Beneficial Exercises for Cancer-Related Fatigue among Women with Breast Cancer: A Systematic Review and Network Meta-Analysis
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction and Article Selection
2.4. Classification in ‘Inter-Treatment’ or ‘Post-Treatment’
2.5. Exercise Classification
2.6. Risk of Bias Assessment
2.7. Statistical Analysis
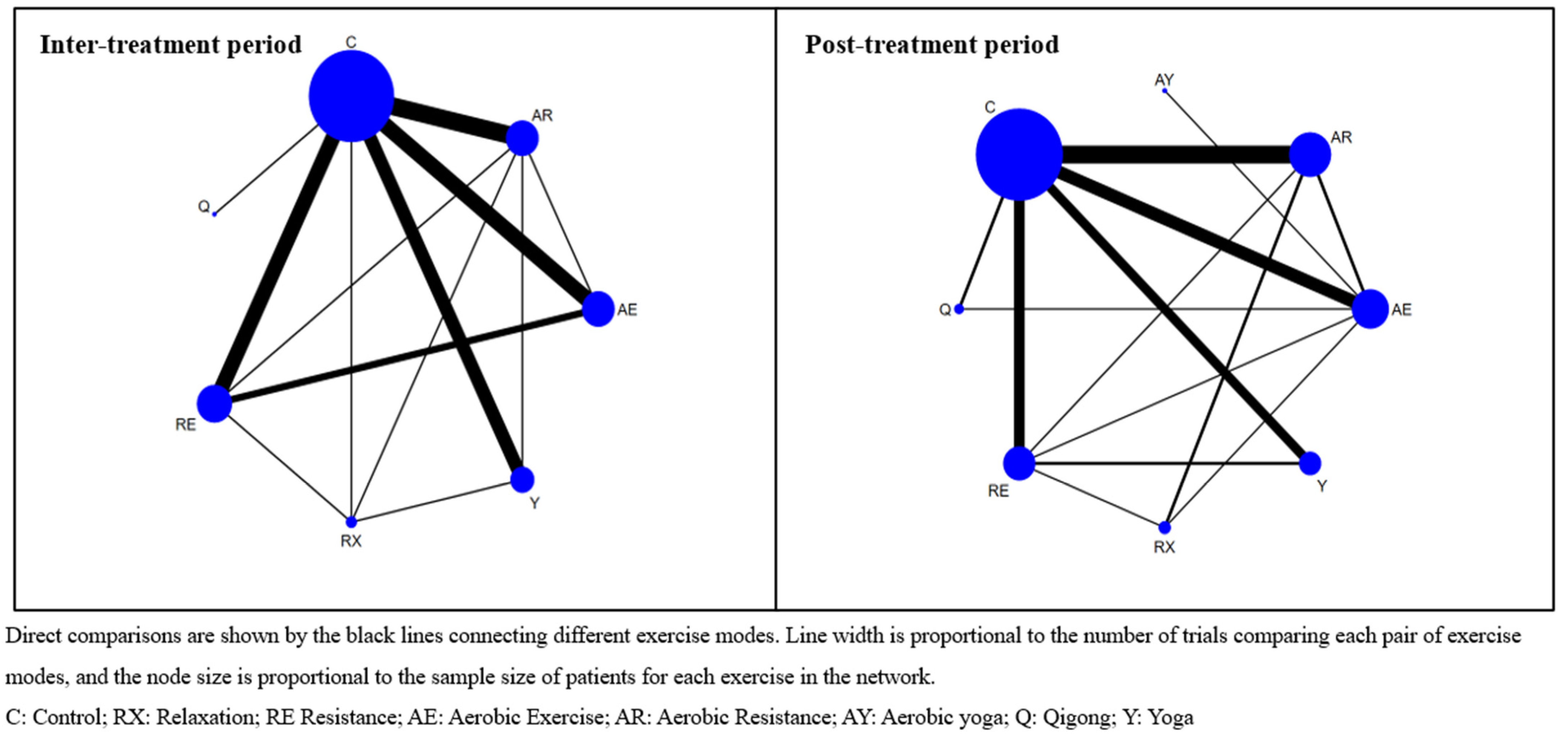
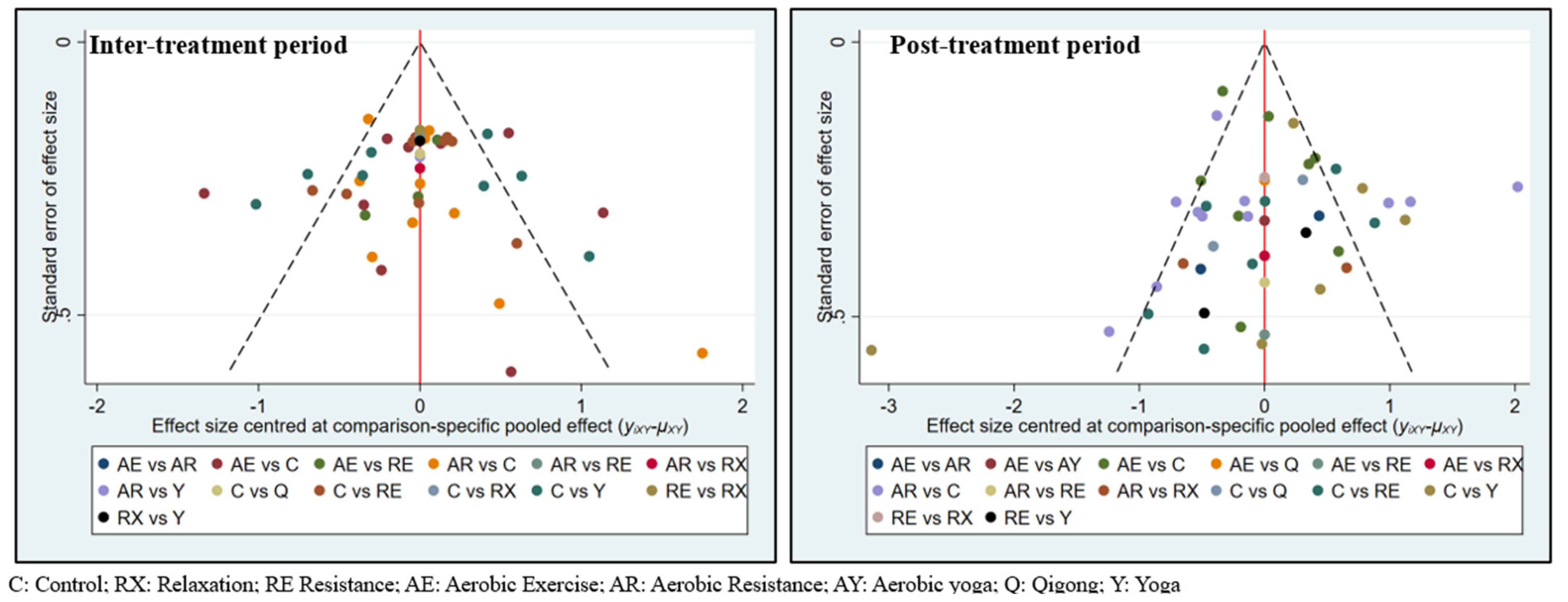
3. Results
3.1. Study Selection and Characteristics
3.2. Rob of Included Trials
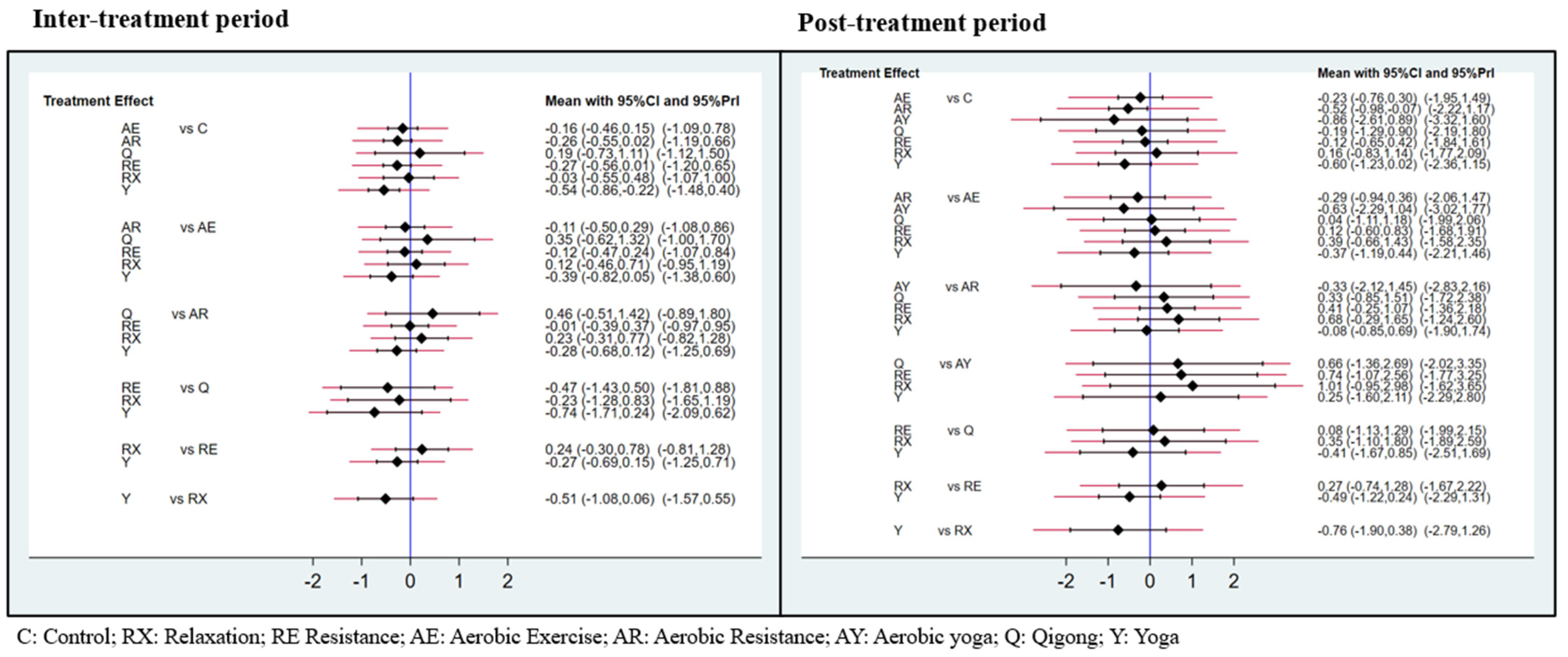
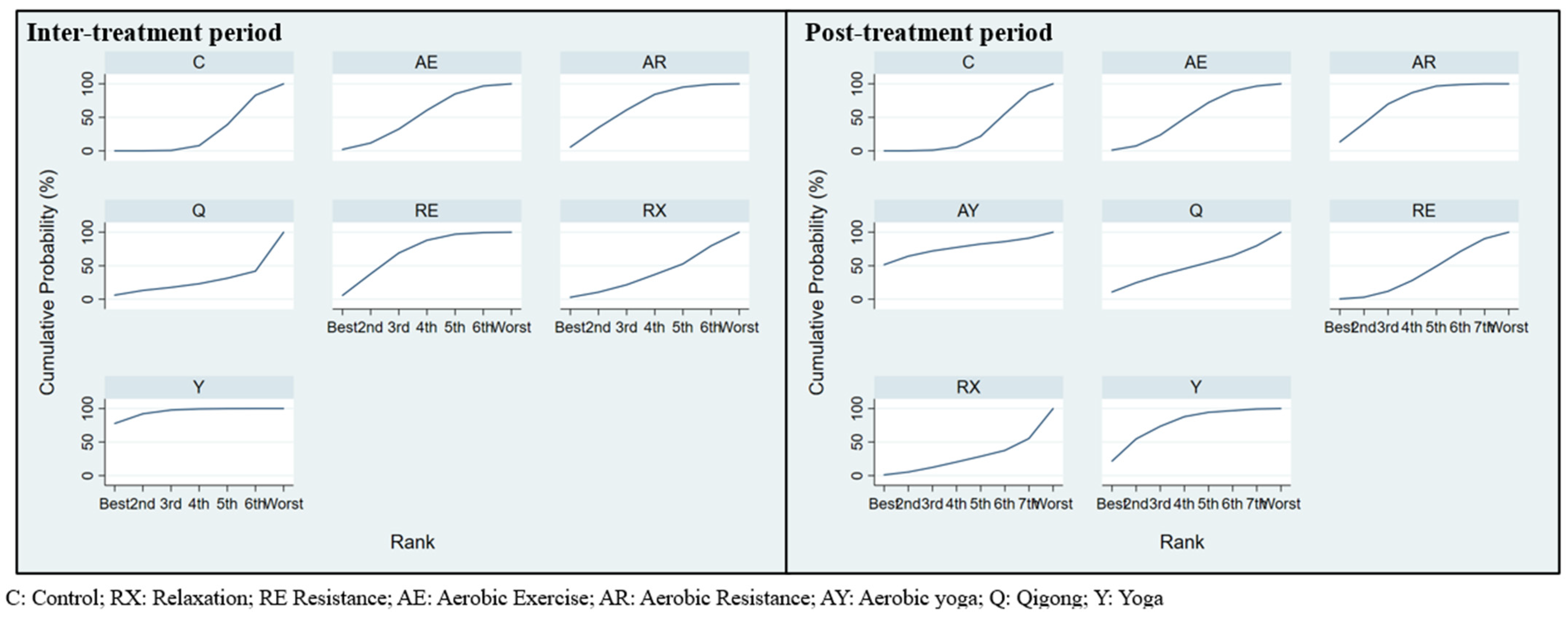
3.3. Exercise Efficacy and Ranking during Inter-Treatment Period
3.4. Exercise Efficacy and Ranking in Post-Treatment
4. Discussion
4.1. Exercise Efficacy and Ranking in Respect of the Inter-Treatment Period
4.2. Exercise Efficacy and Ranking in the Post-Treatment Period
4.3. Study Strengths and Limitations
4.4. Clinical Implications
4.5. Implications for Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ruiz-Casado, A.; Álvarez-Bustos, A.; de Pedro, C.G.; Méndez-Otero, M.; Romero-Elías, M. Cancer-related fatigue in breast cancer survivors: A review. Clin. Breast Cancer 2021, 21, 10–25. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Bustos, A.; de Pedro, C.G.; Romero-Elías, M.; Ramos, J.; Osorio, P.; Cantos, B.; Maximiano, C.; Méndez, M.; Fiuza-Luces, C.; Méndez-Otero, M. Prevalence and correlates of cancer-related fatigue in breast cancer survivors. Support. Care Cancer 2021, 29, 6523–6534. [Google Scholar] [CrossRef] [PubMed]
- Bower, J.E.; Ganz, P.A.; Desmond, K.A.; Bernaards, C.; Rowland, J.H.; Meyerowitz, B.E.; Belin, T.R. Fatigue in long-term breast carcinoma survivors: A longitudinal investigation. Cancer 2006, 106, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Reinertsen, K.V.; Cvancarova, M.; Loge, J.H.; Edvardsen, H.; Wist, E.; Fosså, S.D. Predictors and course of chronic fatigue in long-term breast cancer survivors. J. Cancer Surviv. 2010, 4, 405–414. [Google Scholar] [CrossRef]
- Sanft, T.; Denlinger, C.S.; Armenian, S.; Baker, K.S.; Broderick, G.; Demark-Wahnefried, W.; Friedman, D.L.; Goldman, M.; Hudson, M.; Khakpour, N. NCCN guidelines insights: Survivorship, version 2.2019: Featured updates to the NCCN guidelines. J. Natl. Compr. Cancer Netw. 2019, 17, 784–794. [Google Scholar] [CrossRef]
- Durosini, I.; Triberti, S.; Savioni, L.; Sebri, V.; Pravettoni, G. The Role of Emotion-Related Abilities in the Quality of Life of Breast Cancer Survivors: A Systematic Review. Int. J. Environ. Res. Public Health 2022, 19, 12704. [Google Scholar] [CrossRef]
- Pourfallahi, M.; Gholami, M.; Tarrahi, M.J.; Toulabi, T.; Kordestani Moghadam, P. The effect of informational-emotional support program on illness perceptions and emotional coping of cancer patients undergoing chemotherapy. Support. Care Cancer 2020, 28, 485–495. [Google Scholar] [CrossRef]
- Den Oudsten, B.L.; Van Heck, G.L.; Van der Steeg, A.F.; Roukema, J.A.; De Vries, J. Predictors of depressive symptoms 12 months after surgical treatment of early-stage breast cancer. Psycho-Oncology 2009, 18, 1230–1237. [Google Scholar] [CrossRef]
- Ancoli-Israel, S.; Liu, L.; Rissling, M.; Natarajan, L.; Neikrug, A.B.; Palmer, B.W.; Mills, P.J.; Parker, B.A.; Sadler, G.R.; Maglione, J. Sleep, fatigue, depression, and circadian activity rhythms in women with breast cancer before and after treatment: A 1-year longitudinal study. Support. Care Cancer 2014, 22, 2535–2545. [Google Scholar] [CrossRef]
- Abrahams, H.; Gielissen, M.; Schmits, I.; Verhagen, C.; Rovers, M.; Knoop, H. Risk factors, prevalence, and course of severe fatigue after breast cancer treatment: A meta-analysis involving 12 327 breast cancer survivors. Ann. Oncol. 2016, 27, 965–974. [Google Scholar] [CrossRef]
- Durosini, I.; Triberti, S.; Sebri, V.; Giudice, A.V.; Guiddi, P.; Pravettoni, G. Psychological Benefits of a Sport-Based Program for Female Cancer Survivors: The Role of Social Connections. Front. Psychol. 2021, 12, 751077. [Google Scholar] [CrossRef]
- Northey, J.M.; Pumpa, K.L.; Quinlan, C.; Ikin, A.; Toohey, K.; Smee, D.J.; Rattray, B. Cognition in breast cancer survivors: A pilot study of interval and continuous exercise. J. Sci. Med. Sport 2019, 22, 580–585. [Google Scholar] [CrossRef] [PubMed]
- Neil-Sztramko, S.E.; Winters-Stone, K.M.; Bland, K.A.; Campbell, K.L. Updated systematic review of exercise studies in breast cancer survivors: Attention to the principles of exercise training. Br. J. Sport. Med. 2019, 53, 504–512. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.X.M.; Jung, S.-Y.; Lee, E.-G.; Cho, H.; Cho, J.; Lee, E.; Chang, Y.J.; Cho, H. Long-term trajectory of postoperative health-related quality of life in young breast cancer patients: A 15-year follow-up study. J. Cancer Surviv. 2022. [Google Scholar] [CrossRef] [PubMed]
- Bøhn, S.-K.H.; Vandraas, K.; Kiserud, C.; Dahl, A.; Thorsen, L.; Ewertz, M.; Lie, H.; Falk, R.; Reinertsen, K. Work status changes and associated factors in a nationwide sample of Norwegian long-term breast cancer survivors. J. Cancer Surviv. 2022. [Google Scholar] [CrossRef]
- Carlson, M.A.; Fradgley, E.A.; Bridge, P.; Taylor, J.; Morris, S.; Coutts, E.; Paul, C. The dynamic relationship between cancer and employment-related financial toxicity: An in-depth qualitative study of 21 Australian cancer survivor experiences and preferences for support. Support. Care Cancer 2022, 30, 3093–3103. [Google Scholar] [CrossRef]
- Phillips, S.M.; McAuley, E. Physical Activity and Fatigue in Breast Cancer Survivors: A Panel Model Examining the Role of Self-efficacy and DepressionPhysical Activity and Fatigue in Breast Cancer Survivors. Cancer Epidemiol. Biomark. Prev. 2013, 22, 773–781. [Google Scholar] [CrossRef]
- Cella, D.; Davis, K.; Breitbart, W.; Curt, G.; Coalition, F. Cancer-related fatigue: Prevalence of proposed diagnostic criteria in a United States sample of cancer survivors. J. Clin. Oncol. 2001, 19, 3385–3391. [Google Scholar] [CrossRef]
- Raudonis, B.M.; Kelley, I.H.; Rowe, N.; Ellis, J. A pilot study of proinflammatory cytokines and fatigue in women with breast cancer during chemotherapy. Cancer Nurs. 2017, 40, 323–331. [Google Scholar] [CrossRef]
- Pomykala, K.; Ganz, P.; Bower, J.; Kwan, L.; Castellon, S.; Mallam, S.; Cheng, I.; Ahn, R.; Breen, E.; Irwin, M. The association between pro-inflammatory cytokines, regional cerebral metabolism, and cognitive complaints following adjuvant chemotherapy for breast cancer. Brain Imaging Behav. 2013, 7, 511–523. [Google Scholar] [CrossRef]
- Berger, A.M.; Gerber, L.H.; Mayer, D.K. Cancer-related fatigue: Implications for breast cancer survivors. Cancer 2012, 118, 2261–2269. [Google Scholar] [CrossRef] [PubMed]
- Balachandran, D.D.; Miller, M.A.; Faiz, S.A.; Yennurajalingam, S.; Innominato, P.F. Evaluation and management of sleep and circadian rhythm disturbance in cancer. Curr. Treat. Options Oncol. 2021, 22, 81. [Google Scholar] [CrossRef] [PubMed]
