Quantification of Nanoscale Dose Enhancement in Gold Nanoparticle-Aided External Photon Beam Radiotherapy
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Simulation Details
2.2. Photon Beam Modelling
2.3. AuNPs Modelling
2.4. Dosimetry Details
2.5. Investigated Parameters
2.5.1. AuNPs Size
2.5.2. Photon Field Size and Depth inside the Water Phantom
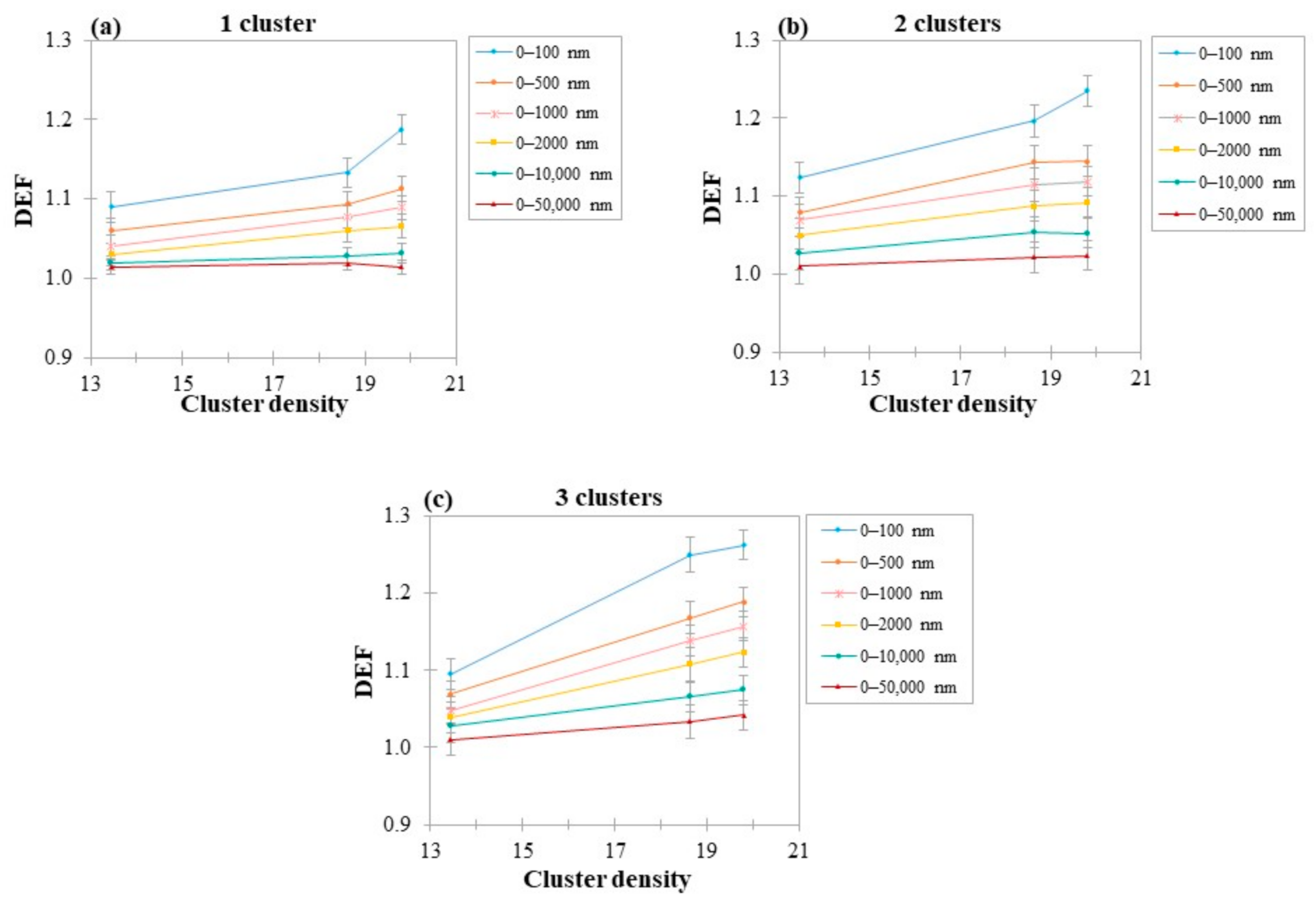
2.5.3. Concentration and Density of Clusters
3. Results
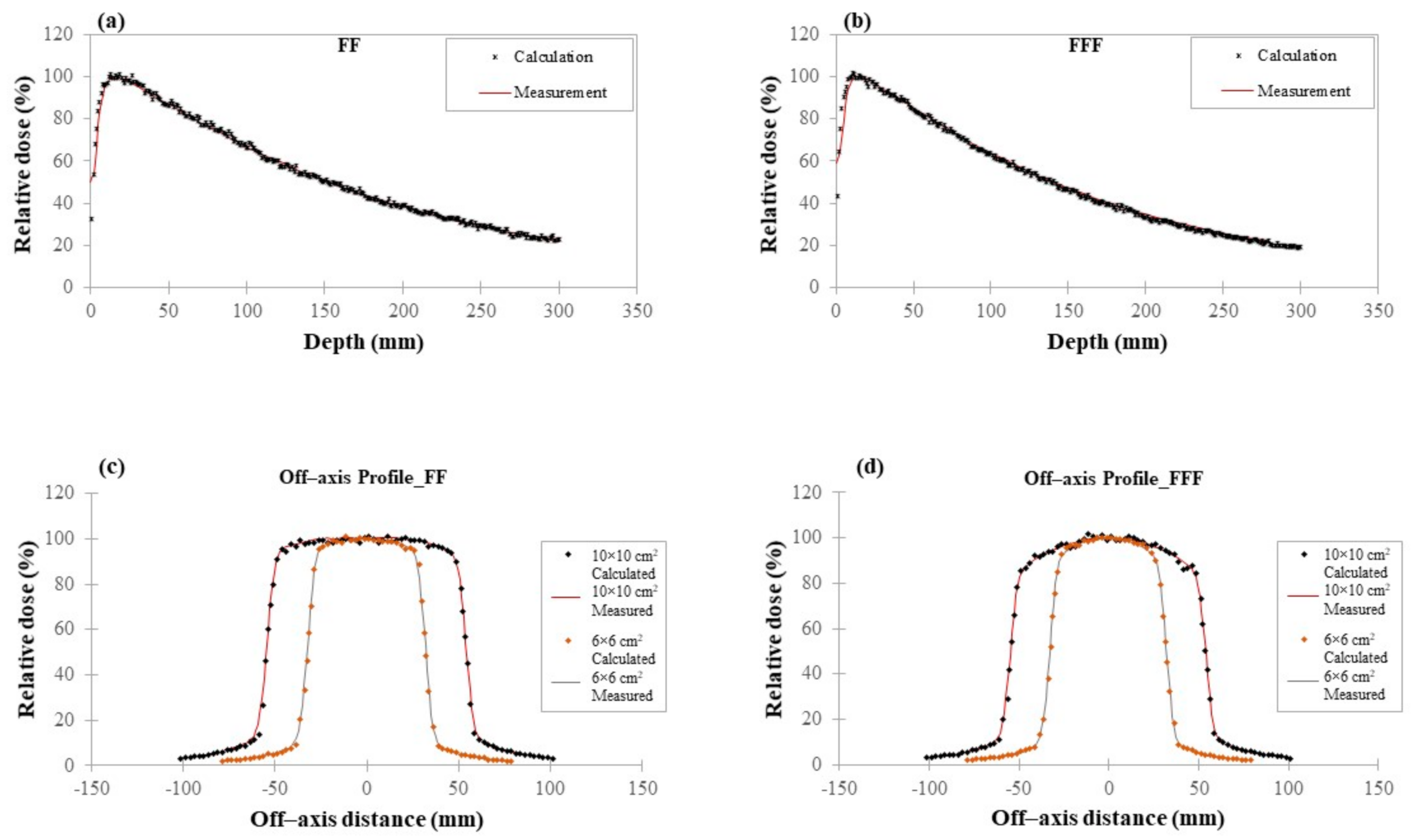
3.1. Photon Beam Model Verification
3.2. DEF for Different AuNPs Sizes as a Function of Distance from AuNPs Cluster
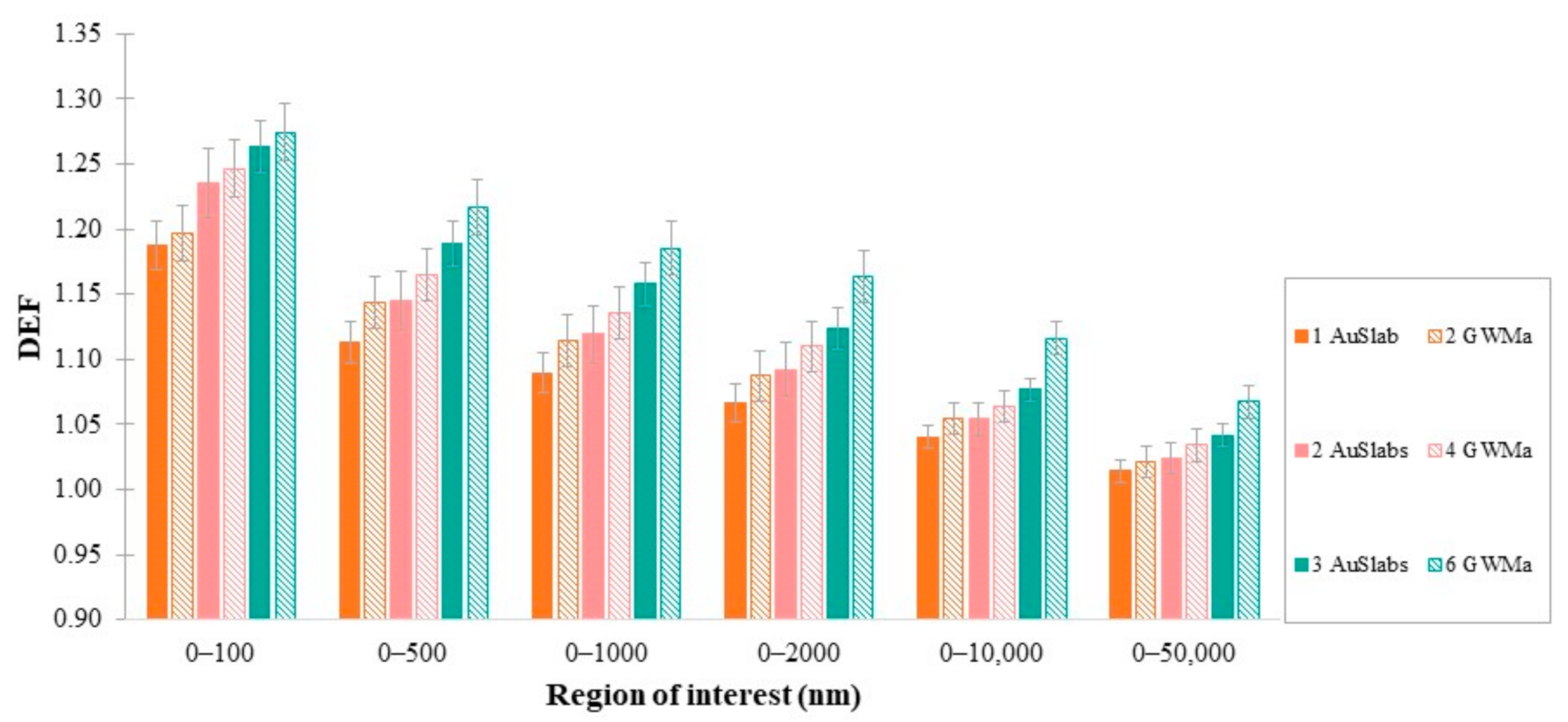
3.3. DEF as a Function of Number of AuSlabs
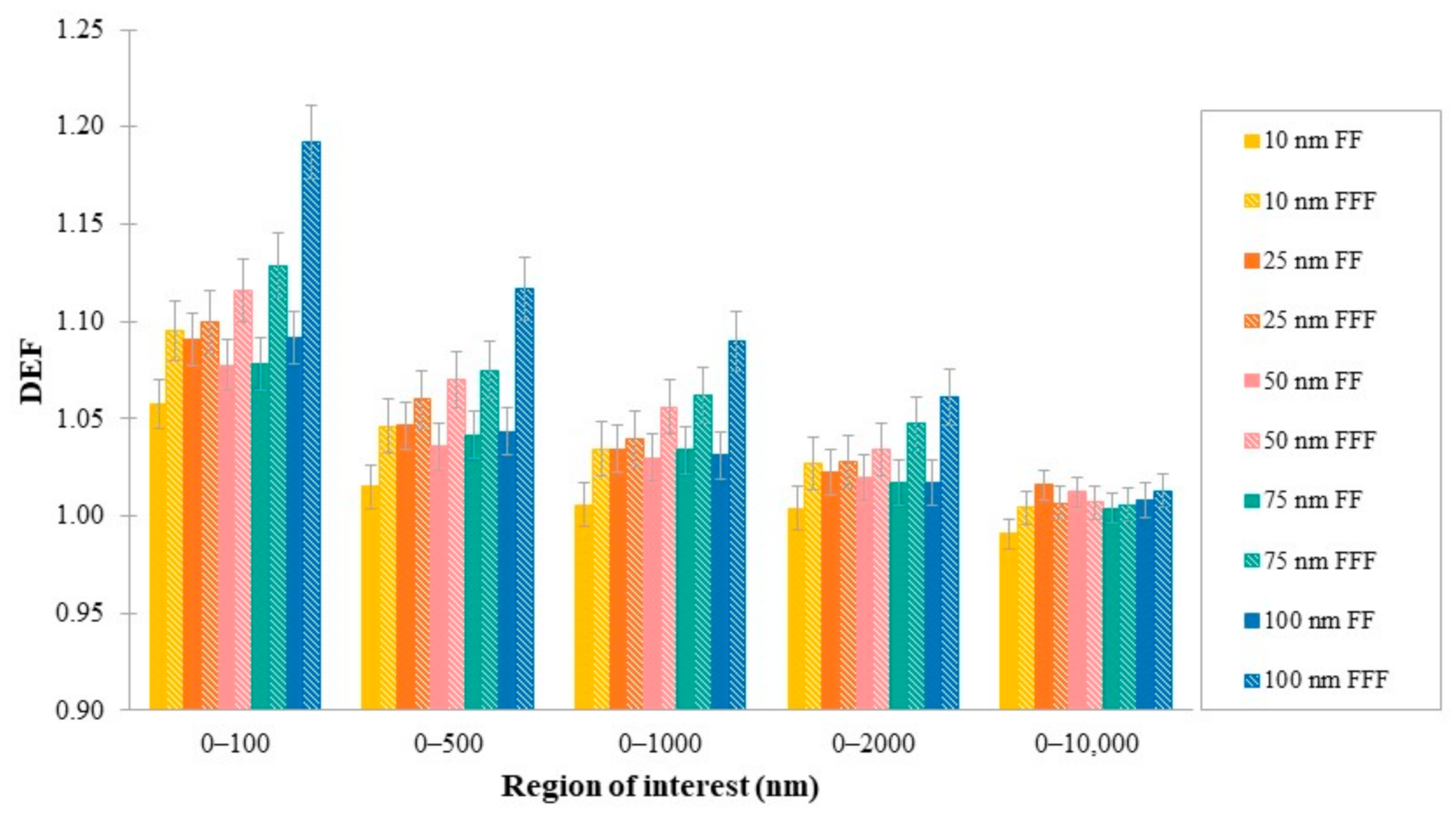
3.4. DEF as a Function of GWM Density
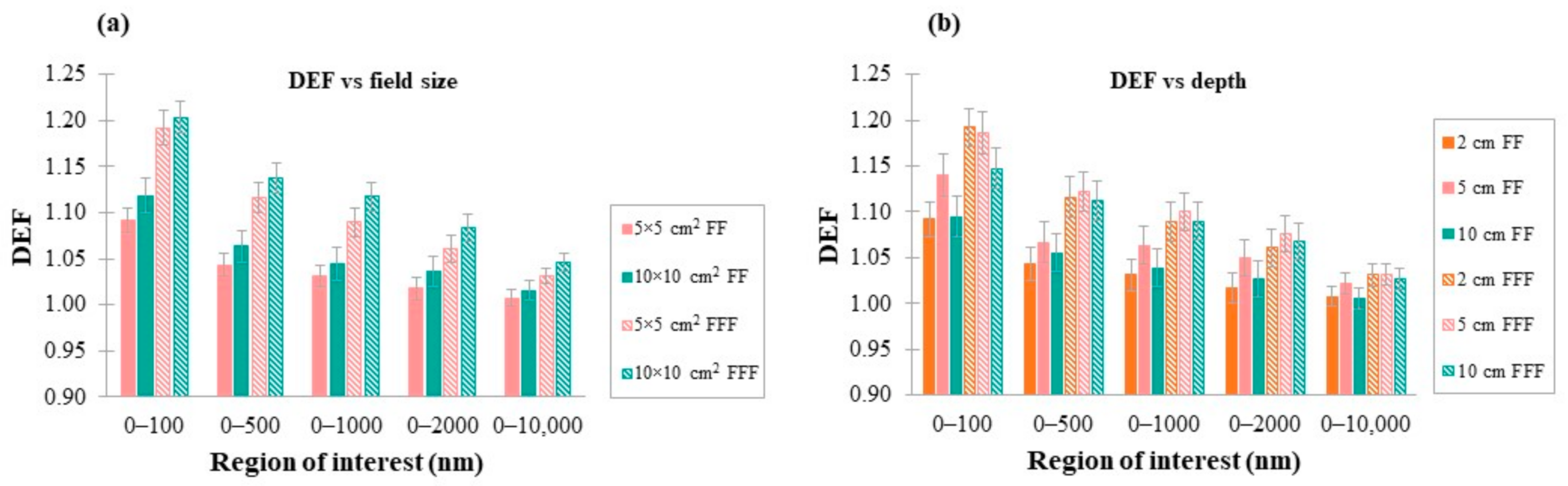
3.5. DEF as a Function of Photon Field Size and Depth of AuNPs Cluster in Water
4. Discussion
4.1. Dependence of DEF on FF
4.2. DEF as a Function of Distance from AuNPs Clusters
4.3. Dependence of DEF on AuNPs Size
4.4. Dependence of DEF on Clusters Density
4.5. Dependence of DEF on the Number of Clusters
4.6. Dependence of DEF on Clusters Modelling
4.7. Dependence of DEF on Photon Field Size and Depth of AuNPs in Water
4.8. Average DEF in Cellular Regions of Interest
4.9. Future Prospects
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hainfeld, J.F.; Slatkin, D.N.; Smilowitz, H.M. The use of gold nanoparticles to enhance radiotherapy in mice. Phys. Med. Biol. 2004, 49, N309–N315. [Google Scholar] [CrossRef] [PubMed]
- Chithrani, D.B.; Jelveh, S.; Jalali, F.; van Prooijen, M.; Allen, C.; Bristow, R.G.; Hill, R.P.; Jaffray, D.A. Gold nanoparticles as radiation sensitizers in cancer therapy. Radiat. Res. 2010, 173, 719–728. [Google Scholar] [CrossRef] [PubMed]
- Leung, M.K.; Chow, J.C.; Chithrani, B.D.; Lee, M.J.; Oms, B.; Jaffray, D.A. Irradiation of gold nanoparticles by X-rays: Monte Carlo simulation of dose enhancements and the spatial properties of the secondary electrons production. Med. Phys. 2011, 38, 624–631. [Google Scholar] [CrossRef] [PubMed]
