; Stacchiotti, S.; Ruggieri, P.; Donati, D.; Casali, P.G.; Palmerini, E.; Collini, P.; Gambarotti, M.; Porcu, L.; Boriani, S.J.S. Sacral chordoma: Long-term outcome of a large series of patients surgically treated at two reference centers. Spine 2016, 41, 1049–1057. [Google Scholar] [CrossRef] [PubMed]
- Fujii, R.; Friedman, E.R.; Richards, J.; Tsang, K.Y.; Heery, C.R.; Schlom, J.; Hodge, J.W. Enhanced killing of chordoma cells by antibody-dependent cell-mediated cytotoxicity employing the novel anti-PD-L1 antibody avelumab. Oncotarget 2016, 7, 33498–33511. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.S.; Schwab, J.H. Immunotherapy as a Potential Treatment for Chordoma: A Review. Curr. Oncol. Rep. 2016, 18, 55. [Google Scholar] [CrossRef] [PubMed]
- Bilusic, M.; Heery, C.R.; Collins, J.M.; Donahue, R.N.; Palena, C.; Madan, R.A.; Karzai, F.; Marte, J.L.; Strauss, J.; Gatti-Mays, M.E.; et al. Phase I trial of HuMax-IL8 (BMS-986253), an anti-IL-8 monoclonal antibody, in patients with metastatic or unresectable solid tumors. J. Immunother. Cancer 2019, 7, 240. [Google Scholar] [CrossRef]
- Blay, J.-Y.; Penel, N.; Ray-Coquard, I.L.; Cousin, S.; Bertucci, F.; Bompas, E.; Eymard, J.-C.; Saada-Bouzid, E.; Soulie, P.; Boudou-Rouquette, P. High clinical activity of pembrolizumab in chordoma, alveolar soft part sarcoma (ASPS) and other rare sarcoma histotypes: The French AcSé pembrolizumab study from Unicancer. J. Clin. Oncol. 2021, 39, 11520. [Google Scholar] [CrossRef]
- Collins, J.M.; Donahue, R.N.; Tsai, Y.T.; Manu, M.; Palena, C.; Gatti-Mays, M.E.; Marte, J.L.; Madan, R.A.; Karzai, F.; Heery, C.R.; et al. Phase I Trial of a Modified Vaccinia Ankara Priming Vaccine Followed by a Fowlpox Virus Boosting Vaccine Modified to Express Brachyury and Costimulatory Molecules in Advanced Solid Tumors. Oncologist 2020, 25, 560-e1006. [Google Scholar] [CrossRef]
- DeMaria, P.J.; Bilusic, M.; Park, D.; Heery, C.R.; Madan, R.A.; Strauss, J.; Donahue, R.N.; Marte, J.; Gilbert, M.R.; Steinberg, S.M.; et al. A randomized, double-blind, phase II clinical trial of GI-6301 (yeast-brachyury vaccine) versus placebo in combination with standard of care definitive radiotherapy in locally advanced, unresectable, chordoma. J. Clin. Oncol. 2020, 38, 11527. [Google Scholar] [CrossRef]
- DeMaria, P.J.; Bilusic, M.; Park, D.M.; Heery, C.R.; Donahue, R.N.; Madan, R.A.; Bagheri, M.H.; Strauss, J.; Shen, V.; Marte, J.L.; et al. Randomized, Double-Blind, Placebo-Controlled Phase II Study of Yeast-Brachyury Vaccine (GI-6301) in Combination with Standard-of-Care Radiotherapy in Locally Advanced, Unresectable Chordoma. Oncologist 2021, 26, E847–E858. [Google Scholar] [CrossRef]
- DeMaria, P.J.; Lee-Wisdom, K.; Donahue, R.N.; Madan, R.A.; Karzai, F.; Schwab, A.; Palena, C.; Jochems, C.; Floudas, C.; Strauss, J.; et al. Phase 1 open-label trial of intravenous administration of MVA-BN-brachyury-TRICOM vaccine in patients with advanced cancer. J. Immunother. Cancer 2021, 9, e003238. [Google Scholar] [CrossRef]
- Dredge, K.; Brennan, T.V.; Hammond, E.; Lickliter, J.D.; Lin, L.; Bampton, D.; Handley, P.; Lankesheer, F.; Morrish, G.; Yang, Y.; et al. A Phase i study of the novel immunomodulatory agent PG545 (pixatimod) in subjects with advanced solid tumours. Br. J. Cancer 2018, 118, 1035–1041. [Google Scholar] [CrossRef]
