Endoscopic Ultrasound-Guided Laser Ablation Using a Diffusing Applicator for Locally Advanced Pancreatic Cancer Treatment
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
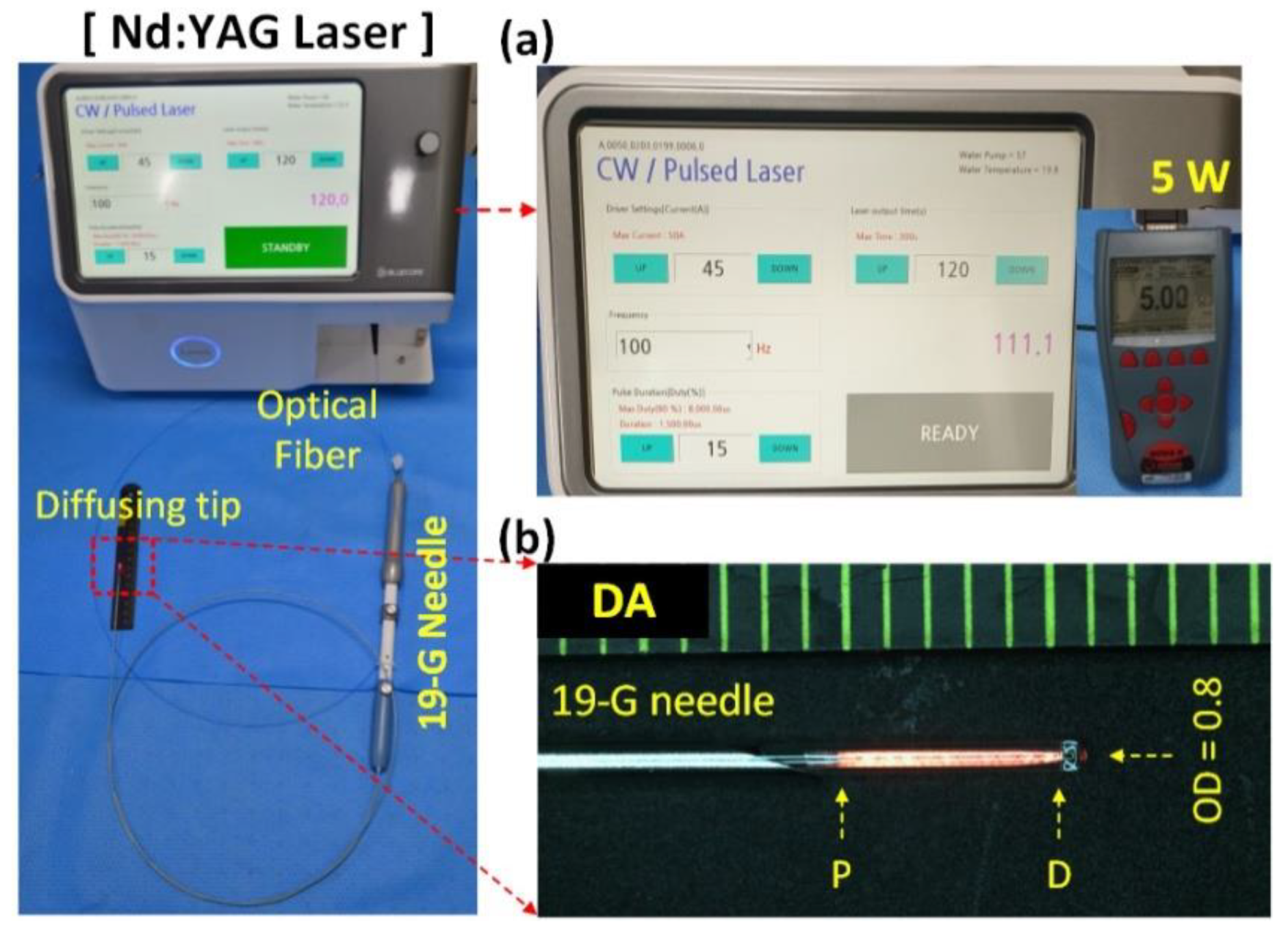
2.1. Devices and Laser System
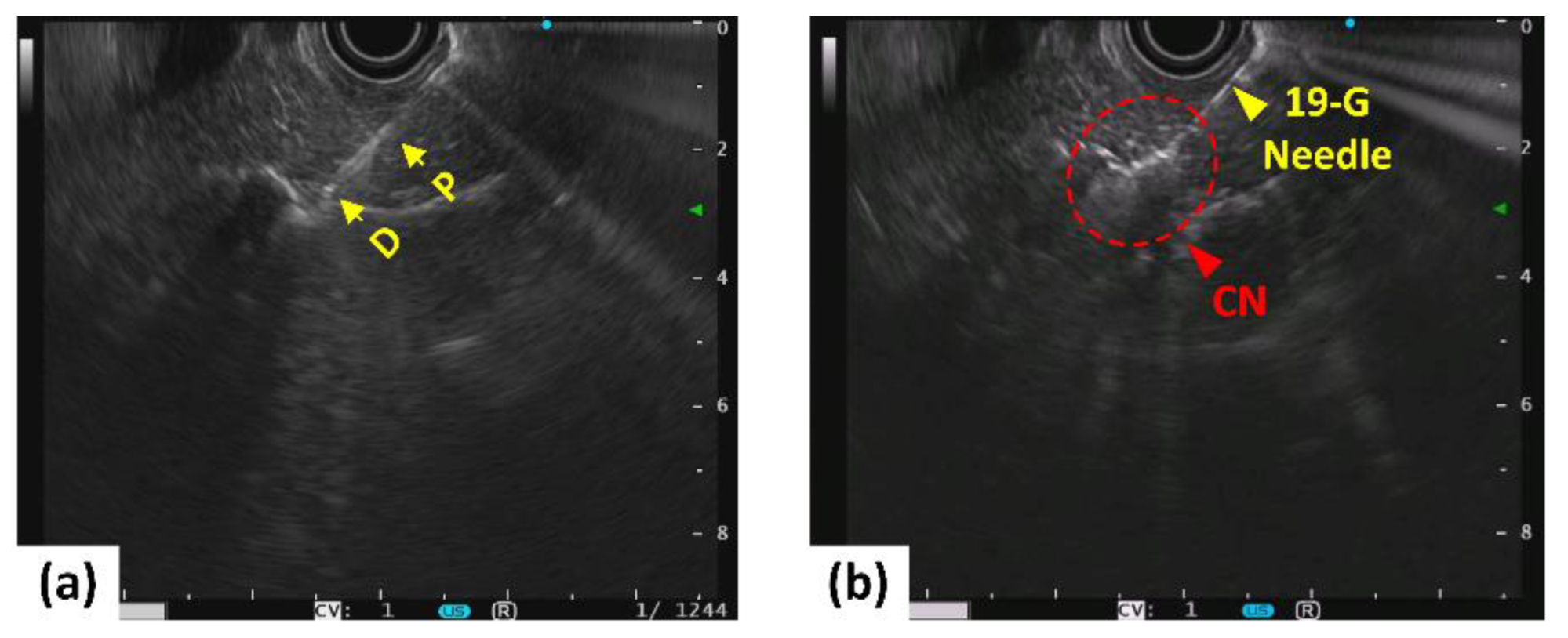
2.2. In Vivo Porcine Tests
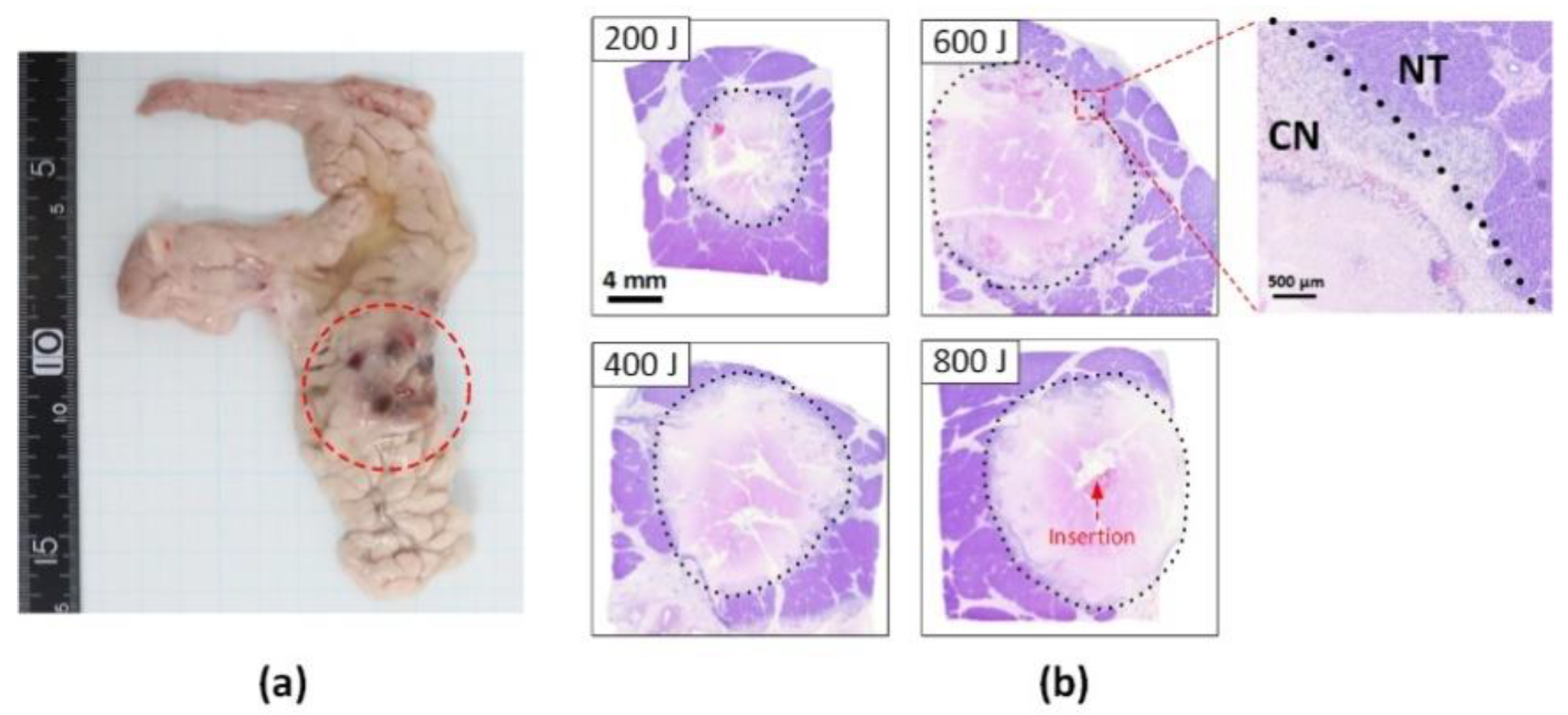
2.3. Histological Analysis
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McGuigan, A.; Kelly, P.; Turkington, R.C.; Jones, C.; Coleman, H.G.; McCain, R.S. Pancreatic cancer: A review of clinical diagnosis, epidemiology, treatment and outcomes. World J. Gastroenterol. 2018, 24, 4846. [Google Scholar] [CrossRef] [PubMed]
- Ishii, Y.; Serikawa, M.; Tsuboi, T.; Kawamura, R.; Tsushima, K.; Nakamura, S.; Hirano, T.; Fukiage, A.; Mori, T.; Ikemoto, J. Role of Endoscopic Ultrasonography and Endoscopic Retrograde Cholangiopancreatography in the Diagnosis of Pancreatic Cancer. Diagnostics 2021, 11, 238. [Google Scholar] [CrossRef] [PubMed]
- Heger, U.; Hackert, T. Can local ablative techniques replace surgery for locally advanced pancreatic cancer? J. Gastrointest. Oncol. 2021, 12, 2536. [Google Scholar] [CrossRef] [PubMed]
- Paiella, S.; Casetti, L.; Ewald, J.; Marchese, U.; D’Onofrio, M.; Garnier, J.; Landoni, L.; Gilabert, M.; Manzini, G.; Esposito, A. Laser treatment of pancreatic cancer with immunostimulating interstitial laser thermotherapy protocol: Safety and feasibility results from two phase 2a studies. J. Surg. Res. 2021, 259, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Suker, M.; Beumer, B.R.; Sadot, E.; Marthey, L.; Faris, J.E.; Mellon, E.A.; El-Rayes, B.F.; Wang-Gillam, A.; Lacy, J.; Hosein, P.J. FOLFIRINOX for locally advanced pancreatic cancer: A systematic review and patient-level meta-analysis. Lancet Oncol. 2016, 17, 801–810. [Google Scholar] [CrossRef]
