Hemodynamic Imaging in Cerebral Diffuse Glioma—Part B: Molecular Correlates, Treatment Effect Monitoring, Prognosis, and Future Directions
Abstract
Simple Summary
Abstract
1. Introduction
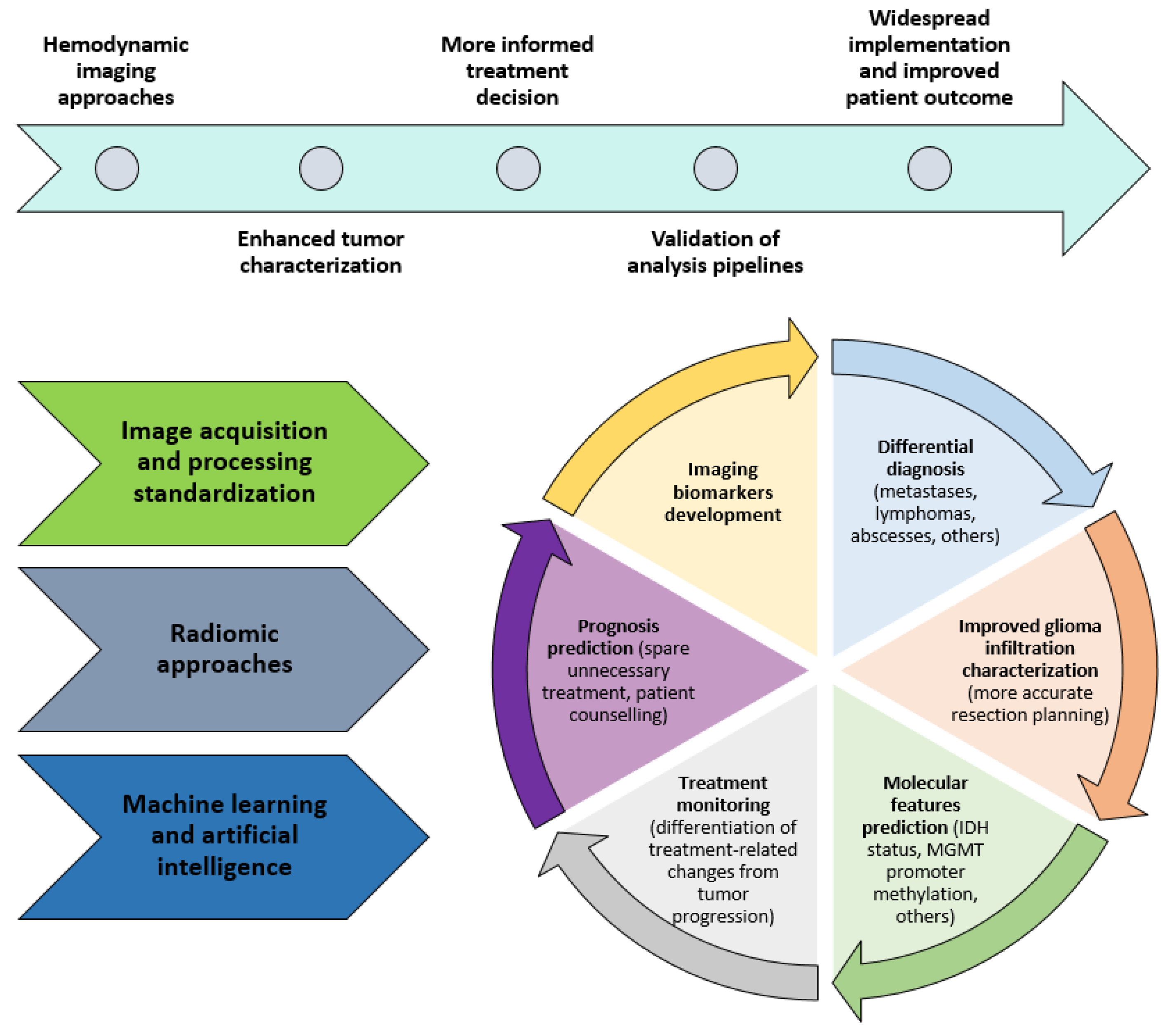
2. Clinical Applications of Hemodynamic Imaging in Gliomas—Part 2
2.1. Molecular Features Prediction
2.2. IDH Mutation Status
2.3. p/19q Codeletion
2.4. MGMT Promoter Methylation
2.5. EGFR Mutation
2.6. Other Markers: Hypoxia, Angiogenesis, Proliferation
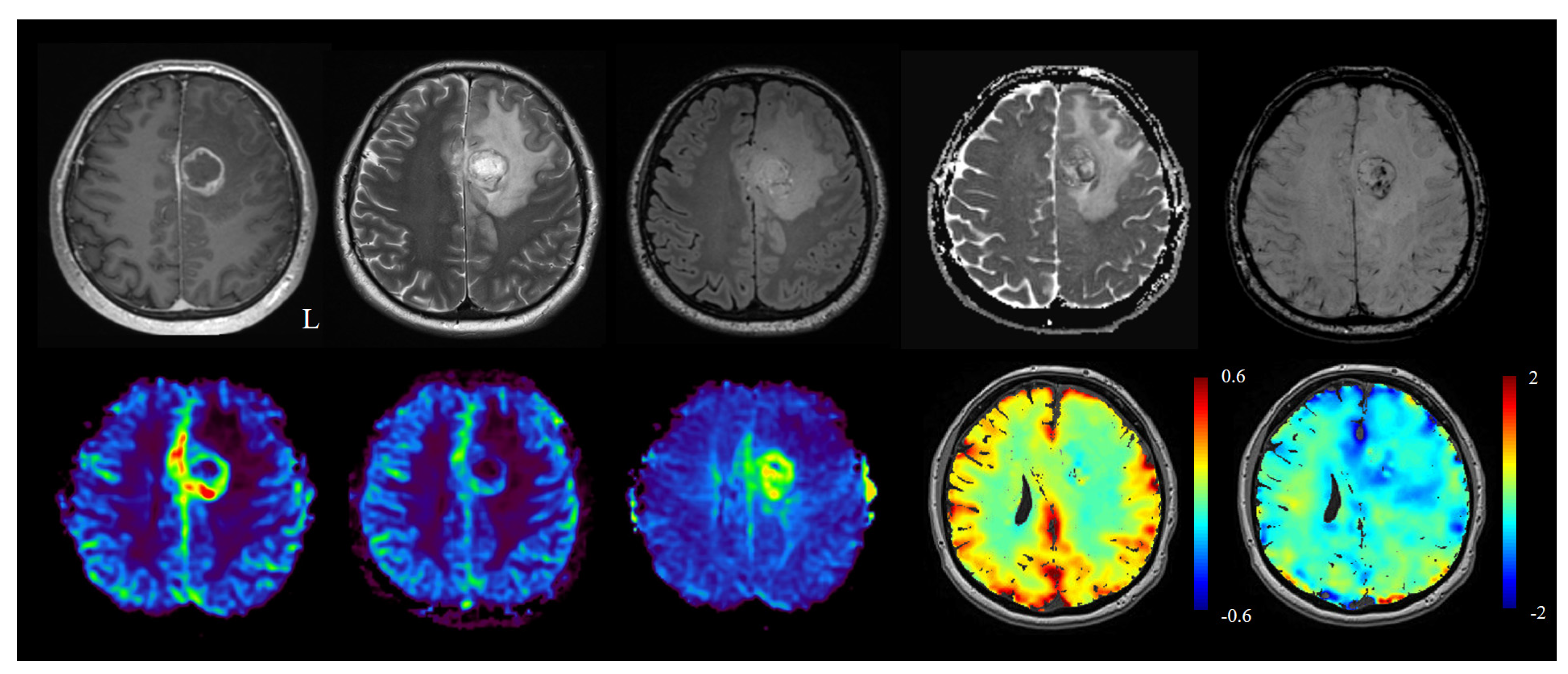
2.7. Differentiation between Tumor Progression/Tumor Recurrence vs Radiation Necrosis/Pseudoprogression/Pseudoresponse
2.8. Prognosis Prediction
3. Future Directions
3.1. New Approaches to Hemodynamic Imaging
3.2. Contrast-Enhanced Ultrasound (CEUS)
3.3. Intravoxel Incoherent Motion (IVIM)-MRI
3.4. Gas Modulation and BOLD Imaging: BOLD-CVR and Oxygen Modulation for Enhanced Lesion Characterization
3.5. Machine-Learning and Radiomics
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ludwig, K.; Kornblum, H.I. Molecular markers in glioma. J. Neuro Oncol. 2017, 134, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro Oncol. 2021, 23, 1231–1251. [Google Scholar] [CrossRef] [PubMed]
- Weller, M.; van den Bent, M.; Preusser, M.; Le Rhun, E.; Tonn, J.C.; Minniti, G.; Bendszus, M.; Balana, C.; Chinot, O.; Dirven, L.; et al. EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat. Rev. Clin. Oncol. 2020, 18, 170–186. [Google Scholar] [CrossRef] [PubMed]
- Smits, M.; van den Bent, M.J. Imaging Correlates of Adult Glioma Genotypes. Radiology 2017, 284, 316–331. [Google Scholar] [CrossRef]
- Zikou, A.; Sioka, C.; Alexiou, G.A.; Fotopoulos, A.; Voulgaris, S.; Argyropoulou, M.I. Radiation Necrosis, Pseudoprogression, Pseudoresponse, and Tumor Recurrence: Imaging Challenges for the Evaluation of Treated Gliomas. Contrast Media Mol. Imaging 2018, 2018, e6828396. [Google Scholar] [CrossRef]
- Guida, L.; Stumpo, V.; Bellomo, J.; van Niftrik, C.H.B.; Sebök, M.; Berhouma, M.; Bink, A.; Weller, M.; Kulcsar, Z.; Regli, L.; et al. Hemodynamic Imaging in Cerebral Diffuse Glioma—Part A: Concept, Differential Diagnosis and Tumor Grading. Cancers 2022. manuscript under peer review. [Google Scholar]
- Phillip Law, W.; Miles, K.A. Incorporating prognostic imaging biomarkers into clinical practice. Cancer Imaging 2013, 13, 332–341. [Google Scholar] [CrossRef]
- Sanvito, F.; Castellano, A.; Falini, A. Advancements in Neuroimaging to Unravel Biological and Molecular Features of Brain Tumors. Cancers 2021, 13, 424. [Google Scholar] [CrossRef]
- Shui, L.; Ren, H.; Yang, X.; Li, J.; Chen, Z.; Yi, C.; Zhu, H.; Shui, P. The Era of Radiogenomics in Precision Medicine: An Emerging Approach to Support Diagnosis, Treatment Decisions, and Prognostication in Oncology. Front. Oncol. 2021, 10, 3195. [Google Scholar] [CrossRef]
- Song, S.; Wang, L.; Yang, H.; Shan, Y.; Cheng, Y.; Xu, L.; Dong, C.; Zhao, G.; Lu, J. Static 18F-FET PET and DSC-PWI based on hybrid PET/MR for the prediction of gliomas defined by IDH and 1p/19q status. Eur. Radiol. 2020, 31, 4087–4096. [Google Scholar] [CrossRef]
- Conte, G.M.; Altabella, L.; Castellano, A.; Cuccarini, V.; Bizzi, A.; Grimaldi, M.; Costa, A.; Caulo, M.; Falini, A.; Anzalone, N. Comparison of T1 mapping and fixed T1 method for dynamic contrast-enhanced MRI perfusion in brain gliomas. Eur. Radiol. 2019, 29, 3467–3479. [Google Scholar] [CrossRef]
- Wang, N.; Xie, S.; Liu, H.; Chen, G.; Zhang, W. Arterial Spin Labeling for Glioma Grade Discrimination: Correlations with IDH1 Genotype and 1p/19q Status. Transl. Oncol. 2019, 12, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Arevalo-Perez, J.; Peck, K.K.; Young, R.J.; Holodny, A.I.; Karimi, S.; Lyo, J.K. Dynamic Contrast-Enhanced Perfusion MRI and Diffusion-Weighted Imaging in Grading of Gliomas. J. Neuroimaging 2015, 25, 792–798. [Google Scholar] [CrossRef] [PubMed]
- Fudaba, H.; Shimomura, T.; Abe, T.; Matsuta, H.; Momii, Y.; Sugita, K.; Ooba, H.; Kamida, T.; Hikawa, T.; Fujiki, M. Comparison of Multiple Parameters Obtained on 3T Pulsed Arterial Spin-Labeling, Diffusion Tensor Imaging, and MRS and the Ki-67 Labeling Index in Evaluating Glioma Grading. Am. J. Neuroradiol. 2014, 35, 2091–2098. [Google Scholar] [CrossRef]
- Alexiou, G.A.; Zikou, A.; Tsiouris, S.; Goussia, A.; Kosta, P.; Papadopoulos, A.; Voulgaris, S.; Kyritsis, A.P.; Fotopoulos, A.D.; Argyropoulou, M.I. Correlation of diffusion tensor, dynamic susceptibility contrast MRI and 99mTc-Tetrofosmin brain SPECT with tumour grade and Ki-67 immunohistochemistry in glioma. Clin. Neurol. Neurosurg. 2014, 116, 41–45. [Google Scholar] [CrossRef]
- Awasthi, R.; Rathore, R.K.S.; Soni, P.; Sahoo, P.; Awasthi, A.; Husain, N.; Behari, S.; Singh, R.K.; Pandey, C.M.; Gupta, R.K. Discriminant analysis to classify glioma grading using dynamic contrast-enhanced MRI and immunohistochemical markers. Neuroradiology 2012, 54, 205–213. [Google Scholar] [CrossRef]
- Emblem, K.E.; Scheie, D.; Due-Tonnessen, P.; Nedregaard, B.; Nome, T.; Hald, J.K.; Beiske, K.; Meling, T.R.; Bjornerud, A. Histogram Analysis of MR Imaging–Derived Cerebral Blood Volume Maps: Combined Glioma Grading and Identification of Low-Grade Oligodendroglial Subtypes. Am. J. Neuroradiol. 2008, 29, 1664–1670. [Google Scholar] [CrossRef]
- Tateishi, K.; Tateishi, U.; Sato, M.; Yamanaka, S.; Kanno, H.; Murata, H.; Inoue, T.; Kawahara, N. Application of 62Cu-Diacetyl-Bis (N4-Methylthiosemicarbazone) PET Imaging to Predict Highly Malignant Tumor Grades and Hypoxia-Inducible Factor-1α Expression in Patients with Glioma. Am. J. Neuroradiol. 2013, 34, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Deng, D.; Yang, Z.; Wang, W.; Cao, M.; Huang, Y.; Shen, J. Pretreatment structural and arterial spin labeling MRI is predictive for p53 mutation in high-grade gliomas. Br. J. Radiol. 2020, 93, 20200661. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Tong, H.; Du, X.; Guo, H.; Ma, Q.; Zhang, Y.; Zhou, X.; Liu, H.; Wang, S.; Fang, J.; et al. Vascular habitat analysis based on dynamic susceptibility contrast perfusion MRI predicts IDH mutation status and prognosis in high-grade gliomas. Eur. Radiol. 2020, 30, 3254–3265. [Google Scholar] [CrossRef]
- Xue, W.; Zhang, J.; Tong, H.; Xie, T.; Chen, X.; Zhou, B.; Wu, P.; Zhong, P.; Du, X.; Guo, Y.; et al. Effects of BMPER, CXCL10, and HOXA9 on Neovascularization During Early-Growth Stage of Primary High-Grade Glioma and Their Corresponding MRI Biomarkers. Front. Oncol. 2020, 10, 10. [Google Scholar] [CrossRef] [PubMed]