- Tsiamas, P.; Liu, B.; Cifter, F.; Ngwa, W.F.; Berbeco, R.I.; Kappas, C.; Theodorou, K.; Marcus, K.; Makrigiorgos, M.G.; Sajo, E.; et al. Impact of beam quality on megavoltage radiotherapy treatment techniques utilizing gold nanoparticles for dose enhancement. Phys. Med. Biol. 2013, 58, 451–464. [Google Scholar] [CrossRef]
- McMahon, S.J.; Hyland, W.B.; Muir, M.F.; Coulter, J.A.; Jain, S.; Butterworth, K.T.; Schettino, G.; Dickson, G.R.; Hounsell, A.R.; O’Sullivan, J.M.; et al. Nanodosimetric effects of gold nanoparticles in megavoltage radiation therapy. Radiother. Oncol. 2011, 100, 412–416. [Google Scholar] [CrossRef]
- Jain, S.; Coulter, J.A.; Hounsell, A.R.; Butterworth, K.T.; McMahon, S.J.; Hyland, W.B.; Muir, M.F.; Dickson, G.R.; Prise, K.M.; Currell, F.J.; et al. Cell-specific radiosensitization by gold nanoparticles at megavoltage radiation energies. Int. J. Radiat. Oncol. Biol. Phys. 2011, 79, 531–539. [Google Scholar] [CrossRef] [Green Version]
- Wolfe, T.; Chatterjee, D.; Lee, J.; Grant, J.D.; Bhattarai, S.; Tailor, R.; Goodrich, G.; Nicolucci, P.; Krishnan, S. Targeted gold nanoparticles enhance sensitization of prostate tumors to megavoltage radiation therapy in vivo. Nanomedicine 2015, 11, 1277–1283. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.; Bromma, K.; Sung, W.; Schuemann, J.; Chithrani, D. Determining the Radiation Enhancement Effects of Gold Nanoparticles in Cells in a Combined Treatment with Cisplatin and Radiation at Therapeutic Megavoltage Energies. Cancers 2018, 10, 150. [Google Scholar] [CrossRef] [Green Version]
- Douglass, M.; Bezak, E.; Penfold, S. Monte Carlo investigation of the increased radiation deposition due to gold nanoparticles using kilovoltage and megavoltage photons in a 3D randomized cell model. Med. Phys. 2013, 40, 071710. [Google Scholar] [CrossRef]
- Mesbahi, A.; Jamali, F.; Garehaghaji, N. Effect of Photon Beam Energy, Gold Nanoparticle Size and Concentration on the Dose Enhancement in Radiation Therapy. BioImpacts 2013, 3, 29–35. [Google Scholar] [CrossRef]
- Keshavarz, S.; Sardari, D. Different distributions of gold nanoparticles on the tumor and calculation of dose enhancement factor by Monte Carlo simulation. Nucl. Energy Technol. 2019, 5, 361–371. [Google Scholar] [CrossRef] [Green Version]
