An Ecologic Study of the Association between 1,3-Dichloropropene and Pancreatic Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources
2.2. Variable Definitions
2.3. Statistical Analysis
3. Results
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boyd, C. Commonly Used Pesticides Explained: 1,3-Dichloropropene. Available online: https://www.chemservice.com/news/commonly-used-pesticides-explained-13-dichloropropene/ (accessed on 1 November 2022).
- Hays, S.M.; Nelson, D.M.; Kirman, C.R. Peer review of a cancer weight of evidence assessment based on updated toxicokinetics, genotoxicity, and carcinogenicity data for 1,3-dichloropropene using a blinded, virtual panel of experts. Crit. Rev. Toxicol. 2020, 50, 861–884. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.J.; Bartels, M.; Gollapudi, B.; Driver, J.; Himmelstein, M.; Gehen, S.; Juberg, D.; van Wesenbeeck, I.; Terry, C.; Rasoulpour, R. Weight of evidence analysis of the tumorigenic potential of 1,3-dichloropropene supports a threshold-based risk assessment. Crit. Rev. Toxicol. 2020, 50, 836–860. [Google Scholar] [CrossRef] [PubMed]
- Clary, T.; Ritz, B. Pancreatic cancer mortality and organochlorine pesticide exposure in California, 1989–1996. Am. J. Ind. Med. 2003, 43, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Thelin, G.P.; Stone, W.W. Estimation of Annual Agricultural Pesticide Use for Counties of the Conterminous United States, 1992–2009; U.S. Geological Survey Scientific Investigations Report 2013-5009; U.S. Geological Survey: Sacramento, CA, USA, 2013; 54p. [Google Scholar]
- Baker, N.T. Agricultural Pesticide Use Estimates for the USGS National Water Quality Network, 1992–2014. Available online: https://www.usgs.gov/data/agricultural-pesticide-use-estimates-usgs-national-water-quality-network-1992-2014 (accessed on 11 November 2022).
- Klein, A.P. Pancreatic cancer epidemiology: Understanding the role of lifestyle and inherited risk factors. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Division of Population Health. BRFSS Prevalence & Trends Data. 2015. Available online: https://www.cdc.gov/brfss/brfssprevalence/ (accessed on 30 October 2022).