- Piccardo, A.; Tortora, D.; Mascelli, S.; Severino, M.; Piatelli, G.; Consales, A.; Pescetto, M.; Biassoni, V.; Schiavello, E.; Massollo, M.; et al. Advanced MR imaging and 18F-DOPA PET characteristics of H3K27M-mutant and wild-type pediatric diffuse midline gliomas. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 1685–1694. [Google Scholar] [CrossRef] [PubMed]
- Pang, H.; Dang, X.; Ren, Y.; Zhuang, D.; Qiu, T.; Chen, H.; Zhang, J.; Ma, N.; Li, G.; Zhang, J.; et al. 3D-ASL perfusion correlates with VEGF expression and overall survival in glioma patients: Comparison of quantitative perfusion and pathology on accurate spatial location-matched basis. J. Magn. Reson. Imaging 2019, 50, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Bekaert, L.; Valable, S.; Lechapt-Zalcman, E.; Ponte, K.; Collet, S.; Constans, J.-M.; Levallet, G.; Bordji, K.; Petit, E.; Branger, P.; et al. [18F]-FMISO PET study of hypoxia in gliomas before surgery: Correlation with molecular markers of hypoxia and angiogenesis. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1383–1392. [Google Scholar] [CrossRef]
- Haris, M.; Husain, N.; Singh, A.; Husain, M.; Srivastava, S.; Srivastava, C.; Behari, S.; Rathore, R.K.S.; Saksena, S.; Gupta, R.K. Dynamic Contrast-Enhanced Derived Cerebral Blood Volume Correlates Better With Leak Correction Than With No Correction for Vascular Endothelial Growth Factor, Microvascular Density, and Grading of Astrocytoma. J. Comput. Assist. Tomogr. 2008, 32, 955–965. [Google Scholar] [CrossRef]
- Maia, A.C.M.; Malheiros, S.M.F.; da Rocha, A.J.; da Silva, C.J.; Gabbai, A.A.; Ferraz, F.A.P.; Stávale, J.N. MR Cerebral Blood Volume Maps Correlated with Vascular Endothelial Growth Factor Expression and Tumor Grade in Nonenhancing Gliomas. Am. J. Neuroradiol. 2005, 26, 777–783. [Google Scholar]
- Li, X.; Vigneron, D.B.; Cha, S.; Graves, E.E.; Crawford, F.; Chang, S.M.; Nelson, S.J. Relationship of MR-Derived Lactate, Mobile Lipids, and Relative Blood Volume for Gliomas In Vivo. Am. J. Neuroradiol. 2005, 26, 760–769. [Google Scholar]
- Aronen, H.J.; Gazit, I.E.; Louis, D.N.; Buchbinder, B.R.; Pardo, F.S.; Weisskoff, R.M.; Harsh, G.R.; Cosgrove, G.R.; Halpern, E.F.; Hochberg, F.H. Cerebral blood volume maps of gliomas: Comparison with tumor grade and histologic findings. Radiology 1994, 191, 41–51. [Google Scholar] [CrossRef]
- Kickingereder, P.; Sahm, F.; Radbruch, A.; Wick, W.; Heiland, S.; von Deimling, A.; Bendszus, M.; Wiestler, B. IDH mutation status is associated with a distinct hypoxia/angiogenesis transcriptome signature which is non-invasively predictable with rCBV imaging in human glioma. Sci. Rep. 2015, 5, 16238. [Google Scholar] [CrossRef]
- Hong, E.K.; Choi, S.H.; Shin, D.J.; Jo, S.W.; Yoo, R.-E.; Kang, K.M.; Yun, T.J.; Kim, J.; Sohn, C.-H.; Park, S.-H.; et al. Comparison of Genetic Profiles and Prognosis of High-Grade Gliomas Using Quantitative and Qualitative MRI Features: A Focus on G3 Gliomas. Korean J. Radiol. 2021, 22, 233–242. [Google Scholar] [CrossRef]
- Ahn, S.S.; Shin, N.-Y.; Chang, J.H.; Kim, S.H.; Kim, E.H.; Kim, D.W.; Lee, S.-K. Prediction of methylguanine methyltransferase promoter methylation in glioblastoma using dynamic contrast-enhanced magnetic resonance and diffusion tensor imaging: Clinical article. J. Neurosurg. 2014, 121, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Choi, S.H.; Ryoo, I.; Yoon, T.J.; Kim, T.M.; Lee, S.-H.; Park, C.-K.; Kim, J.-H.; Sohn, C.-H.; Park, S.-H.; et al. Evaluation of the microenvironmental heterogeneity in high-grade gliomas with IDH1/2 gene mutation using histogram analysis of diffusion-weighted imaging and dynamic-susceptibility contrast perfusion imaging. J. Neuro Oncol. 2015, 121, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Qiao, X.J.; Ellingson, B.M.; Kim, H.J.; Wang, D.J.J.; Salamon, N.; Linetsky, M.; Sepahdari, A.R.; Jiang, B.; Tian, J.J.; Esswein, S.R.; et al. Arterial Spin-Labeling Perfusion MRI Stratifies Progression-Free Survival and Correlates with Epidermal Growth Factor Receptor Status in Glioblastoma. Am. J. Neuroradiol. 2015, 36, 672–677. [Google Scholar] [CrossRef] [PubMed]
- Crisi, G.; Filice, S. Predicting MGMT Promoter Methylation of Glioblastoma from Dynamic Susceptibility Contrast Perfusion: A Radiomic Approach. J. Neuroimaging 2020, 30, 458–462. [Google Scholar] [CrossRef]
- Choi, H.J.; Choi, S.H.; You, S.-H.; Yoo, R.-E.; Kang, K.M.; Yun, T.J.; Kim, J.-H.; Sohn, C.-H.; Park, C.-K.; Park, S.-H. MGMT Promoter Methylation Status in Initial and Recurrent Glioblastoma: Correlation Study with DWI and DSC PWI Features. Am. J. Neuroradiol. 2021, 42, 853–860. [Google Scholar] [CrossRef]
- Waitkus, M.S.; Diplas, B.H.; Yan, H. Isocitrate dehydrogenase mutations in gliomas. Neuro Oncol. 2016, 18, 16–26. [Google Scholar] [CrossRef]
- Yamashita, A.S.; da Costa Rosa, M.; Stumpo, V.; Rais, R.; Slusher, B.S.; Riggins, G.J. The glutamine antagonist prodrug JHU-083 slows malignant glioma growth and disrupts mTOR signaling. Neuro Oncol. Adv. 2021, 3, vdaa149. [Google Scholar] [CrossRef]
- Xing, Z.; Yang, X.; She, D.; Lin, Y.; Zhang, Y.; Cao, D. Noninvasive Assessment of IDH Mutational Status in World Health Organization Grade II and III Astrocytomas Using DWI and DSC-PWI Combined with Conventional MR Imaging. Am. J. Neuroradiol. 2017, 38, 1138–1144. [Google Scholar] [CrossRef]
- Tan, W.; Xiong, J.; Huang, W.; Wu, J.; Zhan, S.; Geng, D. Noninvasively detecting Isocitrate dehydrogenase 1 gene status in astrocytoma by dynamic susceptibility contrast MRI. J. Magn. Reson. Imaging 2017, 45, 492–499. [Google Scholar] [CrossRef]
- Cindil, E.; Sendur, H.N.; Cerit, M.N.; Erdogan, N.; Celebi, F.; Dag, N.; Celtikci, E.; Inan, A.; Oner, Y.; Tali, T. Prediction of IDH Mutation Status in High-grade Gliomas Using DWI and High T1-weight DSC-MRI. Acad. Radiol. 2021. online ahead of print. [Google Scholar] [CrossRef]
- Zhang, H.; lyu, G.; He, W.; Lei, Y.; Lin, F.; Wang, M.; Zhang, H.; Liang, L.; Feng, Y.; Yang, J. DSC and DCE Histogram Analyses of Glioma Biomarkers, Including IDH, MGMT, and TERT, on Differentiation and Survival. Acad. Radiol. 2020, 27, e263–e271. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhao, W.; He, B.; Koh, T.S.; Li, Y.; Zeng, Y.; Zhang, Z.; Zhang, J.; Hou, Z. Application of Distributed Parameter Model to Assessment of Glioma IDH Mutation Status by Dynamic Contrast-Enhanced Magnetic Resonance Imaging. Available online: https://www.hindawi.com/journals/cmmi/2020/8843084/ (accessed on 14 December 2021).
