Together Intra-Tumor Hypoxia and Macrophagic Immunity Are Driven Worst Outcome in Pediatric High-Grade Osteosarcomas
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient and Tumor Data
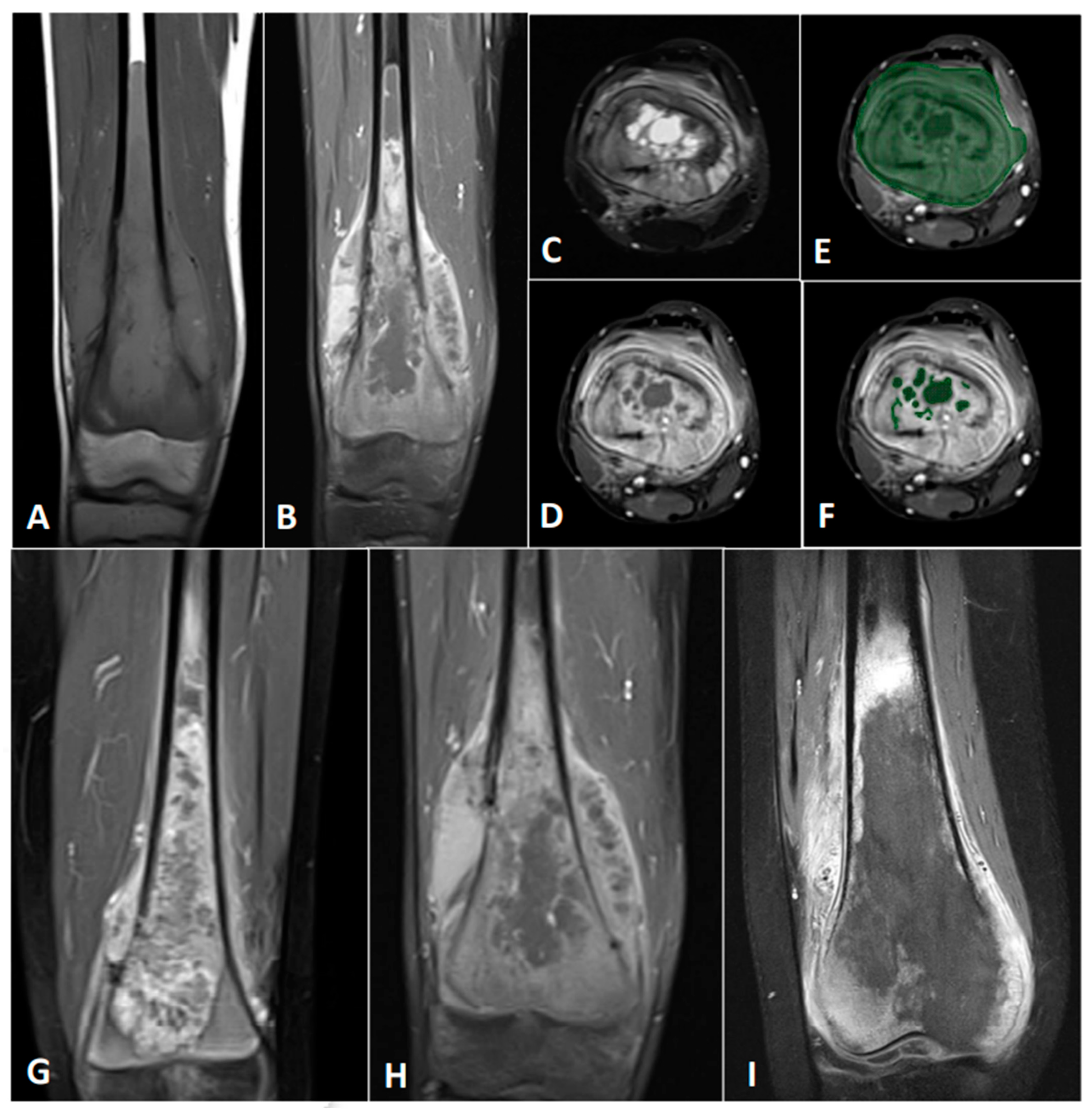
2.2. MRI and Its Processing
2.3. Immunohistochemical Analyses on FFPE Samples
2.4. Statistical Analyses
3. Results
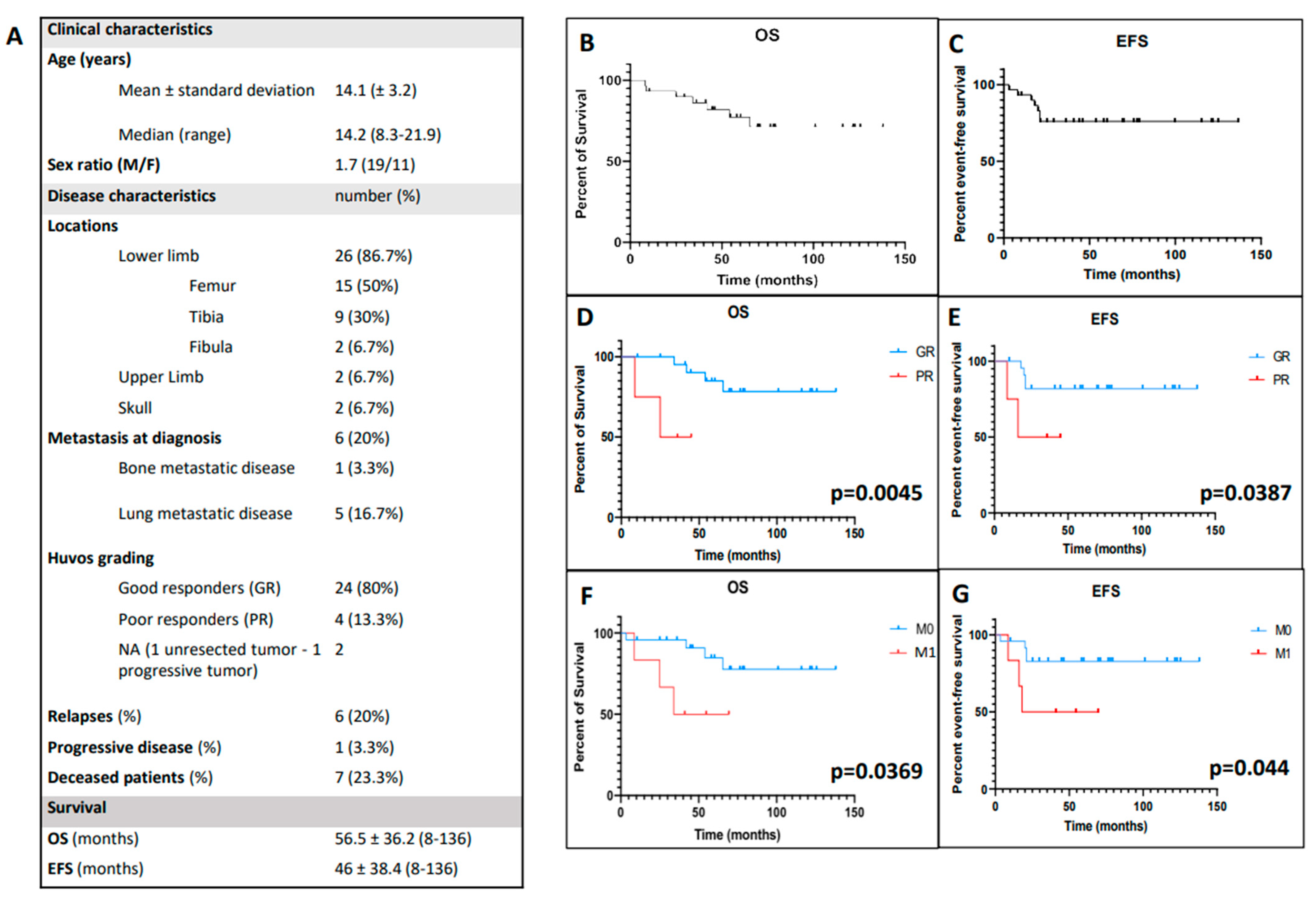
3.1. Clinical Characteristics of the Studied Cohort
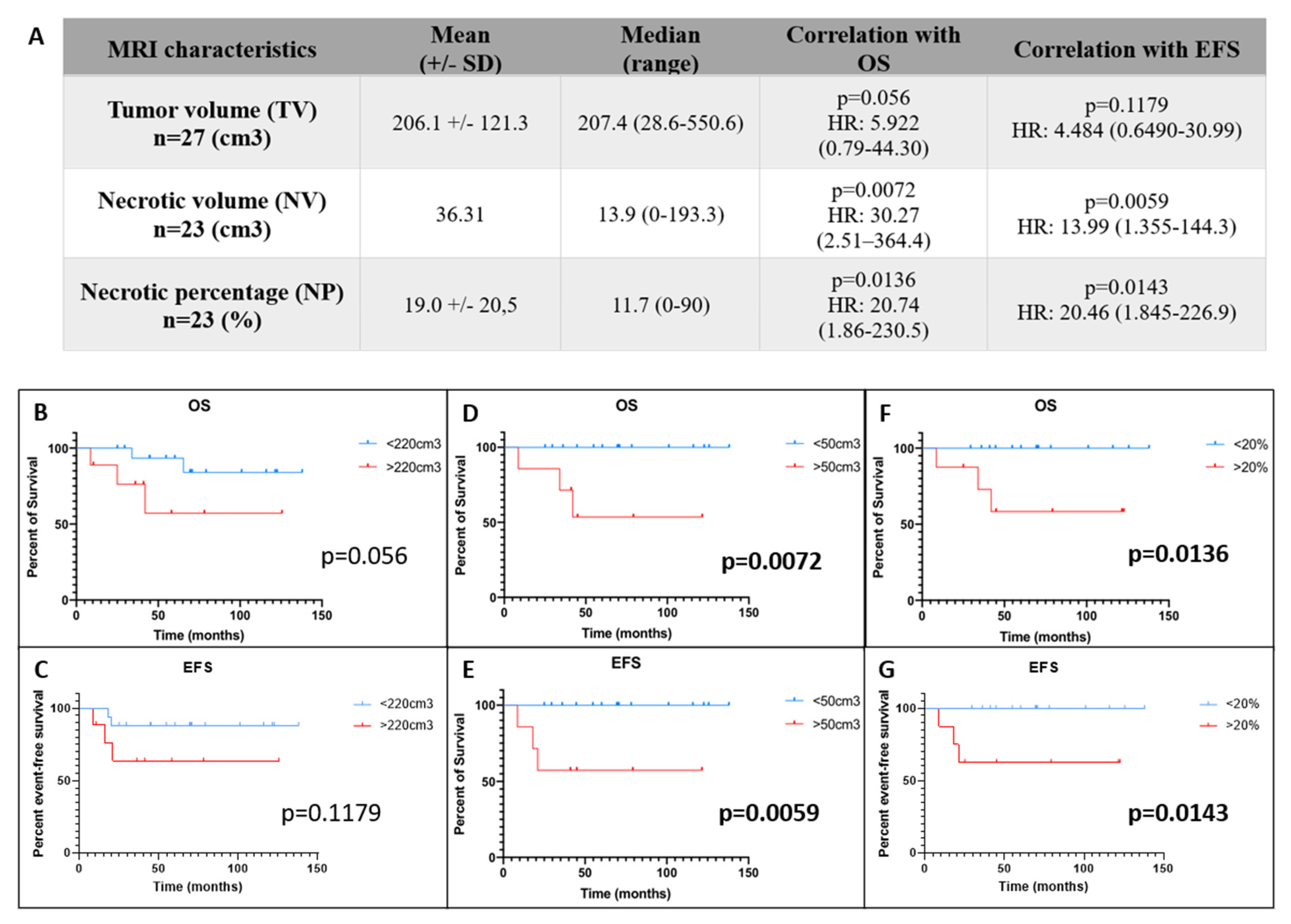
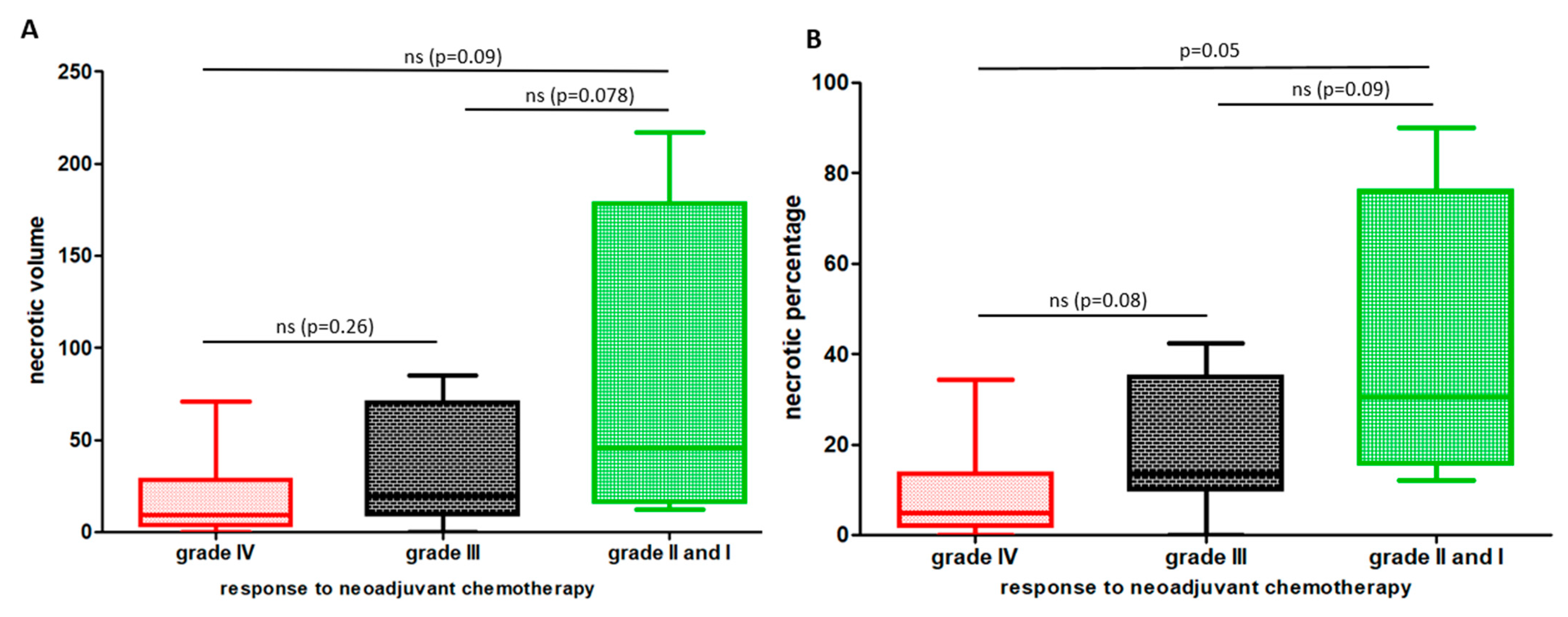
3.2. Entire Necrotic Volume (NV) and the Necrotic Percentage (NP) at Diagnosis Represent New Predictive Radiological Markers of Outcome in OTS
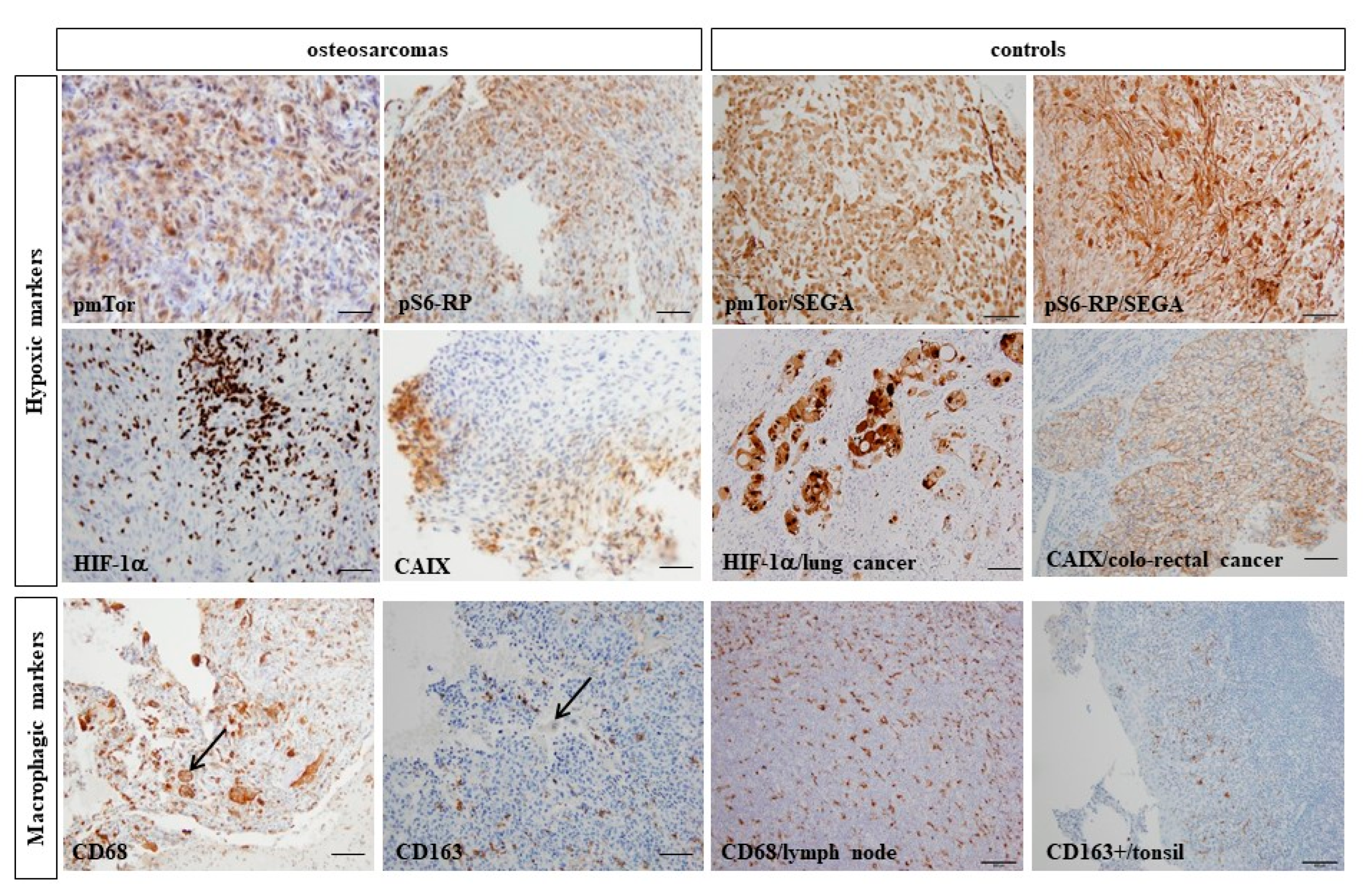
3.3. Hypoxic and Macrophagic Immunohistochemical Biomarkers Correlate with Survivals
3.4. Hypoxic and Macrophagic Immunohistochemical Biomarkers Correlate with Survivals
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smeland, S.; Bielack, S.S.; Whelan, J.; Bernstein, M.; Hogendoorn, P.; Krailo, M.D.; Gorlick, R.; Janeway, K.A.; Ingleby, F.C.; Anninga, J.; et al. Survival and prognosis with osteosarcoma: Outcomes in more than 2000 patients in the EURAMOS-1 (European and American Osteosarcoma Study) cohort. Eur. J. Cancer 2019, 109, 36–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lacour, B.; Guyot-Goubin, A.; Guissou, S.; Bellec, S.; Desandes, E.; Clavel, J. Incidence of childhood cancer in France: National Children Cancer Registries, 2000–2004. Eur. J. Cancer Prev. 2010, 19, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Allison, D.C.; Carney, S.C.; Ahlmann, E.R.; Hendifar, A.; Chawla, S.; Fedenko, A.; Angeles, C.; Menendez, L.R. A meta-analysis of osteosarcoma outcomes in the modern medical era. Sarcoma 2012, 2012, 704872. [Google Scholar] [CrossRef] [Green Version]
- Piperno-Neumann, S.; Le Deley, M.C.; Rédini, F.; Pacquement, H.; Marec-Berard, P.; Petit, P.; Brisse, H.; Lervat, C.; Gentet, J.C.; Entz-Werle, N.; et al. Zoledronate in combination with chemotherapy and surgery to treat osteosarcoma (OS2006): A randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2016, 17, 1070–1080. [Google Scholar] [CrossRef]
