Simple Summary
This literature analysis is focused on the pleiotropic action associated with metformin treatment. Precisely, metformin treatment exerts many health effects, mainly by inhibiting inflammatory processes, increasing antioxidant capacity, and improving glycemic and lipid metabolism, which overall may be particularly useful in cancer patients’ clinical management. Consequently, metformin is the main novel therapeutic extensively studied in various clinical trials, which are also summarized in this review.
Abstract
The molecular mechanism of action and the individual influence of various metabolic pathways related to metformin intervention are under current investigation. The available data suggest that metformin provides many advantages, exhibiting anti-inflammatory, anti-cancer, hepatoprotective, cardioprotective, otoprotective, radioprotective, and radio-sensitizing properties depending on cellular context. This literature review was undertaken to provide novel evidence concerning metformin intervention, with a particular emphasis on cancer treatment and prevention. Undoubtedly, the pleiotropic actions associated with metformin include inhibiting inflammatory processes, increasing antioxidant capacity, and improving glycemic and lipid metabolism. Consequently, these characteristics make metformin an attractive medicament to translate to human trials, the promising results of which were also summarized in this review.
1. Introduction
Metformin, a biguanide derived from galegine (isoamylene guanidine), has been the main initial pharmacological intervention for type 2 diabetes mellitus (T2DM) since the 20th century [1,2]. Metformin administration has been recommended as the first-line glucose-lowering therapy for all newly diagnosed T2DM by both the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) since 2009 [1,3]. This medicine is considered to be safe and efficient in monotherapy and in combination with other anti-diabetic therapies [3]. Moreover, it has been shown that adding metformin to insulin therapy to patients with type 1 diabetes mellitus (T1DM) resulted in greater glycemic control and a greater decrease in HbA1c [1].
In addition to decreasing blood sugar levels, metformin is emerging as a therapy with other numerous beneficial effects, including body weight control; reductions in the incidence of aging-related diseases; reductions in the risks of cardiovascular and neuropsychiatric disorders and metabolic syndrome; and even improving fertility in polycystic ovary syndrome (PCOS) [4,5,6,7]. Recent literature data describe metformin as an endothelial protector, an effective drug in heart failure, and an anti-inflammatory target useful in rheumatological and immunological diseases as well as in many aging-related morbidities [8,9,10]. Metformin’s influence on multiple pathways, including lipid metabolism, deactivation of inflammation-related processes, and oxidative homeostasis, has been established [11,12,13,14]. Interestingly, the anti-cancer potential of metformin was firstly recognized in patients with T2DM. Patients treated with metformin were characterized by a lower cancer incidence compared to patients receiving other anti-diabetic therapies [15,16,17]. Moreover, the evidence suggests that metformin treatment is associated with a decreased incidence of certain type of cancers, such as colon, liver, breast, pancreatic, and lung cancer [18,19]. Simultaneous anti-cancer treatment and metformin administration improved the response to cancer therapy and decreased cancer mortality [15,20]. With the wide pleiotropic effect of metformin, only 20% of side effects occur, mainly in the gastrointestinal tract. Therefore, this therapy could be used in most clinical management protocols. Moreover, metformin is the main novel therapeutic focus of various clinical trials [21].
This literature review was undertaken to summarize novel evidence concerning metformin intervention with a particular emphasis on its antioxidant and anti-cancer properties. Furthermore, the promising results obtained in many clinical trials concerning metformin intervention are also presented in this review [15,22,23,24,25,26].
2. Multifactorial Actions of Metformin
Metformin is generally known for its anti-diabetic effects. Approximately 40–60% of orally administered metformin is absorbed into the blood. The maximum blood levels are reached after approximately 2.5 h for the immediate-release and 7 h for the extended-release tablet forms [27]. Following the oral ingestion of metformin, this therapeutic is absorbed by enterocytes through the plasma monoamine transporter (PMAT; encoded by the gene Solute Carrier Family 29 Member 4 (SLC29A4), and the organic cation transporter 3 (OCT3), encoded by the gene Solute Carrier Family 22 Member 3, SCL22A3) [28,29]. In hepatocytes, the organic cation transporter 1 (OCT1) and OCT3 are responsible for metformin absorption [30]. Metformin is extracted into the bile by multidrug toxin and extrusion 1 (MATE1) (encoded by Solute Carrier Family 47 Member 1, SLC47A1) [31]. Then, metformin metabolites are excreted unchanged into the urine, where the organic cation transporter 2 (OCT2) (encoded by Solute Carrier Family 22 Member 2, SLC22A2) transports it into the renal epithelial cells through the basolateral membrane, and MATE1 and MATE2 (encoded by Solute Carrier Family 47 Member 2, SLC47A2) excrete it into the urine [31,32,33,34].
Metformin is able to directly and indirectly interact with many enzymes, such as mitochondrial electron transport chain (ETC) complex I, adenosine 5′-monophosphate-activated protein kinase (AMPK), glycerol 3-phosphate dehydrogenase, fructose 1-6-bisphosphatase, and glucose 6P-phosphatase, which highlights the metabolic properties of metformin [15,35,36,37,38,39,40]. Metformin’s influence on multiple metabolic pathways is produced by targeting ETC complex I, the inhibition of which leads to subsequent AMPK activation [41,42,43]. AMPK is the most significant regulator of many metabolic pathways related to lipid metabolism, insulin sensitivity, and energy homeostasis [6,15,27,44]. Nevertheless, several other metabolic properties should be also considered as a major contributor to the therapeutic action of metformin, particularly inhibition of fructose 1-6-bisphosphatase, a gluconeogenesis rate-controlling enzyme, via lowering hepatic glucose production [45]. Moreover, metformin decreases glucose 6-phosphatase activity in hepatocytes by activation of glycolysis downstream of glucose phosphorylation [46]. These mechanisms are implicated in the subsequent pentose-phosphate pathway in the endoplasmic reticulum, constituting the principal component involved in cellular proliferation and antioxidant processes [47].
The reduction in insulin resistance after metformin treatment is associated with an increase in plasma insulin-like growth factor-binding protein 1 (IGFBP-1) concentrations and a decrease in the insulin-like growth factor (IGF-I)/IGFBP-1 ratio [48,49]. This biguanide is also implicated in the redistribution of glucose transporters—glucose transporter 1 (GLUT-1) and glucose transporter 4 (GLUT-4)—from the intracellular space to the cell membranes [50,51]. One of metformin’s mechanisms of action is the activation of insulin receptor substrate 2 (IRS-2) in the liver, and the downstream increase in deoxyglucose uptake is mediated via increased translocation of GLUT-1 to the plasma membrane [52].
Importantly, metformin administration is also associated with the regulation of many molecular targets, such as mitogen-activated protein kinases (MAPKs), B-cell CLL (BCL-2), signal transducer and activator of transcription 3 (STAT3), glioma-associated oncogene homolog 1 (GLI1), mitogen-activated protein kinase 1/2 (ERK1/2), and ribosomal S6 kinase (RSK1) [14,53,54,55,56,57,58]. Using gene expression analysis after metformin intervention, the expression of key genes and their associated proteins was assessed to evaluate metformin’s anti-cancer properties (Table 1).

Table 1.
Metformin’s metabolic targets.
Nevertheless, metformin exerts hermetic effects [68]. The metformin dose–response action is characterized by a low dose stimulation and high dose inhibition [69]. This phenomenon can be explained using an example of the activation of mitochondrial complex I by metformin administration [41]. In in vivo studies, the inhibition of respiratory complex I was achieved after 10 mM or 30 mmol/L metformin treatment [70,71,72]. On the other hand, a study performed by Larsen et al. revealed that a high dose of metformin does not impact mitochondrial complex I respiration in skeletal muscle of patients with T2DM [73]. The inhibition of respiratory complex I and AMPK phosphorylation is achieved using concentrations higher than 1 mM, which is above the maximum therapeutic doses used (generally, 500–2500 mg/day). In contrast, therapeutic concentrations of metformin induce activation of mitochondrial energy metabolism with an improvement in cellular energy status [74]. The concept of hormesis can be useful to fully understand the effective tailoring of metformin therapy [69,75,76].
3. Metformin as a Promoter of Antioxidant Defense
Oxidative stress is an imbalance between the production of reactive oxygen species (ROS) and the organism system’s ability to detoxify and may cause a toxic effects through the production of lipid peroxides and ROS, which could result in damage of components, such as proteins, lipids, and DNA [77]. These damages are mostly caused by O2− (superoxide radical), OH– (hydroxyl radical), and H2O2 (hydrogen peroxide) [78,79,80].
While multiple reports based on in vivo and in vitro studies provide evidence that oxidative-stress markers unequivocally decrease in metformin-treated individuals, the details of the mechanisms responsible for these changes are not thoroughly understood [81,82,83,84,85,86]. The in vitro study performed by Javadipour et al., which focused on the antioxidant effects associated with metformin, revealed that metformin protected against increased oxidative stress via the nicotinamide adenine dinucleotide (NAD)-dependent protein deacylase sirtuin-3 (SIRT3)-related pathway [86,87]. The study was conducted on mitochondria isolated from rat pancreases, and increased oxidative stress and insulin resistance were induced through arsenic exposure [86]. Additionally, metformin is a direct Sirtuin 1 (SIRT1) activating compound, which is a crucial cellular survival protein, thus also involved in combatting oxidative stress [88,89]. Experimental studies indicate that SIRT1 may play a role in the pathogenesis of skeletal muscle insulin sensitivity. SIRT1 directly influences the insulin signal transduction pathway. It increases insulin-dependent IRS2 phosphorylation and Akt activation. Moreover, SIRT1 interacts with peroxisome proliferator-activated receptor α coactivator 1 (PGC1α) and AMPK to stimulate muscle glucose uptake and fatty acid oxidation, and thus it can prevent insulin resistance. Therefore, SIRT1 activators might be useful in the treatment of insulin resistance-related diseases [90].
Accordingly, in the following studies, the metformin-dependent mechanisms involved in enhancing the endogenous antioxidant system were also evaluated. Literature data reported the upregulation of the activity of antioxidant enzymes, including glutathione reductase, catalase, and superoxide dismutase (SOD), and GSH through the downregulation of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase [82,91,92,93]. Moreover, metformin’s modulation of oxidative phosphorylation and glycolysis is mediated by mitochondrial-derived glycerophosphate dehydrogenase (MGPDH) by significant MGPDH downregulation [20]. In addition, metformin can reduce oxidative stress and promote autophagic processes through the activation of AMPK and SIRT3 [53,85,94]. Moreover, paraoxonase 1 (PON1) is the antioxidant that circulates in association with high-density lipoprotein (HDL), simultaneously eliminating the lipid peroxides within lipoproteins [95,96,97]. It is considered to be an HDL lipid-oxidation protector. The study, which was performed using diabetic animal models [54,85] and patients with newly diagnosed T2DM [98] treated with metformin, proved that after this drug intervention, the activity of PON1 was increased (Table 2).

Table 2.
Metformin’s antioxidant targets.
In addition, an in vivo animal study suggested that metformin may protect against ROS [83]. Accordingly, the administration of metformin increases the survival of cells exposed to 4–7 Gray of radiation [100]. An additional advantage is a protective effect 24 h after exposure to radiation, which is potentially useful in counteracting the previously received radiation [100,101]. These reports emphasize the necessity of continuing to evaluate metformin-induced antioxidant mechanisms, therefore, to provide protection against ROS and radiation-induced oxidative damage.
4. Anti-Cancer Effect
There is growing interest in the potential benefits of metformin in cancer treatment and prevention [60]. Tumorigenesis is a complex relationship of metabolic processes that requires a number of steps, in which hyperglycemia may be the main modulator. Cancer cells require high glucose uptake for unrestrained cell growth [6,21,102]. Metformin treatment may not only reduce the risk of cancer patient mortality but may also improve the efficacy of cancer treatment [22,103]. A study performed on patients with T2DM treated with metformin revealed that cancer incidence and mortality were decreased by approximately 10% to 40% compared to that in patients not treated with metformin [14,104].