- Hernandez, A.F.; Martin-Rubi, J.C.; Ballesteros, J.L.; Oliver, M.; Pla, A.; Villanueva, E. Clinical and pathological findings in fatal 1,3-dichloropropene intoxication. Hum. Exp. Toxicol. 1994, 13, 303–306. [Google Scholar] [CrossRef] [PubMed]
- Ji, B.T.; Silverman, D.T.; Stewart, P.A.; Blair, A.; Swanson, G.M.; Baris, D.; Greenberg, R.S.; Hayes, R.B.; Brown, L.M.; Lillemoe, K.D.; et al. Occupational exposure to pesticides and pancreatic cancer. Am. J. Ind. Med. 2001, 39, 92–99, Erratum in 2001, 40, 225–226. [Google Scholar] [CrossRef]
- Lo, A.C.; Soliman, A.S.; El-Ghawalby, N.; Abdel-Wahab, M.; Fathy, O.; Khaled, H.M.; Omar, S.; Hamilton, S.R.; Greenson, J.K.; Abbruzzese, J.L. Lifestyle, occupational, and reproductive factors in relation to pancreatic cancer risk. Pancreas 2007, 35, 120–129. [Google Scholar] [CrossRef]
- Andreotti, G.; Freeman, L.E.; Hou, L.; Coble, J.; Rusiecki, J.; Hoppin, J.A.; Silverman, D.T.; Alavanja, M.C. Agricultural pesticide use and pancreatic cancer risk in the Agricultural Health Study Cohort. Int. J. Cancer 2009, 124, 2495–2500. [Google Scholar] [CrossRef]
- Louis, L.M.; Lerro, C.C.; Friesen, M.C.; Andreotti, G.; Koutros, S.; Sandler, D.P.; Blair, A.; Robson, M.G.; Beane Freeman, L.E. A prospective study of cancer risk among Agricultural Health Study farm spouses associated with personal use of organochlorine insecticides. Environ. Health 2017, 16, 95. [Google Scholar] [CrossRef]
- Antwi, S.O.; Eckert, E.C.; Sabaque, C.V.; Leof, E.R.; Hawthorne, K.M.; Bamlet, W.R.; Chaffee, K.G.; Oberg, A.L.; Petersen, G.M. Exposure to environmental chemicals and heavy metals, and risk of pancreatic cancer. Cancer Causes Control 2015, 26, 1583–1591. [Google Scholar] [CrossRef]
- Fryzek, J.P.; Garabrant, D.H.; Harlow, S.D.; Severson, R.K.; Gillespie, B.W.; Schenk, M.; Schottenfeld, D. A case-control study of self-reported exposures to pesticides and pancreas cancer in southeastern Michigan. Int. J. Cancer 1997, 72, 62–67. [Google Scholar] [CrossRef]
- Hoppin, J.A.; Tolbert, P.E.; Holly, E.A.; Brock, J.W.; Korrick, S.A.; Altshul, L.M.; Zhang, R.H.; Bracci, P.M.; Burse, V.W.; Needham, L.L. Pancreatic cancer and serum organochlorine levels. Cancer Epidemiol. Biomark. Prev. 2000, 9, 199–205. [Google Scholar]
