Locoregional Treatments for Metastatic Gastrointestinal Stromal Tumor in British Columbia: A Retrospective Cohort Study from January 2008 to December 2017
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methodology
2.1. Study Subjects
2.2. Data Collection and Analysis
2.3. Statistical Analysis
2.4. Ethics
3. Results
3.1. Cohort Characteristics
3.2. Surgery
3.3. Radiotherapy (RT)
3.4. Local Ablation
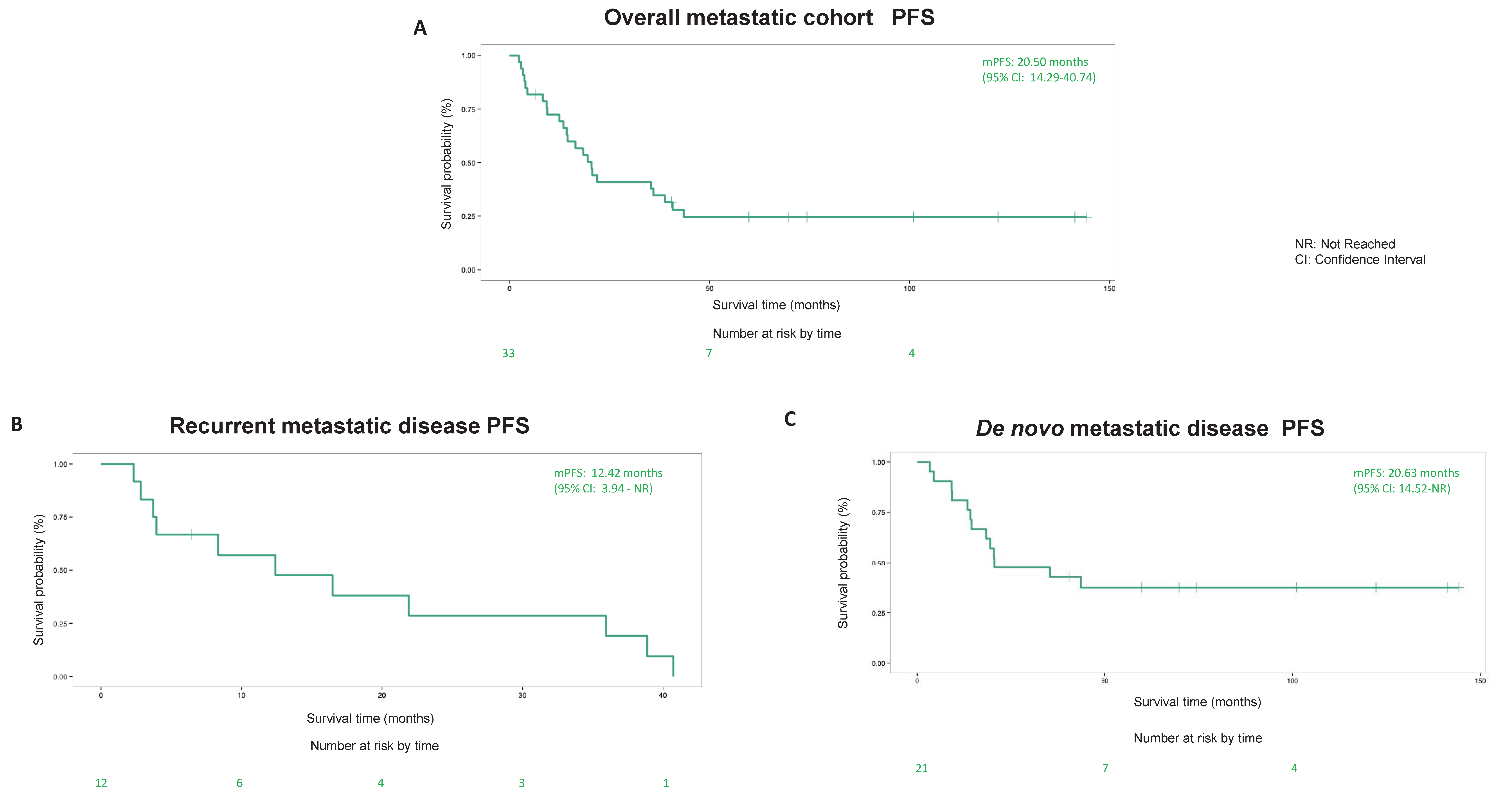
3.5. Survival Outcome
4. Discussions
4.1. Surgery
4.2. Radiotherapy (RT)
4.3. Local Ablation
4.4. Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Demetri, G.D.; Von Mehren, M.; Blanke, C.D.; Van den Abbeele, A.D.; Eisenberg, B.; Roberts, P.J.; Heinrich, M.C.; Tuveson, D.A.; Singer, S.; Janicek, M.; et al. Efficacy and Safety of Imatinib Mesylate in Advanced Gastrointestinal Stromal Tumors. N. Engl. J. Med. 2002, 347, 472–480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joensuu, H.; Roberts, P.J.; Sarlomo-Rikala, M.; Andersson, L.C.; Tervahartiala, P.; Tuveson, D.; Silberman, S.L.; Capdeville, R.; Dimitrijevic, S.; Druker, B.; et al. Effect of the Tyrosine Kinase Inhibitor STI571 in a Patient with a Metastatic Gastrointestinal Stromal Tumor. N. Engl. J. Med. 2001, 344, 1052–1056. [Google Scholar] [CrossRef] [PubMed]
- Patel, S. Long-term efficacy of imatinib for treatment of metastatic GIST. Cancer Chemother. Pharmacol. 2013, 72, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Demetri, G.D.; Reichardt, P.; Kang, Y.-K.; Blay, J.-Y.; Rutkowski, P.; Gelderblom, H.; Hohenberger, P.; Leahy, M.; Von Mehren, M.; Joensuu, H.; et al. Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib (GRID): An international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 2013, 381, 295–302. [Google Scholar] [CrossRef] [Green Version]
- Demetri, G.D.; van Oosterom, A.T.; Garrett, C.R.; Blackstein, M.E.; Shah, M.H.; Verweij, J.; McArthur, G.; Judson, I.R.; Heinrich, M.C.; Morgan, J.A.; et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: A randomised controlled trial. Lancet 2006, 368, 1329–1338. [Google Scholar] [CrossRef]
- Blay, J.-Y.; Serrano, C.; Heinrich, M.C.; Zalcberg, J.; Bauer, S.; Gelderblom, H.; Schöffski, P.; Jones, R.L.; Attia, S.; D’Amato, G.; et al. Ripretinib in patients with advanced gastrointestinal stromal tumours (INVICTUS): A double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2020, 21, 923–934. [Google Scholar] [CrossRef]
- Heinrich, M.C.; Jones, R.L.; von Mehren, M.; Schöffski, P.; Serrano, C.; Kang, Y.-K.; Cassier, P.A.; Mir, O.; Eskens, F.; Tap, W.D.; et al. Avapritinib in advanced PDGFRA D842V-mutant gastrointestinal stromal tumour (NAVIGATOR): A multicentre, open-label, phase 1 trial. Lancet Oncol. 2020, 21, 935–946. [Google Scholar] [CrossRef]