Mallik et al. showed that AMPK, which is a regulator of multiple processes in cells, specifically acts to inhibit cellular proliferation by disrupting cell division in mitosis, thus playing an essential role in cancer pathogenesis [105]. The increase in AMPK activity induced by metformin appears to induce the G1 phase of the cell cycle, which leads to the arrest of cell proliferation without activating the apoptotic mechanism [42]. Moreover, through AMPK activation, metformin indirectly reduces cancer cell viability by inducing apoptosis and inhibits osteoclast differentiation in bone tumors [106,107]. Furthermore, the reduction in blood glucose and insulin levels may impair the cancer’s growth by AMPK activation, which leads to decreased transcription of gluconeogenic genes by inhibition of histone deacetylases (HDAC) and decreased lipogenic gene expression by inhibition of sterol regulatory element binding proteins [108].
The mammalian target of rapamycin (mTOR) is one of the major intracellular factors involved in modulating cell growth, proliferation, and metabolism [109,110,111]. The overexpression of mTOR is often associated with tumor progression, diabetes, and neurodegenerative disorders [19,105]. It has been shown that metformin inhibits mTOR activity, and this may help to inhibit the multiplication of neoplastic cells [37,112,113]. Moreover, mTOR kinase integrates cell-signaling pathways, including the insulin pathway, and growth factors such as insulin-like growth factor 1 (IGF-1) and 2 (IGF-2), and acts as a sensor for the cellular levels of energetic compounds [62]. Typically, under aerobic conditions, energy is produced through glycolysis followed by the citric acid cycle and oxidative phosphorylation [114]. However, the pathway of energy production in cancer cells differs; the citric acid cycle and oxidative phosphorylation are limited, even under aerobic conditions, to favor lactic fermentation, which is called the “Warburg effect”. This process produces significantly less ATP, which leads to increased energy uptake and use of nutrients, such as nucleotides, amino acids, and lipids, which in turn favor the proliferation of cancer cells [115]. Importantly, metformin, by reducing hyperglycemia and normalizing lipid metabolism, inhibits the Warburg effect, thereby hindering cancer proliferation.
Protein 53 (p53) is a tumor suppressor that regulates autophagic and apoptotic processes [116]. Accumulating evidence suggests that p53 activation can transcriptionally inhibit GLUT1 and GLUT4 expression, suppressing glucose uptake [117]. Moreover, p53 induces the expression of TP53-induced glycolysis and apoptosis regulator (TIGAR), a transcription factor involved in the regulation of ROS formation, autophagy, and apoptosis [118]. Accordingly, metformin’s anti-cancer effect could be related to the modulation of AMPK, which leads to subsequent p53 activation [105,116]. In one study, the reduced stability of p53 in metformin-treated individuals led to a decrease in differentiated embryo chondrocyte 1 (DEC1) expression, which plays a crucial role in the DNA-damage response via transcriptional regulation under hypoxic conditions and the induction of cancer cell apoptosis [19,21,119].
It has been shown that the inhibition of epidermal growth factor receptor 2 (HER2) expression leads to reduced expression of vascular endothelial growth factor A (VEGFA), ultimately resulting in lower angiogenesis and anti-cancer effects [120]. Interestingly, Wang et al. reported that metformin could reduce the expression of HER2 [121]. Furthermore, metformin has been shown to reduce the synthesis of lithocholic acid (LCA), which is additionally stimulated by increased oxidative stress [122], leading to an inhibitory effect on NF-κB signaling. NF-κB signaling is essential for the regulation of interleukin 8 (IL-8), which plays an important role in tumor progression and metastasis in a variety of human cancers, especially in lung and colon cancers [123,124]. In addition, metformin inhibits hexokinase (HK) in breast cancer cells, which is an essential glycolytic enzyme that catalyzes the phosphorylation of glucose by ATP to glucose-6-phosphate (G6P). It has been shown that G6P partially impairs glucose metabolism and tumor growth [125]. The study performed by Bonannii et al. showed that in a group of diabetic patients also diagnosed with breast cancer treated with metformin, the tumor size was reduced compared to that in the control group [126]. The trend of decreasing breast cancer proliferation in the diabetes patient group suggests that indirect insulin- and glucose-mediated effects are the main mechanism of anticancer effect of metformin in human breast cancer and attenuate the role of direct effects on specific pathways concerned on the AMPK/mTOR cascade activity [126]. The prognosis of neoplastic disease development can be determined based on the expression of genes encoding angiogenic factors. Research focused on angiogenesis is justified in order to identify useful prognostic factors, find diagnostic markers, and determine new therapeutic targets for introducing potential new-generation medicines.
What should also be noted in the context of the anti-cancer action of metformin is that this therapeutic inhibits the production of leptin and stimulates the production of adiponectin [127]. Increased serum leptin levels have been shown to be associated with increased tumor growth and a greater risk of metastasis. By contrast, adiponectin may present an inhibitory effect on cancer development and appears to exert an anti-proliferative effect in tumor cells.
Overall, the results demonstrate that metformin acts comprehensively on various cancers, such as breast, pancreatic, gastric, colorectal, endometrial, prostate, and bladder cancer, and could represent an independent treatment option or be used in combination with other medicines [18,19,22,98,128,129,130]. In many studies performed in a group of lung cancer patients treated with metformin, decreased proliferation of cancer cells and increased apoptosis were reported [102,131,132,133,134]. Growing evidence from epidemiological and observational studies shows that metformin may be an effective adjuvant anti-cancer therapy [24,60,132].
5. Pleiotropic Use of Metformin in Clinical Trials
5.1. Metabolic Effect of Metformin in Various Diseases
Currently, 2035 clinical trials using metformin are registered as completed or ongoing according to the Protocol Registration and Results System (PRS) and the European Union Clinical Trials Register. This underlines the growing interest in this therapy [135]. Numerous clinical studies have demonstrated that metformin monotherapy is effective in reducing the risk of serious complications associated with T2DM with a median dose of 1500–2000 mg/day [136,137,138,139]. The beneficial effects of metformin in reducing the risk factors of atherosclerosis, improving microcirculatory blood flow, and, in particular, increasing the sensitivity of cells to insulin are well documented [140]. Metformin also improves the function of the heart muscle cells. Increasing glucose metabolism along the glycolysis pathway through the inhibition of fatty acid β-oxidation reduces the oxygen demand of myocardial cells. This has a positive effect on the efficiency of the Na/K/Ca membrane pumps [141,142]. The increased removal of Ca2+ ions from the cytoplasm of cardiomyocytes improves the relaxation of the heart muscle [143]. Additionally, metformin intervention in T2DM patients also resulted in a reduction in the risk of Parkinson’s disease, dementia, and other neurodegenerative diseases [144,145]. The study performed by Hsu et al. reported that T2DM increased the risk of dementia 2.6-fold, and the combined intervention of sulfonylureas and metformin was able to decrease the risk of dementia by 35% in an 8-year observation [145].
It has been shown that obesity is associated with increased risk of the cancer development, whereas metformin treatment is associated with weight reduction [146,147,148,149]. The clinical trial conducted by Ruholamin et al., in a group of women diagnosed with gestational diabetes who received 500 mg metformin once or twice a day, demonstrated that metformin intervention resulted in weight and insulin dose reductions, leading to better glycemic control. Moreover, newborns were observed to have a decreased rate of obesity [150,151,152]. The study conducted by Seifarth et al., which focused on the effect of metformin intervention on weight loss in non-diabetic obese individuals, suggested that this treatment option was an effective tool for weight reduction in both insulin-sensitive and insulin-resistant overweight patients and obese patients [26]. This study included 154 patients with body mass indices ≥27 kg/m2 who received metformin intervention dosages of 2500 mg per day in an outpatient setting over 6 months [26].
5.2. Effect of Metformin on Fertility
There are currently 349 female infertility trials, many of which are focused on the increased efficacy of in vitro fertilization (IVF) when combined with metformin intervention [153]. The clinical trial performed by Foda et al. showed that metformin therapy in patients with endometriosis resulted in an increased pregnancy rate and lower serum cytokine levels [154]. For the purpose of this study, 35 infertile patients with endometriosis were administered with 500 mg metformin three times daily for 6 months plus a multivitamin once daily. A literature review on metformin intervention in the treatment of infertility in PCOS revealed 10 completed clinical trials [153]. The studies demonstrated that metformin was effective in inducing ovulation and reducing the risk of preterm labor in PCOS patients. A comprehensive review of seven randomized clinical trials involving 702 women unsuccessfully trying to become pregnant for a period of 6 months showed an increased clinical pregnancy rate after metformin treatment. Moreover, these studies confirmed that metformin therapy improved blood supply and the thickness of the endometrium in PCOS women [155]. Additionally, Johnson suggested that women with PCOS undergoing IVF should be treated with metformin to reduce the risk of ovarian hyperstimulation syndrome [156]. In 2019, a promising metformin intervention in a group of women with unexplained infertility with anovulatory cycles was begun (NCT03681197) [157].
5.3. Anti-Cancer Effect of Metformin in Cancer in Clinical Trials
Currently, 255 clinical trials concerning the potential use of metformin in cancer treatment are being conducted. There is strong evidence concerning the association between metformin use and decreased pancreatic cancer incidence and increased overall survival in colorectal cancer [158,159]. The study conducted by Miranda et al. on patients with colorectal cancer revealed that 850 mg of metformin, 425 mg/m2 5-fluorouracil, and 50 mg leucovorin twice a day was associated with longer survival in obese patients [160]. In another METAL (metformin in advanced lung cancer) trial, the clinical utility of metformin with erlotinib in second-line therapy of patients with stage IV non-small-cell lung cancer was evaluated. For the purpose of this study, twelve patients were enrolled and administered with 1500 mg metformin with 150 mg erlotinib. The study showed that this combination improved prognoses for patients. This approach could also improve survival and overall outcome [161]. The clinical trial conducted by Bever et al., in which 22 patients with metastatic pancreatic adenocarcinoma were treated with metformin alone (850 mg orally twice a day) or with rapamycin (4 mg daily), showed that the intervention was well tolerated, and that certain patients achieved stable disease, which was further associated with longer survival [162]. Moreover, interim analyses of ongoing studies involving neoadjuvant metformin treatment in newly diagnosed breast cancer patients demonstrate that this intervention is safe and well tolerated. It was proved that metformin can directly affect primary breast cancer in vivo, including the downregulation of phosphodiesterase 3B (PDE3B), a critical regulator of cAMP synthesis, which combined with AMPK activation, can be considered as adjuvant breast cancer therapy [163]. The clinical trial conducted by Goodwin et al. suggested that the administration of 850 mg of metformin led to weight loss and improved metabolic health in early-stage breast cancer patients. Furthermore, support for a potential metformin treatment mediated by reducing serum levels of insulin and other metabolic markers, such as serum levels of glucose, leptin, and high-sensitivity C-reactive protein (hsCRP) in a group of breast cancer patients was provided [164]. Patients receive oral metformin hydrochloride twice a day (once daily in weeks 1–4). Treatment continues for up to 5 years in receptor positive (estrogen receptor and/or progesterone receptor positive) subjects in the absence of disease progression or unacceptable toxicity. Contrarily, the clinical trial of Zhao et al. concerning aromatase-inhibitor treatment combined with 500 mg metformin orally in pre-treated postmenopausal patients with hormone receptor-positive metastatic breast cancer returned negative results although with excellent tolerability [109]. However, the study performed by Monami et al., in which 195 patients were included over a period of 9.6 years, proved that metformin intervention for more than 36 months was associated with a significant reduction in the risk of cancer [165]. According to a cohort study, patients with esophageal cancer (in a study group of 285 patients) and patients with rectal cancer (in a study group of 472 patients) receiving a combination of metformin with radiotherapy/chemotherapy demonstrated increased responses to the anti-cancer treatment and improved prognoses [24].
