Effect of Adjuvant Radiation Dose on Survival in Patients with Esophageal Squamous Cell Carcinoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Patient Population
2.2. Treatment
2.3. Statistical Analyses
3. Results
3.1. Patient Characteristics
3.2. Identification of the Patients Who Potentially Benefit from PORT
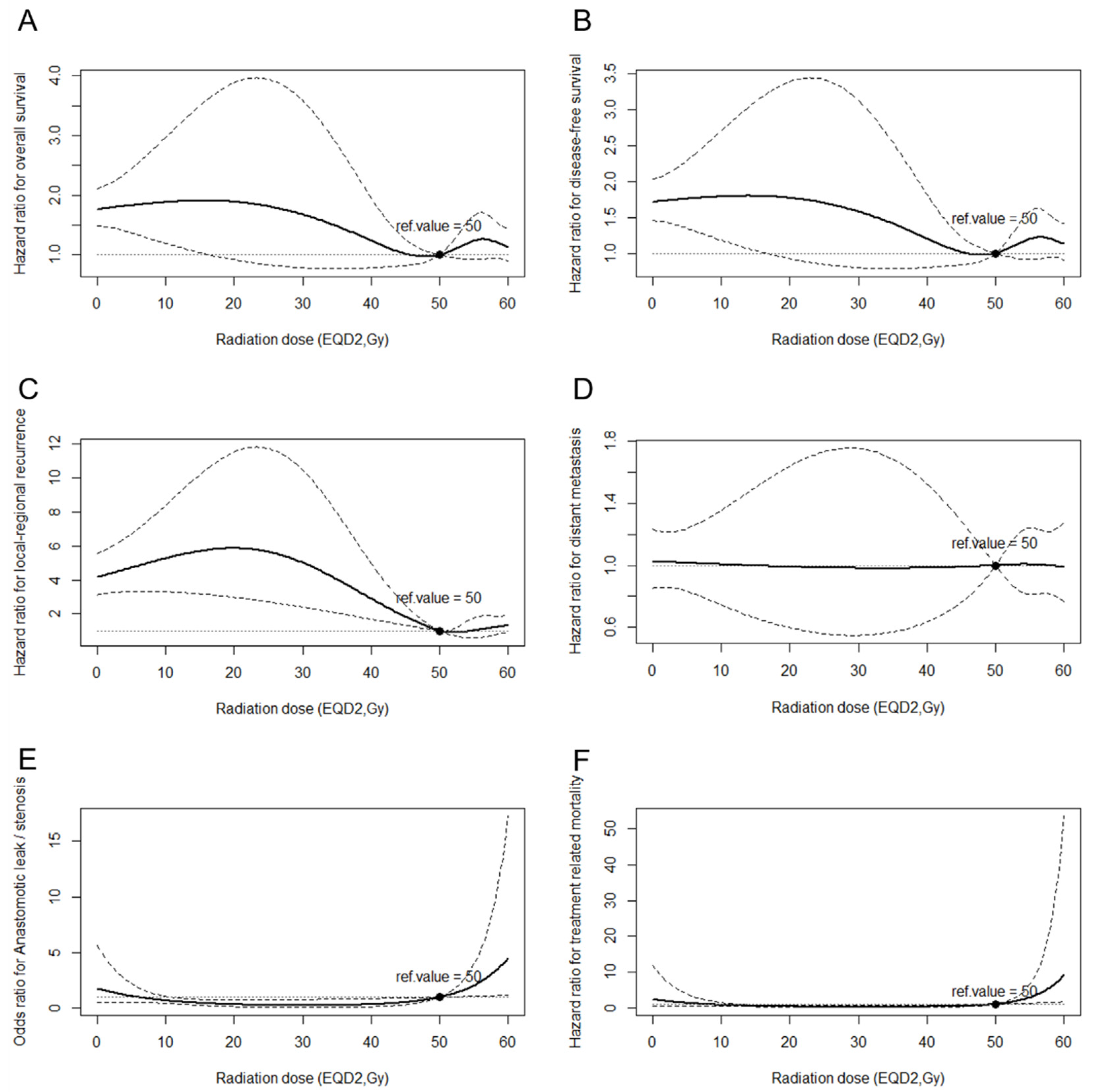
3.3. Relationship between Radiation Dose and Survival/Treatment Toxicities
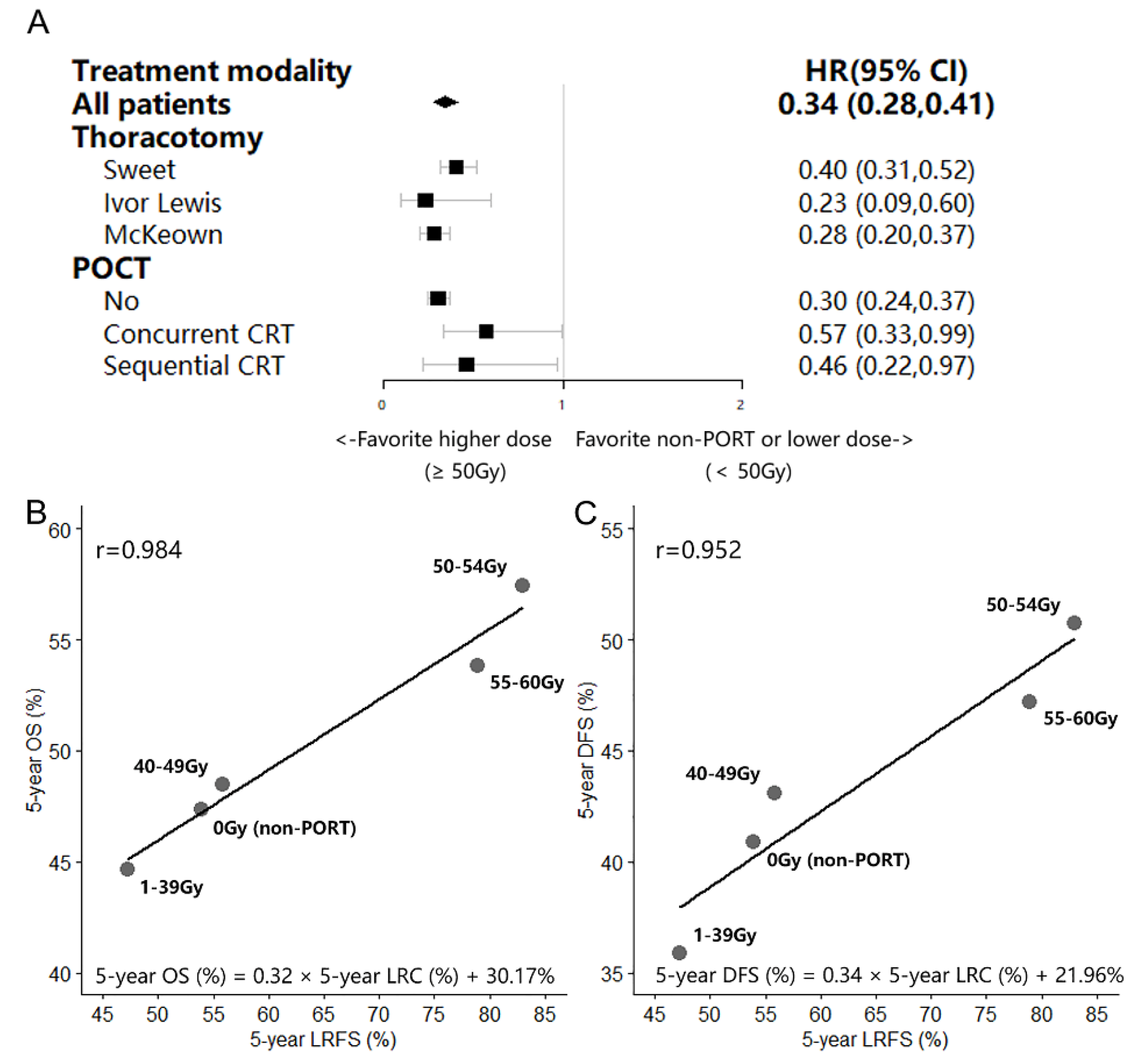
3.4. Effect of the aRTD on LRFS and the Correlation between LRFS and OS/DFS
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- van Hagen, P.; Hulshof, M.C.C.M.; Van Lanschot, J.J.B.; Steyerberg, E.W.; van Berge Henegouwen, M.I.; Wijnhoven, B.P.L.; Richel, D.J.; Nieuwenhuijzen, G.A.P.; Hospers, G.A.P.; Bonenkamp, J.J.; et al. Preoperative Chemoradiotherapy for Esophageal or Junctional Cancer. N. Engl. J. Med. 2012, 366, 2074–2084. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Liu, H.; Chen, Y.; Zhu, C.; Fang, W.; Yu, Z.; Mao, W.; Xiang, J.; Han, Y.; Chen, Z.; et al. Neoadjuvant Chemoradiotherapy Followed by Surgery Versus Surgery Alone for Locally Advanced Squamous Cell Carcinoma of the Esophagus (NEOCRTEC5010): A Phase III Multicenter, Randomized, Open-Label Clinical Trial. J. Clin. Oncol. 2018, 36, 2796–2803. [Google Scholar] [CrossRef] [PubMed]
- Mariette, C.; Dahan, L.; Mornex, F.; Maillard, E.; Thomas, P.; Meunier, B.; Boige, V.; Pezet, D.; Robb, W.B.; Le Brun-Ly, V.; et al. Surgery Alone Versus Chemoradiotherapy Followed by Surgery for Stage I and II Esophageal Cancer: Final Analysis of Randomized Controlled Phase III Trial FFCD. J. Clin. Oncol. 2014, 32, 2416–2422. [Google Scholar] [CrossRef]
- Rice, T.W.; Chen, L.-Q.; Hofstetter, W.L.; Smithers, B.M.; Rusch, V.; Wijnhoven, B.P.L.; Chen, K.L.; Davies, A.R.; D'Journo, X.B.; Kesler, K.A.; et al. Worldwide Esophageal Cancer Collaboration: Pathologic staging data. Dis. Esophagus 2016, 29, 724–733. [Google Scholar] [CrossRef] [PubMed]
- Rice, T.W.; Ishwaran, H.; Hofstetter, W.L.; Kelsen, D.P.; Apperson-Hansen, C.; Blackstone, E.H. Recommendations for pathologic staging (pTNM) of cancer of the esophagus and esophagogastric junction for the 8th edition AJCC/UICC staging manuals. Dis. Esophagus 2016, 29, 897–905. [Google Scholar] [CrossRef]