- Schöffski, P.; Mir, O.; Kasper, B.; Papai, Z.; Blay, J.-Y.; Italiano, A.; Benson, C.; Kopeckova, K.; Ali, N.; Dileo, P.; et al. Activity and safety of the multi-target tyrosine kinase inhibitor cabozantinib in patients with metastatic gastrointestinal stromal tumour after treatment with imatinib and sunitinib: European Organisation for Research and Treatment of Cancer phase II trial 1317 ‘CaboGIST’. Eur. J. Cancer 2020, 134, 62–74. [Google Scholar] [CrossRef]
- Martin-Broto, J.; Moura, D.S. New drugs in gastrointestinal stromal tumors. Curr. Opin. Oncol. 2020, 32, 314–320. [Google Scholar] [CrossRef]
- Napolitano, A.; Vincenzi, B. Secondary KIT mutations: The GIST of drug resistance and sensitivity. Br. J. Cancer 2019, 120, 577–578. [Google Scholar] [CrossRef] [Green Version]
- Timmerman, R.D.; Bizekis, C.S.; Pass, H.I.; Fong, Y.; Dupuy, D.E.; Dawson, L.; Lu, D. Local Surgical, Ablative, and Radiation Treatment of Metastases. CA Cancer J. Clin. 2009, 59, 145–170. [Google Scholar] [CrossRef]
- Gronchi, A.; Guadagnolo, B.A.; Erinjeri, J.P. Local Ablative Therapies to Metastatic Soft Tissue Sarcoma. Am. Soc. Clin. Oncol. Educ. Book 2016, 35, e566–e575. [Google Scholar] [CrossRef]
- de Baere, T.; Tselikas, L.; Gravel, G.; Hakime, A.; Deschamps, F.; Honoré, C.; Mir, O.; Lecesne, A. Interventional radiology: Role in the treatment of sarcomas. Eur. J. Cancer 2018, 94, 148–155. [Google Scholar] [CrossRef]
- Farooqi, A.; Mitra, D.; Guadagnolo, B.A.; Bishop, A.J. The Evolving Role of Radiation Therapy in Patients with Metastatic Soft Tissue Sarcoma. Curr. Oncol. Rep. 2020, 22, 79. [Google Scholar] [CrossRef]
- Rubió-Casadevall, J.; The Spanish Group for Research on Sarcoma (GEIS); Martinez-Trufero, J.; Garcia-Albeniz, X.; Calabuig, S.; Pousa, A.L.; Del Muro, J.G.; Fra, J.; Redondo, A.; Lainez, N.; et al. Role of Surgery in Patients with Recurrent, Metastatic, or Unresectable Locally Advanced Gastrointestinal Stromal Tumors Sensitive to Imatinib: A Retrospective Analysis of the Spanish Group for Research on Sarcoma (GEIS). Ann. Surg. Oncol. 2015, 22, 2948–2957. [Google Scholar] [CrossRef]
- Ollila, D.W.; Caudle, A.S. Surgical Management of Distant Metastases. Surg. Oncol. Clin. N. Am. 2006, 15, 385–398. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Dean, C.B.; Nielsen, J.D. Generalized linear mixed models: A review and some extensions. Lifetime Data Anal. 2007, 13, 497–512. [Google Scholar] [CrossRef]
