Simple Summary
α6β4-integrins and plectin are the key structural components of hemidesmosomes that have been implicated in carcinogenesis and which are thus considered as potential cancer biomarkers and drug targets for anti-cancer therapies. In this review, we elaborate on the current knowledge of the kinase signaling pathways regulated by α6β4-integrins and plectin in the context of prostate cancer. We discuss an emerging scenario where hemidesmosomal α6β4-integrins and plectin function as tumor suppressors but adopt new oncogenic roles upon hemidesmosome disassembly in prostate cancer.
Abstract
Hemidesmosomes (HDs) are adhesive structures that ensure stable anchorage of cells to the basement membrane. They are formed by α6β4-integrin heterodimers and linked to intermediate filaments via plectin. It has been reported that one of the most common events during the pathogenesis of prostate cancer (PCa) is the loss of HD organization. While the expression levels of β4-integrins are strongly reduced, the expression levels of α6-integrins and plectin are maintained or even elevated, and seem to promote tumorigenic properties of PCa cells, such as proliferation, invasion, metastasis, apoptosis- and drug-resistance. In this review, we discuss the potential mechanisms of how HD components might contribute to various cellular signaling pathways to promote prostate carcinogenesis. Moreover, we summarize the current knowledge on the involvement of α6β4-integrins and plectin in PCa initiation and progression.
1. Introduction
PCa is a complex disease that affects millions of men globally [1]. Although improvements in its early detection have reduced the mortality rate, PCa is still one of the leading causes of cancer-related death in men [2]. The 5-year survival rate for men with local PCa is around 98%, whereas the 5-year survival rate for men with metastatic PCa does not exceed 30% [3]. The most preferable metastatic target tissue for PCa are lymph nodes and bone. Since over 20% of PCa patients progress to metastatic disease, a better understanding of the molecular signatures differing between indolent and aggressive tumors is needed.
The metastatic cells that escape from their primary site and circulate in the bloodstream are exposed to many factors inducing cellular stress. These cells have to be able to endure shear forces, adapt to new surroundings at potential metastatic sites and avoid the surveillance and attack of the immune system [4]. Indeed, the invasion of cancer cells into nearby or distant tissue requires the reinforcement of many of their properties, including proliferation, migration, cell cycle progression and apoptosis-, anoikis- and drug-resistance. These features enable cancer cells to first disseminate and invade the primary site, eventually leading to intravasation and circulation in the bloodstream and finally extravasation and colonization of a new niche [5].
The extracellular matrix (ECM) has emerged as a critical driver of tumorigenesis [6,7]. It governs the reversible biochemical modifications that enable the achievement of specific epithelial-mesenchymal phenotype plasticity which is crucial for tumor progression and metastasis [8]. Thus, it is not surprising that the function of integrins, the major family of ECM receptors, is often perturbed in cancer cells [7,9]. Integrins form the basis for two main types of cell-ECM adhesions, the actin-linked focal adhesions (FAs) involved in cell migration, and the HDs that link to the intermediate filament network and form dynamic anchoring structures regulating cell adhesion and cellular differentiation [10]. Although FAs and HDs are distinct complexes, they seem to regulate each other via intricate signaling crosstalk that is not yet thoroughly understood [11,12,13]. The main core of HDs is formed by a heterodimer of α6- and β4-integrins which mediate the attachment of epithelial cells to the laminin-rich basement membrane (BM) and are often considered to function as tumor suppressors [14,15,16,17]. There are two types of HDs: highly organized type I HDs that are typical for squamous epithelia such as the skin epithelium, and less well-defined type II HDs that are found in simple epithelium [18]. The prostate epithelium is considered to express type II HDs and, therefore, in this review we mainly focus on the functions associated with type II HDs.
It has been reported that one of the most frequent events taking place during PCa pathogenesis is the loss of HDs [15,16,19,20]. In line with these observations, a strong reduction in β4-integrin levels is observed in HD-deficient PCa cell lines as well as in tumor tissues in PCa patients [19]. Moreover, the downregulation of ITGB4 leading to HD disassembly is strongly correlated with PCa progression, metastasis, tumor stage, Gleason score, prostate-specific antigen (PSA) level and worse patient survival [19,21]. Furthermore, a reduced expression of β4-integrins was observed during the development of prostate intraepithelial neoplasia (PIN), and subsequent progression to prostate carcinoma [20]. Upon HD disassembly, α6-integrin and plectin are released from HDs and seem to play a key role in the activation and maintenance of tumorigenic properties in PCa cells [19,22,23,24]. To exercise these functions, the HD components activate or cooperate with many cellular kinases. Protein kinases are intracellular enzymes that catalyze the transfer of phosphate from ATP to other proteins and are considered central mediators of cellular signals with critical implications in the PCa progression as well. However, they are involved in the regulation of almost every cellular activity: proliferation, migration, apoptosis and cell cycle control [25]. The different kinase pathways are linked via complex signaling crosstalk and therefore the role of individual kinases and the associated signaling pathways are likely highly context-dependent. The role of kinase signaling cascades in PCa has been recently thoroughly reviewed [26,27,28]. While there are approximately 500 protein kinases in humans [29], in this review, we discuss those that have been reported to be associated with HD functions.
2. Hemidesmosomal Components Regulate the Tumorigenic Properties of Cancer Cells
α6β4-integrins have been studied for decades and modulation of their function appears to be strongly linked to cancer progression. Both oncogenic and tumor-suppressing functions have been reported but the manner in which α6β4-integrin levels correlate with tumorigenesis and metastatic potential appears to be context-dependent and is thus still a point of debate. On the one hand, there are several papers showing the importance of upregulation of α6β4-integrins linked to cancer progression, especially in pancreatic, lung, breast and colon cancer [21,30,31,32]. On the other hand, downregulation of α6- or β4-integrins, associated with loss of HDs, has been positively correlated with poor PCa prognosis and increased metastatic potential of PCa cells [11,15,16,19,20,21,33]. Moreover, one of the most frequent events during PCa pathogenesis is the loss of HDs [15,16,20]. Recent data revealed that strong downregulation of β4-integrin causing HD disassembly is critical for the metastatic potential of PCa cells [19,33]. In line with these observations, reduced expression of β4-integrins was documented during the development of prostate intraepithelial neoplasia and its progression to prostate carcinoma [20]. These findings were validated by an extensive bioinformatics analysis of PCa patient cohorts [19,21]. Importantly, a recent analysis of a PCa tissue microarray (TMA) indicated that the loss of HDs correlated with a strong reduction in β4-subunit levels while significant levels of α6-integrins and plectin were retained in PCa patient tissues, as was seen for most of the PCa cell lines [19]. It was found that this scenario was particularly evident in phosphatase and tensin homolog (PTEN)-negative prostate tumors. In PTEN-positive prostate and breast cancer cells, loss of α6- or β4-integrin expression caused a strong reduction in the other heterodimer partner and plectin, while in PTEN-negative cells both the protein levels of the heterodimer partner and plectin were only modestly affected or even upregulated. Importantly, plectin expression was not only retained but also required for enhanced tumorigenesis [19]. In addition, mutations in locus rs12621278 within the ITGA6 gene region and resulting in reduced ITGA6 expression have been associated with increased susceptibility for the development of PCa, suggesting that, in some cases, HD-disruption via downregulation of α6-integrins might also promote PCa tumorigenesis in vivo [34].
Taken together, it seems that the function of α6β4-integrins in tumorigenesis can be different depending on whether the HDs are intact or not and this could be regulated by β4- or α6-expression levels, ECM composition, the genomic landscape of cancer cells as well as any signaling activities that regulate the activity and/or stability of α6β4-integrins.
2.1. Disassembly of HDs Is a Prerequisite for Initiation of Carcinogenesis Driven by Hemidesmosomal Proteins
α6β4-integrins and plectin play a central role in the progression of several types of cancer. It has been postulated that HD disassembly occurs at and is a critical driver of the cancer initiation stage, especially in PCa. In normal tissues, laminin-332 has been shown to bind to α6β4-integrins, leading to HD assembly and inhibition of cell movement [35]. By contrast, in tumor tissues laminin-332 forms complexes with α3β1-integrins in actively migrating cells [36]. Growth factor receptor-mediated signaling is thought to regulate the integrin receptor switch from α6β4- to α3β1-integrins [37]. Although many studies have shown that laminin-332 expression is up- or downregulated in cancer, its expression levels in prostate, colorectal, and breast carcinoma are consistently strongly reduced in comparison to unchanged tissue [38,39,40]. Therefore, the reduction in laminin-332 levels or the stimulation of α3β1-integrin binding to laminin-332 may lead to loss of interactions between α6β4-integrins and laminin-332 resulting in HDs disassembly. Furthermore, it has been shown that laminin-332 levels are altered by epidermal growth factor (EGF) signaling. Mainiero et al. [41] found that activated EGFR induces phosphorylation of the cytoplasmic tail of β4-integrin, suggesting that the interaction of growth factor receptors with β4-integrin might be directly involved in HD disassembly. In support of such a hypothesis, α6- or β4-integrin knock-out in PTEN-negative PCa cells resulted in plectin upregulation and robust activation of epidermal growth factor receptor (EGFR) phosphorylation [19]. HD disruption might also be a consequence of the epigenetic modification of ITGB4 expression. Recently, Wilkinson et al. [42] found that the ITGB4 promoter region is epigenetically modified by hypermethylation in aggressive androgen receptor (AR)-independent prostate cancer cells. Such modifications caused the production of a shorter form of β4-integrin from the alternative transcription start site [42]. So, far, the role of this variant is unclear, but the shorter β4-integrin might be unable to pair with α6-integrin and thereby contribute to HD disassembly.
Importantly, there is a growing body of evidence showing that an altered stromal microenvironment leads to modified interplay between stromal and epithelial cells which is a central factor promoting cancer progression [43,44]. In a normal prostate gland, the BM separates stromal from epithelial cells but even minor polarity defects, which are frequent during cancer initiation, may lead to compromised BM barrier function and aberrant interactions between the two compartments. Whether and how HD disintegration contributes to BM dysfunctions remains to be studied.
2.2. Regulation of Cell Proliferation
When HDs are intact, the attachment of α6β4-integrins to laminin induces a conformational change enabling tyrosine phosphorylation of the cytoplasmic tail of β4-integrin [45,46,47,48]. This phosphotyrosine (pTyr) residue can be recognized and bound by proteins containing pTyr-binding domains, such as the Src homology-2 (SH2) or pTyr-binding (PTB) domain leading to the activation of the downstream signaling pathways. The adaptor protein Shc has both such domains, and it can interact with the pTyr-residue at the cytoplasmic domain of the β4-subunit [46]. The interaction between β4-integrin and Shc does not affect HD formation, but it is required for the phosphorylation of tyrosine residue in Shc [45]. The phosphorylated Shc can bind to growth factor receptor-bound protein-2 (Grb2), which in turn recruits a cytoplasmic molecule Son of Sevenless (SOS), leading to its translocation to the membrane where it promotes the activation of Ras small GTPase (Ras) [49,50]. Activated Ras recruits rapidly accelerated fibrosarcoma kinase (RAF) to the cell membrane, leading to RAF activation and dimerization. Activated RAF kinase phosphorylates and activates mitogen-activated protein kinase (MEK), which in turn phosphorylates and activates extracellular signal-regulated kinase (ERK). Finally, active ERK translocates to the nucleus, where it phosphorylates transcription factors that promote cell proliferation [51]. In principle, all elements of the Ras/RAF/MEK/ERK pathway can be overexpressed and/or activated in PCa, leading to further activation of the pathway [52,53,54].
It has been shown that α6β4-integrins are involved in the activation of the PI3K/AKT pathway. However, neither α6- nor β4-integrin can directly activate phosphoinositide 3-kinase (PI3K) because they lack the required binding domain in their cytoplasmic tail. However, they can initiate a signal leading to activation of the PI3K/AKT pathway, which is then passed on to other proteins involved in direct interaction with PI3K [55]. Insulin receptor substrates 1 and 2 (IRS-1/2) are cytoplasmic adapter proteins that do not contain intrinsic kinase activity but function by recruiting proteins to cell surface receptors, thereby assembling complexes that function as signaling intermediates linking the activation of PI3K and α6β4-integrins. Ligation of the α6β4-integrins promotes the tyrosine phosphorylation of IRS-1 and -2, increasing their association with PI3K [55]. Similarly, phosphorylated Shc recruited by the β4-integrin cytoplasmic tail not only activates the Ras/RAF/MEK/ERK pathway, but it can also trigger the PI3K/AKT pathway when accompanied by cytokine-inducible phosphorylated GRB2-associated-binding protein 2 (Gab2). This Shc-Grb2-Gab2 complex can activate PI3K [56]. Upon activation, PI3K phosphorylates phosphatidylinositol 4,5-bisphosphate (PIP2) to generate phosphatidylinositol (3,4,5)-trisphosphate (PIP3), which in turn recruits 3-phosphoinositide-dependent kinase 1 (PDK1) leading to AKT phosphorylation [57]. AKT, also known as protein kinase B, is a signaling nexus connecting several downstream pathways. These include the direct phosphorylation of a mammalian target of rapamycin (mTOR), which is highly expressed in PCa [58].