6. Can Metformin Intervention Be Considered as Adjuvant Anti-Cancer Therapy?
6.1. Anti-Cancer Effect of Metformin in Thyroid Cancer
In recent years, the global incidence rates of thyroid cancer (TC) have been steadily rising [15]. Recent reports indicate that metformin may exert anti-tumor activity by inhibiting tumor cell proliferation and inducing apoptosis [62,125,166]. It has been documented that TC diabetic patients treated with metformin are characterized by smaller tumor sizes, higher complete remission rates, and longer progression-free survival compared to diabetic patients not treated with metformin [4]. Ye et al. described the in vitro effects of 10 and 20 mM of metformin on human papillary TC using a human papillary TC cell line (TPC-1). The use of metformin increased the expression of several factors, including the heat shock 70-kDa protein 5 (Hspa5), also known as binding immunoglobulin protein (BIP), C/EBP homologous protein (CHOP), and caspase-12, which activate endoplasmic reticulum (ER) stress conditions, leading to cancer cell apoptosis [55,64]. Using flow cytometry, a significant increase in TPC-1 cell apoptosis after metformin intervention was observed as compared to in the control group. The study performed using in vitro and in vivo models concluded that metformin can promote apoptosis through endoplasmic-reticulum-stress-associated pathways following the increased expression of BiP, DNA damage-inducible transcript 3, and caspase-12 [167]. Han et al. suggested that metformin inhibits TC cell growth, migration, and the epithelial-to-mesenchymal transition by inhibiting the mTOR pathway, where the metformin treatment was given at a concentration of 10 mM concentration [168]. The study performed by Thakur et al. demonstrated that MGPDH regulated human TC cell growth and oxidative phosphorylation (OXPHOS) [20]. Moreover, MGPDH overexpression was associated with an increase in thyroid cell proliferation. Interestingly, downregulation of MGPDH expression and OXPHOS inhibition in TC in vitro was observed after 48 h 5 mM metformin treatment [20]. Using TC cells (FTC133 and BCPAP), Bikas et al. demonstrated that the combination of metformin and other glycolysis inhibitors improved upon current TC treatments. Moreover, due to providing the decrease in cellular ATP, the prolonged activation of AMPK, and the sustained autophagy, adding metformin to already used treatment regimens resulted in a broader cancer-treatment spectrum [55,107].
Radioactive iodine therapy (RAI) is the standard treatment for differentiated thyroid cancer (DTC). The increased oxidative stress, reflected by malondialdehyde (MDA) measurements, further enhanced by RAI in DTC patients may have important implications for future health complications [169,170]. Despite the beneficial therapeutic effect of RAI intervention, exposure to radiation can lead to several oxidative alterations, especially in the metabolism of reproductive system tissue [7,171]. Over the last decade, evidence of the anti-cancer effects of metformin—the most widely prescribed anti-diabetic medicine in the world—shows its usefulness in the clinical management of endocrine malignancies [15,21,61,172,173,174].
6.2. Metformin Intervention in Endometrial Cancer
Endometrial cancer (EC) is the most common gynecological malignancy and is characterized by hypermenorrhea, dysfunctional uterine bleeding, and infertility [175,176]. The current recommendations from the Cancer Genome Atlas (TCGA) define four clinically distinct endometrial cancer types based on their p53 mutational burden, the copy number variations, the exonuclease domain of the DNA polymerase epsilon (POLE) mutations, and the microsatellite instability [177]. Furthermore, EC is usually associated with PCOS, obesity, insulin resistance, and T2DM [156,178]. The impact of insulin and IGF-1 expression have an important role in the development of EC. Furthermore, the aggressiveness of EC has been shown to correspond with elevated levels of circulating insulin and endometrial IGF-1 concentrations. Another potentially important element in the mechanism through which metformin inhibits the development of EC is related to increased GLUT4 activity, which is combined with cell proliferation inhibition and cell cycle arrest and apoptosis induction [178]. Moreover, the data demonstrate that female sex steroids regulate tissue insulin sensitivity and modulate the intracellular glucose pathways [7,179,180]. In this regard, metformin increases the blood levels of sex hormone binding globulin, which leads to a reduction in the circulating estrogen and androgen concentrations [178,181]. It has been reported that 2 mM of metformin improved EC hormonal treatment by causing a regression to histologically normal endometrium, enhancing healing, and reducing any side effects [175,178,182]. Additionally, 500 mg of metformin orally, three times a day, is capable of overcoming progestin resistance in endometrial cancer cells [183]. It has been suggested that metformin’s anti-cancer properties result from its ability to alter glucose metabolism, activating AMPK and inhibiting the PI3K/AKT/mTOR signaling pathway [184,185]. In particular, numerous studies have examined the effectiveness of targeted therapies acting on the PI3K/AKT/mTOR pathway, epidermal growth factor receptor (EGFR), human epidermal growth factor receptor 2 (HER2), and vascular endothelial growth factor (VEGF), which have been shown to be metformin targets [178,186,187]. The use of targeted therapies appears to be key to achieving better responses and survival among women with advanced or recurrent EC [188]. In this regard, the pleiotropic effects of metformin on cellular energy production with intercellular and hormone-based interactions make metformin a potential anti-cancer treatment for EC.
There are 14 clinical trials being conducted to assess the effectiveness of metformin medication in the treatment of endometrial hyperplasia and cancer [189]. Studies of the therapeutic properties of metformin in EC patients have been published, showing that metformin treatment functions as an useful preventive therapy for neoplastic diseases [190,191]. The results from clinical trials suggest that metformin intervention (750–2250 mg/day administered for 24–36 weeks to achieve a complete response) combined with medroxyprogesterone acetate (MPA) improves the regression of cancer cells, providing additional protective effects on fertility in EC patients [192]. Current clinical trials showed that progestin fertility-sparing treatment combined with 500–1500 mg daily of metformin shortened the treatment time, reducing the risk of side effects and endometrial damage during the treatment of EC [178,181,183,189,190,191,193].
7. Materials and Methods
The literature review was performed using the PubMed database and according to the PRISMA and EQUATOR network guidelines [194,195,196,197]. For the purpose of this review, medical papers published in 2004–2021 were analyzed. Systematic review of the current literature about the metformin intervention was performed. The keywords used in the literature search were as follows: metformin, metformin pharmacokinetics, anti-cancer therapy, antioxidant therapy, metformin therapy, potential therapy, clinical trials, thyroid cancer, and endometrial cancer. Studies evaluating the latest reports based on anticancer properties, the impact of oxidative stress, and potential therapeutic targets related to metformin intervention were included. Articles with irrelevant conclusion statements, inappropriate study methods, inadequate reporting, or incomplete reports were excluded from the study (Figure 1). This review has been registered in PROSPERO (CRD42022299568).
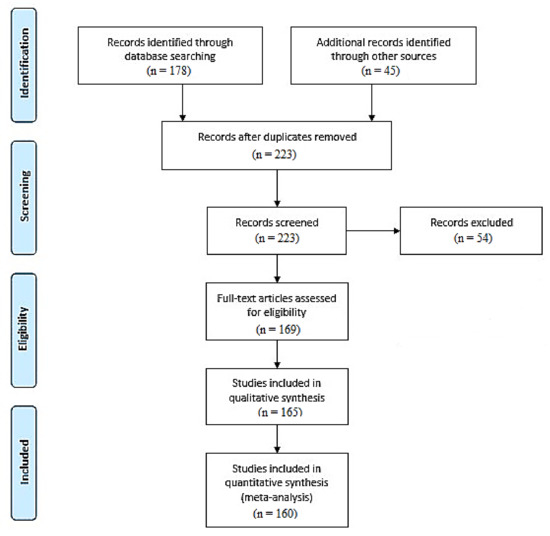
Figure 1.
PRISMA-based flow diagram of the meta-analysis process performed [194,198].
8. Conclusions
Undoubtedly, metformin exerts pleiotropic effects on many metabolic pathways. One of metformin’s most significant potential applications is cancer treatment. Studies using in vitro models focused on metformin’s anti-cancer mechanisms and potential uses produced favorable results. Therefore, based on this evidence, metformin can be used widely in relation to thyroid, endometrial, breast, pancreas, and lung cancers, which are epidemic in modern societies according to the reputably published literature data. However, further randomized clinical trials to assess metformin’s individual metabolic effects and specific molecular mechanisms are warranted. There is a high probability that introduction of metformin to therapeutic protocols could extend the period of cancer non-recurrence and reduce the side effects of chemotherapy and radiotherapy. Thus, the development of novel indications for this therapy is still required.
Author Contributions
Conceptualization, A.B. and A.A.; methodology, A.A.; software, I.S.; validation, A.B., A.J.K. and A.A.; formal analysis, M.Z.-K.; investigation, A.A.; resources, A.A.; data curation, A.B. and I.S.; writing—original draft preparation, A.B. and I.S.; writing—review and editing, A.A.; visualization, M.Z.-K.; supervision, A.A.; funding acquisition, M.Z.-K. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by internal financing of the Medical University of Bialystok (SUB/1/NN/21/001/1210).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sanchez-Rangel, E.; Inzucchi, S.E. Metformin: Clinical use in type 2 diabetes. Diabetologia 2017, 60, 1586–1593. [Google Scholar] [CrossRef] [PubMed]