- Fegrachi, S.; Besselink, M.G.; van Santvoort, H.C.; van Hillegersberg, R.; Molenaar, I.Q. Radiofrequency ablation for unresectable locally advanced pancreatic cancer: A systematic review. HPB 2014, 16, 119–123. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Di Matteo, F.M.; Saccomandi, P.; Martino, M.; Pandolfi, M.; Pizzicannella, M.; Balassone, V.; Schena, E.; Pacella, C.M.; Silvestri, S.; Costamagna, G. Feasibility of EUS-guided Nd: YAG laser ablation of unresectable pancreatic adenocarcinoma. Gastrointest. Endosc. 2018, 88, 168–174.e161. [Google Scholar] [CrossRef]
- Tian, G.; Liu, X.; Zhao, Q.; Xu, D.; Jiang, T.A. Irreversible electroporation in patients with pancreatic cancer: How important is the new weapon? BioMed Res. Int. 2018, 2018, 5193067. [Google Scholar] [CrossRef]
- Truong, V.G.; Jeong, S.; Park, J.-S.; Kim, S.M.; Lee, D.H.; Kang, H.W. Endoscopic ultrasound (EUS)-guided cylindrical interstitial laser ablation (CILA) on in vivo porcine pancreas. Biomed. Opt. Express 2021, 12, 4423–4437. [Google Scholar] [CrossRef]
- Truong, V.G.; Kim, H.; Park, J.-S.; Tran, V.N.; Kang, H.W. Multiple cylindrical interstitial laser ablations (CILAs) of porcine pancreas in ex vivo and in vivo models. Int. J. Hyperth. 2021, 38, 1313–1321. [Google Scholar] [CrossRef]
- Barret, M.; Leblanc, S.; Rouquette, A.; Chaussade, S.; Terris, B.; Prat, F. EUS-guided pancreatic radiofrequency ablation: Preclinical comparison of two currently available devices in a pig model. Endosc. Int. Open 2019, 7, E138–E143. [Google Scholar] [CrossRef] [PubMed]
