Fundus Autofluorescence Imaging in Patients with Choroidal Melanoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Fundus Autofluorescence Imaging
2.1. Basic Principles
2.2. Retinal Pigment Epithelium and Main Fluorophores
2.3. Imaging Devices
2.3.1. Scanning Laser Ophthalmoscopy
2.3.2. Fundus Camera
2.3.3. Wide-Field Imaging
2.4. Interpreting Fundus Autofluorescence Images
3. Choroidal Melanoma
3.1. General Aspects
3.2. To Differentiate between Choroidal Melanoma and Choroidal Nevus
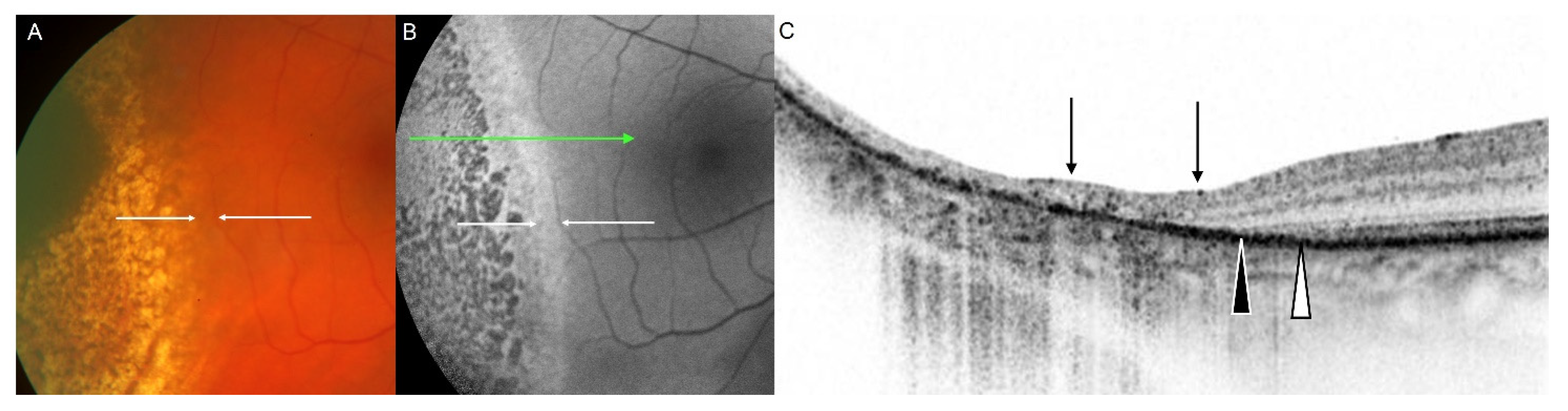
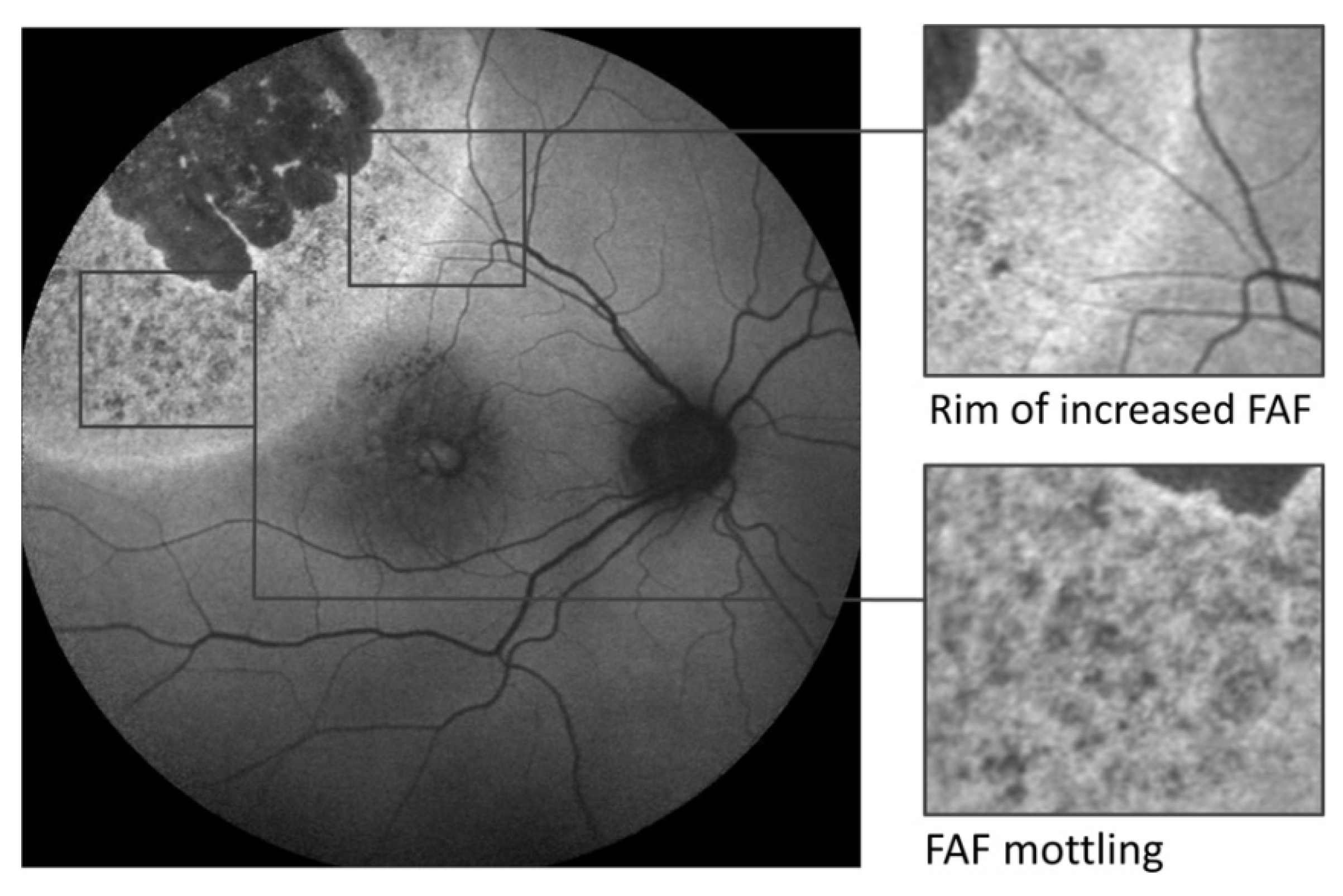
3.3. Tumour-Associated Retinal Pigment Epitheliopathy
3.4. Orange Pigment
3.5. Fundus Autofluorescence Imaging of Choroidal Melanoma
3.6. Fundus Autofluorescence Imaging following Treatment of Choroidal Melanoma
4. Limitations
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kaliki, S.; Shields, C.L. Uveal melanoma: Relatively rare but deadly cancer. Eye 2017, 31, 241–257. [Google Scholar] [CrossRef]
- Jager, M.J.; Shields, C.L.; Cebulla, C.M.; Abdel-Rahman, M.H.; Grossniklaus, H.E.; Stern, M.-H.; Carvajal, R.D.; Belfort, R.N.; Jia, R.; Shields, J.A.; et al. Uveal melanoma. Nat. Rev. Dis. Primers 2020, 6, 24. [Google Scholar] [CrossRef] [PubMed]
- Nürnberg, D.; Seibel, I.; Riechardt, A.I.; Brockmann, C.; Zeitz, O.; Heufelder, J.; Joussen, A.M. Multimodale Bildgebung des Aderhautmelanoms mit seinen Differenzialdiagnosen, Therapie (Bestrahlungsplanung) und Verlaufskontrolle. Klin. Monbl. Augenheilkd. 2018, 235, 1001–1012. [Google Scholar] [CrossRef] [PubMed]
- Dalvin, L.A.; Shields, C.L.; Ancona-Lezama, D.A.; Yu, M.D.; Di Nicola, M.; Williams, B.K.; Lucio-Alvarez, J.A.; Ang, S.M.; Maloney, S.M.; Welch, R.J.; et al. Combination of multimodal imaging features predictive of choroidal nevus transformation into melanoma. Br. J. Ophthalmol. 2019, 103, 1441–1447. [Google Scholar] [CrossRef]
- Shields, C.L.; Lim, L.-A.S.; Dalvin, L.A.; Shields, J.A. Small choroidal melanoma: Detection with multimodal imaging and management with plaque radiotherapy or AU-011 nanoparticle therapy. Curr. Opin. Ophthalmol. 2019, 30, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Shields, C.L.; Lally, S.E.; Dalvin, L.A.; Sagoo, M.S.; Pellegrini, M.; Kaliki, S.; Gündüz, A.K.; Furuta, M.; Mruthyunjaya, P.; Fung, A.T.; et al. White Paper on Ophthalmic Imaging for Choroidal Nevus Identification and Transformation into Melanoma. Transl. Vis. Sci. Technol. 2021, 10, 24. [Google Scholar] [CrossRef] [PubMed]
- Schmitz-Valckenberg, S.; Holz, F.G.; Bird, A.C.; Spaide, R.F. Fundus autofluorescence imaging: Review and perspectives. Retina 2008, 28, 385–409. [Google Scholar] [CrossRef]
- Holz, F.G.; Schmitz-Valckenberg, S.; Spaide, R.F.; Bird, A.C. (Eds.) Atlas of Fundus Autofluorescence Imaging; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Lois, N.; Forrester, J.V. (Eds.) Fundus Autofluorescence, 2nd ed.; Wolters Kluwer: Philadelphia, PA, USA, 2016. [Google Scholar]
- Webb, R.H.; Hughes, G.W.; Pomerantzeff, O. Flying spot TV ophthalmoscope. Appl. Opt. 1980, 19, 2991–2997. [Google Scholar] [CrossRef] [PubMed]
- Webb, R.H.; Hughes, G.W. Scanning laser ophthalmoscope. IEEE Trans. Biomed. Eng. 1981, 28, 488–492. [Google Scholar] [CrossRef] [PubMed]
- Plesch, A.; Klingbeil, U.; Bille, J. Digital laser scanning fundus camera. Appl. Opt. 1987, 26, 1480–1486. [Google Scholar] [CrossRef]
- Delori, F.C.; Dorey, C.K.; Staurenghi, G.; Arend, O.; Goger, D.G.; Weiter, J.J. In vivo fluorescence of the ocular fundus exhibits retinal pigment epithelium lipofuscin characteristics. Investig. Ophthalmol. Vis. Sci. 1995, 36, 718–729. [Google Scholar]
- Delori, F.C.; Staurenghi, G.; Arend, O.; Dorey, C.K.; Goger, D.G.; Weiter, J.J. In vivo measurement of lipofuscin in Stargardt’s disease—Fundus flavimaculatus. Investig. Ophthalmol. Vis. Sci. 1995, 36, 2327–2331. [Google Scholar]
- von Rückmann, A.; Fitzke, F.W.; Bird, A.C. Distribution of fundus autofluorescence with a scanning laser ophthalmoscope. Br. J. Ophthalmol. 1995, 79, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Schmitz-Valckenberg, S.; Pfau, M.; Fleckenstein, M.; Staurenghi, G.; Sparrow, J.R.; Bindewald-Wittich, A.; Spaide, R.F.; Wolf, S.; Sadda, S.R.; Holz, F.G. Fundus autofluorescence imaging. Prog. Retin. Eye Res. 2021, 81, 100893. [Google Scholar] [CrossRef] [PubMed]
