Prolonged Survival after Recurrence in HCC Resected Patients Using Repeated Curative Therapies: Never Give Up!
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Recurrence
2.2. Clinical Variables
2.3. Endpoints
2.4. Statistical Analysis
3. Results
3.1. Overall Survival and Recurrence-Free Survival in the Entire Cohort
3.2. Risk Factor for Recurrence
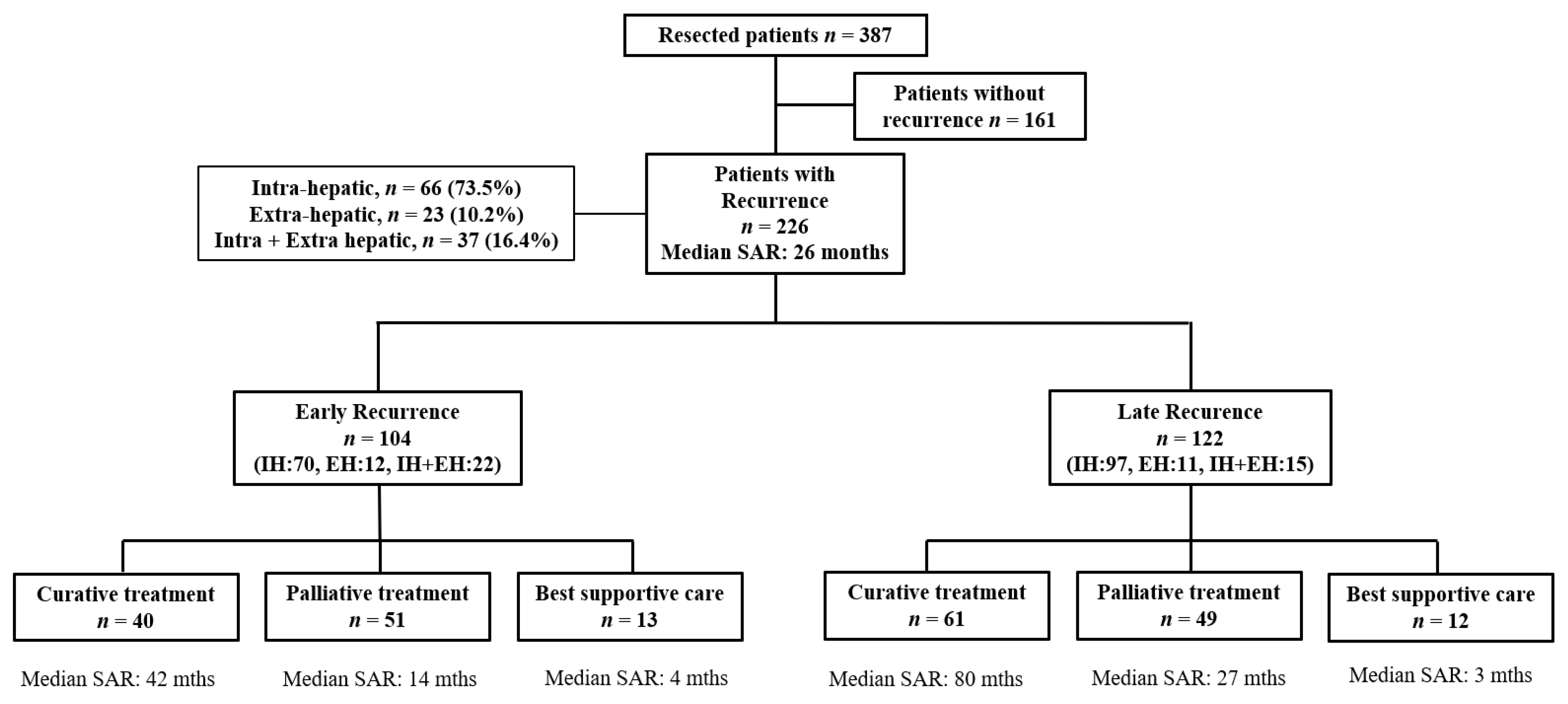
3.3. Patterns of Recurrence
3.4. Predictive Factors of Survival after Recurrence
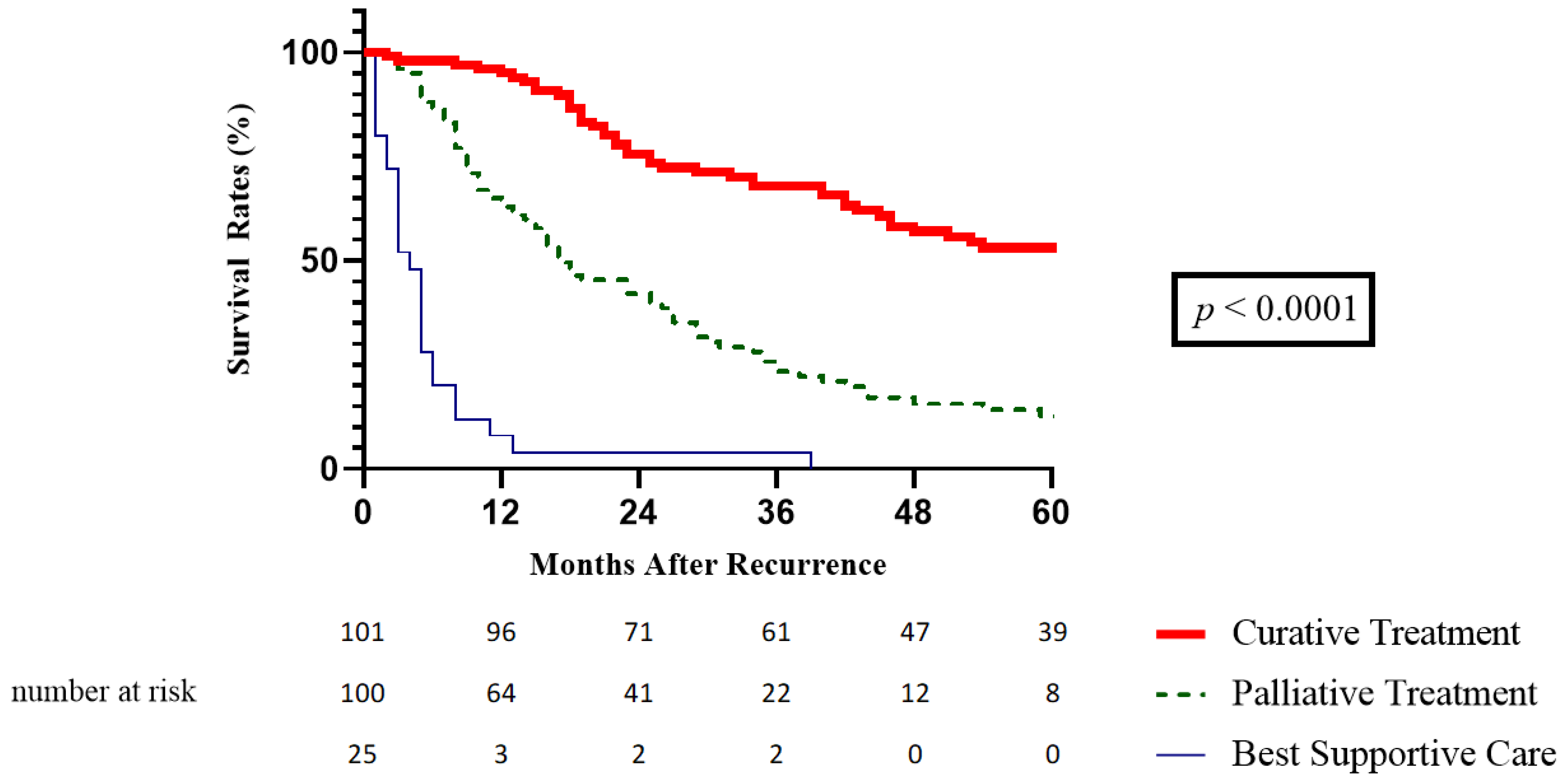
3.5. SAR According to the Treatment of Recurrence
3.6. Survival Analysis According to Time to Recurrence
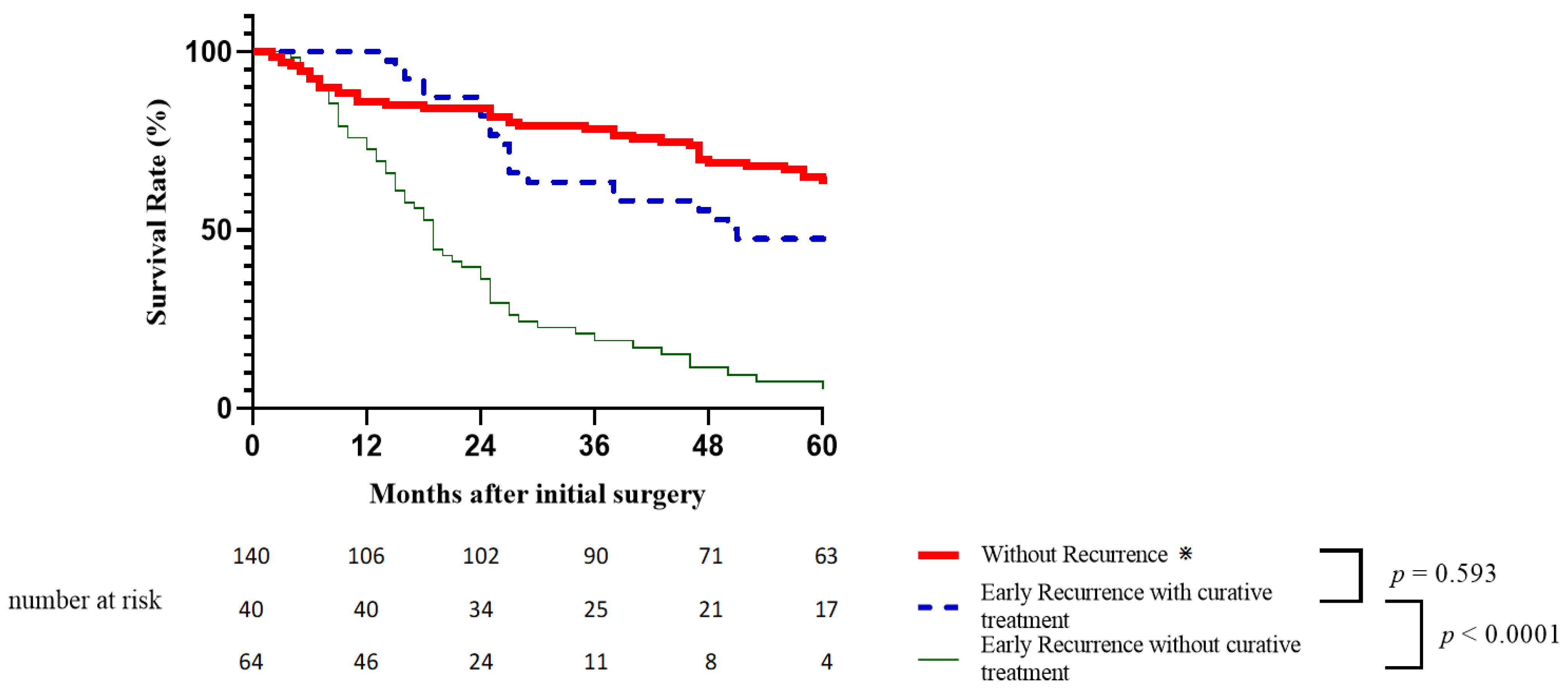
3.6.1. Early vs. Late Recurrence
3.6.2. Impact of Curative Treatments for Early Recurrence on Survival
3.6.3. Impact of Curative Treatments for Late Recurrence on Survival
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Rumgay, H.; Arnold, M.; Ferlay, J.; Lesi, O.; Cabasag, C.J.; Vignat, J.; Laversanne, M.; McGlynn, K.A.; Soerjomataram, I. Global burden of primary liver cancer in 2020 and predictions to 2040. J. Hepatol. 2022, 77, 1598–1606. [Google Scholar] [CrossRef] [PubMed]
- Mazzaferro, V.M.; Regalia, E.; Doci, R.; Andreola, S.; Pulvirenti, A.; Bozzetti, F.; Montalto, F.; Ammatuna, M.; Morabito, A.; Gennari, L. Liver Transplantation for the Treatment of Small Hepatocellular Carcinomas in Patients with Cirrhosis. N. Engl. J. Med. 1996, 334, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Duvoux, C.; Roudot-Thoraval, F.; Decaens, T.; Pessione, F.; Badran, H.; Piardi, T.; Francoz, C.; Compagnon, P.; Vanlemmens, C.; Dumortier, J.; et al. Liver Transplantation for Hepatocellular Carcinoma: A Model Including α-Fetoprotein Improves the Performance of Milan Criteria. Gastroenterology 2012, 143, 986–994.e3. [Google Scholar] [CrossRef]
- Mehta, N.; Dodge, J.L.; Hirose, R.; Roberts, J.P.; Yao, F.Y. Increasing Liver Transplantation Wait-List Dropout for Hepatocellular Carcinoma With Widening Geographical Disparities: Implications for Organ Allocation. Liver Transplant. 2018, 24, 1346–1356. [Google Scholar] [CrossRef] [PubMed]
- Reig, M.; Forner, A.; Rimola, J.; Ferrer-Fàbrega, J.; Burrel, M.; Garcia-Criado, Á.; Kelley, R.K.; Galle, P.R.; Mazzaferro, V.; Salem, R.; et al. BCLC strategy for prognosis prediction and treatment recommendation Barcelona Clinic Liver Cancer (BCLC) staging system: The 2022 update. J. Hepatol. 2022, 76, 681–693. [Google Scholar] [CrossRef]
- Marrero, J.A.; Kulik, L.M.; Sirlin, C.B.; Zhu, A.X.; Finn, R.S.; Abecassis, M.M.; Roberts, L.R.; Heimbach, J.K. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2018, 68, 723–750. [Google Scholar] [CrossRef]
- Galle, P.R.; Forner, A.; Llovet, J.M.; Mazzaferro, V.; Piscaglia, F.; Raoul, J.L.; Schirmacher, P.; Vilgrain, V. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef]