- Xiao, Y.; Kry, S.F.; Popple, R.; Yorke, E.; Papanikolaou, N.; Stathakis, S.; Xia, P.; Huq, S.; Bayouth, J.; Galvin, J.; et al. Flattening filter-free accelerators: A report from the AAPM Therapy Emerging Technology Assessment Work Group. J. Appl. Clin. Med. Phys. 2015, 16, 12–29. [Google Scholar] [CrossRef]
- Detappe, A.; Tsiamas, P.; Ngwa, W.; Zygmanski, P.; Makrigiorgos, M.; Berbeco, R. The effect of flattening filter free delivery on endothelial dose enhancement with gold nanoparticles. Med. Phys. 2013, 40, 031706. [Google Scholar] [CrossRef]
- Pakravan, D.; Ghorbani, M.; Momennezhad, M. Tumor dose enhancement by gold nanoparticles in a 6 MV photon beam: A Monte Carlo study on the size effect of nanoparticles. Nukleonika 2013, 58, 275–280. [Google Scholar]
- Kakade, N.; Sharma, S. Dose enhancement in gold nanoparticle-aided radiotherapy for the therapeutic photon beams using Monte Carlo technique. J. Cancer Res. Ther. 2015, 11, 94–97. [Google Scholar] [CrossRef]
- Khosravi, H.; Mahdavi, A.; Rahmani, F.; Ebadi, A. The Impact of Nano-Sized Gold Particles on the Target Dose Enhancement Based on Photon Beams Using by Monte Carlo Method. Nanomed. Res. J. 2016, 1, 84–89. [Google Scholar] [CrossRef]
- Hwang, C.; Kim, J.M.; Kim, J. Influence of concentration, nanoparticle size, beam energy, and material on dose enhancement in radiation therapy. J. Radiat. Res. 2017, 58, 405–411. [Google Scholar] [CrossRef] [Green Version]
- Vlastou, E.; Diamantopoulos, S.; Efstathopoulos, E.P. Monte Carlo studies in Gold Nanoparticles enhanced radiotherapy: The impact of modelled parameters in dose enhancement. Phys. Med. 2020, 80, 57–64. [Google Scholar] [CrossRef]
- Mousavie Anijdan, S.H.; Mahdavi, S.R.; Shirazi, A.; Zarrinfard, M.A.; Hajati, J. Megavoltage X-ray Dose Enhancement with Gold Nanoparticles in Tumor Bearing Mice. Int. J. Mol. Cell. Med. 2013, 2, 118–123. [Google Scholar]
- Guo, M.; Sun, Y.; Zhang, X.-D. Enhanced Radiation Therapy of Gold Nanoparticles in Liver Cancer. Appl. Sci. 2017, 7, 232. [Google Scholar] [CrossRef] [Green Version]
- Hau, H.; Khanal, D.; Rogers, L.; Suchowerska, N.; Kumar, R.; Sridhar, S.; McKenzie, D.; Chrzanowski, W. Dose enhancement and cytotoxicity of gold nanoparticles in colon cancer cells when irradiated with kilo- and mega-voltage radiation. Bioeng. Transl. Med. 2016, 1, 94–102. [Google Scholar] [CrossRef]