- Pertl, M.M.; Hevey, D.; Boyle, N.T.; Hughes, M.M.; Collier, S.; O’Dwyer, A.-M.; Harkin, A.; Kennedy, M.J.; Connor, T.J. C-reactive protein predicts fatigue independently of depression in breast cancer patients prior to chemotherapy. Brain Behav. Immun. 2013, 34, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Powers, S.K.; Lynch, G.S.; Murphy, K.T.; Reid, M.B.; Zijdewind, I. Disease-induced skeletal muscle atrophy and fatigue. Med. Sci. Sport. Exerc. 2016, 48, 2307. [Google Scholar] [CrossRef] [PubMed]
- Vargas, N.T.; Marino, F. A neuroinflammatory model for acute fatigue during exercise. Sport. Med. 2014, 44, 1479–1487. [Google Scholar] [CrossRef]
- Isanejad, A.; Alizadeh, A.M.; Shalamzari, S.A.; Khodayari, H.; Khodayari, S.; Khori, V.; Khojastehnjad, N. MicroRNA-206, let-7a and microRNA-21 pathways involved in the anti-angiogenesis effects of the interval exercise training and hormone therapy in breast cancer. Life Sci. 2016, 151, 30–40. [Google Scholar] [CrossRef]
- Bower, J.E.; Greendale, G.; Crosswell, A.D.; Garet, D.; Sternlieb, B.; Ganz, P.A.; Irwin, M.R.; Olmstead, R.; Arevalo, J.; Cole, S.W. Yoga reduces inflammatory signaling in fatigued breast cancer survivors: A randomized controlled trial. Psychoneuroendocrinology 2014, 43, 20–29. [Google Scholar] [CrossRef]
- Weis, J. Cancer-related fatigue: Prevalence, assessment and treatment strategies. Expert Rev. Pharm. Outcomes Res. 2011, 11, 441–446. [Google Scholar] [CrossRef]
- Furmaniak, A.C.; Menig, M.; Markes, M.H. Exercise for women receiving adjuvant therapy for breast cancer. Cochrane Database Syst. Rev. 2016, 4, cd005001. [Google Scholar] [CrossRef]
- Lipsett, A.; Barrett, S.; Haruna, F.; Mustian, K.; O’Donovan, A. The impact of exercise during adjuvant radiotherapy for breast cancer on fatigue and quality of life: A systematic review and meta-analysis. Breast 2017, 32, 144–155. [Google Scholar] [CrossRef]
- Courneya, K.S.; McKenzie, D.C.; Mackey, J.R.; Gelmon, K.; Friedenreich, C.M.; Yasui, Y.; Reid, R.D.; Cook, D.; Jespersen, D.; Proulx, C.; et al. Effects of Exercise Dose and Type During Breast Cancer Chemotherapy: Multicenter Randomized Trial. JNCI: J. Natl. Cancer Inst. 2013, 105, 1821–1832. [Google Scholar] [CrossRef] [PubMed]
- Carayol, M.; Bernard, P.; Boiché, J.; Riou, F.; Mercier, B.; Cousson-Gélie, F.; Romain, A.J.; Delpierre, C.; Ninot, G. Psychological effect of exercise in women with breast cancer receiving adjuvant therapy: What is the optimal dose needed? Ann. Oncol. 2013, 24, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Lu, H.J.; Lin, L.; Hu, Y. Effects of aerobic exercise on cancer-related fatigue: A meta-analysis of randomized controlled trials. Support. Care Cancer 2016, 24, 969–983. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Dong, J.; Huang, M.; Zhang, J.-e.; Zhang, X.; Xie, M.; Wefel, J.S. Nonpharmacological interventions for cancer-related cognitive impairment in adult cancer patients: A network meta-analysis. Int. J. Nurs. Stud. 2020, 104, 103514. [Google Scholar] [CrossRef] [PubMed]
- De Zoete, R.M.; Armfield, N.R.; McAuley, J.H.; Chen, K.; Sterling, M. Comparative effectiveness of physical exercise interventions for chronic non-specific neck pain: A systematic review with network meta-analysis of 40 randomised controlled trials. Br. J. Sport. Med. 2021, 55, 730–742. [Google Scholar] [CrossRef] [PubMed]
- McInnes, M.D.; Moher, D.; Thombs, B.D.; McGrath, T.A.; Bossuyt, P.M.; Clifford, T.; Cohen, J.F.; Deeks, J.J.; Gatsonis, C.; Hooft, L. Preferred reporting items for a systematic review and meta-analysis of diagnostic test accuracy studies: The PRISMA-DTA statement. Jama 2018, 319, 388–396. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann. Intern. Med. 2009, 151, 264–269. [Google Scholar] [CrossRef]
- Page, M.J.; Moher, D. Evaluations of the uptake and impact of the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) Statement and extensions: A scoping review. Syst. Rev. 2017, 6, 263. [Google Scholar] [CrossRef]
- Cipriani, A.; Higgins, J.P.; Geddes, J.R.; Salanti, G. Conceptual and technical challenges in network meta-analysis. Ann. Intern. Med. 2013, 159, 130–137. [Google Scholar] [CrossRef]
- White, I.R.; Barrett, J.K.; Jackson, D.; Higgins, J.P. Consistency and inconsistency in network meta-analysis: Model estimation using multivariate meta-regression. Res. Synth. Methods 2012, 3, 111–125. [Google Scholar] [CrossRef]
- Ardern, C.L.; Büttner, F.; Andrade, R.; Weir, A.; Ashe, M.C.; Holden, S.; Impellizzeri, F.M.; Delahunt, E.; Dijkstra, H.P.; Mathieson, S. Implementing the 27 PRISMA 2020 Statement items for systematic reviews in the sport and exercise medicine, musculoskeletal rehabilitation and sports science fields: The PERSiST (implementing Prisma in Exercise, Rehabilitation, Sport medicine and SporTs science) guidance. Br. J. Sport. Med. 2022, 56, 175–195. [Google Scholar]
- Hozo, S.P.; Djulbegovic, B.; Hozo, I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med. Res. Methodol. 2005, 5, 13. [Google Scholar] [CrossRef] [PubMed]
- Gradishar, W.J.; Anderson, B.O.; Balassanian, R.; Blair, S.L.; Burstein, H.J.; Cyr, A.; Elias, A.D.; Farrar, W.B.; Forero, A.; Giordano, S.H. NCCN guidelines insights: Breast cancer, version 1.2017. J. Natl. Compr. Cancer Netw. 2017, 15, 433–451. [Google Scholar] [CrossRef] [PubMed]
- American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2013. [Google Scholar]
- Swain, D.P.; Brawner, C.A.; American College of Sports Medicine. ACSM’s Resource Manual for Guidelines for Exercise Testing And Prescription; Wolters Kluwer Health/Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2014. [Google Scholar]
- Courneya, K.S.; Segal, R.J.; Mackey, J.R.; Gelmon, K.; Reid, R.D.; Friedenreich, C.M.; Ladha, A.B.; Proulx, C.; Vallance, J.; Lane, K. Effects of aerobic and resistance exercise in breast cancer patients receiving adjuvant chemotherapy: A multicenter randomized controlled trial. J. Clin. Oncol. 2007, 25, 4396–4404. [Google Scholar] [CrossRef]
- Lopez, P.; Galvão, D.A.; Taaffe, D.R.; Newton, R.U.; Souza, G.; Trajano, G.S.; Pinto, R.S. Resistance training in breast cancer patients undergoing primary treatment: A systematic review and meta-regression of exercise dosage. Breast Cancer 2021, 28, 16–24. [Google Scholar] [CrossRef]
- Soriano-Maldonado, A.; Díez-Fernández, D.M.; Esteban-Simón, A.; Rodríguez-Pérez, M.A.; Artés-Rodríguez, E.; Casimiro-Artés, M.A.; Moreno-Martos, H.; Toro-de-Federico, A.; Hachem-Salas, N.; Bartholdy, C. Effects of a 12-week supervised resistance training program, combined with home-based physical activity, on physical fitness and quality of life in female breast cancer survivors: The EFICAN randomized controlled trial. J. Cancer Surviv. 2022. [Google Scholar] [CrossRef]
- Batalik, L.; Winnige, P.; Dosbaba, F.; Vlazna, D.; Janikova, A. Home-based aerobic and resistance exercise interventions in cancer patients and survivors: A systematic review. Cancers 2021, 13, 1915. [Google Scholar] [CrossRef]
- Belloni, S.; Arrigoni, C.; Caruso, R. Effects from physical exercise on reduced cancer-related fatigue: A systematic review of systematic reviews and meta-analysis. Acta Oncol. 2021, 60, 1678–1687. [Google Scholar] [CrossRef]