- Heery, C.R.; O’Sullivan-Coyne, G.; Madan, R.A.; Cordes, L.; Rajan, A.; Rauckhorst, M.; Lamping, E.; Oyelakin, I.; Marté, J.L.; Lepone, L.M.; et al. Avelumab for metastatic or locally advanced previously treated solid tumours (JAVELIN Solid Tumor): A phase 1a, multicohort, dose-escalation trial. Lancet Oncol. 2017, 18, 587–598. [Google Scholar] [CrossRef] [PubMed]
- Heery, C.R.; Palena, C.; McMahon, S.; Donahue, R.N.; Lepone, L.M.; Grenga, I.; Dirmeier, U.; Cordes, L.; Marte, J.; Dahut, W.; et al. Phase I study of a poxviral TRICOM-based vaccine directed against the transcription factor brachyury. Clin. Cancer Res. 2017, 23, 6833–6845. [Google Scholar] [CrossRef] [PubMed]
- Heery, C.R.; Singh, B.H.; Rauckhorst, M.; Marte, J.L.; Donahue, R.N.; Grenga, I.; Rodell, T.C.; Dahut, W.; Arlen, P.M.; Madan, R.A.; et al. Phase I Trial of a Yeast-Based Therapeutic Cancer Vaccine (GI-6301) Targeting the Transcription Factor Brachyury. Cancer Immunol. Res. 2015, 3, 1248–1256. [Google Scholar] [CrossRef] [PubMed]
- Ingham, M.; Hu, J.S.; Whalen, G.F.; Thomas, J.S.; El-Khoueiry, A.B.; Hanna, D.L.; Olszanski, A.J.; Meyer, C.F.; Azad, N.S.; Mahmood, S. Early results of intratumoral INT230-6 alone or in combination with ipilimumab in subjects with advanced sarcomas. J. Clin. Oncol. 2021, 39, 15. [Google Scholar] [CrossRef]
- Janku, F.; Zhang, H.H.; Pezeshki, A.; Goel, S.; Murthy, R.; Wang-Gillam, A.; Shepard, D.R.; Helgason, T.; Masters, T.; Hong, D.S. Intratumoral Injection of Clostridium novyi-NT Spores in Patients with Treatment-refractory Advanced Solid TumorsPhase I Study of Intratumoral Clostridium novyi-NT. Clin. Cancer Res. 2021, 27, 96–106. [Google Scholar] [CrossRef]
- Streby, K.A.; Geller, J.I.; Currier, M.A.; Warren, P.S.; Racadio, J.M.; Towbin, A.J.; Vaughan, M.R.; Triplet, M.; Ott-Napier, K.; Dishman, D.J.; et al. Intratumoral injection of HSV1716, an oncolytic herpes virus, is safe and shows evidence of immune response and viral replication in young cancer patients. Clin. Cancer Res. 2017, 23, 3566–3574. [Google Scholar] [CrossRef]
- Bishop, A.J.; Amini, B.; Lin, H.; Raza, S.M.; Patel, S.; Grosshans, D.R.; Ghia, A.; Farooqi, A.; Guadagnolo, B.A.; Mitra, D.; et al. Immune Checkpoint Inhibitors Have Clinical Activity in Patients With Recurrent Chordoma. J. Immunother. 2022, 45, 374–378. [Google Scholar] [CrossRef]
- Jagersberg, M.; El Rahal, A.; Dammann, P.; Merkler, D.; Weber, D.C.; Schaller, K. Clival chordoma: A single-centre outcome analysis. Acta Neurochir. 2017, 159, 1815–1823. [Google Scholar] [CrossRef]
- Ramos-Casals, M.; Maria, A.; Suárez-Almazor, M.E.; Lambotte, O.; Fisher, B.A.; Hernández-Molina, G.; Guilpain, P.; Pundole, X.; Flores-Chávez, A.; Baldini, C.; et al. Sicca/Sjögren’s syndrome triggered by PD-1/PD-L1 checkpoint inhibitors. Data from the International Immunocancer Registry (ICIR). Clin. Exp. Rheumatol. 2019, 37, 114–122. [Google Scholar]
- Chen, Y.; Jia, Y.; Liu, Q.; Shen, Y.; Zhu, H.; Dong, X.; Huang, J.; Lu, J.; Yin, Q. Myocarditis related to immune checkpoint inhibitors treatment: Two case reports and literature review. Ann. Palliat. Med. 2021, 10, 8512–8517. [Google Scholar] [CrossRef]
- Gounder, M.M.; Zhu, G.; Roshal, L.; Lis, E.; Daigle, S.R.; Blakemore, S.J.; Michaud, N.R.; Hameed, M.; Hollmann, T.J. ImmunologicCorrelates of the Abscopal Effect in a SMARCB1/INI1-negative Poorly Differentiated Chordoma after EZH2 Inhibition and Radiotherapy. Clin. Cancer Res. 2019, 25, 2064–2071. [Google Scholar] [CrossRef] [PubMed]