- Yu, J.; Ouyang, W.; Li, C.; Shen, J.; Xu, Y.; Zhang, J.; Xie, C. Mapping patterns of metastatic lymph nodes for postoperative radiotherapy in thoracic esophageal squamous cell carcinoma: A recommendation for clinical target volume definition. BMC Cancer 2019, 19, 1–7. [Google Scholar] [CrossRef]
- Hsu, P.; Chen, H.; Huang, C.; Liu, C.; Hsieh, C.; Hsu, H.; Wu, Y. Patterns of recurrence after oesophagectomy and postoperative chemoradiotherapy versus surgery alone for oesophageal squamous cell carcinoma. Br. J. Surg. 2016, 104, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yang, X.; Ni, J.; Li, Y.; Zou, L.; Chu, L.; Chu, X.; Xia, F.; Zhu, Z. Recommendation for the definition of postoperative radiotherapy target volume based on a pooled analysis of patterns of failure after radical surgery among patients with thoracic esophageal squamous cell carcinoma. Radiat. Oncol. 2018, 13, 255. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Cai, X.-W.; Wu, B.; Zhu, Z.-F.; Chen, H.-Q.; Fu, X.-L. Patterns of Failure after Radical Surgery among Patients with Thoracic Esophageal Squamous Cell Carcinoma: Implications for the Clinical Target Volume Design of Postoperative Radiotherapy. PLoS ONE 2014, 9, e97225. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Parry, K.; Visser, E.; Van Rossum, P.S.N.; Mohammad, N.H.; Ruurda, J.P.; Van Hillegersberg, R. Prognosis and Treatment After Diagnosis of Recurrent Esophageal Carcinoma Following Esophagectomy with Curative Intent. Ann. Surg. Oncol. 2015, 22, 1292–1300. [Google Scholar] [CrossRef] [PubMed]
- Blom, R.L.; Lagarde, S.M.; Van Oudenaarde, K.; Klinkenbijl, J.H.G.; Hulshof, M.C.; Van Laarhoven, H.W.; Bergman, J.J.; Busch, O.R.; Henegouwen, M.I.V.B. Survival After Recurrent Esophageal Carcinoma Has Not Improved Over the Past 18 Years. Ann. Surg. Oncol. 2013, 20, 2693–2698. [Google Scholar] [CrossRef] [PubMed]
- Su, X.-D.; Zhang, D.-K.; Zhang, X.; Lin, P.; Long, H.; Rong, T.-H. Prognostic factors in patients with recurrence after complete resection of esophageal squamous cell carcinoma. J. Thorac. Dis. 2014, 6, 949–957. