Towards Novel Gene and Cell Therapy Approaches for Cervical Cancer
Abstract
Simple Summary
Abstract
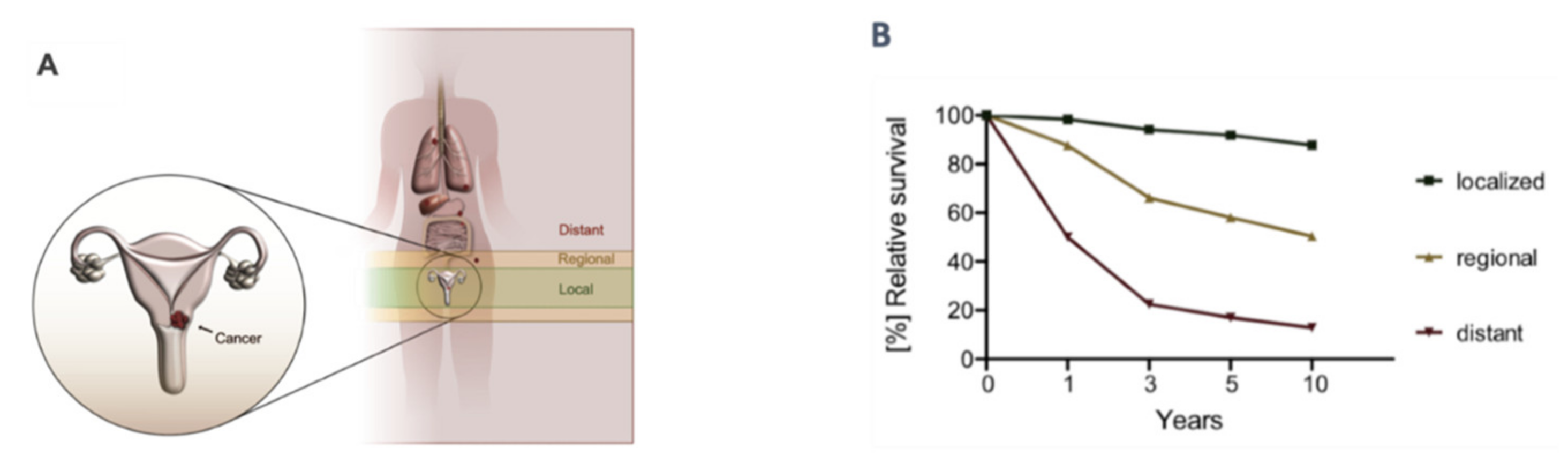
1. Introduction
2. HPV Prevention Vaccine
3. Immune Checkpoint Inhibitors in the Treatment of Cervical Cancer
4. Cell-Based Therapies
4.1. Adoptive T Cell Therapy with Unmodified Cells
4.2. Natural Tumor-Infiltrating Lymphocytes
4.3. Alternative Cell Sources for Immune Therapy
4.4. Modification of Somatic Cells for Therapeutic Use
4.5. Chimeric Antigen Receptor-Based Cell Therapy
4.6. TCR-Modified Cell Therapy
5. Combinatorial Therapies—Chemo-/Radiation Therapy
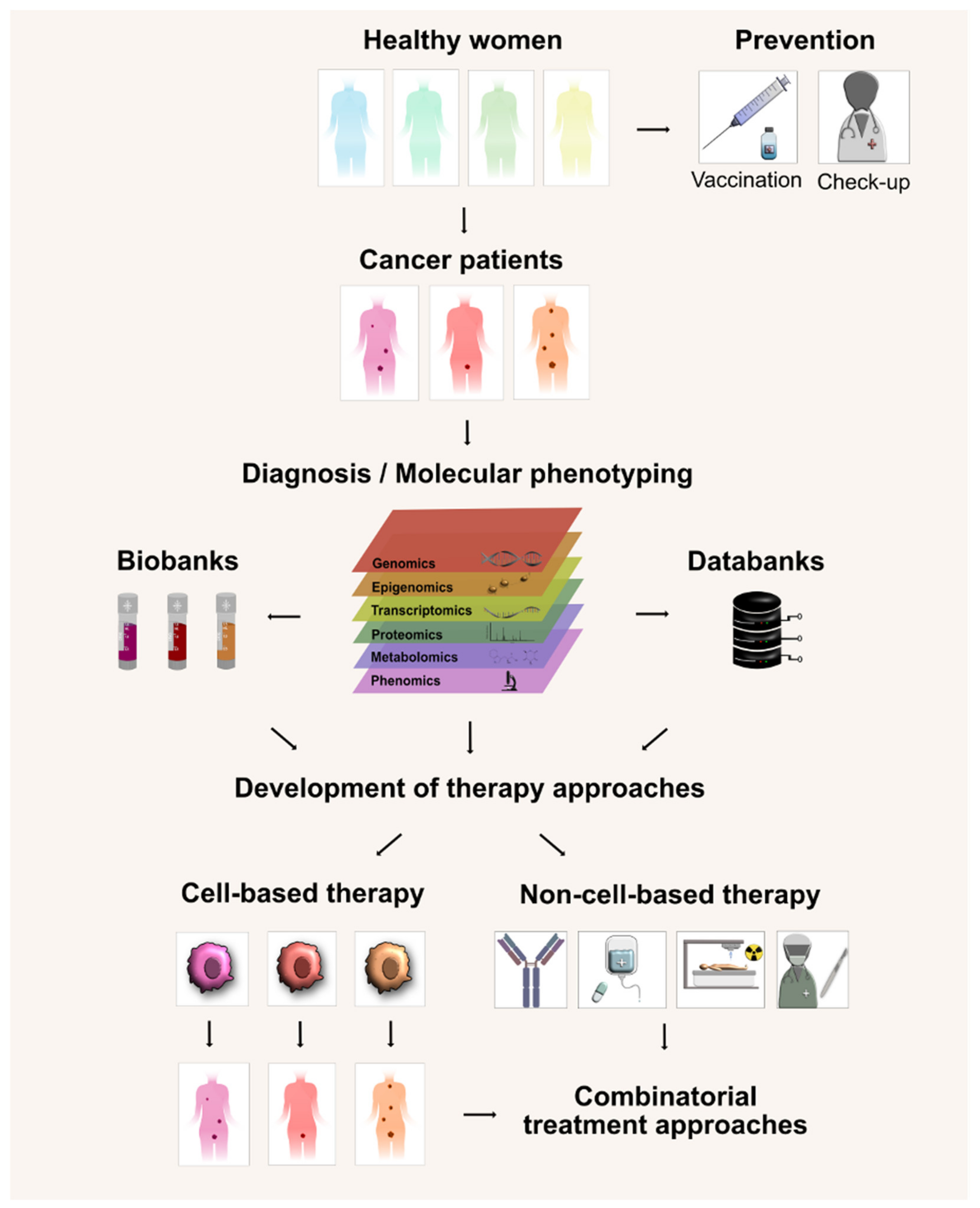
6. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA. Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- The American Cancer Society Cancer.org. 1.800.227.2345. 2020. Available online: https://www.cancer.org/content/dam/CRC/PDF/Public/8893.00.pdf (accessed on 29 December 2022).