- Du, C.-Y.; Zhou, Y.; Song, C.; Wang, Y.-P.; Jie, Z.-G.; He, Y.-L.; Liang, X.-B.; Cao, H.; Yan, Z.-S.; Shi, Y.-Q. Is there a role of surgery in patients with recurrent or metastatic gastrointestinal stromal tumours responding to imatinib: A prospective randomised trial in China. Eur. J. Cancer 2014, 50, 1772–1778. [Google Scholar] [CrossRef]
- Bauer, S.; Rutkowski, P.; Hohenberger, P.; Miceli, R.; Fumagalli, E.R.; Siedlecki, J.; Nguyen, B.-P.; Kerst, M.; Fiore, M.; Nyckowski, P.; et al. Long-term follow-up of patients with GIST undergoing metastasectomy in the era of imatinib—Analysis of prognostic factors (EORTC-STBSG collaborative study). Eur. J. Surg. Oncol. (EJSO) 2014, 40, 412–419. [Google Scholar] [CrossRef]
- Hasegawa, J.; Kanda, T.; Hirota, S.; Fukuda, M.; Nishitani, A.; Takahashi, T.; Kurosaki, I.; Tsutsui, S.; Hatakeyama, K.; Nishida, T. Surgical interventions for focal progression of advanced gastrointestinal stromal tumors during imatinib therapy. Int. J. Clin. Oncol. 2007, 12, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Gronchi, A.; Fiore, M.; Miselli, F.; Lagonigro, M.S.; Coco, P.; Messina, A.; Pilotti, S.; Casali, P.G. Surgery of Residual Disease Following Molecular-targeted Therapy with Imatinib Mesylate in Advanced/Metastatic GIST. Ann. Surg. 2007, 245, 341–346. [Google Scholar] [CrossRef] [PubMed]
- DeMatteo, R.P.; Maki, R.G.; Singer, S.; Gonen, M.; Brennan, M.F.; Antonescu, C.R. Results of Tyrosine Kinase Inhibitor Therapy Followed by Surgical Resection for Metastatic Gastrointestinal Stromal Tumor. Ann. Surg. 2007, 245, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Raut, C.P.; Posner, M.; Desai, J.; Morgan, J.A.; George, S.; Zahrieh, D.; Fletcher, C.D.; Demetri, G.D.; Bertagnolli, M.M. Surgical Management of Advanced Gastrointestinal Stromal Tumors After Treatment with Targeted Systemic Therapy Using Kinase Inhibitors. J. Clin. Oncol. 2006, 24, 2325–2331. [Google Scholar] [CrossRef]
- Fairweather, M.; Balachandran, V.P.; Li, G.Z.; Bertagnolli, M.M.; Antonescu, C.; Tap, W.; Singer, S.; DeMatteo, R.P.; Raut, C.P. Cytoreductive Surgery for Metastatic Gastrointestinal Stromal Tumors Treated with Tyrosine Kinase Inhibitors: A 2-institutional Analysis. Ann. Surg. 2018, 268, 296–302. [Google Scholar] [CrossRef]
- Xia, L.; Zhang, M.-M.; Ji, L.; Li, X.; Wu, X.-T. Resection combined with imatinib therapy for liver metastases of gastrointestinal stromal tumors. Surg. Today 2010, 40, 936–942. [Google Scholar] [CrossRef]
- Patterson, T.; Chai, J.; Li, H.; de Bruyns, A.; Cleversey, C.; Lee, C.-H.; Yip, S.; Simmons, C.; Hart, J.; Pollock, P.; et al. Utilization of Mutational Analysis (MA) in Gastrointestinal Stromal Tumor (GIST) Management in British Columbia (BC) between January 2008 to December 2017: A Retrospective Population-Based Study. J. Gastrointest. Cancer 2021, 1–9. [Google Scholar] [CrossRef]