- Tabrizian, P.; Jibara, G.; Shrager, B.; Schwartz, M.; Roayaie, S. Recurrence of Hepatocellular Cancer After Resection: Patterns, Treatments, and Prognosis. Ann. Surg. 2015, 261, 947–955. [Google Scholar] [CrossRef]
- Tranchart, H.; Chirica, M.; Sepulveda, A.; Massault, P.-P.; Conti, F.; Scatton, O.; Soubrane, O. Long-term Outcomes Following Aggressive Management of Recurrent Hepatocellular Carcinoma After Upfront Liver Resection. World J. Surg. 2012, 36, 2684–2691. [Google Scholar] [CrossRef]
- Poon, R.T.-P.; Fan, S.-T.; Lo, C.-M.; Liu, C.-L.; Wong, J. Intrahepatic Recurrence After Curative Resection of Hepatocellular Carcinoma Long-Term Results of Treatment and Prognostic Factors. Ann. Surg. 1999, 229, 216–222. [Google Scholar] [CrossRef]
- Shah, S.A.; Cleary, S.P.; Wei, A.C.; Yang, I.; Taylor, B.R.; Hemming, A.W.; Langer, B.; Grant, D.R.; Greig, P.D.; Gallinger, S. Recurrence after liver resection for hepatocellular carcinoma: Risk factors, treatment, and outcomes. Surgery 2007, 141, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Magro, B.; Pinelli, D.; De Giorgio, M.; Lucà, M.G.; Ghirardi, A.; Carrobio, A.; Baronio, G.; Del Prete, L.; Nounamo, F.; Gianatti, A.; et al. Pre-Transplant Alpha-Fetoprotein > 25.5 and Its Dynamic on Waitlist Are Predictors of HCC Recurrence after Liver Transplantation for Patients Meeting Milan Criteria. Cancers 2021, 13, 5976. [Google Scholar] [CrossRef] [PubMed]
- Vibert, E.; Azoulay, D.; Hoti, E.; Iacopinelli, S.; Samuel, D.; Salloum, C.; Lemoine, A.; Bismuth, H.; Castaing, D.; Adam, R. Progression of Alphafetoprotein Before Liver Transplantation for Hepatocellular Carcinoma in Cirrhotic Patients: A Critical Factor. Am. J. Transplant. 2010, 10, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Poon, R.T.-P.; Fan, S.-T.; Wong, J. Risk Factors, Prevention, and Management of Postoperative Recurrence After Resection of Hepatocellular Carcinoma. Ann. Surg. 2000, 232, 10–24. [Google Scholar] [CrossRef]
- Lee, H.Y.; Rhim, H.; Lee, M.W.; Kim, Y.-S.; Choi, D.; Park, M.J.; Kim, S.H.; Lim, H.K.; Kim, Y.K. Early diffuse recurrence of hepatocellular carcinoma after percutaneous radiofrequency ablation: Analysis of risk factors. Eur. Radiol. 2013, 23, 190–197. [Google Scholar] [CrossRef]
- Shimoda, M.; Tago, K.; Shiraki, T.; Mori, S.; Kato, M.; Aoki, T.; Kubota, K. Risk Factors for Early Recurrence of Single Lesion Hepatocellular Carcinoma After Curative Resection. World J. Surg. 2016, 40, 2466–2471. [Google Scholar] [CrossRef]
- Calderaro, J.; Couchy, G.; Imbeaud, S.; Amaddeo, G.; Letouzé, E.; Blanc, J.-F.; Laurent, C.; Hajji, Y.; Azoulay, D.; Bioulac-Sage, P.; et al. Histological subtypes of hepatocellular carcinoma are related to gene mutations and molecular tumour classification. J. Hepatol. 2017, 67, 727–738. [Google Scholar] [CrossRef]
- Ziol, M.; Poté, N.; Amaddeo, G.; Laurent, A.; Nault, J.C.; Oberti, F.; Costentin, C.; Michalak, S.; Bouattour, M.; Francoz, C.; et al. Macrotrabecular-massive hepatocellular carcinoma: A distinctive histological subtype with clinical relevance. Hepatology 2018, 68, 103–112. [Google Scholar] [CrossRef]