In PCa, several receptor tyrosine kinases (RTKs), including EGFR, ErbB2, MET and Ron, have been shown to phosphorylate β4-integrin, leading to the activation of the PI3K/AKT pathway and disassembly of HDs. Macrophage-stimulating protein (MSP) activates Ron, which in turn activates PI3K/AKT and, together with 14-3-3 proteins, phosphorylates α6β4-integrins, forming a complex and generating a feedback loop to further promote signaling [59,60,61,62,63,64]. Moreover, the activation of EGFR or protein kinase C (PKC) has been shown to lead to β4-integrin activation via phosphorylation of its S1356 and S1364 by ERK1/2 and ribosomal S6 kinase 1/2 (RSK1/2) [65]. This phosphorylation event prevents the ABD domain of plectin from interacting with β4-integrin, leading to HD disassembly. Plectin released from HDs binds to the receptor for activated kinase C1 (RACK1) and regulates PKC signaling critical for the MAPK/ERK signaling pathway, promoting proliferation [66]. This finding agrees with the observation that plectin is a Pro tumorigenic regulator of cancer cell proliferation in several cancers including PCa [19,24,67]. Plectin knock-down inhibited PCa cell growth both in vitro and in vivo [24]. Moreover, when released from HDs, plectin plays an important role in the modulation of proliferative signaling together with α6- or β4-integrins since plectin knock-out prevented the activation of the PI3K/AKT pathway in PTEN-negative PCa cells with disrupted HDs [19].
PCa cell proliferation can also be promoted through the activation of the AKT/IKK/NF-κB pathway. The nuclear factor kappa B (NF-κB) is normally localized in the cytoplasm as a complex with the inhibitory proteins IκB. IκB can be phosphorylated by the IκB kinase (IKK) complex, leading to its ubiquitination and degradation and release of NF-κB from the complex. NF-κB then translocates to the nucleus and activates the transcription of genes that promote cell survival [68,69]. Zahir et al. [70] reported that the cytoplasmic tail of β4-integrin is required for the activation of the Rac family of small GTPases induced by basal and epidermal growth factors. They found that constitutive activation of Rac through NFκB activation is sufficient to maintain the viability of breast tumor cells lacking functional β1- and β4-integrins [70]. Therefore, autocrine laminin 332–α6β4-integrins–Rac–NFκB signaling might be a pathway that is activated to sustain the proliferation of PCa cells when the contact with BM is lost and/or HDs disassembled.
2.3. Regulation of Cell Migration
HD disassembly appears to be necessary for epithelial cell migration and tumor invasion (Figure 1) [13,71]. The known molecular drivers responsible for the disassembly of HDs are described in Section 2.1. It has been shown that upon disintegration of HDs, retraction fibers containing α6β4-integrins localize to the rear of actively migrating tumor cells and keratinocytes [72,73]. The exact mechanism by which HDs dynamics are controlled in cancer cells is not understood, but it has been suggested that specific phosphorylation and Ca2+ may be involved in this regulation. The specific amino acid residues phosphorylated within the β4-integrin molecule and their functional consequences for cell migration capacity were recently summarized in [74]. Activating the stimulus results in the phosphorylation of the cytosolic tail of β4-integrin, leading to the interruption of plectin binding to β4-integrin [75]. Dissociation of the plakin domain of plectin from the β4-integrin cytoplasmic tail depends on specific threonine phosphorylation at T1736 in the C-terminal region of β4-integrin by RSK1 or protein kinase D2 (PKD2) [76,77]. The phosphorylation of serine residues at S1356 and S1364 located in the connecting segment (CS) of β4-integrin is thought to be catalyzed by ERK and by RSK, respectively, and these events appear to contribute to HD disassembly [65]. After plectin detachment from HD, the exposed tyrosine and serine residues within the β4-integrin molecule become available for possible phosphorylation by RTKs, leading to the activation of PI3K, AKT and Ras homologous small GTPase (Rho)-mediated signaling pathways [30].
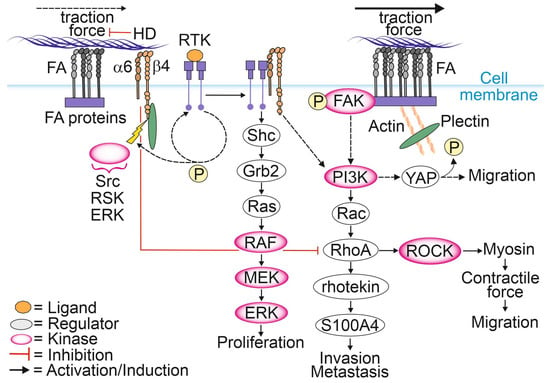
Figure 1.
Involvement of α6β4-integrins and plectin in the regulation of cell proliferation and migration.
Although FAs and HDs are distinct complexes, they seem to dynamically regulate each other via intricate signaling crosstalk that is not yet thoroughly understood [11,12,13]. Several reports find that loss of HDs promotes cell migration by stimulating FA dynamics [11,78,79]. Microscopic analysis of exogenous and endogenous FA marker proteins showed higher FA dynamics and faster diffusion rates for FA-localized vinculin in and out of FAs in HD-depleted PCa cells. Moreover, several FA marker proteins formed larger FAs compared to cells with intact HDs [11]. Furthermore, upon HD disassembly plectin relocated to actin filaments with a concomitant increase in stress fiber formation, focal adhesion kinase (FAK) autophosphorylation and activation of the PI3K/AKT pathway. Another study reported upregulation and activation of FAs leading to increased actomyosin contractility and traction forces stimulating FAK phosphorylation and translocation of Yes-associated protein (YAP) into the nucleus [78,80]. Active YAP subsequently activates the Rho/ROCK/myosin light chain (MLC)-pathway at the transcriptional level, whereas FAK activates multiple signaling pathways promoting cell migration [78,81,82,83,84,85,86,87]. Furthermore, downstream of the PI3K/Rac/RhoA pathway, rhotekin plays an important role by interacting with S100 calcium-binding protein A4 (S100A4) to promote membrane ruffling, lamella formation and the generation of contractile forces facilitating tumor invasion [88,89,90]. Taken together, HD-mediated adhesion to laminin is essential for the suppression of FA maturation and cell spreading, possibly through a reduction in RhoA/ROCK/MLC signaling [78]. Therefore, RhoA remains inactive, contributing to low ROCK activity, thereby preventing myosin phosphorylation, and ultimately leading to a decrease in actomyosin contractility and traction forces [82].
2.4. Regulation of Cell-Death-Associated Pathways
Depending on the cellular context, integrins can promote either cell survival or cell death. As long as cells maintain a proper contact with the ECM, integrins initiate a signaling cascade that promotes cell growth and survival. Loss of integrin signaling in non-adherent cells leads to the inhibition of cell growth and the promotion of a specialized form of cell death known as anoikis (Figure 2) [30,91].
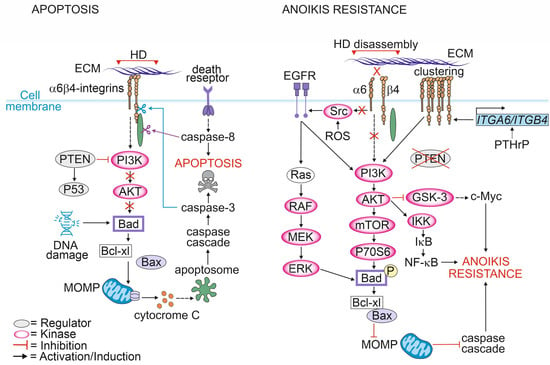
Figure 2.
Mechanisms of apoptosis resistance induced by hemidesmosomal proteins.
The most critical event and point-of-no-return in apoptotic signaling is the mitochondrial outer membrane permeabilization (MOMP) [92]. Under normal conditions, bcl-2-like protein 4 (Bax) forms a complex with the anti-apoptotic protein B-cell lymphoma-extra-large (Bcl-XL) in the mitochondrial outer membrane. Activation of apoptotic signaling induces phosphorylation of the BH3-only protein BAD that interacts with Bcl-XL leading to the release of Bax from the complex. Bax release allows its oligomerization to form pores at the outer mitochondrial membrane [93]. MOMP leads to release of cytochrome c into the cytosol, triggering apoptosome formation and resulting in the activation of the caspase cascade [94].
In adherent cells, cell death via apoptosis can be triggered by intracellular events such as excessive DNA damage, ER stress or hypoxia. The pro-apoptotic signaling triggered by cellular stressors follows the same mitochondrial pathway as described for anoikis and is followed by the activation of caspases, including caspase-3 and -7. It has been shown that the cytoplasmic tail of β4-integrin is cleaved by activated caspase 3 in epithelial cells undergoing apoptosis [95].
Caspases can also be activated by death receptors [96]. Stegh et al. reported that after induction of extrinsic apoptosis by CD95 or TNF-R, initiator caspase-8 bound to and selectively cleaved plectin at Asp2395 [97]. Microscopic analysis revealed translocation of caspase-8 into plectin-rich foci resulting in complete cleavage of plectin upon stimulation of apoptosis in vivo. In another study, plectin was shown to be a target of caspase-3 and -7 cleavage in keratinocytes undergoing staurosporine-induced apoptosis [98]. Furthermore, plectin-deficient primary mouse fibroblasts showed impaired apoptosis-induced actin reorganization after CD95-mediated apoptosis suggesting that plectin is critical for this process [97,99]. Consistent with these findings, Ni et al. found that plectin plays a critical role in protecting podocytes from adriamycin-induced apoptosis and disruption of the F-actin cytoskeleton. Adriamycin treatment led to the downregulation of plectin levels followed by phosphorylation of Y1494 in β4-integrins and activation of FAK/p38 signaling pathway resulting in cell death. Activation of p38 subsequently decreased F-actin levels and inhibited actin polymerization resulting in a disrupted cytoskeleton [100]. In agreement with these findings, a recent study reported that a lack of β4-integrins in PTEN-negative PCa cells resulted in the upregulation of plectin and increased apoptosis- and anoikis-resistance [19]. Given that plectin is often upregulated in PCa cell lines and patients’ tissues representing an aggressive form of PCa it is possible that plectin upregulation might be a strategy adopted by PCa cells to avoid cell death enabling metastasis to other organs.
Metastatic PCa cells inhibit apoptotic pathways upon loss of ECM contact and avoid anoikis by several means. One of them is the redox-mediated pathway [101]. Reactive oxygen species (ROS) can directly oxidate proto-oncogene tyrosine-protein kinase Src at cysteine residues leading to its activation [102]. Activated Src can, in turn, phosphorylate EGFR which activates the AKT pathway [101]. AKT can phosphorylate BAD, leading to the inhibition of apoptosis [103,104]. EGFR can also phosphorylate BAD in a PI3K-independent manner by activating Ras/RAF/MEK/ ERK signaling cascade [105,106].
ROS signaling can disrupt cytoskeletal organization by oxidating actin, resulting in its disassembly and depolymerization and thereby promoting cell death [107]. In fibroblasts, overexpression of plectin was shown to stabilize the actin cytoskeleton, which in turn increased the number of actin filaments, thereby creating more binding sites for plectin [108,109]. A similar observation was made in PCa cells where plectin was released from β4-integrins upon HDs disassembly and associated with actin cytoskeleton. This approach is likely beneficial for cancer cells, as a stable actin cytoskeleton protects cells from ROS-induced damage and apoptosis. In line with these findings, structural studies by Spurny et al. showed that after oxidation, plectin becomes more stable due to the disulfide bond-mediated conformation change. Importantly, this protects not only actin but also vimentin from being oxidized. Plectin, by directly binding to vimentin, sterically blocks the access for ROS and thereby prevents collapse of the vimentin cytoskeleton [110]. Moreover, ROS have been shown to exert direct oxidation and activation of several kinases, including Src tyrosine kinase [111,112,113]. Oxidized Src at positions Cys245 and Cys485 can induce phosphorylation of the Tyr845 residue of EGFR, which leads to the activation of pathways mediated by Erk and Akt. It has been proposed that in PCa such a mechanism protects cancer cells from apoptosis [101].
Parathyroid hormone-related protein (PTHrP) is expressed by human prostate tissues and PCa cell lines [114]. PTHrP has been shown to increase α6β4-integrin expression at the cell surface via a non-canonical intracrine pathway which in turn facilitates growth factor receptor signaling by activating the PI3K/AKT pathway, leading to BAD phosphorylation and reduced apoptosis in an anchorage-independent manner. Moreover, intracrine PTHrP signaling results in the phosphorylation of glycogen synthase kinase-3 (GSK-3), leading to the inhibition of GSK-3 activity [114]. Since GSK-3 is known to phosphorylate MYC proto-oncogene (c-myc) targeting it into the ubiquitin-proteasome pathway, the inactivation of GSK-3 results in increased levels of c-myc and thus decreased apoptosis [115].
An additional escape route from apoptosis is through the activation of the PI3K/AKT/mTOR pathway. mTOR activates the mammalian target of the rapamycin complex 1 (mTORC1) that mediates protein synthesis by phosphorylating and activating ribosomal protein S6 kinase (p70S6), which in turn phosphorylates and inactivates BAD, thereby inhibiting apoptosis [116,117].