- Schmitz-Valckenberg, S.; Fitzke, F.W. Imaging techniques of fundus autofluorescence. In Fundus Autofluorescence, 2nd ed.; Lois, N., Forrester, J.V., Eds.; Wolters Kluwer: Philadelphia, PA, USA, 2016; Chapter 4; pp. 29–35. [Google Scholar]
- Feeney, L. Lipofuscin and melanin of human retinal pigment epithelium. Fluorescence, enzyme cytochemical, and ultrastructural studies. Investig. Ophthalmol. Vis. Sci. 1978, 17, 583–600. [Google Scholar]
- Feeney-Burns, L.; Berman, E.R.; Rothman, H. Lipofuscin of human retinal pigment epithelium. Am. J. Ophthalmol. 1980, 90, 783–791. [Google Scholar] [CrossRef]
- Bindewald-Wittich, A.; Han, M.; Schmitz-Valckenberg, S.; Snyder, S.R.; Giese, G.; Bille, J.F.; Holz, F.G. Two-photon-excited fluorescence imaging of human RPE cells with a femtosecond Ti:Sapphire laser. Investig. Ophthalmol. Vis. Sci. 2006, 47, 4553–4557. [Google Scholar] [CrossRef] [PubMed]
- Ben Ami, T.; Tong, Y.; Bhuiyan, A.; Huisingh, C.; Ablonczy, Z.; Ach, T.; Curcio, C.A.; Smith, R.T. Spatial and spectral characterization of human retinal pigment epithelium fluorophore families by ex vivo hyperspectral autofluorescence imaging. Transl. Vis. Sci. Technol. 2016, 5, 5. [Google Scholar] [CrossRef] [PubMed]
- Pollreisz, A.; Messinger, J.D.; Sloan, K.R.; Mittermueller, T.J.; Weinhandl, A.S.; Benson, E.K.; Kidd, G.J.; Schmidt-Erfurth, U.; Curcio, C.A. Visualizing melanosomes, lipofuscin, and melanolipofuscin in human retinal pigment epithelium using serial block face scanning electron microscopy. Exp. Eye Res. 2018, 166, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Bermond, K.; Wobbe, C.; Tarau, I.-S.; Heintzmann, R.; Hillenkamp, J.; Curcio, C.A.; Sloan, K.R.; Ach, T. Autofluorescent granules of the human retinal pigment epithelium: Phenotypes, intracellular distribution, and age-related topography. Investig. Ophthalmol. Vis. Sci. 2020, 61, 35. [Google Scholar] [CrossRef]
- Marmorstein, A.D. The polarity of the retinal pigment epithelium. Traffic 2001, 2, 867–872. [Google Scholar] [CrossRef] [PubMed]
- Storm, T.; Burgoyne, T.; Futter, C.E. Membrane trafficking in the retinal pigment epithelium at a glance. J. Cell Sci. 2020, 133, jcs238279. [Google Scholar] [CrossRef]
- Strauss, O. The retinal pigment epithelium in visual function. Physiol. Rev. 2005, 85, 845–881. [Google Scholar] [CrossRef]
- Anderson, D.H.; Fisher, S.K.; Steinberg, R.H. Mammalian cones: Disc shedding, phagocytosis, and renewal. Investig. Ophthalmol. Vis. Sci. 1978, 17, 117–133. [Google Scholar]
- Kevany, B.M.; Palczewski, K. Phagocytosis of retinal rod and cone photoreceptors. Physiology 2010, 25, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Kiser, P.D.; Palczewski, K. Retinoids and Retinal Diseases. Annu. Rev. Vis. Sci. 2016, 2, 197–234. [Google Scholar] [CrossRef] [PubMed]
- Kwon, W.; Freeman, S.A. Phagocytosis by the retinal pigment epithelium: Recognition, resolution, recycling. Front. Immunol. 2020, 11, 604205. [Google Scholar] [CrossRef]
- Lakkaraju, A.; Umapathy, A.; Tan, L.X.; Daniele, L.; Philp, N.J.; Boesze-Battaglia, K.; Williams, D.S. The cell biology of the retinal pigment epithelium. Prog. Retin. Eye Res. 2020, 78, 100846. [Google Scholar] [CrossRef] [PubMed]
- Sparrow, J.R.; Wu, Y.; Kim, C.Y.; Zhou, J. Phospholipid meets all-trans-retinal: The making of RPE bisretinoids. J. Lipid Res. 2010, 51, 247–261. [Google Scholar] [CrossRef] [PubMed]
- Sparrow, J.R.; Wu, Y.; Nagasaki, T.; Yoon, K.D.; Yamamoto, K.; Zhou, J. Fundus autofluorescence and the bisretinoids of retina†. Photochem. Photobiol. Sci. 2010, 9, 1480–1489. [Google Scholar] [CrossRef] [PubMed]
- Pole, C.; Ameri, H. Fundus autofluorescence and clinical applications. J. Ophthalmic Vis. Res. 2021, 16, 432–461. [Google Scholar] [CrossRef] [PubMed]
- Fischer, J.; Otto, T.; Delori, F.; Pace, L.; Staurenghi, G. Scanning Laser Ophthalmoscopy (SLO). In High Resolution Imaging in Microscopy and Ophthalmology: New Frontiers in Biomedical Optics; Bille, J.F., Ed.; Springer: Cham, Switzerland, 2019; Chapter 2. [Google Scholar]