- Yoh, T.; Seo, S.; Taura, K.; Iguchi, K.; Ogiso, S.; Fukumitsu, K.; Ishii, T.; Kaido, T.; Uemoto, S. Surgery for Recurrent Hepatocellular Carcinoma: Achieving Long-term Survival. Ann. Surg. 2021, 273, 792–799. [Google Scholar] [CrossRef]
- Yao, L.-Q.; Chen, Z.-L.; Feng, Z.-H.; Diao, Y.-K.; Li, C.; Sun, H.-Y.; Zhong, J.-H.; Chen, T.-H.; Gu, W.-M.; Zhou, Y.-H.; et al. Clinical Features of Recurrence After Hepatic Resection for Early-Stage Hepatocellular Carcinoma and Long-Term Survival Outcomes of Patients with Recurrence: A Multi-institutional Analysis. Ann. Surg. Oncol. 2022, 29, 4291–4303. [Google Scholar] [CrossRef] [PubMed]
- Erridge, S.; Pucher, P.H.; Markar, S.R.; Malietzis, G.; Athanasiou, T.; Darzi, A.; Sodergren, M.H.; Jiao, L.R. Meta-analysis of determinants of survival following treatment of recurrent hepatocellular carcinoma. Br. J. Surg. 2017, 104, 1433–1442. [Google Scholar] [CrossRef]
- Faber, W.; Seehofer, D.; Neuhaus, P.; Stockmann, M.; Denecke, T.; Kalmuk, S.; Warnick, P.; Bahra, M. Repeated liver resection for recurrent hepatocellular carcinoma. J. Gastroenterol. Hepatol. 2011, 26, 1189–1194. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Wu, H.; Huang, D.Q.; Xu, C.; Zheng, H.; Maeda, M.; Zhao, X.; Wang, L.; Xiao, F.; Lv, H.; et al. Radiofrequency ablation versus repeat resection for recurrent hepatocellular carcinoma (≤5 cm) after initial curative resection. Eur. Radiol. 2020, 30, 6357–6368. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Di Bisceglie, A.M.; Bruix, J.; Kramer, B.S.; Lencioni, R.; Zhu, A.X.; Sherman, M.; Schwartz, M.; Lotze, M.; Talwalkar, J.; et al. Design and Endpoints of Clinical Trials in Hepatocellular Carcinoma. J. Natl. Cancer Inst. 2008, 100, 698–711. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Ikoma, H.; Morimura, R.; Konishi, H.; Murayama, Y.; Komatsu, S.; Shiozaki, A.; Kuriu, Y.; Kubota, T.; Nakanishi, M.; et al. Optimal duration of the early and late recurrence of hepatocellular carcinoma after hepatectomy. World J. Gastroenterol. 2015, 21, 1207–1215. [Google Scholar] [CrossRef]
- Xing, H.; Zhang, W.-G.; Cescon, M.; Liang, L.; Li, C.; Wang, M.-D.; Wu, H.; Lau, W.Y.; Zhou, Y.-H.; Gu, W.-M.; et al. Defining and predicting early recurrence after liver resection of hepatocellular carcinoma: A multi-institutional study. Hpb 2020, 22, 677–689. [Google Scholar] [CrossRef]
- Groot, V.P.; Gemenetzis, G.; Blair, A.; Rivero-Soto, R.J.; Yu, J.; Javed, A.A.; Burkhart, R.A.; Rinkes, I.H.M.B.; Molenaar, I.Q.; Cameron, J.L.; et al. Defining and Predicting Early Recurrence in 957 Patients With Resected Pancreatic Ductal Adenocarcinoma. Ann. Surg. 2019, 269, 1154–1162. [Google Scholar] [CrossRef]
- Bedossa, P.; Poynard, T. An algorithm for the grading of activity in chronic hepatitis C. Hepatology 2016, 24, 289–293. [Google Scholar] [CrossRef]