- Kazmi, F.; Vallis, K.A.; Vellayappan, B.A.; Bandla, A.; Yukun, D.; Carlisle, R. Megavoltage Radiosensitization of Gold Nanoparticles on a Glioblastoma Cancer Cell Line Using a Clinical Platform. Int. J. Mol. Sci. 2020, 21, 429. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Wang, H.; Coulter, J.A.; Yang, R. Octaarginine-modified gold nanoparticles enhance the radiosensitivity of human colorectal cancer cell line LS180 to megavoltage radiation. Int. J. Nanomed. 2018, 13, 3541–3552. [Google Scholar] [CrossRef] [Green Version]
- Kyriakou, I.; Ivanchenko, V.; Sakata, D.; Bordage, M.C.; Guatelli, S.; Incerti, S.; Emfietzoglou, D. Influence of track structure and condensed history physics models of Geant4 to nanoscale electron transport in liquid water. Phys. Med. 2019, 58, 149–154. [Google Scholar] [CrossRef] [Green Version]
- Lazarakis, P.; Incerti, S.; Ivanchenko, V.; Kyriakou, I.; Emfietzoglou, D.; Corde, S.; Rosenfeld, A.; Lerch, M.; Tehei, M.; Guatelli, S. Investigation of Track Structure and Condensed History physics models for applications in radiation dosimetry on a micro and nano scale in Geant4. Biomed. Phys. Eng. Express 2018, 4, 024001. [Google Scholar] [CrossRef] [Green Version]
- Zygmanski, P.; Sajo, E. Nanoscale radiation transport and clinical beam modeling for gold nanoparticle dose enhanced radiotherapy (GNPT) using X-rays. Br. J. Radiol. 2016, 89, 20150200. [Google Scholar] [CrossRef] [Green Version]
- Amani, S.; Mehdizadeh, A.; Movahedi, M.M.; Keshavarz, M.; Koosha, F. Investigation of the Dose-Enhancement Effects of Spherical and Rod-Shaped Gold Nanoparticles on the HeLa Cell Line. Galen Med. J. 2020, 9, e1581. [Google Scholar] [CrossRef] [PubMed]
- Berbeco, R.I.; Korideck, H.; Ngwa, W.; Kumar, R.; Patel, J.; Sridhar, S.; Johnson, S.; Price, B.D.; Kimmelman, A.; Makrigiorgos, G.M. DNA damage enhancement from gold nanoparticles for clinical MV photon beams. Radiat. Res. 2012, 178, 604–608. [Google Scholar] [CrossRef] [Green Version]
- Shi, M.; Paquette, B.; Thippayamontri, T.; Gendron, L.; Guérin, B.; Sanche, L. Increased radiosensitivity of colorectal tumors with intra-tumoral injection of low dose of gold nanoparticles. Int. J. Nanomed. 2016, 11, 5323–5333. [Google Scholar] [CrossRef] [Green Version]