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.F.; Yang, Z.Y.; Liang, J.; Miao, Y.J.; Wang, M.; Yin, W.B.; Gu, X.Z.; Zhang, D.C.; Zhang, R.G.; Wang, L.J. Value of radiotherapy after radical surgery for esophageal carcinoma: A report of 495 patients. Ann. Thorac. Surg. 2003, 75, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Yang, J.; Ni, W.; Li, C.; Chang, X.; Han, W.; Zhou, Z.; Chen, D.; Feng, Q.; Liang, J.; et al. Postoperative Radiotherapy in Pathological T2-3N0M0 Thoracic Esophageal Squamous Cell Carcinoma: Interim Report of a Prospective, Phase III, Randomized Controlled Study. Oncologist 2020, 25, e701–e708. [Google Scholar] [CrossRef] [PubMed]
- Ni, W.; Yu, S.; Xiao, Z.; Zhou, Z.; Chen, D.; Feng, Q.; Liang, J.; Lv, J.; Gao, S.; Mao, Y.; et al. Postoperative Adjuvant Therapy Versus Surgery Alone for Stage IIB-III Esophageal Squamous Cell Carcinoma: A Phase III Randomized Controlled Trial. Oncologist 2021, 26, e2151–e2160. [Google Scholar] [CrossRef]
- Chang, X.; Chen, J.; Zhang, W.; Yang, J.; Yu, S.; Deng, W.; Ni, W.; Zhou, Z.; Chen, D.; Feng, Q.; et al. Recurrence risk stratification based on a competing-risks nomogram to identify patients with esophageal cancer who may benefit from postoperative radiotherapy. Ther. Adv. Med. Oncol. 2021, 13, 1–14. [Google Scholar] [CrossRef]
- Chen, J.; Pan, J.; Zheng, X.; Zhu, K.; Li, J.; Chen, M.; Wang, J.; Liao, Z. Number and Location of Positive Nodes, Postoperative Radiotherapy, and Survival After Esophagectomy with Three-Field Lymph Node Dissection for Thoracic Esophageal Squamous Cell Carcinoma. Int. J. Radiat. Oncol. 2012, 82, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, W.; Xiao, Z.; Wang, Q.; Zhou, Z.; Zhang, H.; Chen, D.; Feng, Q.; He, J.; Gao, S.; et al. The Impact of Postoperative Conformal Radiotherapy after Radical Surgery on Survival and Recurrence in Pathologic T3N0M0 Esophageal Carcinoma: A Propensity Score-Matched Analysis. J. Thorac. Oncol. 2017, 12, 1143–1151. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, D.; Rineer, J.; Vongtama, D.; Wortham, A.; Han, P.; Schwartz, D.; Choi, K.; Rotman, M. Impact of Postoperative Radiation after Esophagectomy for Esophageal Cancer. J. Thorac. Oncol. 2010, 5, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.T.; Shao, M.; Rineer, J.; Lee, A.; Schwartz, D.; Schreiber, D. The Impact of Adjuvant Postoperative Radiation Therapy and Chemotherapy on Survival After Esophagectomy for Esophageal Carcinoma. Ann. Surg. 2017, 265, 1146–1151. [Google Scholar] [CrossRef]
