The Clinical Impact of Hepatic Arterial Infusion Chemotherapy New-FP for Hepatocellular Carcinoma with Preserved Liver Function
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Patients
2.3. Evaluation Items
2.4. Propensity Score Matching
2.5. Treatment Protocol
2.5.1. Sorafenib
2.5.2. New-FP
2.6. Statistical Analyses
3. Results
3.1. Patient and Tumor Characteristics
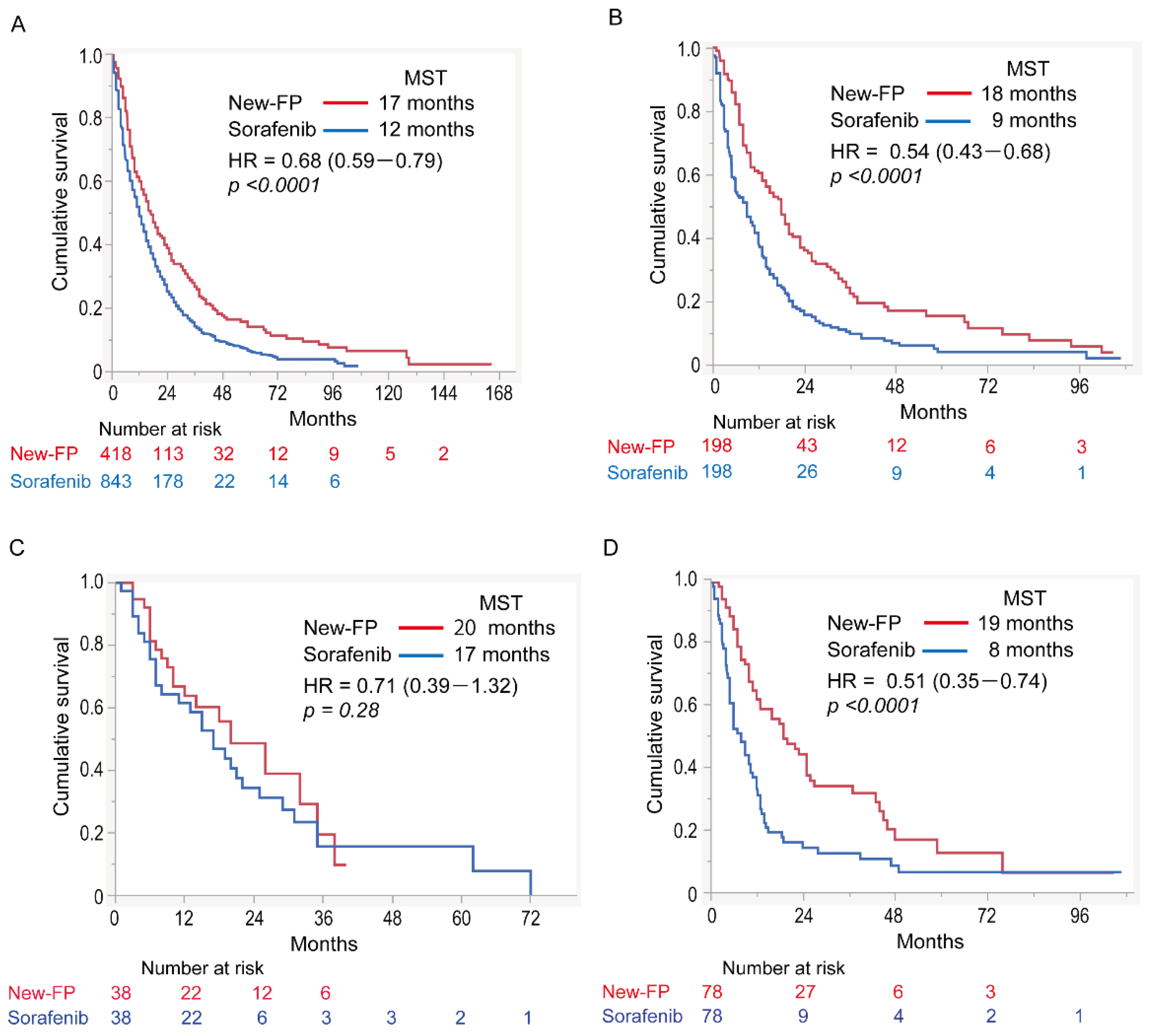
3.2. Overall Survival
3.3. Prognostic Factors
3.4. Subgroup Analyses
3.4.1. Cohort 1: No MVI or EHS
3.4.2. Cohort 2: MVI without EHS
3.4.3. Cohort 3: EHS without MVI
3.4.4. Cohort 4: MVI and EHS
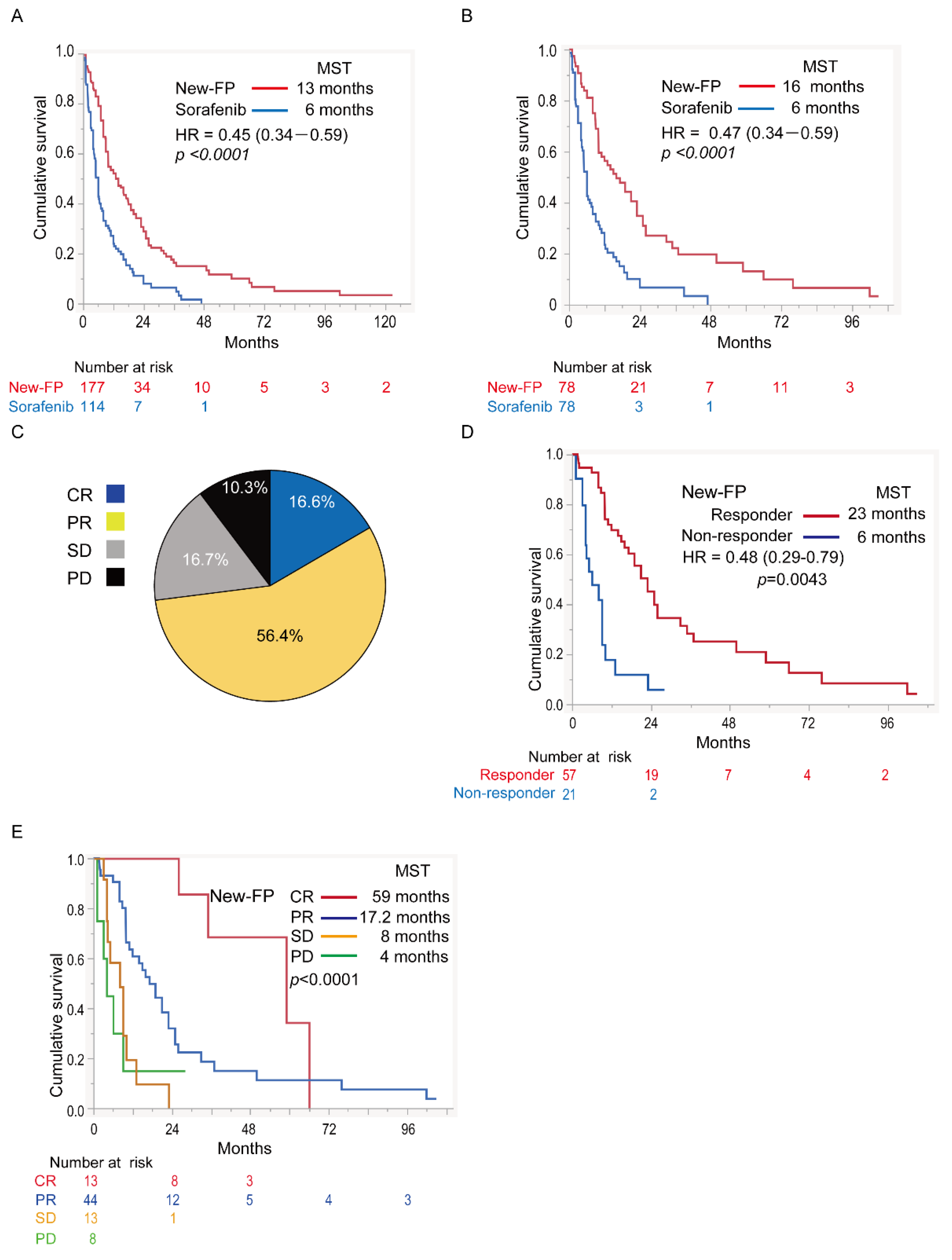
3.5. Assessment in Major PVTT-HCC
3.5.1. Comparison of OS in Major PVTT-HCC
3.5.2. Forest Plot Analysis
3.5.3. Efficacy of New-FP in Major PVTT-HCC
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kudo, M.; Kawamura, Y.; Hasegawa, K.; Tateishi, R.; Kariyama, K.; Shiina, S.; Toyoda, H.; Imai, Y.; Hiraoka, A.; Ikeda, M.; et al. Management of Hepatocellular Carcinoma in Japan: JSH Consensus Statements and Recommendations 2021 Update. Liver Cancer 2021, 10, 181–223. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Ricci, S.; Mazzaferro, V.; Hilgard, P.; Gane, E.; Blanc, J.-F.; De Oliveira, A.C.; Santoro, A.; Raoul, J.-L.; Forner, A.; et al. Sorafenib in Advanced Hepatocellular Carcinoma. N. Engl. J. Med. 2008, 359, 378–390. [Google Scholar] [CrossRef] [PubMed]
- Su, G.L.; Altayar, O.; O’Shea, R.; Shah, R.; Estfan, B.; Wenzell, C.; Sultan, S.; Falck-Ytter, Y. AGA Clinical Practice Guideline on Systemic Therapy for Hepatocellular Carcinoma. Gastroenterology 2022, 162, 920–934. [Google Scholar] [CrossRef]
- Haber, P.K.; Puigvehí, M.; Castet, F.; Lourdusamy, V.; Montal, R.; Tabrizian, P.; Buckstein, M.; Kim, E.; Villanueva, A.; Schwartz, M.; et al. Evidence-Based Management of Hepatocellular Carcinoma: Systematic Review and Meta-analysis of Randomized Controlled Trials (2002–2020). Gastroenterology 2021, 161, 879–898. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Brú, C.; Bruix, J. Prognosis of hepatocellular carcinoma: The BCLC staging classification. Semin. Liver Dis. 1999, 19, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Ju-Xian, S.; Jie, S.; Nan, L.; Wei-Xing, G.; Meng-Chao, W.; Wan-Yee, L.; Shu-Qun, C. Portal vein tumor thrombus is a bottleneck in the treatment of hepatocellular carcinoma. Cancer Biol. Med. 2016, 13, 452–458. [Google Scholar] [CrossRef]