- Graham, G.G.; Punt, J.; Arora, M.; Day, R.O.; Doogue, M.P.; Duong, J.K.; Furlong, T.J.; Greenfield, J.R.; Greenup, L.C.; Kirkpatrick, C.; et al. Clinical pharmacokinetics of metformin. Clin. Pharmacokinet. 2011, 50, 81–98. [Google Scholar] [CrossRef] [PubMed]
- Foretz, M.; Guigas, B.; Bertrand, L.; Pollak, M.; Viollet, B. Metformin: From mechanisms of action to therapies. Cell Metab. 2014, 20, 953–966. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, S.; Jinnouchi, H.; Hieshima, K.; Kurinami, N.; Jinnouchi, K. Type 2 diabetes remission and substantial body weight reduction achieved with metformin and a sodium-glucose cotransporter 2 inhibitor. Cureus 2020, 12, e7110. [Google Scholar] [CrossRef]
- Soukas, A.A.; Hao, H.; Wu, L. Metformin as anti-aging therapy: Is it for everyone? Trends Endocrinol. Metab. 2019, 30, 745–755. [Google Scholar] [CrossRef]
- Lv, Z.; Guo, Y. Metformin and its benefits for various diseases. Front. Endocrinol. 2020, 11, 191. [Google Scholar] [CrossRef]
- Shpakov, A.O.; Sechenov, I.M. Improvement effect of metformin on female and male reproduction in endocrine pathologies and its mechanisms. Pharmaceuticals 2021, 14, 42. [Google Scholar] [CrossRef]
- Salvatore, T.; Pafundi, P.C.; Galiero, R.; Rinaldi, L.; Caturano, A.; Vetrano, E.; Aprea, C.; Albanese, G.; Di Martino, A.; Ricozzi, C.; et al. Can metformin exert as an active drug on endothelial dysfunction in diabetic subjects? Biomedicines 2021, 9, 3. [Google Scholar] [CrossRef]
- Salvatore, T.; Galiero, R.; Caturano, A.; Vetrano, E.; Rinaldi, L.; Coviello, F.; Di Martino, A.; Albanese, G.; Marfella, R.; Sardu, C.; et al. Effects of metformin in heart failure: From pathophysiological rationale to clinical evidence. Biomolecules 2021, 11, 1834. [Google Scholar] [CrossRef]
- Salvatore, T.; Pafundi, P.C.; Galiero, R.; Gjeloshi, K.; Masini, F.; Acierno, C.; Di Martino, A.; Albanese, G.; Alfano, M.; Rinaldi, L.; et al. Metformin: A potential therapeutic tool for rheumatologists. Pharmaceuticals 2020, 13, 234. [Google Scholar] [CrossRef]
- Apostolova, N.; Iannantuoni, F.; Gruevska, A.; Muntane, J.; Rocha, M.; Victor, V.M. Mechanisms of action of metformin in type 2 diabetes: Effects on mitochondria and leukocyte-endothelium interactions. Redox Biol. 2020, 34, 101517. [Google Scholar] [CrossRef]
- Yuan, T.; Yang, T.; Chen, H.; Fu, D.; Hu, Y.; Wang, J.; Yuan, Q.; Yu, H.; Xu, W.; Xie, X. New insights into oxidative stress and inflammation during diabetes mellitus-accelerated atherosclerosis. Redox Biol. 2019, 20, 247. [Google Scholar] [CrossRef]
- Bai, B.; Chen, H. Metformin: A novel weapon against inflammation. Front. Pharmacol. 2021, 12, 15. [Google Scholar] [CrossRef]
- Drzewoski, J.; Hanefeld, M. The current and potential therapeutic use of metformin—The good old drug. Pharmaceuticals 2021, 14, 122. [Google Scholar] [CrossRef]
- Thakur, S.; Daley, B.; Klubo-Gwiezdzinska, J. The role of the antidiabetic drug metformin in the treatment of endocrine tumors. J. Mol. Endocrinol. 2019, 63, R17. [Google Scholar] [CrossRef]
- Park, Y.M.M.; Bookwalter, D.B.; O’Brien, K.M.; Jackson, C.L.; Weinberg, C.R.; Sandler, D.P. A prospective study of type 2 diabetes, metformin use, and risk of breast cancer. Ann. Oncol. 2021, 32, 351–359. [Google Scholar] [CrossRef]
- Zhang, K.; Bai, P.; Dai, H.; Deng, Z. Metformin and risk of cancer among patients with type 2 diabetes mellitus: A systematic review and meta-analysis. Prim. Care Diabetes 2021, 15, 52–58. [Google Scholar] [CrossRef]
- Suissa, S.; Azoulay, L. Metformin and cancer: Mounting evidence against an association. Diabetes Care 2014, 37, 1786–1788. [Google Scholar] [CrossRef]
- Saraei, P.; Asadi, I.; Kakar, M.A.; Moradi-Kor, N. The beneficial effects of metformin on cancer prevention and therapy: A comprehensive review of recent advances. Cancer Manag. Res. 2019, 11, 3295. [Google Scholar] [CrossRef]
- Thakur, S.; Daley, B.; Gaskins, K.; Vasko, V.V.; Boufraqech, M.; Patel, D.; Sourbier, C.; Reece, J.M.; Cheng, S.-Y.; Kebebew, E.; et al. Translational cancer mechanisms and therapy metformin targets mitochondrial glycerophosphate dehydrogenase to control rate of oxidative phosphorylation and growth of thyroid cancer in vitro and in vivo. Clin. Cancer Res. 2018, 24, 4030. [Google Scholar] [CrossRef]
- Morales, D.R.; Morris, A.D. Metformin in cancer treatment and prevention. Annu. Rev. Med. 2015, 66, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Hawkes, A.; Quinn, M.; Gebski, V.; Armes, J.; Brennan, D.; Janda, M.; Obermair, A. Improving treatment for obese women with early stage cancer of the uterus: Rationale and design of the levonorgestrel intrauterine device ± metformin ± weight loss in endometrial cancer (feMME) trial. Contemp. Clin. Trials 2014, 39, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Graff, S.K.; Mario, F.M.; Ziegelmann, P.; Spritzer, P.M. Effects of orlistat vs. metformin on weight loss-related clinical variables in women with PCOS: Systematic review and meta-analysis. Int. J. Clin. Pract. 2016, 70, 450–461. [Google Scholar] [CrossRef] [PubMed]
- Samsuri, N.A.B.; Leech, M.; Marignol, L. Metformin and improved treatment outcomes in radiation therapy—A review. Cancer Treat. Rev. 2017, 55, 150–162. [Google Scholar] [CrossRef]
- Zisser, H.C. Polycystic ovary syndrome and pregnancy: Is metformin the magic bullet? Diabetes Spectr. 2007, 20, 85–89. [Google Scholar] [CrossRef]
- Seifarth, C.; Schehler, B.; Schneider, H.J. Effectiveness of metformin on weight loss in non-diabetic individuals with obesity. Exp. Clin. Endocrinol. Diabetes 2013, 121, 27–31. [Google Scholar] [CrossRef]
- Jeong, Y.-S.; Jusko, W. Meta-assessment of metformin absorption and disposition pharmacokinetics in nine species. Pharmaceuticals 2021, 14, 545. [Google Scholar] [CrossRef]
- Zhou, M.; Xia, L.; Wang, J. Metformin transport by a newly cloned proton-stimulated organic cation transporter (plasma membrane monoamine transporter) expressed in human intestine. Drug Metab. Dispos. 2007, 35, 1956–1962. [Google Scholar] [CrossRef]
- Dawed, A.Y.; Zhou, K.; van Leeuwen, N.; Mahajan, A.; Robertson, N.; Koivula, R.; Elders, P.J.; Rauh, S.P.; Jones, A.G.; Holl, R.W.; et al. Variation in the plasma membrane monoamine transporter (PMAT) (encoded by SLC29A4) and organic cation transporter 1 (OCT1) (encoded by SLC22A1) and gastrointestinal intolerance to metformin in type 2 diabetes: An IMI DIRECT study. Diabetes Care 2019, 42, 1027–1033. [Google Scholar] [CrossRef]
- Meyer, M.J.; Tuerkova, A.; Römer, S.; Wenzel, C.; Seitz, T.; Gaedcke, J.; Oswald, S.; Brockmöller, J.; Zdrazil, B.; Tzvetkov, M.V. Differences in metformin and thiamine uptake between human and mouse organic cation transporter 1: Structural determinants and potential consequences for intrahepatic concentrations. Drug Metab. Dispos. 2020, 48, 1380–1392. [Google Scholar] [CrossRef]
- Zu Schwabedissen, H.E.M.; Verstuyft, C.; Kroemer, H.K.; Becquemont, L.; Kim, R.B. Human multidrug and toxin extrusion 1 (MATE1/SLC47A1) transporter: Functional characterization, interaction with OCT2 (SLC22A2), and single nucleotide polymorphisms. Am. J. Physiol. Physiol. 2010, 298, F997–F1005. [Google Scholar] [CrossRef]
- Arruda, A.C.; Perilhão, M.S.; Santos, W.A.; Gregnani, M.F.; Budu, A.; Neto, J.C.R.; Estrela, G.R.; Araujo, R.C. PPARα-dependent modulation by metformin of the expression of OCT-2 and MATE-1 in the kidney of mice. Molecules 2020, 25, 392. [Google Scholar] [CrossRef]
- Stocker, S.; Morrissey, K.M.; Yee, S.W.; Castro, R.A.; Xu, L.; Dahlin, A.; Ramirez, A.H.; Roden, D.M.; Wilke, R.A.; McCarty, C.; et al. The effect of novel promoter variants in MATE1 and MATE2 on the pharmacokinetics and pharmacodynamics of metformin. Clin. Pharmacol. Ther. 2013, 93, 186. [Google Scholar] [CrossRef]
- Yonezawa, A.; Inui, K.-I. Importance of the multidrug and toxin extrusion MATE/SLC47A family to pharmacokinetics, pharmacodynamics/toxicodynamics and pharmacogenomics. Br. J. Pharmacol. 2011, 164, 1817. [Google Scholar] [CrossRef]
- Van Stee, M.F.; De Graaf, A.A.; Groen, A.K. Actions of metformin and statins on lipid and glucose metabolism and possible benefit of combination therapy. Cardiovasc. Diabetol. 2018, 17, 94. [Google Scholar] [CrossRef]
- Vilela, D.D.; Peixoto, L.G.; Teixeira, R.R.; Baptista, N.B.; Caixeta, D.C.; De Souza, A.V.; Machado, H.L.; Pereira, M.N.; Sabino-Silva, R.; Espindola, F.S. The role of metformin in controlling oxidative stress in muscle of diabetic rats. Oxid. Med. Cell. Longev. 2016, 2016, 6978625. [Google Scholar] [CrossRef]
- Faria, J.; Negalha, G.; Azevedo, A.; Martel, F. Metformin and breast cancer: Molecular targets. J. Mammary Gland Biol. Neoplasia 2019, 24, 111–123. [Google Scholar] [CrossRef]
- E LaMoia, T.; I Shulman, G. Cellular and molecular mechanisms of metformin action. Endocr. Rev. 2021, 42, 77. [Google Scholar] [CrossRef]
- Allende-Vega, N.; Brualla, J.M.; Falvo, P.; Alexia, C.; Constantinides, M.; de Maudave, A.F.; Coenon, L.; Gitenay, D.; Mitola, G.; Massa, P.; et al. Metformin sensitizes leukemic cells to cytotoxic lymphocytes by increasing expression of intercellular adhesion molecule-1 (ICAM-1). Sci. Rep. 2022, 12, 1341. [Google Scholar] [CrossRef]
- Marini, C.; Cossu, V.; Bauckneht, M.; Lanfranchi, F.; Raffa, S.; Orengo, A.M.; Ravera, S.; Bruno, S.; Sambuceti, G. Metformin and cancer glucose metabolism: At the bench or at the bedside? Biomolecules 2021, 11, 1231. [Google Scholar] [CrossRef]
- Vial, G.; Detaille, D.; Guigas, B. Role of mitochondria in the mechanism(s) of action of metformin. Front. Endocrinol. 2019, 10, 294. [Google Scholar] [CrossRef]