- Gasparini, R.; Panatto, D. Cervical cancer: From Hippocrates through Rigoni-Stern to zur Hausen. Vaccine 2009, 27. [Google Scholar] [CrossRef] [PubMed]
- Hajdu, S.I. A note from history: Landmarks in history of cancer, part 1. Cancer 2011, 117, 1097–1102. [Google Scholar] [CrossRef] [PubMed]
- Wertheim, E. The extended abdominal operation for carcinoma uteri (based on 500 operative cases). Am. J. Obstet. Dis. Women Child. 1912, 66, 169–232. [Google Scholar]
- Giannini, A.; Bogani, G.; Vizza, E.; Chiantera, V.; Laganà, A.S.; Muzii, L.; Salerno, M.G.; Caserta, D.; D’Oria, O. Advances on Prevention and Screening of Gynecologic Tumors: Are We Stepping Forward? Healthcare 2022, 10, 1605. [Google Scholar] [CrossRef]
- Swailes, A.L.; Hossler, C.E.; Kesterson, J.P. Pathway to the Papanicolaou smear: The development of cervical cytology in twentieth-century America and implications in the present day. Gynecol. Oncol. 2019, 154, 3–7. [Google Scholar] [CrossRef]
- Kokka, F.; Bryant, A.; Brockbank, E.; Powell, M.; Oram, D. Hysterectomy with radiotherapy or chemotherapy or both for women with locally advanced cervical cancer. Cochrane Database Syst. Rev. 2015, 7, CD010260. [Google Scholar] [CrossRef]
- Forman, D.; de Martel, C.; Lacey, C.J.; Soerjomatarama, I.; Lortet-Tieulent, J.; Bruni, L.; Vignat, J.; Ferlay, J.; Bray, F.; Plummer, M.; et al. Global burden of human papillomavirus and related diseases. Vaccine 2012, 30, 12–23. [Google Scholar] [CrossRef]
- Roberts, J.N.; Buck, C.B.; Thompson, C.D.; Kines, R.; Bernardo, M.; Choyke, P.L.; Lowy, D.R.; Schiller, J.T. Genital transmission of HPV in a mouse model is potentiated by nonoxynol-9 and inhibited by carrageenan. Nat. Med. 2007, 13, 857–861. [Google Scholar] [CrossRef]
- Van Doorslaer, K.; Li, Z.; Xirasagar, S.; Maes, P.; Kaminsky, D.; Liou, D.; Sun, Q.; Kaur, R.; Huyen, Y.; McBride, A.A. The Papillomavirus Episteme: A major update to the papillomavirus sequence database. Nucleic Acids Res. 2017, 45, 499–506. [Google Scholar] [CrossRef]
- Mühr, L.S.A.; Eklund, C.; Dillner, J. Towards quality and order in human papillomavirus research. Virology 2018, 519, 74–76. [Google Scholar] [CrossRef] [PubMed]
- Gissmann, L.; Hausen, H. Zur Partial characterization of viral DNA from human genital warts (condylomata acuminata). Int. J. Cancer 1980, 25, 605–609. [Google Scholar] [CrossRef] [PubMed]
- Tommasino, M. The human papillomavirus family and its role in carcinogenesis. Semin. Cancer Biol. 2014, 26, 13–21. [Google Scholar] [CrossRef] [PubMed]
- de Sanjose, S.; Quint, W.G.V.; Alemany, L.; Geraets, D.T.; Klaustermeier, J.E.; Lloveras, B.; Tous, S.; Felix, A.; Bravo, L.E.; Shin, H.R.; et al. Human papillomavirus genotype attribution in invasive cervical cancer: A retrospective cross-sectional worldwide study. Lancet Oncol. 2010, 11, 1048–1056. [Google Scholar] [CrossRef]
- de Martel, C.; Plummer, M.; Vignat, J.; Franceschi, S. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int. J. Cancer 2017, 141, 664–670. [Google Scholar] [CrossRef]
- Hebner, C.M.; Laimins, L.A. Human papillomaviruses: Basic mechanisms of pathogenesis and oncogenicity. Rev. Med. Virol. 2006, 16, 83–97. [Google Scholar] [CrossRef]
- McCredie, M.R.; Sharples, K.J.; Paul, C.; Baranyai, J.; Medley, G.; Jones, R.W.; Skegg, D.C. Natural history of cervical neoplasia and risk of invasive cancer in women with cervical intraepithelial neoplasia 3: A retrospective cohort study. Lancet Oncol. 2008, 9, 425–434. [Google Scholar] [CrossRef]
- Kirnbauer, R.; Booy, F.; Cheng, N.; Lowy, D.R.; Schiller, J.T. Papillomavirus L1 major capsid protein self-assembles into virus-like particles that are highly immunogenic. Proc. Natl. Acad. Sci. USA 1992, 89, 12180–12184. [Google Scholar] [CrossRef]
- Reisinger, K.S.; Block, S.L.; Lazcano-Ponce, E.; Samakoses, R.; Esser, M.T.; Erick, J.; Puchalski, D.; Giacoletti, K.E.D.; Sings, H.L.; Lukac, S.; et al. Safety and persistent immunogenicity of a quadrivalent human papillomavirus types 6, 11, 16, 18 L1 virus-like particle vaccine in preadolescents and adolescents: A randomized controlled trial. Pediatr. Infect. Dis. J. 2007, 26, 201–209. [Google Scholar] [CrossRef]
- Romanowski, B.; Schwarz, T.F.; Ferguson, L.M.; Peters, K.; Dionne, M.; Schulze, K.; Ramjattan, B.; Hillemanns, P.; Catteau, G.; Dobbelaere, K.; et al. Immunogenicity and safety of the HPV-16/18 AS04-adjuvanted vaccine administered as a 2-dose schedule compared with the licensed 3-dose schedule: Results from a randomized study. Hum. Vaccin. 2011, 7, 1374–1386. [Google Scholar] [CrossRef]
- Joura, E.A.; Ulied, A.; Vandermeulen, C.; Rua Figueroa, M.; Seppä, I.; Hernandez Aguado, J.J.; Ahonen, A.; Reich, O.; Virta, M.; Perino, A.; et al. Immunogenicity and safety of a nine-valent human papillomavirus vaccine in women 27–45 years of age compared to women 16–26 years of age: An open-label phase 3 study. Vaccine 2021, 39, 2800–2809. [Google Scholar] [CrossRef] [PubMed]
- World Health, O. Human papillomavirus vaccines: WHO position paper, May 2017–Recommendations. Vaccine 2017, 35, 5753–5755. [Google Scholar] [CrossRef]
- Munoz, N.; Kjaer, S.K.; Sigurdsson, K.; Iversen, O.E.; Hernandez-Avila, M.; Wheeler, C.M.; Perez, G.; Brown, D.R.; Koutsky, L.A.; Tay, E.H.; et al. Impact of Human Papillomavirus (HPV)-6/11/16/18 Vaccine on All HPV-Associated Genital Diseases in Young Women. J. Natl. Cancer Inst. 2010, 102, 325–339. [Google Scholar] [CrossRef] [PubMed]
- Donovan, B.; Franklin, N.; Guy, R.; Grulich, A.E.; Regan, D.G.; Ali, H.; Wand, H.; Fairley, C.K. Quadrivalent human papillomavirus vaccination and trends in genital warts in Australia: Analysis of national sentinel surveillance data. Lancet Infect. Dis. 2011, 11, 39–44. [Google Scholar] [CrossRef]
- Ali, H.; Guy, R.J.; Wand, H.; Read, T.R.H.; Regan, D.G.; Grulich, A.E.; Fairley, C.K.; Donovan, B. Decline in in-patient treatments of genital warts among young Australians following the national HPV vaccination program. BMC Infect. Dis. 2013, 13, 140. [Google Scholar] [CrossRef]
- Lei, J.; Ploner, A.; Elfström, K.M.; Wang, J.; Roth, A.; Fang, F.; Sundström, K.; Dillner, J.; Sparén, P. HPV Vaccination and the Risk of Invasive Cervical Cancer. N. Engl. J. Med. 2020, 383, 1340–1348. [Google Scholar] [CrossRef]
- Dorji, T.; Nopsopon, T.; Tamang, S.T.; Pongpirul, K. Human papillomavirus vaccination uptake in low-and middle-income countries: A meta-analysis. EClinicalMedicine 2021, 34, 100836. [Google Scholar] [CrossRef]
- Torre, L.A.; Islami, F.; Siegel, R.L.; Ward, E.M.; Jemal, A. Global cancer in women: Burden and trends. Cancer Epidemiol. Biomarkers Prev. 2017, 26, 44–457. [Google Scholar] [CrossRef]
- Rumfield, C.S.; Roller, N.; Pellom, S.T.; Schlom, J.; Jochems, C. Therapeutic Vaccines for HPV-Associated Malignancies. ImmunoTargets Ther. 2020, 9, 167–200. [Google Scholar] [CrossRef]