- Lin, H.-N.; Chen, L.-Q.; Shang, Q.-X.; Yuan, Y.; Yang, Y.-S. A meta-analysis on surgery with or without postoperative radiotherapy to treat squamous cell esophageal carcinoma. Int. J. Surg. 2020, 80, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.-W.; Zeng, Y.; Feng, W.; Liu, M.-N.; Yu, W.; Zhang, Q.; Liu, J.; Wang, J.-M.; Lv, C.-X.; Fu, X.-L. Randomized phase II trial comparing tumor bed alone with tumor bed and elective nodal postoperative radiotherapy in patients with locoregionally advanced thoracic esophageal squamous cell carcinoma. Dis. Esophagus 2019, 32, doz013. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.-C.; Tao, H.; Zhang, Y.-Q.; Zha, W.-W.; Qian, P.-D.; Li, F.; Xu, K.-X. Extent of prophylactic postoperative radiotherapy after radical surgery of thoracic esophageal squamous cell carcinoma. Dis. Esophagus 2008, 21, 502–507. [Google Scholar] [CrossRef]
- Daiko, H.; Hayashi, R.; Sakuraba, M.; Ebihara, M.; Miyazaki, M.; Shinozaki, T.; Saikawa, M.; Zenda, S.; Kawashima, M.; Tahara, M.; et al. A Pilot Study of Post-operative Radiotherapy with Concurrent Chemotherapy for High-risk Squamous Cell Carcinoma of the Cervical Esophagus. Jpn. J. Clin. Oncol. 2011, 41, 508–513. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Adelstein, D.J.; Rice, T.W.; Rybicki, L.A.; Saxton, J.P.; Videtic, G.M.; Murthy, S.C.; Mason, D.P.; Rodriguez, C.P.; Ives, D.I. Mature Results from a Phase II Trial of Postoperative Concurrent Chemoradiotherapy for Poor Prognosis Cancer of the Esophagus and Gastroesophageal Junction. J. Thorac. Oncol. 2009, 4, 1264–1269. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wang, Z.; Yang, Z.; Shang, B.; Liu, X.; Chen, G. Prospective Study of Adjuvant Radiotherapy on Preventing Lymph Node Metastasis After Ivor-Lewis Esophagectomy in Esophageal Cancer. Ann. Surg. Oncol. 2013, 20, 2721–2726. [Google Scholar] [CrossRef]
- Tachibana, M.; Yoshimura, H.; Kinugasa, S.; Shibakita, M.; Dhar, D.; Ueda, S.; Fujii, T.; Nagasue, N. Postoperative chemotherapy vs. chemoradiotherapy for thoracic esophageal cancer: A prospective randomized clinical trial. Eur. J. Surg. Oncol. 2003, 29, 580–587. [Google Scholar] [CrossRef]