- Brard, C.; Piperno-Neumann, S.; Delaye, J.; Brugieres, L.; Hampson, L.V.; Le Teuff, G.; Le Deley, M.C.; Gaspar, N. Sarcome-13/OS2016 trial protocol: A multicentre, randomised, open-label, phase II trial of mifamurtide combined with postoperative chemotherapy for patients with newly diagnosed high-risk osteosarcoma. BMJ Open 2019, 9, e025877. [Google Scholar] [CrossRef]
- Marina, N.M.; Smeland, S.; Bielack, S.S.; Bernstein, M.; Jovic, G.; Krailo, M.D.; Hook, J.M.; Arndt, C.; van den Berg, H.; Brennan, B.; et al. Comparison of MAPIE versus MAP in patients with a poor response to preoperative chemotherapy for newly diagnosed high-grade osteosarcoma (EURAMOS-1): An open-label, international, randomised controlled trial. Lancet Oncol. 2016, 17, 1396–1408. [Google Scholar] [CrossRef] [Green Version]
- Gaspar, N.; Occean, B.V.; Pacquement, H.; Bompas, E.; Bouvier, C.; Brisse, H.J.; Castex, M.P.; Cheurfa, N.; Corradini, N.; Delaye, J.; et al. Results of methotrexate-etoposide-ifosfamide based regimen (M-EI) in osteosarcoma patients included in the French OS2006/sarcome-09 study. Eur. J. Cancer 2018, 88, 57–66. [Google Scholar] [CrossRef]
- Bielack, S.S.; Smeland, S.; Whelan, J.S.; Marina, N.; Jovic, G.; Hook, J.M.; Krailo, M.D.; Gebhardt, M.; Pápai, Z.; Meyer, J.; et al. Methotrexate, Doxorubicin, and Cisplatin (MAP) Plus Maintenance Pegylated Interferon Alfa-2b Versus MAP Alone in Patients With Resectable High-Grade Osteosarcoma and Good Histologic Response to Preoperative MAP: First Results of the EURAMOS-1 Good Response Randomized Controlled Trial. J. Clin. Oncol. 2015, 33, 2279–2287. [Google Scholar]
- Cianchi, F.; Vinci, M.C.; Supuran, C.T.; Peruzzi, B.; De Giuli, P.; Fasolis, G.; Perigli, G.; Pastorekova, S.; Papucci, L.; Pini, A.; et al. Selective Inhibition of Carbonic Anhydrase IX Decreases Cell Proliferation and Induces Ceramide-Mediated Apoptosis in Human Cancer Cells. J. Pharmacol. Exp. Ther. 2010, 334, 710–719. [Google Scholar] [CrossRef] [Green Version]
- Shin, H.J.; Rho, S.B.; Jung, D.C.; Han, I.O.; Oh, E.S.; Kim, J.Y. Carbonic anhydrase IX (CA9) modulates tumor-associated cell migration and invasion. J. Cell Sci. 2011, 124, 1077–1087. [Google Scholar] [CrossRef] [Green Version]