- Waldman, A.D.; Fritz, J.M.; Lenardo, M.J. A guide to cancer immunotherapy: From T cell basic science to clinical practice. Nat. Rev. Immunol. 2020, 20, 651–668. [Google Scholar] [CrossRef]
- Naimi, A.; Mohammed, R.N.; Raji, A.; Chupradit, S.; Yumashev, A.V.; Suksatan, W.; Shalaby, M.N.; Thangavelu, L.; Kamrava, S.; Shomali, N.; et al. Tumor immunotherapies by immune checkpoint inhibitors (ICIs); the pros and cons. Cell Commun. Signal. 2022, 20, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Xu, L.; Yi, M.; Yu, S.; Wu, K.; Luo, S. Novel immune checkpoint targets: Moving beyond PD-1 and CTLA-4. Mol. Cancer 2019, 18. [Google Scholar] [CrossRef] [PubMed]
- Jacob, J.B.; Jacob, M.K.; Parajuli, P. Review of immune checkpoint inhibitors in immuno-oncology. Adv. Pharmacol. 2021, 91, 111–139. [Google Scholar]
- Mezache, L.; Paniccia, B.; Nyinawabera, A.; Nuovo, G.J. Enhanced expression of PD L1 in cervical intraepithelial neoplasia and cervical cancers. Mod. Pathol. 2015, 28, 1594–1602. [Google Scholar] [CrossRef]
- Heeren, A.M.; Punt, S.; Bleeker, M.C.; Gaarenstroom, K.N.; Van Der Velden, J.; Kenter, G.G.; De Gruijl, T.D.; Jordanova, E.S. Prognostic effect of different PD-L1 expression patterns in squamous cell carcinoma and adenocarcinoma of the cervix. Mod. Pathol. 2016, 29, 753–763. [Google Scholar] [CrossRef]
- Meng, Y.; Liang, H.; Hu, J.; Liu, S.; Hao, X.; Wong, M.S.K.; Li, X.; Hu, L. PD-L1 expression correlates with tumor infiltrating lymphocytes and response to neoadjuvant chemotherapy in cervical cancer. J. Cancer 2018, 9, 2938–2945. [Google Scholar] [CrossRef]
- de Bono, J.S.; Concin, N.; Hong, D.S.; Thistlethwaite, F.C.; Machiels, J.P.; Arkenau, H.T.; Plummer, R.; Jones, R.H.; Nielsen, D.; Windfeld, K.; et al. Tisotumab vedotin in patients with advanced or metastatic solid tumours (InnovaTV 201): A first-in-human, multicentre, phase 1–2 trial. Lancet Oncol. 2019, 20, 383–393. [Google Scholar] [CrossRef]
- Chung, H.C.; Ros, W.; Delord, J.P.; Perets, R.; Italiano, A.; Shapira-Frommer, R.; Manzuk, L.; Piha-Paul, S.A.; Xu, L.; Zeigenfuss, S.; et al. Efficacy and safety of pembrolizumab in previously treated advanced cervical cancer: Results from the phase II KEYNOTE-158 study. J. Clin. Oncol. 2019, 37, 1470–1478. [Google Scholar] [CrossRef]
- Chung, H.; Delord, J.-P.; Perets, R.; Italiano, A.; Shapira-Frommer, R.; Manzuk, L.; Piha-Paul, S.; Xu, L.; Jin, F.; Norwood, K.; et al. Pembrolizumab treatment of advanced cervical cancer: Updated results from the phase II KEYNOTE-158 study. Gynecol. Oncol. 2021, 162, S1. [Google Scholar] [CrossRef]
- Colombo, N.; Dubot, C.; Lorusso, D.; Caceres, M.V.; Hasegawa, K.; Shapira-Frommer, R.; Tewari, K.S.; Salman, P.; Hoyos Usta, E.; Yañez, E.; et al. Pembrolizumab for Persistent, Recurrent, or Metastatic Cervical Cancer. N. Engl. J. Med. 2021, 385, 1856–1867. [Google Scholar] [CrossRef]
- Tewari, K.S.; Monk, B.J.; Vergote, I.; Miller, A.; de Melo, A.C.; Kim, H.-S.; Kim, Y.M.; Lisyanskaya, A.; Samouëlian, V.; Lorusso, D.; et al. Survival with Cemiplimab in Recurrent Cervical Cancer. N. Engl. J. Med. 2022, 386, 544–555. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.C.; Roane, B.M.; Leath, C.A. Novel Therapeutics for Recurrent Cervical Cancer: Moving Towards Personalized Therapy. Drugs 2020, 80, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Kagabu, M.; Nagasawa, T.; Sato, C.; Fukagawa, Y.; Kawamura, H.; Tomabechi, H.; Takemoto, S.; Shoji, T.; Baba, T. Immunotherapy for uterine cervical cancer using checkpoint inhibitors: Future directions. Int. J. Mol. Sci. 2020, 21, 2335. [Google Scholar] [CrossRef] [PubMed]
- Villemin, C.; Six, A.; Neville, B.A.; Lawley, T.D.; Robinson, M.J.; Bakdash, G. The heightened importance of the microbiome in cancer immunotherapy. Trends Immunol. 2022, 44, 44–59. [Google Scholar] [CrossRef]
- Hayase, E.; Jenq, R.R. Role of the intestinal microbiome and microbial-derived metabolites in immune checkpoint blockade immunotherapy of cancer. Genome Med. 2021, 13, 107. [Google Scholar] [CrossRef]
- Sivan, A.; Corrales, L.; Hubert, N.; Williams, J.B.; Aquino-Michaels, K.; Earley, Z.M.; Benyamin, F.W.; Lei, Y.M.; Jabri, B.; Alegre, M.L.; et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science 2015, 350, 1084–1089. [Google Scholar] [CrossRef]
- Xu, H.; Cao, C.; Ren, Y.; Weng, S.; Liu, L.; Guo, C.; Wang, L.; Han, X.; Ren, J.; Liu, Z. Antitumor effects of fecal microbiota transplantation: Implications for microbiome modulation in cancer treatment. Front. Immunol. 2022, 13, 949490. [Google Scholar] [CrossRef]
- Chitapanarux, I.; Chitapanarux, T.; Traisathit, P.; Kudumpee, S.; Tharavichitkul, E.; Lorvidhaya, V. Randomized controlled trial of live lactobacillus acidophilus plus bifidobacterium bifidum in prophylaxis of diarrhea during radiotherapy in cervical cancer patients. Radiat. Oncol. 2010, 5, 31. [Google Scholar] [CrossRef]
- Ramos-Casals, M.; Brahmer, J.R.; Callahan, M.K.; Flores-Chávez, A.; Keegan, N.; Khamashta, M.A.; Lambotte, O.; Mariette, X.; Prat, A.; Suárez-Almazor, M.E. Immune-related adverse events of checkpoint inhibitors. Nat. Rev. Dis. Prim. 2020, 6, 38. [Google Scholar] [CrossRef]
- Chmielewski, M.; Abken, H. TRUCKS, the fourth-generation CAR T cells: Current developments and clinical translation. Adv. Cell Gene Ther. 2020, 3, 1–9. [Google Scholar] [CrossRef]
- Huynh, D.; Winter, P.; Märkl, F.; Endres, S.; Kobold, S. Beyond direct killing—Novel cellular immunotherapeutic strategies to reshape the tumor microenvironment. Semin. Immunopathol. 2022. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Cao, Y.J. Engineered T Cell Therapy for Cancer in the Clinic. Front. Immunol. 2019, 10, 2250. [Google Scholar] [CrossRef] [PubMed]
- Laskowski, T.J.; Biederstädt, A.; Rezvani, K. Natural killer cells in antitumour adoptive cell immunotherapy. Nat. Rev. Cancer 2022, 22, 557–575. [Google Scholar] [CrossRef] [PubMed]
- Kantoff, P.W.; Higano, C.S.; Shore, N.D.; Berger, E.R.; Small, E.J.; Penson, D.F.; Redfern, C.H.; Ferrari, A.C.; Dreicer, R.; Sims, R.B.; et al. Sipuleucel-T Immunotherapy for Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2010, 363, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Neelapu, S.S.; Locke, F.L.; Bartlett, N.L.; Lekakis, L.J.; Miklos, D.B.; Jacobson, C.A.; Braunschweig, I.; Oluwole, O.O.; Siddiqi, T.; Lin, Y.; et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N. Engl. J. Med. 2017, 377, 2531–2544. [Google Scholar] [CrossRef]
- Maude, S.L.; Laetsch, T.W.; Buechner, J.; Rives, S.; Boyer, M.; Bittencourt, H.; Bader, P.; Verneris, M.R.; Stefanski, H.E.; Myers, G.D.; et al. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. N. Engl. J. Med. 2018, 378, 439–448. [Google Scholar] [CrossRef]