- Casali, P.G.; Abecassis, N.; Aro, H.T.; Bauer, S.; Biagini, R.; Bielack, S.; Boukovinas, I.; Bovee, J.V.M.N.; Brodowicz, T.; Broto, J.M.; et al. Gastrointestinal stromal tumours: ESMO-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018, 29 (Suppl. S4), iv68–iv78. [Google Scholar] [CrossRef]
- Nour, A.A.; Alaradi, A.; Mohamed, A.; Altuwaijri, S.; Rudat, V. Intensity modulated radiotherapy of upper abdominal malignancies: Dosimetric comparison with 3D conformal radiotherapy and acute toxicity. Radiat. Oncol. 2013, 8, 207. [Google Scholar] [CrossRef] [Green Version]
- Taremi, M.; Ringash, J.; Dawson, L.A. Upper Abdominal Malignancies: Intensity-Modulated Radiation Therapy. Front. Radiat. Ther. Oncol. 2007, 40, 272–288. [Google Scholar] [CrossRef]
- Joensuu, H.; Eriksson, M.; Collan, J.; Balk, M.H.; Leyvraz, S.; Montemurro, M. Radiotherapy for GIST progressing during or after tyrosine kinase inhibitor therapy: A prospective study. Radiother. Oncol. 2015, 116, 233–238. [Google Scholar] [CrossRef] [Green Version]
- Cuaron, J.J.; Goodman, K.A.; Lee, N.; Wu, A.J. External beam radiation therapy for locally advanced and metastatic gastrointestinal stromal tumors. Radiat. Oncol. 2013, 8, 274. [Google Scholar] [CrossRef] [Green Version]
- Ciresa, M.; D’Angelillo, R.M.; Ramella, S.; Cellini, F.; Gaudino, D.; Stimato, G.; Fiore, M.; Greco, C.; Nudo, R.; Trodella, L. Molecularly Targeted Therapy and Radiotherapy in the Management of Localized Gastrointestinal Stromal Tumor (GIST) of the Rectum: A Case Report. Tumori J. 2009, 95, 236–239. [Google Scholar] [CrossRef]
- Boruban, C.; Sencan, O.; Akmansu, M.; Atik, E.T.; Ozbek, S. Metastatic gastrointestinal stromal tumor with long-term response after treatment with concomitant radiotherapy and imatinib mesylate. Anti-Cancer Drugs 2007, 18, 969–972. [Google Scholar] [CrossRef]
- Lolli, C.; Pantaleo, M.A.; Nannini, M.; Saponara, M.; Pallotti, M.C.; Di Scioscio, V.; Barbieri, E.; Mandrioli, A.; Biasco, G. Successful radiotherapy for local control of progressively increasing metastasis of gastrointestinal stromal tumor. Rare Tumors 2011, 3, 153–154. [Google Scholar] [CrossRef]
- Dileo, P.; Randhawa, R.; Vansonnenberg, E.; Shankar, S.; Desai, J.; Morgan, J.A.; Van Den Abbeele, A.; Silverman, S.G.; Demetri, G.D. Safety and efficacy of percutaneous radio-frequency ablation (RFA) in patients (pts) with metastatic gastrointestinal stromal tumor (GIST) with clonal evolution of lesions refractory to imatinib mesylate (IM). J. Clin. Oncol. 2004, 22, 9024. [Google Scholar] [CrossRef]