- Kefas, B.A.; Cai, Y.; Kerckhofs, K.; Ling, Z.; Martens, G.; Heimberg, H.; Pipeleers, D.; Van de Casteele, M. Metformin-induced stimulation of AMP-activated protein kinase in β-cells impairs their glucose responsiveness and can lead to apoptosis. Biochem. Pharmacol. 2004, 68, 409–416. [Google Scholar] [CrossRef]
- Ouyang, J.; Parakhia, R.A.; Ochs, R.S. Metformin activates AMP kinase through inhibition of AMP deaminase. J. Biol. Chem. 2011, 286, 1. [Google Scholar] [CrossRef]
- Ruderman, N.B.; Carling, D.; Prentki, M.; Cacicedo, J.M. AMPK, insulin resistance, and the metabolic syndrome. J. Clin. Investig. 2013, 123, 2764. [Google Scholar] [CrossRef]
- Hunter, R.W.; Hughey, C.C.; Lantier, L.; Sundelin, E.I.; Peggie, M.; Zeqiraj, E.; Sicheri, F.; Jessen, N.; Wasserman, D.H.; Sakamoto, K. Metformin reduces liver glucose production by inhibition of fructose-1-6-bisphosphatase. Nat. Med. 2018, 24, 1395–1406. [Google Scholar] [CrossRef]
- Moonira, T.; Chachra, S.S.; Ford, B.E.; Marin, S.; Alshawi, A.; Adam-Primus, N.S.; Arden, C.; Al-Oanzi, Z.H.; Foretz, M.; Viollet, B.; et al. Metformin lowers glucose 6-phosphate in hepatocytes by activation of glycolysis downstream of glucose phosphorylation. J. Biol. Chem. 2020, 295, 3330–3346. [Google Scholar] [CrossRef]
- Kowalik, M.A.; Columbano, A.; Perra, A. Emerging role of the pentose phosphate pathway in hepatocellular carcinoma. Front. Oncol. 2017, 7, 87. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, J.-L.; Ji, M.; Yuan, Z.-F.; Peng, Z.; Zhang, Y.; Wen, J.-G.; Shi, H.-R. Regulation of insulin-like growth factor signaling by metformin in endometrial cancer cells. Oncol. Lett. 2014, 8, 1993–1999. [Google Scholar] [CrossRef]
- Huhtala, M.S.; Tertti, K.; Juhila, J.; Sorsa, T.; Rönnemaa, T. Metformin and insulin treatment of gestational diabetes: Effects on inflammatory markers and IGF-binding protein-1—Secondary analysis of a randomized controlled trial. BMC Pregnancy Childbirth 2020, 20, 401. [Google Scholar] [CrossRef]
- Vargas, E.; Podder, V.; Sepulveda, M.A.C. Physiology, glucose transporter type 4. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Lee, J.O.; Lee, S.K.; Kim, J.H.; Kim, N.; You, G.Y.; Moon, J.W.; Kim, S.J.; Park, S.H.; Kim, H.S. Metformin regulates glucose transporter 4 (GLUT4) translocation through AMP-activated protein kinase (AMPK)-mediated Cbl/CAP signaling in 3T3-L1 preadipocyte cells. J. Biol. Chem. 2012, 287, 44121. [Google Scholar] [CrossRef]
- Rice, S.; Pellatt, L.J.; Bryan, S.J.; Whitehead, S.A.; Mason, H.D. Action of metformin on the insulin-signaling pathway and on glucose transport in human granulosa cells. J. Clin. Endocrinol. Metab. 2011, 96, E427–E435. [Google Scholar] [CrossRef] [PubMed]
- Alhaider, A.A.; Korashy, H.M.; Sayed-Ahmed, M.M.; Mobark, M.; Kfoury, H.; Mansour, M.A. Metformin attenuates streptozotocin-induced diabetic nephropathy in rats through modulation of oxidative stress genes expression. Chem. Biol. Interact. 2011, 192, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Jia, D.; Wu, X.; Shi, K.; Ren, C.; Dou, Y.; Guo, M.; Wang, J.; Ma, M.; Wu, Z.; et al. A novel metformin derivative showed improvement of lipid metabolism in obese rats with type 2 diabetes. Clin. Exp. Pharmacol. Physiol. 2020, 47, 1382–1392. [Google Scholar] [CrossRef] [PubMed]
- Bikas, A.; Jensen, K.; Patel, A.; Costello, J.; McDaniel, D.; Klubo-Gwiezdzinska, J.; Larin, O.; Hoperia, V.; Burman, K.D.; Boyle, L.; et al. Glucose-deprivation increases thyroid cancer cells sensitivity to metformin. Endocr.-Relat. Cancer 2015, 22, 919–932. [Google Scholar] [CrossRef]
- Gonnissen, A.; Isebaert, S.; McKee, C.M.; Muschel, R.J.; Haustermans, K. The effect of metformin and GANT61 combinations on the radiosensitivity of prostate cancer cells. Int. J. Mol. Sci. 2017, 18, 399. [Google Scholar] [CrossRef]
- Wu, N.; Gu, C.; Gu, H.; Hu, H.; Han, Y.; Li, Q. Metformin induces apoptosis of lung cancer cells through activating JNK/p38 MAPK pathway and GADD153. Neoplasma 2011, 58, 482–490. [Google Scholar] [CrossRef]
- Klein, J.; Westphal, S.; Kraus, D.; Meier, B.; Perwitz, N.; Ott, V.; Fasshauer, M.; Klein, H.H. Metformin inhibits leptin secretion via a mitogen-activated protein kinase signalling pathway in brown adipocytes. J. Endocrinol. 2004, 183, 299–307. [Google Scholar] [CrossRef]
- He, X.; Gao, F.; Hou, J.; Li, T.; Tan, J.; Wang, C.; Liu, X.; Wang, M.; Liu, H.; Chen, Y.; et al. Metformin inhibits MAPK signaling and rescues pancreatic aquaporin 7 expression to induce insulin secretion in type 2 diabetes mellitus. J. Biol. Chem. 2021, 297, 101002. [Google Scholar] [CrossRef]
- Tsou, Y.-A.; Chang, W.-C.; Lin, C.-D.; Chang, R.-L.; Tsai, M.-H.; Shih, L.-C.; Staniczek, T.; Wu, T.-F.; Hsu, H.-Y.; Chang, W.-D.; et al. Metformin increases survival in hypopharyngeal cancer patients with diabetes mellitus: Retrospective cohort study and cell-based analysis. Pharmaceuticals 2021, 14, 191. [Google Scholar] [CrossRef]
- Tseng, S.-C.; Huang, Y.-C.; Chen, H.-J.; Chiu, H.-C.; Huang, Y.-J.; Wo, T.-Y.; Weng, S.-H.; Lin, Y.-W. Metformin-mediated downregulation of p38 mitogen-activated protein kinase-dependent excision repair cross-complementing 1 decreases DNA repair capacity and sensitizes human lung cancer cells to paclitaxel. Biochem. Pharmacol. 2013, 85, 583–594. [Google Scholar] [CrossRef]
- Deng, X.-S.; Wang, S.; Deng, A.; Liu, B.; Edgerton, S.M.; Lind, S.E.; Wahdan-Alaswad, R.; Thor, A.D. Metformin targets Stat3 to inhibit cell growth and induce apoptosis in triple-negative breast cancers. Cell Cycle 2012, 11, 367–376. [Google Scholar] [CrossRef]
- Della Corte, C.M.; Ciaramella, V.; Di Mauro, C.; Castellone, M.; Papaccio, F.; Fasano, M.; Sasso, F.C.; Martinelli, E.; Troiani, T.; De Vita, F.; et al. Metformin increases antitumor activity of MEK inhibitors through GLI1 downregulation in LKB1 positive human NSCLC cancer cells. Oncotarget 2016, 7, 4265. [Google Scholar] [CrossRef]
- Ko, J.-C.; Huang, Y.-C.; Chen, H.-J.; Tseng, S.-C.; Chiu, H.-C.; Wo, T.-Y.; Huang, Y.-J.; Weng, S.-H.; Chiou, R.Y.Y.; Lin, Y.-W. Metformin induces cytotoxicity by down-regulating thymidine phosphorylase and excision repair cross-complementation 1 expression in non-small cell lung cancer cells. Basic Clin. Pharmacol. Toxicol. 2013, 113, 56–65. [Google Scholar] [CrossRef]
- Chen, X.; Ma, J.; Yao, Y.; Zhu, J.; Zhou, Z.; Zhao, R.; Dong, X.; Gao, W.; Zhang, S.; Huang, S.; et al. Metformin prevents BAFF activation of Erk1/2 from B-cell proliferation and survival by impeding mTOR-PTEN/Akt signaling pathway. Int. Immunopharmacol. 2021, 96, 107771. [Google Scholar] [CrossRef]
- Martin, M.J.; Hayward, R.; Viros, A.; Marais, R. Metformin accelerates the growth of BRAFV600E-driven melanoma by upregulating VEGF-A. Cancer Discov. 2012, 2, 344–355. [Google Scholar] [CrossRef]
- Ni, H.-Z.; Liu, Z.; Sun, L.-L.; Zhou, M.; Liu, C.; Li, W.-D.; Li, X.-Q. Metformin inhibits angiogenesis of endothelial progenitor cells via miR-221-mediated p27 expression and autophagy. Future Med. Chem. 2019, 11, 2263–2272. [Google Scholar] [CrossRef]
- Mohammed, I.; Hollenberg, M.D.; Ding, H.; Triggle, C.R. A critical review of the evidence that metformin is a putative anti-aging drug that enhances healthspan and extends lifespan. Front. Endocrinol. 2021, 12, 933. [Google Scholar] [CrossRef]
- Luengo, A.; Sullivan, L.B.; Heiden, M.G.V. Understanding the complex-I-ty of metformin action: Limiting mitochondrial respiration to improve cancer therapy. BMC Biol. 2014, 12, 82. [Google Scholar] [CrossRef]
- Brunmair, B.; Staniek, K.; Gras, F.; Scharf, N.; Althaym, A.; Clara, R.; Roden, M.; Gnaiger, E.; Nohl, H.; Waldhäusl, W.; et al. Thiazolidinediones, like metformin, inhibit respiratory complex I. Diabetes 2004, 53, 1052–1059. [Google Scholar] [CrossRef]
- Carvalho, C.; Correia, S.; Santos, M.S.; Seiça, R.; Oliveira, C.R.; Moreira, P.I. Metformin promotes isolated rat liver mitochondria impairment. Mol. Cell. Biochem. 2008, 308, 75–83. [Google Scholar] [CrossRef]
- Victor, V.M.; Rovira-Llopis, S.; Bañuls, C.; Diaz-Morales, N.; Castelló, R.; Falcón, R.; Gómez, M.; Rocha, M.; Hernández-Mijares, A. Effects of metformin on mitochondrial function of leukocytes from polycystic ovary syndrome patients with insulin resistance. Eur. J. Endocrinol. 2015, 173, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Larsen, S.; Rabøl, R.; Hansen, C.N.; Madsbad, S.; Helge, J.; Dela, F. Metformin-treated patients with type 2 diabetes have normal mitochondrial complex I respiration. Diabetologia 2012, 55, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Viollet, B.; Guigas, B.; Garcia, N.S.; Leclerc, J.; Foretz, M.; Andreelli, F. Cellular and molecular mechanisms of metformin: An overview. Clin. Sci. 2012, 122, 253. [Google Scholar] [CrossRef] [PubMed]
- Panfoli, I.; Puddu, A.; Bertola, N.; Ravera, S.; Maggi, D. The hormetic effect of metformin: “Less is more”? Int. J. Mol. Sci. 2021, 22, 6297. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, E.J.; Agathokleous, E.; Kapoor, R.; Dhawan, G.; Kozumbo, W.J.; Calabrese, V. Metformin-enhances resilience via hormesis. Ageing Res. Rev. 2021, 71, 101418. [Google Scholar] [CrossRef] [PubMed]
- Izzo, A.; Mollo, N.; Nitti, M.; Paladino, S.; Calì, G.; Genesio, R.; Bonfiglio, F.; Cicatiello, R.; Barbato, M.; Sarnataro, V.; et al. Mitochondrial dysfunction in Down syndrome: Molecular mechanisms and therapeutic targets. Mol. Med. 2018, 24, 2. [Google Scholar] [CrossRef]
- Perrone, S.; Longini, M.; Bellieni, C.; Centini, G.; Kenanidis, A.; De Marco, L.; Petraglia, F.; Buonocore, G. Early oxidative stress in amniotic fluid of pregnancies with Down syndrome. Clin. Biochem. 2007, 40, 177–180. [Google Scholar] [CrossRef]