- Johnson, P.J.; Berhane, S.; Kagebayashi, C.; Satomura, S.; Teng, M.; Reeves, H.L.; O’Beirne, J.; Fox, R.; Skowronska, A.; Palmer, D.; et al. Assessment of Liver Function in Patients With Hepatocellular Carcinoma: A New Evidence-Based Approach—The ALBI Grade. J. Clin. Oncol. 2015, 33, 550–558. [Google Scholar] [CrossRef]
- Slankamenac, K.; Graf, R.; Barkun, J.; Puhan, M.A.; Clavien, P.-A. The Comprehensive Complication Index: A novel continuous scale to measure surgical morbidity. Ann. Surg. 2013, 258, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ronnie, T.P.P.; Sheung-Tat, F.; Irene, O.L.N.; John, W. Significance of Resection Margin in Hepatectomy for Hepatocellular Carcinoma. Ann. Surg. 2000, 231, 544–551. [Google Scholar] [CrossRef]
- Portolani, N.; Coniglio, A.; Ghidoni, S.; Giovanelli, M.; Benetti, A.; Tiberio, G.A.M.; Giulini, S.M. Early and Late Recurrence After Liver Resection for Hepatocellular Carcinoma: Prognostic and therapeutic implications. Ann. Surg. 2006, 243, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Imamura, H.; Matsuyama, Y.; Tanaka, E.; Ohkubo, T.; Hasegawa, K.; Miyagawa, S.; Sugawara, Y.; Minagawa, M.; Takayama, T.; Kawasaki, S.; et al. Risk factors contributing to early and late phase intrahepatic recurrence of hepatocellular carcinoma after hepatectomy. J. Hepatol. 2003, 38, 200–207. [Google Scholar] [CrossRef]
- Xu, X.-F.; Xing, H.; Han, J.; Li, Z.-L.; Lau, W.-Y.; Zhou, Y.-H.; Gu, W.-M.; Wang, H.; Chen, T.-H.; Zeng, Y.-Y.; et al. Risk Factors, Patterns, and Outcomes of Late Recurrence After Liver Resection for Hepatocellular Carcinoma: A Multicenter Study from China. JAMA Surg. 2019, 154, 209–217. [Google Scholar] [CrossRef]
- Tribillon, E.; Barbier, L.; Goumard, C.; Irtan, S.; Perdigao-Cotta, F.; Durand, F.; Paradis, V.; Belghiti, J.; Scatton, O.; Soubrane, O. When Should We Propose Liver Transplant After Resection of Hepatocellular Carcinoma? A Comparison of Salvage and De Principe Strategies. J. Gastrointest. Surg. 2016, 20, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Guerrini, G.P.; Esposito, G.; Olivieri, T.; Magistri, P.; Ballarin, R.; Di Sandro, S.; Di Benedetto, F. Salvage versus Primary Liver Transplantation for Hepatocellular Carcinoma: A Twenty-Year Experience Meta-Analysis. Cancers 2022, 14, 3465. [Google Scholar] [CrossRef]
- Fuks, D.; Dokmak, S.; Paradis, V.; Diouf, M.; Durand, F.; Belghiti, J. Benefit of initial resection of hepatocellular carcinoma followed by transplantation in case of recurrence: An intention-to-treat analysis. Hepatology 2012, 55, 132–140. [Google Scholar] [CrossRef]
- Ferrer-Fàbrega, J.; Forner, A.; Liccioni, A.; Miquel, R.; Molina, V.; Navasa, M.; Fondevila, C.; García-Valdecasas, J.C.; Bruix, J.; Fuster, J. Prospective validation ofab initioliver transplantation in hepatocellular carcinoma upon detection of risk factors for recurrence after resection. Hepatology 2016, 63, 839–849. [Google Scholar] [CrossRef]