- Jones, R.; McCall, J.; Adam, A.; O’Donnell, D.; Ashley, S.; Al-Muderis, O.; Thway, K.; Fisher, C.; Judson, I. Radiofrequency ablation is a feasible therapeutic option in the multi modality management of sarcoma. Eur. J. Surg. Oncol. (EJSO) 2010, 36, 477–482. [Google Scholar] [CrossRef] [Green Version]
- Jung, J.-H.; Won, H.J.; Shin, Y.M.; Kim, P.N. Safety and Efficacy of Radiofrequency Ablation for Hepatic Metastases from Gastrointestinal Stromal Tumor. J. Vasc. Interv. Radiol. 2015, 26, 1797–1802. [Google Scholar] [CrossRef]
- Kobayashi, K.; Gupta, S.; Trent, J.C.; Vauthey, J.N.; Krishnamurthy, S.; Ensor, J.; Ahrar, K.; Wallace, M.J.; Madoff, D.C.; Murthy, R.; et al. Hepatic artery chemoembolization for 110 gastrointestinal stromal tumors: Response, survival, and prognostic factors. Cancer 2006, 107, 2833–2841. [Google Scholar] [CrossRef]
- Cao, G.; Li, J.; Shen, L.; Zhu, X. Transcatheter arterial chemoembolization for gastrointestinal stromal tumors with liver metastases. World J. Gastroenterol. 2012, 18, 6134–6140. [Google Scholar] [CrossRef]
- Takaki, H.; Litchman, T.; Covey, A.; Cornelis, F.; Maybody, M.; Getrajdman, G.I.; Sofocleous, C.T.; Brown, K.; Solomon, S.B.; Alago, W.; et al. Hepatic Artery Embolization for Liver Metastasis of Gastrointestinal Stromal Tumor following Imatinib and Sunitinib Therapy. J. Gastrointest. Cancer 2014, 45, 494–499. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, K.; Szklaruk, J.; Trent, J.C.; Ensor, J.; Ahrar, K.; Wallace, M.J.; Madoff, D.C.; Murthy, R.; Hicks, M.E.; Gupta, S. Hepatic Arterial Embolization and Chemoembolization for Imatinib-Resistant Gastrointestinal Stromal Tumors. Am. J. Clin. Oncol. 2009, 32, 574–581. [Google Scholar] [CrossRef]
- Rathmann, N.; Diehl, S.J.; Dinter, D.; Schütte, J.; Pink, D.; Schoenberg, S.O.; Hohenberger, P. Radioembolization in Patients with Progressive Gastrointestinal Stromal Tumor Liver Metastases Undergoing Treatment with Tyrosine Kinase Inhibitors. J. Vasc. Interv. Radiol. 2014, 26, 231–238. [Google Scholar] [CrossRef]


| Patient Characteristics | Rec Met w/o LRT (%) n = 55 | Rec Met with LRT (%) n = 20 | De Novo Met w/o LRT (%) n = 26 | De Novo Met with LRT (%) n = 26 | |
|---|---|---|---|---|---|
| Age Median (min–max) | 66 (31–84) | 66 (31–75) | 67.5 (23–90) | 63 (36–82) | |
| Gender Female Male | 21 (38.2%) 34 (61.8%) | 9 (45%) 11 (55%) | 15 (57.7%) 11 (42.3%) | 8 (30.8%) 18 (69.2%) | |
| Treatment Center Vancouver Fraser Valley Victoria Interior Northern Community/unknown | 18 (32.7%) 18 (32.7%) 10 (18.2%) 7 (12.7%) 1 (1.8%) 1 (1.8%) | 12 (60%) 2 (10%) 1 (5%) 4 (20%) 1 (5%) 0 (0%) | 17 (65.4%) 4 (15.4%) 2 (7.7%) 3 (11.5%) 0 (0%) 0 (0%) | 7 (26.9%) 9 (34.6%) 4 (15.4%) 5 (19.2%) 1 (3.8%) 0 (0%) | |