- Larghi, A.; Rimbaș, M.; Rizzatti, G.; Carbone, C.; Gasbarrini, A.; Costamagna, G.; Alfieri, S.; Tortora, G. Endoscopic ultrasound-guided therapies for pancreatic solid tumors: An overview. Semin. Oncol. 2021, 48, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Crinò, S.F.; D’Onofrio, M.; Bernardoni, L.; Frulloni, L.; Iannelli, M.; Malleo, G.; Paiella, S.; Larghi, A.; Gabbrielli, A. EUS-guided radiofrequency ablation (EUS-RFA) of solid pancreatic neoplasm using an 18-gauge needle electrode: Feasibility, safety, and technical success. J. Gastrointestin. Liver Dis. 2018, 27, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Saccomandi, P.; Lapergola, A.; Longo, F.; Schena, E.; Quero, G. Thermal ablation of pancreatic cancer: A systematic literature review of clinical practice and pre-clinical studies. Int. J. Hyperth. 2018, 35, 398–418. [Google Scholar] [CrossRef]
- Di Matteo, F.; Martino, M.; Rea, R.; Pandolfi, M.; Rabitti, C.; Masselli, G.M.P.; Silvestri, S.; Pacella, C.M.; Papini, E.; Panzera, F. EUS-guided Nd: YAG laser ablation of normal pancreatic tissue: A pilot study in a pig model. Gastrointest. Endosc. 2010, 72, 358–363. [Google Scholar] [CrossRef]
- Di Matteo, F.; Martino, M.; Rea, R.; Pandolfi, M.; Panzera, F.; Stigliano, E.; Schena, E.; Saccomandi, P.; Silvestri, S.; Pacella, C.M. US-guided application of Nd: YAG laser in porcine pancreatic tissue: An ex vivo study and numerical simulation. Gastrointest. Endosc. 2013, 78, 750–755. [Google Scholar] [CrossRef]
- Jiang, T.A.; Chai, W. Endoscopic ultrasonography (EUS)-guided laser ablation (LA) of adrenal metastasis from pancreatic adenocarcinoma. Lasers Med. Sci. 2018, 33, 1613–1616. [Google Scholar] [CrossRef]
- Lekht, I.; Gulati, M.; Nayyar, M.; Katz, M.D.; Ter-Oganesyan, R.; Marx, M.; Cen, S.Y.; Grant, E. Role of contrast-enhanced ultrasound (CEUS) in evaluation of thermal ablation zone. Abdom. Radiol. 2016, 41, 1511–1521. [Google Scholar] [CrossRef]