- Csaderova, L.; Debreova, M.; Radvak, P.; Stano, M.; Vrestiakova, M.; Kopacek, J.; Pastorekova, S.; Svastova, E. The effect of carbonic anhydrase IX on focal contacts during cell spreading and migration. Front. Physiol. 2013, 4, 271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsubara, T.; Diresta, G.R.; Kakunaga, S.; Li, D.; Healey, J.H. Additive Influence of Extracellular pH, Oxygen Tension, and Pressure on Invasiveness and Survival of Human Osteosarcoma Cells. Front. Oncol. 2013, 3, 199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bristow, R.G.; Hill, R.P. Hypoxia and metabolism. Hypoxia, DNA repair and genetic instability. Nat. Rev. Cancer 2008, 8, 180–192. [Google Scholar] [CrossRef] [PubMed]
- Moeller, B.J.; Richardson, R.A.; Dewhirst, M.W. Hypoxia and radiotherapy: Opportunities for improved outcomes in cancer treatment. Cancer Metastasis Rev. 2007, 26, 241–248. [Google Scholar] [CrossRef]
- Vaupel, P.; Mayer, A. Hypoxia in cancer: Significance and impact on clinical outcome. Cancer Metastasis Rev. 2007, 26, 225–239. [Google Scholar] [CrossRef]
- Brahimi-Horn, M.C.; Chiche, J.; Pouysségur, J. Hypoxia and cancer. J. Mol. Med. 2007, 85, 1301–1307. [Google Scholar] [CrossRef] [Green Version]
- Semenza, G.L. Hypoxia-Inducible Factors in Physiology and Medicine. Cell 2012, 148, 399–408. [Google Scholar] [CrossRef] [Green Version]
- Ferrara, N.; Kerbel, R.S. Angiogenesis as a therapeutic target. Nature 2005, 438, 967–974. [Google Scholar] [CrossRef]
- Laplante, M.; Sabatini, D.M. Regulation of mTORC1 and its impact on gene expression at a glance. J. Cell Sci. 2013, 126, 1713–1719. [Google Scholar] [CrossRef] [Green Version]
- Baptista, A.M.; Camargo, A.F.D.F.; Filippi, R.Z.; Oliveira, C.R.; Azevedo Neto, R.S.; Camargo, O.P. Correlation between the expression of vegf and survival in osteosarcoma. Acta Ortop. Bras. 2014, 22, 250–255. [Google Scholar] [CrossRef]
- Mizobuchi, H.; García-Castellano, J.M.; Philip, S.; Healey, J.H.; Gorlick, R. Hypoxia markers in human osteosarcoma: An exploratory study. Clin. Orthop. Relat. Res. 2008, 466, 2052–2059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Hu, Z.; Wang, Z.; Cui, Y.; Cui, X. Angiopoietin-like protein 2 is an important facilitator of tumor proliferation, metastasis, angiogenesis and glycolysis in osteosarcoma. Am. J. Transl. Res. 2019, 11, 6341–6355. [Google Scholar] [PubMed]