- Tao, Z.-W.; Cheng, B.-Q.; Zhou, T.; Gao, Y.-J. Management of hepatocellular carcinoma patients with portal vein tumor thrombosis: A narrative review. Hepatobiliary Pancreat. Dis. Int. 2021, 21, 134–144. [Google Scholar] [CrossRef]
- Liu, M.; Shi, J.; Mou, T.; Wang, Y.; Wu, Z.; Shen, A. Systematic review of hepatic arterial infusion chemotherapy versus sorafenib in patients with hepatocellular carcinoma with portal vein tumor thrombosis. J. Gastroenterol. Hepatol. 2020, 35, 1277–1287. [Google Scholar] [CrossRef]
- Ueshima, K.; Komemushi, A.; Aramaki, T.; Iwamoto, H.; Obi, S.; Sato, Y.; Tanaka, T.; Matsueda, K.; Moriguchi, M.; Saito, H.; et al. Clinical Practice Guidelines for Hepatic Arterial Infusion Chemotherapy with a Port System Proposed by the Japanese Society of Interventional Radiology and Japanese Society of Implantable Port Assisted Treatment. Liver Cancer 2022, 11, 407–425. [Google Scholar] [CrossRef]
- Ueshima, K.; Kudo, M.; Takita, M.; Nagai, T.; Tatsumi, C.; Ueda, T.; Kitai, S.; Ishikawa, E.; Yada, N.; Inoue, T.; et al. Hepatic Arterial Infusion Chemotherapy Using Low-Dose 5-Fluorouracil and Cisplatin for Advanced Hepatocellular Carcinoma. Oncology 2010, 78, 148–153. [Google Scholar] [CrossRef]
- Kudo, M.; Matsui, O.; Izumi, N.; Iijima, H.; Kadoya, M.; Imai, Y.; Okusaka, T.; Miyayama, S.; Tsuchiya, K.; Ueshima, K.; et al. JSH Consensus-Based Clinical Practice Guidelines for the Management of Hepatocellular Carcinoma: 2014 Update by the Liver Cancer Study Group of Japan. Liver Cancer 2014, 3, 458–468. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Bao, Q.; Cao, G.; Zhu, X.; Yang, R.; Ji, X.; Xu, L.; Zheng, K.; Li, W.; Xing, B.; et al. Hepatic Arterial Infusion Chemotherapy Using Oxaliplatin Plus 5-Fluorouracil Versus Transarterial Chemoembolization/Embolization for the Treatment of Advanced Hepatocellular Carcinoma with Major Portal Vein Tumor Thrombosis. Cardiovasc. Interv. Radiol. 2020, 43, 996–1005. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, M.; Shimizu, S.; Sato, T.; Morimoto, M.; Kojima, Y.; Inaba, Y.; Hagihara, A.; Kudo, M.; Nakamori, S.; Kaneko, S.; et al. Sorafenib plus hepatic arterial infusion chemotherapy with cisplatin versus sorafenib for advanced hepatocellular carcinoma: Randomized phase II trial. Ann. Oncol. 