- Li, X.; Cheng, Y.; Cheng, Y.; Shi, H. Transcriptome Analysis Reveals the Immune Infiltration Profiles in Cervical Cancer and Identifies KRT23 as an Immunotherapeutic Target. Front. Oncol. 2022, 12, 779356. [Google Scholar] [CrossRef]
- Schwartz, R.S. Paul Ehrlich’s Magic Bullets. N. Engl. J. Med. 2004, 350, 1079–1080. [Google Scholar] [CrossRef]
- Balkwill, F.; Mantovani, A. Inflammation and cancer: Back to Virchow? Lancet 2001, 357, 539–545. [Google Scholar] [CrossRef]
- Beaugerie, L.; Svrcek, M.; Seksik, P.; Bouvier, A.M.; Simon, T.; Allez, M.; Brixi, H.; Gornet, J.M.; Altwegg, R.; Beau, P.; et al. Risk of colorectal high-grade dysplasia and cancer in a prospective observational cohort of patients with inflammatory bowel disease. Gastroenterology 2013, 145, 166–175. [Google Scholar] [CrossRef]
- Coussens, L.M.; Werb, Z. Inflammation and Cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.P.; Amstad, P.; Raja, K.; Ambs, S.; Nagashima, M.; Bennett, W.P.; Shields, P.G.; Ham, A.J.; Swenberg, J.A.; Marrogi, A.J.; et al. Increased p53 mutation load in noncancerous colon tissue from ulcerative colitis: A cancer-prone chronic inflammatory disease. Cancer Res. 2000, 60, 3333–3337. [Google Scholar] [PubMed]
- Askeland, E.J.; Newton, M.R.; O’Donnell, M.A.; Luo, Y. Bladder cancer immunotherapy: BCG and beyond. Adv. Urol. 2012, 2012, 181987. [Google Scholar] [CrossRef] [PubMed]
- Welniak, L.A.; Blazar, B.R.; Murphy, W.J. Immunobiology of allogeneic hematopoietic stem cell transplantation. Annu. Rev. Immunol. 2007, 25, 139–170. [Google Scholar] [CrossRef]
- Goldman, J.M.; Gale, R.P.; Horowitz, M.M.; Biggs, J.C.; Champlin, R.E.; Gluckman, E.; Hoffmann, R.G.; Jacobsen, S.J.; Marmont, A.M.; McGlave, P.B.; et al. Bone marrow transplantation for chronic myelogenous leukemia in chronic phase. Increased risk for relapse associated with T-cell depletion. Ann. Intern. Med. 1988, 108, 806–814. [Google Scholar] [CrossRef]
- Clemente, C.G.; Mihm, M.C.; Bufalino, R.; Zurrida, S.; Collini, P.; Cascinelli, N. Prognostic value of tumor infiltrating lymphocytes in the vertical growth phase of primary cutaneous melanoma. Cancer 1996, 77, 1303–1310. [Google Scholar] [CrossRef]
- Dieu-Nosjean, M.C.; Antoine, M.; Danel, C.; Heudes, D.; Wislez, M.; Poulot, V.; Rabbe, N.; Laurans, L.; Tartour, E.; De Chaisemartin, L.; et al. Long-term survival for patients with non-small-cell lung cancer with intratumoral lymphoid structures. J. Clin. Oncol. 2008, 26, 4410–4417. [Google Scholar] [CrossRef]
- Fridman, W.H.; Pagès, F.; Sautès-Fridman, C.; Galon, J. The immune contexture in human tumours: Impact on clinical outcome. Nat. Rev. Cancer 2012, 12, 298–306. [Google Scholar] [CrossRef]
- Kitamura, T.; Qian, B.Z.; Pollard, J.W. Immune cell promotion of metastasis. Nat. Rev. Immunol. 2015, 15, 73–86. [Google Scholar] [CrossRef]
- Kondo, T.; Nakazawa, H.; Ito, F.; Hashimoto, Y.; Osaka, Y.; Futatsuyama, K.; Toma, H.; Tanabe, K. Favorable prognosis of renal cell carcinoma with increased expression of chemokines associated with a Th1-type immune response. Cancer Sci. 2006, 97, 780–786. [Google Scholar] [CrossRef]
- Oldford, S.A.; Robb, J.D.; Codner, D.; Gadag, V.; Watson, P.H.; Drover, S. Tumor cell expression of HLA-DM associates with a Th1 profile and predicts improved survival in breast carcinoma patients. Int. Immunol. 2006, 18, 1591–1602. [Google Scholar] [CrossRef] [PubMed]
- Tosolini, M.; Kirilovsky, A.; Mlecnik, B.; Fredriksen, T.; Mauger, S.; Bindea, G.; Berger, A.; Bruneval, P.; Fridman, W.H.; Pagès, F.; et al. Clinical impact of different classes of infiltrating T cytotoxic and helper cells (Th1, Th2, Treg, Th17) in patients with colorectal cancer. Cancer Res. 2011, 71, 1263–1271. [Google Scholar] [CrossRef] [PubMed]
- Ubukata, H.; Motohashi, G.; Tabuchi, T.; Nagata, H.; Konishi, S.; Tabuchi, T. Evaluations of interferon-γ/interleukin-4 ratio and neutrophil/lymphocyte ratio as prognostic indicators in gastric cancer patients. J. Surg. Oncol. 2010, 102, 742–747. [Google Scholar] [CrossRef] [PubMed]
- Vesalainen, S.; Lipponen, P.; Talja, M.; Syrjänen, K. Histological grade, perineural infiltration, tumour-infiltrating lymphocytes and apoptosis as determinants of long-term prognosis in prostatic adenocarcinoma. Eur. J. Cancer 1994, 30A, 1797–1803. [Google Scholar] [CrossRef] [PubMed]
- Zloza, A.; Al-Harthi, L. Multiple populations of T lymphocytes are distinguished by the level of CD4 and CD8 coexpression and require individual consideration. J. Leukoc. Biol. 2006, 79, 4–6. [Google Scholar] [CrossRef] [PubMed]
- Bethwaite, P.B.; Holloway, L.J.; Thornton, A.; Delahunt, B. Infiltration by immunocompetent cells in early stage invasive carcinoma of the uterine cervix: A prognostic study. Pathology 1996, 28, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Piersma, S.J.; Jordanova, E.S.; Van Poelgeest, M.I.E.; Kwappenberg, K.M.C.; Van Der Hulst, J.M.; Drijfhout, J.W.; Melief, C.J.M.; Kenter, G.G.; Fleuren, G.J.; Offringa, R.; et al. High number of intraepithelial CD8+ tumor-infiltrating lymphocytes is associated with the absence of lymph node metastases in patients with large early-stage cervical cancer. Cancer Res. 2007, 67, 354–361. [Google Scholar] [CrossRef]
- Ohno, A.; Iwata, T.; Katoh, Y.; Taniguchi, S.; Tanaka, K.; Nishio, H.; Nakamura, M.; Morisada, T.; Chen, G.; Saito, M.; et al. Tumor-infiltrating lymphocytes predict survival outcomes in patients with cervical cancer treated with concurrent chemoradiotherapy. Gynecol. Oncol. 2020, 159, 329–334. [Google Scholar] [CrossRef]
- Reits, E.A.; Hodge, J.W.; Herberts, C.A.; Groothuis, T.A.; Chakraborty, M.; Wansley, E.K.; Camphausen, K.; Luiten, R.M.; De Ru, A.H.; Neijssen, J.; et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J. Exp. Med. 2006, 203, 1259–1271. [Google Scholar] [CrossRef]
- Yoneyama, H.; Narumi, S.; Zhang, Y.; Murai, M.; Baggiolini, M.; Lanzavecchia, A.; Ichida, T.; Asakura, H.; Matsushima, K. Pivotal role of dendritic cell-derived CXCL10 in the retention of T helper cell 1 lymphocytes in secondary lymph nodes. J. Exp. Med. 2002, 195, 1257–1266. [Google Scholar] [CrossRef]
- Gardner, A.; Ruffell, B. Dendritic Cells and Cancer Immunity. Trends Immunol. 2016, 37, 855–865. [Google Scholar] [CrossRef] [PubMed]
- Abakushina, E.V.; Popova, L.I.; Zamyatnin, A.A.; Werner, J.; Mikhailovsky, N.V.; Bazhin, A.V. The advantages and challenges of anticancer dendritic cell vaccines and nk cells in adoptive cell immunotherapy. Vaccines 2021, 9, 1363. [Google Scholar] [CrossRef] [PubMed]