- Palesh, O.; Scheiber, C.; Kesler, S.; Mustian, K.; Koopman, C.; Schapira, L. Management of side effects during and post-treatment in breast cancer survivors. Breast J. 2018, 24, 167–175. [Google Scholar] [CrossRef]
- Vardar Yağlı, N.; Şener, G.; Arıkan, H.; Sağlam, M.; İnal İnce, D.; Savcı, S.; Çalık Kutukcu, E.; Altundağ, K.; Kaya, E.B.; Kutluk, T. Do yoga and aerobic exercise training have impact on functional capacity, fatigue, peripheral muscle strength, and quality of life in breast cancer survivors? Integr. Cancer Ther. 2015, 14, 125–132. [Google Scholar] [CrossRef]
- Franklin, B.A. Evolution of the ACSM Guidelines: Historical Perspectives, New Insights, and Practical Implications. ACSM’s Health Fit. J. 2021, 25, 26–32. [Google Scholar] [CrossRef]
- Wang, G.; Wang, S.; Jiang, P.; Zeng, C. Effect of Yoga on cancer related fatigue in breast cancer patients with chemotherapy. Zhong Nan Da Xue Xue Bao Yi Xue Ban/J. Cent. South Univ. Med. Sci. 2014, 39, 1077–1082. [Google Scholar]
- Zhu, G.; Zhang, X.; Wang, Y.; Xiong, H.; Zhao, Y.; Sun, F. Effects of exercise intervention in breast cancer survivors: A meta-analysis of 33 randomized controlled trails. OncoTargets Ther. 2016, 9, 2153. [Google Scholar] [CrossRef]
- Jardim, P.S.J.; Rose, C.J.; Ames, H.M.; Echavez, J.F.M.; Van de Velde, S.; Muller, A.E. Automating risk of bias assessment in systematic reviews: A real-time mixed methods comparison of human researchers to a machine learning system. BMC Med. Res. Methodol. 2022, 22, 167. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons: Hoboken, NJ, USA, 2019. [Google Scholar]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef]
- Deeks, J.J.; Higgins, J.P.; Altman, D.G.; Group, C.S.M. Analysing data and undertaking meta-analyses. Cochrane Handb. Syst. Rev. Interv. 2019, 17, 241–284. [Google Scholar]
- Cumpston, M.; Li, T.; Page, M.J.; Chandler, J.; Welch, V.A.; Higgins, J.P.; Thomas, J. Updated guidance for trusted systematic reviews: A new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst. Rev. 2019, 10, 14651858. [Google Scholar] [CrossRef] [PubMed]
- van Valkenhoef, G.; Dias, S.; Ades, A.; Welton, N.J. Automated generation of node-splitting models for assessment of inconsistency in network meta-analysis. Res. Synth. Methods 2016, 7, 80–93. [Google Scholar] [CrossRef]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control. Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Tu, Y.-K. Using Generalized Linear Mixed Models to Evaluate Inconsistency within a Network Meta-Analysis. Value Health 2015, 18, 1120–1125. [Google Scholar] [CrossRef]
- Chiarito, M.; Sanz-Sánchez, J.; Cannata, F.; Cao, D.; Sturla, M.; Panico, C.; Godino, C.; Regazzoli, D.; Reimers, B.; De Caterina, R. Monotherapy with a P2Y12 inhibitor or aspirin for secondary prevention in patients with established atherosclerosis: A systematic review and meta-analysis. Lancet 2020, 395, 1487–1495. [Google Scholar] [CrossRef] [PubMed]
- Owen, P.J.; Miller, C.T.; Mundell, N.L.; Verswijveren, S.J.; Tagliaferri, S.D.; Brisby, H.; Bowe, S.J.; Belavy, D.L. Which specific modes of exercise training are most effective for treating low back pain? Network meta-analysis. Br. J. Sport. Med. 2020, 54, 1279–1287. [Google Scholar] [CrossRef]
- Salanti, G.; Ades, A.; Ioannidis, J.P. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: An overview and tutorial. J. Clin. Epidemiol. 2011, 64, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Dechartres, A.; Trinquart, L.; Boutron, I.; Ravaud, P. Influence of trial sample size on treatment effect estimates: Meta-epidemiological study. BMJ 2013, 346, f2304. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, T.A.; Miura, T.; Chaimani, A.; Leucht, S.; Cipriani, A.; Noma, H.; Mitsuyasu, H.; Kanba, S.; Salanti, G. Using the contribution matrix to evaluate complex study limitations in a network meta-analysis: A case study of bipolar maintenance pharmacotherapy review. BMC Res. Notes 2016, 9, 218. [Google Scholar] [CrossRef] [PubMed]
- Ehlers, D.K.; DuBois, K.; Salerno, E.A. The effects of exercise on cancer-related fatigue in breast cancer patients during primary treatment: A meta-analysis and systematic review. Expert Rev. Anticancer Ther. 2020, 20, 865–877. [Google Scholar] [CrossRef] [PubMed]
- Cramer, H.; Lauche, R.; Klose, P.; Lange, S.; Langhorst, J.; Dobos, G.J. Yoga for improving health-related quality of life, mental health and cancer-related symptoms in women diagnosed with breast cancer. Cochrane Database Syst. Rev. 2017, 1, cd010802. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.-Y.; Hu, Y.-T.; Chang, K.-J.; Lin, H.-F.; Tsauo, J.-Y. Effects of yoga on psychological health, quality of life, and physical health of patients with cancer: A meta-analysis. Evid.-Based Complement. Altern. Med. 2011, 2011, 659876. [Google Scholar] [CrossRef]
- Pan, Y.; Yang, K.; Wang, Y.; Zhang, L.; Liang, H. Could yoga practice improve treatment-related side effects and quality of life for women with breast cancer? A systematic review and meta-analysis. Asia-Pac. J. Clin. Oncol. 2017, 13, e79–e95. [Google Scholar] [CrossRef]
- Chandwani, K.D.; Perkins, G.; Nagendra, H.R.; Raghuram, N.V.; Spelman, A.; Nagarathna, R.; Johnson, K.; Fortier, A.; Arun, B.; Wei, Q. Randomized, controlled trial of yoga in women with breast cancer undergoing radiotherapy. J. Clin. Oncol. 2014, 32, 1058. [Google Scholar] [CrossRef]
- Chaoul, A.; Milbury, K.; Spelman, A.; Basen-Engquist, K.; Hall, M.H.; Wei, Q.; Shih, Y.C.T.; Arun, B.; Valero, V.; Perkins, G.H. Randomized trial of Tibetan yoga in patients with breast cancer undergoing chemotherapy. Cancer 2018, 124, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Taso, C.-J.; Lin, H.-S.; Lin, W.-L.; Chen, S.-M.; Huang, W.-T.; Chen, S.-W. The effect of yoga exercise on improving depression, anxiety, and fatigue in women with breast cancer: A randomized controlled trial. J. Nurs. Res. 2014, 22, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Vadiraja, H.; Rao, R.M.; Nagarathna, R.; Nagendra, H.; Patil, S.; Diwakar, R.B.; Shashidhara, H.; Gopinath, K.; Ajaikumar, B. Effects of yoga in managing fatigue in breast cancer patients: A randomized controlled trial. Indian J. Palliat. Care 2017, 23, 247. [Google Scholar] [PubMed]
- Meneses-Echávez, J.F.; González-Jiménez, E.; Ramírez-Vélez, R. Effects of supervised exercise on cancer-related fatigue in breast cancer survivors: A systematic review and meta-analysis. BMC Cancer 2015, 15, 77. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Spence, R.R.; Steele, M.L.; Sandler, C.X.; Peake, J.M.; Hayes, S.C. A systematic review and meta-analysis of the safety, feasibility, and effect of exercise in women with stage II+ breast cancer. Arch. Phys. Med. Rehabil. 2018, 99, 2621–2636. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.C.; Huedo-Medina, T.B.; Pescatello, L.S.; Pescatello, S.M.; Ferrer, R.A.; Johnson, B.T. Efficacy of Exercise Interventions in Modulating Cancer-Related Fatigue among Adult Cancer Survivors: A Meta-AnalysisExercise and Cancer-Related Fatigue: A Meta-Analysis. Cancer Epidemiol. Biomark. Prev. 2011, 20, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Doyle, C.; Kushi, L.H.; Byers, T.; Courneya, K.S.; Demark-Wahnefried, W.; Grant, B.; McTiernan, A.; Rock, C.L.; Thompson, C.; Gansler, T. Nutrition and physical activity during and after cancer treatment: An American Cancer Society guide for informed choices. CA Cancer J. Clin. 2006, 56, 323–353. [Google Scholar] [CrossRef]