- Moon, S.; Kim, H.; Chie, E.; Kim, J.; Park, C. Positive impact of radiation dose on disease free survival and locoregional control in postoperative radiotherapy for squamous cell carcinoma of esophagus. Dis. Esophagus 2009, 22, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Chen, L.; Zhang, S.; Wu, Q.; Jiang, X.; Zhu, H.; Wang, J.; Li, Z.; Xu, Y.; Zhang, Y.J.; et al. Predictive factors for acute radiation pneumonitis in postoperative intensity modulated radiation therapy and volumetric modulated arc therapy of esophageal cancer. Thorac. Cancer 2015, 6, 49–57. [Google Scholar] [CrossRef]
- Mao, Y.-S.; Gao, S.-G.; Wang, Q.; Shi, X.-T.; Li, Y.; Gao, W.-J.; Guan, F.-S.; Li, X.F.; Han, Y.-T.; Liu, Y.-Y.; et al. Analysis of a registry database for esophageal cancer from high-volume centers in China. Dis. Esophagus 2019, 33, doz091. [Google Scholar] [CrossRef]
- The Cancer Genome Atlas Research Network; Kim, J.; Bowlby, R.; Mungall, A.J.; Robertson, A.G.; Odze, R.D.; Cherniack, A.D.; Shih, J.; Pedamallu, C.S.; Cibulskis, C.; et al. Integrated genomic characterization of oesophageal carcinoma. Nature 2017, 541, 169–175. [Google Scholar] [CrossRef]
- Dale, R.G. The application of the linear-quadratic dose-effect equation to fractionated and protracted radiotherapy. Br. J. Radiol. 1985, 58, 515–528. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lee, K.-E.; Im, Y.-H.; Kang, W.K.; Park, K.; Kim, K.; Shim, Y.M. Adjuvant Chemotherapy with 5-Fluorouracil and Cisplatin in Lymph Node-Positive Thoracic Esophageal Squamous Cell Carcinoma. Ann. Thorac. Surg. 2005, 80, 1170–1175. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liao, Z.; Jin, J.; Ajani, J.; Chang, J.Y.; Jeter, M.; Guerrero, T.; Stevens, C.W.; Swisher, S.; Ho, L.; et al. Dose-response relationship in locoregional control for patients with stage II-III esophageal cancer treated with concurrent chemotherapy and radiotherapy. Int. J. Radiat. Oncol. 2005, 61, 656–664. [Google Scholar] [CrossRef] [PubMed]
- Semenkovich, T.R.; Samson, P.P.; Hudson, J.L.; Subramanian, M.; Meyers, B.F.; Kozower, B.D.; Kreisel, D.; Patterson, G.A.; Robinson, C.G.; Bradley, J.D.; et al. Induction Radiation Therapy for Esophageal Cancer: Does Dose Affect Outcomes? Ann. Thorac. Surg. 2018, 107, 903–911. [Google Scholar] [CrossRef] [PubMed]
- Herskovic, A.; Martz, K.; Al-Sarraf, M.; Leichman, L.; Brindle, J.; Vaitkevicius, V.; Cooper, J.; Byhardt, R.; Davis, L.; Emami, B. Combined Chemotherapy and Radiotherapy Compared with Radiotherapy Alone in Patients with Cancer of the Esophagus. N. Engl. J. Med. 1992, 326, 1593–1598. [Google Scholar] [CrossRef] [PubMed]