- Brendle, C.; Hempel, J.-M.; Schittenhelm, J.; Skardelly, M.; Tabatabai, G.; Bender, B.; Ernemann, U.; Klose, U. Glioma Grading and Determination of IDH Mutation Status and ATRX loss by DCE and ASL Perfusion. Clin. Neuroradiol. 2018, 28, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Yoo, R.-E.; Yun, T.J.; Hwang, I.; Hong, E.K.; Kang, K.M.; Choi, S.H.; Park, C.-K.; Won, J.-K.; Kim, J.; Sohn, C.-H. Arterial spin labeling perfusion-weighted imaging aids in prediction of molecular biomarkers and survival in glioblastomas. Eur. Radiol. 2020, 30, 1202–1211. [Google Scholar] [CrossRef]
- Wang, K.; Li, Y.; Cheng, H.; Li, S.; Xiang, W.; Ming, Y.; Chen, L.; Zhou, J. Perfusion CT detects alterations in local cerebral flow of glioma related to IDH, MGMT and TERT status. BMC Neurol. 2021, 21, 460. [Google Scholar] [CrossRef] [PubMed]
- Polívka, J., Jr.; Pešta, M.; Pitule, P.; Hes, O.; Holubec, L.; Polívka, J.; Kubíková, T.; Tonar, Z. IDH1 mutation is associated with lower expression of VEGF but not microvessel formation in glioblastoma multiforme. Oncotarget 2018, 9, 16462–16476. [Google Scholar] [CrossRef]
- Bou Zerdan, M.; Assi, H.I. Oligodendroglioma: A Review of Management and Pathways. Front. Mol. Neurosci. 2021, 14, 225. [Google Scholar] [CrossRef]
- Latysheva, A.; Emblem, K.E.; Brandal, P.; Vik-Mo, E.O.; Pahnke, J.; Røysland, K.; Hald, J.K.; Server, A. Dynamic susceptibility contrast and diffusion MR imaging identify oligodendroglioma as defined by the 2016 WHO classification for brain tumors: Histogram analysis approach. Neuroradiology 2019, 61, 545–555. [Google Scholar] [CrossRef]
- Mair, M.J.; Geurts, M.; van den Bent, M.J.; Berghoff, A.S. A basic review on systemic treatment options in WHO grade II-III gliomas. Cancer Treat. Rev. 2021, 92, 102124. [Google Scholar] [CrossRef]
- Sunwoo, L.; Choi, S.H.; Yoo, R.-E.; Kang, K.M.; Yun, T.J.; Kim, T.M.; Lee, S.-H.; Park, C.-K.; Kim, J.; Park, S.-W.; et al. Paradoxical perfusion metrics of high-grade gliomas with an oligodendroglioma component: Quantitative analysis of dynamic susceptibility contrast perfusion MR imaging. Neuroradiology 2015, 57, 1111–1120. [Google Scholar] [CrossRef]
- Lee, J.Y.; Ahn, K.J.; Lee, Y.S.; Jang, J.H.; Jung, S.L.; Kim, B.S. Differentiation of grade II and III oligodendrogliomas from grade II and III astrocytomas: A histogram analysis of perfusion parameters derived from dynamic contrast-enhanced (DCE) and dynamic susceptibility contrast (DSC) MRI. Acta Radiol. 2018, 59, 723–731. [Google Scholar] [CrossRef]
- Yoon, H.J.; Ahn, K.J.; Lee, S.; Jang, J.H.; Choi, H.S.; Jung, S.L.; Kim, B.S.; Jeun, S.S.; Hong, Y.K. Differential diagnosis of oligodendroglial and astrocytic tumors using imaging results: The added value of perfusion MR imaging. Neuroradiology 2017, 59, 665–675. [Google Scholar] [CrossRef] [PubMed]
- Delgado, A.F.; Delgado, A.F. Discrimination between Glioma Grades II and III Using Dynamic Susceptibility Perfusion MRI: A Meta-Analysis. Am. J. Neuroradiol. 2017, 38, 1348–1355. [Google Scholar] [CrossRef] [PubMed]
- Narang, J.; Jain, R.; Scarpace, L.; Saksena, S.; Schultz, L.R.; Rock, J.P.; Rosenblum, M.; Patel, S.C.; Mikkelsen, T. Tumor vascular leakiness and blood volume estimates in oligodendrogliomas using perfusion CT: An analysis of perfusion parameters helping further characterize genetic subtypes as well as differentiate from astroglial tumors. J. Neurooncol. 2011, 102, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, A.; Hachem, L.D.; Mansouri, S.; Nassiri, F.; Laperriere, N.J.; Xia, D.; Lindeman, N.I.; Wen, P.Y.; Chakravarti, A.; Mehta, M.P.; et al. MGMT promoter methylation status testing to guide therapy for glioblastoma: Refining the approach based on emerging evidence and current challenges. Neuro Oncol. 2019, 21, 167–178. [Google Scholar] [CrossRef]
- Moon, W.-J.; Choi, J.W.; Roh, H.G.; Lim, S.D.; Koh, Y.-C. Imaging parameters of high grade gliomas in relation to the MGMT promoter methylation status: The CT, diffusion tensor imaging, and perfusion MR imaging. Neuroradiology 2012, 54, 555–563. [Google Scholar] [CrossRef]
- Han, Y.; Yan, L.-F.; Wang, X.-B.; Sun, Y.-Z.; Zhang, X.; Liu, Z.-C.; Nan, H.-Y.; Hu, Y.-C.; Yang, Y.; Zhang, J.; et al. Structural and advanced imaging in predicting MGMT promoter methylation of primary glioblastoma: A region of interest based analysis. BMC Cancer 2018, 18, 215. [Google Scholar] [CrossRef]
- Mikkelsen, V.E.; Dai, H.Y.; Stensjøen, A.L.; Berntsen, E.M.; Salvesen, Ø.; Solheim, O.; Torp, S.H. MGMT Promoter Methylation Status Is Not Related to Histological or Radiological Features in IDH Wild-type Glioblastomas. J. Neuropathol. Exp. Neurol. 2020, 79, 855–862. [Google Scholar] [CrossRef]
- Ozturk, K.; Soylu, E.; Cayci, Z. Correlation between dynamic susceptibility contrast perfusion MRI and genomic alterations in glioblastoma. Neuroradiology 2021, 63, 1801–1810. [Google Scholar] [CrossRef]
- Lu, J.; Li, X.; Li, H. Perfusion parameters derived from MRI for preoperative prediction of IDH mutation and MGMT promoter methylation status in glioblastomas. Magn. Reson. Imaging 2021, 83, 189–195. [Google Scholar] [CrossRef]
- Ryoo, I.; Choi, S.H.; Kim, J.-H.; Sohn, C.-H.; Kim, S.C.; Shin, H.S.; Yeom, J.A.; Jung, S.C.; Lee, A.L.; Yun, T.J.; et al. Cerebral Blood Volume Calculated by Dynamic Susceptibility Contrast-Enhanced Perfusion MR Imaging: Preliminary Correlation Study with Glioblastoma Genetic Profiles. PLoS ONE 2013, 8, e71704. [Google Scholar] [CrossRef][Green Version]
- Fuster-Garcia, E.; Lorente Estellés, D.; Álvarez-Torres, M.D.M.; Juan-Albarracín, J.; Chelebian, E.; Rovira, A.; Acosta, C.A.; Pineda, J.; Oleaga, L.; Mollá-Olmos, E.; et al. MGMT methylation may benefit overall survival in patients with moderately vascularized glioblastomas. Eur. Radiol. 2021, 31, 1738–1747. [Google Scholar] [CrossRef] [PubMed]
- Oprita, A.; Baloi, S.-C.; Staicu, G.-A.; Alexandru, O.; Tache, D.E.; Danoiu, S.; Micu, E.S.; Sevastre, A.-S. Updated Insights on EGFR Signaling Pathways in Glioma. Int. J. Mol. Sci. 2021, 22, 587. [Google Scholar] [CrossRef]
- Eskilsson, E.; Røsland, G.V.; Solecki, G.; Wang, Q.; Harter, P.N.; Graziani, G.; Verhaak, R.G.W.; Winkler, F.; Bjerkvig, R.; Miletic, H. EGFR heterogeneity and implications for therapeutic intervention in glioblastoma. Neuro Oncol. 2018, 20, 743–752. [Google Scholar] [CrossRef]
- Gupta, A.; Young, R.J.; Shah, A.D.; Schweitzer, A.D.; Graber, J.J.; Shi, W.; Zhang, Z.; Huse, J.; Omuro, A.M.P. Pretreatment Dynamic Susceptibility Contrast MRI Perfusion in Glioblastoma: Prediction of EGFR Gene Amplification. Clin. Neuroradiol. 2015, 25, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Arevalo-Perez, J.; Thomas, A.A.; Kaley, T.; Lyo, J.; Peck, K.K.; Holodny, A.I.; Mellinghoff, I.K.; Shi, W.; Zhang, Z.; Young, R.J. T1-Weighted Dynamic Contrast-Enhanced MRI as a Noninvasive Biomarker of Epidermal Growth Factor Receptor vIII Status. Am. J. Neuroradiol. 2015, 36, 2256–2261. [Google Scholar] [CrossRef]
- Tykocinski, E.S.; Grant, R.A.; Kapoor, G.S.; Krejza, J.; Bohman, L.-E.; Gocke, T.A.; Chawla, S.; Halpern, C.H.; Lopinto, J.; Melhem, E.R.; et al. Use of magnetic perfusion-weighted imaging to determine epidermal growth factor receptor variant III expression in glioblastoma. Neuro Oncol. 2012, 14, 613–623. [Google Scholar] [CrossRef]
- Oughourlian, T.C.; Yao, J.; Hagiwara, A.; Nathanson, D.A.; Raymond, C.; Pope, W.B.; Salamon, N.; Lai, A.; Ji, M.; Nghiemphu, P.L.; et al. Relative oxygen extraction fraction (rOEF) MR imaging reveals higher hypoxia in human epidermal growth factor receptor (EGFR) amplified compared with non-amplified gliomas. Neuroradiology 2021, 63, 857–868. [Google Scholar] [CrossRef] [PubMed]
- Hino-Shishikura, A.; Tateishi, U.; Shibata, H.; Yoneyama, T.; Nishii, T.; Torii, I.; Tateishi, K.; Ohtake, M.; Kawahara, N.; Inoue, T. Tumor hypoxia and microscopic diffusion capacity in brain tumors: A comparison of 62Cu-Diacetyl-Bis (N4-Methylthiosemicarbazone) PET/CT and diffusion-weighted MR imaging. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 1419–1427. [Google Scholar] [CrossRef]
- Jensen, R.L.; Mumert, M.L.; Gillespie, D.L.; Kinney, A.Y.; Schabel, M.C.; Salzman, K.L. Preoperative dynamic contrast-enhanced MRI correlates with molecular markers of hypoxia and vascularity in specific areas of intratumoral microenvironment and is predictive of patient outcome. Neuro Oncol. 2014, 16, 280–291. [Google Scholar] [CrossRef]
- Liu, X.; Mangla, R.; Tian, W.; Qiu, X.; Li, D.; Walter, K.A.; Ekholm, S.; Johnson, M.D. The preliminary radiogenomics association between MR perfusion imaging parameters and genomic biomarkers, and their predictive performance of overall survival in patients with glioblastoma. J. Neurooncol. 2017, 135, 553–560. [Google Scholar] [CrossRef]
- Strauss, S.B.; Meng, A.; Ebani, E.J.; Chiang, G.C. Imaging Glioblastoma Posttreatment: Progression, Pseudoprogression, Pseudoresponse, Radiation Necrosis. Radiol. Clin. N. Am. 2019, 57, 1199–1216. [Google Scholar] [CrossRef] [PubMed]
- Thust, S.C.; van den Bent, M.J.; Smits, M. Pseudoprogression of brain tumors. J. Magn. Reson. Imaging 2018, 48, 571–589. [Google Scholar] [CrossRef] [PubMed]
- Leao, D.J.; Craig, P.G.; Godoy, L.F.; Leite, C.C.; Policeni, B. Response Assessment in Neuro-Oncology Criteria for Gliomas: Practical Approach Using Conventional and Advanced Techniques. Am. J. Neuroradiol. 2020, 41, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Ren, X.; Dong, G.; Wang, J.; Jiang, H.; Yang, C.; Zhao, X.; Zhu, Q.; Cui, Y.; Yu, K.; et al. Distinguishing Pseudoprogression From True Early Progression in Isocitrate Dehydrogenase Wild-Type Glioblastoma by Interrogating Clinical, Radiological, and Molecular Features. Front. Oncol. 2021, 11, 601. [Google Scholar] [CrossRef]
- Farid, N.; Almeida-Freitas, D.B.; White, N.S.; McDonald, C.R.; Kuperman, J.M.; Almutairi, A.A.; Muller, K.A.; VandenBerg, S.R.; Kesari, S.; Dale, A.M. Combining diffusion and perfusion differentiates tumor from bevacizumab-related imaging abnormality (bria). J. Neurooncol. 2014, 120, 539–546. [Google Scholar] [CrossRef]