- Bingle, L.; Brown, N.J.; Lewis, C.E. The role of tumour-associated macrophages in tumour progression: Implications for new anticancer therapies. J. Pathol. 2002, 196, 254–265. [Google Scholar] [CrossRef] [PubMed]
- Leek, R.D.; Lewis, C.E.; Whitehouse, R.; Greenall, M.; Clarke, J.; Harris, A.L. Association of macrophage infiltration with angiogenesis and prognosis in invasive breast carcinoma. Cancer Res. 1996, 56, 4625–4629. [Google Scholar] [PubMed]
- Rogers, T.L.; Holen, I. Tumour macrophages as potential targets of bisphosphonates. J. Transl. Med. 2011, 9, 177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dumars, C.; Ngyuen, J.M.; Gaultier, A.; Lanel, R.; Corradini, N.; Gouin, F.; Heymann, D.; Heymann, M.F. Dysregulation of macrophage polarization is associated with the metastatic process in osteosarcoma. Oncotarget 2016, 7, 78343–78354. [Google Scholar] [CrossRef] [Green Version]
- Gomez-Brouchet, A.; Illac, C.; Gilhodes, J.; Bouvier, C.; Aubert, S.; Guinebretiere, J.M.; Marie, B.; Larousserie, F.; Entz-Werle, N.; de Pinieux, G.; et al. CD163-positive tumor-associated macrophages and CD8-positive cytotoxic lymphocytes are powerful diagnostic markers for the therapeutic stratification of osteosarcoma patients: An immunohistochemical analysis of the biopsies from the French OS2006 phase 3 trial. OncoImmunology 2017, 6, e1331193. [Google Scholar]
- Dehne, N.; Mora, J.; Namgaladze, D.; Weigert, A.; Brüne, B. Cancer cell and macrophage cross-talk in the tumor microenvironment. Curr. Opin. Pharmacol. 2017, 35, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Kaste, S.C. Imaging pediatric bone sarcomas. Radiol. Clin. N. Am. 2011, 49, 749–765. [Google Scholar] [CrossRef] [Green Version]
- Silva, F.D.; Pinheiro, L.; Cristofano, C.; de Oliveira Schiavon, J.L.; Lederman, H.M. Magnetic Resonance Imaging in Pediatric Bone Tumors. Curr. Radiol. Rep. 2014, 2, 77. [Google Scholar] [CrossRef]