- Santin, A.; Bellone, S.; Roman, J.; Burnett, A.; Cannon, M.; Pecorelli, S. Therapeutic Vaccines for Cervical Cancer: Dendritic Cell-Based Immunotherapy. Curr. Pharm. Des. 2005, 11, 3485–3500. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhou, H.; Wang, W.; Fu, Y.X.; Zhu, M. A novel dendritic cell targeting HPV16 E7 synthetic vaccine in combination with PD-L1 blockade elicits therapeutic antitumor immunity in mice. Oncoimmunology 2016, 5, e1147641. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, R. Immunotherapy Targeting Tumor-Associated Macrophages. Front. Med. 2020, 7, 75. [Google Scholar] [CrossRef]
- Zhang, B.; Yao, G.; Zhang, Y.; Gao, J.; Yang, B.; Rao, Z.; Gao, J. M2-Polarized tumor-associated macrophages are associated with poor prognoses resulting from accelerated lymphangiogenesis in lung adenocarcinoma. Clinics 2011, 66, 1879–1886. [Google Scholar] [CrossRef]
- Wang, H.; Yung, M.M.H.; Ngan, H.Y.S.; Chan, K.K.L.; Chan, D.W. The impact of the tumor microenvironment on macrophage polarization in cancer metastatic progression. Int. J. Mol. Sci. 2021, 22. [Google Scholar] [CrossRef]
- Liu, Y.; Li, L.; Li, Y.; Zhao, X. Research Progress on Tumor-Associated Macrophages and Inflammation in Cervical Cancer. Biomed Res. Int. 2020, 2020, 6842963. [Google Scholar] [CrossRef]
- Cendrowicz, E.; Sas, Z.; Bremer, E.; Rygiel, T.P. The role of macrophages in cancer development and therapy. Cancers 2021, 13, 1946. [Google Scholar] [CrossRef]
- Heusinkveld, M.; de Vos van Steenwijk, P.J.; Goedemans, R.; Ramwadhdoebe, T.H.; Gorter, A.; Welters, M.J.P.; van Hall, T.; van der Burg, S.H. M2 Macrophages Induced by Prostaglandin E 2 and IL-6 from Cervical Carcinoma Are Switched to Activated M1 Macrophages by CD4 + Th1 Cells. J. Immunol. 2011, 187, 1157–1165. [Google Scholar] [CrossRef]
- Anfray, C.; Ummarino, A.; Andón, F.T.; Allavena, P. Current strategies to target tumor-associated-macrophages to improve anti-tumor immune responses. Cells 2020, 9, 46. [Google Scholar] [CrossRef]
- Ren, J.; Li, L.; Yu, B.; Xu, E.; Sun, N.; Li, X.; Xing, Z.; Han, X.; Cui, Y.; Wang, X.; et al. Extracellular vesicles mediated proinflammatory macrophage phenotype induced by radiotherapy in cervical cancer. BMC Cancer 2022, 22, 88. [Google Scholar] [CrossRef]
- Brempelis, K.J.; Cowan, C.M.; Kreuser, S.A.; Labadie, K.P.; Prieskorn, B.M.; Lieberman, N.A.P.; Ene, C.I.; Moyes, K.W.; Chinn, H.; Degolier, K.R.; et al. Genetically engineered macrophages persist in solid tumors and locally deliver therapeutic proteins to activate immune responses. J. Immunother. Cancer 2020, 8, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Kiessling, R.; Klein, E.; Wigzell, H. “Natural” killer cells in the mouse. I. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Specificity and distribution according to genotype. Eur. J. Immunol. 1975, 5, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Malmberg, K.J.; Carlsten, M.; Björklund, A.; Sohlberg, E.; Bryceson, Y.T.; Ljunggren, H.G. Natural killer cell-mediated immunosurveillance of human cancer. Semin. Immunol. 2017, 31, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Garrido, F.; Aptsiauri, N. Cancer immune escape: MHC expression in primary tumours versus metastases. Immunology 2019, 158, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Dibbern, M.E.; Bullock, T.N.; Jenkins, T.M.; Duska, L.R.; Stoler, M.H.; Mills, A.M. Loss of MHC class i expression in HPV-associated cervical and vulvar neoplasia: A potential mechanism of resistance to checkpoint inhibition. Am. J. Surg. Pathol. 2020, 44, 1184–1191. [Google Scholar] [CrossRef] [PubMed]
- Guerra, N.; Tan, Y.X.; Joncker, N.T.; Choy, A.; Gallardo, F.; Xiong, N.; Knoblaugh, S.; Cado, D.; Greenberg, N.R.; Raulet, D.H. NKG2D-Deficient Mice Are Defective in Tumor Surveillance in Models of Spontaneous Malignancy. Immunity 2008, 28, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Imai, K.; Matsuyama, S.; Miyake, S.; Suga, K.; Nakachi, K. Natural cytotoxic activity of peripheral-blood lymphocytes and cancer incidence: An 11-year follow-up study of a general population. Lancet 2000, 356, 1795–1799. [Google Scholar] [CrossRef]
- López-Soto, A.; Gonzalez, S.; Smyth, M.J.; Galluzzi, L. Control of Metastasis by NK Cells. Cancer Cell 2017, 32, 135–154. [Google Scholar] [CrossRef]
- Lanier, L.L. Up on the tightrope: Natural killer cell activation and inhibition. Nat. Immunol. 2008, 9, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Brodin, P.; Kärre, K.; Höglund, P. NK cell education: Not an on-off switch but a tunable rheostat. Trends Immunol. 2009, 30, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Burshtyn, D.N.; Long, E.O. Regulation through inhibitory receptors: Lessons from natural killer cells. Trends Cell Biol. 1997, 7, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Joncker, N.T.; Shifrin, N.; Delebecque, F.; Raulet, D.H. Mature natural killer cells reset their responsiveness when exposed to an altered MHC environment. J. Exp. Med. 2010, 207, 2065–2072. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, W.M.; Kim, S. Licensing of natural killer cells by self-major histocompatibility complex class I. Immunol. Rev. 2006, 214, 143–154. [Google Scholar] [CrossRef]
- Wang, W.; Erbe, A.K.; Hank, J.A.; Morris, Z.S.; Sondel, P.M. NK cell-mediated antibody-dependent cellular cytotoxicity in cancer immunotherapy. Front. Immunol. 2015, 6, 1–15. [Google Scholar] [CrossRef]
- Prager, I.; Watzl, C. Mechanisms of natural killer cell-mediated cellular cytotoxicity. J. Leukoc. Biol. 2019, 105, 1319–1329. [Google Scholar] [CrossRef]
- Park, M.H.; Lee, J.S.; Yoon, J.H. High expression of CX3CL1 by tumor cells correlates with a good prognosis and increased tumor-infiltrating CD8+ T cells, natural killer cells, and dendritic cells in breast carcinoma. J. Surg. Oncol. 2012, 106, 386–392. [Google Scholar] [CrossRef]
- Ponzetta, A.; Benigni, G.; Antonangeli, F.; Sciumè, G.; Sanseviero, E.; Zingoni, A.; Ricciardi, M.R.; Petrucci, M.T.; Santoni, A.; Bernardini, G. Multiple myeloma impairs bone marrow localization of effector natural killer cells by altering the chemokine microenvironment. Cancer Res. 2015, 75, 4766–4777. [Google Scholar] [CrossRef]
- Veluchamy, J.P.; Heeren, A.M.; Spanholtz, J.; van Eendenburg, J.D.H.; Heideman, D.A.M.; Kenter, G.G.; Verheul, H.M.; van der Vliet, H.J.; Jordanova, E.S.; de Gruijl, T.D. High-efficiency lysis of cervical cancer by allogeneic NK cells derived from umbilical cord progenitors is independent of HLA status. Cancer Immunol. Immunother. 2017, 66, 51–61. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, J.; Cui, Z.M.; Zhao, J.; Zheng, Y. Expression of the CXCl12/CXCR4 and CXCL16/CXCR6 axes in cervical intraepithelial neoplasia and cervical cancer. Chin. J. Cancer 2013, 32, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Yong, S.B.; Chung, J.Y.; Song, Y.; Kim, Y.H. Recent challenges and advances in genetically-engineered cell therapy. J. Pharm. Investig. 2018, 48, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, R.S.; Beitzel, B.F.; Schroder, A.R.W.; Shinn, P.; Chen, H.; Berry, C.C.; Ecker, J.R.; Bushman, F.D. Retroviral DNA integration: ASLV, HIV, and MLV show distinct target site preferences. PLoS Biol. 2004, 2, E234. [Google Scholar] [CrossRef]