2016, 27, 2090–2096. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, H.; Niizeki, T.; Nagamatsu, H.; Ueshima, K.; Nomura, T.; Kuzuya, T.; Kasai, K.; Kooka, Y.; Hiraoka, A.; Sugimoto, R.; et al. Survival Benefit of Hepatic Arterial Infusion Chemotherapy over Sorafenib in the Treatment of Locally Progressed Hepatocellular Carcinoma. Cancers 2021, 13, 646. [Google Scholar] [CrossRef] [PubMed]
- Quirk, M.; Kim, Y.H.; Saab, S.; Lee, E.W. Management of hepatocellular carcinoma with portal vein thrombosis. World J. Gastroenterol. 2015, 21, 3462–3471. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.L.; Chong, C.; Chan, A.; Poon, D.M.C.; Chok, K.S.H. Management of hepatocellular carcinoma with portal vein tumor thrombosis: Review and update at 2016. World J. Gastroenterol. 2016, 22, 7289–7300. [Google Scholar] [CrossRef] [PubMed]
- Lencioni, R.; Llovet, J.M. Modified RECIST (mRECIST) Assessment for Hepatocellular Carcinoma. Semin. Liver Dis. 2010, 30, 52–60. [Google Scholar] [CrossRef]
- Ueshima, K.; Ogasawara, S.; Ikeda, M.; Yasui, Y.; Terashima, T.; Yamashita, T.; Obi, S.; Sato, S.; Aikata, H.; Ohmura, T.; et al. Hepatic Arterial Infusion Chemotherapy versus Sorafenib in Patients with Advanced Hepatocellular Carcinoma. Liver Cancer 2020, 9, 583–595. [Google Scholar] [CrossRef]
- Niizeki, T.; Iwamoto, H.; Shirono, T.; Shimose, S.; Nakano, M.; Okamura, S.; Noda, Y.; Kamachi, N.; Hiroyuki, S.; Sakai, M.; et al. Clinical Importance of Regimens in Hepatic Arterial Infusion Chemotherapy for Advanced Hepatocellular Carcinoma with Macrovascular Invasion. Cancers 2021, 13, 4450. [Google Scholar] [CrossRef]
- Gordan, J.D.; Kennedy, E.B.; Abou-Alfa, G.K.; Beg, M.S.; Brower, S.T.; Gade, T.P.; Goff, L.; Gupta, S.; Guy, J.; Harris, W.P.; et al. Systemic Therapy for Advanced Hepatocellular Carcinoma: ASCO Guideline. J. Clin. Oncol. 2020, 38, 4317–4345. [Google Scholar] [CrossRef]
- Nakano, M.; Tanaka, M.; Kuromatsu, R.; Nagamatsu, H.; Tajiri, N.; Satani, M.; Niizeki, T.; Aino, H.; Okamura, S.; Iwamoto, H.; et al. Sorafenib for the treatment of advanced hepatocellular carcinoma with extrahepatic metastasis: A prospective multicenter cohort study. Cancer Med. 2015, 4, 1836–1843. [Google Scholar] [CrossRef] [PubMed]