Role of p53 and PTEN in the Regulation of Hemidesmosomal-Components-Mediated Cell Death
p53 is one of the most prominent tumor suppressors, halting cell division upon the detection of DNA damage. Mutations in TP53 gene encoding for the p53 protein are common in PCa [118,119,120,121]. The mechanisms by which the different mutated forms of p53 promote the development of PCa remain incompletely understood but it is evident that p53 mutants can modulate the activity of many signaling pathways including those associated with α6β4-integrins. Studies in colon and breast carcinoma have shown that p53 inhibits α6β4-integrins-mediated activation of AKT by promoting the caspase-3-dependent cleavage of this kinase [122,123]. Bachelder et al. postulated that the function of α6β4-integrins depends on the p53 status. In p53-deficient cells, α6β4-integrins promoted AKT-dependent survival of carcinoma cells, whereas in carcinomas expressing functional p53, α6β4-integrins stimulated the cleavage and inactivation of serine/threonine kinase AKT in caspase-3-dependent manner, indicating that p53 can inhibit survival signals emanating from α6β4-integrins [123].
Inactivation of the PTEN is among the most common genomic aberrations in PCa. Loss of PTEN function leads to the activation of the AKT pathway via enhanced phosphorylation of Thr-308 and Ser-473 residues in AKT [124]. PTEN is a dual-specificity lipid and protein phosphatase that has the ability to remove the phosphate group from both phospho-tyrosine and phospho-serine/threonine residues. It converts PIP3 into PIP2 and removes phosphate groups from the tyrosine residues of several substrate proteins. PIP3-to-PIP2 conversion results in the activation of PI3K and AKT, key factors regulating cell survival [125]. PTEN is involved in a complex network of interactions with p53 as it harms p53 stability while p53 enhances PTEN transcription. When PTEN is lost, the p53 pathway is strongly activated [126]. Recently, the loss of PTEN and HDs were shown to synergistically promote the tumorigenesis of PCa cells [19]. The dual loss of PTEN and HDs induced apoptosis- and anoikis-resistance via the activation of the EGFR/PI3K/AKT-cascade. A bioinformatic analysis of several PCa patient cohorts revealed a strong negative correlation between plectin and PTEN expression, suggesting that plectin was essential for tumorigenicity in PTEN-negative PCa cells with disrupted HDs [19].
2.5. Regulation of Cell Cycle
The involvement of HD components in cell cycle regulation has been documented (Figure 3). However, there are only a few papers specifically focusing on the involvement of α6β4-integrins and plectin in the regulation of cell cycle in PCa. Here, we compile these studies and correlate them with evidence for the role of HD components in the cell cycle in other cancer types.
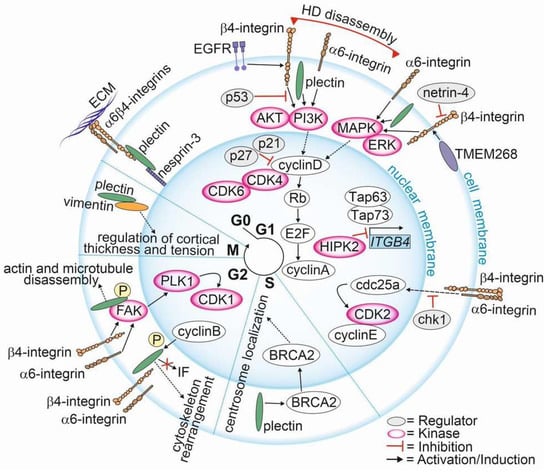
Figure 3.
Involvement of hemidesmosomal proteins in cell cycle regulation.
The upregulation of plectin in PTEN-negative cells with disrupted HDs was associated with differential expression of signaling pathways regulating the cell cycle. Plectin knockout cancelled these effects, suggesting that in PTEN-negative PCa cells plectin when released from HDs regulates the cell cycle [19]. In line with this finding, Hong et al. showed that transmembrane protein 268 (TMEM268) induces an S-phase cell cycle arrest and disrupts cytoskeletal remodeling by interacting with β4-integrin [127]. They found that the depletion of TMEM268 led to polyubiquitination and subsequent degradation of β4-integrins, accompanied by a strong reduction in α6-integrin levels. This event was associated with a cell cycle arrest in the S-phase caused by the inactivation of the cyclin B1/cyclin-dependent kinase 1 (CDK1) complex [127]. TMEM268-KO gastric cancer cells had reduced viability, proliferation rate and adhesion, all contributing to observed reduced tumorigenicity in a xenograft mouse model. Moreover, the disruption of TMEM268-β4-integrin interaction in gastric cancer cells led to the displacement of plectin from the cytoplasmic tail of β4-integrins, leading to plectin binding to the actin cytoskeleton.
The current data propose a model where, upon HDs disassembly, α6β4-integrins adopt new signaling functions and activate FAK/Src- and EGFR/PI3K/AKT/ERK-cascades, leading to the upregulation of cyclin D and the degradation of CDK inhibitors (p21 and p27) (Figure 3) [19,128,129]. Cyclin D is a sensor that integrates extracellular signals to regulate the cell cycle machinery as it plays a critical role in allowing the G1 to S phase transition [130]. The CCND1 expression is upregulated by ERK, Ets-like factors, AP-1 and KLF8 [131,132]. Cyclin D binds to and activates cyclin-dependent kinase 4 and -6 (CDK4 and CDK6), which then phosphorylate retinoblastoma protein (Rb) leading to the dissociation of the Rb-E2F complex freeing E2F transcription factors to activate cyclin A expression and entry into the S phase. Furthermore, PI3K/AKT-mediated inhibition of GSK-3β increases the stability of cyclin D by preventing its degradation [133]. During the G2 phase, integrin-mediated signaling leads to the activation of FAK and polo-like kinase 1 (PLK1) [131]. PLK1 phosphorylates the activating phosphatase cell division cycle 25c (Cdc25c) that together with inhibiting kinase Wee1 regulate the activity of CDK1 and cyclin B at the late G2 phase. Cdc25c triggers a rapid activation of the CDK1 and cyclin B to allow entry to mitosis [131,134,135]. Moreover, plectin interacts with CDK1 to regulate the marked cytoskeletal rearrangements necessary for mitosis [136]. Upon phosphorylation of plectin at Thr4542 by the p34cdc2 kinase that is activated during the M phase, plectin rapidly dissociates from IFs and becomes diffusely distributed in the cytoplasm [136]. This leads to the disassembly of F-actin microtubule networks and causes the collapse of keratin IF networks in prophase [136].
The crosstalk between α6β4-integrins and the activation of p53 followed by the induction of p21 has been reported [137,138,139,140]. p21 inhibits cell cycle progression by binding to CDK2, cyclin D and cyclin E and by regulating the expression of Rb and E2F proteins [141,142]. Furthermore, members of the p53 family are central to the regulation of integrin expression. Bon et al. [143] revealed that homeodomain-interacting protein kinase 2 (HIPK2), which is involved in p53-mediated cellular apoptosis and regulation of the cell cycle, modulates ITGB4 expression [143,144]. HIPK2 depletion activates tumor protein p63 and p73 (TAp63 and TAp73) functions, leading to p53-dependent activation of ITGB4 transcription [145]. TAp73, a tumor suppressor inducing apoptosis and cell cycle arrest [146], has been shown to bind the ITGB4 promoter region to stimulate ITGB4 transcription [145]. In addition to transcriptional regulation, β4-integrin levels can be altered by netrin-4 [147] and human leukocyte antigens (HLA), both of which have been implicated in cell cycle progression [148]. However, the mechanistic details of these interactions are still elusive.
It has been demonstrated that the knock-down of ITGA6 upregulates cyclin-dependent kinase inhibitor p27 in the G1/S phase and transcription factor E2F1 that subsequently downregulates cdc25a and cyclin E/CDK2, leading to a deceleration of cell cycle progression by inhibition entry into the S phase [149]. Activated cyclin E enables passage through the G1/S phase by binding to CDK2, leading to phosphorylation and inactivation of Rb and release of E2F transcription factors [150]. Furthermore, the downregulation of α6-integrins was associated with upregulation of checkpoint kinase 1 (chk1), resulting in cdc25a downregulation and inhibition of Rb phosphorylation [149]. chk1 phosphorylates cdc25a, generating a binding site for inhibitory 14-3-3 protein. The binding of 14-3-3 regulates the S-phase checkpoint in response to genotoxic stress induced by DNA-damaging agents or irradiation [150,151,152]. These data suggest that α6-integrin depletion prevents cells from passing through the G1/S. In line with this finding, Hu et al. showed that depletion of α6-integrin affected arrested cells in the G2/M checkpoint only when the cells had been treated with infrared radiation. α6-integrin expression was associated with elevated DNA repair activity in irradiated cells, and this correlated with the activation of PI3K/AKT- and MEK/ERK-signaling pathways [153].
Plectin knock-out was associated with the downregulation of CCND1 [154]. By interacting with breast cancer susceptibility protein (BRCA2), which is involved in S phase checkpoint activation and centrosome duplication, plectin regulated the perinuclear positioning of the centrosome during the S phase [155]. Furthermore, at the beginning of the M phase, plectin is phosphorylated by CDK1, resulting in cytoskeletal rearrangements and the dissociation of plectin from the IF network to promote centrosome positioning [155,156]. In line with this finding, plectin downregulation was associated with centrosome dislocation and abnormal nuclear morphology [155]. Interestingly, the depletion of a specific plectin isoform, 1c, led to mitotic spindle defects in primary basal keratinocytes [157]. Plectin appears to be involved in the maintenance of genomic stability and proper cell divisions. Moreover, studies using plectin-binding peptides resembling a natural degradation product of cyclin D2 revealed that cells treated with the peptide became stuck at the G1/S checkpoint, resulting in collapse of the cytoskeleton and cell death [158]. Accordingly, Perez et al. recently showed that a monoclonal antibody (1H11) specifically targeting a cancer-specific plectin isoform inhibited the plectin activity and caused cell cycle arrest at the G0/G1 checkpoint in the antibody-treated cells [159].
Intriguingly, HDs can directly affect the organization of the nucleus. Using a yeast two-hybrid screen assay, nesprin-3 was identified as plectin interacting protein binding to its plakin domain [160]. Nesprin-3 is an integral transmembrane protein of the outer nuclear membrane, and it has been implicated in flow-induced centrosome polarization. Plectin function can also be modified by phosphorylation by PKC and cyclin adenosine monophosphate (cAMP)-dependent protein kinase A (PKA), which regulates the interaction between plectin and an IF protein vimentin as well as the association of plectin with laminin B [161]. Recently, Serres et al. showed that vimentin is recruited to the cell cortex in mitotic cells in a plectin-dependent manner [162]. They found that during the interphase, plectin orchestrated the assembly of cytoplasmic networks by interlinking IF, microtubules and actin filaments into a cortical layer after entry into mitosis [136,162]. The depletion of plectin caused loss of vimentin at the cortex and led to vimentin accumulation in cytoplasmic bundles and an increase in the thickness of the cortical networks. Furthermore, plectin depletion also affected the mechanical properties of the actin cortex, resulting in increased cortical tension driving mitotic rounding. Hence, plectin seems to be critical for vimentin recruitment to the cell cortex in mitotic cells, thereby regulating thinning of the cortical actin network and cortical tension [162].
3. Drugs Targeting Hemidesmosomal Components as Potential Anti-Cancer Therapy
Because the key HD proteins, α6β4-integrins and plectin, play multiple roles in both normal cell differentiation and tumor progression, their use as drug targets in cancer therapy is a very challenging task. Their involvement in the development of multidrug resistance and their role in cell adhesion and signaling makes this aim even more complicated [31,163]. The upregulation of ITGA6 is central to the development of cisplatin-resistant ovarian cell lines, and drug-resistant tumors in patients [163]. Furthermore, increased expression of α6-integrin correlates with drug resistance to leukemia [164]. Cancer-specific plectin isoform was reported to mitigate drug sensitivity and reduced plectin levels correlated with better efficacy for several drugs such as sorafenib [34,159].
The activity of α6β4-integrins might be dynamically modulated by miRNAs that inhibit translation or direct mRNA degradation by binding to target mRNAs, often at their 3′-untranslated regions (3′-UTRs) [121]. miRNAs can regulate multiple target genes and play multidimensional roles in cancer as tumor suppressors or oncogenes, which has led to increased interest in using miRNAs as therapeutic tools [165]. ITGA6 is a direct target gene for miR-25 that has been shown to regulate the invasiveness of PCa cells. Zoni et al. found that miR-25 is expressed at low levels in normal and transformed prostate stem cells but is rapidly upregulated upon luminal differentiation, leading to the downregulation of α6- and αV-integrins and thereby inhibiting invasive properties and PCa cell extravasation of tumor stem/progenitor cell subpopulations [166].
ITGA6 expression is suppressed also by another miR, miR-143-3p. The downregulation of ITGA6 in gallbladder carcinoma by miR-143-3p decreased tumor growth by reducing PLGF expression in a PI3K/AKT-dependent manner [167]. In addition, p53-induced miR-30e-5p is involved in the modulation of ITGA6 levels. Laudato et al. found that miR-30e-5p inhibits colorectal cancer invasion and metastasis, and upregulates p21 and p27, leading to G1/S cell cycle arrest by targeting ITGA6 and ITGB1 [168]. Furthermore, aberrant expression of ITGA6 might be induced by the miR-92a [169] and miR-29-3p, which negatively regulate the transcription activator of ITGA6/ITGB1 by SP1 [170].