- Muscas, G.; van Niftrik, C.H.B.; Sebök, M.; Della Puppa, A.; Seystahl, K.; Andratschke, N.; Brown, M.; Weller, M.; Regli, L.; Piccirelli, M.; et al. Distinct Cerebrovascular Reactivity Patterns for Brain Radiation Necrosis. Cancers 2021, 13, 1840. [Google Scholar] [CrossRef]
- Pyatigorskaya, N.; Sgard, B.; Bertaux, M.; Yahia-Cherif, L.; Kas, A. Can FDG-PET/MR help to overcome limitations of sequential MRI and PET-FDG for differential diagnosis between recurrence/progression and radionecrosis of high-grade gliomas? J. Neuroradiol. 2020. [Google Scholar] [CrossRef]
- Qiao, Z.; Zhao, X.; Wang, K.; Zhang, Y.; Fan, D.; Yu, T.; Shen, H.; Chen, Q.; Ai, L. Utility of Dynamic Susceptibility Contrast Perfusion-Weighted MR Imaging and 11C-Methionine PET/CT for Differentiation of Tumor Recurrence from Radiation Injury in Patients with High-Grade Gliomas. Am. J. Neuroradiol. 2019, 40, 253–259. [Google Scholar] [CrossRef]
- Sacconi, B.; Raad, R.A.; Lee, J.; Fine, H.; Kondziolka, D.; Golfinos, J.G.; Babb, J.S.; Jain, R. Concurrent functional and metabolic assessment of brain tumors using hybrid PET/MR imaging. J. Neurooncol. 2016, 127, 287–293. [Google Scholar] [CrossRef]
- Prager, A.J.; Martinez, N.; Beal, K.; Omuro, A.; Zhang, Z.; Young, R.J. Diffusion and Perfusion MRI to Differentiate Treatment-Related Changes Including Pseudoprogression from Recurrent Tumors in High-Grade Gliomas with Histopathologic Evidence. Am. J. Neuroradiol. 2015, 36, 877–885. [Google Scholar] [CrossRef]
- Shin, K.E.; Ahn, K.J.; Choi, H.S.; Jung, S.L.; Kim, B.S.; Jeon, S.S.; Hong, Y.G. DCE and DSC MR perfusion imaging in the differentiation of recurrent tumour from treatment-related changes in patients with glioma. Clin. Radiol. 2014, 69, e264–e272. [Google Scholar] [CrossRef] [PubMed]
- Alexiou, G.A.; Zikou, A.; Tsiouris, S.; Goussia, A.; Kosta, P.; Papadopoulos, A.; Voulgaris, S.; Tsekeris, P.; Kyritsis, A.P.; Fotopoulos, A.D.; et al. Comparison of diffusion tensor, dynamic susceptibility contrast MRI and 99mTc-Tetrofosmin brain SPECT for the detection of recurrent high-grade glioma. Magn. Reson. Imaging 2014, 32, 854–859. [Google Scholar] [CrossRef] [PubMed]
- Seeger, A.; Braun, C.; Skardelly, M.; Paulsen, F.; Schittenhelm, J.; Ernemann, U.; Bisdas, S. Comparison of Three Different MR Perfusion Techniques and MR Spectroscopy for Multiparametric Assessment in Distinguishing Recurrent High-Grade Gliomas from Stable Disease. Acad. Radiol. 2013, 20, 1557–1565. [Google Scholar] [CrossRef] [PubMed]
- Gahramanov, S.; Muldoon, L.L.; Varallyay, C.G.; Li, X.; Kraemer, D.F.; Fu, R.; Hamilton, B.E.; Rooney, W.D.; Neuwelt, E.A. Pseudoprogression of Glioblastoma after Chemo- and Radiation Therapy: Diagnosis by Using Dynamic Susceptibility-weighted Contrast-enhanced Perfusion MR Imaging with Ferumoxytol versus Gadoteridol and Correlation with Survival. Radiology 2013, 266, 842–852. [Google Scholar] [CrossRef]
- Larsen, V.A.; Simonsen, H.J.; Law, I.; Larsson, H.B.W.; Hansen, A.E. Evaluation of dynamic contrast-enhanced T1-weighted perfusion MRI in the differentiation of tumor recurrence from radiation necrosis. Neuroradiology 2013, 55, 361–369. [Google Scholar] [CrossRef]
- Baek, H.J.; Kim, H.S.; Kim, N.; Choi, Y.J.; Kim, Y.J. Percent Change of Perfusion Skewness and Kurtosis: A Potential Imaging Biomarker for Early Treatment Response in Patients with Newly Diagnosed Glioblastomas. Radiology 2012, 264, 834–843. [Google Scholar] [CrossRef]
- Bisdas, S.; Naegele, T.; Ritz, R.; Dimostheni, A.; Pfannenberg, C.; Reimold, M.; Koh, T.S.; Ernemann, U. Distinguishing Recurrent High-grade Gliomas from Radiation Injury: A Pilot Study Using Dynamic Contrast-enhanced MR Imaging. Acad. Radiol. 2011, 18, 575–583. [Google Scholar] [CrossRef]
- Kim, Y.H.; Oh, S.W.; Lim, Y.J.; Park, C.-K.; Lee, S.-H.; Kang, K.W.; Jung, H.-W.; Chang, K.H. Differentiating radiation necrosis from tumor recurrence in high-grade gliomas: Assessing the efficacy of 18F-FDG PET, 11C-methionine PET and perfusion MRI. Clin. Neurol. Neurosurg. 2010, 112, 758–765. [Google Scholar] [CrossRef]
- Prat, R.; Galeano, I.; Lucas, A.; Martínez, J.C.; Martín, M.; Amador, R.; Reynés, G. Relative value of magnetic resonance spectroscopy, magnetic resonance perfusion, and 2-(18F) fluoro-2-deoxy-D-glucose positron emission tomography for detection of recurrence or grade increase in gliomas. J. Clin. Neurosci. 2010, 17, 50–53. [Google Scholar] [CrossRef]
- Dandois, V.; Rommel, D.; Renard, L.; Jamart, J.; Cosnard, G. Substitution of 11C-methionine PET by perfusion MRI during the follow-up of treated high-grade gliomas: Preliminary results in clinical practice. J. Neuroradiol. 2010, 37, 89–97. [Google Scholar] [CrossRef]
- Hu, L.S.; Baxter, L.C.; Smith, K.A.; Feuerstein, B.G.; Karis, J.P.; Eschbacher, J.M.; Coons, S.W.; Nakaji, P.; Yeh, R.F.; Debbins, J.; et al. Relative Cerebral Blood Volume Values to Differentiate High-Grade Glioma Recurrence from Posttreatment Radiation Effect: Direct Correlation between Image-Guided Tissue Histopathology and Localized Dynamic Susceptibility-Weighted Contrast-Enhanced Perfusion MR Imaging Measurements. Am. J. Neuroradiol. 2009, 30, 552–558. [Google Scholar] [CrossRef] [PubMed]
- Barajas, R.F.; Chang, J.S.; Segal, M.R.; Parsa, A.T.; McDermott, M.W.; Berger, M.S.; Cha, S. Differentiation of Recurrent Glioblastoma Multiforme from Radiation Necrosis after External Beam Radiation Therapy with Dynamic Susceptibility-weighted Contrast-enhanced Perfusion MR Imaging. Radiology 2009, 253, 486–496. [Google Scholar] [CrossRef] [PubMed]
- Sugahara, T.; Korogi, Y.; Kochi, M.; Ushio, Y.; Takahashi, M. Perfusion-sensitive MR Imaging of Gliomas: Comparison between Gradient-echo and Spin-echo Echo-planar Imaging Techniques. Am. J. Neuroradiol. 2001, 22, 1306–1315. [Google Scholar] [PubMed]
- Hatzoglou, V.; Yang, T.J.; Omuro, A.; Gavrilovic, I.; Ulaner, G.; Rubel, J.; Schneider, T.; Woo, K.M.; Zhang, Z.; Peck, K.K.; et al. A prospective trial of dynamic contrast-enhanced MRI perfusion and fluorine-18 FDG PET-CT in differentiating brain tumor progression from radiation injury after cranial irradiation. Neuro Oncol. 2016, 18, 873–880. [Google Scholar] [CrossRef] [PubMed]
- Yun, T.J.; Park, C.-K.; Kim, T.M.; Lee, S.-H.; Kim, J.-H.; Sohn, C.-H.; Park, S.-H.; Kim, I.H.; Choi, S.H. Glioblastoma Treated with Concurrent Radiation Therapy and Temozolomide Chemotherapy: Differentiation of True Progression from Pseudoprogression with Quantitative Dynamic Contrast-enhanced MR Imaging. Radiology 2014, 274, 830–840. [Google Scholar] [CrossRef] [PubMed]
- Masch, W.R.; Wang, P.I.; Chenevert, T.L.; Junck, L.; Tsien, C.; Heth, J.A.; Sundgren, P.C. Comparison of Diffusion Tensor Imaging and Magnetic Resonance Perfusion Imaging in Differentiating Recurrent Brain Neoplasm From Radiation Necrosis. Acad. Radiol. 2016, 23, 569–576. [Google Scholar] [CrossRef]
- Tsien, C.; Galbán, C.J.; Chenevert, T.L.; Johnson, T.D.; Hamstra, D.A.; Sundgren, P.C.; Junck, L.; Meyer, C.R.; Rehemtulla, A.; Lawrence, T.; et al. Parametric Response Map As an Imaging Biomarker to Distinguish Progression From Pseudoprogression in High-Grade Glioma. J. Clin. Oncol. 2010, 28, 2293–2299. [Google Scholar] [CrossRef]
- Suh, C.H.; Kim, H.S.; Choi, Y.J.; Kim, N.; Kim, S.J. Prediction of Pseudoprogression in Patients with Glioblastomas Using the Initial and Final Area Under the Curves Ratio Derived from Dynamic Contrast-Enhanced T1-Weighted Perfusion MR Imaging. Am. J. Neuroradiol. 2013, 34, 2278–2286. [Google Scholar] [CrossRef]
- Vöglein, J.; Tüttenberg, J.; Weimer, M.; Gerigk, L.; Kauczor, H.-U.; Essig, M.; Weber, M.-A. Treatment Monitoring in Gliomas: Comparison of Dynamic Susceptibility-Weighted Contrast-Enhanced and Spectroscopic MRI Techniques for Identifying Treatment Failure. Investig. Radiol. 2011, 46, 390–400. [Google Scholar] [CrossRef]
- Hu, C.; Fang, X.; Hu, X.; Cui, L. Analysis of the mismatched manifestation between rCBF and rCBV maps in cerebral astrocytomas. Clin. Imaging 2009, 33, 417–423. [Google Scholar] [CrossRef]
- Mangla, R.; Singh, G.; Ziegelitz, D.; Milano, M.T.; Korones, D.N.; Zhong, J.; Ekholm, S.E. Changes in Relative Cerebral Blood Volume 1 Month after Radiation-Temozolomide Therapy Can Help Predict Overall Survival in Patients with Glioblastoma. Radiology 2010, 256, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Iv, M.; Liu, X.; Lavezo, J.; Gentles, A.J.; Ghanem, R.; Lummus, S.; Born, D.E.; Soltys, S.G.; Nagpal, S.; Thomas, R.; et al. Perfusion MRI-Based Fractional Tumor Burden Differentiates between Tumor and Treatment Effect in Recurrent Glioblastomas and Informs Clinical Decision-Making. Am. J. Neuroradiol. 2019, 40, 1649–1657. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wei, L.; Wang, J.; Li, N.; Gao, Y.; Ma, H.; Qu, X.; Zhang, M. Evaluation of perfusion MRI value for tumor progression assessment after glioma radiotherapy: A systematic review and meta-analysis. Medicine 2020, 99, e23766. [Google Scholar] [CrossRef] [PubMed]
- Chuang, M.-T.; Liu, Y.-S.; Tsai, Y.-S.; Chen, Y.-C.; Wang, C.-K. Differentiating Radiation-Induced Necrosis from Recurrent Brain Tumor Using MR Perfusion and Spectroscopy: A Meta-Analysis. PLoS ONE 2016, 11, e0141438. [Google Scholar] [CrossRef]
- Patel, P.; Baradaran, H.; Delgado, D.; Askin, G.; Christos, P.; John Tsiouris, A.; Gupta, A. MR perfusion-weighted imaging in the evaluation of high-grade gliomas after treatment: A systematic review and meta-analysis. Neuro Oncol. 2017, 19, 118–127. [Google Scholar] [CrossRef]
- Tsakiris, C.; Siempis, T.; Alexiou, G.A.; Zikou, A.; Sioka, C.; Voulgaris, S.; Argyropoulou, M.I. Differentiation Between True Tumor Progression of Glioblastoma and Pseudoprogression Using Diffusion-Weighted Imaging and Perfusion-Weighted Imaging: Systematic Review and Meta-analysis. World Neurosurg. 2020, 144, e100–e109. [Google Scholar] [CrossRef]