- Kurabayashi, N.; Nguyen, M.D.; Sanada, K. Triple play of DYRK1A kinase in cortical progenitor cells of Trisomy 21. Neurosci. Res. 2019, 138, 19–25. [Google Scholar] [CrossRef]
- Barone, E.; Head, E.; Butterfield, D.A.; Perluigi, M. HNE-modified proteins in Down syndrome: Involvement in development of Alzheimer disease neuropathology. Free Radic. Biol. Med. 2017, 111, 262–269. [Google Scholar] [CrossRef]
- Zemgulyte, G.; Tanaka, S.; Hide, I.; Sakai, N.; Pampuscenko, K.; Borutaite, V.; Rastenyte, D. Evaluation of the effectiveness of post-stroke metformin treatment using permanent middle cerebral artery occlusion in rats. Pharmaceuticals 2021, 14, 312. [Google Scholar] [CrossRef]
- Buldak, L.; Łabuzek, K.; Bułdak, R.J.; Machnik, G.; Bołdys, A.; Basiak, M.; Bogusław, O. Metformin reduces the expression of NADPH oxidase and increases the expression of antioxidative enzymes in human monocytes/macrophages cultured in vitro. Exp. Ther. Med. 2016, 11, 1095–1103. [Google Scholar] [CrossRef][Green Version]
- Obi, B.; Okoye, T.C.; Okpashi, V.E.; Igwe, C.N.; Alumanah, E.O. Comparative study of the antioxidant effects of metformin, glibenclamide, and repaglinide in alloxan-induced diabetic rats. J. Diabetes Res. 2016, 2016, 9060649. [Google Scholar] [CrossRef]
- Yahyapour, R.; Amini, P.; Saffar, H.; Motevaseli, E.; Farhood, B.; Pooladvand, V.; Shabeeb, D.; Musa, A.E.; Najafi, M. Protective effect of metformin, resveratrol and alpha-lipoic acid on radiation- induced pneumonitis and fibrosis: A histopathological study. Curr. Drug Res. Rev. 2019, 11, 111–117. [Google Scholar] [CrossRef]
- Ren, H.; Shao, Y.; Wu, C.; Ma, X.; Lv, C.; Wang, Q. Metformin alleviates oxidative stress and enhances autophagy in diabetic kidney disease via AMPK/SIRT1-FoxO1 pathway. Mol. Cell. Endocrinol. 2020, 500, 110628. [Google Scholar] [CrossRef]
- Javadipour, M.; Rezaei, M.; Keshtzar, E.; Khodayar, M.J. Metformin in contrast to berberine reversed arsenic-induced oxidative stress in mitochondria from rat pancreas probably via Sirt3-dependent pathway. J. Biochem. Mol. Toxicol. 2019, 33, e22368. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, J.; Lin, Y.; Lei, Q.; Guan, K.-L.; Zhao, S.; Xiong, Y. Tumour suppressor SIRT3 deacetylates and activates manganese superoxide dismutase to scavenge ROS. EMBO Rep. 2011, 12, 534–541. [Google Scholar] [CrossRef]
- Xue, F.; Huang, J.-W.; Ding, P.-Y.; Zang, H.-G.; Kou, Z.-J.; Li, T.; Fan, J.; Peng, Z.-W.; Yan, W.-J. Nrf2/antioxidant defense pathway is involved in the neuroprotective effects of Sirt1 against focal cerebral ischemia in rats after hyperbaric oxygen preconditioning. Behav. Brain Res. 2016, 309, 1–8. [Google Scholar] [CrossRef]
- Singh, C.K.; Chhabra, G.; Ndiaye, M.A.; Garcia-Peterson, L.M.; Mack, N.J.; Ahmad, N. The role of sirtuins in antioxidant and redox signaling. Antioxid. Redox Signal. 2018, 28, 643. [Google Scholar] [CrossRef]
- Cuyàs, E.; Verdura, S.; Llorach-Pares, L.; Fernández-Arroyo, S.; Joven, J.; Martin-Castillo, B.; Barrera, J.B.; Brunet, J.; Nonell-Canals, A.; Sanchez-Martinez, M.; et al. Metformin is a direct SIRT1-activating compound: Computational modeling and experimental validation. Front. Endocrinol. 2018, 9, 657. [Google Scholar] [CrossRef]
- Warkad, M.S.; Kim, C.-H.; Kang, B.-G.; Park, S.-H.; Jung, J.-S.; Feng, J.-H.; Inci, G.; Kim, S.-C.; Suh, H.-W.; Lim, S.S.; et al. Metformin-induced ROS upregulation as amplified by apigenin causes profound anticancer activity while sparing normal cells. Sci. Rep. 2021, 111, 14002. [Google Scholar] [CrossRef]
- Picone, P.; Nuzzo, D.; Caruana, L.; Messina, E.; Barera, A.; Vasto, S.; Di Carlo, M. Metformin increases APP expression and processing via oxidative stress, mitochondrial dysfunction and NF-κB activation: Use of insulin to attenuate metformin’s effect. Biochim. Biophys. Acta Mol. Cell Res. 2015, 1853, 1046–1059. [Google Scholar] [CrossRef]
- Karise, I.; Ornellas, F.; Barbosa-Da-Silva, S.; Matsuura, C.; del Sol, M.; Aguila, M.B.; Mandarim-De-Lacerda, C.A. Liver and metformin: Lessons of a fructose diet in mice. Biochim. Open 2017, 4, 19–30. [Google Scholar] [CrossRef]
- Li, J.; Gui, Y.; Ren, J.; Liu, X.; Feng, Y.; Zeng, Z.; He, W.; Yang, J.; Dai, C. Metformin protects against cisplatin-induced tubular cell apoptosis and acute kidney injury via AMPKα-regulated autophagy induction. Sci. Rep. 2016, 6, 23975. [Google Scholar] [CrossRef]
- Yavuz, D.G.; Yüksel, M.; Deyneli, O.; Ozen, Y.; Aydin, H.; Akalin, S.; Aydın, H.; Akalın, S. Association of serum paraoxonase activity with insulin sensitivity and oxidative stress in hyperthyroid and TSH-suppressed nodular goitre patients. Clin. Endocrinol. 2004, 61, 515–521. [Google Scholar] [CrossRef]
- Brites, F.; Martin, M.; Guillas, I.; Kontush, A. Antioxidative activity of high-density lipoprotein (HDL): Mechanistic insights into potential clinical benefit. BBA Clin. 2017, 8, 66. [Google Scholar] [CrossRef]
- Meneses, M.J.; Silvestre, R.; Sousa-Lima, I.; Macedo, M.P.; Lima, S. Paraoxonase-1 as a regulator of glucose and lipid homeostasis: Impact on the onset and progression of metabolic disorders. Int. J. Mol. Sci. 2019, 20, 4049. [Google Scholar] [CrossRef]
- Jang, E.K.; Kim, W.G.; Kwon, H.; Choi, Y.M.; Jeon, M.J.; Kim, T.Y.; Shong, Y.K.; Kim, E.Y. Metformin is associated with a favorable outcome in diabetic patients with cervical lymph node metastasis of differentiated thyroid cancer. Eur. Thyroid J. 2015, 4, 181. [Google Scholar] [CrossRef]
- Xu, J.; Liu, L.; Xu, L.; Xing, Y.; Ye, S. Metformin alleviates renal injury in diabetic rats by inducing Sirt1/FoxO1 autophagic signal axis. Clin. Exp. Pharmacol. Physiol. 2020, 47, 599–608. [Google Scholar] [CrossRef]
- Miller, R.C.; Murley, J.S.; Grdina, D.J. Metformin exhibits radiation countermeasures efficacy when used alone or in combination with sulfhydryl containing drugs. Radiat. Res. 2014, 181, 464. [Google Scholar] [CrossRef]
- Brown, S.L.; Kolozsvary, A.; Isrow, D.M.; Al Feghali, K.; Lapanowski, K.; Jenrow, K.A.; Kim, J.H. A novel mechanism of high dose radiation sensitization by metformin. Front. Oncol. 2019, 9, 247. [Google Scholar] [CrossRef]
- Skinner, H.; Hu, C.; Tsakiridis, T.; Santana-Davila, R.; Lu, B.; Erasmus, J.J.; Doemer, A.J.; Videtic, G.M.M.; Coster, J.; Yang, A.X.; et al. Addition of metformin to concurrent chemoradiation in patients with locally advanced non–small cell lung cancer: The NRG-LU001 phase 2 randomized clinical trial. JAMA Oncol. 2021, 7, 1324–1332. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Li, Y.; Guo, Z.; Zeng, Y.; Zhang, W.; Wang, H. Metformin: Current clinical applications in nondiabetic patients with cancer. Aging 2020, 12, 3993. [Google Scholar] [CrossRef] [PubMed]
- Aroda, V.R.; for the Diabetes Prevention Program Research Group; Knowler, W.C.; Crandall, J.P.; Perreault, L.; Edelstein, S.L.; Jeffries, S.L.; Molitch, M.E.; Pi-Sunyer, X.; Darwin, C.; et al. Metformin for diabetes prevention: Insights gained from the Diabetes Prevention Program/Diabetes Prevention Program Outcomes Study. Diabetologia 2017, 60, 1601–1611. [Google Scholar] [CrossRef] [PubMed]
- Mallik, R.; Chowdhury, T.A. Metformin in cancer. Diabetes Res. Clin. Pract. 2018, 143, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.S.; Sun, H.J.; Whang, Y.M.; Park, Y.J.; Park, D.J.; Cho, S.W. Metformin reduces thyroid cancer tumor growth in the metastatic niche of bone by inhibiting osteoblastic RANKL productions. Thyroid 2021, 31, 760–771. [Google Scholar] [CrossRef] [PubMed]
- Bikas, A.; Vachhani, S.; Jensen, K.; Vasko, V.; Burman, K.D. Targeted therapies in thyroid cancer: An extensive review of the literature. Expert Rev. Clin. Pharmacol. 2016, 9, 1299–1313. [Google Scholar] [CrossRef]
- Andrzejewski, S.; Siegel, P.M.; St-Pierre, J. Metabolic profiles associated with metformin efficacy in cancer. Front. Endocrinol. 2018, 9, 372. [Google Scholar] [CrossRef]
- Zhao, Y.; Gong, C.; Wang, Z.; Zhang, J.; Wang, L.; Zhang, S.; Cao, J.; Tao, Z.; Li, T.; Wang, B.; et al. A randomized phase II study of aromatase inhibitors plus metformin in pre-treated postmenopausal patients with hormone receptor positive metastatic breast cancer. Oncotarget 2017, 8, 84224. [Google Scholar] [CrossRef]
- Guertin, D.A.; Sabatini, D.M. Defining the role of mTOR in cancer. Cancer Cell 2007, 12, 9–22. [Google Scholar] [CrossRef]
- Mahmoud, A.R.; Ali, F.E.; Abd-Elhamid, T.; Hassanein, E. Coenzyme Q10 protects hepatocytes from ischemia reperfusion-induced apoptosis and oxidative stress via regulation of Bax/Bcl-2/PUMA and Nrf-2/FOXO-3/Sirt-1 signaling pathways. Tissue Cell 2019, 60, 1–13. [Google Scholar] [CrossRef]
- Yap, T.A.; Garrett, M.D.; Walton, M.I.; Raynaud, F.; De Bono, J.S.; Workman, P. Targeting the PI3K–AKT–mTOR pathway: Progress, pitfalls, and promises. Curr. Opin. Pharmacol. 2008, 8, 393–412. [Google Scholar] [CrossRef]
- Pecinová, A.; Brázdová, A.; Drahota, Z.; Houštěk, J.; Mráček, T. Mitochondrial targets of metformin—Are they physiologically relevant? BioFactors 2019, 45, 703–711. [Google Scholar] [CrossRef]
- Sarkar, S.; Horn, G.; Moulton, K.; Oza, A.; Byler, S.; Kokolus, S.; Longacre, M. Cancer development, progression, and therapy: An epigenetic overview. Int. J. Mol. Sci. 2013, 14, 21087. [Google Scholar] [CrossRef]
- Liberti, M.V.; Locasale, J.W. The Warburg effect: How does it benefit cancer cells? Trends Biochem. Sci. 2016, 41, 211. [Google Scholar] [CrossRef]
- Yi, Y.; Zhang, W.; Yi, J.; Xiao, Z.-X. Role of p53 family proteins in metformin anti-cancer activities. J. Cancer 2019, 10, 2434. [Google Scholar] [CrossRef]