The most studied approach for interfering with integrin functions is to use antibodies. The activity of α6-integrin can be blocked by monoclonal GoH3 and J1B5 antibodies, which are specific for α6-integrin and selectively inhibit both adhesion and signaling functions, whereas another monoclonal α6-targeting antibody J8H efficiently inhibits the processing of α6-integrin to the truncated form ItgA6p without affecting adhesion to laminin-511 in vitro [171,172,173]. The J8H antibody specifically recognizes and binds to the extracellular domain of full-length α6-integrin and might be useful as a less cytotoxic strategy to improve current therapies for patients with advanced PCa and extensive bone metastasis. In support of such a hypothesis, Landowski et al. reported that J8H antibody-mediated blocking of α6-integrin cleavage inhibited PCa progression and metastasis to the bone in mice. Such additional treatment enhancements could thus help with disease control without inducing significant systemic toxicity [171].
Other strategies have been developed to block plectin activity. Chen et al. designed plectin-1 targeting dual-modality nanoparticles (plectin-SPION-Cy7). Such particles have a high affinity for cancer-specific plectin (CSP) and accumulate within tumors but not in normal pancreatic tissue [174]. Similarly, Bausch et al. generated a CSP-targeting agent to visualize tumor localization and distribution using single-photon emission/CT (SPECT) [175]. Iron oxide nanoparticles can be applied for magnetic techniques such as magnetic resonance imaging (MRI), magnetic particle imaging (MPI), and magnetic hyperthermia. Bovine serum albumin superparamagnetic iron oxide nanoparticles (BSA·SPIONs) conjugated with near infra-red fluorescent dyes, Cy5 or Cy7, and monoclonal anti-plectin antibody specifically were shown to associate with CSF-plectin in pancreatic cancer cells and mice tissue [174]. Moreover, the metallodrugs have been tested. Plecstatin-1 is an organoruthenium drug that specifically targets plectin [176,177,178]. In colon and breast cancer cell lines, plecstatin-1 induced G0/G1 checkpoint arrest, leading to increased phosphorylation of eIF2α, increased ROS levels and reduced spheroid growth in vitro and tumor growth in mice in vivo [176,177,178]. 1H11 monoclonal antibody that selectively binds to cancer-specific plectin with a high specificity has been generated [159]. Treatment with this antibody resulted in JAK2-STAT3 pathway inhibition, upregulation of p21 and p27 and differential expression of EMT markers, leading to cell cycle arrest, decreased migration, proliferation, and tumor growth of ovarian tumor cells. The 1H11 antibody has remarkably low toxicity and it has shown synergistic activity with currently used chemotherapeutics. The administration of 1H11 together with cisplatin resulted in significantly greater tumor inhibition than cisplatin alone [159]. Taken together, plectin appears to be a valuable therapeutic target to improve current therapies.
Summing up, current approaches focusing on simultaneous targeting of integrin-mediated adhesion and signaling in most cases result in high toxicity. This is especially evident when targeting α6β4-integrins, which have tissue-limited distribution in the epithelium but which is critical to maintain the integrity of the epithelium [179,180]. Thus, targeting the downstream effectors of integrin signaling could be considered to reduce potential toxic effects, e.g., by using PI3K inhibitors that decrease integrin α6-integrin expression, thereby reducing tumorigenic potential without completely blocking integrin functions [181].
4. Conclusions and Future Directions
The role of HDs in carcinogenesis is complex and still incompletely understood. However, the oncogenic functions of plectin and α6-integrin upon HD disassembly appear to be consistent in many cancer types. β4-integrin, due to its unique cytoplasmic tail and ability to induce multiple signaling pathways, is more controversial and its cancer-related functions are likely context-dependent and vary between different cancer types. Therefore, the role of α6β4-integrins might vary significantly even within different PCa subtypes, depending on the genomic background, and the functions reported in other cancer types such as breast, colon, and pancreatic cancer need to be confirmed using a PCa model. However, the reduction of β4-integrin levels leading to HD disassembly seems to be a key tumor-promoting factor in PCa. More knowledge about the exact mechanisms, how and when HDs disassemble needs to be accumulated. Moreover, careful mechanistic studies are needed to understand how the lack of β4-integrin drives PCa tumorigenesis and how common this is in PCa and other tumor types in general.
Multiple studies have emerged implicating plectin as an oncogenic factor due to its involvement in the regulation of the tumorigenic properties of cancer cells. In several cancer types, including pancreatic and ovarian cancers, plectin has been shown to relocate from the cytoskeleton to the cell surface. The increased level of plectin has been positively correlated with PCa progression and metastasis [19,24]. Moreover, the CSP cell surface translocation has been proposed as a prognostic biomarker in several cancer types and serves as one of the most promising targets for improved anti-cancer therapies [23]. Unfortunately, the mechanisms regulating plectin translocation are still elusive. It is also unclear which plectin isoform undergoes this cancer-specific translocation and how this contributes to tumorigenesis.
Finally, one key feature of PCa pathogenesis is the dependence on androgen signaling. How α6β4-integrins and plectin may regulate or contribute to AR-signaling remains to be investigated in detail in future studies.
Author Contributions
Conceptualization, T.W. and A.M.; writing—original draft preparation, S.K., A.S., T.W. and A.M.; writing—review and editing, S.K., A.S., T.W. and A.M.; visualization—S.K. and T.W.; supervision, A.M.; funding acquisition, A.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Jane and Aatos Erkko Foundation (190046; AM), Cancer Foundation Finland (61-6158; AM), Biocenter Oulu and by the University of Oulu and Academy of Finland PROFI3 strategic program (AM).
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
AKT/PKB, protein kinase B; AR, androgen receptor; BAD-BH3, only protein; Bax, Bcl-2-associated X protein; Bcl-XL, B-cell lymphoma-extra-large; BM, basement membrane; BRCA2, breast cancer susceptibility protein; CD95, cluster of differentiation 95; Cdc25c, cell division cycle 25c; CDK, cyclin-dependent kinase; c-myc, MYC proto-oncogene; CS, connecting segment; ECM, extracellular matrix; EGF, epidermal growth factor; EGFR, epidermal growth factor receptor; ER, endoplasmic reticulum; ERK, extracellular signal-regulated kinase; FA, focal adhesion; FAK, focal adhesion kinase; Gab2, GRB2-associated-binding protein 2; Grb2, growth factor receptor binding protein 2; GSK3, glycogen synthase kinase 3; HD, hemidesmosome; HIPK2, homeodomain-interacting protein kinase 2; HLA, human leucocyte antigens; IF, intermediate filament; IKK, IκB kinase; IRS1/2, insulin receptor substrates 1,2; MAPK, mitogen-activated protein kinase; MLC, myosin light chain; MOMP, mitochondrial outer membrane permeabilization; MSP, macrophage simulating protein; mTOR, mammalian target of rapamycin; mTORC1, mammalian target of rapamycin complex 1; NFκB, nuclear factor κB; p70S6, ribosomal protein S6 kinase; PCa, prostate cancer; PDK1, 3-phosphoinositide-dependent kinase 1; PIN, prostate intraepithelial neoplasia; PI3K, phosphoinositide 3-kinase; PIP2, phosphatidylinositol 4,5-bisphosphate; PIP3, phosphatidylinositol (3,4,5)-trisphosphate; PKC, protein kinase C; PKD2, protein kinase D2; PLK1, polo-like kinase 1; PTEN, phosphatase and tensin homolog; PTHrP, parathyroid hormone-related protein; Rac, Rac family small GTPase; RAF, rapidly accelerated fibrosarcoma; Ras, Ras small GTPase; Rb, retinoblastoma protein; Rho, Ras homologous small GTPase; RhoA, Ras homolog family member A; ROCK1, Rho-associated protein kinase; ROS, reactive oxygen species; RSK, ribosomal S6 kinase; RTK, receptor tyrosine kinase; SOS, Son of Sevenless; Src, proto-oncogene tyrosine-protein kinase Src; Tap63, tumor protein p63; Tap73, tumor protein p73; TMA, tissue microarray; TMEM268, transmembrane protein 268; TNFR, tumor necrosis factor receptor; Wee1, Wee1 G2 checkpoint kinase; YAP, Yes-associated protein; S100A4, calcium binding protein A4.
References
- Rebello, R.J.; Oing, C.; Knudsen, K.E.; Loeb, S.; Johnson, D.C.; Reiter, R.E.; Gillessen, S.; Van der Kwast, T.; Bristow, R.G. Prostate Cancer. Nat. Rev. Dis. Primer 2021, 7, 9. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, B.S.; Vasioukhin, V. Mechanisms of Prostate Cancer Initiation and Progression. In Advances in Cancer Research; Elsevier: Amsterdam, The Netherlands, 2010; Volume 109, pp. 1–50. ISBN 978-0-12-380890-5. [Google Scholar]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Estimating the Global Cancer Incidence and Mortality in 2018: GLOBOCAN Sources and Methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef] [PubMed]
- Lambert, A.W.; Pattabiraman, D.R.; Weinberg, R.A. Emerging Biological Principles of Metastasis. Cell 2017, 168, 670–691. [Google Scholar] [CrossRef] [PubMed]
- Fares, J.; Fares, M.Y.; Khachfe, H.H.; Salhab, H.A.; Fares, Y. Molecular Principles of Metastasis: A Hallmark of Cancer Revisited. Signal Transduct. Target. Ther. 2020, 5, 28. [Google Scholar] [CrossRef] [PubMed]
- Izzi, V.; Heljasvaara, R.; Heikkinen, A.; Karppinen, S.-M.; Koivunen, J.; Pihlajaniemi, T. Exploring the Roles of MACIT and Multiplexin Collagens in Stem Cells and Cancer. Semin. Cancer Biol. 2020, 62, 134–148. [Google Scholar] [CrossRef]
- Hamidi, H.; Ivaska, J. Every Step of the Way: Integrins in Cancer Progression and Metastasis. Nat. Rev. Cancer 2018, 18, 533–548. [Google Scholar] [CrossRef]
- Ye, X.; Weinberg, R.A. Epithelial-Mesenchymal Plasticity: A Central Regulator of Cancer Progression. Trends Cell Biol. 2015, 25, 675–686. [Google Scholar] [CrossRef]
- Cooper, J.; Giancotti, F.G. Integrin Signaling in Cancer: Mechanotransduction, Stemness, Epithelial Plasticity, and Therapeutic Resistance. Cancer Cell 2019, 35, 347–367. [Google Scholar] [CrossRef]