- De Basea, M.B.; Porta, M.; Alguacil, J.; Puigdomènech, E.; Gasull, M.; Garrido, J.A.; López, T.; PANKRAS II Study Group. Relationships between occupational history and serum concentrations of organochlorine compounds in exocrine pancreatic cancer. Occup. Environ. Med. 2011, 68, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Luo, J. KRAS mutation in pancreatic cancer. Semin. Oncol. 2021, 48, 10–18. [Google Scholar] [CrossRef]
- Porta, M.; Pumarega, J.; Amaral, A.F.; Genkinger, J.M.; Camargo, J.; Mucci, L.; Alguacil, J.; Gasull, M.; Zhang, X.; Morales, E.; et al. Influence of KRAS mutations, persistent organic pollutants, and trace elements on survival from pancreatic ductal adenocarcinoma. Environ. Res. 2020, 190, 109781. [Google Scholar] [CrossRef]
- Porta, M.; Malats, N.; Jariod, M.; Grimalt, J.O.; Rifà, J.; Carrato, A.; Guarner, L.; Salas, A.; Santiago-Silva, M.; Corominas, J.M.; et al. Serum concentrations of organochlorine compounds and K-ras mutations in exocrine pancreatic cancer. PANKRAS II Study Group. Lancet 1999, 354, 2125–2129. [Google Scholar] [CrossRef]
- López, T.; Pumarega, J.A.; Pollack, A.Z.; Lee, D.H.; Richiardi, L.; Jacobs, D.R., Jr.; Schisterman, E.F.; Porta, M. Adjusting serum concentrations of organochlorine compounds by lipids and symptoms: A causal framework for the association with K-ras mutations in pancreatic cancer. Chemosphere 2014, 114, 219–225. [Google Scholar] [CrossRef]
- Schneider, M.; Quistad, G.B.; Casida, J.E. 1,3-Dichloropropene epoxides: Intermediates in bioactivation of the promutagen 1,3-dichloropropene. Chem. Res. Toxicol. 1998, 11, 1137–1144. [Google Scholar] [CrossRef]
- Veith, A.; Moorthy, B. Role of Cytochrome P450s in the Generation and Metabolism of Reactive Oxygen Species. Curr. Opin. Toxicol. 2018, 7, 44–51. [Google Scholar] [CrossRef]
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative Stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef] [PubMed]
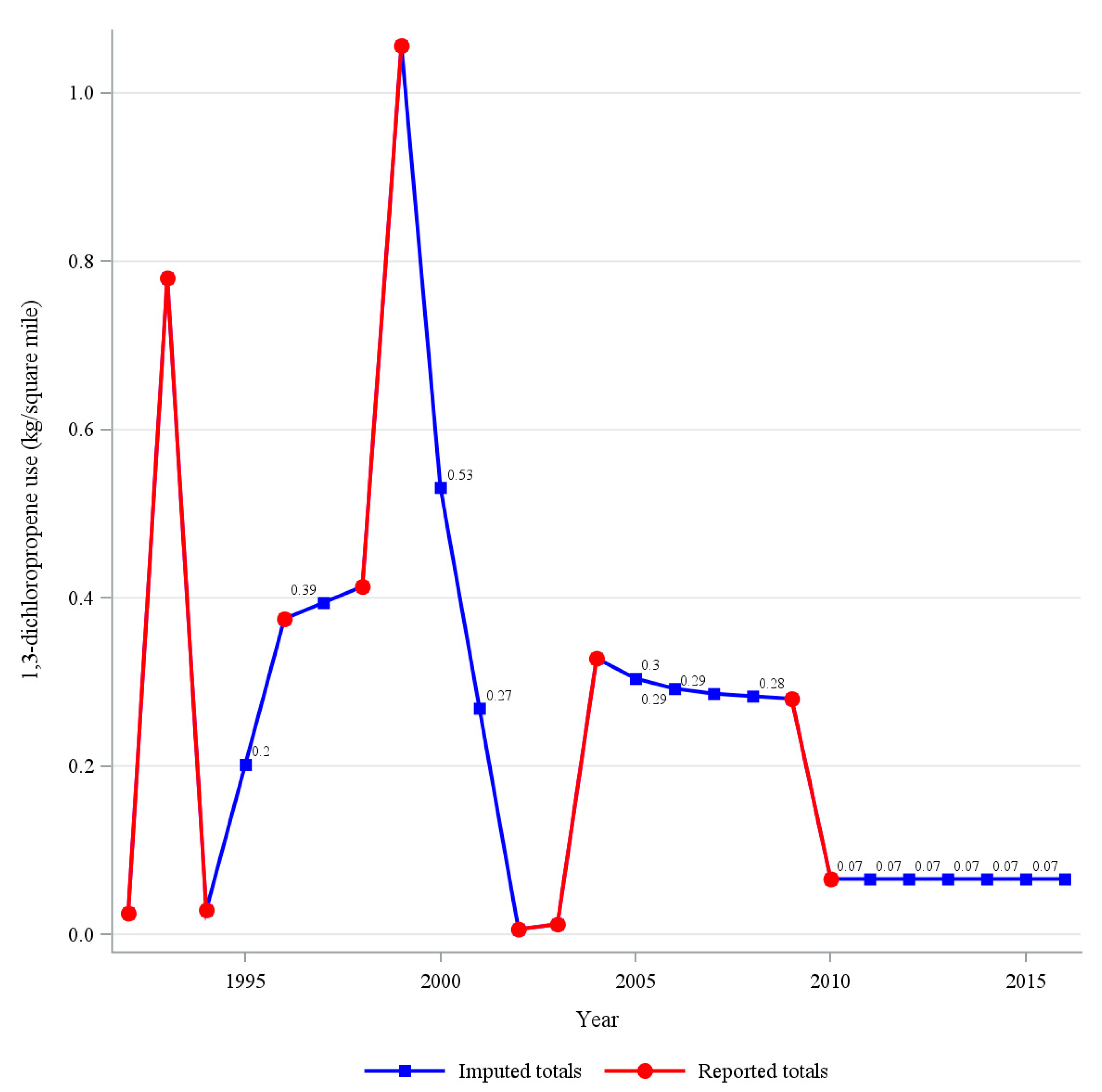
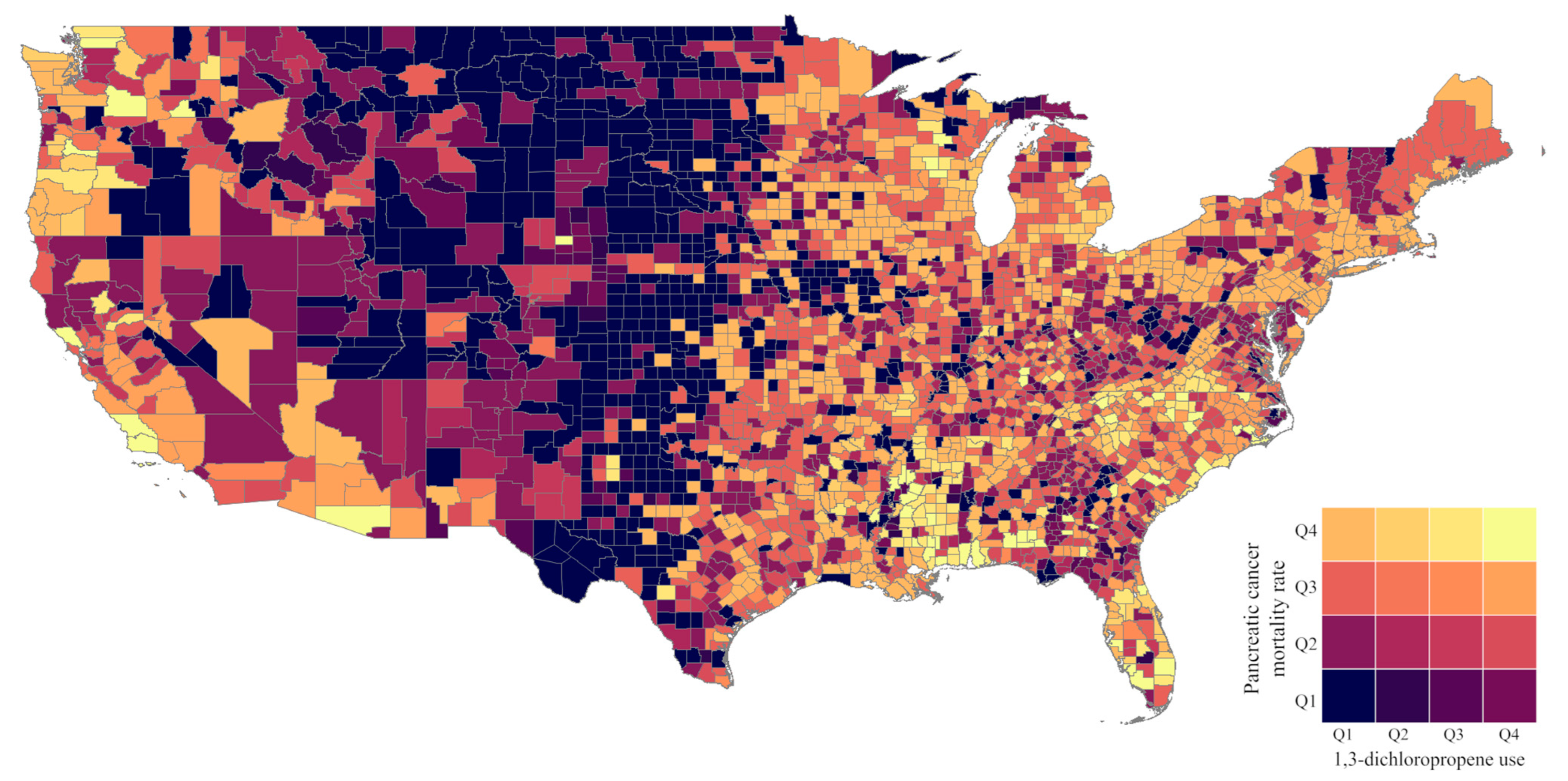