- Hilfiker, R.; Meichtry, A.; Eicher, M.; Balfe, L.N.; Knols, R.H.; Verra, M.L.; Taeymans, J. Exercise and other non-pharmaceutical interventions for cancer-related fatigue in patients during or after cancer treatment: A systematic review incorporating an indirect-comparisons meta-analysis. Br. J. Sport. Med. 2018, 52, 651–658. [Google Scholar] [CrossRef]
- Islam, M.R.; Islam, F.; Nafady, M.H.; Akter, M.; Mitra, S.; Das, R.; Urmee, H.; Shohag, S.; Akter, A.; Chidambaram, K. Natural small molecules in breast cancer treatment: Understandings from a therapeutic viewpoint. Molecules 2022, 27, 2165. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Weiderpass, E.; Soerjomataram, I. The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer 2021, 127, 3029–3030. [Google Scholar] [CrossRef]
- Zhang, L.-L.; Wang, S.-Z.; Chen, H.-L.; Yuan, A.-Z. Tai chi exercise for cancer-related fatigue in patients with lung cancer undergoing chemotherapy: A randomized controlled trial. J. Pain Symptom Manag. 2016, 51, 504–511. [Google Scholar] [CrossRef]
- Song, S.; Yu, J.; Ruan, Y.; Liu, X.; Xiu, L.; Yue, X. Ameliorative effects of Tai Chi on cancer-related fatigue: A meta-analysis of randomized controlled trials. Support. Care Cancer 2018, 26, 2091–2102. [Google Scholar] [CrossRef] [PubMed]
- Thongteratham, N.; Pongthavornkamol, K.; Olson, K.; Ratanawichitrasin, A.; Nityasuddhi, D.; Wattanakitkrilert, D. Effectiveness of Tai Chi Qi Qong program for Thai women with breast cancer: A randomized control trial. Pac. Rim Int. J. Nurs. Res. 2015, 19, 280–294. [Google Scholar]
- Nunes, P.R.; Martins, F.M.; Souza, A.P.; Carneiro, M.A.; Orsatti, C.L.; Michelin, M.A.; Murta, E.F.; de Oliveira, E.P.; Orsatti, F.L. Effect of high-intensity interval training on body composition and inflammatory markers in obese postmenopausal women: A randomized controlled trial. Menopause 2019, 26, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Khosravi, N.; Stoner, L.; Farajivafa, V.; Hanson, E.D. Exercise training, circulating cytokine levels and immune function in cancer survivors: A meta-analysis. Brain Behav. Immun. 2019, 81, 92–104. [Google Scholar] [CrossRef]
- Liguori, G.; American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2020. [Google Scholar]
- Tomlinson, D.; Diorio, C.; Beyene, J.; Sung, L. Effect of exercise on cancer-related fatigue: A meta-analysis. Am. J. Phys. Med. Rehabil. 2014, 93, 675–686. [Google Scholar] [CrossRef]
- Figueiras, M.J.; Neto, D.D.; Marôco, J. Understanding the relationship between illness perceptions of breast cancer and perceived risk in a sample of UAE female university students: The role of comparative risk. BMC Women’s Health 2022, 22, 193. [Google Scholar] [CrossRef]
- Hamed, E.; Alemrayat, B.; Syed, M.A.; Daher-Nashif, S.; Rasheed, H.M.A.; Kane, T. Breast Cancer Knowledge, Attitudes and Practices amongst Women in Qatar. Int. J. Environ. Res. Public Health 2022, 19, 3995. [Google Scholar] [CrossRef]
- Schmidt, M.E.; Wiskemann, J.; Armbrust, P.; Schneeweiss, A.; Ulrich, C.M.; Steindorf, K. Effects of resistance exercise on fatigue and quality of life in breast cancer patients undergoing adjuvant chemotherapy: A randomized controlled trial. Int. J. Cancer 2015, 137, 471–480. [Google Scholar] [CrossRef]
- Lambert, C.P.; Wright, N.R.; Finck, B.N.; Villareal, D.T. Exercise but not diet-induced weight loss decreases skeletal muscle inflammatory gene expression in frail obese elderly persons. J. Appl. Physiol. 2008, 105, 473–478. [Google Scholar] [CrossRef]
- Dieli-Conwright, C.M.; Courneya, K.S.; Demark-Wahnefried, W.; Sami, N.; Lee, K.; Buchanan, T.A.; Spicer, D.V.; Tripathy, D.; Bernstein, L.; Mortimer, J.E. Effects of aerobic and resistance exercise on metabolic syndrome, sarcopenic obesity, and circulating biomarkers in overweight or obese survivors of breast cancer: A randomized controlled trial. J. Clin. Oncol. 2018, 36, 875. [Google Scholar] [CrossRef] [PubMed]
- Ortega, M.A.; Fraile-Martínez, O.; García-Montero, C.; Pekarek, L.; Guijarro, L.G.; Castellanos, A.J.; Sanchez-Trujillo, L.; García-Honduvilla, N.; Álvarez-Mon, M.; Buján, J. Physical activity as an imperative support in breast cancer management. Cancers 2020, 13, 55. [Google Scholar] [CrossRef] [PubMed]
- Dimauro, I.; Grazioli, E.; Antinozzi, C.; Duranti, G.; Arminio, A.; Mancini, A.; Greco, E.A.; Caporossi, D.; Parisi, A.; Di Luigi, L. Estrogen-receptor-positive breast cancer in postmenopausal women: The role of body composition and physical exercise. Int. J. Environ. Res. Public Health 2021, 18, 9834. [Google Scholar] [CrossRef] [PubMed]
- Booth, F.W.; Roberts, C.K.; Laye, M.J. Lack of exercise is a major cause of chronic diseases. Compr. Physiol. 2012, 2, 1143. [Google Scholar]
- Huang, H.-P.; Wen, F.-H.; Tsai, J.-C.; Lin, Y.-C.; Shun, S.-C.; Chang, H.-K.; Wang, J.-S.; Jane, S.-W.; Chen, M.-C.; Chen, M.-L. Adherence to prescribed exercise time and intensity declines as the exercise program proceeds: Findings from women under treatment for breast cancer. Support. Care Cancer 2015, 23, 2061–2071. [Google Scholar] [CrossRef]
- Jiang, M.; Ma, Y.; Yun, B.; Wang, Q.; Huang, C.; Han, L. Exercise for fatigue in breast cancer patients: An umbrella review of systematic reviews. Int. J. Nurs. Sci. 2020, 7, 248–254. [Google Scholar] [CrossRef]
- Gebruers, N.; Camberlin, M.; Theunissen, F.; Tjalma, W.; Verbelen, H.; Van Soom, T.; van Breda, E. The effect of training interventions on physical performance, quality of life, and fatigue in patients receiving breast cancer treatment: A systematic review. Support. Care Cancer 2019, 27, 109–122. [Google Scholar] [CrossRef]
- Jones, L.W.; Courneya, K.S.; Peddle, C.; Mackey, J.R. Oncologists’ opinions towards recommending exercise to patients with cancer: A Canadian national survey. Support. Care Cancer 2005, 13, 929–937. [Google Scholar] [CrossRef]
- Jones, L.W.; Courneya, K.S. Exercise discussions during cancer treatment consultations. Cancer Pract. 2002, 10, 66–74. [Google Scholar] [CrossRef]
- Jones, L.W.; Courneya, K.S. Exercise counseling and programming preferences of cancer survivors. Cancer Pract. 2002, 10, 208–215. [Google Scholar] [CrossRef]
- Courneya, K.S.; Segal, R.J.; Reid, R.D.; Jones, L.W.; Malone, S.C.; Venner, P.M.; Parliament, M.B.; Scott, C.G.; Quinney, H.A.; Wells, G.A. Three independent factors predicted adherence in a randomized controlled trial of resistance exercise training among prostate cancer survivors. J. Clin. Epidemiol. 2004, 57, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Fuller, J.T.; Hartland, M.C.; Maloney, L.T.; Davison, K. Therapeutic effects of aerobic and resistance exercises for cancer survivors: A systematic review of meta-analyses of clinical trials. Br. J. Sport. Med. 2018, 52, 1311. [Google Scholar] [CrossRef] [PubMed]