- Schwartzenberg-Bar-Yoseph, F.; Armoni, M.; Karnieli, E. The tumor suppressor p53 down-regulates glucose transporters GLUT1 and GLUT4 gene expression. Cancer Res. 2004, 64, 2627–2633. [Google Scholar] [CrossRef]
- Bensaad, K.; Tsuruta, A.; Selak, M.A.; Vidal, M.N.C.; Nakano, K.; Bartrons, R.; Gottlieb, E.; Vousden, K.H. TIGAR, a p53-inducible regulator of glycolysis and apoptosis. Cell 2006, 126, 107–120. [Google Scholar] [CrossRef]
- Nakamura, H.; Bono, H.; Hiyama, K.; Kawamoto, T.; Kato, Y.; Nakanishi, T.; Nishiyama, M.; Hiyama, E.; Hirohashi, N.; Sueoka, E.; et al. Differentiated embryo chondrocyte plays a crucial role in DNA damage response via transcriptional regulation under hypoxic conditions. PLoS ONE 2018, 13, e0192136. [Google Scholar] [CrossRef]
- Ren, Y.; Luo, H. Metformin: The next angiogenesis panacea? SAGE Open Med. 2021, 9, 20503121211001641. [Google Scholar] [CrossRef]
- Wang, J.-C.; Li, G.; Wang, Y.; Tang, S.; Sun, X.; Feng, X.; Li, Y.; Bao, G.; Li, P.; Mao, X.; et al. Suppression of tumor angiogenesis by metformin treatment via a mechanism linked to targeting of HER2/HIF-1α/VEGF secretion axis. Oncotarget 2015, 6, 44579–44592. [Google Scholar] [CrossRef]
- Bravard, A.; Gérard, C.; Defois, C.; Benoit, B.; Makki, K.; Meugnier, E.; Rainteau, D.; Rieusset, J.; Godet, M.; Vidal, H. Metformin treatment for 8 days impacts multiple intestinal parameters in high-fat high-sucrose fed mice. Sci. Rep. 2021, 111, 16684. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.-J.; Xu, J.-M.; Xu, W.-L.; Gu, D.-H.; Li, P.-W. Diagnostic value of interleukin-8 in colorectal cancer: A case-control study and meta-analysis. World J. Gastroenterol. 2014, 20, 16334. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.M.; Webster, S.J.; Flower, D.; Woll, P.J. Interleukin-8/CXCL8 is a growth factor for human lung cancer cells. Br. J. Cancer 2004, 91, 1970–1976. [Google Scholar] [CrossRef] [PubMed]
- Marini, C.; Salani, B.; Massollo, M.; Amaro, A.A.; Esposito, A.I.; Orengo, A.M.; Capitanio, S.; Emionite, L.; Riondato, M.; Bottoni, G.; et al. Direct inhibition of hexokinase activity by metformin at least partially impairs glucose metabolism and tumor growth in experimental breast cancer. Cell Cycle 2013, 12, 3490–3499. [Google Scholar] [CrossRef]
- Bonanni, B.; Puntoni, M.; Cazzaniga, M.; Pruneri, G.; Serrano, D.; Gonzaga, A.G.; Gennari, A.; Trabacca, M.S.; Galimberti, V.; Veronesi, P.; et al. Dual effect of metformin on breast cancer proliferation in a randomized presurgical trial. J. Clin. Oncol. 2012, 30, 2593–2600. [Google Scholar] [CrossRef]
- Stern, J.H.; Rutkowski, J.M.; Scherer, P.E. Adiponectin, leptin, and fatty acids in the maintenance of metabolic homeostasis through adipose tissue crosstalk. Cell Metab. 2016, 23, 770. [Google Scholar] [CrossRef]
- He, X.; Wu, D.; Hu, C.; Xu, T.; Liu, Y.; Liu, C.; Xu, B.; Tang, W. Role of metformin in the treatment of patients with thyroid nodules and insulin resistance: A systematic review and meta-analysis. Thyroid 2019, 29, 359–367. [Google Scholar] [CrossRef]
- Hassan, M.M.; Phan, A.; Li, D.; Dagohoy, C.G.; Leary, C.; Yao, J.C. Risk factors associated with neuroendocrine tumors: A U.S.-based case-control study. Int. J. Cancer 2008, 123, 867–873. [Google Scholar] [CrossRef]
- Ugwueze, C.V.; Ogamba, O.J.; Young, E.E.; Onyenekwe, B.M.; Ezeokpo, B.C. Metformin: A possible option in cancer chemotherapy. Anal. Cell. Pathol. 2020, 2020, 7180923. [Google Scholar] [CrossRef]
- Yousef, M.; Tsiani, E. Metformin in lung cancer: Review of in vitro and in vivo animal studies. Cancers 2017, 9, 45. [Google Scholar] [CrossRef]
- Tsakiridis, T.; Pond, G.R.; Wright, J.; Ellis, P.M.; Ahmed, N.; Abdulkarim, B.; Roa, W.; Robinson, A.; Swaminath, A.; Okawara, G.; et al. Metformin in combination with chemoradiotherapy in locally advanced non–small cell lung cancer. JAMA Oncol. 2021, 7, 1333–1341. [Google Scholar] [CrossRef]
- Chun, S.G.; Liao, Z.; Jeter, M.D.; Chang, J.Y.; Lin, S.H.; Komaki, R.U.; Guerrero, T.M.; Mayo, R.C.; Korah, B.M.; Koshy, S.M.; et al. Metabolic responses to metformin in inoperable early-stage non–small cell lung cancer treated with stereotactic radiotherapy. Am. J. Clin. Oncol. 2020, 43, 231–235. [Google Scholar] [CrossRef]
- Eze, C.; Belka, C.; Manapov, F. Forging a path for metformin use in inoperable locally advanced non–small cell lung cancer. JAMA Oncol. 2021, 7, 1341–1342. [Google Scholar] [CrossRef]
- Mayo-Wilson, E.; on behalf of the National Clinical Trials Registration and Results Reporting Taskforce Survey Subcommittee; Heyward, J.; Keyes, A.; Reynolds, J.; White, S.; Atri, N.; Alexander, G.C.; Omar, A.; Ford, D.E. Clinical trial registration and reporting: A survey of academic organizations in the United States. BMC Med. 2018, 16, 60. [Google Scholar] [CrossRef]
- Marín-Peñalver, J.J.; Martín-Timón, I.; Sevillano-Collantes, C.; Del Cañizo-Gómez, F.J. Update on the treatment of type 2 diabetes mellitus. World J. Diabetes 2016, 7, 354. [Google Scholar] [CrossRef]
- Inzucchi, S.E.; Bergenstal, R.M.; Buse, J.B.; Diamant, M.; Ferrannini, E.; Nauck, M.; Peters, A.L.; Tsapas, A.; Wender, R.; Matthews, D.R.; et al. Management of hyperglycemia in type 2 diabetes: A patient-centered approach: Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2012, 35, 1364–1379. [Google Scholar] [CrossRef]
- Currie, C.J.; Poole, C.D.; Jenkins-Jones, S.; Gale, E.A.; Johnson, J.A.; Morgan, C.L. Mortality after incident cancer in people with and without type 2 diabetes. Diabetes Care 2012, 35, 299–304. [Google Scholar] [CrossRef]
- Li, T.; Providencia, R.; Mu, N.; Yin, Y.; Chen, M.; Wang, Y.; Liu, M.; Yu, L.; Gu, C.; Ma, H. Association of metformin monotherapy or combined therapy with cardiovascular risks in patients with type 2 diabetes mellitus. Cardiovasc. Diabetol. 2021, 20, 30. [Google Scholar] [CrossRef]
- Luo, F.; Das, A.; Chen, J.; Wu, P.; Li, X.; Fang, Z. Metformin in patients with and without diabetes: A paradigm shift in cardiovascular disease management. Cardiovasc. Diabetol. 2019, 18, 54. [Google Scholar] [CrossRef]
- Xiao, H.; Ma, X.; Feng, W.; Fu, Y.; Lu, Z.; Xu, M.; Shen, Q.; Zhu, Y.; Zhang, Y. Metformin attenuates cardiac fibrosis by inhibiting the TGFβ1–Smad3 signalling pathway. Cardiovasc. Res. 2010, 87, 504–513. [Google Scholar] [CrossRef]
- Dziubak, A.; Wójcicka, G.; Wojtak, A.; Bełtowski, J. Metabolic effects of metformin in the failing heart. Int. J. Mol. Sci. 2018, 19, 2869. [Google Scholar] [CrossRef] [PubMed]
- Fearnley, C.J.; Roderick, H.L.; Bootman, M.D. Calcium signaling in cardiac myocytes. Cold Spring Harb. Perspect. Biol. 2011, 3, a004242. [Google Scholar] [CrossRef] [PubMed]
- Wahlqvist, M.L.; Lee, M.-S.; Hsu, C.-C.; Chuang, S.-Y.; Lee, J.-T.; Tsai, H.-N. Metformin-inclusive sulfonylurea therapy reduces the risk of Parkinson’s disease occurring with Type 2 diabetes in a Taiwanese population cohort. Park. Relat. Disord. 2012, 18, 753–758. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.-C.; Wahlqvist, M.L.; Lee, M.-S.; Tsai, H.-N. Incidence of dementia is increased in type 2 diabetes and reduced by the use of sulfonylureas and metformin. J. Alzheimer’s Dis. 2011, 24, 485–493. [Google Scholar] [CrossRef]
- Bray, G.A.; Greenway, F.L. Pharmacological treatment of the overweight patient. Pharmacol. Rev. 2007, 59, 151–184. [Google Scholar] [CrossRef]
- Yerevanian, A.; Soukas, A.A. Metformin: Mechanisms in human obesity and weight loss. Curr. Obes. Rep. 2019, 8, 156. [Google Scholar] [CrossRef]
- Kahn, S.E.; Haffner, S.M.; Heise, M.A.; Herman, W.H.; Holman, R.R.; Jones, N.P.; Kravitz, B.G.; Lachin, J.M.; O’Neill, M.C.; Zinman, B.; et al. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N. Engl. J. Med. 2009, 355, 2427–2443. [Google Scholar] [CrossRef]
- The Diabetes Prevention Program Research Group. Long-term safety, tolerability, and weight loss associated with metformin in the diabetes prevention program outcomes study. Diabetes Care 2012, 35, 731–737. [Google Scholar] [CrossRef]
- Feig, D.S.; Donovan, L.E.; Zinman, B.; Sanchez, J.J.; Asztalos, E.; Ryan, E.A.; Fantus, I.G.; Hutton, E.; Armson, A.B.; Lipscombe, L.L.; et al. Metformin in women with type 2 diabetes in pregnancy (MiTy): A multicentre, international, randomised, placebo-controlled trial. Lancet Diabetes Endocrinol. 2020, 8, 834–844. [Google Scholar] [CrossRef]
- Ruholamin, S.; Eshaghian, S.; Allame, Z. Neonatal outcomes in women with gestational diabetes mellitus treated with metformin in compare with insulin: A randomized clinical trial. J. Res. Med Sci. 2014, 19, 970. [Google Scholar]
- Priya, G.; Kalra, S. Metformin in the management of diabetes during pregnancy and lactation. Drugs Context 2018, 7, 212523. [Google Scholar] [CrossRef]
- Clinical Trials. Available online: https://clinicaltrials.gov/ (accessed on 10 January 2022).
- Foda, A.A.; Aal, I.A.A. Metformin as a new therapy for endometriosis, its effects on both clinical picture and cytokines profile. Middle East Fertil. Soc. J. 2012, 17, 262–267. [Google Scholar] [CrossRef]
- Khalaf, W.M.; Akl, S.A.; Ramadan, R.R. Effect of metformin on endometrial thickness and subendometrial flow patterns in anovulatory patients with polycystic ovarian syndrome. Open J. Obstet. Gynecol. 2018, 8, 1465–1475. [Google Scholar] [CrossRef]
- Johnson, N.P. Metformin use in women with polycystic ovary syndrome. Ann. Transl. Med. 2014, 2, 56. [Google Scholar] [CrossRef]
- Metformin Use and Clinical Pregnancy Rate in Women with Unexplained Infertility—Full Text View. Available online: https://clinicaltrials.gov/ct2/show/NCT03681197 (accessed on 23 November 2021).