- Fayad, L.M.; Jacobs, M.A.; Wang, X.; Carrino, J.A.; Bluemke, D.A. Musculoskeletal tumors: How to use anatomic, functional, and metabolic MR techniques. Radiology 2012, 265, 340–356. [Google Scholar] [CrossRef] [Green Version]
- Holscher, H.C.; Bloem, J.L.; van der Woude, H.J.; Hermans, J.; Nooy, M.A.; Taminiau, A.H.; Hogendoorn, P.C. Can MRI predict the histopathological response in patients with osteosarcoma after the first cycle of chemotherapy? Clin. Radiol. 1995, 50, 384–390. [Google Scholar] [CrossRef]
- Lin, P.; Yang, P.F.; Chen, S.; Shao, Y.Y.; Xu, L.; Wu, Y.; Teng, W.; Zhou, X.Z.; Li, B.H.; Luo, C.; et al. A Delta-radiomics model for preoperative evaluation of Neoadjuvant chemotherapy response in high-grade osteosarcoma. Cancer Imaging 2020, 20, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dufau, J.; Bouhamama, A.; Leporq, B.; Malaureille, L.; Beuf, O.; Gouin, F.; Pilleul, F.; Marec-Berard, P. Prédiction de la réponse à la chimiothérapie des ostéosarcomes à partir des données radiomiques issues des IRM diagnostiques. Bull. Cancer 2019, 106, 983–999. [Google Scholar] [CrossRef] [PubMed]
- Le Deley, M.C.; Guinebretière, J.M.; Gentet, J.C.; Pacquement, H.; Pichon, F.; Marec-Bérard, P.; Entz-Werlé, N.; Schmitt, C.; Brugières, L.; Vanel, D.; et al. SFOP OS94: A randomised trial comparing preoperative high-dose methotrexate plus doxorubicin to high-dose methotrexate plus etoposide and ifosfamide in osteosarcoma patients. Eur. J. Cancer 2007, 43, 752–761. [Google Scholar] [CrossRef] [PubMed]
- Therasse, P.; Arbuck, S.G.; Eisenhauer, E.A.; Wanders, J.; Kaplan, R.S.; Rubinstein, L.; Verweij, J.; van Glabbeke, M.; van Oosterom, A.T.; Christian, M.C.; et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J. Natl. Cancer Inst. 2000, 92, 205–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, J.A.; Babyn, P.S.; Chan, H.S.; Thorner, P.S.; Pron, G.E.; Krajbich, I.J. Extremity osteosarcoma in childhood: Prognostic value of radiologic imaging. Radiology 1993, 189, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Holscher, H.C.; Bloem, J.L.; Nooy, M.A.; Taminiau, A.H.; Eulderink, F.; Hermans, J. The value of MR imaging in monitoring the effect of chemotherapy on bone sarcomas. Am. J. Roentgenol. 1990, 154, 763–769. [Google Scholar] [CrossRef] [Green Version]