- Jan, S.; Benoit, D.; Becheva, E.; Carlier, T.; Cassol, F.; Descourt, P.; Frisson, T.; Grevillot, L.; Guigues, L.; Maigne, L.; et al. GATE V6: A major enhancement of the GATE simulation platform enabling modelling of CT and radiotherapy. Phys. Med. Biol. 2011, 56, 881–901. [Google Scholar] [CrossRef]
- Dingfelder, M.; Ritchie, R.H.; Turner, J.E.; Friedland, W.; Paretzke, H.G.; Hamm, R.N. Comparisons of Calculations with PARTRAC and NOREC: Transport of Electrons in Liquid Water. Radiat. Res. 2008, 169, 584–594, 511. [Google Scholar] [CrossRef] [Green Version]
- Incerti, S.; Kyriakou, I.; Bernal, M.A.; Bordage, M.C.; Francis, Z.; Guatelli, S.; Ivanchenko, V.; Karamitros, M.; Lampe, N.; Lee, S.B.; et al. Geant4-DNA example applications for track structure simulations in liquid water: A report from the Geant4-DNA Project. Med. Phys. 2018, 45, e722–e739. [Google Scholar] [CrossRef] [Green Version]
- Sakata, D.; Kyriakou, I.; Tran, H.N.; Bordage, M.-C.; Rosenfeld, A.; Ivanchenko, V.; Incerti, S.; Emfietzoglou, D.; Guatelli, S. Electron track structure simulations in a gold nanoparticle using Geant4-DNA. Phys. Med. Eur. J. Med. Phys. 2019, 63, 98–104. [Google Scholar] [CrossRef]
- Engels, E.; Bakr, S.; Bolst, D.; Sakata, D.; Li, N.; Lazarakis, P.; McMahon, S.J.; Ivanchenko, V.; Rosenfeld, A.B.; Incerti, S.; et al. Advances in modelling gold nanoparticle radiosensitization using new Geant4-DNA physics models. Phys. Med. Biol. 2020, 65, 225017. [Google Scholar] [CrossRef]
- Sakata, D.; Kyriakou, I.; Okada, S.; Tran, H.N.; Lampe, N.; Guatelli, S.; Bordage, M.C.; Ivanchenko, V.; Murakami, K.; Sasaki, T.; et al. Geant4-DNA track-structure simulations for gold nanoparticles: The importance of electron discrete models in nanometer volumes. Med. Phys. 2018, 45, 2230–2242. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Li, Q.; Liang, L.; Li, J.; Wang, K.; Li, J.; Lv, M.; Chen, N.; Song, H.; Lee, J.; et al. Real-time visualization of clustering and intracellular transport of gold nanoparticles by correlative imaging. Nat. Commun. 2017, 8, 15646. [Google Scholar] [CrossRef] [Green Version]