- Ibodeng, G.O.E.; Alkharabsheh, O.; Thanikachalam, K. A case of refractory chordoma of the clivus with a review of therapeutic targets. Curr. Probl. Cancer Case Rep. 2022, 8, 100194. [Google Scholar] [CrossRef]
- Migliorini, D.; Mach, N.; Aguiar, D.; Vernet, R.; Landis, B.N.; Becker, M.; McKee, T.; Dutoit, V.; Dietrich, P.Y. First report of clinical responses to immunotherapy in 3 relapsing cases of chordoma after failure of standard therapies. Oncoimmunology 2017, 6, e1338235. [Google Scholar] [CrossRef] [PubMed]
- Williamson, L.M.; Rive, C.M.; Di Francesco, D.; Titmuss, E.; Chun, H.E.; Brown, S.D.; Milne, K.; Pleasance, E.; Lee, A.F.; Yip, S.; et al. Clinical response to nivolumab in an INI1-deficient pediatric chordoma correlates with immunogenic recognition of brachyury. NPJ Precis Oncol. 2021, 5, 103. [Google Scholar] [CrossRef]
- Wu, X.; Lin, X.; Chen, Y.; Kong, W.; Xu, J.; Yu, Z. Response of metastatic chordoma to the immune checkpoint inhibitor pembrolizumab: A case report. Front. Oncol. 2020, 10, 565945. [Google Scholar] [CrossRef]
- Somaiah, N.; Conley, A.P.; Parra, E.R.; Lin, H.; Amini, B.; Solis Soto, L.; Salazar, R.; Barreto, C.; Chen, H.; Gite, S.; et al. Durvalumab plus tremelimumab in advanced or metastatic soft tissue and bone sarcomas: A single-centre phase 2 trial. Lancet Oncol. 2022, 23, 1156–1166. [Google Scholar] [CrossRef]
- Choi, H.; Charnsangavej, C.; Faria, S.C.; Macapinlac, H.A.; Burgess, M.A.; Patel, S.R.; Chen, L.L.; Podoloff, D.A.; Benjamin, R.S. Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: Proposal of new computed tomography response criteria. J. Clin. Oncol. 2007, 25, 1753–1759. [Google Scholar] [CrossRef]
- Traylor, J.I.; Pernik, M.N.; Plitt, A.R.; Lim, M.; Garzon-Muvdi, T. Immunotherapy for Chordoma and Chondrosarcoma: Current Evidence. Cancers 2021, 13, 2408. [Google Scholar] [CrossRef]
- Akinduro, O.O.; Suarez-Meade, P.; Garcia, D.; Brown, D.A.; Sarabia-Estrada, R.; Attia, S.; Gokaslan, Z.L.; Quinones-Hinojosa, A. Targeted Therapy for Chordoma: Key Molecular Signaling Pathways and the Role of Multimodal Therapy. Target. Oncol. 2021, 16, 325–337. [Google Scholar] [CrossRef]
- Chen, M.; Wu, Y.H.; Zhang, H.; Li, S.Y.; Zhou, J.D.; Shen, J. The Roles of Embryonic Transcription Factor BRACHYURY in Tumorigenesis and Progression. Front. Oncol. 2020, 10, 961. [Google Scholar] [CrossRef]
- Colia, V.; Stacchiotti, S. Medical treatment of advanced chordomas. Eur. J. Cancer 2017, 83, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Shen, J.; Gao, Y.; Liao, Y.F.; Cote, G.; Choy, E.; Chebib, I.; Mankin, H.; Hornicek, F.; Duan, Z.F. Expression of programmed cell death ligand 1 (PD-L1) and prevalence of tumor-infiltrating lymphocytes (TILs) in chordoma. Oncotarget 2015, 6, 11139–11149. [Google Scholar] [CrossRef] [PubMed]
- Thanindratarn, P.; Dean, D.C.; Nelson, S.D.; Hornicek, F.J.; Duan, Z.F. Advances in immune checkpoint inhibitors for bone sarcoma therapy. J. Bone Oncol. 2019, 15, 100221. [Google Scholar] [CrossRef]
- Dridi, M.; Krebs-Drouot, L.; Meyronet, D.; Dumollard, J.M.; Vassal, F.; Jouanneau, E.; Jacquesson, T.; Barrey, C.; Grange, S.; Boutonnat, J.; et al. The Immune Microenvironment of Chordomas: An Immunohistochemical Analysis. Cancers 2021, 13, 3335. [Google Scholar] [CrossRef] [PubMed]