- Yu, H.; Zhong, X.; Gao, P.; Shi, J.; Wu, Z.; Guo, Z.; Wang, Z.; Song, Y. The potential effect of metformin on cancer: An umbrella review. Front. Endocrinol. 2019, 10, 617. [Google Scholar] [CrossRef]
- Hosono, K.; Endo, H.; Takahashi, H.; Sugiyama, M.; Sakai, E.; Uchiyama, T.; Suzuki, K.; Iida, H.; Sakamoto, Y.; Yoneda, K.; et al. Metformin suppresses colorectal aberrant crypt foci in a short-term clinical trial. Cancer Prev. Res. 2010, 3, 1077–1083. [Google Scholar] [CrossRef]
- Miranda, V.C.; Braghiroli, M.I.; Faria, L.D.; Bariani, G.; Alex, A.; Neto, J.E.B.; Capareli, F.C.; Sabbaga, J.; dos Santos, J.F.L.; Hoff, P.M.; et al. Phase 2 trial of metformin combined with 5-fluorouracil in patients with refractory metastatic colorectal cancer. Clin. Color. Cancer 2016, 15, 321–328. [Google Scholar] [CrossRef]
- Morgillo, F.; Fasano, M.; Della Corte, C.M.; Sasso, F.C.; Papaccio, F.; Viscardi, G.; Esposito, G.; Di Liello, R.; Normanno, N.; Capuano, A.; et al. Results of the safety run-in part of the METAL (METformin in advanced lung cancer) study: A multicentre, open-label phase I–II study of metformin with erlotinib in second-line therapy of patients with stage IV non-small-cell lung cancer. ESMO Open 2017, 2, e000132. [Google Scholar] [CrossRef]
- Bever, K.M.; Borazanci, E.H.; Thompson, E.A.; Durham, J.N.; Pinero, K.; Jameson, G.S.; Vrana, A.; Liu, M.; Wilt, C.; Wu, A.A.; et al. An exploratory study of metformin with or without rapamycin as maintenance therapy after induction chemotherapy in patients with metastatic pancreatic adenocarcinoma. Oncotarget 2020, 11, 1929–1941. [Google Scholar] [CrossRef]
- Hadad, S.M.; Dewar, J.A.; Elseedawy, E.; Jordan, L.; Purdie, C.; Bray, S.E.; Thompson, A.M. Gene signature of metformin actions on primary breast cancer within a window of opportunity randomized clinical trial. J. Clin. Oncol. 2010, 28, 560. [Google Scholar] [CrossRef]
- Goodwin, P.J.; Dowling, R.J.O.; Ennis, M.; Chen, B.E.; Parulekar, W.R.; Shepherd, L.E.; Burnell, M.J.; Meer, R.V.; Molckovsky, A.; Gurjal, A.; et al. Effect of metformin versus placebo on metabolic factors in the MA.32 randomized breast cancer trial. npj Breast Cancer 2021, 7, 74. [Google Scholar] [CrossRef]
- Monami, M.; Lamanna, C.; Balzi, D.; Marchionni, N.; Mannucci, E. Sulphonylureas and cancer: A case–control study. Acta Diabetol. 2009, 46, 279–284. [Google Scholar] [CrossRef]
- Amin, S.; Lux, A.; O’Callaghan, F. The journey of metformin from glycaemic control to mTOR inhibition and the suppression of tumour growth. Br. J. Clin. Pharmacol. 2019, 85, 37. [Google Scholar] [CrossRef]
- Ye, J.; Qi, L.; Chen, K.; Li, R.; Song, S.; Zhou, C.; Zhai, W. Metformin induces TPC-1 cell apoptosis through endoplasmic reticulum stress-associated pathways in vitro and in vivo. Int. J. Oncol. 2019, 55, 331–339. [Google Scholar] [CrossRef]
- Hanzhi, C.; Cui, H.; Kang, L.; Zhang, X.; Jin, Z.; Lu, L.; Fan, Z. Metformin inhibits thyroid cancer cell growth, migration, and EMT through the mTOR pathway. Tumor Biol. 2015, 36, 6295–6304. [Google Scholar] [CrossRef]
- Piek, M.W.; Postma, E.L.; van Leeuwaarde, R.; de Boer, J.P.; Bos, A.M.; Lok, C.; Stokkel, M.; Filipe, M.D.; van der Ploeg, I.M. The effect of radioactive iodine therapy on ovarian function and fertility in female thyroid cancer patients: A systematic review and meta-analysis. Thyroid 2021, 31, 658–668. [Google Scholar] [CrossRef]
- Buczyńska, A.; Sidorkiewicz, I.; Rogucki, M.; Siewko, K.; Adamska, A.; Kościuszko, M.; Maliszewska, K.; Kozłowska, G.; Szumowski, P.; Myśliwiec, J.; et al. Oxidative stress and radioiodine treatment of differentiated thyroid cancer. Sci. Rep. 2021, 11, 17126. [Google Scholar] [CrossRef]
- Nies, M.; Cantineau, A.E.; Arts, E.G.; Berg, M.H.V.D.; Van Leeuwen, F.E.; Kobold, A.C.M.; Hesselink, M.S.K.; Burgerhof, J.G.; Brouwers, A.H.; Van Dam, E.W.; et al. Long-term effects of radioiodine treatment on female fertility in survivors of childhood differentiated thyroid carcinoma. Thyroid 2020, 30, 1169–1176. [Google Scholar] [CrossRef]
- Franciosi, M.; Lucisano, G.; Lapice, E.; Strippoli, G.F.M.; Pellegrini, F.; Nicolucci, A. Metformin therapy and risk of cancer in patients with type 2 diabetes: Systematic review. PLoS ONE 2013, 8, e71583. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Ung, T.T.; Li, S.; Lian, S.; Xia, Y.; Park, S.Y.; Jung, Y.D. Metformin inhibits lithocholic acid-induced interleukin 8 upregulation in colorectal cancer cells by suppressing ROS production and NF-kB activity. Sci. Rep. 2019, 9, 2709. [Google Scholar] [CrossRef]
- Vancura, A.; Bu, P.; Bhagwat, M.; Zeng, J.; Vancurova, I. Metformin as an anticancer agent. Trends Pharmacol. Sci. 2018, 39, 867–878. [Google Scholar] [CrossRef] [PubMed]
- Meireles, C.G.; Pereira, S.A.; Valadares, L.P.; Rêgo, D.F.; Simeoni, L.A.; Guerra, N.S.; Lofrano-Porto, A. Effects of metformin on endometrial cancer: Systematic review and meta-analysis. Gynecol. Oncol. 2017, 147, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Morice, P.; Leary, A.; Creutzberg, C.; Abu-Rustum, N.; Darai, E. Endometrial cancer. Lancet 2016, 387, 1094–1108. [Google Scholar] [CrossRef]
- Travaglino, A.; Raffone, A.; Gencarelli, A.; Mollo, A.; Guida, M.; Insabato, L.; Santoro, A.; Zannoni, G.F.; Zullo, F. TCGA Classification of endometrial cancer: The place of carcinosarcoma. Pathol. Oncol. Res. 2020, 26, 2067–2073. [Google Scholar] [CrossRef]
- Shao, R.; Li, X.; Feng, Y.; Lin, J.-F.; Billig, H. Direct effects of metformin in the endometrium: A hypothetical mechanism for the treatment of women with PCOS and endometrial carcinoma. J. Exp. Clin. Cancer Res. 2014, 33, 41. [Google Scholar] [CrossRef]
- Vieira, G.D.L.T.; Lossie, A.C.; Lay, D.C.; Radcliffe, J.S.; Garner, J.P. Preventing, treating, and predicting barbering: A fundamental role for biomarkers of oxidative stress in a mouse model of Trichotillomania. PLoS ONE 2017, 12, e0175222. [Google Scholar] [CrossRef]
- Agarwal, A.; Gupta, S.; Sharma, R.K. Role of oxidative stress in female reproduction. Reprod. Biol. Endocrinol. 2005, 3, 28. [Google Scholar] [CrossRef]
- Mu, N.; Xu, T.; Gao, M.; Dong, M.; Tang, Q.; Hao, L.; Wang, G.; Li, Z.; Wang, W.; Yang, Y.; et al. Therapeutic effect of metformin in the treatment of endometrial cancer. Oncol. Lett. 2020, 20, 156. [Google Scholar] [CrossRef]
- Zou, J.; Hong, L.; Luo, C.; Liangli, H.; Zhu, Y.; Huang, T.; Zhang, Y.; Yuan, H.; Chaohuan, L.; Wen, T.; et al. Metformin inhibits estrogen-dependent endometrial cancer cell growth by activating the AMPK–FOXO 1 signal pathway. Cancer Sci. 2016, 107, 1806. [Google Scholar] [CrossRef]
- Yang, B.; Gulinazi, Y.; Du, Y.; Ning, C.; Cheng, Y.; Shan, W.; Luo, X.; Zhang, H.; Zhu, Q.; Ma, F.; et al. Metformin plus megestrol acetate compared with megestrol acetate alone as fertility-sparing treatment in patients with atypical endometrial hyperplasia and well-differentiated endometrial cancer: A randomised controlled trial. BJOG Int. J. Obst. Gynaecol. 2020, 127, 848–857. [Google Scholar] [CrossRef]
- Zhuo, Z.; Wang, A.; Yu, H. Metformin targeting autophagy overcomes progesterone resistance in endometrial carcinoma. Arch. Gynecol. Obstet. 2016, 294, 1055–1061. [Google Scholar] [CrossRef]
- Nozhat, Z.; Mohammadi-Yeganeh, S.; Azizi, F.; Zarkesh, M.; Hedayati, M. Effects of metformin on the PI3K/AKT/FOXO1 pathway in anaplastic thyroid cancer cell lines. DARU J. Pharm. Sci. 2018, 26, 93–103. [Google Scholar] [CrossRef]
- Shaw, R.J.; Lamia, K.A.; Vasquez, D.; Koo, S.-H.; Bardeesy, N.; Depinho, R.A.; Montminy, M.; Cantley, L.C. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science 2005, 310, 1642–1646. [Google Scholar] [CrossRef]
- Lee, T.Y.; Martinez-Outschoorn, U.; Schilder, R.J.; Kim, C.H.; Richard, S.D.; Rosenblum, N.G.; Johnson, J.M. Metformin as a therapeutic target in endometrial cancers. Front. Oncol. 2018, 8, 341. [Google Scholar] [CrossRef]
- Westin, S.N.; Broaddus, R.R. Personalized therapy in endometrial cancer: Challenges and opportunities. Cancer Biol. Ther. 2012, 13, 1. [Google Scholar] [CrossRef]
- Kitson, S.; Maskell, Z.; Sivalingam, V.N.; Allen, J.L.; Ali, S.; Burns, S.; Gilmour, K.; Latheef, R.; Slade, R.J.; Pemberton, P.W.; et al. PRE-surgical metformin in uterine malignancy (PREMIUM): A multi-center, randomized double-blind, placebo-controlled phase III trial. Clin. Cancer Res. 2019, 25, 2424. [Google Scholar] [CrossRef]
- Sivalingam, V.; Kitson, S.; McVey, R.; Roberts, C.; Pemberton, P.; Gilmour, K.; Ali, S.; Renehan, A.G.; Kitchener, H.C.; Crosbie, E.J. Measuring the biological effect of presurgical metformin treatment in endometrial cancer. Br. J. Cancer 2016, 114, 281–289. [Google Scholar] [CrossRef]
- Yates, M.S.; Coletta, A.M.; Zhang, Q.; Schmandt, R.E.; Medepalli, M.; Nebgen, D.; Soletsky, B.; Milbourne, A.; Levy, E.; Fellman, B.M.; et al. Prospective randomized biomarker study of metformin and lifestyle intervention for prevention in obese women at increased risk for endometrial cancer. Cancer Prev. Res. 2018, 11, 477. [Google Scholar] [CrossRef]
- Mitsuhashi, A.; Habu, Y.; Kobayashi, T.; Kawarai, Y.; Ishikawa, H.; Usui, H.; Shozu, M. Long-term outcomes of progestin plus metformin as a fertility-sparing treatment for atypical endometrial hyperplasia and endometrial cancer patients. J. Gynecol. Oncol. 2019, 30, e90. [Google Scholar] [CrossRef]
- Mitsuhashi, A.; Kawasaki, Y.; Hori, M.; Fujiwara, T.; Hanaoka, H.; Shozu, M. Medroxyprogesterone acetate plus metformin for fertility-sparing treatment of atypical endometrial hyperplasia and endometrial carcinoma: Trial protocol for a prospective, randomised, open, blinded-endpoint design, dose-response trial (FELICIA trial). BMJ Open 2020, 10, e035416. [Google Scholar] [CrossRef]
- Hutton, B.; Salanti, G.; Caldwell, D.M.; Chaimani, A.; Schmid, C.H.; Cameron, C.; Ioannidis, J.P.A.; Straus, S.; Thorlund, K.; Jansen, J.P.; et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: Checklist and explanations. Ann. Intern. Med. 2015, 162, 777–784. [Google Scholar] [CrossRef]
- Calvert, M.; Blazeby, J.; Altman, D.G.; Revicki, D.A.; Moher, D.; Brundage, M.D. Reporting of patient-reported outcomes in randomized trials: The CONSORT PRO extension. JAMA J. Am. Med. Assoc. 2013, 309, 814–822. [Google Scholar] [CrossRef]
- Schiavo, J.H. PROSPERO: An international register of systematic review protocols. Med. Ref. Serv. Q. 2019, 38, 171–180. [Google Scholar] [CrossRef]
- Booth, A.; Clarke, M.; Dooley, G.; Ghersi, D.; Moher, D.; Petticrew, M.; Stewart, L. The nuts and bolts of PROSPERO: An international prospective register of systematic reviews. Syst. Rev. 2012, 1, 2. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).