- Cepeda-Pérez, E.; de Jonge, N. Dynamics of gold nanoparticle clusters observed with liquid-phase electron microscopy. Micron 2019, 117, 68–75. [Google Scholar] [CrossRef]
- Gholami, Y.H.; Maschmeyer, R.; Kuncic, Z. Radio-enhancement effects by radiolabeled nanoparticles. Sci. Rep. 2019, 9, 14346. [Google Scholar] [CrossRef] [Green Version]
- Kirkby, C.; Koger, B.; Suchowerska, N.; McKenzie, D.R. Dosimetric consequences of gold nanoparticle clustering during photon irradiation. Med. Phys. 2017, 44, 6560–6569. [Google Scholar] [CrossRef]
- Hainfeld, J.F.; Dilmanian, F.A.; Zhong, Z.; Slatkin, D.N.; Kalef-Ezra, J.A.; Smilowitz, H.M. Gold nanoparticles enhance the radiation therapy of a murine squamous cell carcinoma. Phys. Med. Biol. 2010, 55, 3045–3059. [Google Scholar] [CrossRef]
- Rabus, H.; Li, W.B.; Villagrasa, C.; Schuemann, J.; Hepperle, P.A.; de la Fuente Rosales, L.; Beuve, M.; Di Maria, S.; Klapproth, A.P.; Li, C.Y.; et al. Intercomparison of Monte Carlo calculated dose enhancement ratios for gold nanoparticles irradiated by X-rays: Assessing the uncertainty and correct methodology for extended beams. Phys. Med. 2021, 84, 241–253. [Google Scholar] [CrossRef]
- Zygmanski, P.; Liu, B.; Tsiamas, P.; Cifter, F.; Petersheim, M.; Hesser, J.; Sajo, E. Dependence of Monte Carlo microdosimetric computations on the simulation geometry of gold nanoparticles. Phys. Med. Biol. 2013, 58, 7961–7977. [Google Scholar] [CrossRef]
- Zhang, S.X.; Gao, J.; Buchholz, T.A.; Wang, Z.; Salehpour, M.R.; Drezek, R.A.; Yu, T.K. Quantifying tumor-selective radiation dose enhancements using gold nanoparticles: A monte carlo simulation study. Biomed. Microdevices 2009, 11, 925–933. [Google Scholar] [CrossRef]
- Lechtman, E.; Chattopadhyay, N.; Cai, Z.; Mashouf, S.; Reilly, R.; Pignol, J.P. Implications on clinical scenario of gold nanoparticle radiosensitization in regards to photon energy, nanoparticle size, concentration and location. Phys. Med. Biol. 2011, 56, 4631–4647. [Google Scholar] [CrossRef]
- Villagomez-Bernabe, B.; Ramos-Méndez, J.; Currell, F.J. On the Equivalence of the Biological Effect Induced by Irradiation of Clusters of Heavy Atom Nanoparticles and Homogeneous Heavy Atom-Water Mixtures. Cancers 2021, 13, 2034. [Google Scholar] [CrossRef]