- Tsuruta, D.; Hashimoto, T.; Hamill, K.J.; Jones, J.C.R. Hemidesmosomes and Focal Contact Proteins: Functions and Cross-Talk in Keratinocytes, Bullous Diseases and Wound Healing. J. Dermatol. Sci. 2011, 62, 1–7. [Google Scholar] [CrossRef][Green Version]
- Schmidt, A.; Kaakinen, M.; Wenta, T.; Manninen, A. Loss of A6β4 Integrin-Mediated Hemidesmosomes Promotes Prostate Epithelial Cell Migration by Stimulating Focal Adhesion Dynamics. Front. Cell Dev. Biol. 2022, 10, 886569. [Google Scholar] [CrossRef]
- Myllymäki, S.-M.; Kämäräinen, U.-R.; Liu, X.; Cruz, S.P.; Miettinen, S.; Vuorela, M.; Varjosalo, M.; Manninen, A. Assembly of the B4-Integrin Interactome Based on Proximal Biotinylation in the Presence and Absence of Heterodimerization. Mol. Cell. Proteom. MCP 2019, 18, 277–293. [Google Scholar] [CrossRef] [PubMed]
- Pora, A.; Yoon, S.; Windoffer, R.; Leube, R.E. Hemidesmosomes and Focal Adhesions Treadmill as Separate but Linked Entities during Keratinocyte Migration. J. Investig. Dermatol. 2019, 139, 1876–1888.e4. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.C.R.; Hopkinson, S.B.; Goldfinger, L.E. Structure and Assembly of Hemidesmosomes. BioEssays 1998, 20, 488–494. [Google Scholar] [CrossRef]
- Knox, J.D.; Cress, A.E.; Clark, V.; Manriquez, L.; Affinito, K.S.; Dalkin, B.L.; Nagle, R.B. Differential Expression of Extracellular Matrix Molecules and the Alpha 6-Integrins in the Normal and Neoplastic Prostate. Am. J. Pathol. 1994, 145, 167–174. [Google Scholar]
- Allen, M.V.; Smith, G.J.; Juliano, R.; Maygarden, S.J.; Mohler, J.L. Downregulation of the B4 Integrin Subunit in Prostatic Carcinoma and Prostatic Intraepithelial Neoplasia. Hum. Pathol. 1998, 29, 311–318. [Google Scholar] [CrossRef]
- Nievers, M.G.; Schaapveld, R.Q.; Sonnenberg, A. Biology and Function of Hemidesmosomes. Matrix Biol. J. Int. Soc. Matrix Biol. 1999, 18, 5–17. [Google Scholar] [CrossRef]
- Walko, G.; Castañón, M.J.; Wiche, G. Molecular Architecture and Function of the Hemidesmosome. Cell Tissue Res. 2015, 360, 529–544. [Google Scholar] [CrossRef]
- Wenta, T.; Schmidt, A.; Zhang, Q.; Devarajan, R.; Singh, P.; Yang, X.; Ahtikoski, A.; Vaarala, M.; Wei, G.-H.; Manninen, A. Disassembly of A6β4-Mediated Hemidesmosomal Adhesions Promotes Tumorigenesis in PTEN-Negative Prostate Cancer by Targeting Plectin to Focal Adhesions. Oncogene 2022, 41, 3804–3820. [Google Scholar] [CrossRef]
- Davis, T.L.; Cress, A.E.; Dalkin, B.L.; Nagle, R.B. Unique Expression Pattern of the Alpha6beta4 Integrin and Laminin-5 in Human Prostate Carcinoma. Prostate 2001, 46, 240–248. [Google Scholar] [CrossRef]
- Huang, W.; Fan, L.; Tang, Y.; Chi, Y.; Li, J. A Pan-Cancer Analysis of the Oncogenic Role of Integrin Beta4 (ITGB4) in Human Tumors. Int. J. Gen. Med. 2021, 14, 9629–9645. [Google Scholar] [CrossRef]
- Bigoni-Ordóñez, G.D.; Czarnowski, D.; Parsons, T.; Madlambayan, G.J.; Villa-Diaz, L.G. Integrin A6 (CD49f), The Microenvironment and Cancer Stem Cells. Curr. Stem Cell Res. Ther. 2019, 14, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Perez, S.M.; Brinton, L.T.; Kelly, K.A. Plectin in Cancer: From Biomarker to Therapeutic Target. Cells 2021, 10, 2246. [Google Scholar] [CrossRef] [PubMed]
- Buckup, M.; Rice, M.A.; Hsu, E.-C.; Garcia-Marques, F.; Liu, S.; Aslan, M.; Bermudez, A.; Huang, J.; Pitteri, S.J.; Stoyanova, T. Plectin Is a Regulator of Prostate Cancer Growth and Metastasis. Oncogene 2021, 40, 663–676. [Google Scholar] [CrossRef] [PubMed]
- Theivendren, P.; Kunjiappan, S.; Hegde, Y.M.; Vellaichamy, S.; Gopal, M.; Dhramalingam, S.R.; Kumar, S. Importance of Protein Kinase and Its Inhibitor: A Review. In Biochemistry; Kumar Singh, R., Ed.; IntechOpen: London, UK, 2021; Volume 24, ISBN 978-1-83880-906-5. [Google Scholar]
- Bagheri, S.; Rahban, M.; Bostanian, F.; Esmaeilzadeh, F.; Bagherabadi, A.; Zolghadri, S.; Stanek, A. Targeting Protein Kinases and Epigenetic Control as Combinatorial Therapy Options for Advanced Prostate Cancer Treatment. Pharmaceutics 2022, 14, 515. [Google Scholar] [CrossRef]
- Miller, K.J.; Asim, M. Unravelling the Role of Kinases That Underpin Androgen Signalling in Prostate Cancer. Cells 2022, 11, 952. [Google Scholar] [CrossRef]
- Whitworth, H.; Bhadel, S.; Ivey, M.; Conaway, M.; Spencer, A.; Hernan, R.; Holemon, H.; Gioeli, D. Identification of Kinases Regulating Prostate Cancer Cell Growth Using an RNAi Phenotypic Screen. PLoS ONE 2012, 7, e38950. [Google Scholar] [CrossRef]
- Manning, G.; Plowman, G.D.; Hunter, T.; Sudarsanam, S. Evolution of Protein Kinase Signaling from Yeast to Man. Trends Biochem. Sci. 2002, 27, 514–520. [Google Scholar] [CrossRef]
- Stewart, R.L.; O’Connor, K.L. Clinical Significance of the Integrin A6β4 in Human Malignancies. Lab. Investig. 2015, 95, 976–986. [Google Scholar] [CrossRef]
- Yang, H.; Xu, Z.; Peng, Y.; Wang, J.; Xiang, Y. Integrin B4 as a Potential Diagnostic and Therapeutic Tumor Marker. Biomolecules 2021, 11, 1197. [Google Scholar] [CrossRef]
- Masugi, Y.; Yamazaki, K.; Emoto, K.; Effendi, K.; Tsujikawa, H.; Kitago, M.; Itano, O.; Kitagawa, Y.; Sakamoto, M. Upregulation of Integrin B4 Promotes Epithelial–Mesenchymal Transition and Is a Novel Prognostic Marker in Pancreatic Ductal Adenocarcinoma. Lab. Investig. 2015, 95, 308–319. [Google Scholar] [CrossRef]
- Dalton, G.N.; Massillo, C.; Scalise, G.D.; Duca, R.; Porretti, J.; Farré, P.L.; Gardner, K.; Paez, A.; Gueron, G.; De Luca, P.; et al. CTBP1 Depletion on Prostate Tumors Deregulates MiRNA/MRNA Expression and Impairs Cancer Progression in Metabolic Syndrome Mice. Cell Death Dis. 2019, 10, 299. [Google Scholar] [CrossRef] [PubMed]
- Cheng, I.; Plummer, S.J.; Neslund-Dudas, C.; Klein, E.A.; Casey, G.; Rybicki, B.A.; Witte, J.S. Prostate Cancer Susceptibility Variants Confer Increased Risk of Disease Progression. Cancer Epidemiol. Biomark. Prev. 2010, 19, 2124–2132. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Aumailley, M.; Bruckner-Tuderman, L.; Carter, W.G.; Deutzmann, R.; Edgar, D.; Ekblom, P.; Engel, J.; Engvall, E.; Hohenester, E.; Jones, J.C.R.; et al. A Simplified Laminin Nomenclature. Matrix Biol. J. Int. Soc. Matrix Biol. 2005, 24, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Litjens, S.H.M.; de Pereda, J.M.; Sonnenberg, A. Current Insights into the Formation and Breakdown of Hemidesmosomes. Trends Cell Biol. 2006, 16, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Décline, F.; Okamoto, O.; Mallein-Gerin, F.; Helbert, B.; Bernaud, J.; Rigal, D.; Rousselle, P. Keratinocyte Motility Induced by TGF-Beta1 Is Accompanied by Dramatic Changes in Cellular Interactions with Laminin 5. Cell Motil. Cytoskelet. 2003, 54, 64–80. [Google Scholar] [CrossRef]
- Henning, K.; Berndt, A.; Katenkamp, D.; Kosmehl, H. Loss of Laminin-5 in the Epithelium-Stroma Interface: An Immunohistochemical Marker of Malignancy in Epithelial Lesions of the Breast: Laminin-5 in Epithelial Tumours of the Breast. Histopathology 1999, 34, 305–309. [Google Scholar] [CrossRef]
- Shinto, E.; Tsuda, H.; Ueno, H.; Hashiguchi, Y.; Hase, K.; Tamai, S.; Mochizuki, H.; Inazawa, J.; Matsubara, O. Prognostic Implication of Laminin-5 Gamma 2 Chain Expression in the Invasive Front of Colorectal Cancers, Disclosed by Area-Specific Four-Point Tissue Microarrays. Lab. Investig. 2005, 85, 257–266. [Google Scholar] [CrossRef]
- Hao, J.; Jackson, L.; Calaluce, R.; McDaniel, K.; Dalkin, B.L.; Nagle, R.B. Investigation into the Mechanism of the Loss of Laminin 5 (A3β3γ2) Expression in Prostate Cancer. Am. J. Pathol. 2001, 158, 1129–1135. [Google Scholar] [CrossRef]
- Mainiero, F.; Pepe, A.; Yeon, M.; Ren, Y.; Giancotti, F.G. The Intracellular Functions of Alpha6beta4 Integrin Are Regulated by EGF. J. Cell Biol. 1996, 134, 241–253. [Google Scholar] [CrossRef]
- Wilkinson, E.J.; Woodworth, A.M.; Parker, M.; Phillips, J.L.; Malley, R.C.; Dickinson, J.L.; Holloway, A.F. Epigenetic Regulation of the ITGB4 Gene in Prostate Cancer. Exp. Cell Res. 2020, 392, 112055. [Google Scholar] [CrossRef]
- Tuxhorn, J.A.; Ayala, G.E.; Rowley, D.R. Reactive Stroma in Prostate Cancer Progression. J. Urol. 2001, 166, 2472–2483. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Franco, O.E.; Hayward, S.W. Interaction of Prostate Carcinoma-Associated Fibroblasts with Human Epithelial Cell Lines In Vivo. Differentiation 2017, 96, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Dans, M.; Gagnoux-Palacios, L.; Blaikie, P.; Klein, S.; Mariotti, A.; Giancotti, F.G. Tyrosine Phosphorylation of the B4 Integrin Cytoplasmic Domain Mediates Shc Signaling to Extracellular Signal-Regulated Kinase and Antagonizes Formation of Hemidesmosomes. J. Biol. Chem. 2001, 276, 1494–1502. [Google Scholar] [CrossRef] [PubMed]
- Giancotti, F.G. Signal Transduction by the A6β4 Integrin: Charting the Path between Laminin Binding and Nuclear Events. J. Cell Sci. 1996, 109, 1165–1172. [Google Scholar] [CrossRef]
- Luo, B.-H.; Carman, C.V.; Springer, T.A. Structural Basis of Integrin Regulation and Signaling. Annu. Rev. Immunol. 2007, 25, 619–647. [Google Scholar] [CrossRef]
- Askari, J.A.; Buckley, P.A.; Mould, A.P.; Humphries, M.J. Linking Integrin Conformation to Function. J. Cell Sci. 2009, 122, 165–170. [Google Scholar] [CrossRef]
- Mainiero, F.; Pepe, A.; Wary, K.K.; Spinardi, L.; Mohammadi, M.; Schlessinger, J.; Giancotti, F.G. Signal Transduction by the Alpha 6 Beta 4 Integrin: Distinct Beta 4 Subunit Sites Mediate Recruitment of Shc/Grb2 and Association with the Cytoskeleton of Hemidesmosomes. EMBO J. 1995, 14, 4470–4481. [Google Scholar] [CrossRef]
- Guo, Y.; Pan, W.; Liu, S.; Shen, Z.; Xu, Y.; Hu, L. ERK/MAPK Signalling Pathway and Tumorigenesis (Review). Exp. Ther. Med. 2020, 19, 1997–2007. [Google Scholar] [CrossRef]
- Tran, T.H.; Chan, A.H.; Young, L.C.; Bindu, L.; Neale, C.; Messing, S.; Dharmaiah, S.; Taylor, T.; Denson, J.-P.; Esposito, D.; et al. KRAS Interaction with RAF1 RAS-Binding Domain and Cysteine-Rich Domain Provides Insights into RAS-Mediated RAF Activation. Nat. Commun. 2021, 12, 1176. [Google Scholar] [CrossRef]
- Weinstein-Oppenheimer, C.R.; Blalock, W.L.; Steelman, L.S.; Chang, F.; McCubrey, J.A. The Raf Signal Transduction Cascade as a Target for Chemotherapeutic Intervention in Growth Factor-Responsive Tumors. Pharmacol. Ther. 2000, 88, 229–279. [Google Scholar] [CrossRef]