- Gill, C.M.; Fowkes, M.; Shrivastava, R.K. Emerging Therapeutic Targets in Chordomas: A Review of the Literature in the Genomic Era. Neurosurgery 2020, 86, E118–E123. [Google Scholar] [CrossRef]
- Fujii, R.; Schlom, J.; Hodge, J.W. A potential therapy for chordoma via antibody-dependent cell-mediated cytotoxicity employing NK or high-affinity NK cells in combination with cetuximab. J. Neurosurg. 2018, 128, 1419–1427. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, G.; Rath, B. Avelumab: Combining immune checkpoint inhibition and antibody-dependent cytotoxicity. Expert Opin. Biol. Ther. 2017, 17, 515–523. [Google Scholar] [CrossRef]
- He, G.; Liu, X.; Pan, X.; Ma, Y.; Liu, X. Cytotoxic T lymphocyte antigen-4 (CTLA-4) expression in chordoma and tumor-infiltrating lymphocytes (TILs) predicts prognosis of spinal chordoma. Clin. Transl. Oncol. Off. Publ. Fed. Span. Oncol. Soc. Natl. Cancer Inst. Mex. 2020, 22, 2324–2332. [Google Scholar] [CrossRef]
- Hoke, A.T.K.; Padget, M.R.; Fabian, K.P.; Nandal, A.; Gallia, G.L.; Bilusic, M.; Soon-Shiong, P.; Hodge, J.W.; London, N.R., Jr. Combinatorial Natural Killer Cell-based Immunotherapy Approaches Selectively Target Chordoma Cancer Stem Cells. Cancer Res. Commun. 2021, 1, 127–139. [Google Scholar] [CrossRef]
- Cogdill, A.P.; Andrews, M.C.; Wargo, J.A. Hallmarks of response to immune checkpoint blockade. Br. J. Cancer 2017, 117, 1–7. [Google Scholar] [CrossRef]
- Meftahpour, V.; Aghebati-Maleki, A.; Fotouhi, A.; Safarzadeh, E.; Aghebati-Maleki, L. Prognostic significance and therapeutic potentials of immune checkpoints in osteosarcoma. Excli J. 2022, 21, 250–268. [Google Scholar] [CrossRef] [PubMed]
- Hargadon, K.M.; Johnson, C.E.; Williams, C.J. Immune checkpoint blockade therapy for cancer: An overview of FDA-approved immune checkpoint inhibitors. Int. Immunopharmacol. 2018, 62, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Xia, C.; Huang, W.; Chen, Y.-L.; Fu, H.-B.; Tang, M.; Zhang, T.-L.; Li, J.; Lv, G.-H.; Yan, Y.-G.; Ouyang, Z.-H.; et al. Coexpression of HHLA2 and PD-L1 on Tumor Cells Independently Predicts the Survival of Spinal Chordoma Patients. Front. Immunol. 2022, 12, 797407. [Google Scholar] [CrossRef] [PubMed]
- Zou, M.X.; Lv, G.H.; Wang, X.B.; Huang, W.; Li, J.; Jiang, Y.; She, X.L. Clinical Impact of the Immune Microenvironment in Spinal Chordoma: Immunoscore as an Independent Favorable Prognostic Factor. Neurosurgery 2019, 84, E318–E333. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.P.; Jiang, Y.; Zhang, H.Y.; Chen, L.; Luo, P.; Li, L.; Zhao, J.S.; Lv, F.; Zou, D.; Zhang, Y.; et al. Clinicopathological implications of TIM3(+) tumor-infiltrating lymphocytes and the miR-455-5p/Galectin-9 axis in skull base chordoma patients. Cancer Immunol. Immunother. 2019, 68, 1157–1169. [Google Scholar] [CrossRef]
- Dancsok, A.R.; Gao, D.X.; Lee, A.N.F.; Steigen, S.E.; Blay, J.Y.; Thomas, D.M.; Maki, R.G.; Nielsen, T.O.; Demicco, E.G. Tumor-associated macrophages and macrophage-related immune checkpoint expression in sarcomas. Oncoimmunology 2020, 9, 1747340. [Google Scholar] [CrossRef] [PubMed]
- Kushlinskii, N.E.; Kovaleva, O.V.; Kuzmin, Y.B.; Korotkova, E.A.; Gershtein, E.S.; Boulytcheva, I.V.; Kozlova, E.V.; Kudlay, D.A.; Podlesnaya, P.A.; Gratchev, A.N.; et al. Clinical and prognostic significance of the soluble form of the VISTA immunity control point in patients with primary bone tumors. Klin. Lab. Diagn. 2021, 66, 533–538. [Google Scholar] [CrossRef]