- Wang, C.; Jiang, Y.; Li, X.; Hu, L. Thioglucose-bound gold nanoparticles increase the radiosensitivity of a triple-negative breast cancer cell line (MDA-MB-231). Breast Cancer 2015, 22, 413–420. [Google Scholar] [CrossRef]
- Rahman, W.; Rahman, A.; Ab Rashid, R.; Muhammad, M.; Dollah, N.; Razak, K.; Geso, M. Dose Enhancement Effects by Different Size of Gold Nanoparticles under Irradiation of Megavoltage Photon Beam. J. Sains Nukl. Malays. 2018, 30, 23–29. [Google Scholar]
- Behrouzkia, Z.; Zohdiaghdam, R.; Khalkhali, H.R.; Mousavi, F. Evaluation of Gold Nanoparticle Size Effect on Dose Enhancement Factor in Megavoltage Beam Radiotherapy Using MAGICA Polymer Gel Dosimeter. J. Biomed. Phys. Eng. 2019, 9, 89–96. [Google Scholar] [CrossRef]
- Abolaban, F.; Taha, E.; Alhawsawi, A.; Djouider, F.; Banoqitah, E.; Nisbet, A. Estimation of Dose Enhancement for Inhomogeneous Distribution of Nanoparticles: A Monte Carlo Study. Appl. Sci. 2021, 11, 4900. [Google Scholar] [CrossRef]
- Smith, C.L.; Best, S.P.; Gagliardi, F.; Tominaga, T.; Geso, M. The effects of gold nanoparticles concentrations and beam quality/LET on dose enhancement when irradiated with X-rays and protons using alanine/EPR dosimetry. Radiat. Meas. 2017, 106, 352–356. [Google Scholar] [CrossRef]
- Chow, J.C.L. Depth Dose Enhancement on Flattening-Filter-Free Photon Beam: A Monte Carlo Study in Nanoparticle-Enhanced Radiotherapy. Appl. Sci. 2020, 10, 7052. [Google Scholar] [CrossRef]
- Zhang, X.D.; Wu, D.; Shen, X.; Chen, J.; Sun, Y.M.; Liu, P.X.; Liang, X.J. Size-dependent radiosensitization of PEG-coated gold nanoparticles for cancer radiation therapy. Biomaterials 2012, 33, 6408–6419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
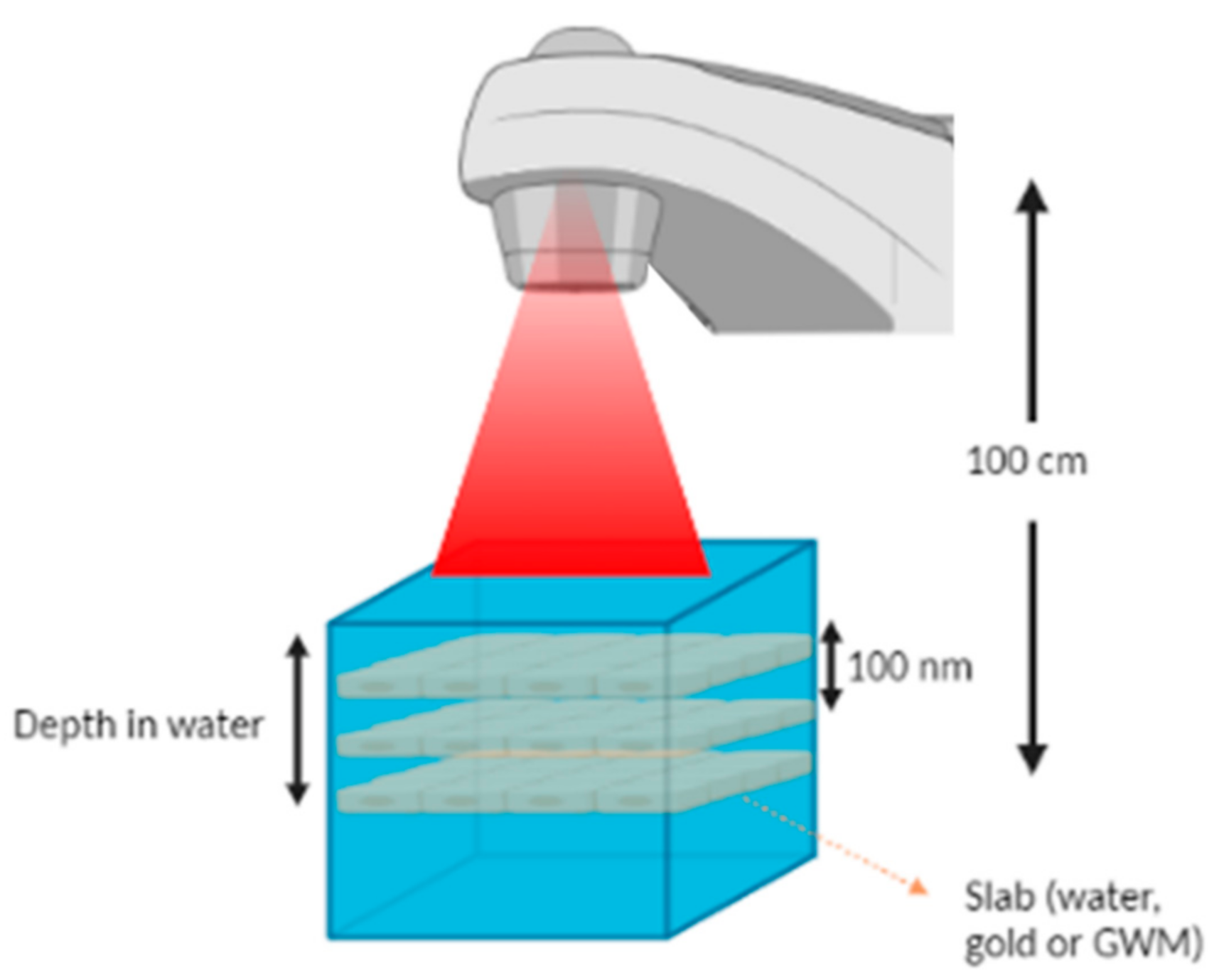



| AuSlab Size (nm) | Field Size | Depth | Photon Beam |
|---|---|---|---|
| 10 | 5 × 5 cm2 | 2 cm | FF, FFF |
| 25 | |||
| 50 | |||
| 75 | |||
| 100 |
| Field Size (cm2) | Depth (cm) | Photon Beam | AuSlab Size |
|---|---|---|---|
| 5 × 5 | 2, 5, 10 | FF, FFF | 100 nm |
| 10 × 10 | 2 |
| Cluster ID | Concentration by Mass (%) | Au Mass (mg) | Density (g/cm3) | Photon Beam | Number of Clusters | |
|---|---|---|---|---|---|---|
| Water | Au | |||||