- Koelwyn, G.J.; Wennerberg, E.; Demaria, S.; Jones, L.W. Exercise in regulation of inflammation-immune axis function in cancer initiation and progression. Oncology 2015, 29, 908–912. [Google Scholar]
- Sebri, V.; Durosini, I.; Mazzoni, D.; Pravettoni, G. Breast Cancer Survivors’ Motivation to Participate in a Tailored Physical and Psychological Intervention: A Qualitative Thematic Analysis. Behav. Sci. 2022, 12, 271. [Google Scholar] [CrossRef] [PubMed]
- Savioni, L.; Triberti, S.; Durosini, I.; Sebri, V.; Pravettoni, G. Cancer patients’ participation and commitment to psychological interventions: A scoping review. Psychol. Health 2022, 37, 1022–1055. [Google Scholar] [CrossRef] [PubMed]
- Ezzatvar, Y.; Ramírez-Vélez, R.; Izquierdo, M.; Garcia-Hermoso, A. Physical activity and risk of infection, severity and mortality of COVID-19: A systematic review and non-linear dose–response meta-analysis of data from 1,853,610 adults. Br. J. Sport. Med. 2022, 2022, 105733. [Google Scholar] [CrossRef] [PubMed]
| Author Year | Treatment Phase | Country | Stage | Age Mean ± SD | CRF Measurement | Sample Size & Exercise Interventions | Findings Mean (SD) of CRF Score Effect Size (95% CI) |
|---|---|---|---|---|---|---|---|
| Al-Majid 2015 | Inter-treatment (CT) | USA | I–II | AE: 47.9 ± 10.4 C: 52.7 ± 10.7 | Revised Piper Fatigue Scale (PFS) (SD is calculated by SE) | AE (n = 6) Length: 20–40 min/session Frequency: two to three sessions/week Duration: 12 weeks Supervised: no Control (n = 6) Usual care | AE: 3 (1.96) C: 4.6 (2.2) Hedges’ g: −0.71 (−1.89, 0.47) |
| Battaglini 2006 | Inter-treatment (OP, CT, or RT) | USA | NI | AE+RE: 57.5 ± 23 C: 56.6 ± 16 | PFS | AE+RE (n = 10) Length: 60 min/session Frequency: two sessions/week Duration: 15 weeks Supervised: yes Control (n = 10) Usual care | AE+RE: 0.84 (1.13) C: 3.23 (1.16) Hedges’ g: −2.00 (−3.12, −0.88) |
| Bolam 2019 | Inter-treatment (CT) | Sweden | I–IIIA | RE: 52.7 ± 10.3 AE: 60 ± 10.3 C: 57 ± 10.2 | PFS | RE (n = 65) Length: 60 min/session Frequency: two session/week Duration: 16 weeks Supervised: yes AE (n = 60) Length: 20 min/session Frequency: two session/week Duration: 16 months Supervised: yes Control (n = 57) Usual care | RE: 3.12 (3.03) AE: 3.18 (2.77) C: 3.98 (3.05) Hedges’ g: RE:C −0.28 (−0.64, 0.07) AE:C −0.27 (−0.64, 0.09) RE:AE −0.02 (−0.37, 0.33) |
| Campbell 2005 | Inter-treatment (OP, CT, or RT) | UK | Early stage | AE+RE: 48 ± 10 C: 47 ± 5 | PFS (Using changed score) | AE+RE (n = 10) Length: 10–20 min/session Frequency: two sessions/week Duration: 12 weeks Supervised: yes Control (n = 9) Usual care | AE+RE: −2.11 (2.3) C: −0.25 (2.5) Hedges’ g: −0.74 (−1.68, 0.20) |
| Cešeiko 2019 | Inter-treatment (OP, CT, or RT) | Latvia | I–III | RE: 48.2 ± 6.7 C: 49.0 ± 8.0 | European Organization for the Research and Treatment of Cancer—Quality of Life (EORTC QoL C30) | RE (n = 27) Length: 20 min/session Frequency: two session/week Duration: 12 weeks Supervised: yes Control (n = 28) Usual care | RE: 25.5 (15.5) C: 36.8 (16.7) Hedges’ g: −0.69 (−1.24, −0.15) |
| Chandwani 2010 | Inter-treatment (RT) | USA | 0–III | Y: 51.4 ± 8.0 C: 4.0 ± 10.0 | Brief Fatigue Inventory (BFI) (SD is calculated by SE) | Yoga (n = 27) Length: 60 min/session Frequency: two sessions/week Duration: six weeks Supervised: yes Control (n = 31) Waiting list | Y: 1.9 (3.64) C: 2.5 (4.45) Hedges’ g: −0.14 (−0.66, 0.37) |
| Chandwani 2014 | Inter-treatment (RT) | USA | 0–III | Y: 52.4 ± 9.8 C: 52.1 ± 9.8 | BFI | Yoga (n = 53) Length: 60 min/session Frequency: three sessions/week Duration: six weeks Supervised: yes Control (n = 54) Waiting list | Y: 2.9 (0.3) C: 3.2 (0.4) Hedges’ g: −0.84 (−1.24, −0.45) |
| Chaoul 2018 | Inter-treatment (CT) | USA | I–III | Y: 49.5 ± 9.8 RX: 50.4 ± 10.3 C: 49.0 ± 10.1 | BFI | Yoga (n = 64) Length: 75–90 min/session Frequency: four sessions/12 weeks Duration: 12 weeks Supervised: yes Relaxation (n = 59) Length: 75–90 min/session Frequency: four sessions/week Duration: 12 weeks Supervised: yes Control (n = 79) Usual care | Y: 3.2 (2.4) RX: 3.7 (2.3) C: 3.5 (2.5) Hedges’ g: Y:C −0.12 (−0.45, 0.21) RX:C 0.08 (−0.26, 0.42) Y:RX −0.21 (−0.57, 0.14) |
| Chen 2013 | Inter-treatment (RT) | China | 0–III | Q: 45.3 ± 6.3 C: 44.7 ± 9.7 | BFI | Qigong (n = 49) Length: 40 min/session Frequency: one sessions/week Duration: five to six weeks Supervised: yes Control (n = 47) Waiting list | Q: 3.1 (2.0) C: 2.7 (2.1) Hedges’ g: 0.19 (−0.21, 0.59) |
| Cornette 2016 | Inter-treatment (CT) | USA | I–IIIB | (Median age) AE+RE: 52 C: 49 | Multidimensional Fatigue Inventory (MFI) | AE+RE (n = 20) Length: 20–40 min/session Frequency: three sessions/week Duration: 27 weeks Supervised: no (home based) Control (n = 22) Usual care | AE+RE: 38 (12.3) C: 44.2 (13.9) Hedges’ g: −0.46 (−1.08, 0.15) |
| Courneya 2007 | Inter-treatment (CT) | Canada | I–IIIA | Total Participants Range: 25–78 Mean: 49 | Functional Assessment of Cancer Therapy—Anemia (FACT—An) | AE (n = 68) Length: 15–45 min/session Frequency: three sessions/week Duration: 18 weeks Supervised: yes RE (n = 68) Length: No information Frequency: three sessions/week Duration: 18 weeks Supervised: yes Control (n = 60) Usual care | AE: 42.1 (10.5) RE: 40.8 (10.5) C: 41.5 (9.8) Hedges’ g: AE:C 0.06 (−0.29, 0.41) RE:C −0.07 (−0.41, 0.27) AE:RE 0.12 (−0.21, 0.45) |
| Danhauer 2015 | Inter-treatment (CT) | USA | Any stage | Y: 54.3 ± 9.6 C: 57.2 ± 10.2 | Functional Assessment of Cancer Therapy—Fatigue (FACT—F) | Yoga (n = 22) Length: 75 min/session Frequency: one session/week Duration: 10 weeks Homework: 45 min/twice a week Supervised: yes Control education (n = 18) Length: 75 min/session Frequency: one sessions/week Duration: 10 weeks | Y: 39.8 (11.5) C: 32.6 (15.5) Hedges’ g: 0.51 (−0.26, 1.28) |
| Gokal 2016 | Inter-treatment (CT) | UK | I–III | AE: 52.1 ± 11.7 C: 52.36 ± 8.9 | FACT—F | AE (n = 25) Length: Began by completing 10 min of walking and then increased to 30 min/session Frequency: five sessions/week Duration: 12 weeks Supervised: no Control (n = 25) Usual care | AE: 26.04 (3.8) C: 33.6 (7.29) Hedges’ g: −1.28 (−1.89, −0.67) |
| Hu 2013 | Inter-treatment (Immediate post-OP) | Taiwan | 0–III | Total: 46.8 ± 9.7 AE: 46.5 ± 10.4 C: 47.1 ± 9.2 | Functional Assessment of Chronic Illness Therapy—Fatigue (FACIT—F) | AE (n = 30) Length: 30–50 min/session Frequency: three to five sessions/week Duration: 5 weeks Supervised: no Control (n = 25) Usual care | AE: 38.2 (8.9) C: 36.9 (11.6) Hedges’ g: −0.12 (−0.62, 0.37) |
| Huang 2019 | Inter-treatment (CT) | Taiwan | I–III | RE: 48.3 ± 7.9 C: 48.3 ± 8.7 | BFI | RE (n = 81) Length: 30–40 min/session Frequency: five session/week Duration: 12 weeks Supervised: no Control (n = 78) Usual care | RE: 1.0 (2.2) C: 1.19 (2.3) Hedges’ g: −0.08 (−0.44, 0.27) |
| Husebø 2014 | Inter-treatment (CT) | Norway | I–III | Total: 52.2 ± 9.3 AE+RE: 50.8 ± 9.7 C: 53.6 ± 8.8 | Schwartz Cancer Fatigue Scale (SCFS-6) | AE+RE Length: 30 min/session Frequency: RE—three sessions/week AE—seven sessions/week Duration: 17 weeks Supervised: no Control (n = 78) Usual care | AE+RE: 12.01 (4.38) C: 13.13 (4.47) Hedges’ g: −0.25 (−0.76, 0.26) |