- Court, C.; Briand, S.; Mir, O.; Le Pechoux, C.; Lazure, T.; Missenard, G.; Bouthors, C. Management of chordoma of the sacrum and mobile spine. Orthop. Traumatol.-Surg. Res. 2022, 108, 103169. [Google Scholar] [CrossRef]
- Keung, E.Z.; Burgess, M.; Salazar, R.; Parra, E.R.; Rodrigues-Canales, J.; Bolejack, V.; Van Tine, B.A.; Schuetze, S.M.; Attia, S.; Riedel, R.F. Correlative Analyses of the SARC028 Trial Reveal an Association Between Sarcoma-Associated Immune Infiltrate and Response to PembrolizumabSarcoma-Associated Immune Infiltrate and Anti-PD1 Therapy. Clin. Cancer Res. 2020, 26, 1258–1266. [Google Scholar] [CrossRef]
- Zou, M.X.; Pan, Y.; Huang, W.; Zhang, T.L.; Escobar, D.; Wang, X.B.; Jiang, Y.; She, X.L.; Lv, G.H.; Li, J. A four-factor immune risk score signature predicts the clinical outcome of patients with spinal chordoma. Clin. Transl. Med. 2020, 10, 224–237. [Google Scholar] [CrossRef]
- Williamson, L.; Rive, C.; Di Francesco, D.; Titmuss, E.; Chun, E.; Brown, S.; Milne, K.; Pleasance, E.; Lee, A.F.; Yip, S.; et al. Response to nivolumab in a pediatric chordoma with overexpression of brachyury. Cancer Res. 2021, 81, 630. [Google Scholar] [CrossRef]
- Quiroga, D.; Liebner, D.A.; Philippon, J.S.; Hoffman, S.; Tan, Y.; Chen, J.L.; Lenobel, S.; Wakely, P.E., Jr.; Pollock, R.; Tinoco, G. Activity of PD1 inhibitor therapy in advanced sarcoma: A single-center retrospective analysis. BMC Cancer 2020, 20, 527. [Google Scholar] [CrossRef] [PubMed]
- Chalmers, Z.R.; Connelly, C.F.; Fabrizio, D.; Gay, L.; Ali, S.M.; Ennis, R.; Schrock, A.; Campbell, B.; Shlien, A.; Chmielecki, J.; et al. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med. 2017, 9, 34. [Google Scholar] [CrossRef] [PubMed]
- Killian, J.K.; Yang, X.R.; Danziger, N.; Elvin, J.A.; Vergilio, J.-A.; Lin, D.I.; Williams, E.A.; Ramkissoon, S.H.; Severson, E.A.; Hemmerich, A. Differential genomic landscape of clinically advanced/metastatic chordomas (mChor) based on primary tumor site. J. Clin. Oncol. 2020, 38, 11521. [Google Scholar] [CrossRef]
- Abro, B.; Kaushal, M.; Chen, L.; Wu, R.; Dehner, L.P.; Pfeifer, J.D.; He, M.J.P.-R.; Practice. Tumor mutation burden, DNA mismatch repair status and checkpoint immunotherapy markers in primary and relapsed malignant rhabdoid tumors. Pathol.-Res. Pract. 2019, 215, 152395. [Google Scholar] [CrossRef]
- Forrest, S.J.; Al-Ibraheemi, A.; Doan, D.; Ward, A.; Clinton, C.M.; Putra, J.; Pinches, R.S.; Kadoch, C.; Chi, S.N.; DuBois, S.G. Genomic and immunologic characterization of INI1-deficient pediatric cancers. Clin. Cancer Res. 2020, 26, 2882–2890. [Google Scholar] [CrossRef]
- Jelinic, P.; Ricca, J.; Van Oudenhove, E.; Olvera, N.; Merghoub, T.; Levine, D.A.; Zamarin, D. Immune-active microenvironment in small cell carcinoma of the ovary, hypercalcemic type: Rationale for immune checkpoint blockade. JNCI J. Natl. Cancer Inst. 2018, 110, 787–790. [Google Scholar] [CrossRef]
- Forrest, S.J.; Yi, J.; Kline, C.; Cash, T.; Reddy, A.T.; Cote, G.M.; Merriam, P.; Czaplinski, J.; Bhushan, K.; DuBois, S.G. Phase II study of nivolumab and ipilimumab in children and young adults with INI1-negative cancers. J. Clin. Oncol. 2021, 39, 15. [Google Scholar] [CrossRef]