- Delori, F.C.; Webb, R.H.; Sliney, D.H. Maximum permissible exposures for ocular safety (ANSI 2000), with emphasis on ophthalmic devices. J. Opt. Soc. Am. A Opt. Image Sci. Vis. 2007, 24, 1250–1265. [Google Scholar] [CrossRef]
- Delori, F.C.; Fleckner, M.R.; Goger, D.G.; Weiter, J.J.; Dorey, C.K. Autofluorescence distribution associated with drusen in age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2000, 41, 496–504. [Google Scholar]
- Spaide, R.F. Fundus autofluorescence and age-related macular degeneration. Ophthalmology 2003, 110, 392–399. [Google Scholar] [CrossRef]
- Kernt, M.; Schaller, U.C.; Stumpf, C.; Ulbig, M.W.; Kampik, A.; Neubauer, A.S. Choroidal pigmented lesions imaged by ultra-wide-field scanning laser ophthalmoscopy with two laser wavelengths (Optomap). Clin. Ophthalmol. 2010, 4, 829–836. [Google Scholar] [CrossRef]
- Nagiel, A.; Lalane, R.A.; Sadda, S.R.; Schwartz, S.D. Ultra-widefield fundus imaging: A review of clinical applications and future trends. Retina 2016, 36, 660–678. [Google Scholar] [CrossRef] [PubMed]
- Xu, A.; Chen, C. Clinical application of ultra-widefield fundus autofluorescence. Int. Ophthalmol. 2021, 41, 727–741. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Surve, A.; Kumawat, D.; Takkar, B.; Azad, S.; Chawla, R.; Shroff, D.; Arora, A.; Singh, R.; Venkatesh, P. Ultra-wide field retinal imaging: A wider clinical perspective. Indian J. Ophthalmol. 2021, 69, 824–835. [Google Scholar] [CrossRef] [PubMed]
- Sawa, M.; Ober, M.D.; Spaide, R.F. Autofluorescence and retinal pigment epithelial atrophy after subretinal hemorrhage. Retina 2006, 26, 119–120. [Google Scholar] [CrossRef]
- Spaide, R.F.; Klancnik, J.M. Fundus autofluorescence and central serous chorioretinopathy. Ophthalmology 2005, 112, 825–833. [Google Scholar] [CrossRef]
- Bird, A.C. Interpreting fundus autofluorescence. In Fundus Autofluorescence, 2nd ed.; Lois, N., Forrester, J.V., Eds.; Wolters Kluwer: Philadelphia, PA, USA, 2016; Chapter 6; pp. 45–51. [Google Scholar]
- Singh, A.D.; Topham, A. Incidence of uveal melanoma in the United States: 1973–1997. Ophthalmology 2003, 110, 956–961. [Google Scholar] [CrossRef]
- Virgili, G.; Gatta, G.; Ciccolallo, L.; Capocaccia, R.; Biggeri, A.; Crocetti, E.; Lutz, J.-M.; Paci, E. Incidence of uveal melanoma in Europe. Ophthalmology 2007, 114, 2309–2315. [Google Scholar] [CrossRef]
- Manchegowda, P.; Singh, A.D.; Shields, C.; Kaliki, S.; Shah, P.; Gopal, L.; Rishi, P. Uveal melanoma in Asians: A review. Ocul. Oncol. Pathol. 2021, 7, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Shields, C.L.; Furuta, M.; Berman, E.L.; Zahler, J.D.; Hoberman, D.M.; Dinh, D.H.; Mashayekhi, A.; Shields, J.A. Choroidal nevus transformation into melanoma: Analysis of 2514 consecutive cases. Arch. Ophthalmol. 2009, 127, 981–987. [Google Scholar] [CrossRef]
- Diener-West, M.; Hawkins, B.S.; Markowitz, J.A.; Schachat, A.P. A review of mortality from choroidal melanoma. II. A meta-analysis of 5-year mortality rates following enucleation, 1966 through 1988. Arch. Ophthalmol. 1992, 110, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Kujala, E.; Mäkitie, T.; Kivelä, T. Very long-term prognosis of patients with malignant uveal melanoma. Investig. Ophthalmol. Vis. Sci. 2003, 44, 4651–4659. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.D.; Turell, M.E.; Topham, A.K. Uveal melanoma: Trends in incidence, treatment, and survival. Ophthalmology 2011, 118, 1881–1885. [Google Scholar] [CrossRef] [PubMed]
- Martel, A.; Baillif, S.; Nahon-Esteve, S.; Gastaud, L.; Bertolotto, C.; Roméo, B.; Mograbi, B.; Lassalle, S.; Hofman, P. Liquid biopsy for solid ophthalmic malignancies: An updated review and perspectives. Cancers 2020, 12, 3284. [Google Scholar] [CrossRef] [PubMed]
- Shields, C.L.; Dalvin, L.A.; Ancona-Lezama, D.; Yu, M.D.; Di Nicola, M.; Williams, B.K.; Lucio-Alvarez, J.A.; Ang, S.M.; Maloney, S.; Welch, R.J.; et al. Choroidal nevus imaging features in 3806 cases and risk factors for transformation into melanoma in 2355 cases: The 2020 Taylor R. Smith and Victor T. Curtin Lecture. Retina 2019, 39, 1840–1851. [Google Scholar] [CrossRef]