| Hwang 2008 | Inter-treatment (RT) | Korea | NI | AE+RE: 46.3 ± 7.5 C: 46.3 ± 9.5 | BFI | AE+RE (n = 17) Length: 50 min/session Frequency: three sessions/week Duration: five weeks Supervised: yes Control (n = 20) Usual care | AE+RE: 3.5 (1.7) C: 3.9 (2.1) Hedges’ g: −0.20 (−0.85, 0.45) |
| Jong 2018 | Inter-treatment (CT) | Netherlands | I–III | Y: 51 ± 8.0 C: 51 ± 7.3 | MFI | Yoga (n = 39) Length: 75 min/session Frequency: one session/week Duration: 12 weeks Homework: 5–20 min/daily Supervised: yes Control (n = 29) Usual care | Y: 14.6 (4.5) C: 14.2 (4.2) Hedges’ g: 0.09 (−0.39, 0.57) |
| Kirkham 2020 | Inter-treatment (CT) | Canada | I–III | AE+RE: 51 ± 8.1 C: 49.5 ± 11 | PFS | AE+RE (n = 12) Length: 25–40 min/session Frequency: three sessions/week Duration: 12 weeks Supervised: yes Control (n = 15) Waiting list | AE+RE: 4 (2.3) C: 3.9 (1.9) Hedges’ g: 0.05 (−0.72, 0.82) |
| Lee 2021 | Inter-treatment (CT) | USA | I–III | Total: 46.9 ± 9.8 | MFI−20 | RE (n = 15) Length: 30 min/session Frequency: one session/week Duration: eight weeks Supervised: yes Control (n = 15) Usual care | RE: 54.3 (14.1) C: 49.3 (12.6) Hedges’ g: 0.36 (−0.36, 1.08) |
| Lötzke 2016 | Inter-treatment (CT, HT, or RT) | Germany | I–III | Y: 51.0 ± 11.0 AE+RE: 51.4 ± 11.1 | Cancer Fatigue Scale (CFS) | Yoga (n = 45) Length: 60 min/session Frequency: one to two sessions/week Duration: 12 weeks Supervised: yes AE+RE (n = 47) Length: 60 min per week Frequency: one session/week Duration: 12 weeks Supervised: yes | Y: 21.04 (9.91) AE+RE: 24.32 (10.63) Hedges’ g: −0.32 (−0.73, 0.10) |
| Mijwel 2018 | Inter-treatment (CT) | Sweden | I–IIIA | RE: 54.4 ± 10.3 AE+RE: 52.7 ± 10.3 C: 52.6 ± 10.2 | PFS | RE (n = 70) Length: 60 min/session Frequency: two sessions/week Duration: 16 weeks Supervised: yes AE+RE (n = 74) Length: 60 min/session Frequency: two sessions/week Duration: 16 weeks Supervised: yes Control (n = 60) Usual care | RE: 3.16 (2.92) AE+RE: 3.16 (2.61) C: 3.94 (2.95) Hedges’ g: RE:C −0.26 (−0.61, 0.08) AE+RE:C −0.28 (−0.63, 0.07) RE:AE+RE 0 (−0.37, 0.37) |
| Mock 2005 | Inter-treatment (CT or RT) | USA | 0–III | Total: 51.5 ± 9.3 AE: 51.3 ± 8.9 C: 51.6 ± 9.7 | PFS | AE (n = 60) Length: 15–30 min/session Frequency: five to six sessions/week Duration: During treatments Supervised: no Control (n = 59) Usual care | AE: 3.5 (2.4) C: 3.7 (3.0) Hedges’ g: −0.07 (−0.45, 0.30) |
| Møller 2020 | Inter-treatment (CT) | Denmark | I–III | Total: 51.7 ± 9.4 RE: 51.5 ± 9.6 C: 52.0 ± 9.3 | EORTC QLQ-C30 | RE (n = 62) Length: 20 min/session Frequency: two sessions/week Duration: 12 weeks Supervised: yes Control Education (n = 59) Health counseling and symptom guidance | RE: 58 (27) C: 59 (25) Hedges’ g: −0.04 (−0.39, 0.32) |
| Mostafaei 2021 | Inter-treatment (CT) | Iran | 0–III | RE: 48.5 ± 5.7 C: 49.6 ± 7.5 | Fatigue Severity Scale (FSS) | RE (n = 30) Length: 20–30 min/session Frequency: three sessions/week Duration: six weeks Supervised: no Control (n = 30) Usual care | RE: 41.3 (9.4) C: 50.16 (9.96) Hedges’ g: −0.90 (−1.43, −0.37) |
| Mutrie 2007 | Inter-treatment (CT or RT) | Scotland | 0–III | Total: 51.6 ± 9.5 AE+RE: 51.3 ± 10.3 C: 51.8 ± 8.7 | FACT—F | AE+RE (n = 99) Length: 45 min/session Frequency: 12 sessions/week Duration: During treatments Supervised: yes Control (n = 102) Usual care | AE+RE: 4 (10.4) C: 3.2 (12.1) Hedges’ g: 0.07 (−0.21, 0.35) |
| Naraphong 2015 | Inter-treatment (CT) | USA | I–IIIA | AE: 46.4 ± 9.4 C: 47.2 ± 6.9 | Piper Fatigue Scale—Revised (PFS—R) | AE (n = 11) Length: 30–40 min/session Frequency: three to five sessions/week Duration: 10 weeks Supervised: no Control (n = 12) Usual care | AE: 3.62 (2.07) C: 3.38 (2.75) Hedges’ g: 0.09 (−0.72, 0.91) |
| Schmidt 2015 | Inter-treatment (CT) | Germany | I–IV | Total: 52.7 ± 10.0 AE+RE: 52.2 ± 9.9 RX: 53.3 ± 10.2 | Fatigue Assessment Questionnaire (FAQ) | AE+RE (n = 45) Length: 60 min/session Frequency: two sessions/week Duration: 12 weeks Supervised: yes Relaxation (n = 33) Length: 60 min/session Frequency: two sessions/week Duration: 12 weeks | AE: 33.7 (18.8) RX: 41 (21.2) Hedges’ g: AE+RE:RX −0.36 (−0.81, 0.09) |
| Schmidt 2016 | Inter-treatment (CT) | Germany | Early stage | RE: 53 ± 12.6 AE: 56 ± 10.2 C: 54 ± 11.2 | MFI | RE (n = 21) Length: 60 min/session Frequency: two sessions/week Duration: 12 weeks Supervised: yes AE (n = 20) Length: 60 min/session Frequency: two sessions/week Duration: 12 weeks Supervised: yes Control (n = 26) Usual care | RE: 38.62 (17.43) AE: 48 (21.77) C: 43.52 (21.46) Hedges’ g: RE:C −0.24 (−0.82, 0.33) AE:C 0.20 (−0.38, 0.79) RE:AE −0.47 (−1.09, 0.15) |
| Steindorf 2014 | Inter-treatment (RT) | Germany | 0–III | Total: 55.8 ± 9.1 RE: 55.2 ± 9.5 RX: 56.4 ± 8.7 | FAQ | RE (n = 77) Length: 60 min/session Frequency: two sessions/week Duration: 12 weeks Supervised: yes Relaxation (n = 78) Length: 60 min/session Frequency: two sessions/week Duration: 12 weeks | RE: 5.4 (2.3) RX: 5.9 (1.9) Hedges’ g: −0.24 (−0.55, 0.08) |
| Taso 2014 | Inter-treatment (CT) | Taiwan | I–III | Total: 49.3 ± 10.2 | BFI | Yoga (n = 30) Length: 60 min/session Frequency: two sessions/week Duration: eight weeks Supervised: yes Control (n = 30) Usual care | Y: 10.9 (6.9) C: 20.4 (5) Hedges’ g: −1.56 (−2.14, −0.97) |
| van Waart 2015 | Inter-treatment (CT) | Netherlands | I–III | Total: 50.7 ± 9.1 AE+RE: 49.9 ± 8.4 RE: 50.5 ± 10.1 C: 51.6 ± 8.8 | MFI | AE+RE (n = 76) Length: 50 min/session Frequency: two sessions/week Duration: 20 weeks Supervised: yes AE (n = 77) Length: 30 min/session Frequency: five sessions/week Duration: 20 weeks Supervised: no Control (n = 77) Usual care | AE+RE: 13.3 (4.7) AE: 11.7 (4.2) C: 14.7 (4.4) Hedges’ g: AE+RE:C: −0.31 (−0.62, 0.01) AE:C −0.69 (−1.02, −0.37) AE+RE:AE 0.36 (0.04, 0.68) |
| VanderWalde 2020 | Inter-treatment (RT) | USA | 0–III | (Median [range]) RE: 69 [66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84] AE: 68 [65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83] | FSI | RE (n = 25) Length: 30 min/session Frequency: four session/week Duration: six weeks Supervised: no AE (n = 25) Usual care | RE: 0 (8.9) AE: 0 (−1.7) Hedges’ g: −0.14 (−0.69, 0.42) |
| Vadiraja 2009 | Inter-treatment (RT) | India | II–III | NA | EORTC QoL C30 | Yoga (n = 42) Length: 60 min/session Frequency: three sessions/week Duration: six weeks Supervised: yes Control education (n = 33) Length: 15 min/session Frequency: one sessions/10 days Duration: six weeks | Y: 31.37 (21.79) C: 52.09 (24.24) Hedges’ g: −0.90 (−1.37, −0.42) |
| Wang 2011 | Inter-treatment (CT) | USA | I–II | Total: 50.4 ± 9.6 AE: 48.4 ± 10.2 C: 52.3 ±8.8 | FACIT—F | AE (n = 35) Length: 40 min/session Frequency: four sessions/week Duration: six weeks Supervised: no Control (n = 37) Usual care | AE: 45.81 (4.29) C: 39.91 (5.38) Hedges’ g: 1.19 (0.64, 1.74) |
| Wang 2014 | Inter-treatment (CT) | China | NA | NA | CFS | Yoga (n = 40) Length: 50 min/session Frequency: four sessions/week Duration: 12 weeks Supervised: no Control (n = 42) Usual care | Y: 10.22 (2.06) C: 12.79 (2.06) Hedges’ g: −1.24 (−1.71, −0.76) |