- Lin, C.-C.; Elsarawy, A.M.; Li, W.-F.; Lin, T.-L.; Yong, C.-C.; Wang, S.-H.; Wang, C.-C.; Kuo, F.-Y.; Cheng, Y.-F.; Chen, C.-L. Liver Transplantation for High Risk Hepatocellular Carcinoma After Liver Resection: A Sequential or Salvage Approach? Ann. Transplant. 2017, 22, 602–610. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.; Li, H.; Zheng, T.; Jiang, H.; Li, M.; Song, B. Performance of LI-RADS version 2018 CT treatment response algorithm in tumor response evaluation and survival prediction of patients with single hepatocellular carcinoma after radiofrequency ablation. Ann. Transl. Med. 2020, 8, 388. [Google Scholar] [CrossRef]
- Chan, A.C.Y.; Poon, R.T.P.; Cheung, T.T.; Chok, K.S.H.; Chan, S.C.; Fan, S.T.; Lo, C.M. Survival Analysis of Re-resection Versus Radiofrequency Ablation for Intrahepatic Recurrence After Hepatectomy for Hepatocellular Carcinoma. World J. Surg. 2012, 36, 151–156. [Google Scholar] [CrossRef]
- Liang, H.-H.; Chen, M.-S.; Peng, Z.-W.; Zhang, Y.-J.; Zhang, Y.-Q.; Li, J.-Q.; Lau, W.Y. Percutaneous Radiofrequency Ablation Versus Repeat Hepatectomy for Recurrent Hepatocellular Carcinoma: A Retrospective Study. Ann. Surg. Oncol. 2008, 15, 3484–3493. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Li, J.; Liu, G.; Wang, K.; Qian, G.; Lu, Z.; Yang, T.; Yan, Z.; Lei, Z.; Si, A.; et al. Long-term Effects of Repeat Hepatectomy vs Percutaneous Radiofrequency Ablation Among Patients With Recurrent Hepatocellular Carcinoma: A Randomized Clinical Trial. JAMA Oncol. 2020, 6, 255–263. [Google Scholar] [CrossRef]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.-Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef]
- Hack, S.P.; Spahn, J.; Chen, M.; Cheng, A.-L.; Kaseb, A.; Kudo, M.; Lee, H.C.; Yopp, A.; Chow, P.; Qin, S. IMbrave 050: A Phase III trial of atezolizumab plus bevacizumab in high-risk hepatocellular carcinoma after curative resection or ablation. Futur. Oncol. 2020, 16, 975–989. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Li, Y.; Geng, L.; Shen, W.; Sui, C.; Dai, B.; Lu, J.; Pan, M.; Yang, J. Adjuvant stereotactic body radiotherapy after marginal resection for hepatocellular carcinoma with microvascular invasion: A randomised controlled trial. Eur. J. Cancer 2022, 166, 176–184. [Google Scholar] [CrossRef]
- Esagian, S.; Kakos, C.; Giorgakis, E.; Burdine, L.; Barreto, J.; Mavros, M. Adjuvant Transarterial Chemoembolization Following Curative-Intent Hepatectomy Versus Hepatectomy Alone for Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Cancers 2021, 13, 2984. [Google Scholar] [CrossRef] [PubMed]
- Shen, A.; Liu, M.; Zheng, D.; Chen, Q.; Wu, Z. Adjuvant transarterial chemoembolization after curative hepatectomy for hepatocellular carcinoma with microvascular invasion: A systematic review and meta-analysis. Clin. Res. Hepatol. Gastroenterol. 2020, 44, 142–154. [Google Scholar] [CrossRef] [PubMed]