- Okuchi, S.; Rojas-Garcia, A.; Ulyte, A.; Lopez, I.; Ušinskienė, J.; Lewis, M.; Hassanein, S.M.; Sanverdi, E.; Golay, X.; Thust, S.; et al. Diagnostic accuracy of dynamic contrast-enhanced perfusion MRI in stratifying gliomas: A systematic review and meta-analysis. Cancer Med. 2019, 8, 5564–5573. [Google Scholar] [CrossRef]
- Miyoshi, F.; Shinohara, Y.; Kambe, A.; Kuya, K.; Murakami, A.; Kurosaki, M.; Ogawa, T. Utility of intravoxel incoherent motion magnetic resonance imaging and arterial spin labeling for recurrent glioma after bevacizumab treatment. Acta Radiol. 2018, 59, 1372–1379. [Google Scholar] [CrossRef]
- Nyberg, E.; Honce, J.; Kleinschmidt-DeMasters, B.K.; Shukri, B.; Kreidler, S.; Nagae, L. Arterial spin labeling: Pathologically proven superiority over conventional MRI for detection of high-grade glioma progression after treatment. Neuroradiol. J. 2016, 29, 377–383. [Google Scholar] [CrossRef]
- Jain, R.; Narang, J.; Schultz, L.; Scarpace, L.; Saksena, S.; Brown, S.; Rock, J.P.; Rosenblum, M.; Gutierrez, J.; Mikkelsen, T. Permeability Estimates in Histopathology-Proved Treatment-Induced Necrosis Using Perfusion CT: Can These Add to Other Perfusion Parameters in Differentiating from Recurrent/Progressive Tumors? Am. J. Neuroradiol. 2011, 32, 658–663. [Google Scholar] [CrossRef]
- Jain, R.; Scarpace, L.; Ellika, S.; Schultz, L.R.; Rock, J.P.; Rosenblum, M.L.; Patel, S.C.; Lee, T.-Y.; Mikkelsen, T. First-pass perfusion computed tomography: Initial experience in differentiating recurrent brain tumors from radiation effects and radiation necrosis. Neurosurgery 2007, 61, 778–787. [Google Scholar] [CrossRef] [PubMed]
- Baker, S.G.; Kramer, B.S. Evaluating surrogate endpoints, prognostic markers, and predictive markers: Some simple themes. Clin. Trials 2015, 12, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Ulyte, A.; Katsaros, V.K.; Liouta, E.; Stranjalis, G.; Boskos, C.; Papanikolaou, N.; Usinskiene, J.; Bisdas, S. Prognostic value of preoperative dynamic contrast-enhanced MRI perfusion parameters for high-grade glioma patients. Neuroradiology 2016, 58, 1197–1208. [Google Scholar] [CrossRef] [PubMed]
- Ellingson, B.M.; Yao, J.; Raymond, C.; Nathanson, D.A.; Chakhoyan, A.; Simpson, J.; Garner, J.S.; Olivero, A.G.; Mueller, L.U.; Rodon, J.; et al. Multiparametric MR-PET Imaging Predicts Pharmacokinetics and Clinical Response to GDC-0084 in Patients with Recurrent High-Grade Glioma. Clin. Cancer Res. 2020, 26, 3135–3144. [Google Scholar] [CrossRef] [PubMed]
- White, M.L.; Zhang, Y.; Yu, F.; Shonka, N.; Aizenberg, M.R.; Adapa, P.; Kazmi, S.A.J. Post-operative perfusion and diffusion MR imaging and tumor progression in high-grade gliomas. PLoS ONE 2019, 14, e0213905. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.; Poisson, L.M.; Gutman, D.; Scarpace, L.; Hwang, S.N.; Holder, C.A.; Wintermark, M.; Rao, A.; Colen, R.R.; Kirby, J.; et al. Outcome Prediction in Patients with Glioblastoma by Using Imaging, Clinical, and Genomic Biomarkers: Focus on the Nonenhancing Component of the Tumor. Radiology 2014, 272, 484–493. [Google Scholar] [CrossRef] [PubMed]
- Law, M.; Young, R.J.; Babb, J.S.; Peccerelli, N.; Chheang, S.; Gruber, M.L.; Miller, D.C.; Golfinos, J.G.; Zagzag, D.; Johnson, G. Gliomas: Predicting Time to Progression or Survival with Cerebral Blood Volume Measurements at Dynamic Susceptibility-weighted Contrast-enhanced Perfusion MR Imaging. Radiology 2008, 247, 490–498. [Google Scholar] [CrossRef]
- Burth, S.; Kickingereder, P.; Eidel, O.; Tichy, D.; Bonekamp, D.; Weberling, L.; Wick, A.; Löw, S.; Hertenstein, A.; Nowosielski, M.; et al. Clinical parameters outweigh diffusion- and perfusion-derived MRI parameters in predicting survival in newly diagnosed glioblastoma. Neuro Oncol. 2016, 18, 1673–1679. [Google Scholar] [CrossRef]
- Kim, H.S.; Kwon, S.L.; Choi, S.H.; Hwang, I.; Kim, T.M.; Park, C.-K.; Park, S.-H.; Won, J.-K.; Kim, I.H.; Lee, S.T. Prognostication of anaplastic astrocytoma patients: Application of contrast leakage information of dynamic susceptibility contrast-enhanced MRI and dynamic contrast-enhanced MRI. Eur. Radiol. 2020, 30, 2171–2181. [Google Scholar] [CrossRef]
- Stecco, A.; Amatuzzo, P.; Sponghini, A.P.; Platini, F.; Quagliozzi, M.; Buemi, F.; Guenzi, E.; Carriero, A. Prognostic value of relative cerebral blood volume in patients with recurrent glioblastoma multiforme treated with bevacizumab. J. Neurosurg. Sci. 2019, 63, 394–401. [Google Scholar] [CrossRef]
- Lucas, J.T., Jr.; Knapp, B.J.; Uh, J.; Hua, C.-H.; Merchant, T.E.; Hwang, S.N.; Patay, Z.; Broniscer, A. Posttreatment DSC-MRI is Predictive of Early Treatment Failure in Children with Supratentorial High-Grade Glioma Treated with Erlotinib. Clin. Neuroradiol. 2018, 28, 393–400. [Google Scholar] [CrossRef] [PubMed]
- McCullough, B.J.; Ader, V.; Aguedan, B.; Feng, X.; Susanto, D.; Benkers, T.L.; Henson, J.W.; Mayberg, M.; Cobbs, C.S.; Gwinn, R.P.; et al. Preoperative relative cerebral blood volume analysis in gliomas predicts survival and mitigates risk of biopsy sampling error. J. Neurooncol. 2018, 136, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Requena, R.; Revert-Ventura, A.J.; García-Martí, G.; Salamé-Gamarra, F.; Pérez-Girbés, A.; Mollá-Olmos, E.; Martí-Bonmatí, L. Post-treatment changes of tumour perfusion parameters can help to predict survival in patients with high-grade astrocytoma. Eur. Radiol. 2017, 27, 3392–3400. [Google Scholar] [CrossRef] [PubMed]
- Vajapeyam, S.; Brown, D.; Billups, C.; Patay, Z.; Vezina, G.; Shiroishi, M.S.; Law, M.; Baxter, P.; Onar-Thomas, A.; Fangusaro, J.R.; et al. Advanced ADC Histogram, Perfusion, and Permeability Metrics Show an Association with Survival and Pseudoprogression in Newly Diagnosed Diffuse Intrinsic Pontine Glioma: A Report from the Pediatric Brain Tumor Consortium. Am. J. Neuroradiol. 2020, 41, 718–724. [Google Scholar] [CrossRef]
- Spampinato, M.V.; Schiarelli, C.; Cianfoni, A.; Giglio, P.; Welsh, C.T.; Bisdas, S.; Rumboldt, Z. Correlation between Cerebral Blood Volume Measurements by Perfusion-Weighted Magnetic Resonance Imaging and Two-Year Progression-Free Survival in Gliomas. Neuroradiol. J. 2013, 26, 385–395. [Google Scholar] [CrossRef]
- Mangla, R.; Ginat, D.T.; Kamalian, S.; Milano, M.T.; Korones, D.N.; Walter, K.A.; Ekholm, S. Correlation between progression free survival and dynamic susceptibility contrast MRI perfusion in WHO grade III glioma subtypes. J. Neurooncol. 2014, 116, 325–331. [Google Scholar] [CrossRef]
- Bonekamp, D.; Mouridsen, K.; Radbruch, A.; Kurz, F.T.; Eidel, O.; Wick, A.; Schlemmer, H.-P.; Wick, W.; Bendszus, M.; Østergaard, L.; et al. Assessment of tumor oxygenation and its impact on treatment response in bevacizumab-treated recurrent glioblastoma. J. Cereb. Blood Flow Metab. 2017, 37, 485–494. [Google Scholar] [CrossRef]
- Jain, R.; Poisson, L.; Narang, J.; Gutman, D.; Scarpace, L.; Hwang, S.N.; Holder, C.; Wintermark, M.; Colen, R.R.; Kirby, J.; et al. Genomic Mapping and Survival Prediction in Glioblastoma: Molecular Subclassification Strengthened by Hemodynamic Imaging Biomarkers. Radiology 2013, 267, 212–220. [Google Scholar] [CrossRef]
- Jenkinson, M.D.; Smith, T.S.; Joyce, K.A.; Fildes, D.; Broome, J.; du Plessis, D.G.; Haylock, B.; Husband, D.J.; Warnke, P.C.; Walker, C. Cerebral blood volume, genotype and chemosensitivity in oligodendroglial tumours. Neuroradiology 2006, 48, 703–713. [Google Scholar] [CrossRef]
- Jabehdar Maralani, P.; Melhem, E.R.; Wang, S.; Herskovits, E.H.; Voluck, M.R.; Kim, S.J.; Learned, K.O.; O’Rourke, D.M.; Mohan, S. Association of dynamic susceptibility contrast enhanced MR Perfusion parameters with prognosis in elderly patients with glioblastomas. Eur. Radiol. 2015, 25, 2738–2744. [Google Scholar] [CrossRef]
- Çoban, G.; Mohan, S.; Kural, F.; Wang, S.; O’Rourke, D.M.; Poptani, H. Prognostic Value of Dynamic Susceptibility Contrast-Enhanced and Diffusion-Weighted MR Imaging in Patients with Glioblastomas. Am. J. Neuroradiol. 2015, 36, 1247–1252. [Google Scholar] [CrossRef] [PubMed]
- Fong, C.; Parpia, S.; Yemen, B.; Tsai, S.; Greenspoon, J. Using Magnetic Resonance Perfusion to Stratify Overall Survival in Treated High-Grade Gliomas. Can. J. Neurol. Sci. 2019, 46, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Juan-Albarracín, J.; Fuster-Garcia, E.; Pérez-Girbés, A.; Aparici-Robles, F.; Alberich-Bayarri, Á.; Revert-Ventura, A.; Martí-Bonmatí, L.; García-Gómez, J.M. Glioblastoma: Vascular Habitats Detected at Preoperative Dynamic Susceptibility-weighted Contrast-enhanced Perfusion MR Imaging Predict Survival. Radiology 2018, 287, 944–954. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Tsien, C.I.; Nagesh, V.; Junck, L.; Ten Haken, R.; Ross, B.D.; Chenevert, T.L.; Lawrence, T.S. Clinical investigation survival prediction in high-grade gliomas by MRI perfusion before and during early stage of RT. Int. J. Radiat. Oncol. Biol. Phys. 2006, 64, 876–885. [Google Scholar] [CrossRef] [PubMed]
- Bag, A.K.; Cezayirli, P.C.; Davenport, J.J.; Gaddikeri, S.; Fathallah-Shaykh, H.M.; Cantor, A.; Han, X.S.; Nabors, L.B. Survival analysis in patients with newly diagnosed primary glioblastoma multiforme using pre- and post-treatment peritumoral perfusion imaging parameters. J. Neurooncol. 2014, 120, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Danchaivijitr, N.; Waldman, A.D.; Tozer, D.J.; Benton, C.E.; Brasil Caseiras, G.; Tofts, P.S.; Rees, J.H.; Jäger, H.R. Low-Grade Gliomas: Do Changes in rCBV Measurements at Longitudinal Perfusion-weighted MR Imaging Predict Malignant Transformation? Radiology 2008, 247, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Schmainda, K.M.; Zhang, Z.; Prah, M.; Snyder, B.S.; Gilbert, M.R.; Sorensen, A.G.; Barboriak, D.P.; Boxerman, J.L. Dynamic susceptibility contrast MRI measures of relative cerebral blood volume as a prognostic marker for overall survival in recurrent glioblastoma: Results from the ACRIN 6677/RTOG 0625 multicenter trial. Neuro Oncol. 2015, 17, 1148–1156. [Google Scholar] [CrossRef]