| Years 1,3-D Use Reported | Median (kg/sq Mile) | 25th Percentile (kg/sq Mile) | 75th Percentile (kg/sq Mile) | |
|---|---|---|---|---|
| Overall | 5-year lag (n = 646) | 2.12 | 0.40 | 7.94 |
| 10-year lag (n = 583) | 2.03 | 0.41 | 7.63 | |
| 15-year lag (n = 425) | 2.17 | 0.47 | 7.30 | |
| 20-year lag (n = 271) | 2.52 | 0.47 | 7.88 | |
| 0–9 | 5-year lag (n = 36) | 0.99 | 0.09 | 6.23 |
| 10-year lag (n = 43) | 1.01 | 0.12 | 7.57 | |
| 15-year lag (n = 35) | 0.96 | 0.12 | 7.57 | |
| 20-year lag (n = 26) | 0.42 | 0.05 | 7.57 | |
| 10–14 | 5-year lag (n = 48) | 0.27 | 0.08 | 0.72 |
| 10-year lag (n = 51) | 0.27 | 0.11 | 0.84 | |
| 15-year lag (n = 37) | 0.30 | 0.14 | 1.47 | |
| 20-year lag (n = 26) | 0.29 | 0.14 | 0.84 | |
| 15–19 | 5-year lag (n = 75) | 0.42 | 0.09 | 1.64 |
| 10-year lag (n = 68) | 0.51 | 0.12 | 1.78 | |
| 15-year lag (n = 44) | 0.71 | 0.12 | 2.85 | |
| 20-year lag (n = 20) | 1.08 | 0.09 | 5.80 | |
| 20–24 | 5-year lag (n = 113) | 2.02 | 0.70 | 2.83 |
| 10-year lag (n = 98) | 2.03 | 0.75 | 4.04 | |
| 15-year lag (n = 71) | 1.97 | 0.77 | 2.59 | |
| 20-year lag (n = 46) | 1.84 | 0.93 | 3.77 | |
| 25 | 5-year lag (n = 374) | 4.77 | 0.68 | 13.32 |
| 10-year lag (n = 323) | 4.88 | 0.74 | 12.54 | |
| 15-year lag (n = 238) | 4.55 | 0.80 | 12.11 | |
| 20-year lag (n = 153) | 4.33 | 1.12 | 11.68 |
| Years 1,3-D Use Reported | Median (per 100,000 Persons) | 25th Percentile (per 100,000 Persons) | 75th Percentile (per 100,000 Persons) |
|---|---|---|---|
| Overall | 12.75 | 11.32 | 14.27 |
| Years of Use Reported | |||
| 0–9 | 13.50 | 12.43 | 14.85 |
| 10–14 | 13.68 | 12.09 | 15.21 |
| 15–19 | 12.62 | 11.59 | 14.16 |
| 20–24 | 12.34 | 11.28 | 14.25 |
| 25 | 11.61 | 10.23 | 13.24 |
| 1,3-D Use Quartiles- | All States | States Reporting 20+ Years of 1,3-D Use | ||
|---|---|---|---|---|
| Complete † Data | Imputed Data | Complete Data | Imputed Data | |
| RR (95% CI) | RR (95% CI) | RR (95% CI) | RR (95% CI) | |
| 5-YEAR LAG | ||||
| ≤0.40 kg/sq mi | Referent | Referent | Referent | Referent |
| 0.41–2.2 kg/sq mile | 0.97 (0.94–1.00) | 0.98 (0.95–1.00) | 0.98 (0.93–1.03) | 0.99 (0.94–1.03) |