- Patel, S.S.; Nota, S.P.; Sabbatino, F.; Nielsen, G.P.; Deshpande, V.; Wang, X.; Ferrone, S.; Schwab, J.H. Defective HLA Class I Expression and Patterns of Lymphocyte Infiltration in Chordoma Tumors. Clin. Orthop. Relat. Res. 2021, 479, 1373–1382. [Google Scholar] [CrossRef]
- Bourbon, E.; Ghesquières, H.; Bachy, E.J. CAR-T cells, from principle to clinical applications. Bull. Cancer 2021, 108, S4–S17. [Google Scholar] [CrossRef]
- Schwab, J.H.; Boland, P.J.; Agaram, N.P.; Socci, N.D.; Guo, T.; O’Toole, G.C.; Wang, X.; Ostroumov, E.; Hunter, C.J.; Block, J.A.; et al. Chordoma and chondrosarcoma gene profile: Implications for immunotherapy. Cancer Immunol. Immunother. CII 2009, 58, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Schoenfeld, A.J.; Wang, X.; Wang, Y.; Hornicek, F.J.; Nielsen, G.P.; Duan, Z.; Ferrone, S.; Schwab, J.H. CSPG4 as a prognostic biomarker in chordoma. Spine J. 2016, 16, 722–727. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, Y.; Yu, L.; Sakakura, K.; Visus, C.; Schwab, J.H.; Ferrone, C.R.; Favoino, E.; Koya, Y.; Campoli, M.R.; et al. CSPG4 in cancer: Multiple roles. Curr. Mol. Med. 2010, 10, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Beard, R.E.; Zheng, Z.; Lagisetty, K.H.; Burns, W.R.; Tran, E.; Hewitt, S.M.; Abate-Daga, D.; Rosati, S.F.; Fine, H.A.; Ferrone, S.; et al. Multiple chimeric antigen receptors successfully target chondroitin sulfate proteoglycan 4 in several different cancer histologies and cancer stem cells. J. Immunother. Cancer 2014, 2, 25. [Google Scholar] [CrossRef] [PubMed]
- Long, C.; Li, G.; Zhang, C.; Jiang, T.; Li, Y.; Duan, X.; Zhong, G. B7-H3 as a Target for CAR-T Cell Therapy in Skull Base Chordoma. Front. Oncol. 2021, 11, 659662. [Google Scholar] [CrossRef]

| Year | Study Design | Levels of Evidence | Sample Size | Tumor Site | Treatment History | Drug | Median Treatment Time (m) | AEs | Choi’s Criteria | RECIST/irRECIST | Median PFS (m) | Median OS (m) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 022 | case series | IV | 9 | 2 clival 5 spine, and 10 sacrum, | radiotherapy | pembrolizumab 200 mg | 12 | dermatologic (n = 2), endocrine (n = 2), or Sicca syndrome related (n = 2). grade 3 myocarditis and myositis (n = 1), grade 4 potentially attributable pneumonitis versus infectious reaction (n = 1) | 1CR,6SD,2PR, | 14 (95% CI, 5–17) | 1-year OS was 87% | |
| 2 | FAZ053 | 2SD | ||||||||||
| 1 | Nivolumab + bempegaldesleukin | PD | ||||||||||
| 2021 | phase2 | V | 34 | pembrolizumab 200mg |
The side effect profile of pembrolizumab was similar to other tumor type | 3PR | 6.6 | not reached | ||||
| 2022 | case report | V | 1 | clival | Surgery and radiotherapy | Pembrolizumab (200mg) | 3 | hypotension, severe fatigue, and dyspnea | ||||
| 2020 | case report | V | 1 | sacrum | surgery | pembrolizumab | grades 1–2, liver function and hyperglycemia | PR | 9.3 | |||
| 2017 | case report | V | 1 | spine | Surgery and radiotherapy | Pembrolizumab 200 mg | vitiligo | controlled for 6 months | ||||
| 1 | clival | Surgery and radiotherapy | Nivolumab 3 mg/kg | controlled for 9 months | ||||||||
| 2019 | case report | V | 1 | sacrum | radiotherapy | nivolumab, followed by nivolumab + ipilimumab | >4 | |||||
| 2017 | case series | IV | 2 | clival | radiotherapy | pazopanib + nivolumab | 161 | |||||
| 2021 | case report | V | 1 | clival | nivolumab | PR | ||||||