- Aino, H.; Sumie, S.; Niizeki, T.; Kuromatsu, R.; Tajiri, N.; Nakano, M.; Satani, M.; Yamada, S.; Okamura, S.; Shimose, S.; et al. Clinical characteristics and prognostic factors for advanced hepatocellular carcinoma with extrahepatic metastasis. Mol. Clin. Oncol. 2014, 2, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Minagawa, M.; Makuuchi, M. Treatment of hepatocellular carcinoma accompanied by portal vein tumor thrombus. World J. Gastroenterol. 2006, 12, 7561–7567. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Zhu, X.; Fu, S.; Cao, G.; Li, W.-Q.; Xu, L.; Chen, H.; Wu, D.; Yang, R.; Wang, K.; et al. Sorafenib Plus Hepatic Arterial Infusion Chemotherapy versus Sorafenib for Hepatocellular Carcinoma with Major Portal Vein Tumor Thrombosis: A Randomized Trial. Radiology 2022, 303, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Chuma, M.; Uojima, H.; Hiraoka, A.; Kobayashi, S.; Toyoda, H.; Tada, T.; Hidaka, H.; Iwabuchi, S.; Numata, K.; Itobayashi, E.; et al. Analysis of efficacy of lenvatinib treatment in highly advanced hepatocellular carcinoma with tumor thrombus in the main trunk of the portal vein or tumor with more than 50% liver occupation: A multicenter analysis. Hepatol. Res. 2020, 51, 201–215. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M.; Ueshima, K.; Yokosuka, O.; Ogasawara, S.; Obi, S.; Izumi, N.; Aikata, H.; Nagano, H.; Hatano, E.; Sasaki, Y.; et al. Sorafenib plus low-dose cisplatin and fluorouracil hepatic arterial infusion chemotherapy versus sorafenib alone in patients with advanced hepatocellular carcinoma (SILIUS): A randomised, open label, phase 3 trial. Lancet Gastroenterol. Hepatol. 2018, 3, 424–432. [Google Scholar] [CrossRef]
- Kudo, M.; Ueshima, K.; Chiba, Y.; Ogasawara, S.; Obi, S.; Izumi, N.; Aikata, H.; Nagano, H.; Hatano, E.; Sasaki, Y.; et al. Objective Response by mRECIST Is an Independent Prognostic Factor for Overall Survival in Hepatocellular Carcinoma Treated with Sorafenib in the SILIUS Trial. Liver Cancer 2019, 8, 505–519. [Google Scholar] [CrossRef]
- Kudo, M. Systemic Therapy for Hepatocellular Carcinoma: Latest Advances. Cancers 2018, 10, 412. [Google Scholar] [CrossRef]