- Minsky, B.D.; Pajak, T.F.; Ginsberg, R.J.; Pisansky, T.M.; Martenson, J.; Komaki, R.; Okawara, G.; Rosenthal, S.A.; Kelsen, D.P. INT 0123 (Radiation Therapy Oncology Group 94-05) Phase III Trial of Combined-Modality Therapy for Esophageal Cancer: High-Dose Versus Standard-Dose Radiation Therapy. J. Clin. Oncol. 2002, 20, 1167–1174. [Google Scholar] [CrossRef]
- Yu, W.; Cai, X.-W.; Liu, Q.; Zhu, Z.-F.; Feng, W.; Zhang, Q.; Zhang, Y.-J.; Yao, Z.-F.; Fu, X.-L. Safety of dose escalation by simultaneous integrated boosting radiation dose within the primary tumor guided by 18FDG-PET/CT for esophageal cancer. Radiother. Oncol. 2015, 114, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Welsh, J.W.; Seyedin, S.N.; Allen, P.K.; Hofstetter, W.L.; Ajani, J.A.; Chang, J.Y.; Gomez, D.R.; Amini, A.; Swisher, S.G.; Blum, M.A.; et al. Local Control and Toxicity of a Simultaneous Integrated Boost for Dose Escalation in Locally Advanced Esophageal Cancer: Interim Results from a Prospective Phase I/II Trial. J. Thorac. Oncol. 2016, 12, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Menon, H.; Verma, V.; Seyedin, S.N.; Ajani, J.A.; Hofstetter, W.L.; Nguyen, Q.-N.; Chang, J.Y.; Gomez, D.R.; Amini, A.; et al. Results of a Phase 1/2 Trial of Chemoradiotherapy with Simultaneous Integrated Boost of Radiotherapy Dose in Unresectable Locally Advanced Esophageal Cancer. JAMA Oncol. 2019, 5, 1597–1604. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Ni, W.; Wang, X.; Zhou, Z.; Deng, W.; Chang, X.; Chen, D.; Feng, Q.; Liang, J.; Wang, X.; et al. A phase I/II radiation dose escalation trial using simultaneous integrated boost technique with elective nodal irradiation and concurrent chemotherapy for unresectable esophageal Cancer. Radiat. Oncol. 2019, 14, 48. [Google Scholar] [CrossRef] [PubMed]
- Hulshof, M.C.C.M.; Geijsen, E.D.; Rozema, T.; Oppedijk, V.; Buijsen, J.; Neelis, K.J.; Nuyttens, J.J.M.E.; van der Sangen, M.J.C.; Jeene, P.M.; Reinders, J.G.; et al. Randomized Study on Dose Escalation in Definitive Chemoradiation for Patients with Locally Advanced Esophageal Cancer (ARTDECO Study). J. Clin. Oncol. 2021, 39, 2816–2824. [Google Scholar] [CrossRef] [PubMed]