- Shields, C.L.; Shields, J.A.; Kiratli, H.; de Potter, P.; Cater, J.R. Risk factors for growth and metastasis of small choroidal melanocytic lesions. Ophthalmology 1995, 102, 1351–1361. [Google Scholar]
- Shields, C.L.; Cater, J.; Shields, J.A.; Singh, A.D.; Santos, M.C.; Carvalho, C. Combination of clinical factors predictive of growth of small choroidal melanocytic tumors. Arch. Ophthalmol. 2000, 118, 360–364. [Google Scholar] [CrossRef]
- Shields, C.L.; Furuta, M.; Thangappan, A.; Nagori, S.; Mashayekhi, A.; Lally, D.R.; Kelly, C.C.; Rudich, D.S.; Nagori, A.V.; Wakade, O.A.; et al. Metastasis of uveal melanoma millimeter-by-millimeter in 8033 consecutive eyes. Arch. Ophthalmol. 2009, 127, 989–998. [Google Scholar] [CrossRef] [PubMed]
- Almeida, A.; Kaliki, S.; Shields, C.L. Autofluorescence of intraocular tumours. Curr. Opin. Ophthalmol. 2013, 24, 222–232. [Google Scholar] [CrossRef] [PubMed]
- Lohmann, W.; Wiegand, W.; Stolwijk, T.R.; van Delft, J.L.; van Best, J.A. Endogenous fluorescence of ocular malignant melanomas. Ophthalmologica 1995, 209, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Gündüz, K.; Pulido, J.S.; Ezzat, K.; Salomao, D.; Hann, C. Review of fundus autofluorescence in choroidal melanocytic lesions. Eye 2009, 23, 497–503. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wallow, I.H.; Ts’o, M.O. Proliferation of the retinal pigment epithelium over malignant choroidal tumors. A light and electron microscopic study. Am. J. Ophthalmol. 1972, 73, 914–926. [Google Scholar] [CrossRef]
- Damato, B.E.; Foulds, W.S. Tumour-associated retinal pigment epitheliopathy. Eye 1990, 4, 382–387. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.Y.; Gunduz, K.; Shields, C.L.; Shields, J.A. Choroidal melanoma-associated retinal and retinal pigment epithelial changes. J. Ophthalmic Nurs. Technol. 1998, 17, 110–114. [Google Scholar]
- Garcia, M.D.; Salomao, D.R.; Marmorstein, A.D.; Pulido, J.S. Histopathologic findings in the areas of orange pigment overlying choroidal melanomas. Transl. Vis. Sci. Technol. 2016, 5, 4. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lavinsky, D.; Belfort, R.N.; Navajas, E.; Torres, V.; Martins, M.C.; Belfort, R. Fundus autofluorescence of choroidal nevus and melanoma. Br. J. Ophthalmol. 2007, 91, 1299–1302. [Google Scholar] [CrossRef]
- Shields, C.L.; Bianciotto, C.; Pirondini, C.; Materin, M.A.; Harmon, S.A.; Shields, J.A. Autofluorescence of orange pigment overlying small choroidal melanoma. Retina 2007, 27, 1107–1111. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.T.; Irvine, A.R. Diagnostic significance of orange pigment accumulation over choroidal tumors. Am. J. Ophthalmol. 1973, 76, 212–216. [Google Scholar] [CrossRef]
- Font, R.L.; Zimmerman, L.E.; Armaly, M.F. The nature of the orange pigment over a choroidal melanoma. Histochemical and electron microscopical observations. Arch. Ophthalmol. 1974, 91, 359–362. [Google Scholar] [CrossRef] [PubMed]
- Shields, J.A.; Rodrigues, M.M.; Sarin, L.K.; Tasman, W.S.; Annesley, W.H. Lipofuscin pigment over benign and malignant choroidal tumors. Trans. Sect. Ophthalmol. Am. Acad. Ophthalmol. Otolaryngol. 1976, 81, 871–881. [Google Scholar] [PubMed]
- Elner, S.G.; Elner, V.M.; Nielsen, J.C.; Torczynski, E.; Yu, R.; Franklin, W.A. CD68 antigen expression by human retinal pigment epithelial cells. Exp. Eye Res. 1992, 55, 21–28. [Google Scholar] [CrossRef]
- Riechardt, A.I.; Gundlach, E.; Joussen, A.M.; Willerding, G.D. The development of orange pigment overlying choroidal metastasis. Ocul. Oncol. Pathol. 2015, 1, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Ramasubramanian, A.; Shields, C.L.; Harmon, S.A.; Shields, J.A. Autofluorescence of choroidal hemangioma in 34 consecutive eyes. Retina 2010, 30, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Shields, C.L.; Shields, J.A.; Kiratli, H.; de Potter, P.; Cater, J.R. Risk factors for growth and metastasis of small choroidal melanocytic lesions. Trans. Am. Ophthalmol. Soc. 1995, 93, 259–279. [Google Scholar]
- Factors predictive of growth and treatment of small choroidal melanoma: COMS Report No.5. The Collaborative Ocular Melanoma Study Group. Arch. Ophthalmol. 1997, 115, 1537–1544.