- Schmainda, K.M.; Prah, M.A.; Marques, H.; Kim, E.; Barboriak, D.P.; Boxerman, J.L. Value of dynamic contrast perfusion MRI to predict early response to bevacizumab in newly diagnosed glioblastoma: Results from ACRIN 6686 multicenter trial. Neuro Oncol. 2021, 23, 314–323. [Google Scholar] [CrossRef]
- Kang, Y.; Hong, E.K.; Rhim, J.H.; Yoo, R.-E.; Kang, K.M.; Yun, T.J.; Kim, J.-H.; Sohn, C.-H.; Park, S.-W.; Choi, S.H. Prognostic Value of Dynamic Contrast-Enhanced MRI-Derived Pharmacokinetic Variables in Glioblastoma Patients: Analysis of Contrast-Enhancing Lesions and Non-Enhancing T2 High-Signal Intensity Lesions. Korean J. Radiol. 2020, 21, 707–716. [Google Scholar] [CrossRef]
- Hwang, I.; Choi, S.H.; Park, C.-K.; Kim, T.M.; Park, S.-H.; Won, J.K.; Kim, I.H.; Lee, S.-T.; Yoo, R.-E.; Kang, K.M.; et al. Dynamic Contrast-Enhanced MR Imaging of Nonenhancing T2 High-Signal-Intensity Lesions in Baseline and Posttreatment Glioblastoma: Temporal Change and Prognostic Value. Am. J. Neuroradiol. 2020, 41, 49–56. [Google Scholar] [CrossRef]
- Ly, K.I.; Vakulenko-Lagun, B.; Emblem, K.E.; Ou, Y.; Da, X.; Betensky, R.A.; Kalpathy-Cramer, J.; Duda, D.G.; Jain, R.K.; Chi, A.S.; et al. Probing tumor microenvironment in patients with newly diagnosed glioblastoma during chemoradiation and adjuvant temozolomide with functional MRI. Sci. Rep. 2018, 8, 17062. [Google Scholar] [CrossRef] [PubMed]
- Hilario, A.; Hernandez-Lain, A.; Sepulveda, J.M.; Lagares, A.; Perez-Nuñez, A.; Ramos, A. Perfusion MRI grading diffuse gliomas: Impact of permeability parameters on molecular biomarkers and survival. Neurocirugía 2019, 30, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Mills, S.J.; Patankar, T.A.; Haroon, H.A.; Balériaux, D.; Swindell, R.; Jackson, A. Do Cerebral Blood Volume and Contrast Transfer Coefficient Predict Prognosis in Human Glioma? Am. J. Neuroradiol. 2006, 27, 853–858. [Google Scholar] [PubMed]
- Nguyen, T.B.; Cron, G.O.; Mercier, J.F.; Foottit, C.; Torres, C.H.; Chakraborty, S.; Woulfe, J.; Jansen, G.H.; Caudrelier, J.M.; Sinclair, J.; et al. Preoperative Prognostic Value of Dynamic Contrast-Enhanced MRI–Derived Contrast Transfer Coefficient and Plasma Volume in Patients with Cerebral Gliomas. Am. J. Neuroradiol. 2015, 36, 63–69. [Google Scholar] [CrossRef]
- Bonekamp, D.; Deike, K.; Wiestler, B.; Wick, W.; Bendszus, M.; Radbruch, A.; Heiland, S. Association of overall survival in patients with newly diagnosed glioblastoma with contrast-enhanced perfusion MRI: Comparison of intraindividually matched T1- and T2*-based bolus techniques. J. Magn. Reson. Imaging 2015, 42, 87–96. [Google Scholar] [CrossRef]
- Kickingereder, P.; Wiestler, B.; Burth, S.; Wick, A.; Nowosielski, M.; Heiland, S.; Schlemmer, H.-P.; Wick, W.; Bendszus, M.; Radbruch, A. Relative cerebral blood volume is a potential predictive imaging biomarker of bevacizumab efficacy in recurrent glioblastoma. Neuro Oncol. 2015, 17, 1139–1147. [Google Scholar] [CrossRef]
- Larsson, C.; Groote, I.; Vardal, J.; Kleppestø, M.; Odland, A.; Brandal, P.; Due-Tønnessen, P.; Holme, S.S.; Hope, T.R.; Meling, T.R.; et al. Prediction of survival and progression in glioblastoma patients using temporal perfusion changes during radiochemotherapy. Magn. Reson. Imaging 2020, 68, 106–112. [Google Scholar] [CrossRef]
- Bisdas, S.; Smrdel, U.; Bajrovic, F.F.; Surlan-Popovic, K. Assessment of Progression-Free-Survival in Glioblastomas by Intratreatment Dynamic Contrast-Enhanced MRI. Clin. Neuroradiol. 2016, 26, 39–45. [Google Scholar] [CrossRef]
- O’Neill, A.F.; Qin, L.; Wen, P.Y.; de Groot, J.F.; Van den Abbeele, A.D.; Yap, J.T. Demonstration of DCE-MRI as an early pharmacodynamic biomarker of response to VEGF Trap in glioblastoma. J. Neurooncol. 2016, 130, 495–503. [Google Scholar] [CrossRef]
- Møller, S.; Lundemann, M.; Law, I.; Poulsen, H.S.; Larsson, H.B.W.; Engelholm, S.A. Early changes in perfusion of glioblastoma during radio- and chemotherapy evaluated by T1-dynamic contrast enhanced magnetic resonance imaging. Acta Oncol. 2015, 54, 1521–1528. [Google Scholar] [CrossRef]
- Park, Y.W.; Ahn, S.S.; Moon, J.H.; Kim, E.H.; Kang, S.-G.; Chang, J.H.; Kim, S.H.; Lee, S.-K. Dynamic contrast-enhanced MRI may be helpful to predict response and prognosis after bevacizumab treatment in patients with recurrent high-grade glioma: Comparison with diffusion tensor and dynamic susceptibility contrast imaging. Neuroradiology 2021, 63, 1811–1822. [Google Scholar] [CrossRef]
- Choi, Y.S.; Ahn, S.S.; Lee, H.-J.; Chang, J.H.; Kang, S.-G.; Kim, E.H.; Kim, S.H.; Lee, S.-K. The Initial Area Under the Curve Derived from Dynamic Contrast-Enhanced MRI Improves Prognosis Prediction in Glioblastoma with Unmethylated MGMT Promoter. Am. J. Neuroradiol. 2017, 38, 1528–1535. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.H.; Jung, S.C.; Kim, K.W.; Lee, J.Y.; Choi, Y.; Park, S.H.; Kim, H.S. Perfusion MRI as the predictive/prognostic and pharmacodynamic biomarkers in recurrent malignant glioma treated with bevacizumab: A systematic review and a time-to-event meta-analysis. J. Neurooncol. 2016, 128, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Furtner, J.; Bender, B.; Braun, C.; Schittenhelm, J.; Skardelly, M.; Ernemann, U.; Bisdas, S. Prognostic Value of Blood Flow Measurements Using Arterial Spin Labeling in Gliomas. PLoS ONE 2014, 9, e99616. [Google Scholar] [CrossRef]
- Jain, R.; Narang, J.; Griffith, B.; Bagher-Ebadian, H.; Scarpace, L.; Mikkelsen, T.; Littenberg, B.; Schultz, L.R. Prognostic vascular imaging biomarkers in high-grade gliomas: Tumor permeability as an adjunct to blood volume estimates. Acad Radiol. 2013, 20, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.; Griffith, B.; Alotaibi, F.; Zagzag, D.; Fine, H.; Golfinos, J.; Schultz, L. Glioma Angiogenesis and Perfusion Imaging: Understanding the Relationship between Tumor Blood Volume and Leakiness with Increasing Glioma Grade. Am. J. Neuroradiol. 2015, 36, 2030–2035. [Google Scholar] [CrossRef]
- Yeung, T.P.C.; Wang, Y.; He, W.; Urbini, B.; Gafà, R.; Ulazzi, L.; Yartsev, S.; Bauman, G.; Lee, T.-Y.; Fainardi, E.; et al. Survival prediction in high-grade gliomas using CT perfusion imaging. J. Neuro Oncol. 2015, 123, 93–102. [Google Scholar] [CrossRef]
- Stadlbauer, A.; Kinfe, T.M.; Eyüpoglu, I.; Zimmermann, M.; Kitzwögerer, M.; Podar, K.; Buchfelder, M.; Heinz, G.; Oberndorfer, S.; Marhold, F. Tissue Hypoxia and Alterations in Microvascular Architecture Predict Glioblastoma Recurrence in Humans. Clin. Cancer Res. 2020. [Google Scholar] [CrossRef]
- Stadlbauer, A.; Zimmermann, M.; Doerfler, A.; Oberndorfer, S.; Buchfelder, M.; Coras, R.; Kitzwögerer, M.; Roessler, K. Intratumoral heterogeneity of oxygen metabolism and neovascularization uncovers 2 survival-relevant subgroups of IDH1 wild-type glioblastoma. Neuro Oncol. 2018, 20, 1536–1546. [Google Scholar] [CrossRef]
- Stadlbauer, A.; Eyüpoglu, I.; Buchfelder, M.; Dörfler, A.; Zimmermann, M.; Heinz, G.; Oberndorfer, S. Vascular architecture mapping for early detection of glioblastoma recurrence. Neurosurg. Focus. 2019, 47, E14. [Google Scholar] [CrossRef]
- Fuster-Garcia, E.; Juan-Albarracín, J.; García-Ferrando, G.A.; Martí-Bonmatí, L.; Aparici-Robles, F.; García-Gómez, J.M. Improving the estimation of prognosis for glioblastoma patients by MR based hemodynamic tissue signatures. NMR Biomed. 2018, 31, e4006. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Torres, M.D.M.; Juan-Albarracín, J.; Fuster-Garcia, E.; Bellvís-Bataller, F.; Lorente, D.; Reynés, G.; de Mora, J.F.; Aparici-Robles, F.; Botella, C.; Muñoz-Langa, J.; et al. Robust association between vascular habitats and patient prognosis in glioblastoma: An international multicenter study. J. Magn. Reson. Imaging 2020, 51, 1478–1486. [Google Scholar] [CrossRef] [PubMed]
- Chelebian, E.; Fuster-Garcia, E.; Álvarez-Torres, M.D.M.; Juan-Albarracín, J.; García-Gómez, J.M. Higher vascularity at infiltrated peripheral edema differentiates proneural glioblastoma subtype. PLoS ONE 2020, 15, e0232500. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Torres, M.D.M.; Fuster-García, E.; Balaña, C.; Puig, J.; García-Gómez, J.M. Lack of Benefit of Extending Temozolomide Treatment in Patients with High Vascular Glioblastoma with Methylated MGMT. Cancers 2021, 13, 5420. [Google Scholar] [CrossRef]
- Prada, F.; Vetrano, I.G.; Gennari, A.G.; Mauri, G.; Martegani, A.; Solbiati, L.; Sconfienza, L.M.; Quaia, E.; Kearns, K.N.; Kalani, M.Y.S.; et al. How to Perform Intra-Operative Contrast-Enhanced Ultrasound of the Brain—A WFUMB Position Paper. Ultrasound Med. Biol. 2021, 47, 2006–2016. [Google Scholar] [CrossRef]
- Prada, F.; Vitale, V.; Del Bene, M.; Boffano, C.; Sconfienza, L.M.; Pinzi, V.; Mauri, G.; Solbiati, L.; Sakas, G.; Kolev, V.; et al. Contrast-enhanced MR Imaging versus Contrast-enhanced US: A Comparison in Glioblastoma Surgery by Using Intraoperative Fusion Imaging. Radiology 2017, 285, 242–249. [Google Scholar] [CrossRef]
- Della Pepa, G.M.; Menna, G.; Ius, T.; Di Bonaventura, R.; Altieri, R.; Marchese, E.; Olivi, A.; Sabatino, G.; La Rocca, G. Contrast enhanced ultrasound (CEUS) applications in neurosurgical and neurological settings–New scenarios for brain and spinal cord ultrasonography. A systematic review. Clin. Neurol. Neurosurg. 2020, 198, 106105. [Google Scholar] [CrossRef]
- Kearns, K.N.; Sokolowski, J.D.; Chadwell, K.; Chandler, M.; Kiernan, T.; Prada, F.; Kalani, M.Y.S.; Park, M.S. The role of contrast-enhanced ultrasound in neurosurgical disease. Neurosurg. Focus 2019, 47, E8. [Google Scholar] [CrossRef]
- Wang, J.; Yang, Y.; Liu, X.; Duan, Y. Intraoperative contrast-enhanced ultrasound for cerebral glioma resection and the relationship between microvascular perfusion and microvessel density. Clin. Neurol. Neurosurg. 2019, 186, 105512. [Google Scholar] [CrossRef]
- Cheng, L.-G.; He, W.; Zhang, H.-X.; Song, Q.; Ning, B.; Li, H.-Z.; He, Y.; Lin, S. Intraoperative Contrast Enhanced Ultrasound Evaluates the Grade of Glioma. Available online: https://www.hindawi.com/journals/bmri/2016/2643862/ (accessed on 7 December 2021).