- Sudo, K.; Xiao, L.; Wadhwa, R.; Shiozaki, H.; Elimova, E.; Taketa, T.; Blum, M.A.; Lee, J.H.; Bhutani, M.S.; Weston, B.; et al. Importance of Surveillance and Success of Salvage Strategies After Definitive Chemoradiation in Patients with Esophageal Cancer. J. Clin. Oncol. 2014, 32, 3400–3405. [Google Scholar] [CrossRef] [PubMed]
- Kelly, R.J.; Ajani, J.A.; Kuzdzal, J.; Zander, T.; Van Cutsem, E.; Piessen, G.; Mendez, G.; Feliciano, J.; Motoyama, S.; Lièvre, A.; et al. Adjuvant Nivolumab in Resected Esophageal or Gastroesophageal Junction Cancer. N. Engl. J. Med. 2021, 384, 1191–1203. [Google Scholar] [CrossRef] [PubMed]
- Teniere, P.; Hay, J.M.; Fingerhut, A.; Fagniez, P.L. Postoperative radiation therapy does not increase survival after curative resection for squamous cell carcinoma of the middle and lower esophagus as shown by a multicenter controlled trial. French University Association for Surgical Research. Surg. Gynecol. Obs. 1991, 173, 123–130. [Google Scholar]
- Fok, M.; Sham, J.S.; Choy, D.; Cheng, S.W.; Wong, J. Postoperative radiotherapy for carcinoma of the esophagus: A prospective, randomized controlled study. Surgery 1993, 113, 138–147. [Google Scholar]
- Zieren, H.U.; Jacobi, C.A.; Pichlmaier, H.; Staar, S. Adjuvant postoperative radiation therapy after curative resection of squamous cell carcinoma of the thoracic esophagus: A prospective randomized study. World J. Surg. 1995, 19, 444–449. [Google Scholar] [CrossRef]

| Characteristics | No. (%) (All 3591 Cases) |
|---|---|
| Age | |
| <70 years | 3196 (89.00%) |
| ≥70 years | 395 (11.00%) |
| Median (IQR) [years] | 58 (52–65) |
| Sex | |
| Male | 2777 (77.33%) |
| Female | 814 (22.67%) |
| Thoracotomy | |
| Sweet | 1697 (47.25%) |
| Ivor–Lewis | 104 (2.90%) |
| McKeown | 1790 (49.85%) |
| Location | |
| Upper third | 338 (9.41%) |
| Middle third | 2174 (60.54%) |
| Lower third | 1079 (30.05%) |
| Length | |
| ≤5 cm | 2121 (59.06%) |
| >5 cm | 1470 (40.94%) |
| Median (IQR) [cm] | 5 (4–6) |
| Differentiation | |
| Well | 676 (18.82%) |
| Moderate | 2062 (57.42%) |
| Poorly | 853 (23.75%) |
| LVSI | |
| no | 3108 (86.55%) |
| yes | 483 (13.45%) |
| T stage (AJCC 8th) | |
| T1b | 191 (5.32%) |
| T2 | 710 (19.77%) |
| T3 | 2492 (69.40%) |
| T4a | 198 (5.51%) |
| N stage (AJCC 8th) | |
| N0 | 1711 (47.65%) |
| N1 | 1035 (28.82%) |
| N2 | 622 (17.32%) |
| N3 | 223 (6.21%) |