- Dietrich, C.F.; Averkiou, M.; Nielsen, M.B.; Barr, R.G.; Burns, P.N.; Calliada, F.; Cantisani, V.; Choi, B.; Chammas, M.C.; Clevert, D.-A. How to perform contrast-enhanced ultrasound (CEUS). Ultrasound Int. Open 2018, 4, E2–E15. [Google Scholar] [CrossRef]
- Hank, T.; Hinz, U.; Tarantino, I.; Kaiser, J.; Niesen, W.; Bergmann, F.; Hackert, T.; Büchler, M.W.; Strobel, O. Validation of at least 1 mm as cut-off for resection margins for pancreatic adenocarcinoma of the body and tail. J. Br. Surg. 2018, 105, 1171–1181. [Google Scholar] [CrossRef]
- Sung, H.Y.; Jung, S.E.; Cho, S.H.; Zhou, K.; Han, J.-Y.; Han, S.T.; Kim, J.I.; Kim, J.K.; Choi, J.Y.; Yoon, S.K. Long-term outcome of high-intensity focused ultrasound in advanced pancreatic cancer. Pancreas 2011, 40, 1080–1086. [Google Scholar] [CrossRef]
- Lee, J.M.; Choi, H.S.; Chun, H.J.; Kim, E.S.; Keum, B.; Seo, Y.S.; Jeen, Y.T.; Lee, H.S.; Um, S.H.; Kim, C.D. EUS-guided irreversible electroporation using endoscopic needle-electrode in porcine pancreas. Surg. Endosc. 2019, 33, 658–662. [Google Scholar] [CrossRef] [PubMed]
- Giardino, A.; Girelli, R.; Frigerio, I.; Regi, P.; Cantore, M.; Alessandra, A.; Lusenti, A.; Salvia, R.; Bassi, C.; Pederzoli, P. Triple approach strategy for patients with locally advanced pancreatic carcinoma. HPB 2013, 15, 623–627. [Google Scholar] [CrossRef] [PubMed]
- D’Onofrio, M.; Barbi, E.; Girelli, R.; Martini, P.T.; De Robertis, R.; Ciaravino, V.; Salvia, R.; Butturini, G.; Frigerio, I.; Milazzo, T. Variation of tumoral marker after radiofrequency ablation of pancreatic adenocarcinoma. J. Gastrointest. Oncol. 2016, 7, 213. [Google Scholar] [PubMed]


| Mean (Range) | |||
|---|---|---|---|
| Pre-op | Post-op | Before Euthanasia | |