- Chernyak, V.; Fowler, K.J.; Kamaya, A.; Kielar, A.Z.; Elsayes, K.M.; Bashir, M.R.; Kono, Y.; Do, R.K.; Mitchell, D.G.; Singal, A.G.; et al. Liver Imaging Reporting and Data System (LI-RADS) Version 2018: Imaging of Hepatocellular Carcinoma in At-Risk Patients. Radiology 2018, 289, 816–830. [Google Scholar] [CrossRef]
- Chaudhry, M.; McGinty, K.A.; Mervak, B.; Lerebours, R.; Li, C.; Shropshire, E.; Ronald, J.; Commander, L.; Hertel, J.; Luo, S.; et al. The LI-RADS Version 2018 MRI Treatment Response Algorithm: Evaluation of Ablated Hepatocellular Carcinoma. Radiology 2020, 294, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Cools, K.S.; Moon, A.M.; Burke, L.M.B.; McGinty, K.A.; Strassle, P.D.; Gerber, D.A. Validation of the Liver Imaging Reporting and Data System Treatment Response Criteria After Thermal Ablation for Hepatocellular Carcinoma. Liver Transplant. 2020, 26, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Rimola, J.; Forner, A.; Sapena, V.; Llarch, N.; Darnell, A.; Díaz, A.; García-Criado, A.; Bianchi, L.; Vilana, R.; Díaz-González, Á.; et al. Performance of gadoxetic acid MRI and diffusion-weighted imaging for the diagnosis of early recurrence of hepatocellular carcinoma. Eur. Radiol. 2020, 30, 186–194. [Google Scholar] [CrossRef]
- Zucman-Rossi, J.; Villanueva, A.; Nault, J.C.; Llovet, J.M. Genetic Landscape and Biomarkers of Hepatocellular Carcinoma. Gastroenterology 2015, 149, 1226–1239.e4. [Google Scholar] [CrossRef]
- Nault, J.; De Reyniès, A.; Villanueva, A.; Calderaro, J.; Rebouissou, S.; Couchy, G.; Decaens, T.; Franco, D.; Imbeaud, S.; Rousseau, F.; et al. A Hepatocellular Carcinoma 5-Gene Score Associated With Survival of Patients After Liver Resection. Gastroenterology 2013, 145, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Hoshida, Y.; Villanueva, A.; Kobayashi, M.; Peix, J.; Chiang, D.Y.; Camargo, A.; Gupta, S.; Moore, J.; Wrobel, M.J.; Lerner, J.; et al. Gene Expression in Fixed Tissues and Outcome in Hepatocellular Carcinoma. N. Engl. J. Med. 2008, 359, 1995–2004. [Google Scholar] [CrossRef] [PubMed]
- Pantel, K.; Alix-Panabières, C. Circulating tumour cells in cancer patients: Challenges and perspectives. Trends Mol. Med. 2010, 16, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Cui, K.; Ou, Y.; Shen, Y.; Li, S.; Sun, Z. Clinical value of circulating tumor cells for the diagnosis and prognosis of hepatocellular carcinoma (HCC): A systematic review and meta-analysis. Medicine 2020, 99, e22242. [Google Scholar] [CrossRef]
- Sun, C.; Liao, W.; Deng, Z.; Li, E.; Feng, Q.; Lei, J.; Yuan, R.; Zou, S.; Mao, Y.; Shao, J.; et al. The diagnostic value of assays for circulating tumor cells in hepatocellular carcinoma: A meta-analysis. Medicine 2017, 96, e7513. [Google Scholar] [CrossRef]
- Fan, J.-L.; Yang, Y.-F.; Yuan, C.-H.; Chen, H.; Wang, F.-B. Circulating Tumor Cells for Predicting the Prognostic of Patients with Hepatocellular Carcinoma: A Meta Analysis. Cell. Physiol. Biochem. 2015, 37, 629–640. [Google Scholar] [CrossRef]