- Holscher, H.C.; Bloem, J.L.; Vanel, D.; Hermans, J.; Nooy, M.A.; Taminiau, A.H.; Henry-Amar, M. Osteosarcoma: Chemotherapy-induced changes at MR imaging. Radiology 1992, 182, 839–844. [Google Scholar] [CrossRef]
- Baunin, C.; Aschero, A.; Gorincour, G.; Desvignes, C.; Boulière, B.; Gentet, J.C.; Bollini, G.; Bouvier, C.; Simeoni, M.C.; Petit, P. IRM de diffusion: Evaluation de la reponse a la chimiotherapie dans l’osteosarcome. J. Radiol. 2008, 89, 1422–1423. [Google Scholar] [CrossRef]
- Dyke, J.P.; Panicek, D.M.; Healey, J.H.; Meyers, P.A.; Huvos, A.G.; Schwartz, L.H.; Thaler, H.T.; Tofts, P.S.; Gorlick, R.; Koutcher, J.A.; et al. Osteogenic and Ewing sarcomas: Estimation of necrotic fraction during induction chemotherapy with dynamic contrast-enhanced MR imaging. Radiology 2003, 228, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Reddick, W.E.; Taylor, J.S.; Fletcher, B.D. Dynamic MR imaging (DEMRI) of microcirculation in bone sarcoma. J. Magn. Reson. Imaging 1999, 10, 277–285. [Google Scholar] [CrossRef]
- Monsky, W.L.; Jin, B.; Molloy, C.; Canter, R.J.; Li, C.S.; Lin, T.C.; Borys, D.; Mack, W.; Kim, I.; Buonocore, M.H.; et al. Semi-automated volumetric quantification of tumor necrosis in soft tissue sarcoma using contrast-enhanced MRI. Anticancer Res. 2012, 32, 4951–4961. [Google Scholar]
- McErlean, A.; Panicek, D.M.; Zabor, E.C.; Moskowitz, C.S.; Bitar, R.; Motzer, R.J.; Hricak, H.; Ginsberg, M.S. Intra- and interobserver variability in CT measurements in oncology. Radiology 2013, 269, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, Y.; Li, H.; Bu, J.; Li, X.; Chen, Z.; Xiao, T. Hypoxia-inducible factor-1 expression predicts osteosarcoma patients’ survival: A meta-analysis. Int. J. Biol. Markers 2016, 31, e229–e234. [Google Scholar] [CrossRef]
- Okuno, K.; Matsubara, T.; Nakamura, T.; Iino, T.; Kakimoto, T.; Asanuma, K.; Matsumine, A.; Sudo, A. Carbonic anhydrase IX enhances tumor cell proliferation and tumor progression in osteosarcoma. Onco Targets Ther. 2018, 11, 6879–6886. [Google Scholar] [CrossRef] [Green Version]