| TNM stage (AJCC 8th) | |
| IB | 191 (5.32%) |
| IIA | 896 (24.95%) |
| IIB | 662 (18.43%) |
| IIIA | 203 (5.65%) |
| IIIB | 1353 (37.68%) |
| IVA | 286 (7.96%) |
| POCT | |
| No | 3371 (93.87%) |
| POCT without RT | 63 (1.76%) |
| Concurrent CRT | 106 (2.95%) |
| Subsequent CRT | 51 (1.42%) |
| PORT | |
| No | 2677 (74.55%) |
| Yes | 914 (25.45%) |
| Radiation technique | |
| Conventional RT (2D-RT) | 191 (20.90%) |
| 3D-CRT | 41 (4.48%) |
| IMRT | 678 (74.62%) |
| Radiation dose (EQD2) | |
| 1–39Gy | 22 (2.41%) |
| 40–49Gy | 38 (4.16%) |
| 50–54Gy | 450 (49.23%) |
| 55–60Gy | 404 (44.20%) |
| Median (IQR) [Gy] | 54 (50–60) |
| Radiation Dose (EQD2) | No. | 5-Year OS (%) | Log-Rank p-Value | 5-Year DFS (%) | Log-Rank p-Value | 5-Year LRFS (%) | Log-Rank p-Value |
|---|---|---|---|---|---|---|---|
| 0 Gy (non-PORT) | 1987 | 47.37 | 0.001 | 40.88 | 0.001 | 53.89 | <0.001 |
| 1–39 Gy | 16 | 44.64 | 35.90 | 47.20 | |||
| 40–49 Gy | 37 | 48.50 | 43.07 | 55.85 | |||
| 50–54 Gy | 369 | 57.44 | 50.74 | 83.03 | |||
| 55–60 Gy | 356 | 53.80 | 47.17 | 78.90 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, W.; Chang, X.; Zhang, W.; Yang, J.; Yu, S.; Deng, W.; Ni, W.; Zhou, Z.; Chen, D.; Feng, Q.; et al. Effect of Adjuvant Radiation Dose on Survival in Patients with Esophageal Squamous Cell Carcinoma. Cancers 2022, 14, 5879. https://doi.org/10.3390/cancers14235879
Han W, Chang X, Zhang W, Yang J, Yu S, Deng W, Ni W, Zhou Z, Chen D, Feng Q, et al. Effect of Adjuvant Radiation Dose on Survival in Patients with Esophageal Squamous Cell Carcinoma. Cancers. 2022; 14(23):5879. https://doi.org/10.3390/cancers14235879
Chicago/Turabian StyleHan, Weiming, Xiao Chang, Wencheng Zhang, Jingsong Yang, Shufei Yu, Wei Deng, Wenjie Ni, Zongmei Zhou, Dongfu Chen, Qinfu Feng, and et al. 2022. "Effect of Adjuvant Radiation Dose on Survival in Patients with Esophageal Squamous Cell Carcinoma" Cancers 14, no. 23: 5879. https://doi.org/10.3390/cancers14235879
APA StyleHan, W., Chang, X., Zhang, W., Yang, J., Yu, S., Deng, W., Ni, W., Zhou, Z., Chen, D., Feng, Q., Liang, J., Hui, Z., Wang, L., Gao, S., Lin, Y., Chen, X., Chen, J., & Xiao, Z. (2022). Effect of Adjuvant Radiation Dose on Survival in Patients with Esophageal Squamous Cell Carcinoma. Cancers, 14(23), 5879. https://doi.org/10.3390/cancers14235879