- Suerth, J.D.; Maetzig, T.; Galla, M.; Baum, C.; Schambach, A. Self-Inactivating Alpharetroviral Vectors with a Split-Packaging Design. J. Virol. 2010, 84, 6626–6635. [Google Scholar] [CrossRef]
- Suerth, J.D.; Labenski, V.; Schambach, A. Alpharetroviral vectors: From a cancer-causing agent to a useful tool for human gene therapy. Viruses 2014, 6, 4811–4838. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.F.; Wu, T.C.; Monie, A.; Roden, R. Antigen-specific immunotherapy of cervical and ovarian cancer. Immunol. Rev. 2008, 222, 43–69. [Google Scholar] [CrossRef]
- Kuwana, Y.; Asakura, Y.; Usunomiya, N.; Nakanishi, M.; Arata, Y.; Itoh, S.; Nagase, F.; Kurosawa, Y. Expression of chimeric receptor composed of immunoglobulin-derived V regions and T-cell receptor-derived C regions. Biochem. Biophys. Res. Commun. 1987, 149, 960–968. [Google Scholar] [CrossRef]
- Morgan, M.A.; Büning, H.; Sauer, M.; Schambach, A. Use of Cell and Genome Modification Technologies to Generate Improved “Off-the-Shelf” CAR T and CAR NK Cells. Front. Immunol. 2020, 11, 1965. [Google Scholar] [CrossRef]
- Paasch, D.; Meyer, J.; Stamopoulou, A.; Lenz, D.; Kuehle, J.; Kloos, D.; Buchegger, T.; Holzinger, A.; Falk, C.S.; Kloth, C.; et al. Ex Vivo Generation of CAR Macrophages from Hematopoietic Stem and Progenitor Cells for Use in Cancer Therapy. Cells 2022, 11, 994. [Google Scholar] [CrossRef]
- Lindner, S.E.; Johnson, S.M.; Brown, C.E.; Wang, L.D. Chimeric antigen receptor signaling: Functional consequences and design implications. Sci. Adv. 2020, 6, 2–9. [Google Scholar] [CrossRef]
- Brentjens, R.; Davila, M.L.; Riviere, I.; Park, J.; Cowell, L.G.; Bartido, S.; Stefanski, J.; Taylor, C.; Borquez-ojeda, O.; Qu, J.; et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci. Transl. Med. 2013, 5, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.W.; Kochenderfer, J.N.; Stetler-Stevenson, M.; Cui, Y.K.; Delbrook, C.; Feldman, S.A.; Fry, T.J.; Orentas, R.; Sabatino, M.; Shah, N.N.; et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: A phase 1 dose-escalation trial. Lancet 2015, 385, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Sharpe, M.; Mount, N. Genetically modified T cells in cancer therapy: Opportunities and challenges. DMM Dis. Model. Mech. 2015, 8, 337–350. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Ren, J.; Luo, Y.; Keith, B.; Young, R.M.; Scholler, J.; Zhao, Y.; June, C.H. Augmentation of Antitumor Immunity by Human and Mouse CAR T Cells Secreting IL-18. Cell Rep. 2017, 20, 3025–3033. [Google Scholar] [CrossRef] [PubMed]
- Chmielewski, M.; Abken, H. CAR T Cells Releasing IL-18 Convert to T-Bethigh FoxO1low Effectors that Exhibit Augmented Activity against Advanced Solid Tumors. Cell Rep. 2017, 21, 3205–3219. [Google Scholar] [CrossRef]
- Chmielewski, M.; Kopecky, C.; Hombach, A.A.; Abken, H. IL-12 release by engineered T cells expressing chimeric antigen receptors can effectively muster an antigen-independent macrophage response on tumor cells that have shut down tumor antigen expression. Cancer Res. 2011, 71, 5697–5706. [Google Scholar] [CrossRef]
- Adachi, K.; Kano, Y.; Nagai, T.; Okuyama, N.; Sakoda, Y.; Tamada, K. IL-7 and CCL19 expression in CAR-T cells improves immune cell infiltration and CAR-T cell survival in the tumor. Nat. Biotechnol. 2018, 36, 346–351. [Google Scholar] [CrossRef]
- Liu, E.; Tong, Y.; Dotti, G.; Shaim, H.; Savoldo, B.; Mukherjee, M.; Orange, J.; Wan, X.; Lu, X.; Reynolds, A.; et al. Cord blood NK cells engineered to express IL-15 and a CD19- targeted CAR show long-term persistence and potent anti-tumor activity. Leukemia 2018, 32, 520–531. [Google Scholar] [CrossRef]
- Richards, K.H.; Doble, R.; Wasson, C.W.; Haider, M.; Blair, G.E.; Wittmann, M.; Macdonald, A. Human Papillomavirus E7 Oncoprotein Increases Production of the Anti-Inflammatory Interleukin-18 Binding Protein in Keratinocytes. J. Virol. 2014, 88, 4173–4179. [Google Scholar] [CrossRef][Green Version]
- Sukumaran, S.; Watanabe, N.; Bajgain, P.; Raja, K.; Mohammed, S.; Fisher, W.E.; Brenner, M.K.; Leen, A.M.; Vera, J.F. Enhancing the potency and specificity of engineered T cells for cancer treatment. Cancer Discov 2018, 8, 972–987. [Google Scholar] [CrossRef]
- Prosser, M.E.; Brown, C.E.; Shami, A.F.; Forman, S.J.; Jensen, M.C. Tumor PD-L1 co-stimulates primary human CD8+ cytotoxic T cells modified to express a PD1: CD28 chimeric receptor. Mol. Immunol. 2012, 51, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Rafiq, S.; Yeku, O.O.; Jackson, H.J.; Purdon, T.J.; Dayenne, G.; Leeuwen, V.; Drakes, D.J.; Song, M.; Miele, M.M.; Li, Z.; et al. Targeted delivery of a PD-1-blocking scFv by CAR-T cells enhances anti-tumor efficacy in vivo. Nat. Biotechnol. 2018, 36, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Lohmueller, J.J.; Ham, J.D.; Kvorjak, M.; Finn, O.J. mSA2 affinity-enhanced biotin-binding CAR T cells for universal tumor targeting. Oncoimmunology 2018, 7, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Urbanska, K.; Lanitis, E.; Poussin, M.; Lynn, R.; Gavin, B.P.; Kelderman, S.; Yu, J.; Scholler, N.; Jr, D.J.P. A universal strategy for adoptive immunotherapy of cancer through use of a novel T cell antigen receptor. Cancer Res. 2012, 72, 1844–1852. [Google Scholar] [CrossRef]
- Hirabayashi, K.; Du, H.; Xu, Y.; Shou, P.; Zhou, X.; Fucá, G.; Landoni, E.; Sun, C.; Chen, Y.; Savoldo, B.; et al. Dual-targeting CAR-T cells with optimal co-stimulation and metabolic fitness enhance antitumor activity and prevent escape in solid tumors. Nat. Cancer 2021, 2, 904–918. [Google Scholar] [CrossRef]
- Spiegel, J.Y.; Patel, S.; Muffly, L.; Hossain, N.M.; Oak, J.; Baird, J.H.; Frank, M.J.; Shiraz, P.; Sahaf, B.; Craig, J.; et al. CAR T cells with dual targeting of CD19 and CD22 in adult patients with recurrent or refractory B cell malignancies: A phase 1 trial. Nat. Med. 2021, 27, 1419–1431. [Google Scholar] [CrossRef]
- Xu, Y.; Morales, A.J.; Cargill, M.J.; Towlerton, A.M.H.; Coffey, D.G.; Warren, E.H.; Tykodi, S.S. Preclinical development of T-cell receptor-engineered T-cell therapy targeting the 5T4 tumor antigen on renal cell carcinoma. Cancer Immunol. Immunother. 2019, 68, 1979–1993. [Google Scholar] [CrossRef]
- Zhao, Q.; Jiang, Y.; Xiang, S.; Kaboli, P.J.; Shen, J.; Zhao, Y.; Wu, X.; Du, F.; Li, M.; Cho, C.H.; et al. Engineered TCR-T Cell Immunotherapy in Anticancer Precision Medicine: Pros and Cons. Front. Immunol. 2021, 12, 1–12. [Google Scholar] [CrossRef]