- Singh, A.D.; Mokashi, A.A.; Bena, J.F.; Jacques, R.; Rundle, P.A.; Rennie, I.G. Small choroidal melanocytic lesions: Features predictive of growth. Ophthalmology 2006, 113, 1032–1039. [Google Scholar] [CrossRef]
- Gündüz, K.; Pulido, J.S.; Bakri, S.J.; Petit-Fond, E. Fundus autofluorescence in choroidal melanocytic lesions. Retina 2007, 27, 681–687. [Google Scholar] [CrossRef]
- Materin, M.A.; Raducu, R.; Bianciotto, C.; Shields, C.L. Fundus autofluorescence and optical coherence tomography findings in choroidal melanocytic lesions. Middle East Afr. J. Ophthalmol. 2010, 17, 201–206. [Google Scholar] [CrossRef]
- Hashmi, F.; Rojanaporn, D.; Kaliki, S.; Shields, C.L. Orange pigment sediment overlying small choroidal melanoma. Arch. Ophthalmol. 2012, 130, 937–939. [Google Scholar] [CrossRef]
- Cennamo, G.; Romano, M.R.; Velotti, N.; Breve, M.A.; de Crecchio, G.; Cennamo, G. Fundus autofluorescence of choroidal nevi and melanoma. Acta Ophthalmol. 2018, 96, e102–e104. [Google Scholar] [CrossRef] [PubMed]
- Gündüz, K.; Pulido, J.S.; Pulido, J.E.; Link, T. Correlation of fundus autofluorescence with fluorescein and indocyanine green angiography in choroidal melanocytic lesions. Retina 2008, 28, 1257–1264. [Google Scholar] [CrossRef] [PubMed]
- Samuelsson, D.; Sznage, M.; Engelsberg, K.; Wittström, E. Clinical, optical coherence tomography, and fundus autofluorescence findings in patients with intraocular tumors. Clin. Ophthalmol. 2016, 10, 1953–1964. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shields, C.L.; Pirondini, C.; Bianciotto, C.; Materin, M.A.; Harmon, S.A.; Shields, J.A. Autofluorescence of choroidal nevus in 64 cases. Retina 2008, 28, 1035–1043. [Google Scholar] [CrossRef] [PubMed]
- Shields, C.L.; Bianciotto, C.; Pirondini, C.; Materin, M.A.; Harmon, S.A.; Shields, J.A. Autofluorescence of choroidal melanoma in 51 cases. Br. J. Ophthalmol. 2008, 92, 617–622. [Google Scholar] [CrossRef] [PubMed]
- Reznicek, L.; Stumpf, C.; Seidensticker, F.; Kampik, A.; Neubauer, A.S.; Kernt, M. Role of wide-field autofluorescence imaging and scanning laser ophthalmoscopy in differentiation of choroidal pigmented lesions. Int. J. Ophthalmol. 2014, 7, 697–703. [Google Scholar] [PubMed]
- Chin, K.; Finger, P.T. Autofluorescence characteristics of suspicious choroidal nevi. Optometry 2009, 80, 126–130. [Google Scholar] [CrossRef] [PubMed]