- Prada, F.; Mattei, L.; Del Bene, M.; Aiani, L.; Saini, M.; Casali, C.; Filippini, A.; Legnani, F.G.; Perin, A.; Saladino, A.; et al. Intraoperative Cerebral Glioma Characterization with Contrast Enhanced Ultrasound. BioMed Res. Int. 2014, 2014, e484261. [Google Scholar] [CrossRef]
- Berhouma, M.; Picart, T.; Dumot, C.; Pelissou-Guyotat, I.; Meyronet, D.; Ducray, F.; Honnorat, J.; Eker, O.; Guyotat, J.; Lukaszewicz, A.-C.; et al. Alterations of cerebral microcirculation in peritumoral edema: Feasibility of in vivo sidestream dark-field imaging in intracranial meningiomas. Neuro Oncol. Adv. 2020, 2, vdaa108. [Google Scholar] [CrossRef] [PubMed]
- Tahhan, N.; Balanca, B.; Fierstra, J.; Waelchli, T.; Picart, T.; Dumot, C.; Eker, O.; Marinesco, S.; Radovanovic, I.; Cotton, F.; et al. Intraoperative cerebral blood flow monitoring in neurosurgery: A review of contemporary technologies and emerging perspectives. Neurochirurgie 2021. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Le Bihan, D.; Breton, E.; Lallemand, D.; Grenier, P.; Cabanis, E.; Laval-Jeantet, M. MR imaging of intravoxel incoherent motions: Application to diffusion and perfusion in neurologic disorders. Radiology 1986, 161, 401–407. [Google Scholar] [CrossRef]
- Le Bihan, D.; Breton, E.; Lallemand, D.; Aubin, M.L.; Vignaud, J.; Laval-Jeantet, M. Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology 1988, 168, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Federau, C. Measuring Perfusion: Intravoxel Incoherent Motion MR Imaging. Magn. Reson. Imaging Clin. N. Am. 2021, 29, 233–242. [Google Scholar] [CrossRef]
- Suh, C.H.; Kim, H.S.; Lee, S.S.; Kim, N.; Yoon, H.M.; Choi, C.-G.; Kim, S.J. Atypical Imaging Features of Primary Central Nervous System Lymphoma That Mimics Glioblastoma: Utility of Intravoxel Incoherent Motion MR Imaging. Radiology 2014, 272, 504–513. [Google Scholar] [CrossRef]
- Yamashita, K.; Hiwatashi, A.; Togao, O.; Kikuchi, K.; Kitamura, Y.; Mizoguchi, M.; Yoshimoto, K.; Kuga, D.; Suzuki, S.O.; Baba, S.; et al. Diagnostic utility of intravoxel incoherent motion mr imaging in differentiating primary central nervous system lymphoma from glioblastoma multiforme. J. Magn. Reson. Imaging 2016, 44, 1256–1261. [Google Scholar] [CrossRef]
- Keil, V.C.; Mädler, B.; Gielen, G.H.; Pintea, B.; Hiththetiya, K.; Gaspranova, A.R.; Gieseke, J.; Simon, M.; Schild, H.H.; Hadizadeh, D.R. Intravoxel incoherent motion MRI in the brain: Impact of the fitting model on perfusion fraction and lesion differentiability. J. Magn. Reson. Imaging 2017, 46, 1187–1199. [Google Scholar] [CrossRef]
- Wang, X.; Chen, X.-Z.; Shi, L.; Dai, J.-P. Glioma grading and IDH1 mutational status: Assessment by intravoxel incoherent motion MRI. Clin. Radiol. 2019, 74, 651.e7–651.e14. [Google Scholar] [CrossRef]
- Wang, C.; Dong, H. Ki-67 labeling index and the grading of cerebral gliomas by using intravoxel incoherent motion diffusion-weighted imaging and three-dimensional arterial spin labeling magnetic resonance imaging. Acta Radiol. 2020, 61, 1057–1063. [Google Scholar] [CrossRef]
- Hu, Y.-C.; Yan, L.-F.; Wu, L.; Du, P.; Chen, B.-Y.; Wang, L.; Wang, S.-M.; Han, Y.; Tian, Q.; Yu, Y.; et al. Intravoxel incoherent motion diffusion-weighted MR imaging of gliomas: Efficacy in preoperative grading. Sci. Rep. 2014, 4, 7208. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Niu, C.; Shakir, T.M.; Chen, T.; Zhang, M.; Wang, Z. An evidence-based approach to assess the accuracy of intravoxel incoherent motion imaging for the grading of brain tumors. Medicine 2018, 97, e13217. [Google Scholar] [CrossRef] [PubMed]
- Jabehdar Maralani, P.; Myrehaug, S.; Mehrabian, H.; Chan, A.K.M.; Wintermark, M.; Heyn, C.; Conklin, J.; Ellingson, B.M.; Rahimi, S.; Lau, A.Z.; et al. Intravoxel incoherent motion (IVIM) modeling of diffusion MRI during chemoradiation predicts therapeutic response in IDH wildtype glioblastoma. Radiother. Oncol. 2021, 156, 258–265. [Google Scholar] [CrossRef]
- Liu, Z.-C.; Yan, L.-F.; Hu, Y.-C.; Sun, Y.-Z.; Tian, Q.; Nan, H.-Y.; Yu, Y.; Sun, Q.; Wang, W.; Cui, G.-B. Combination of IVIM-DWI and 3D-ASL for differentiating true progression from pseudoprogression of Glioblastoma multiforme after concurrent chemoradiotherapy: Study protocol of a prospective diagnostic trial. BMC Med. Imaging 2017, 17, 10. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Xu, D.; Zhou, J.; Wang, S.-C.; Cai, Y.-X.; Li, H.; Xu, H.-B. Monitoring Bevacizumab-Induced Tumor Vascular Normalization by Intravoxel Incoherent Motion Diffusion-Weighted MRI. J. Magn. Reson. Imaging 2021. [Google Scholar] [CrossRef] [PubMed]
- Puig, J.; Sánchez-González, J.; Blasco, G.; Daunis-i-Estadella, P.; Federau, C.; Alberich-Bayarri, Á.; Biarnes, C.; Nael, K.; Essig, M.; Jain, R.; et al. Intravoxel Incoherent Motion Metrics as Potential Biomarkers for Survival in Glioblastoma. PLoS ONE 2016, 11, e0158887. [Google Scholar] [CrossRef] [PubMed]
- Federau, C.; Cerny, M.; Roux, M.; Mosimann, P.J.; Maeder, P.; Meuli, R.; Wintermark, M. IVIM perfusion fraction is prognostic for survival in brain glioma. Clin. Neuroradiol. 2017, 27, 485–492. [Google Scholar] [CrossRef]
- Zhu, L.; Wu, J.; Zhang, H.; Niu, H.; Wang, L. The value of intravoxel incoherent motion imaging in predicting the survival of patients with astrocytoma. Acta Radiol. 2021, 62, 423–429. [Google Scholar] [CrossRef]
- Hsu, Y.-Y.; Chang, C.-N.; Jung, S.-M.; Lim, K.-E.; Huang, J.-C.; Fang, S.-Y.; Liu, H.-L. Blood oxygenation level-dependent MRI of cerebral gliomas during breath holding. J. Magn. Reson. Imaging 2004, 19, 160–167. [Google Scholar] [CrossRef]
- Iranmahboob, A.; Peck, K.K.; Brennan, N.P.; Karimi, S.; Fisicaro, R.; Hou, B.; Holodny, A.I. Vascular Reactivity Maps in Patients with Gliomas Using Breath-Holding BOLD fMRI. J. Neuroimaging 2016, 26, 232–239. [Google Scholar] [CrossRef]
- Agarwal, S.; Sair, H.I.; Pillai, J.J. The Problem of Neurovascular Uncoupling. Neuroimaging Clin. North. Am. 2021, 31, 53–67. [Google Scholar] [CrossRef] [PubMed]
- Slessarev, M.; Han, J.; Mardimae, A.; Prisman, E.; Preiss, D.; Volgyesi, G.; Ansel, C.; Duffin, J.; Fisher, J.A. Prospective targeting and control of end-tidal CO2 and O2 concentrations. J. Physiol. 2007, 581, 1207–1219. [Google Scholar] [CrossRef]
- Fisher, J.A.; Mikulis, D.J. Cerebrovascular Reactivity: Purpose, Optimizing Methods, and Limitations to Interpretation–A Personal 20-Year Odyssey of (Re)searching. Front. Physiol. 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Fierstra, J.; van Niftrik, B.; Piccirelli, M.; Burkhardt, J.K.; Pangalu, A.; Kocian, R.; Valavanis, A.; Weller, M.; Regli, L.; Bozinov, O. Altered intraoperative cerebrovascular reactivity in brain areas of high-grade glioma recurrence. Magn. Reson. Imaging 2016, 34, 803–808. [Google Scholar] [CrossRef] [PubMed]
- Muscas, G.; van Niftrik, C.H.B.; Sebök, M.; Seystahl, K.; Piccirelli, M.; Stippich, C.; Weller, M.; Regli, L.; Fierstra, J. Hemodynamic investigation of peritumoral impaired blood oxygenation-level dependent cerebrovascular reactivity in patients with diffuse glioma. Magn. Reson. Imaging 2020, 70, 50–56. [Google Scholar] [CrossRef]
- Sebök, M.; van Niftrik, C.H.B.; Muscas, G.; Pangalu, A.; Seystahl, K.; Weller, M.; Regli, L.; Fierstra, J. Hypermetabolism and impaired cerebrovascular reactivity beyond the standard MRI-identified tumor border indicate diffuse glioma extended tissue infiltration. Neuro Oncol. Adv. 2021, 3, vdab048. [Google Scholar] [CrossRef] [PubMed]
- Fierstra, J.; van Niftrik, C.; Piccirelli, M.; Bozinov, O.; Pangalu, A.; Krayenbühl, N.; Valavanis, A.; Weller, M.; Regli, L. Diffuse gliomas exhibit whole brain impaired cerebrovascular reactivity. Magn. Reson. Imaging 2018, 45, 78–83. [Google Scholar] [CrossRef]
- Sebök, M.; van Niftrik, C.H.B.; Halter, M.; Hiller, A.; Seystahl, K.; Pangalu, A.; Weller, M.; Stippich, C.; Regli, L.; Fierstra, J. Crossed Cerebellar Diaschisis in Patients with Diffuse Glioma Is Associated with Impaired Supratentorial Cerebrovascular Reactivity and Worse Clinical Outcome. Cerebellum 2020, 19, 824–832. [Google Scholar] [CrossRef]
- Bashat, D.B.; Artzi, M.; Ami, H.B.; Aizenstein, O.; Blumenthal, D.T.; Bokstein, F.; Corn, B.W.; Ram, Z.; Kanner, A.A.; Lifschitz-Mercer, B.; et al. Hemodynamic Response Imaging: A Potential Tool for the Assessment of Angiogenesis in Brain Tumors. PLoS ONE 2012, 7, e49416. [Google Scholar] [CrossRef]