- Davenport, A.J.; Cross, R.S.; Watson, K.A.; Liao, Y.; Shi, W.; Prince, H.M.; Beavis, P.A.; Trapani, J.A.; Kershaw, M.H.; Ritchie, D.S.; et al. Chimeric antigen receptor T cells form nonclassical and potent immune synapses driving rapid cytotoxicity. Proc. Natl. Acad. Sci. USA 2018, 115, E2068–E2076. [Google Scholar] [CrossRef]
- Baeuerle, P.A.; Ding, J.; Patel, E.; Thorausch, N.; Horton, H.; Gierut, J.; Scarfo, I.; Choudhary, R.; Kiner, O.; Krishnamurthy, J.; et al. Synthetic TRuC receptors engaging the complete T cell receptor for potent anti-tumor response. Nat. Commun. 2019, 10, 2087. [Google Scholar] [CrossRef]
- Hardy, I.R.; Schamel, W.W.; Baeuerle, P.A.; Getts, D.R.; Hofmeister, R. Implications of T cell receptor biology on the development of new T cell therapies for cancer. Immunotherapy 2020, 12, 89–103. [Google Scholar] [CrossRef] [PubMed]
- Helsen, C.W.; Hammill, J.A.; Lau, V.W.C.; Mwawasi, K.A.; Afsahi, A.; Bezverbnaya, K.; Newhook, L.; Hayes, D.L.; Aarts, C.; Bojovic, B.; et al. The chimeric TAC receptor co-opts the T cell receptor yielding robust anti-tumor activity without toxicity. Nat. Commun. 2018, 9, 3049. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Yang, Z.; Horan, L.H.; Zhang, P.; Liu, L.; Zimdahl, B.; Green, S.; Lu, J.; Morales, J.F.; Barrett, D.M.; et al. A novel antibody-TCR (AbTCR) platform combines Fab-based antigen recognition with gamma/delta-TCR signaling to facilitate T-cell cytotoxicity with low cytokine release. Cell Discov. 2018, 4, 62. [Google Scholar] [CrossRef] [PubMed]
- Harper, J.; Adams, K.J.; Bossi, G.; Wright, D.E.; Stacey, A.R.; Bedke, N.; Martinez-Hague, R.; Blat, D.; Humbert, L.; Buchanan, H.; et al. An approved in vitro approach to preclinical safety and efficacy evaluation of engineered T cell receptor anti-CD3 bispecific (ImmTAC) molecules. PLoS ONE 2018, 13, e0205491. [Google Scholar] [CrossRef] [PubMed]
- Walseng, E.; Köksal, H.; Sektioglu, I.M.; Fåne, A.; Skorstad, G.; Kvalheim, G.; Gaudernack, G.; Inderberg, E.M.; Wälchli, S. A TCR-based Chimeric Antigen Receptor. Sci. Rep. 2017, 7, 10713. [Google Scholar] [CrossRef]
- Kombe Kombe, A.J.; Li, B.; Zahid, A.; Mengist, H.M.; Bounda, G.A.; Zhou, Y.; Jin, T. Epidemiology and Burden of Human Papillomavirus and Related Diseases, Molecular Pathogenesis, and Vaccine Evaluation. Front. Public Health 2021, 8, 552028. [Google Scholar] [CrossRef]
- Yim, E.-K.; Park, J.-S. The Role of HPV E6 and E7 Oncoproteins in HPV-associated Cervical Carcinogenesis. Cancer Res. Treat. 2005, 37, 319–324. [Google Scholar] [CrossRef]
- Cone, R.W.; Minson, A.C.; Smith, M.R.; McDougall, J.K. Conservation of HPV-16 E6/E7 ORF sequences in a cervical carcinoma. J. Med. Virol. 1992, 37, 99–107. [Google Scholar] [CrossRef]
- Jin, B.Y.; Campbell, T.E.; Draper, L.; Stevanovic, S.; Weissbrich, B.; Yu, Z.; Restifo, N.P.; Rosenberg, S.A.; Trimble, C.; Hinrichs, C.S. Engineered t cells targeting E7 mediate regression of human papillomavirus cancers in a murine model. J. Clin. Oncol. 2018, 3, e99488. [Google Scholar] [CrossRef]
- Nagarsheth, N.B.; Norberg, S.M.; Sinkoe, A.L.; Adhikary, S.; Meyer, T.J.; Lack, J.B.; Warner, A.C.; Schweitzer, C.; Doran, S.L.; Korrapati, S.; et al. TCR-engineered T cells targeting E7 for patients with metastatic HPV-associated epithelial cancers. Nat. Med. 2021, 27, 419–425. [Google Scholar] [CrossRef]
- Doran, S.L.; Stevanović, S.; Adhikary, S.; Gartner, J.J.; Jia, L.; Kwong, M.L.M.; Faquin, W.C.; Hewitt, S.M.; Sherry, R.M.; Yang, J.C.; et al. T-cell receptor gene therapy for human papillomavirus-associated epithelial cancers: A first-in-human, phase I/II study. J. Clin. Oncol. 2019, 37, 2759–2768. [Google Scholar] [CrossRef] [PubMed]
- Michaud, M.; Martins, I.; Sukkurwala, A.Q.; Adjemian, S.; Ma, Y.; Pellegatti, P.; Shen, S.; Kepp, O.; Scoazec, M.; Mignot, G.; et al. Autophagy-dependent anticancer immune responses induced by chemotherapeutic agents in mice. Science 2011, 334, 1573–1577. [Google Scholar] [CrossRef] [PubMed]
- Muranski, P.; Boni, A.; Wrzesinski, C.; Citrin, D.E.; Rosenberg, S.A.; Childs, R.; Restifo, N.P. Increased intensity lymphodepletion and adoptive immunotherapy—How far can we go? Nat. Clin. Pract. Oncol. 2006, 3, 668–681. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, S.A.; Dudley, M.E. Adoptive Cell Therapy for the Treatment of Patients with Metastatic Melanoma Steven. Curr. Opin Immunol 2009, 21, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wang, Y.; Shi, J.; Liu, J.; Li, Q.; Chen, L. Combination therapy: A feasibility strategy for car-t cell therapy in the treatment of solid tumors (review). Oncol. Lett. 2018, 16, 2063–2070. [Google Scholar] [CrossRef]
- Parente-Pereira, A.C.; Whilding, L.M.; Brewig, N.; van der Stegen, S.J.C.; Davies, D.M.; Wilkie, S.; van Schalkwyk, M.C.I.; Ghaem-Maghami, S.; Maher, J. Synergistic Chemoimmunotherapy of Epithelial Ovarian Cancer Using ErbB-Retargeted T Cells Combined with Carboplatin. J. Immunol. 2013, 191, 2437–2445. [Google Scholar] [CrossRef]
- Santin, A.D.; Hermonat, P.L.; Hiserodt, J.C.; Chiriva-Internati, M.; Woodliff, J.; Theus, J.W.; Barclay, D.; Pecorelli, S.; Parham, G.P. Effects of irradiation on the expression of major histocompatibility complex class I antigen and adhesion costimulation molecules ICAM-1 in human cervical cancer. Int. J. Radiat. Oncol. Biol. Phys. 1997, 39, 737–742. [Google Scholar] [CrossRef]
- Liao, Y.-P.; Wang, C.-C.; Butterfield, L.H.; Economou, J.S.; Ribas, A.; Meng, W.S.; Iwamoto, K.S.; McBride, W.H. Ionizing Radiation Affects Human MART-1 Melanoma Antigen Processing and Presentation by Dendritic Cells. J. Immunol. 2004, 173, 2462–2469. [Google Scholar] [CrossRef]
- Wan, S.; Pestka, S.; Jubin, R.G.; Lyu, Y.L.; Tsai, Y.C.; Liu, L.F. Chemotherapeutics and radiation stimulate MHC class i expression through elevated interferon-beta signaling in breast cancer cells. PLoS ONE 2012, 7, e32542. [Google Scholar] [CrossRef]
- Phi, L.T.H.; Sari, I.N.; Yang, Y.G.; Lee, S.H.; Jun, N.; Kim, K.S.; Lee, Y.K.; Kwon, H.Y. Cancer stem cells (CSCs) in drug resistance and their therapeutic implications in cancer treatment. Stem Cells Int. 2018, 2018, 5416923. [Google Scholar] [CrossRef]
- Zhao, J. Cancer Stem Cells and Chemoresistance: The Smartest Survives the Raid. Pharmacol. Ther. 2016, 160, 145–158. [Google Scholar] [CrossRef] [PubMed]
- Klapdor, R.; Wang, S.; Hacker, U.; Büning, H.; Morgan, M.; Dörk, T.; Hillemanns, P.; Schambach, A. Improved Killing of Ovarian Cancer Stem Cells by Combining a Novel Chimeric Antigen Receptor-Based Immunotherapy and Chemotherapy. Hum. Gene Ther. 2017, 28, 886–896. [Google Scholar] [CrossRef] [PubMed]