| Aydin 2021 | Post-treatment | Turkey | All stage | Total: 45.0 ± 2.2 | EORTC QLQ—C30 | AE+RE (n = 24) Length: AE—50 min/session RE—60 min/session Frequency: AE—three sessions/week RE—two sessions/week Duration: 12 weeks Supervised: yes (AE) Control (n = 24) Usual care | AE+RE: 34.2 (18.2) C: 38.4 (22.9) Hedges’ g: −0.20 (−0.77, −0.37) |
| Baglia 2019 | Post-treatment | USA | 0–III | AE: 62.0 ± 7.0 C: 60.5 ± 7.0 | FACIT—Fatigue (Changed mean and SD are calculated by 95% CI) | AE (n = 48) Length: 50 min/session Frequency: three sessions/week Duration: 12 months Supervised: yes Control Usual care | AE: 0.5 (7.23) C: 5.7 (6.72) Hedges’ g: −0.74 (−1.15, −0.32) |
| Banasik 2011 | Post-treatment | USA | II–IV | Y: 63.3 ± 6.9 C: 62.4 ± 7.3 | Fatigue Score (Likert 0–4) | Yoga (n = 7) Length: 90 min/session Frequency: two sessions/week Duration: eight weeks Supervised: yes Control (n = 7) Regular routine | Y: 1 (0.89) C: 1.57 (0.98) Hedges’ g: −0.57 (−1.65, 0.51) |
| Bower 2012 | Post-treatment | USA | 0–II | Y: 54.4 ± 5.7 C: 53.3 ± 4.9 | FSI | Yoga (n = 16) Length: 90 min/session Frequency: two sessions/week Duration: 12 weeks Supervised: yes Control education (n = 15) Length: 120 min/session Frequency: one session/week Duration: 12 weeks | Y: 3.4 (1.8) C: 4.9 (1.3) Hedges’ g: −0.93 (−1.67, −0.18) |
| Carson 2009 | Post-treatment | USA | IA–IIB | Total: 54.4 ± 7.5 Y: 53.9 ± 9.0 C: 54.9 ± 6.2 | Fatigue Subscale of Daily Menopausal Symptoms (SD is calculated via the t-value) | Yoga (n = 17) Length: 120 min/session Frequency: one session/week Duration: eight weeks Supervised: yes Control (n = 20) Waiting list | Y: 2.87 (0.39) C: 4.34 (0.39) Hedges’ g: −3.69 (−4.79, −2.59) |
| Cohen 2021 | Post-treatment | USA | I–III | Total: 57.3 ± 8.8 RX: 59.7 ± 7.0 AE: 58.6 ± 10.4 AE+RE: 53.6 ± 8.0 | PFS | AE (n = 14) Length: 20 min/session Frequency: three sessions/week Duration: 16 weeks Supervised: yes AE+RE (n = 13) Length: 20 min/session Frequency: three sessions/week Duration: 16 weeks Supervised: yes Relaxation (n = 13) Length: 20 min/session Frequency: three sessions/week Duration: 16 weeks | RX: 3.54 (1.56) AE: 4.15 (1.62) AE+RE: 2.65 (1.2) Hedges’ g: AE:RX 0.37 (−0.39, 1.13) AE+AR:RX −0.62 (−1.40, 0.17) AE+AR:AE 1.01 (0.20, 1.82) |
| Cramer 2015 | Post-treatment | Germany | I–III | Total: 49.2 ± 5.0 Y: 48.3 ± 4.8 C: 50.0 ± 6.7 | FACIT—F | Yoga (n = 19) Length: 90 min/session Frequency: one session/week Duration: 12 weeks Supervised: yes Control (n = 21) Usual care | Y: 42.8 (11.1) C: 37 (8.7) Hedges’ g: 0.57 (−0.06, 1.21) |
| Demello 2018 | Post-treatment | USA | 0–III | Total: 55.6 ± 9.6 | FACIT-F | RE (n = 38) Length: 30 min/session Frequency: one session/week Duration: 12 weeks Supervised: yes Control (n = 21) Usual care | RE: 43.83 (7.28) C: 41.22 (8.49) Hedges’ g: 0.33 (−0.13, 0.78) |
| Dieli-Conwright 2018 | Post-treatment | USA | 0–III | Total: 53.5 ± 10.4 | BFI | AE+RE (n = 50) Length: 30–50 min/session Frequency: three sessions/week Duration: 16 weeks Supervised: yes Control (n = 50) Usual care | AE+RE: 2.9 (1.5) C: 7.7 (2.4) Hedges’ g: −2.38 (−2.90, −1.86) |
| Do 2015 | Post-treatment | Korea | 0–III | AE+RE: 47.1 ± 8.5 C: 48.3 ± 8.2 | FSS | AE+RE (n = 32) Length: 80 min/session Frequency: five sessions/week Duration: four weeks Supervised: yes Control (n = 30) Waiting list | AE: 17.8 (9.6) C: 37.1 (15) Hedges’ g: −1.52 (−2.0, −0.95) |
| Ergun 2013 | Post-treatment | Tukey | Early stage | AE+RE: 49.6 ± 8.3 AE: 55.1 ± 6.9 C: 50.3 ± 10.4 | BFI | AE+RE (n = 20) Length: 75 min/session Frequency: three sessions/week Duration: 12 weeks Supervised: yes AE (n = 18) Length: 30 min/session Frequency: three sessions/week Duration: 12 weeks Supervised: no Control (n = 20) Usual care | AE+RE: 2.86 (2.02) AE: 3.02 (2.5) C: 3.3 (1.79) Hedges’ g: AE+RE:C −0.23 (−0.85, 0.40) AE:C −0.13 (−0.75, 0.49) AE+RE:AE −0.07 (−0.69, 0.55) |
| Gal 2021 | Post-treatment | Netherlands | NA | AE+RE: 58.0 ± 9.8 C:58.3 ± 9.5 | MFI-20 | AE+RE (n = 127) Length: 30 min/session Frequency: two sessions/week Duration: 12 weeks Supervised: yes Control (n = 130) Usual care | AE+RE: 10.4 (4.7) C: 10.3 (4.6) Hedges’ g: 0.02 (−0.24, 0.28) |
| Hagstrom 2016 | Post-treatment | Australia | I–IIIA | Total: 59.1 ± 8.8 RE: 51.2 ± 8.5 C: 52.7 ± 9.4 | FACIT-F | RE (n = 19) Length: 60 min/session Frequency: three sessions/week Duration: 16 weeks Supervised: yes Control (n = 20) Usual care | RE: 45.7 (7.57) C: 39.79 (10.36) Hedges’ g: 0.64 (−0.01, 1.28) |
| Jang 2021 | Post-treatment | Korea | I–III | AE+RE: 49.9 ± 7.9 C: 47.6 ± 7.0 | Korean version of the Revised Piper Fatigue Scale (K-R-PFS) | AE+RE (n = 24) Length: 60 min/session Frequency: one session/week Duration: 12 weeks Supervised: yes Control (n = 20) Usual care | AE+RE: 4.52 (1.93) C: 4.23 (2.18) Hedges’ g: 0.14 (−0.48, 0.76) |
| Kiecolt-Glaser 2014 | Post-treatment | USA | 0–IIIA | Total: 51.6 ± 9.2 Y: 51.8 ± 9.8 C: 51.3 ± 8.7 | Multidimensional Fatigue Symptom Inventory Short Form (MFSI-SF) | Yoga (n = 96) Length: 90 min/session Frequency: two sessions/week Duration: 12 weeks Supervised: yes Control (n = 90) Waiting list | Y: 6.3 (20) C: 12.7 (20) Hedges’ g: −0.32 (−0.61, 0.03) |
| Kim 2020 | Post-treatment | Korea | I–III | RE: 49.9 ± 7.6 C: 48.5 ± 6.8 | K-R-PFS | RE (n = 23) Length: not mentioned Frequency: one session/week Duration: 12 weeks Supervised: yes Control (n = 20) Usual care | RE: 3.89 (1.19) C: 4.88 (1.52) Hedges’ g: −0.71 (−1.30, −0.12) |
| Littman 2012 | Post-treatment | USA | 0–III | Y: 60.6 ± 7.1 C: 58.2 ± 8.8 | FACIT-F | Yoga (n = 30) Length: 75 min/session Frequency: five sessions/week Duration: 26 weeks Supervised: yes Control (n = 27) Usual care | Y: 45 (5.3) C: 43.1 (10.3) Hedges’ g: 0.23 (−0.29, 0.75) |
| Loh 2014 | Post-treatment | Malaysia | I–II | NI (18–65 years) | FACIT-F | Qigong (n = 32) Length: 90 min/session Frequency: one session/week Duration: eight weeks Homework: 30 min/twice a week Supervised: yes AE (n = 31) Length: 90 min/session Frequency: one session/week Duration: eight weeks Supervised: yes Control (n = 32) Usual care | Q: 42.06 (6.04) AE: 41.81 (7.03) C: 40.38 (9.08) Hedges’ g: Q:C 0.22 (−0.28, 0.71) AE:C 0.17 (−0.32, 0.67) Q:AE 0.04 (−0.46, 0.53) |
| Milne 2008 | Post-treatment | Australia | I–II | Total: 55.1 ± 8.2 AE+RE: 55.2 ± 8.4 C: 55.1 ± 8.0 | Schwartz Cancer Fatigue Scale (SCFS) | AE+RE (n = 29) Length: 30 min/session (Aerobic), no information about resistance Frequency: three sessions/week Duration: 12 weeks Supervised: yes Control (n = 29) Waiting list | AE+RE: 11.9 (3.2) C: 17.4 (4.7) Hedges’ g: −1.35 (−1.92, −1.77) |
| Moraes 2021 | Post-treatment | Brazil | NA | Total: 55.1 ± 8.2 RE: 55.2 ± 8.4 C: 55.1 ± 8.0 | PFS | RE (n = 12) Length: not mentioned Frequency: one session/week Duration: eight weeks Supervised: yes Control (n = 13) Waiting list | RE: 2.3 (1.4) C: 3 (2.4) Hedges’ g: −0.34 (−1.13, 0.45) |
| Name 2015 | Post-treatment | Thailand | 0–IIIb | Total: ≤ 60 (n = 13) > 60 (n = 17) | FSI | Qigong (n = 15) Length: 60 min/session Frequency: four sessions/week Duration: 12 weeks Supervised: yes Control (n = 15) Usual care | Q: 17.33 (16.45) C: 28.8 (26.97) Hedges’ g: −0.50 (−1.23, 0.23) |