- Poublanc, J.; Sobczyk, O.; Shafi, R.; Sayin, E.S.; Schulman, J.; Duffin, J.; Uludag, K.; Wood, J.C.; Vu, C.; Dharmakumar, R.; et al. Perfusion MRI using endogenous deoxyhemoglobin as a contrast agent: Preliminary data. Magn. Reson. Med. 2021, 86, 3012–3021. [Google Scholar] [CrossRef]
- Vu, C.; Chai, Y.; Coloigner, J.; Nederveen, A.J.; Borzage, M.; Bush, A.; Wood, J.C. Quantitative perfusion mapping with induced transient hypoxia using BOLD MRI. Magn. Reson. Med. 2021, 85, 168–181. [Google Scholar] [CrossRef]
- Sayin, E.S.; Schulman, J.; Poublanc, J.; Levine, H.; Venkatraghavan, L.; Uludag, K.; Duffin, J.; Fisher, J.A.; Mikulis, D.J.; Sobczyk, O. Cerebral perfusion imaging: Hypoxia-induced deoxyhemoglobin or gadolinium? bioRxiv 2021. [Google Scholar] [CrossRef]
- Kiviniemi, A.; Gardberg, M.; Ek, P.; Frantzén, J.; Bobacka, J.; Minn, H. Gadolinium retention in gliomas and adjacent normal brain tissue: Association with tumor contrast enhancement and linear/macrocyclic agents. Neuroradiology 2019, 61, 535–544. [Google Scholar] [CrossRef]
- Stumpo, V.; Sebök, M.; van Niftrik, C.H.B.; Seystahl, K.; Hainc, N.; Kulcsar, Z.; Weller, M.; Regli, L.; Fierstra, J. Feasibility of glioblastoma tissue response mapping with physiologic BOLD imaging using precise oxygen and carbon dioxide challenge. Magn. Reson. Mater. Phy. 2021. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Stumpo, V.; Kernbach, J.M.; van Niftrik, C.H.B.; Sebök, M.; Fierstra, J.; Regli, L.; Serra, C.; Staartjes, V.E. Machine Learning Algorithms in Neuroimaging: An Overview. Acta Neurochir. Suppl. 2022, 134, 125–138. [Google Scholar] [CrossRef] [PubMed]
- Lambin, P.; Leijenaar, R.T.H.; Deist, T.M.; Peerlings, J.; de Jong, E.E.C.; van Timmeren, J.; Sanduleanu, S.; Larue, R.T.H.M.; Even, A.J.G.; Jochems, A.; et al. Radiomics: The bridge between medical imaging and personalized medicine. Nat. Rev. Clin. Oncol. 2017, 14, 749–762. [Google Scholar] [CrossRef]
- Staartjes, V.E.; Stumpo, V.; Kernbach, J.M.; Klukowska, A.M.; Gadjradj, P.S.; Schröder, M.L.; Veeravagu, A.; Stienen, M.N.; van Niftrik, C.H.B.; Serra, C.; et al. Machine learning in neurosurgery: A global survey. Acta Neurochir. 2020, 162, 3081–3091. [Google Scholar] [CrossRef]
- Wagner, M.W.; Namdar, K.; Biswas, A.; Monah, S.; Khalvati, F.; Ertl-Wagner, B.B. Radiomics, machine learning, and artificial intelligence—what the neuroradiologist needs to know. Neuroradiology 2021, 63, 1957–1967. [Google Scholar] [CrossRef]
- Park, C.J.; Han, K.; Kim, H.; Ahn, S.S.; Choi, D.; Park, Y.W.; Chang, J.H.; Kim, S.H.; Cha, S.; Lee, S.-K. MRI Features May Predict Molecular Features of Glioblastoma in Isocitrate Dehydrogenase Wild-Type Lower-Grade Gliomas. Am. J. Neuroradiol. 2021, 42, 448–456. [Google Scholar] [CrossRef]
- Gusev, Y.; Bhuvaneshwar, K.; Song, L.; Zenklusen, J.-C.; Fine, H.; Madhavan, S. The rembrandt study, a large collection of genomic data from brain cancer patients. Sci. Data 2018, 5, 180158. [Google Scholar] [CrossRef]
- Sudre, C.H.; Panovska-Griffiths, J.; Sanverdi, E.; Brandner, S.; Katsaros, V.K.; Stranjalis, G.; Pizzini, F.B.; Ghimenton, C.; Surlan-Popovic, K.; Avsenik, J.; et al. Machine learning assisted DSC-MRI radiomics as a tool for glioma classification by grade and mutation status. BMC Med. Inform. Decis. Mak. 2020, 20, 149. [Google Scholar] [CrossRef] [PubMed]
- Pak, E.; Choi, K.S.; Choi, S.H.; Park, C.-K.; Kim, T.M.; Park, S.-H.; Lee, J.H.; Lee, S.-T.; Hwang, I.; Yoo, R.-E.; et al. Prediction of Prognosis in Glioblastoma Using Radiomics Features of Dynamic Contrast-Enhanced MRI. Korean J. Radiol. 2021, 22, 1514–1524. [Google Scholar] [CrossRef] [PubMed]
- Hashido, T.; Saito, S.; Ishida, T. A radiomics-based comparative study on arterial spin labeling and dynamic susceptibility contrast perfusion-weighted imaging in gliomas. Sci. Rep. 2020, 10, 6121. [Google Scholar] [CrossRef] [PubMed]
- Manikis, G.C.; Ioannidis, G.S.; Siakallis, L.; Nikiforaki, K.; Iv, M.; Vozlic, D.; Surlan-Popovic, K.; Wintermark, M.; Bisdas, S.; Marias, K. Multicenter DSC–MRI-Based Radiomics Predict IDH Mutation in Gliomas. Cancers 2021, 13, 3965. [Google Scholar] [CrossRef]
- Peng, H.; Huo, J.; Li, B.; Cui, Y.; Zhang, H.; Zhang, L.; Ma, L. Predicting Isocitrate Dehydrogenase (IDH) Mutation Status in Gliomas Using Multiparameter MRI Radiomics Features. J. Magn. Reson. Imaging 2021, 53, 1399–1407. [Google Scholar] [CrossRef]
- Choi, K.S.; Choi, S.H.; Jeong, B. Prediction of IDH genotype in gliomas with dynamic susceptibility contrast perfusion MR imaging using an explainable recurrent neural network. Neuro Oncol. 2019, 21, 1197–1209. [Google Scholar] [CrossRef]
- Bisdas, S.; Sanverdi, E.; Sudre, C.; Roettger, D.; Brandner, S.; Katsaros, V. The role of dynamic susceptibility contrast perfusion- weighted MRI in the estimation of IDH mutation in gliomas. J. Clin. Oncol. 2018, 36, 12063. [Google Scholar] [CrossRef]
- Priya, S.; Liu, Y.; Ward, C.; Le, N.H.; Soni, N.; Pillenahalli Maheshwarappa, R.; Monga, V.; Zhang, H.; Sonka, M.; Bathla, G. Machine learning based differentiation of glioblastoma from brain metastasis using MRI derived radiomics. Sci. Rep. 2021, 11, 10478. [Google Scholar] [CrossRef]
- Jeong, J.; Wang, L.; Ji, B.; Lei, Y.; Ali, A.; Liu, T.; Curran, W.J.; Mao, H.; Yang, X. Machine-learning based classification of glioblastoma using delta-radiomic features derived from dynamic susceptibility contrast enhanced magnetic resonance images. Quant. Imaging Med. Surg. 2019, 9, 1201213. [Google Scholar] [CrossRef]
- Kim, J.Y.; Park, J.E.; Jo, Y.; Shim, W.H.; Nam, S.J.; Kim, J.H.; Yoo, R.-E.; Choi, S.H.; Kim, H.S. Incorporating diffusion- and perfusion-weighted MRI into a radiomics model improves diagnostic performance for pseudoprogression in glioblastoma patients. Neuro Oncol. 2019, 21, 404–414. [Google Scholar] [CrossRef]
- Elshafeey, N.; Kotrotsou, A.; Hassan, A.; Elshafei, N.; Hassan, I.; Ahmed, S.; Abrol, S.; Agarwal, A.; El Salek, K.; Bergamaschi, S.; et al. Multicenter study demonstrates radiomic features derived from magnetic resonance perfusion images identify pseudoprogression in glioblastoma. Nat. Commun. 2019, 10, 3170. [Google Scholar] [CrossRef] [PubMed]
- Siakallis, L.; Sudre, C.H.; Mulholland, P.; Fersht, N.; Rees, J.; Topff, L.; Thust, S.; Jager, R.; Cardoso, M.J.; Panovska-Griffiths, J.; et al. Longitudinal structural and perfusion MRI enhanced by machine learning outperforms standalone modalities and radiological expertise in high-grade glioma surveillance. Neuroradiology 2021, 63, 2047–2056. [Google Scholar] [CrossRef] [PubMed]
- Park, J.E.; Kim, H.S.; Jo, Y.; Yoo, R.-E.; Choi, S.H.; Nam, S.J.; Kim, J.H. Radiomics prognostication model in glioblastoma using diffusion- and perfusion-weighted MRI. Sci. Rep. 2020, 10, 4250. [Google Scholar] [CrossRef] [PubMed]
- Shim, K.Y.; Chung, S.W.; Jeong, J.H.; Hwang, I.; Park, C.-K.; Kim, T.M.; Park, S.-H.; Won, J.K.; Lee, J.H.; Lee, S.-T.; et al. Radiomics-based neural network predicts recurrence patterns in glioblastoma using dynamic susceptibility contrast-enhanced MRI. Sci. Rep. 2021, 11, 9974. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stumpo, V.; Guida, L.; Bellomo, J.; Van Niftrik, C.H.B.; Sebök, M.; Berhouma, M.; Bink, A.; Weller, M.; Kulcsar, Z.; Regli, L.; et al. Hemodynamic Imaging in Cerebral Diffuse Glioma—Part B: Molecular Correlates, Treatment Effect Monitoring, Prognosis, and Future Directions. Cancers 2022, 14, 1342. https://doi.org/10.3390/cancers14051342
Stumpo V, Guida L, Bellomo J, Van Niftrik CHB, Sebök M, Berhouma M, Bink A, Weller M, Kulcsar Z, Regli L, et al. Hemodynamic Imaging in Cerebral Diffuse Glioma—Part B: Molecular Correlates, Treatment Effect Monitoring, Prognosis, and Future Directions. Cancers. 2022; 14(5):1342. https://doi.org/10.3390/cancers14051342
Chicago/Turabian StyleStumpo, Vittorio, Lelio Guida, Jacopo Bellomo, Christiaan Hendrik Bas Van Niftrik, Martina Sebök, Moncef Berhouma, Andrea Bink, Michael Weller, Zsolt Kulcsar, Luca Regli, and et al. 2022. "Hemodynamic Imaging in Cerebral Diffuse Glioma—Part B: Molecular Correlates, Treatment Effect Monitoring, Prognosis, and Future Directions" Cancers 14, no. 5: 1342. https://doi.org/10.3390/cancers14051342
APA StyleStumpo, V., Guida, L., Bellomo, J., Van Niftrik, C. H. B., Sebök, M., Berhouma, M., Bink, A., Weller, M., Kulcsar, Z., Regli, L., & Fierstra, J. (2022). Hemodynamic Imaging in Cerebral Diffuse Glioma—Part B: Molecular Correlates, Treatment Effect Monitoring, Prognosis, and Future Directions. Cancers, 14(5), 1342. https://doi.org/10.3390/cancers14051342