- Gomez-Brouchet, A.; Gilhodes, J.; Acker, N.V.; Brion, R.; Bouvier, C.; Assemat, P.; Gaspar, N.; Aubert, S.; Guinebretiere, J.M.; Marie, B.; et al. Characterization of macrophages and osteoclasts in the osteosarcoma tumor microenvironment at diagnosis: New perspective for osteosarcoma treatment? Cancers 2021, 13, 423. [Google Scholar] [CrossRef]
- Marchais, A.; Marques Da Costa, M.E.; Job, B.; Abbas, R.; Drubay, D.; Piperno-Neumann, S.; Fromigué, O.; Gomez-Brouchet, A.; Redini, F.; Droit, R.; et al. Immune infiltrate and tumor microenvironement transcriptional programs stratify pediatric osteosarcoma into prognostic groups at diagnosis. Cancer Res. 2022. CAN-20-4189-A.2020. [Google Scholar] [CrossRef]
- Venturella, M.; Criscuoli, M.; Carraro, F.; Naldini, A.; Zocco, D. Interplay between Hypoxia and Extracellular Vesicles in Cancer and Inflammation. Biology 2021, 10, 606. [Google Scholar] [CrossRef]
- Park, J.E.; Dutta, B.; Tse, S.W.; Gupta, N.; Tan, C.F.; Low, J.K.; Yeoh, K.W.; Kon, O.L.; Tam, J.P.; Sze, S.K. Hypoxia-induced tumor exosomes promote M2-like macrophage polarization of infiltrating myeloid cells and microRNA-mediated metabolic shift. Oncogene 2019, 38, 5158–5173. [Google Scholar] [CrossRef] [PubMed]
- Wolf-Dennen, K.; Kleinerman, E.S. Exosomes: Dynamic Mediators of Extracellular Communication in the Tumor Microenvironment. Adv. Exp. Med. Biol. 2020, 1258, 189–197. [Google Scholar] [PubMed]

| IHC Targets | Positive Cells Median (Range) | Hyper/No Expression | OS Correlation | EFS Correlation | Correlation with Necrotic Percentage | Correlation with CD68+ Cells | Correlation with CD163+ Cells |
|---|---|---|---|---|---|---|---|
| pS6-RP (n = 29) | 40 (0–90) | 25/4 | p = 0.0657 HR: 4.533 (0.9063–22.67) | p = 0.0564 HR: 4.848 (0.9579–24.53) | Rho: 0.044 p = 0.839 | Rho: 0.3904 p = 0.0441 | Rho: 0.6008 p = 0.0012 |
| pmTor (n = 27) | 10 (0–80) | 19/8 | p = 0.5865 HR: 1.208 (0.2405–6.072) | p = 0.9989 HR: 1.001 (0.1994–5.027) | Rho: 0.286 p = 0.197 | Rho: 0.6021 p = 0.0014 | Rho: 0.7374 p < 0.0001 |
| HIF-1α (n = 28) | 1 (0–80) | 14/14 | p = 0.0029 HR: 22.86 (2.910–179.5) | p = 0.0172 HR: 9.676 (1.496–62.59) | Rho: 0.115 p = 0.61 | Rho: 0.5838 p = 0.0017 | Rho: 0.3021 p = 0.1422 |