- Fu, Z.; Smith, P.C.; Zhang, L.; Rubin, M.A.; Dunn, R.L.; Yao, Z.; Keller, E.T. Effects of Raf Kinase Inhibitor Protein Expression on Suppression of Prostate Cancer Metastasis. JNCI J. Natl. Cancer Inst. 2003, 95, 878–889. [Google Scholar] [CrossRef] [PubMed]
- Cho, N.-Y.; Choi, M.; Kim, B.-H.; Cho, Y.-M.; Moon, K.C.; Kang, G.H. BRAF AndKRAS Mutations in Prostatic Adenocarcinoma. Int. J. Cancer 2006, 119, 1858–1862. [Google Scholar] [CrossRef] [PubMed]
- Shaw, L.M. Identification of Insulin Receptor Substrate 1 (IRS-1) and IRS-2 as Signaling Intermediates in the A6β4 Integrin-Dependent Activation of Phosphoinositide 3-OH Kinase and Promotion of Invasion. Mol. Cell. Biol. 2001, 21, 5082–5093. [Google Scholar] [CrossRef]
- Gu, H.; Maeda, H.; Moon, J.J.; Lord, J.D.; Yoakim, M.; Nelson, B.H.; Neel, B.G. New Role for Shc in Activation of the Phosphatidylinositol 3-Kinase/Akt Pathway. Mol. Cell. Biol. 2000, 20, 7109–7120. [Google Scholar] [CrossRef]
- Liu, P.; Cheng, H.; Roberts, T.M.; Zhao, J.J. Targeting the Phosphoinositide 3-Kinase Pathway in Cancer. Nat. Rev. Drug Discov. 2009, 8, 627–644. [Google Scholar] [CrossRef] [PubMed]
- Vivanco, I.; Chen, Z.C.; Tanos, B.; Oldrini, B.; Hsieh, W.-Y.; Yannuzzi, N.; Campos, C.; Mellinghoff, I.K. A Kinase-Independent Function of AKT Promotes Cancer Cell Survival. eLife 2014, 3, e03751. [Google Scholar] [CrossRef]
- Danilkovitch-Miagkova, A. Oncogenic Signaling Pathways Activated by RON Receptor Tyrosine Kinase. Curr. Cancer Drug Targets 2003, 3, 31–40. [Google Scholar] [CrossRef]
- Benight, N.M.; Waltz, S.E. Ron Receptor Tyrosine Kinase Signaling as a Therapeutic Target. Expert Opin. Ther. Targets 2012, 16, 921–931. [Google Scholar] [CrossRef][Green Version]
- Yin, B.; Liu, Z.; Wang, Y.; Wang, X.; Liu, W.; Yu, P.; Duan, X.; Liu, C.; Chen, Y.; Zhang, Y.; et al. RON and C-Met Facilitate Metastasis through the ERK Signaling Pathway in Prostate Cancer Cells. Oncol. Rep. 2017, 37, 3209–3218. [Google Scholar] [CrossRef]
- O’Toole, J.M.; Rabenau, K.E.; Burns, K.; Lu, D.; Mangalampalli, V.; Balderes, P.; Covino, N.; Bassi, R.; Prewett, M.; Gottfredsen, K.J.; et al. Therapeutic Implications of a Human Neutralizing Antibody to the Macrophage-Stimulating Protein Receptor Tyrosine Kinase (RON), a c-MET Family Member. Cancer Res. 2006, 66, 9162–9170. [Google Scholar] [CrossRef]
- Thobe, M.N.; Gurusamy, D.; Pathrose, P.; Waltz, S.E. The Ron Receptor Tyrosine Kinase Positively Regulates Angiogenic Chemokine Production in Prostate Cancer Cells. Oncogene 2010, 29, 214–226. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Ye, H.; Gerrin, S.; Wang, H.; Sharma, A.; Chen, S.; Patnaik, A.; Sowalsky, A.G.; Voznesensky, O.; Han, W.; et al. ErbB2 Signaling Increases Androgen Receptor Expression in Abiraterone-Resistant Prostate Cancer. Clin. Cancer Res. 2016, 22, 3672–3682. [Google Scholar] [CrossRef] [PubMed]
- Frijns, E.; Sachs, N.; Kreft, M.; Wilhelmsen, K.; Sonnenberg, A. EGF-Induced MAPK Signaling Inhibits Hemidesmosome Formation through Phosphorylation of the Integrin B4. J. Biol. Chem. 2010, 285, 37650–37662. [Google Scholar] [CrossRef] [PubMed]
- Osmanagic-Myers, S.; Wiche, G. Plectin-RACK1 (Receptor for Activated C Kinase 1) Scaffolding. J. Biol. Chem. 2004, 279, 18701–18710. [Google Scholar] [CrossRef]
- Burch, T.C.; Watson, M.T.; Nyalwidhe, J.O. Variable Metastatic Potentials Correlate with Differential Plectin and Vimentin Expression in Syngeneic Androgen Independent Prostate Cancer Cells. PLoS ONE 2013, 8, e65005. [Google Scholar] [CrossRef]
- Solt, L.A.; May, M.J. The IκB Kinase Complex: Master Regulator of NF-ΚB Signaling. Immunol. Res. 2008, 42, 3–18. [Google Scholar] [CrossRef]
- Albensi, B.C. What Is Nuclear Factor Kappa B (NF-ΚB) Doing in and to the Mitochondrion? Front. Cell Dev. Biol. 2019, 7, 154. [Google Scholar] [CrossRef]
- Zahir, N.; Lakins, J.N.; Russell, A.; Ming, W.; Chatterjee, C.; Rozenberg, G.I.; Marinkovich, M.P.; Weaver, V.M. Autocrine Laminin-5 Ligates A6β4 Integrin and Activates RAC and NFκB to Mediate Anchorage-Independent Survival of Mammary Tumors. J. Cell Biol. 2003, 163, 1397–1407. [Google Scholar] [CrossRef]
- Elaimy, A.L.; Sheel, A.; Brown, C.W.; Walker, M.R.; Wang, M.; Amante, J.J.; Xue, W.; Chan, A.; Baer, C.E.; Goel, H.L.; et al. Real-Time Imaging of Integrin B4 Dynamics Using a Reporter Cell Line Generated by Crispr/Cas9 Genome Editing. J. Cell Sci. 2019, 132, jcs231241. [Google Scholar] [CrossRef]
- Rabinovitz, I.; Mercurio, A.M. The Integrin A6β4 Functions in Carcinoma Cell Migration on Laminin-1 by Mediating the Formation and Stabilization of Actin-Containing Motility Structures. J. Cell Biol. 1997, 139, 1873–1884. [Google Scholar] [CrossRef]
- Geuijen, C.A.W.; Sonnenberg, A. Dynamics of the A6β4 Integrin in Keratinocytes. Mol. Biol. Cell 2002, 13, 3845–3858. [Google Scholar] [CrossRef] [PubMed]
- te Molder, L.; de Pereda, J.M.; Sonnenberg, A. Regulation of Hemidesmosome Dynamics and Cell Signaling by Integrin A6β4. J. Cell Sci. 2021, 134, jcs259004. [Google Scholar] [CrossRef] [PubMed]
- Lipscomb, E.A.; Mercurio, A.M. Mobilization and Activation of a Signaling Competent A6β4integrin Underlies Its Contribution to Carcinoma Progression. Cancer Metastasis Rev. 2005, 24, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Frijns, E.; Kuikman, I.; Litjens, S.; Raspe, M.; Jalink, K.; Ports, M.; Wilhelmsen, K.; Sonnenberg, A. Phosphorylation of Threonine 1736 in the C-Terminal Tail of Integrin B4 Contributes to Hemidesmosome Disassembly. Mol. Biol. Cell 2012, 23, 1475–1485. [Google Scholar] [CrossRef]
- te Molder, L.; Sonnenberg, A. PKD2 and RSK1 Regulate Integrin B4 Phosphorylation at Threonine 1736. PLoS ONE 2015, 10, e0143357. [Google Scholar] [CrossRef]
- Wang, W.; Zuidema, A.; te Molder, L.; Nahidiazar, L.; Hoekman, L.; Schmidt, T.; Coppola, S.; Sonnenberg, A. Hemidesmosomes Modulate Force Generation via Focal Adhesions. J. Cell Biol. 2020, 219, e201904137. [Google Scholar] [CrossRef]
- Ozawa, T.; Tsuruta, D.; Jones, J.C.R.; Ishii, M.; Ikeda, K.; Harada, T.; Aoyama, Y.; Kawada, A.; Kobayashi, H. Dynamic Relationship of Focal Contacts and Hemidesmosome Protein Complexes in Live Cells. J. Investig. Dermatol. 2010, 130, 1624–1635. [Google Scholar] [CrossRef]
- Lachowski, D.; Cortes, E.; Robinson, B.; Rice, A.; Rombouts, K.; Del Río Hernández, A.E. FAK Controls the Mechanical Activation of YAP, a Transcriptional Regulator Required for Durotaxis. FASEB J. 2018, 32, 1099–1107. [Google Scholar] [CrossRef]
- Kim, N.-G.; Gumbiner, B.M. Adhesion to Fibronectin Regulates Hippo Signaling via the FAK–Src–PI3K Pathway. J. Cell Biol. 2015, 210, 503–515. [Google Scholar] [CrossRef]
- Lessey, E.C.; Guilluy, C.; Burridge, K. From Mechanical Force to RhoA Activation. Biochemistry 2012, 51, 7420–7432. [Google Scholar] [CrossRef]
- Amano, M.; Ito, M.; Kimura, K.; Fukata, Y.; Chihara, K.; Nakano, T.; Matsuura, Y.; Kaibuchi, K. Phosphorylation and Activation of Myosin by Rho-Associated Kinase (Rho-Kinase). J. Biol. Chem. 1996, 271, 20246–20249. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, S.; Matsui, T.S.; Ohashi, K.; Deguchi, S.; Mizuno, K. Solo, a RhoA-Targeting Guanine Nucleotide Exchange Factor, Is Critical for Hemidesmosome Formation and Acinar Development in Epithelial Cells. PLoS ONE 2018, 13, e0195124. [Google Scholar] [CrossRef] [PubMed]
- Nardone, G.; Oliver-De La Cruz, J.; Vrbsky, J.; Martini, C.; Pribyl, J.; Skládal, P.; Pešl, M.; Caluori, G.; Pagliari, S.; Martino, F.; et al. YAP Regulates Cell Mechanics by Controlling Focal Adhesion Assembly. Nat. Commun. 2017, 8, 15321. [Google Scholar] [CrossRef] [PubMed]
- Halder, G.; Dupont, S.; Piccolo, S. Transduction of Mechanical and Cytoskeletal Cues by YAP and TAZ. Nat. Rev. Mol. Cell Biol. 2012, 13, 591–600. [Google Scholar] [CrossRef]
- Dupont, S.; Morsut, L.; Aragona, M.; Enzo, E.; Giulitti, S.; Cordenonsi, M.; Zanconato, F.; Le Digabel, J.; Forcato, M.; Bicciato, S.; et al. Role of YAP/TAZ in Mechanotransduction. Nature 2011, 474, 179–183. [Google Scholar] [CrossRef]
- Chen, M.; Bresnick, A.R.; O’Connor, K.L. Coupling S100A4 to Rhotekin Alters Rho Signaling Output in Breast Cancer Cells. Oncogene 2013, 32, 3754–3764. [Google Scholar] [CrossRef]
- Chen, M.; Sinha, M.; Luxon, B.A.; Bresnick, A.R.; O’Connor, K.L. Integrin A6β4 Controls the Expression of Genes Associated with Cell Motility, Invasion, and Metastasis, Including S100A4/Metastasin. J. Biol. Chem. 2009, 284, 1484–1494. [Google Scholar] [CrossRef]
- O’Connor, K.L.; Nguyen, B.-K.; Mercurio, A.M. Rhoa Function in Lamellae Formation and Migration Is Regulated by the A6β4 Integrin and Camp Metabolism. J. Cell Biol. 2000, 148, 253–258. [Google Scholar] [CrossRef]
- Gilmore, A.P.; Owens, T.W.; Foster, F.M.; Lindsay, J. How Adhesion Signals Reach a Mitochondrial Conclusion—ECM Regulation of Apoptosis. Curr. Opin. Cell Biol. 2009, 21, 654–661. [Google Scholar] [CrossRef]
- Popgeorgiev, N.; Jabbour, L.; Gillet, G. Subcellular Localization and Dynamics of the Bcl-2 Family of Proteins. Front. Cell Dev. Biol. 2018, 6, 13. [Google Scholar] [CrossRef]
- Driak, D.; Dvorska, M.; Bolehovska, P.; Svandova, I.; Novotny, J.; Halaska, M. Bad and Bid—Potential Background Players in Preneoplastic to Neoplastic Shift in Human Endometrium. Neoplasma 2014, 61, 411–415. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, J.C.; Muñoz-Pinedo, C.; Ricci, J.-E.; Adams, S.R.; Kelekar, A.; Schuler, M.; Tsien, R.Y.; Green, D.R. Cytochrome c Is Released in a Single Step during Apoptosis. Cell Death Differ. 2005, 12, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Werner, M.E.; Chen, F.; Moyano, J.V.; Yehiely, F.; Jones, J.C.R.; Cryns, V.L. Caspase Proteolysis of the Integrin B4 Subunit Disrupts Hemidesmosome Assembly, Promotes Apoptosis, and Inhibits Cell Migration. J. Biol. Chem. 2007, 282, 5560–5569. [Google Scholar] [CrossRef] [PubMed]
- Eskandari, E.; Eaves, C.J. Paradoxical Roles of Caspase-3 in Regulating Cell Survival, Proliferation, and Tumorigenesis. J. Cell Biol. 2022, 221, e202201159. [Google Scholar] [CrossRef] [PubMed]
- Stegh, A.H.; Herrmann, H.; Lampel, S.; Weisenberger, D.; Andrä, K.; Seper, M.; Wiche, G.; Krammer, P.H.; Peter, M.E. Identification of the Cytolinker Plectin as a Major Early In Vivo Substrate for Caspase 8 during CD95- and Tumor Necrosis Factor Receptor-Mediated Apoptosis. Mol. Cell. Biol. 2000, 20, 5665–5679. [Google Scholar] [CrossRef]