- Ikeda, M.; Okusaka, T.; Sato, Y.; Furuse, J.; Mitsunaga, S.; Ueno, H.; Morizane, C.; Inaba, Y.; Kobayashi, T.; Arai, Y. A Phase I/II trial of continuous hepatic intra-arterial infusion of 5-fluorouracil, mitoxantrone and cisplatin for advanced hepatocellular carcinoma. Jpn. J. Clin. Oncol. 2017, 47, 512–519. [Google Scholar] [CrossRef]
- Shimose, S.; Iwamoto, H.; Tanaka, M.; Niizeki, T.; Shirono, T.; Noda, Y.; Kamachi, N.; Okamura, S.; Nakano, M.; Suga, H.; et al. Alternating Lenvatinib and Trans-Arterial Therapy Prolongs Overall Survival in Patients with Inter-Mediate Stage HepatoCellular Carcinoma: A Propensity Score Matching Study. Cancers 2021, 13, 160. [Google Scholar] [CrossRef]
- Kudo, M. Changing the Treatment Paradigm for Hepatocellular Carcinoma Using Atezolizumab plus Bevacizumab Combination Therapy. Cancers 2021, 13, 5475. [Google Scholar] [CrossRef] [PubMed]
- Shimose, S.; Iwamoto, H.; Tanaka, M.; Niizeki, T.; Shirono, T.; Kajiwara, A.; Noda, Y.; Kamachi, N.; Okamura, S.; Nakano, M.; et al. Multimolecular-Targeted Agents for Intermediate-Stage Hepatocellular Carcinoma Influence Time to Stage Progression and Overall Survival. Oncology 2021, 99, 756–765. [Google Scholar] [CrossRef] [PubMed]



| Before Matching n = 1262 | After Matching n = 396 | |||||
|---|---|---|---|---|---|---|
| Patient Characteristics | New-FP n = 418 | Sorafenib n = 844 | p-Value | New-FP n = 198 | Sorafenib n = 198 | p-Value |
| Age (years) | 68.6 ± 10.9 | 70.1 ± 9.61 | 0.9398 | 66.6 ± 10.9 | 65.3 ± 10.7 | 0.8046 |
| Sex (male/female) | 327/91 | 677/167 | 0.4154 | 62/16 | 60/18 | 0.8127 |
| HCV/HBV/non-viral | 181/77/160 | 465/154/225 | <0.0001 | 37/19/22 | 27/28/23 | 0.8074 |
| Child–Pugh score (5/6) | 251/167 | 526/317 | 0.0563 | 0.1263 | ||
| Tumor characteristics | ||||||
| Tumor size (cm) | 8.29 ± 4.60 | 3.59 ± 3.39 | <0.0001 | 7.62 ± 4.21 | 7.26 ± 4.75 | 0.8107 |
| MVI (+/−) | 357/61 | 605/239 | <0.0001 | 143/55 | 146/52 | 0.7342 |
| Severe PVTT (+/−) | 177/180 | 114/491 | <0.0001 | 117/81 | 78/120 | 0.2304 |
| EHS (+/−) | 76/342 | 429/415 | <0.0001 | 24/54 | 21/57 | 0.7385 |
| BCLC stage (B/C) | 52/366 | 305/532 | <0.0001 | 46/152 | 50/148 | 0.6390 |
| AFP (ng/mL) | 27,729.0 ± 154,561.1 | 17,735.3 ± 110,114.8 | 0.1886 | 48,645.45 ± 248,475.48 | 52,073.81 ± 163,296.82 | 0.6153 |