- Bindewald, A.; Bird, A.C.; Dandekar, S.S.; Dolar-Szczasny, J.; Dreyhaupt, J.; Fitzke, F.W.; Einbock, W.; Holz, F.G.; Jorzik, J.J.; Keilhauer, C.; et al. Classification of fundus autofluorescence patterns in early age-related macular disease. Investig. Ophthalmol. Vis. Sci. 2005, 46, 3309–3314. [Google Scholar] [CrossRef] [PubMed]
- Gündüz, K.; Pulido, J.S.; Bakri, S.J.; Amselem, L.; Petit-Fond, E.; Link, T. Fundus autofluorescence of choroidal melanocytic lesions and the effect of treatment. Trans. Am. Ophthalmol. Soc. 2007, 105, 172–178. [Google Scholar] [PubMed]
- Amselem, L.; Pulido, J.S.; Gunduz, K.; Bakri, S.J.; Diaz-Llopis, M.; Dolan, J.W.; Morgan, K.; de Souza, S.; Link, T.; Rosvold, J. Changes in fundus autofluorescence of choroidal melanomas following treatment. Eye 2009, 23, 428–434. [Google Scholar] [CrossRef][Green Version]
- Maheshwari, A.; Finger, P.T. Regression patterns of choroidal melanoma: After palladium-103 (103Pd) plaque brachytherapy. Eur. J. Ophthalmol. 2018, 28, 722–730. [Google Scholar] [CrossRef] [PubMed]
- Navaratnam, J.; Bærland, T.P.; Eide, N.A.; Faber, R.T.; Rekstad, B.L.; Vavvas, D.G.; Bragadóttir, R. Fundus autofluorescence change as an early indicator of treatment effect of brachytherapy for choroidal melanomas. Ocul. Oncol. Pathol. 2019, 5, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Bindewald-Wittich, A.; Swenshon, T.; Carasco, E.; Dreyhaupt, J.; Willerding, G.D. Blue-light fundus autofluorescence imaging following Ruthenium-106 brachytherapy for choroidal melanoma. Ophthalmologica 2020, 243, 303–315. [Google Scholar] [CrossRef] [PubMed]
- Psomiadi, A.; Haas, G.; Edlinger, M.; Bechrakis, N.E.; Blatsios, G. Ultra-wide-field imaging of choroidal melanoma before and after proton beam radiation therapy. Eur. J. Ophthalmol. 2020, 30, 1397–1402. [Google Scholar] [CrossRef]
- Frampton, G.K.; Kalita, N.; Payne, L.; Colquitt, J.L.; Loveman, E.; Downes, S.M.; Lotery, A.J. Fundus autofluorescence imaging: Systematic review of test accuracy for the diagnosis and monitoring of retinal conditions. Eye 2017, 31, 995–1007. [Google Scholar] [CrossRef] [PubMed]
- Delori, F.; Greenberg, J.P.; Woods, R.L.; Fischer, J.; Duncker, T.; Sparrow, J.; Smith, R.T. Quantitative measurements of autofluorescence with the scanning laser ophthalmoscope. Investig. Ophthalmol. Vis. Sci. 2011, 52, 9379–9390. [Google Scholar] [CrossRef]