- Aho, S. Plakin Proteins Are Coordinately Cleaved during Apoptosis but Preferentially through the Action of Different Caspases. Exp. Dermatol. 2004, 13, 700–707. [Google Scholar] [CrossRef]
- Liu, Y.-H.; Cheng, C.-C.; Ho, C.-C.; Chao, W.-T.; Pei, R.-J.; Hsu, Y.-H.; Yeh, K.-T.; Ho, L.-C.; Tsai, M.-C.; Lai, Y.-S. Degradation of Plectin with Modulation of Cytokeratin 18 in Human Liver Cells during Staurosporine-Induced Apoptosis. Vivo Athens Greece 2008, 22, 543–548. [Google Scholar]
- Ni, Y.; Wang, X.; Yin, X.; Li, Y.; Liu, X.; Wang, H.; Liu, X.; Zhang, J.; Gao, H.; Shi, B.; et al. Plectin Protects Podocytes from Adriamycin-Induced Apoptosis and F-Actin Cytoskeletal Disruption through the Integrin A6β4/FAK/P38 MAPK Pathway. J. Cell. Mol. Med. 2018, 22, 5450–5467. [Google Scholar] [CrossRef]
- Giannoni, E.; Fiaschi, T.; Ramponi, G.; Chiarugi, P. Redox Regulation of Anoikis Resistance of Metastatic Prostate Cancer Cells: Key Role for Src and EGFR-Mediated pro-Survival Signals. Oncogene 2009, 28, 2074–2086. [Google Scholar] [CrossRef]
- Heppner, D.E.; Dustin, C.M.; Liao, C.; Hristova, M.; Deng, B.; Lam, Y.-W.; Li, J.; van der Vliet, A. Molecular Basis for the Redox Regulation of the Src Kinase. Free Radic. Biol. Med. 2016, 100, S40. [Google Scholar] [CrossRef]
- Datta, S.R.; Dudek, H.; Tao, X.; Masters, S.; Fu, H.; Gotoh, Y.; Greenberg, M.E. Akt Phosphorylation of BAD Couples Survival Signals to the Cell-Intrinsic Death Machinery. Cell 1997, 91, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Goc, A.; Kochuparambil, S.T.; Al-Husein, B.; Al-Azayzih, A.; Mohammad, S.; Somanath, P.R. Simultaneous Modulation of the Intrinsic and Extrinsic Pathways by Simvastatin in Mediating Prostate Cancer Cell Apoptosis. BMC Cancer 2012, 12, 409. [Google Scholar] [CrossRef] [PubMed]
- Chao, O.S.P.; Clément, M.-V. Epidermal Growth Factor and Serum Activate Distinct Pathways to Inhibit the BH3 Only Protein BAD in Prostate Carcinoma LNCaP Cells. Oncogene 2006, 25, 4458–4469. [Google Scholar] [CrossRef] [PubMed]
- Sastry, K.S.R.; Karpova, Y.; Kulik, G. Epidermal Growth Factor Protects Prostate Cancer Cells from Apoptosis by Inducing BAD Phosphorylation via Redundant Signaling Pathways. J. Biol. Chem. 2006, 281, 27367–27377. [Google Scholar] [CrossRef]
- Balta, E.; Kramer, J.; Samstag, Y. Redox Regulation of the Actin Cytoskeleton in Cell Migration and Adhesion: On the Way to a Spatiotemporal View. Front. Cell Dev. Biol. 2021, 8, 618261. [Google Scholar] [CrossRef] [PubMed]
- Ketema, M.; Wilhelmsen, K.; Kuikman, I.; Janssen, H.; Hodzic, D.; Sonnenberg, A. Requirements for the Localization of Nesprin-3 at the Nuclear Envelope and Its Interaction with Plectin. J. Cell Sci. 2007, 120, 3384–3394. [Google Scholar] [CrossRef]
- Allison, S.J. Benefits of Actin Cytoskeleton Stabilization. Nat. Rev. Nephrol. 2022, 18, 413. [Google Scholar] [CrossRef]
- Spurny, R.; Abdoulrahman, K.; Janda, L.; Ruönzler, D.; Koöhler, G.; Castañón, M.J.; Wiche, G. Oxidation and Nitrosylation of Cysteines Proximal to the Intermediate Filament (IF)-Binding Site of Plectin. J. Biol. Chem. 2007, 282, 8175–8187. [Google Scholar] [CrossRef]
- Giannoni, E.; Buricchi, F.; Raugei, G.; Ramponi, G.; Chiarugi, P. Intracellular Reactive Oxygen Species Activate Src Tyrosine Kinase during Cell Adhesion and Anchorage-Dependent Cell Growth. Mol. Cell. Biol. 2005, 25, 6391–6403. [Google Scholar] [CrossRef]
- Basuroy, S.; Dunagan, M.; Sheth, P.; Seth, A.; Rao, R.K. Hydrogen Peroxide Activates Focal Adhesion Kinase and C-Src by a Phosphatidylinositol 3 Kinase-Dependent Mechanism and Promotes Cell Migration in Caco-2 Cell Monolayers. Am. J. Physiol.-Gastrointest. Liver Physiol. 2010, 299, G186–G195. [Google Scholar] [CrossRef]
- Heppner, D.E.; Dustin, C.M.; Liao, C.; Hristova, M.; Veith, C.; Little, A.C.; Ahlers, B.A.; White, S.L.; Deng, B.; Lam, Y.-W.; et al. Direct Cysteine Sulfenylation Drives Activation of the Src Kinase. Nat. Commun. 2018, 9, 4522. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, V.; Mula, R.V.; Weigel, N.L.; Falzon, M. Parathyroid Hormone-Related Protein Regulates Cell Survival Pathways via Integrin A6β4-Mediated Activation of Phosphatidylinositol 3-Kinase/Akt Signaling. Mol. Cancer Res. 2009, 7, 1119–1131. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Thrasher, J.B.; Terranova, P. Glycogen Synthase Kinase-3: A Potential Preventive Target for Prostate Cancer Management. Urol. Oncol. Semin. Orig. Investig. 2015, 33, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Hahn-Windgassen, A.; Nogueira, V.; Chen, C.-C.; Skeen, J.E.; Sonenberg, N.; Hay, N. Akt Activates the Mammalian Target of Rapamycin by Regulating Cellular ATP Level and AMPK Activity. J. Biol. Chem. 2005, 280, 32081–32089. [Google Scholar] [CrossRef]
- Harada, H.; Andersen, J.S.; Mann, M.; Terada, N.; Korsmeyer, S.J. P70S6 Kinase Signals Cell Survival as Well as Growth, Inactivating the pro-Apoptotic Molecule BAD. Proc. Natl. Acad. Sci. USA 2001, 98, 9666–9670. [Google Scholar] [CrossRef]
- Robinson, D.; Van Allen, E.M.; Wu, Y.-M.; Schultz, N.; Lonigro, R.J.; Mosquera, J.-M.; Montgomery, B.; Taplin, M.-E.; Pritchard, C.C.; Attard, G.; et al. Integrative Clinical Genomics of Advanced Prostate Cancer. Cell 2015, 161, 1215–1228. [Google Scholar] [CrossRef]
- Beltran, H.; Yelensky, R.; Frampton, G.M.; Park, K.; Downing, S.R.; MacDonald, T.Y.; Jarosz, M.; Lipson, D.; Tagawa, S.T.; Nanus, D.M.; et al. Targeted Next-Generation Sequencing of Advanced Prostate Cancer Identifies Potential Therapeutic Targets and Disease Heterogeneity. Eur. Urol. 2013, 63, 920–926. [Google Scholar] [CrossRef]
- van Dessel, L.F.; van Riet, J.; Smits, M.; Zhu, Y.; Hamberg, P.; van der Heijden, M.S.; Bergman, A.M.; van Oort, I.M.; de Wit, R.; Voest, E.E.; et al. The Genomic Landscape of Metastatic Castration-Resistant Prostate Cancers Reveals Multiple Distinct Genotypes with Potential Clinical Impact. Nat. Commun. 2019, 10, 5251. [Google Scholar] [CrossRef]
- Hamid, A.A.; Gray, K.P.; Shaw, G.; MacConaill, L.E.; Evan, C.; Bernard, B.; Loda, M.; Corcoran, N.M.; Van Allen, E.M.; Choudhury, A.D.; et al. Compound Genomic Alterations of TP53, PTEN, and RB1 Tumor Suppressors in Localized and Metastatic Prostate Cancer. Eur. Urol. 2019, 76, 89–97. [Google Scholar] [CrossRef]
- Vinall, R.L.; Chen, J.Q.; Hubbard, N.E.; Sulaimon, S.S.; DeVere White, R.W.; Borowsky, A.D. Evidence for an Alternate Molecular Progression in Prostate Cancer. Dis. Model. Mech. 2012, dmm-008995. [Google Scholar] [CrossRef]
- Bachelder, R.E.; Ribick, M.J.; Marchetti, A.; Falcioni, R.; Soddu, S.; Davis, K.R.; Mercurio, A.M. P53 Inhibits A6β4 Integrin Survival Signaling by Promoting the Caspase 3–Dependent Cleavage of Akt/PKB. J. Cell Biol. 1999, 147, 1063–1072. [Google Scholar] [CrossRef]
- Persad, S.; Attwell, S.; Gray, V.; Delcommenne, M.; Troussard, A.; Sanghera, J.; Dedhar, S. Inhibition of Integrin-Linked Kinase (ILK) Suppresses Activation of Protein Kinase B/Akt and Induces Cell Cycle Arrest and Apoptosis of PTEN-Mutant Prostate Cancer Cells. Proc. Natl. Acad. Sci. USA 2000, 97, 3207–3212. [Google Scholar] [CrossRef] [PubMed]
- Jamaspishvili, T.; Berman, D.M.; Ross, A.E.; Scher, H.I.; De Marzo, A.M.; Squire, J.A.; Lotan, T.L. Clinical Implications of PTEN Loss in Prostate Cancer. Nat. Rev. Urol. 2018, 15, 222–234. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, A.; Kitagishi, Y.; Ogura, Y.; Matsuda, S. The Tumor Suppressor PTEN Interacts with P53 in Hereditary Cancer. Int. J. Oncol. 2014, 44, 1813–1819. [Google Scholar] [CrossRef] [PubMed]
- Hong, D.; Zhang, X.; Li, R.; Yu, J.; Lou, Y.; He, Q.; Li, X.; Xu, D.; Lv, P.; Lin, J.; et al. Deletion of TMEM268 Inhibits Growth of Gastric Cancer Cells by Downregulating the ITGB4 Signaling Pathway. Cell Death Differ. 2019, 26, 1453–1466. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Ohtsubo, M.; Böhmer, R.M.; Roberts, J.M.; Assoian, R.K. Adhesion-Dependent Cell Cycle Progression Linked to the Expression of Cyclin D1, Activation of Cyclin E-Cdk2, and Phosphorylation of the Retinoblastoma Protein. J. Cell Biol. 1996, 133, 391–403. [Google Scholar] [CrossRef] [PubMed]
- Shanmugasundaram, K.; Block, K.; Nayak, B.K.; Livi, C.B.; Venkatachalam, M.A.; Sudarshan, S. PI3K Regulation of the SKP-2/P27 Axis through MTORC2. Oncogene 2013, 32, 2027–2036. [Google Scholar] [CrossRef]
- Guo, Z.-Y.; Hao, X.-H.; Tan, F.-F.; Pei, X.; Shang, L.-M.; Jiang, X.-L.; Yang, F. The Elements of Human Cyclin D1 Promoter and Regulation Involved. Clin. Epigenet. 2011, 2, 63–76. [Google Scholar] [CrossRef]
- Kamranvar, S.A.; Rani, B.; Johansson, S. Cell Cycle Regulation by Integrin-Mediated Adhesion. Cells 2022, 11, 2521. [Google Scholar] [CrossRef]
- Zhao, J.; Bian, Z.C.; Yee, K.; Chen, B.P.C.; Chien, S.; Guan, J.-L. Identification of Transcription Factor KLF8 as a Downstream Target of Focal Adhesion Kinase in Its Regulation of Cyclin D1 and Cell Cycle Progression. Mol. Cell 2003, 11, 1503–1515. [Google Scholar] [CrossRef]
- Diehl, J.A.; Cheng, M.; Roussel, M.F.; Sherr, C.J. Glycogen Synthase Kinase-3β Regulates Cyclin D1 Proteolysis and Subcellular Localization. Genes Dev. 1998, 12, 3499–3511. [Google Scholar] [CrossRef] [PubMed]
- Kamranvar, S.A.; Gupta, D.K.; Wasberg, A.; Liu, L.; Roig, J.; Johansson, S. Integrin-Mediated Adhesion Promotes Centrosome Separation in Early Mitosis. Cells 2022, 11, 1360. [Google Scholar] [CrossRef] [PubMed]
- Gheghiani, L.; Loew, D.; Lombard, B.; Mansfeld, J.; Gavet, O. PLK1 Activation in Late G2 Sets Up Commitment to Mitosis. Cell Rep. 2017, 19, 2060–2073. [Google Scholar] [CrossRef] [PubMed]
- Steinböck, F.A.; Wiche, G. Plectin: A Cytolinker by Design. Biol. Chem. 1999, 380, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Dong, Z.; Zhang, Y.; Miao, J. The Roles of Integrin B4 in Vascular Endothelial Cells. J. Cell. Physiol. 2012, 227, 474–478. [Google Scholar] [CrossRef]
- Lewis, J.M.; Truong, T.N.; Schwartz, M.A. Integrins Regulate the Apoptotic Response to DNA Damage through Modulation of P53. Proc. Natl. Acad. Sci. USA 2002, 99, 3627–3632. [Google Scholar] [CrossRef]
- Giancotti, F.G. Targeting Integrin Beta4 for Cancer and Anti-Angiogenic Therapy. Trends Pharmacol. Sci. 2007, 28, 506–511. [Google Scholar] [CrossRef]
- Yuan, L.; Du, X.; Tang, S.; Wu, S.; Wang, L.; Xiang, Y.; Qu, X.; Liu, H.; Qin, X.; Liu, C. ITGB 4 Deficiency Induces Senescence of Airway Epithelial Cells through P53 Activation. FEBS J. 2019, 286, 1191–1203. [Google Scholar] [CrossRef]