| Primary Tumour Characteristics | Tumour Size (cm) >2 ≥2 and ≤5 >5 and ≤10 10 | 1 (1.8%) 9 (16.4%) 16 (29.1%) 29 (52.7%) | 0 (0%) 4 (20%) 8 (40%) 8 (40%) | 1 (3.8%) 2 (7.7%) 8 (30.8%) 15 (57.7%) | 0 (0%) 5 (19.2%) 14 (53.8%) 7 (26.9%) |
| Mitotic Rate (/50HPF) <5 ≥5 and ≤10 >10 Unreported/unknown | 16 (29.1%) 11 (20%) 23 (41.8%) 5 (9.1%) | 3 (15%) 7 (35%) 10 (50%) 0 (0%) | 10 (38.5%) 3 (11.5%) 5 (19.2%) 8 (30.8%) | 7 (26.9%) 3 (11.5%) 12 (46.2%) 4 (15.4%) | |
| Tumour Location Stomach Small Bowel Rectum/Pelvis Other | 23 (41.8%) 26 (47.3%) 3 (5.5%) 3 (5.6%) | 5 (25%) 6 (30%) 6 (30%) 3 (15%) | 7 (26.9%) 4 (15.4%) 4 (15.4%) 11 (42.3%) | 7 (26.9%) 15 (57.7%) 1 (3.8%) 3 (11.6%) | |
| Tumour Rupture No Yes | 49 (89.1%) 6 (10.9%) | 19 (95%) 1 (5%) | 26 (100%) 0 (0%) | 22 (84.6%) 4 (15.4%) | |
| Mutational Status KIT Exon 11 KIT Exon 9 Wt PDGFRA Exon 18 D842V PDGFRA Exon 12 KIT Exon 9 and Exon 11 KIT Exon 11 and Exon 13 KIT Exon 11 and SDHA Unknown/failed | n = 41 (ordered) 20 (48.8%) 7 (17.1%) 7 (17.1%) 2 (4.9%) 1 (2.4%) 1 (2.4%) 1 (2.4%) 0 (0%) 2 (4.9%) | n = 16 (ordered) 11 (68.7%) 2 (12.5%) 3 (18.8%) 0 (0%) 0 (0%) 0 (0%) 0 (0%) 0 (0%) 0 (0%) | n = 15 (ordered) 8 (53.3%) 0 (0%) 3 (20.0%) 0 (0%) 0 (0%) 0 (0%) 0 (0%) 1 (6.7%) 3 (20.0%) | n = 17 (ordered) 13 (76.4%) 1 (5.9%) 2 (11.8%) 0 (0%) 0 (0%) 0 (0%) 0 (0%) 0 (0%) 1 (5.9%) | |
| Systemic treatment median (range) | 1 (1–4) | 2 (1–5) | 2 (1–5) | 1 (1–4) |
| Patient Characteristics | Primary Tumour Characteristics | Treatment Characteristics and Outcome | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Patient ID | Age at Metastatic Diagnosis | Gender | Recurrence/De Novo | Size (cm) | Location | Mitotic Count (/50HPF) | Tumor Rupture (Yes/No) | Systemic Treatment * | Reason for RT | Clinical Outcome |
| 22 | 79 | Male | Recurrence | 10.5 | Stomach | 5 | No | I, S 1 | Bleeding | SI |
| 27 | 59 | Female | Recurrence | 7 | Rectum | >10 | No | I, S, R, C 1, Ri, | Pain | NSI |
| 38 | 71 | Female | de novo | 5 | Stomach | 10–15 | No | I 1, S | Bleeding | SI |
| 89 | 63 | Male | de novo | 3.7 | Stomach | 150 | No | I, S 1 | Preventative bleeding ** | SI |
| 97 | 67 | Male | Recurrence | 6.3 | Rectum | 20 | No | I ¹ | Local control for progressive bone disease | SD for 6 months |
| 99 | 57 | Male | Recurrence | 16.9 | Rectum | 100 | No | I (Adjuvant) I 1, R, S | Pseudo-adjuvant RT after metastectomy | Local control not achieved |
| 106 | 72 | Female | de novo | 9.2 | Small bowel | Not reported | No | I, S 1 | Pain | SI |
| 143 | 73 | Female | Recurrence | 5.1 | Large bowel | 3–4 | No | I (Adjuvant) I 1, S | Pain | SI |