| 2021 | case report | V | 1 | sacrum | Surgery and radiotherapy | sintilimab + anlotinib | ICIs-related myocarditis | <1 | ||||
| 2017 | phase1 | V | 1 | avelumab (1,3,10,20mg/kg) | grade1/grade2 | |||||||
| 2019 | case series | IV | 1 | Durvalumab | Sicca/Sjögren’s syndrome | |||||||
| 2022 | phase2 | V | 5 | durvalumab + tremelimumab | Colitis, pneumonitis, abdominal pain, myocarditis | 1PR+CR,3SD,1PD | 13.57 (2.76, 17.81) |
| Year | Study Design | Levels of Evidence | Sample Size | Tumor Site | Treatment History | Drug | Median Treatment Time (m) | AEs | Choi’s Criteria | RECIST/irRECIST | Median PFS (m) | Median OS (m) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 22 | case series | IV | 5 | Durvalumab + tremelimumab | 12 | Colitis, pneumonitis, abdominal pain, myocarditis | 1PD,3SD,1PR | |||||
| 21 | phase1/2 | V | 1 | INT230-6 + ipilimumab | grade1/grade2, anemia, colitis | |||||||
| 19 | case report | V | 1 | sacrum | Radiotherapy | Nivolumab, followed by nivolumab + ipilimumab | >4 |
| Year | Study Design | Levels of Evidence | Sample Size | Tumor Site | Treatment History | Drug | Median Treatment Time (m) | AEs | Choi’s Criteria | RECIST/irRECIST | Median PFS (m) | Median OS (m) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 20 | phase1 | IV | 3 | MVA-brachyury s.c., 8 × 108 infectious units (IU), followed by FPV-brachyury s.c., 1 × 109 IU, | 24 | grade1/grade2, injection-site reaction, fever, fatigue | 2SD, 1PD | one patient for 52 weeks | ||||
| 20 | phase2 | III | 11 | 5 clival, 3 spine and 3 sacrum | GI-6301 (yeast-brachyury vaccine) +RT | injection-site reaction, lymphocyte count decreased, fever | 5PD,3SD,1 PR, | 20.6 | 37.5 | |||
| 21 | phase1 | IV | 10 | MVA-BN-brachyury-TRICOM vaccine (1 × 107, 1 × 108, 1 × 109) | grade1/grade2, injection-site reaction, fever | 4SD,5PD,1PR | 253 day | |||||
| 17 | phase1 | IV | 13 | MVA-brachyury-TRICOM (5 × 108, 1 × 109, 2 × 109) | grade1/grade2 (fever, diarrhea) injection-site reaction, lymphocyte count decreased, flu-like symptoms, fever, and diarrhea | |||||||
| 15 | phase1 | IV | 11 | 3 clival, 2 spine and 6 sacrum. | Surgery and radiotherapy | GI-6301 (yeast-brachyury vaccine) (4, 16, 40, and 80 yeast units (YU)) | grade1/grade2, injection-site reaction, fever | 3PD,1PR,1mixed response, 5SD | 8.3 |
| Year | Study Design | Levels of Evidence | Sample Size | Tumor Site | Treatment History | Drug | Median Treatment Time (m) | AEs | Choi’s Criteria | RECIST/irRECIST | Median PFS (m) | Median OS(m) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2019 | phase1 | V | 5 | HuMax-IL8 (4, 8, 16, 32mg/kg) | 6 | constipation (33.3%), nausea (26.7%) and anemia (26.7%) | SD = 4, PD = 1 | 5.5 | ||||
| 2018 | phase1 | V | 1 | PG545 150mg | Hypertension, Chills, Fatigue | SD | ||||||
| 2021 | phase1 | V | 1 | C. novyi NT spores 1 × 106 | Pyrexia | SD | ||||||
| 2017 | case series | IV | 1 | clival | Surgery and radiotherapy | MVX-ONCO-1 | controlled >19 months | |||||
| 2017 | phase1 | V | 1 | clival | radiotherapy | HSV1716 (2 × 106 i.u.) | fever, chills, anemia and leukopenia | SD | 14day | 2.5 |
| Clinical Trial | Trial registration Number | Phase | Medical Condition: | Sites | Status |
|---|---|---|---|---|---|
| Talimogene Laherparepvec, Nivolumab and Trabectedin for Sarcoma | NCT03886311 | 2 | Talimogene Laherparepvec, Nivolumab, Trabectedin | USA | Recruiting |