| Variables | Total n (%) n = 387 (100) | Without Recurrence n (%) n = 161 (41.6) | With Recurrence n (%) n = 226 (58.4) | p |
|---|---|---|---|---|
| Demographic Data | ||||
| Age > 70 years | 117 (30.2) | 45 (28) | 72 (31,9) | 0.409 |
| Male sex | 319 (82.4) | 132 (82) | 187 (82.7) | 0.847 |
| BMI > 30 | 68 (17.6) | 30 (18.6) | 38 (16.8) | 0.601 |
| F3-F4 Fibrosis | 258 (66.6) | 103 (63.9) | 155 (68.6) | 0.343 |
| Child–Pugh Score A | 346 (89.4) | 143 (88.8) | 203 (89.8) | 0.621 |
| HBV | 17 (4.4) | 5 (3.1) | 12 (5.3) | 0.297 |
| HCV | 97 (25.1) | 32 (19.9) | 65 (28.8) | 0.047 |
| Alcohol | 131 (33.9) | 57 (35.4) | 74 (32.7) | 0.586 |
| NASH | 21 (5.4) | 11 (6.8) | 10 (4.4) | 0.303 |
| Tumor Characteristics | ||||
| AFP > 200 ng/mL | 49 (12.7) | 20 (12.4) | 29 (12.8) | 0.803 |
| Single nodule | 307 (79.3) | 133 (82.6) | 174 (77) | 0.103 |
| Max Tumor size > 50 mm | 140 (36.2) | 53 (32.9) | 87 (38.5) | 0.261 |
| Score AFP ≤ 2 | 234 (60.5) | 98 (60.9) | 136 (60.2) | 0.879 |
| Milan criteria in | 232 (60) | 96 (59.6) | 136 (60.2) | 0.913 |
| Unilobar | 353 (91.2) | 152 (94.4) | 201 (88.9) | 0.010 |
| Operative Data | ||||
| Pre-operative treatment (1) | 86 (22.2) | 39 (24.2) | 47 (17.7) | 0.500 |
| Major hepatectomy | 124 (32) | 54 (33.5) | 70 (31) | 0.594 |
| Anatomical resection | 254 (65.9) | 110 (68.3) | 144 (63.7) | 0.290 |
| Blood transfusion | 123 (31.8) | 53 (32.9) | 70 (31) | 0.685 |
| Laparoscopic approach | 56 (14.5) | 23 (14.3) | 33 (14.6) | 0.914 |
| CCI > 26.2 | 131 (33.9) | 61 (37.9) | 70 (31) | 0.191 |
| Histological Data | ||||
| Satellite nodule | 79 (20.4) | 27 (16.8) | 52 (23) | 0.133 |
| Poor differentiation | 47 (12.1) | 16 (9.9) | 31 (13.7) | 0.257 |
| Microvascular invasion | 129 (33.3) | 44 (27.3) | 85 (37.6) | 0.028 |
| R1 resection | 25 (6.5) | 5 (3.1) | 20 (8.8) | 0.022 |
| Capsule | 188 (48.6) | 79 (49.1) | 109 (48.2) | 0.958 |
| Adjacent organs invasion | 8 (2.1) | 0 (0) | 8 (3.5) | 0.023 |
| Variables | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| Survival, Median (SE), Months | 95% CI | p | HR | 95% CI | p | |
| Variables at Primary Surgery | ||||||
| Sex | 0.095 | |||||
| Female | 35 (5.3) | 24.7–45.3 | ||||
| Male | 23 (3.1) | 16.9–29.2 | 1.92 | 1.14–3.24 | 0.014 | |
| Albumin (g/L) | 0.012 | |||||
| >35 | 29 (3.6) | 22.0–36.0 | 0.050 | |||
| ≤35 | 18 (5.5) | 7.2–28.8 | 1.74 | 1.00–3.04 | ||
| Platelet Count | 0.037 | |||||
| >100,000 | 29 (5.5) | 18.2–39.8 | ||||
| ≤100,000 | 23 (3.5) | 16.2–29.8 | 1.76 | 1.07–2.91 | 0.026 | |
| AFP (ng/mL) | 0.020 | |||||
| >200 | 29 (4.3) | 20.56–37.44 | ||||
| ≤200 | 16 (5.4) | 5.45–26.55 | 2.16 | 1.27–3.52 | 0.004 | |
| Hepatectomy | 0.03 | |||||
| Minor | 36 (5.9) | 24.45–47.55 | ||||
| Major | 16 (3.6) | 8.99–23.01 | 1.38 | 0.87–2.20 | 0.174 | |
| Per operative Transfusion | 0.043 | |||||
| No | 29 (5.3) | 18.68–39.32 | ||||
| Yes | 18 (2.4) | 13.22–22.78 | 1.15 | 0.77–1.72 | 0.503 | |