| 149 | 78 | Female | Recurrence | 8.5 | Rectum | 50 | No | I (Neoadjuvant) S 1 | Pain | SI |
| 269 | 76 | Male | Recurrence | 14 | Stomach | 10 | No | I (Adjuvant) I 1, S, R, I, A | Bleeding | SI |
| 358 | 63 | Male | Recurrence | 18 | Small bowel | 100 | No | I (Neoadjuvant) I 1, R, A 1 | Pain | SI |
| 414 | 36 | Male | Recurrence | 5.5 | Small bowel | 30 | No | I, S, R, N, | Local control for progressive visceral disease | Local control not achieved |
| Patient Characteristics | Primary Tumour Characteristics | Treatment Characteristics | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient ID | Age at Metastatic Diagnosis | Gender | Reccurence/De Novo | Size (cm) | Location | Mitotic Count (/50HPF) | Tumour rupture (Yes/No) | Systemic Treatment * | Reason for Ablation | Best Response | Duration of Response |
| 185 | 80 | Female | Recurrence | 4 | Small bowel | <5 | No | I ¹ | MWA–2 liver lesions | PR | 17 mos |
| 214 | 48 | Male | de novo | 9.5 | Small bowel | <5 | No | I (Neoadjuvant) I 1, S, R, I | RFA–1 liver lesion | PR | 12 mos |
| 413 | 57 | Female | Recurrence | 7 | Stomach | 12–16 | No | I 1, S, So, I | RFA–2 liver lesions | PR | 1 mos |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patterson, T.; Li, H.; Chai, J.; Debruyns, A.; Simmons, C.; Hart, J.; Pollock, P.; Holloway, C.L.; Truong, P.T.; Feng, X. Locoregional Treatments for Metastatic Gastrointestinal Stromal Tumor in British Columbia: A Retrospective Cohort Study from January 2008 to December 2017. Cancers 2022, 14, 1477. https://doi.org/10.3390/cancers14061477
Patterson T, Li H, Chai J, Debruyns A, Simmons C, Hart J, Pollock P, Holloway CL, Truong PT, Feng X. Locoregional Treatments for Metastatic Gastrointestinal Stromal Tumor in British Columbia: A Retrospective Cohort Study from January 2008 to December 2017. Cancers. 2022; 14(6):1477. https://doi.org/10.3390/cancers14061477
Chicago/Turabian StylePatterson, Tiffany, Haocheng Li, Jocelyn Chai, Angeline Debruyns, Christine Simmons, Jason Hart, Phil Pollock, Caroline L. Holloway, Pauline T. Truong, and Xiaolan Feng. 2022. "Locoregional Treatments for Metastatic Gastrointestinal Stromal Tumor in British Columbia: A Retrospective Cohort Study from January 2008 to December 2017" Cancers 14, no. 6: 1477. https://doi.org/10.3390/cancers14061477
APA StylePatterson, T., Li, H., Chai, J., Debruyns, A., Simmons, C., Hart, J., Pollock, P., Holloway, C. L., Truong, P. T., & Feng, X. (2022). Locoregional Treatments for Metastatic Gastrointestinal Stromal Tumor in British Columbia: A Retrospective Cohort Study from January 2008 to December 2017. Cancers, 14(6), 1477. https://doi.org/10.3390/cancers14061477