- Abbas, T.; Dutta, A. P21 in Cancer: Intricate Networks and Multiple Activities. Nat. Rev. Cancer 2009, 9, 400–414. [Google Scholar] [CrossRef]
- Sun, X.; Liu, B.; Wang, J.; Li, J.; Ji, W.-Y. Inhibition of P21-Activated Kinase 4 Expression Suppresses the Proliferation of Hep-2 Laryngeal Carcinoma Cells via Activation of the ATM/Chk1/2/P53 Pathway. Int. J. Oncol. 2013, 42, 683–689. [Google Scholar] [CrossRef]
- Bon, G.; Di Carlo, S.E.; Folgiero, V.; Avetrani, P.; Lazzari, C.; D’Orazi, G.; Brizzi, M.F.; Sacchi, A.; Soddu, S.; Blandino, G.; et al. Negative Regulation of B4 Integrin Transcription by Homeodomain-Interacting Protein Kinase 2 and P53 Impairs Tumor Progression. Cancer Res. 2009, 69, 5978–5986. [Google Scholar] [CrossRef] [PubMed]
- Sombroek, D.; Hofmann, T.G. How Cells Switch HIPK2 on and Off. Cell Death Differ. 2009, 16, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Xie, N.; Vikhreva, P.; Annicchiarico-Petruzzelli, M.; Amelio, I.; Barlev, N.; Knight, R.A.; Melino, G. Integrin-B4 Is a Novel Transcriptional Target of TAp73. Cell Cycle 2018, 17, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Maas, A.-M.; Bretz, A.C.; Mack, E.; Stiewe, T. Targeting P73 in Cancer. Cancer Lett. 2013, 332, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Ylivinkka, I.; Chen, P.; Li, L.; Hautaniemi, S.; Nyman, T.A.; Keski-Oja, J.; Hyytiäinen, M. Netrin-4 Promotes Glioblastoma Cell Proliferation through Integrin B4 Signaling. Neoplasia 2012, 14, 219IN23. [Google Scholar] [CrossRef]
- Zhang, X.; Rozengurt, E.; Reed, E.F. HLA Class I Molecules Partner with Integrin β 4 to Stimulate Endothelial Cell Proliferation and Migration. Sci. Signal. 2010, 3, ra85. [Google Scholar] [CrossRef]
- Wang, Y.; Shenouda, S.; Baranwal, S.; Rathinam, R.; Jain, P.; Bao, L.; Hazari, S.; Dash, S.; Alahari, S.K. Integrin Subunits Alpha5 and Alpha6 Regulate Cell Cycle by Modulating the Chk1 and Rb/E2F Pathways to Affect Breast Cancer Metastasis. Mol. Cancer 2011, 10, 84. [Google Scholar] [CrossRef]
- Inoue, Y.; Kitagawa, M.; Taya, Y. Phosphorylation of PRB at Ser612 by Chk1/2 Leads to a Complex between PRB and E2F-1 after DNA Damage. EMBO J. 2007, 26, 2083–2093. [Google Scholar] [CrossRef]
- Kreutzer, J.; Guerra, B. The Regulatory Beta-Subunit of Protein Kinase CK2 Accelerates the Degradation of CDC25A Phosphatase through the Checkpoint Kinase Chk1. Int. J. Oncol. 2007, 31, 1251–1259. [Google Scholar]
- Chen, M.-S.; Ryan, C.E.; Piwnica-Worms, H. Chk1 Kinase Negatively Regulates Mitotic Function of Cdc25A Phosphatase through 14-3-3 Binding. Mol. Cell. Biol. 2003, 23, 7488–7497. [Google Scholar] [CrossRef]
- Hu, T.; Zhou, R.; Zhao, Y.; Wu, G. Integrin A6/Akt/Erk Signaling Is Essential for Human Breast Cancer Resistance to Radiotherapy. Sci. Rep. 2016, 6, 33376. [Google Scholar] [CrossRef] [PubMed]
- Mizuta, K.; Matsubara, T.; Goto, A.; Addison, W.N.; Nakatomi, M.; Matsuo, K.; Tada-Shigeyama, Y.; Yaginuma, T.; Honda, H.; Yoshioka, I.; et al. Plectin Promotes Tumor Formation by B16 Mouse Melanoma Cells via Regulation of Rous Sarcoma Oncogene Activity. BMC Cancer 2022, 22, 936. [Google Scholar] [CrossRef] [PubMed]
- Niwa, T.; Saito, H.; Imajoh-ohmi, S.; Kaminishi, M.; Seto, Y.; Miki, Y.; Nakanishi, A. BRCA2 Interacts with the Cytoskeletal Linker Protein Plectin to Form a Complex Controlling Centrosome Localization. Cancer Sci. 2009, 100, 2115–2125. [Google Scholar] [CrossRef] [PubMed]
- Foisner, R.; Malecz, N.; Dressel, N.; Stadler, C.; Wiche, G. M-Phase-Specific Phosphorylation and Structural Rearrangement of the Cytoplasmic Cross-Linking Protein Plectin Involve P34cdc2 Kinase. Mol. Biol. Cell 1996, 7, 273–288. [Google Scholar] [CrossRef]
- Valencia, R.G.; Walko, G.; Janda, L.; Novacek, J.; Mihailovska, E.; Reipert, S.; Andrä-Marobela, K.; Wiche, G. Intermediate Filament-Associated Cytolinker Plectin 1c Destabilizes Microtubules in Keratinocytes. Mol. Biol. Cell 2013, 24, 768–784. [Google Scholar] [CrossRef]
- Burgstaller, G.; Gregor, M.; Winter, L.; Wiche, G. Keeping the Vimentin Network under Control: Cell-Matrix Adhesion-Associated Plectin 1f Affects Cell Shape and Polarity of Fibroblasts. Mol. Biol. Cell 2010, 21, 3362–3375. [Google Scholar] [CrossRef]
- Perez, S.M.; Dimastromatteo, J.; Landen, C.N.; Kelly, K.A. A Novel Monoclonal Antibody Targeting Cancer-Specific Plectin Has Potent Antitumor Activity in Ovarian Cancer. Cells 2021, 10, 2218. [Google Scholar] [CrossRef]
- Wilhelmsen, K.; Litjens, S.H.M.; Kuikman, I.; Tshimbalanga, N.; Janssen, H.; van den Bout, I.; Raymond, K.; Sonnenberg, A. Nesprin-3, a Novel Outer Nuclear Membrane Protein, Associates with the Cytoskeletal Linker Protein Plectin. J. Cell Biol. 2005, 171, 799–810. [Google Scholar] [CrossRef]
- Foisner, R.; Traub, P.; Wiche, G. Protein Kinase A- and Protein Kinase C-Regulated Interaction of Plectin with Lamin B and Vimentin. Proc. Natl. Acad. Sci. USA 1991, 88, 3812–3816. [Google Scholar] [CrossRef]
- Serres, M.P.; Samwer, M.; Quang, B.A.T.; Lavoie, G.; Perera, U.; Görlich, D.; Charras, G.; Petronczki, M.; Roux, P.P.; Paluch, E.K. F-Actin Interactome Reveals Vimentin as a Key Regulator of Actin Organization and Cell Mechanics in Mitosis. Dev. Cell 2020, 52, 210–222. [Google Scholar] [CrossRef]
- Wei, L.; Yin, F.; Chen, C.; Li, L. Expression of Integrin A-6 Is Associated with Multi Drug Resistance and Prognosis in Ovarian Cancer. Oncol. Lett. 2019, 17, 3974–3980. [Google Scholar] [CrossRef] [PubMed]
- Yamakawa, N.; Kaneda, K.; Saito, Y.; Ichihara, E.; Morishita, K. The Increased Expression of Integrin A6 (ITGA6) Enhances Drug Resistance in EVI1high Leukemia. PLoS ONE 2012, 7, e30706. [Google Scholar] [CrossRef] [PubMed]
- Rupaimoole, R.; Calin, G.A.; Lopez-Berestein, G.; Sood, A.K. MiRNA Deregulation in Cancer Cells and the Tumor Microenvironment. Cancer Discov. 2016, 6, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Zoni, E.; van der Horst, G.; van de Merbel, A.F.; Chen, L.; Rane, J.K.; Pelger, R.C.M.; Collins, A.T.; Visakorpi, T.; Snaar-Jagalska, B.E.; Maitland, N.J.; et al. MiR-25 Modulates Invasiveness and Dissemination of Human Prostate Cancer Cells via Regulation of Av- and A6-Integrin Expression. Cancer Res. 2015, 75, 2326–2336. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.-P.; Hu, Y.-P.; Wu, X.-S.; Wu, Y.-S.; Ye, Y.-Y.; Li, H.-F.; Liu, Y.-C.; Jiang, L.; Liu, F.-T.; Zhang, Y.-J.; et al. MiR-143-3p Targeting of ITGA6 Suppresses Tumour Growth and Angiogenesis by Downregulating PLGF Expression via the PI3K/AKT Pathway in Gallbladder Carcinoma. Cell Death Dis. 2018, 9, 182. [Google Scholar] [CrossRef]
- Laudato, S.; Patil, N.; Abba, M.L.; Leupold, J.H.; Benner, A.; Gaiser, T.; Marx, A.; Allgayer, H. P53-Induced MiR-30e-5p Inhibits Colorectal Cancer Invasion and Metastasis by Targeting ITGA6 and ITGB1: MiR-30e-5p Inhibits CRC Metastasis. Int. J. Cancer 2017, 141, 1879–1890. [Google Scholar] [CrossRef]
- Wang, Y.; Li, L.; Zhang, X.; Zhao, X. Inhibited Long Non-Coding RNA OIP5-AS1 Elevated MicroRNA-92a to Suppress Proliferation and Metastasis of Ovarian Cancer Cells by Silencing ITGA6. 2020; in review. [Google Scholar]
- Hozaka, Y.; Seki, N.; Tanaka, T.; Asai, S.; Moriya, S.; Idichi, T.; Wada, M.; Tanoue, K.; Kawasaki, Y.; Mataki, Y.; et al. Molecular Pathogenesis and Regulation of the MiR-29-3p-Family: Involvement of ITGA6 and ITGB1 in Intra-Hepatic Cholangiocarcinoma. Cancers 2021, 13, 2804. [Google Scholar] [CrossRef]
- Landowski, T.H.; Gard, J.; Pond, E.; Pond, G.D.; Nagle, R.B.; Geffre, C.P.; Cress, A.E. Targeting Integrin A6 Stimulates Curative-Type Bone Metastasis Lesions in a Xenograft Model. Mol. Cancer Ther. 2014, 13, 1558–1566. [Google Scholar] [CrossRef]
- Hogervorst, F.; Kuikman, I.; Noteboom, E.; Sonnenberg, A. The Role of Phosphorylation in Activation of the Alpha 6A Beta 1 Laminin Receptor. J. Biol. Chem. 1993, 268, 18427–18430. [Google Scholar] [CrossRef]
- Damsky, C.H.; Librach, C.; Lim, K.H.; Fitzgerald, M.L.; McMaster, M.T.; Janatpour, M.; Zhou, Y.; Logan, S.K.; Fisher, S.J. Integrin Switching Regulates Normal Trophoblast Invasion. Development 1994, 120, 3657–3666. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, H.; Li, X.; Duan, N.; Hu, S.; Liu, Y.; Yue, Y.; Song, L.; Zhang, Y.; Li, D.; et al. Plectin-1 Targeted Dual-Modality Nanoparticles for Pancreatic Cancer Imaging. EBioMedicine 2018, 30, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Bausch, D.; Thomas, S.; Mino-Kenudson, M.; Fernández-del, C.C.; Bauer, T.W.; Williams, M.; Warshaw, A.L.; Thayer, S.P.; Kelly, K.A. Plectin-1 as a Novel Biomarker for Pancreatic Cancer. Clin. Cancer Res. 2011, 17, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Wernitznig, D.; Meier-Menches, S.M.; Cseh, K.; Theiner, S.; Wenisch, D.; Schweikert, A.; Jakupec, M.A.; Koellensperger, G.; Wernitznig, A.; Sommergruber, W.; et al. Plecstatin-1 Induces an Immunogenic Cell Death Signature in Colorectal Tumour Spheroids. Metallomics 2020, 12, 2121–2133. [Google Scholar] [CrossRef] [PubMed]
- Meier, S.M.; Kreutz, D.; Winter, L.; Klose, M.H.M.; Cseh, K.; Weiss, T.; Bileck, A.; Alte, B.; Mader, J.C.; Jana, S.; et al. An Organoruthenium Anticancer Agent Shows Unexpected Target Selectivity For Plectin. Angew. Chem. Int. Ed. 2017, 56, 8267–8271. [Google Scholar] [CrossRef] [PubMed]
- Meier-Menches, S.M.; Zappe, K.; Bileck, A.; Kreutz, D.; Tahir, A.; Cichna-Markl, M.; Gerner, C. Time-Dependent Shotgun Proteomics Revealed Distinct Effects of an Organoruthenium Prodrug and Its Activation Product on Colon Carcinoma Cells. Metallomics 2019, 11, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Dowling, J.; Yu, Q.C.; Fuchs, E. Beta4 Integrin Is Required for Hemidesmosome Formation, Cell Adhesion and Cell Survival. J. Cell Biol. 1996, 134, 559–572. [Google Scholar] [CrossRef] [PubMed]
- Murgia, C.; Blaikie, P.; Kim, N.; Dans, M.; Petrie, H.T.; Giancotti, F.G. Cell Cycle and Adhesion Defects in Mice Carrying a Targeted Deletion of the Integrin B4 Cytoplasmic Domain. EMBO J. 1998, 17, 3940–3951. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Price, T.T.; Cantelli, G.; Ngo, B.; Warner, M.J.; Olivere, L.; Ridge, S.M.; Jablonski, E.M.; Therrien, J.; Tannheimer, S.; et al. Leukaemia Hijacks a Neural Mechanism to Invade the Central Nervous System. Nature 2018, 560, 55–60. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).