| Nivolumab and Ipilimumab in Treating Patients With Rare Tumors | NCT02834013 | 2 | Ipilimumab, Nivolumab | USA | Recruiting |
| Study of Nivolumab and Ipilimumab in Children and Young Adults With INI1-Negative Cancers | NCT04416568 | 2 | Ipilimumab, Nivolumab | USA | Recruiting |
| Multi-Arm Study to Test the Efficacy of Immunotherapeutic Agents in Multiple Sarcoma Subtypes | NCT02815995 | 2 | Durvalumab, Tremelimumab | USA | Active, not recruiting |
| A randomised, comparative, prospective, multicentre study of the efficacy of nivolumab + ipilimumab versus pazopanib alone in patients with metastatic or unresectable advanced sarcoma of rare subtype | EudraCT2020-002821-28 | 2 | nivolumab + ipilimumab versus pazopanib | France | Ongoing |
| A randomized phase II study of Durvalumab (MEDI4736) and Tremelimumab compared to doxorubicin in patients with advanced or metastatic soft tissue sarcoma. | EudraCT 2016-004750-15 | 2 | Durvalumab (MEDI4736) and Tremelimumab compared to doxorubicin | Germany | Ongoing |
| Multi-Arm Study to Test the Efficacy of Immunotherapeutic Agents in Multiple Sarcoma Subtypes | NCT02815995 | 2 | Durvalumab, Tremelimumab | USA | Active, not recruiting |
| Nivolumab and Relatlimab in Treating Participants With Advanced Chordoma | NCT03623854 | 2 | Nivolumab, Relatlimab | USA | Recruiting |
| Tiragolumab and Atezolizumab for the Treatment of Relapsed or Refractory SMARCB1 or SMARCA4 Deficient Tumors | NCT05286801 | 2 | Atezolizumab, Tiragolumab | USA | Recruiting |
| Phase II trial of the immune checkpoint inhibitor nivolumab in patients with select rare CNS cancers | NCT03173950 | 2 | nivolumab | USA | Recruiting |
| Phase I safety study of stereotactic radiosurgery with concurrent and adjuvant PD-1 antibody nivolumab in subjects with recurrent or advanced chordoma | NCT02989636 | 1 | nivolumab | USA | Recruiting |
| TAEK-VAC-HerBy Vaccine for Brachyury and HER2 Expressing Cancer | NCT04246671 | 1 | TAEK-VAC-HerBy Vaccine | Recruiting | |
| BN Brachyury and Radiation in Chordoma | NCT03595228 | 2 | BN-Brachyury plus radiation | ||
| A Study of FAZ053 Single Agent and in Combination with PDR001 in Patients With Advanced Malignancies. | NCT02936102 | 1 | FAZ053 PDR001 | Completed Active, not recruiting | |
| Nivolumab (Opdivo®) Plus ABI-009 (Nab-rapamycin) for Advanced Sarcoma and Certain Cancers | NCT03190174 | 2 | Nab-Rapamycin Nivolumab | Completed |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Chen, Z.; Li, B.; Fan, J.; Xu, W.; Xiao, J. Immunotherapy as a Promising Option for the Treatment of Advanced Chordoma: A Systemic Review. Cancers 2023, 15, 264. https://doi.org/10.3390/cancers15010264
Wang X, Chen Z, Li B, Fan J, Xu W, Xiao J. Immunotherapy as a Promising Option for the Treatment of Advanced Chordoma: A Systemic Review. Cancers. 2023; 15(1):264. https://doi.org/10.3390/cancers15010264
Chicago/Turabian StyleWang, Xiang, Zhaoyu Chen, Bo Li, Jiefu Fan, Wei Xu, and Jianru Xiao. 2023. "Immunotherapy as a Promising Option for the Treatment of Advanced Chordoma: A Systemic Review" Cancers 15, no. 1: 264. https://doi.org/10.3390/cancers15010264
APA StyleWang, X., Chen, Z., Li, B., Fan, J., Xu, W., & Xiao, J. (2023). Immunotherapy as a Promising Option for the Treatment of Advanced Chordoma: A Systemic Review. Cancers, 15(1), 264. https://doi.org/10.3390/cancers15010264