| AuSlab | 0 | 100 | 3 | 19.3 | FF, FFF | 1, 2, 3 |
| GWMa | 5 | 95 | 1.5 | 18.4 | FFF | 1, 2, 3, 4, 6 |
| GWMb | 68 | 32 | 0.3 | 13.4 | FFF | 1, 2, 3 |
| Cluster Size (nm) | Region of Interest (nm) | ||||
|---|---|---|---|---|---|
| 0–100 | 0–500 | 0–1000 | 0–2000 | 0–10,000 | |
| FF | |||||
| 10 | 1.06 ± 0.01 | 1.01 ± 0.01 | 1.01 ± 0.01 | 1 ± 0.01 | 0.99 ± 0.01 |
| 25 | 1.09 ± 0.01 | 1.05 ± 0.01 | 1.03 ± 0.01 | 1.02 ± 0.01 | 1.02 ± 0.01 |
| 50 | 1.08 ± 0.01 | 1.04 ± 0.01 | 1.03 ± 0.01 | 1.02 ± 0.01 | 1.01 ± 0.01 |
| 75 | 1.08 ± 0.01 | 1.04 ± 0.01 | 1.03 ± 0.01 | 1.02 ± 0.01 | 1 ± 0.01 |
| 100 | 1.09 ± 0.01 | 1.04 ± 0.01 | 1.03 ± 0.01 | 1.02 ± 0.01 | 1.01 ± 0.01 |
| FFF | |||||
| 10 | 1.10 ± 0.02 | 1.05 ± 0.01 | 1.03 ± 0.01 | 1.03 ± 0.01 | 1.02 ± 0.01 |
| 25 | 1.10 ± 0.02 | 1.06 ± 0.01 | 1.04 ± 0.01 | 1.03 ± 0.01 | 1.01 ± 0.01 |
| 50 | 1.12 ± 0.02 | 1.07 ± 0.01 | 1.06 ± 0.01 | 1.03 ± 0.01 | 1.02 ± 0.01 |
| 75 | 1.13 ± 0.02 | 1.07 ± 0.01 | 1.06 ± 0.01 | 1.05 ± 0.01 | 1.02 ± 0.01 |
| 100 | 1.19 ± 0.02 | 1.12 ± 0.02 | 1.09 ± 0.02 | 1.06 ± 0.01 | 1.03 ± 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vlastou, E.; Pantelis, E.; Efstathopoulos, E.P.; Karaiskos, P.; Kouloulias, V.; Platoni, K. Quantification of Nanoscale Dose Enhancement in Gold Nanoparticle-Aided External Photon Beam Radiotherapy. Cancers 2022, 14, 2167. https://doi.org/10.3390/cancers14092167
Vlastou E, Pantelis E, Efstathopoulos EP, Karaiskos P, Kouloulias V, Platoni K. Quantification of Nanoscale Dose Enhancement in Gold Nanoparticle-Aided External Photon Beam Radiotherapy. Cancers. 2022; 14(9):2167. https://doi.org/10.3390/cancers14092167
Chicago/Turabian StyleVlastou, Elena, Evaggelos Pantelis, Efstathios P. Efstathopoulos, Pantelis Karaiskos, Vasileios Kouloulias, and Kalliopi Platoni. 2022. "Quantification of Nanoscale Dose Enhancement in Gold Nanoparticle-Aided External Photon Beam Radiotherapy" Cancers 14, no. 9: 2167. https://doi.org/10.3390/cancers14092167
APA StyleVlastou, E., Pantelis, E., Efstathopoulos, E. P., Karaiskos, P., Kouloulias, V., & Platoni, K. (2022). Quantification of Nanoscale Dose Enhancement in Gold Nanoparticle-Aided External Photon Beam Radiotherapy. Cancers, 14(9), 2167. https://doi.org/10.3390/cancers14092167