| 2.3–7.9 kg/sq mile | 1.00 (0.97–1.03) | 0.97 (0.95–1.00) | 1.03 (0.98–1.09) | 1.05 (1.00–1.10) |
| ≥8 kg/sq mile | 1.06 (1.02–1.09) | 1.02 (0.99–1.05) | 1.10 (1.05–1.15) | 1.11 (1.06–1.16) |
| 10-YEAR LAG | ||||
| ≤0.40 kg/sq mi | Referent | Referent | Referent | Referent |
| 0.41–2.2 kg/sq mile | 0.96 (0.93–0.99) | 0.99 (0.96–1.01) | 0.99 (0.94–1.04) | 0.99 (0.95–1.04) |
| 2.3–7.9 kg/sq mile | 0.96 (0.93–1.00) | 0.96 (0.94–0.99) | 1.02 (0.97–1.08) | 1.03 (0.98–1.08) |
| ≥8 kg/sq mile | 1.03 (0.99–1.07) | 1.02 (0.99–1.05) | 1.11 (1.05–1.16) | 1.11 (1.06–1.16) |
| 15-YEAR LAG | ||||
| ≤0.40 kg/sq mi | Referent | Referent | Referent | Referent |
| 0.41–2.2 kg/sq mile | 0.96 (0.93–1.00) | 0.98 (0.95–1.01) | 0.98 (0.92–1.04) | 0.98 (0.93–1.04) |
| 2.3–7.9 kg/sq mile | 0.98 (0.95–1.02) | 0.97 (0.95–1.00) | 1.03 (0.98–1.10) | 1.04 (0.98–1.09) |
| ≥8 kg/sq mile | 1.01 (0.97–1.05) | 1.00 (0.97–1.04) | 1.07 (1.01–1.14) | 1.08 (1.02–1.14) |
| 20-YEAR LAG | ||||
| ≤0.40 kg/sq mi | Referent | Referent | Referent | Referent |
| 0.41–2.2 kg/sq mile | 0.92 (0.88–0.96) | 0.94 (0.91–0.97) | 0.98 (0.91–1.05) | 0.98 (0.92–1.04) |
| 2.3–7.9 kg/sq mile | 0.98 (0.94–1.03) | 0.98 (0.95–1.01) | 1.08 (1.00–1.15) | 1.07 (1.00–1.14) |
| ≥8 kg/sq mile | 1.00 (0.96–1.04) | 0.99 (0.95–1.02) | 1.09 (1.02–1.17) | 1.09 (1.02–1.16) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McGwin, G., Jr.; Griffin, R.L. An Ecologic Study of the Association between 1,3-Dichloropropene and Pancreatic Cancer. Cancers 2023, 15, 150. https://doi.org/10.3390/cancers15010150
McGwin G Jr., Griffin RL. An Ecologic Study of the Association between 1,3-Dichloropropene and Pancreatic Cancer. Cancers. 2023; 15(1):150. https://doi.org/10.3390/cancers15010150
Chicago/Turabian StyleMcGwin, Gerald, Jr., and Russell L Griffin. 2023. "An Ecologic Study of the Association between 1,3-Dichloropropene and Pancreatic Cancer" Cancers 15, no. 1: 150. https://doi.org/10.3390/cancers15010150
APA StyleMcGwin, G., Jr., & Griffin, R. L. (2023). An Ecologic Study of the Association between 1,3-Dichloropropene and Pancreatic Cancer. Cancers, 15(1), 150. https://doi.org/10.3390/cancers15010150





