Taraxasterol Inhibits Tumor Growth by Inducing Apoptosis and Modulating the Tumor Microenvironment in Non-Small Cell Lung Cancer
Abstract
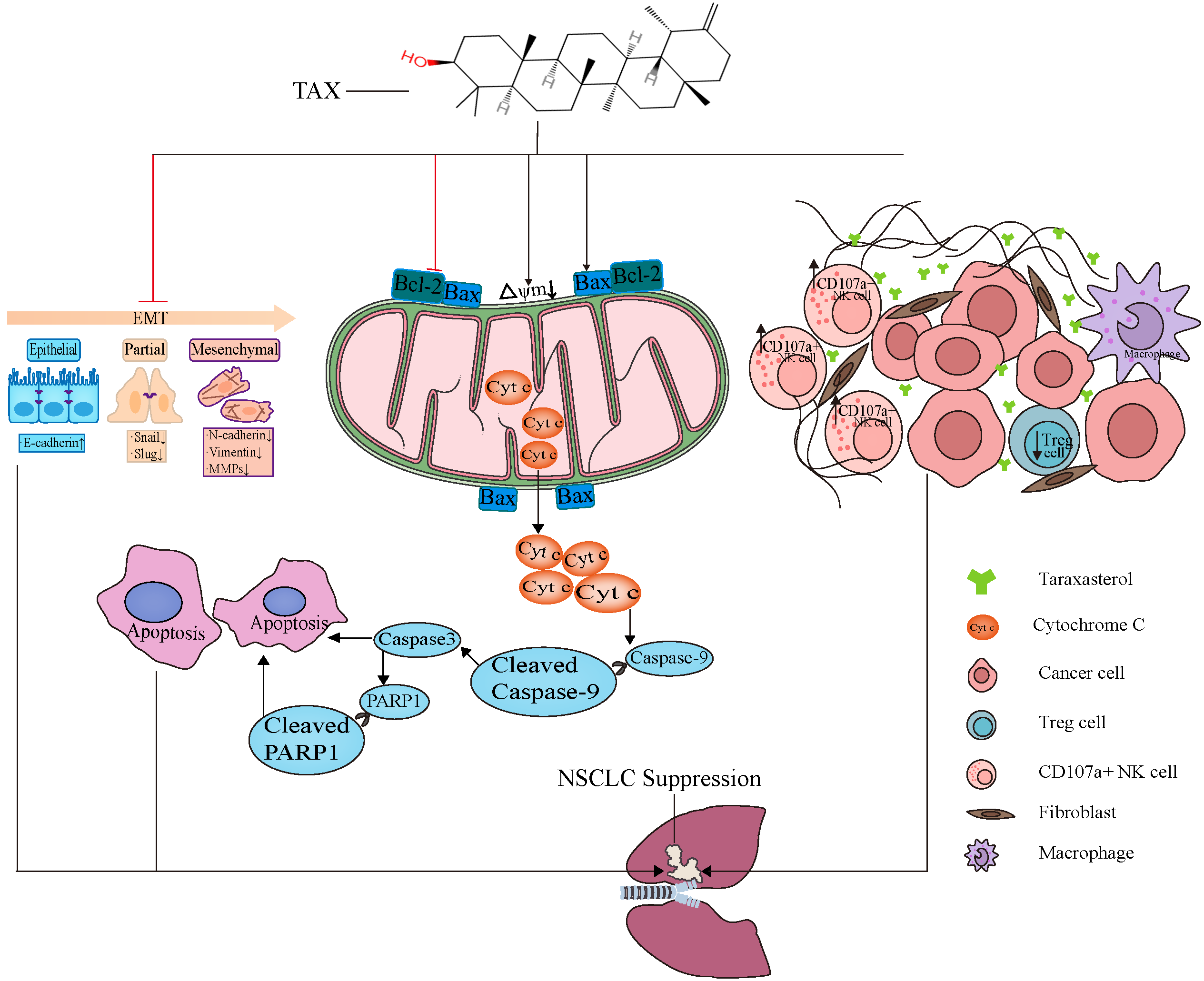
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines and Reagents
2.2. CCK-8 Assay
2.3. LDH Release Assay
2.4. Cell Cycle Assay
2.5. Measurement of the Cell Apoptosis
2.6. Measurement of ROS
2.7. Mitochondrial Membrane Potential Detection
2.8. Wound-Healing Assay
2.9. Colony-Formation Assay