| Primary Tumor Size | 0.001 | |||||
| ≤50 mm | 40 (6.6) | 27.06–52.94 | ||||
| >50 mm | 17 (3.5) | 10.17–23.83 | 1.61 | 1.05–2.46 | 0.03 | |
| Microvascular Invasion | < 0.0001 | |||||
| No | 40 (3.8) | 32.63–47.37 | ||||
| Yes | 17 (2.1) | 12.83–21.17 | 1.72 | 1.17–2.53 | 0.006 | |
| Macrovascular Invasion | ||||||
| No | 29 (3.7) | 21.73–36.27 | ||||
| Yes | 11 (5.3) | 0.61–21.39 | 0.009 | 0.84 | 0.43–1.64 | 0.608 |
| Complete Resection | ||||||
| R0 | 27 (3.4) | 20.37–33.63 | ||||
| R1 | 9 (3.7) | 1.70–16.30 | 0.024 | 0.61 | 0.28–1.34 | 0.221 |
| Adjacent Organ Invasion | ||||||
| No | 27 (3.4) | 20.39–33.62 | ||||
| Yes | 9 (3.8) | 1.61–16.39 | 0.072 | 1.52 | 0.44–5.30 | 0.511 |
| Variables at Recurrence | ||||||
| Number | ||||||
| single | 44 (7.4) | 29.56–58.44 | ||||
| multinodular | 19 (2.8) | 13.47–24.53 | <0.0001 | 1.12 | 0.78–1.61 | 0.526 |
| Size | ||||||
| ≤50 mm | 34 (4.8) | 24.51–43.49 | ||||
| >50 mm | 7 (1.9) | 3.33–10.67 | <0.0001 | 2.15 | 1.13–4.10 | 0.019 |
| Location of Recurrence | ||||||
| Intra-hepatically (IH) | 40 (5.6) | 29.07–50.93 | ||||
| Extra-hepatically ± IH | 14 (3.9) | 6.41–21.60 | <0.0001 | 1.53 | 1.01–2.32 | 0.043 |
| Timing of Recurrence | ||||||
| Late | 40 (5.4) | 29.46–50.55 | ||||
| Early | 17 (1.7) | 13.67–20.33 | 0.003 | 1.50 | 1.07–2.09 | 0.019 |
| Child–Pugh Score at Recurrence | ||||||
| A | 32 (4.3) | 23.61–40.39 | ||||
| B or C | 5 (2.5) | 0.10–9.90 | <0.0001 | 4.47 | 2.71–7.38 | <0.0001 |
| Treatment at Recurrence | ||||||
| Curative | 61 (13.3) | 34.87–87.13 | ||||
| Not curative | 14 (2.1) | 9.92–18.08 | <0.0001 | 2.98 | 1.99–4.46 | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toubert, C.; Guiu, B.; Al Taweel, B.; Assenat, E.; Panaro, F.; Souche, F.-R.; Ursic-Bedoya, J.; Navarro, F.; Herrero, A. Prolonged Survival after Recurrence in HCC Resected Patients Using Repeated Curative Therapies: Never Give Up! Cancers 2023, 15, 232. https://doi.org/10.3390/cancers15010232
Toubert C, Guiu B, Al Taweel B, Assenat E, Panaro F, Souche F-R, Ursic-Bedoya J, Navarro F, Herrero A. Prolonged Survival after Recurrence in HCC Resected Patients Using Repeated Curative Therapies: Never Give Up! Cancers. 2023; 15(1):232. https://doi.org/10.3390/cancers15010232
Chicago/Turabian StyleToubert, Cyprien, Boris Guiu, Bader Al Taweel, Eric Assenat, Fabrizio Panaro, François-Regis Souche, Jose Ursic-Bedoya, Francis Navarro, and Astrid Herrero. 2023. "Prolonged Survival after Recurrence in HCC Resected Patients Using Repeated Curative Therapies: Never Give Up!" Cancers 15, no. 1: 232. https://doi.org/10.3390/cancers15010232
APA StyleToubert, C., Guiu, B., Al Taweel, B., Assenat, E., Panaro, F., Souche, F.-R., Ursic-Bedoya, J., Navarro, F., & Herrero, A. (2023). Prolonged Survival after Recurrence in HCC Resected Patients Using Repeated Curative Therapies: Never Give Up! Cancers, 15(1), 232. https://doi.org/10.3390/cancers15010232