| Naumann 2012 | Post-treatment | USA | I–III | AE+RE: 49.0 ± 8.2 C: 51.8 ± 11.5 | PFS (Changed score, SD is calculated by SE) | AE+RE (n = 11) Length: 50 min/session Frequency: three sessions/week Duration: eight weeks Supervised: yes Control (n = 10) Usual care | AE+RE: −0.69 (1.49) C: −1.43 (1.33) Hedges’ g: 0.50 (−0.37, 1.37) |
| Ochi 2021 | Post-treatment | Japan | I–II | RE: 49.0 ± 8.2 C: 51.8 ± 11.5 | Cancer Fatigue Scale | RE (n = 24) Length: 30 min/session Frequency: three sessions/week Duration: 12 weeks Supervised: yes Control (n = 24) Usual care | RE: 17.5 (9.84) C: 19.96 (10.29) Hedges’ g: −0.24 (−0.81, 0.33) |
| Pagola 2020 | Post-treatment | Spain | NA | AE+RE: 47 ± 7 RE: 51 ± 6 | PERFORM questionnaire | AE+RE (n = 13) Length: 35 min/session Frequency: one to four sessions/week Duration: 16 weeks Supervised: yes RE (n = 10) Length: 70 min/session Frequency: one to four sessions/week Duration: 16 weeks Supervised: yes | AE+RE: 42 (12) RE: 50 (9) Hedges’ g: 0.74 (−0.12, 1.60) |
| Paulo 2019 | Post-treatment | Brazil | I–III | AE+RE: 63.2 ± 7.1 RX: 66.6 ± 9.6 | EORCT-QLQ-C30 | AE+RE (n = 18) Length: 45 min/session Frequency: two sessions/week Duration: 36 weeks Supervised: yes RX (n = 18) Usual care Invited to participate in stretching and relaxation exercises | AE+RE: 0.6 (2.7) RX: 22.9 (15.8) Hedges’ g: −1.92 (−2.73, −1.12) |
| Pinto 2003 | Post-treatment | USA | 0–II | NA | Profile of Mood States (POMS) | AE+RE (n = 12) Length: 50 min/session Frequency: three sessions/week Duration: 12 weeks Supervised: yes Control (n = 6) Usual care | AE+RE: 9 (6.4) C: 4 (1.8) Hedges’ g: 0.88 (−0.15, 1.91) |
| Pinto 2008 | Post-treatment | USA | 0–II | AE: 53.2 ± 9.1 C: 52.9 ± 10.4 | Fatigue 10 cm linear analog scale (Changed score, SD is calculated by SE) | AE (n = 43) Length: 30 min Frequency: five sessions/week Duration: 12 weeks Supervised: yes Control (n = 43) Usual care | AE: −14.93 (24.72) C: 1.79 (23.48) Hedges’ g: −0.69 (−1.12, −0.25) |
| Rogers 2014 | Post-treatment | USA | I–II | Total: 56.2 ± 7.7 AE+RE: 57.2 ± 5.5 C: 55.2 ± 9.1 | FSI | AE+RE (n = 20) Length: 40 min/session (Aerobic); no information about resistance Frequency: four sessions/week (Aerobic); 2 sessions/week (Resistance) Duration: 12 weeks Supervised: yes Control (n = 22) Usual care | AE+RE: 52.7 (5.4) C: 51.6 (6.9) Hedges’ g: 0.17 (−0.43, 0.78) |
| Rogers 2017 | Post-treatment | USA | I–IIIA | Total: 54.4 ± 8.5 | FSI | AE (n = 110) Length: 50 min/session Frequency: three sessions/week Duration: 12 weeks Supervised: yes Control (n = 112) Usual care | AE: 4 (1.8) C: 4.7 (2) Hedges’ g: −0.37 (−0.63, −0.10) |
| Saarto 2012 | Post-treatment | Finland | NA | AE: 52.3 (range 32–68) C: 52.4 (range 35–68) | FACIT-F (Changed score, SD is calculated by 95% CI) | AE (n = 263) Length: 60 min/session Frequency: one session/week Duration: 52 weeks Supervised: yes Control (n = 237) Usual care | AE: 2.4 (8.69) C: 2.4 (8.64) Hedges’ g: 0 (−0.18, 0.18) |
| Santagenello 2020 | Post-treatment | Brazil | I–III | RE: 52.1 ± 10.1 C: 59.0 ± 9.2 | BFI | RE (n = 11) Length: 40 min/session Frequency: three sessions/week Duration: 12 weeks Supervised: yes Control (n = 9) Usual care | RE: 2.8 (2.1) C: 5.6 (2.5) Hedges’ g: −1.17 (−2.14, −0.20) |
| Schmidt 2017 | Post-treatment | Germany | NA | AE+RE: 61.7 ± 10.0 C: 53.0 ± 10.7 | FACIT-F | AE+RE (n = 21) Length: 60 min/session Frequency: two sessions/week Duration: 12 weeks Supervised: yes Control (n = 28) Usual care | AE+RE: 11.29 (4.15) C: 9.89 (3.82) Hedges’ g: 0.35 (−0.22, 0.92) |
| Stan 2016 | Post-treatment | USA | 0–II | Total: 62.1 ± 8.1 Y: 61.4 ± 7.0 RE: 63.0 ± 9.3 | MFSI-SF (Changed score) | Yoga (n = 18) Length: 88 min/session Frequency: 3(−5) sessions/week Duration: 12 weeks Supervised: no RE (n = 16) Length: 26 min/session Frequency: three to five sessions/week Duration: 12 weeks Supervised: no | Y: −12.3 (14.5) RE: −7.4 (11.1) Hedges’ g: −0.37 (−1.05, 0.31) |
| Taylor 2018 | Post-treatment | USA | Early stage | Y: 54.9 ± 8.8 C: 52.6 ± 8.2 | BFI | Yoga (n = 9) Length: 75 min/session Frequency: one session/week Duration: eight weeks Supervised: yes Control (n = 11) Waiting list | Y: 1.85 (1.61) C: 2.1 (2.68) Hedges’ g: −0.11 (−0.99, 0.78) |
| Winters-Stone 2012 | Post-treatment | USA | 0–IIIA | Y: 68.6 ± 6.2 RX: 68.9 ± 2.9 | Schwartz Cancer Fatigue (SCF) | RE (n = 36) Length: 60 min/session Frequency: three sessions/week (two 1 hr sessions were supervised; 1 hr session was home-based) Duration: 52 weeks Supervised: yes Relaxation (n = 31) Length: 60 min/session Frequency: three sessions/week (two 1 hr sessions were supervised and 1 hr session was home-based) Duration: 52 weeks | RE: 10.1 (4.7) RX: 9 (3.21) Hedges’ g: 0.27 (−0.22, 0.75) |
| Yagi 2015 | Post-treatment | Turkey | I–II | RE: 62.3 ± 6.7 RX: 62.2 ± 2.9 | Fatigue Visual Analog Scale (VAS) | Yoga (n = 10) Length: 60 min/session Frequency: one session/week Duration: eight weeks Supervised: yes RE (n = 10) Length: 60 min/session Frequency: one session/week Duration: eight weeks | Y: 2.86 (1.31) RE: 4.28 (0.97) Hedges’ g: −1.18 (−2.15, −0.21) |
| Yaʇli 2015 | Post-treatment | Turkey | I–II | AE: 47.4 ± 7.6 Y+AE: 49.9 ± 4.7 | FSS | AE (n = 21) Length: 30 min/session Frequency: three sessions/week Duration: six weeks Supervised: yes AE + Yoga (n = 19) Length: 30 min/session (Aerobic); 30 min/session Frequency: three sessions/week Duration: six weeks Supervised: yes | AE: 40.14 (7.58) Y+AE: 35.74 (5.99) Hedges’ g: 0.63 (−0.01, 1.26) |
| Yuen 2007 | Post-treatment | USA | NA | AE: 53.1 ± 13.5 RE: 53.7 ± 11.3 C: 55.0 ± 13.4 | PFS | AE (n = 8) Length: 20–40 min/session Frequency: three sessions/week Duration: 12 weeks Supervised: yes RE (n = 7) Length: No information Frequency: three sessions/week Duration: 12 weeks Supervised: yes Control (n = 7) Usual care | AE: 3.9 (1.71) RE: 2.79 (1.85) C: 4.16 (1.67) Hedges’ g: AE:C −0.14 (−1.16, 0.87) RE:C −0.73 (−1.82, 0.37) AE:RE 0.59 (−0.46, 1.63) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.-C.; Hung, T.-T.; Konara Mudiyanselage, S.P.; Wang, C.-J.; Lin, M.-F. Beneficial Exercises for Cancer-Related Fatigue among Women with Breast Cancer: A Systematic Review and Network Meta-Analysis. Cancers 2023, 15, 151. https://doi.org/10.3390/cancers15010151
Liu Y-C, Hung T-T, Konara Mudiyanselage SP, Wang C-J, Lin M-F. Beneficial Exercises for Cancer-Related Fatigue among Women with Breast Cancer: A Systematic Review and Network Meta-Analysis. Cancers. 2023; 15(1):151. https://doi.org/10.3390/cancers15010151
Chicago/Turabian StyleLiu, Yu-Chen, Tsai-Tzu Hung, Sriyani Padmalatha Konara Mudiyanselage, Chi-Jane Wang, and Mei-Feng Lin. 2023. "Beneficial Exercises for Cancer-Related Fatigue among Women with Breast Cancer: A Systematic Review and Network Meta-Analysis" Cancers 15, no. 1: 151. https://doi.org/10.3390/cancers15010151
APA StyleLiu, Y.-C., Hung, T.-T., Konara Mudiyanselage, S. P., Wang, C.-J., & Lin, M.-F. (2023). Beneficial Exercises for Cancer-Related Fatigue among Women with Breast Cancer: A Systematic Review and Network Meta-Analysis. Cancers, 15(1), 151. https://doi.org/10.3390/cancers15010151