- Sparrow, J.R.; Duncker, T.; Schuerch, K.; Paavo, M.; de Carvalho, J.R.L. Lessons learned from quantitative fundus autofluorescence. Prog. Retin. Eye Res. 2020, 74, 100774. [Google Scholar] [CrossRef] [PubMed]
- Dysli, C.; Wolf, S.; Berezin, M.Y.; Sauer, L.; Hammer, M.; Zinkernagel, M.S. Fluorescence lifetime imaging ophthalmoscopy. Prog Retin Eye Res. 2017, 60, 120–143. [Google Scholar] [CrossRef]
- Sauer, L.; Vitale, A.S.; Modersitzki, N.K.; Bernstein, P.S. Fluorescence lifetime imaging ophthalmoscopy: Autofluorescence imaging and beyond. Eye 2021, 35, 93–109. [Google Scholar] [CrossRef] [PubMed]
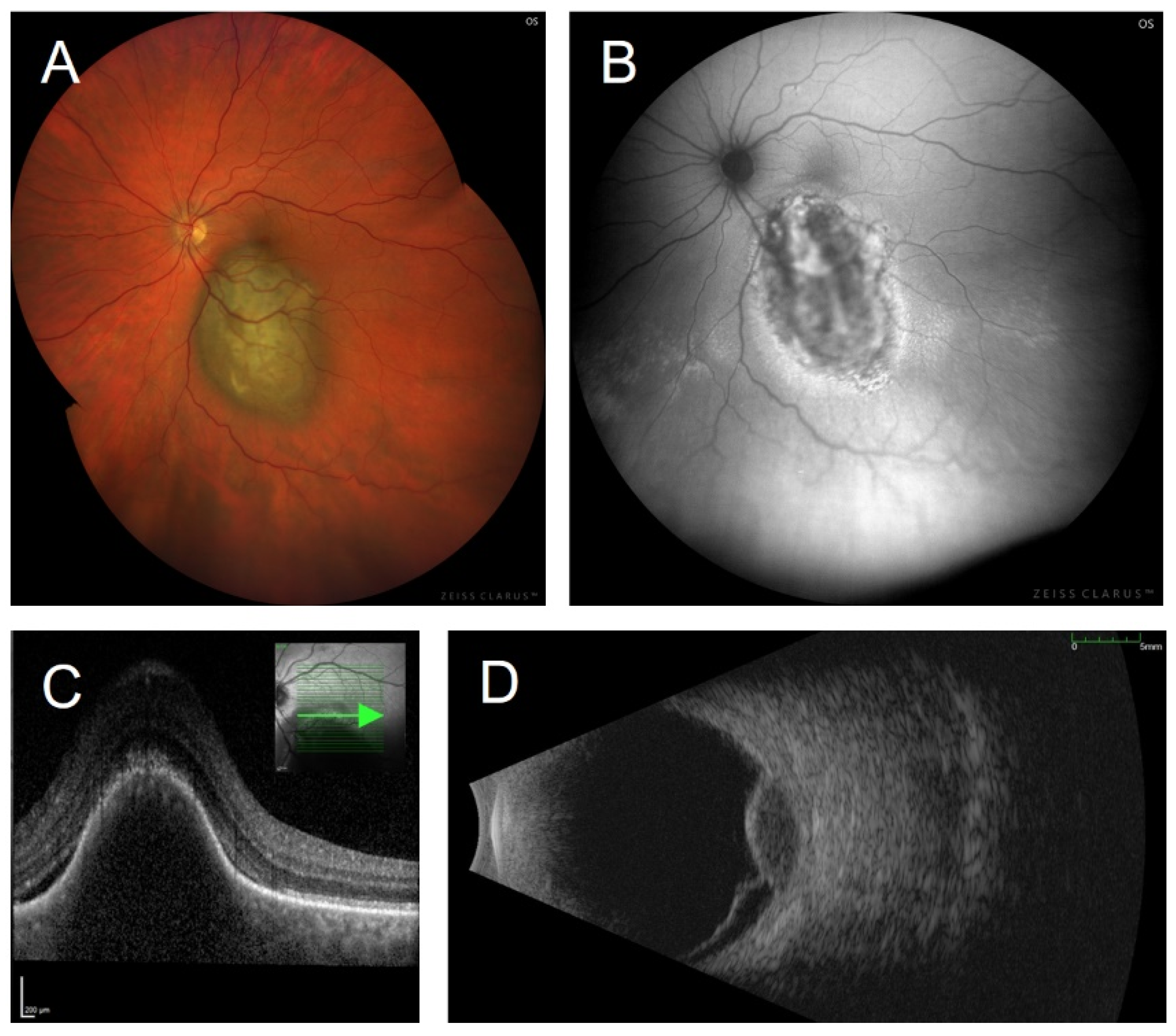
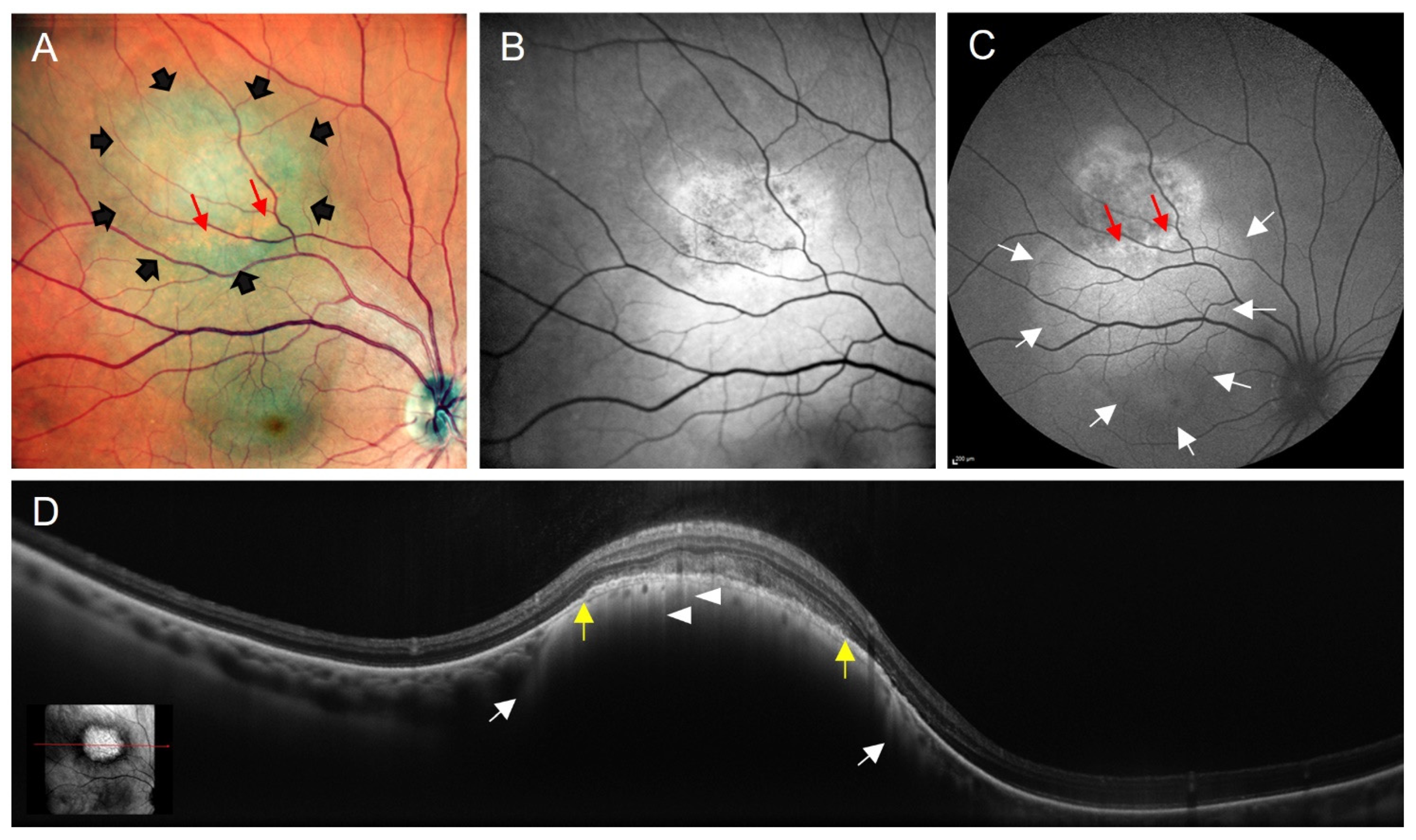
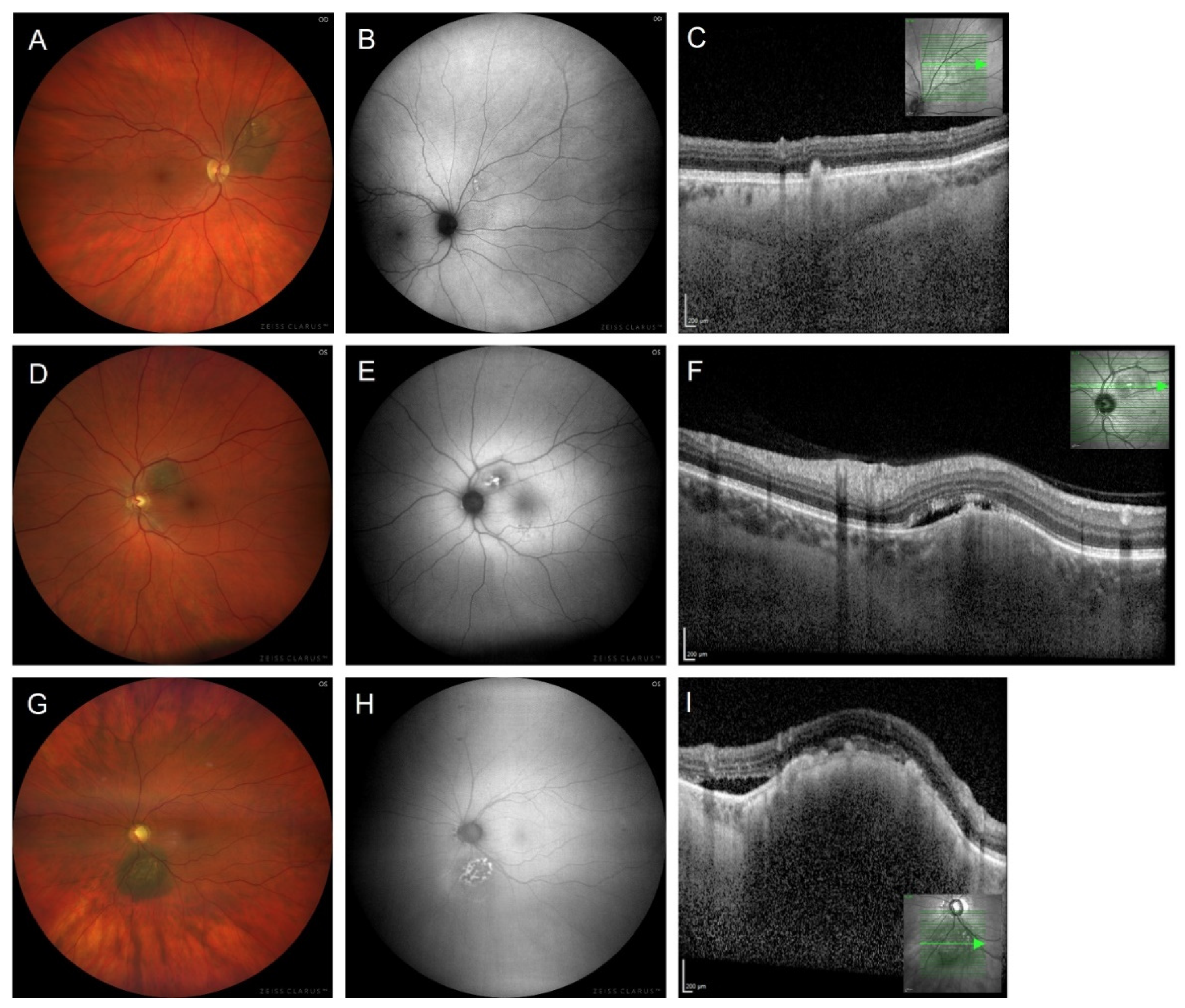
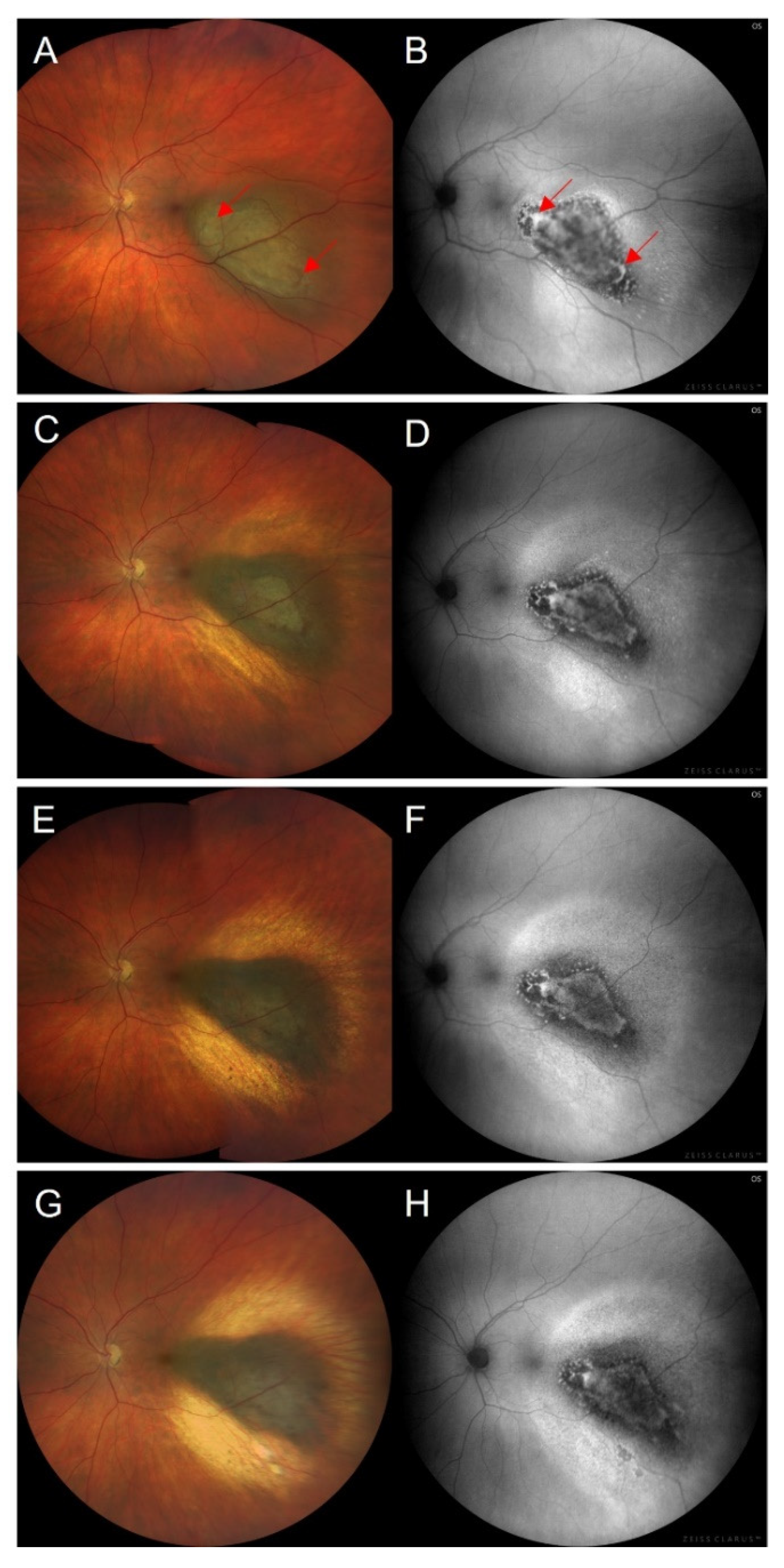
| Study | Technical Device | Excitation Wavelength | Detection of Emission | Number of Eyes | Design | Major FAF Findings |
|---|---|---|---|---|---|---|
| Shields et al. [66] | Fundus camera (ZEISS) | 580 nm | >695 nm | 4 small melanomas (2 pigmented, 2 nonpigmented) | Four case reports | Tumour itself isoautofluorescent. Orange pigment with discrete, bright increased autofluorescence. |
| Gündüz et al. [76] | cSLO (HRA, Heidelberg Engineering) | 488 nm | >500 nm | 23 (12 melanomas, 11 nevi) | Retrospective chart review | ~90% of tumours showed at least 1 focus of increased FAF. Orange pigment in 13 eyes was correlated with increased FAF: completely in 8 tumours (61.5%), partially in 3 tumours (23.1%), not in 2 tumours (15.4%). |
| Gündüz et al. [80] | cSLO, (HRA, Heidelberg Engineering) | 488 nm | >500 nm | 30 (19 melanomas, 11 nevi) | Retrospective chart review | Patchy FAF pattern: areas of increased FAF between areas of normal FAF. Diffuse FAF pattern: increased FAF with indistinct borders. Nevi (n = 11) presented with patchy FAF pattern. Melanomas presented with patchy (n = 6) and diffuse (n = 13) FAF pattern. Orange pigment was present in 15 eyes. |
| Samuelsson et al. [81] | Fundus camera (TRC-50DX, Topcon) | 535–585 | 605–715 nm | 49 (19 nevi, 10 IML, 10 melanomas, 10 other choroidal tumours) | Retrospective study | Melanomas revealed a patchy (n = 6) and a diffuse (n = 4) FAF pattern. Significant differences were observed between choroidal nevi, IML, choroidal melanomas, and other choroidal tumours regarding FAF findings. |