| CAIX (n = 26) | 0 (0–40) | 12/14 | p = 0.0082 HR: 25.34 (2.308–278.1) | p = 0.0459 HR: 8.357 (1.04–67.16) | Rho: 0.397 p = 0.083 | Rho: 0.5255 p = 0.0084 | Rho: 0.3698 p = 0.0824 |
| CD163 (n = 26) | 20 (0–60) | 19/9 | p = 0.6259 HR: 1.551 (0.2658–9.045) | p = 0.4467 HR: 1.989 (0.3384–11.69) | Rho: 0.34 p = 0.142 | Rho: 0.6927 p < 0.0001 | |
| CD68 (n = 28) | 10 (1–40) | 15/13 | p = 0.0214 HR: 8.947 (1.384–57.83) | p = 0.0813 HR: 4.583 (0.8274–25.39) | Rho: 0.307 p = 0.164 | / | Rho: 0.6927 p < 0.0001 |
| CD68+/CD163+ (n = 28) | / | 16/12 | p = 0.3526 | p = 0.6123 | / | / | / |
| CD68+/CD163+/GC+ (n = 28) | / | 8/20 | p = 0.0515 | p = 0.0372 | / | / | / |
| Giant cells (GC) (n = 29) | / | 9/20 | p = 0.0882 | p = 0.0178 | / | / | / |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nazon, C.; Pierrevelcin, M.; Willaume, T.; Lhermitte, B.; Weingertner, N.; Marco, A.D.; Bund, L.; Vincent, F.; Bierry, G.; Gomez-Brouchet, A.; et al. Together Intra-Tumor Hypoxia and Macrophagic Immunity Are Driven Worst Outcome in Pediatric High-Grade Osteosarcomas. Cancers 2022, 14, 1482. https://doi.org/10.3390/cancers14061482
Nazon C, Pierrevelcin M, Willaume T, Lhermitte B, Weingertner N, Marco AD, Bund L, Vincent F, Bierry G, Gomez-Brouchet A, et al. Together Intra-Tumor Hypoxia and Macrophagic Immunity Are Driven Worst Outcome in Pediatric High-Grade Osteosarcomas. Cancers. 2022; 14(6):1482. https://doi.org/10.3390/cancers14061482
Chicago/Turabian StyleNazon, Charlotte, Marina Pierrevelcin, Thibault Willaume, Benoît Lhermitte, Noelle Weingertner, Antonio Di Marco, Laurent Bund, Florence Vincent, Guillaume Bierry, Anne Gomez-Brouchet, and et al. 2022. "Together Intra-Tumor Hypoxia and Macrophagic Immunity Are Driven Worst Outcome in Pediatric High-Grade Osteosarcomas" Cancers 14, no. 6: 1482. https://doi.org/10.3390/cancers14061482
APA StyleNazon, C., Pierrevelcin, M., Willaume, T., Lhermitte, B., Weingertner, N., Marco, A. D., Bund, L., Vincent, F., Bierry, G., Gomez-Brouchet, A., Redini, F., Gaspar, N., Dontenwill, M., & Entz-Werle, N. (2022). Together Intra-Tumor Hypoxia and Macrophagic Immunity Are Driven Worst Outcome in Pediatric High-Grade Osteosarcomas. Cancers, 14(6), 1482. https://doi.org/10.3390/cancers14061482