| WBCB (×103 cells/μL) | 22.0 (15.1–31.6) | 21.1 (13.6–29.6) | 22.6 (15.4–32.2) |
| RBC (×106 cells/μL) | 6.1 (5.6–6.7) | 5.9 (5.1–6.7) | 6.2 (5.5–6.9) |
| measHGB (g/dL) | 9.6 (8.8–11.1) | 9.3 (8.5–10.3) | 9.8 (8.3–11.5) |
| PLT (×103 cells/μL) | 451.4 (249.0–686.0) | 400.1 (155.0–603.0) | 421.1 (168.0–595.0) |
| Creatinine (mg/dL) | 0.9 (0.7–1.1) | 1.1 (0.8–1.7) | 1.2 (0.2–3.0) |
| T. Bil (mg/dL) | 0.6 (0.2–1.0) | 0.5 (0.2–0.9) | 0.6 (0.2–1.1) |
| Amylase (U/L) | 2234.1 (1539.0–3310.0) | 1998.0 (1511.0–2780.0) | 3789.4 (1435.0–9410.0) |
| Lipase (U/L) | 7.0 (1.2–11.7) | 6.5 (1.6–17.0) | 156.8 (10.5–617.4) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, S.; Truong, V.G.; Choi, J.; Jeong, H.J.; Oh, S.-J.; Park, J.-S.; Kang, H.W. Endoscopic Ultrasound-Guided Laser Ablation Using a Diffusing Applicator for Locally Advanced Pancreatic Cancer Treatment. Cancers 2022, 14, 2274. https://doi.org/10.3390/cancers14092274
Lim S, Truong VG, Choi J, Jeong HJ, Oh S-J, Park J-S, Kang HW. Endoscopic Ultrasound-Guided Laser Ablation Using a Diffusing Applicator for Locally Advanced Pancreatic Cancer Treatment. Cancers. 2022; 14(9):2274. https://doi.org/10.3390/cancers14092274
Chicago/Turabian StyleLim, Seonghee, Van Gia Truong, Jongman Choi, Hye Jung Jeong, Sun-Ju Oh, Jin-Seok Park, and Hyun Wook Kang. 2022. "Endoscopic Ultrasound-Guided Laser Ablation Using a Diffusing Applicator for Locally Advanced Pancreatic Cancer Treatment" Cancers 14, no. 9: 2274. https://doi.org/10.3390/cancers14092274
APA StyleLim, S., Truong, V. G., Choi, J., Jeong, H. J., Oh, S.-J., Park, J.-S., & Kang, H. W. (2022). Endoscopic Ultrasound-Guided Laser Ablation Using a Diffusing Applicator for Locally Advanced Pancreatic Cancer Treatment. Cancers, 14(9), 2274. https://doi.org/10.3390/cancers14092274