| Lavinsky et al. [65] | cSLO (HRA2, Heidelberg Engineering) | 488 nm | >500 nm | 20 (5 melanomas, 15 nevi) | Retrospective observational study/consecutive case series | Nevi were associated with normal FAF. Melanomas were associated with confluent to plaque-like increased FAF due to orange pigment. |
| Cennamo et al. [79] | cSLO (SPECTRALIS HRA+OCT, Heidelberg Engineering) | 488 nm | >500 nm | 165 (65 melanomas, 100 nevi) | Letter to the editor | Nevi: normal (n = 40) or decreased (n = 60) FAF. Melanomas: plaque-like increased FAF and surrounding decreased FAF. Orange pigment: brightly increased FAF with adjacent dark areas with decreased FAF. |
| Shields et al. [82] | Fundus camera (Carl Zeiss Meditec) | 580 nm | >695 nm | 64 nevi | Consecutive case series | Decreased FAF in 56%, normal FAF in 19%, and increased FAF in 25%. |
| Shields et al. [83] | Fundus camera (Carl Zeiss Meditec) | 580 nm | >695 nm | 51 melanomas | Non-comparative case series | Decreased FAF in 39%, normal FAF in 6%, and increased FAF in 55%. |
| Reznicek et al. [84] | SLO (Panoramic 200Tx, Optos) | 532 nm | 540–800 nm | 139 (101 melanomas, 38 nevi) | Consecutive case series | Mean FAF intensity of choroidal melanomas was significantly lower than the mean FAF intensity of choroidal nevi. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bindewald-Wittich, A.; Holz, F.G.; Ach, T.; Fiorentzis, M.; Bechrakis, N.E.; Willerding, G.D. Fundus Autofluorescence Imaging in Patients with Choroidal Melanoma. Cancers 2022, 14, 1809. https://doi.org/10.3390/cancers14071809
Bindewald-Wittich A, Holz FG, Ach T, Fiorentzis M, Bechrakis NE, Willerding GD. Fundus Autofluorescence Imaging in Patients with Choroidal Melanoma. Cancers. 2022; 14(7):1809. https://doi.org/10.3390/cancers14071809
Chicago/Turabian StyleBindewald-Wittich, Almut, Frank G. Holz, Thomas Ach, Miltiadis Fiorentzis, Nikolaos E. Bechrakis, and Gregor D. Willerding. 2022. "Fundus Autofluorescence Imaging in Patients with Choroidal Melanoma" Cancers 14, no. 7: 1809. https://doi.org/10.3390/cancers14071809
APA StyleBindewald-Wittich, A., Holz, F. G., Ach, T., Fiorentzis, M., Bechrakis, N. E., & Willerding, G. D. (2022). Fundus Autofluorescence Imaging in Patients with Choroidal Melanoma. Cancers, 14(7), 1809. https://doi.org/10.3390/cancers14071809