- Ng, H.Y.; Li, J.; Tao, L.; Lam, A.K.Y.; Chan, K.W.; Ko, J.M.Y.; Yu, V.Z.; Wong, M.; Li, B.; Lung, M.L. Chemotherapeutic Treatments Increase PD-L1 Expression in Esophageal Squamous Cell Carcinoma through EGFR/ERK Activation. Transl. Oncol. 2018, 11, 1323–1333. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Hamanishi, J.; Matsumura, N.; Abiko, K.; Murat, K.; Baba, T.; Yamaguchi, K.; Horikawa, N.; Hosoe, Y.; Murphy, S.K.; et al. Chemotherapy induces programmed cell death-ligand 1 overexpression via the nuclear factor-κBto foster an immunosuppressive tumor microenvironment in Ovarian Cancer. Cancer Res. 2015, 75, 5034–5045. [Google Scholar] [CrossRef] [PubMed]
- da Silva, D.M.; Enserro, D.M.; Mayadev, J.S.; Skeate, J.G.; Matsuo, K.; Pham, H.Q.; Lankes, H.A.; Moxley, K.M.; Ghamande, S.A.; Lin, Y.G.; et al. Immune activation in patients with locally advanced cervical cancer treated with ipilimumab following definitive chemoradiation (GOG-9929). Clin. Cancer Res. 2021, 26, 5621–5630. [Google Scholar] [CrossRef]
- Integrated genomic and molecular characterization of cervical cancer. Nature 2017, 543, 378–384. [CrossRef]
- Evans, A.M.; Salnikov, M.; Tessier, T.M.; Mymryk, J.S. Reduced MHC Class I and II Expression in HPV-Negative vs. HPV-Positive Cervical Cancers. Cells 2022, 11, 3911. [Google Scholar] [CrossRef]
- Evans, A.M.; Salnikov, M.; Gameiro, S.F.; Maleki Vareki, S.; Mymryk, J.S. HPV-Positive and -Negative Cervical Cancers Are Immunologically Distinct. J. Clin. Med. 2022, 11, 540850. [Google Scholar] [CrossRef]
- D’Oria, O.; Corrado, G.; Laganà, A.S.; Chiantera, V.; Vizza, E.; Giannini, A. New Advances in Cervical Cancer: From Bench to Bedside. Int. J. Environ. Res. Public Health 2022, 19, 7094. [Google Scholar] [CrossRef]
- Bogani, G.; Lalli, L.; Sopracordevole, F.; Ciavattini, A.; Ghelardi, A.; Simoncini, T.; Plotti, F.; Casarin, J.; Serati, M.; Pinelli, C.; et al. Development of a Nomogram Predicting the Risk of Persistence/Recurrence of Cervical Dysplasia. Vaccines 2022, 10, 579. [Google Scholar] [CrossRef]
- Frank, N.Y.; Schatton, T.; Frank, M.H. The therapeutic promise of the cancer stem cell concept. J. Clin. Investig. 2010, 120, 41–50. [Google Scholar] [CrossRef]
- Deuse, T.; Tediashvili, G.; Hu, X.; Gravina, A.; Tamenang, A.; Wang, D.; Connolly, A.; Mueller, C.; Mallavia, B.; Looney, M.R.; et al. Hypoimmune induced pluripotent stem cell–derived cell therapeutics treat cardiovascular and pulmonary diseases in immunocompetent allogeneic mice. Proc. Natl. Acad. Sci. USA. 2021, 118, e2022091118. [Google Scholar] [CrossRef]
- Deuse, T.; Hu, X.; Gravina, A.; Wang, D.; Tediashvili, G.; De, H.; Schrepfer, S. Hypoimmunogenic derivatives of induced pluripotent stem cells evade immune rejection in fully immunocompetent allogeneic recipients. Nat. Biotechnol. 2019, 37, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Liu, X.; Fang, C.; Jiang, S.; June, C.H.; Zhao, Y. Multiplex genome editing to generate universal CAR T cells resistant to PD1 inhibition. Clin. Cancer Res. 2017, 23, 2255–2266. [Google Scholar] [CrossRef]
- Stadtmauer, E.A.; Fraietta, J.A.; Davis, M.M.; Cohen, A.D.; Weber, K.L.; Lancaster, E.; Mangan, P.A.; Kulikovskaya, I.; Gupta, M.; Chen, F.; et al. CRISPR-engineered T cells in patients with refractory cancer. Science 2020, 367, eaba7365. [Google Scholar] [CrossRef]
- Georgiadis, C.; Rasaiyaah, J.; Gkazi, S.A.; Preece, R.; Etuk, A.; Christi, A.; Qasim, W. Base-edited CAR T cells for combinational therapy against T cell malignancies. Leukemia 2021, 35, 3466–3481. [Google Scholar] [CrossRef] [PubMed]
- Georgiadis, C.; Preece, R.; Nickolay, L.; Etuk, A.; Petrova, A.; Ladon, D.; Danyi, A.; Humphryes-Kirilov, N.; Ajetunmobi, A.; Kim, D.; et al. Long Terminal Repeat CRISPR-CAR-Coupled “Universal” T Cells Mediate Potent Anti-leukemic Effects. Mol. Ther. 2018, 26, 1215–1227. [Google Scholar] [CrossRef] [PubMed]

| Trial No. (Location) | Target | Condition | Phase | Patient No. | Start | Status | Results |
|---|---|---|---|---|---|---|---|
| NCT01583686 (United States, Maryland) | Mesothelin | Metastatic/unresectable Mesothelin-positive cancer | I/II | 15 | 2012 | Term. (2018) | CR: 0 PR: 0 SD: 1 PD: 14 SA: 6 |
| NCT03356795 (China, Guangdong) | GD2, PSMA, MUC1, Mesothelin | Patients with stage III, IV or relapsed cervical cancer | I/II | 20 (est.) | 2017 | Pending | |
| NCT04556669 (China, Hebei) | CD22 + PD-L1 | Advanced malignant solid tumors | I | 30 (est.) | 2020 | Pending Est. compl.: 2025 | |
| NCT05518253 (China, Zhejiang) | CD70 | CD70-positive advanced/metastatic solid tumors | I | 36 | 2022 | Pending Est. compl.: 2025 | |
| NCT05468190 (China, Henan) | CD70 | Advanced/metastatic CD70-positive solid tumors | I | 48 | 2022 | Pending Est. compl.: 2025 |
| Trial No. (Location) | Target | Condition | Phase | Patient No. | Start | Status | Results |
|---|---|---|---|---|---|---|---|
| NCT02280811 (United States, Maryland) | E6 | HLA-A*02:01-positive HPV-16-associated metastatic/recurrent cancer | I/II | 12 | 2014 | Compl. (2016) | CR: 0 PR: 2 DLT: 0 SA: 5 |
| NCT02153905 (United States, Maryland) | MAGE-A3 | HLA-A 01-positive metastatic/recurrent cancer expressing MAGE-A3 | I/II | 3 | 2014 | Compl. (2018) | CR: 0 PR: 1 DLT: 1 SA: 2 |
| NCT02111850 (United States, Maryland) | MAGE-A3 | (HLA)-DP0401/0402-positive metastatic/recurrent cancer expressing MAGE-A3-DP4 | I/II | 21 | 2014 | Compl. (2021) | CR: 1 PR: 3 SD: 0 PD: 16 |
| NCT02858310 (United States, Maryland) | E7 | HLA-A*02:01-positive HPV-16-associated metastatic/recurrent cancer | I/II | 180 (est.) | 2017 | Pending Est. compl.: 2026 | |
| NCT03578406 (China, Chongqing) | E6 + PD-1 | Metastatic/recurrent HPV-16-positive cancers | I | 20 | 2018 | Pending | |
| NCT05357027 (China, Chongqing) | E6 | HLA-A*02- and HPV16-positive metastatic/recurrent positive cervical cancer | I/II | 18 | 2022 | Pending Est. compl.: 2024 | |
| NCT05122221 (China, Henan) | E7 | HLA-A*02- and HPV16-positive cancer | I | 12 | 2022 | Pending Est. compl.: 2024 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Polten, R.; Kutle, I.; Hachenberg, J.; Klapdor, R.; Morgan, M.; Schambach, A. Towards Novel Gene and Cell Therapy Approaches for Cervical Cancer. Cancers 2023, 15, 263. https://doi.org/10.3390/cancers15010263
Polten R, Kutle I, Hachenberg J, Klapdor R, Morgan M, Schambach A. Towards Novel Gene and Cell Therapy Approaches for Cervical Cancer. Cancers. 2023; 15(1):263. https://doi.org/10.3390/cancers15010263
Chicago/Turabian StylePolten, Robert, Ivana Kutle, Jens Hachenberg, Rüdiger Klapdor, Michael Morgan, and Axel Schambach. 2023. "Towards Novel Gene and Cell Therapy Approaches for Cervical Cancer" Cancers 15, no. 1: 263. https://doi.org/10.3390/cancers15010263
APA StylePolten, R., Kutle, I., Hachenberg, J., Klapdor, R., Morgan, M., & Schambach, A. (2023). Towards Novel Gene and Cell Therapy Approaches for Cervical Cancer. Cancers, 15(1), 263. https://doi.org/10.3390/cancers15010263