| DCP (mAU/mL) | 23,513.0 ± 70,955.4 | 14,373.38 ± 71,899.8 | 0.0339 | 30,379.13 ± 95,631.52 | 27,714.78 ± 61,755.77 | 0.8773 |
| Variable | Univariate Analysis (p-Value) | Multivariate Analysis (p-Value) | Hazard Ratio (95% CI) |
|---|---|---|---|
| Sex (male/female) | 0.1681 | ||
| HBV (+/−) | 0.3909 | ||
| HCV (+/−) | 0.1851 | ||
| Child–Pugh score (5/6) | 0.0002 | 0.0068 | 0.72 (0.57–0.91) |
| BCLC stage (B/C) | 0.2621 | ||
| MVI (+/−) | 0.9679 | ||
| Major PVTT (+/−) | 0.0213 | 0.0002 | 1.57 (1.24–1.99) |
| EHS (+/−) | <0.0001 | 0.0011 | 1.76 (1.37–2.26) |
| AFP (≥400 ng/mL/<400 ng/mL) | 0.1811 | ||
| Better therapeutic response | <0.0001 | 0.7901 | |
| Treatment (New-FP/sorafenib) | 0.0350 | <0.0001 | 0.52 (0.41–0.65) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iwamoto, H.; Niizeki, T.; Nagamatsu, H.; Ueshima, K.; Tani, J.; Kuzuya, T.; Kasai, K.; Kooka, Y.; Hiraoka, A.; Sugimoto, R.; et al. The Clinical Impact of Hepatic Arterial Infusion Chemotherapy New-FP for Hepatocellular Carcinoma with Preserved Liver Function. Cancers 2022, 14, 4873. https://doi.org/10.3390/cancers14194873
Iwamoto H, Niizeki T, Nagamatsu H, Ueshima K, Tani J, Kuzuya T, Kasai K, Kooka Y, Hiraoka A, Sugimoto R, et al. The Clinical Impact of Hepatic Arterial Infusion Chemotherapy New-FP for Hepatocellular Carcinoma with Preserved Liver Function. Cancers. 2022; 14(19):4873. https://doi.org/10.3390/cancers14194873
Chicago/Turabian StyleIwamoto, Hideki, Takashi Niizeki, Hiroaki Nagamatsu, Kazuomi Ueshima, Joji Tani, Teiji Kuzuya, Kazuhiro Kasai, Youhei Kooka, Atsushi Hiraoka, Rie Sugimoto, and et al. 2022. "The Clinical Impact of Hepatic Arterial Infusion Chemotherapy New-FP for Hepatocellular Carcinoma with Preserved Liver Function" Cancers 14, no. 19: 4873. https://doi.org/10.3390/cancers14194873
APA StyleIwamoto, H., Niizeki, T., Nagamatsu, H., Ueshima, K., Tani, J., Kuzuya, T., Kasai, K., Kooka, Y., Hiraoka, A., Sugimoto, R., Yonezawa, T., Tanaka, S., Deguchi, A., Shimose, S., Shirono, T., Sakai, M., Suzuki, H., Moriyama, E., Koga, H., ... Kurume Liver Cancer Study Group of Japan. (2022). The Clinical Impact of Hepatic Arterial Infusion Chemotherapy New-FP for Hepatocellular Carcinoma with Preserved Liver Function. Cancers, 14(19), 4873. https://doi.org/10.3390/cancers14194873