2.10. Western Blot Analysis
2.11. mRNA Expression Analysis
2.12. Immunofluorescence
2.13. TUNEL Fluorescence Detection
2.14. Determination of Serum ALT, AST, Cre, and BUN in Mice
2.15. Detection of the Immune Microenvironment in Tumor
2.16. In Vivo Tumor Model
2.17. Statistical Analysis
3. Results
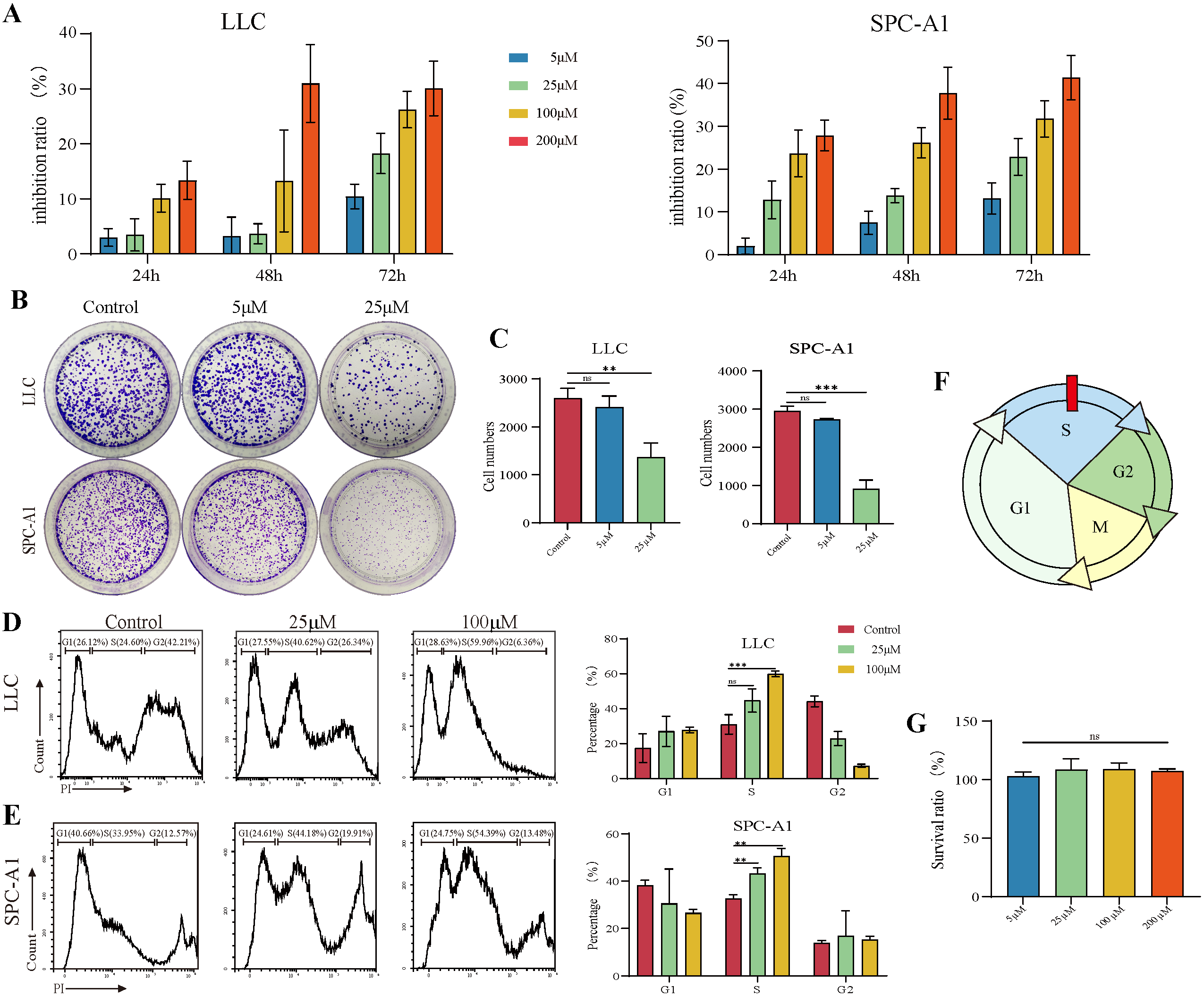
3.1. TAX Inhibits the Proliferation of Lung Cancer Cells
3.2. TAX Inhibits Lung Cancer Cell Migration by Interfering with EMT
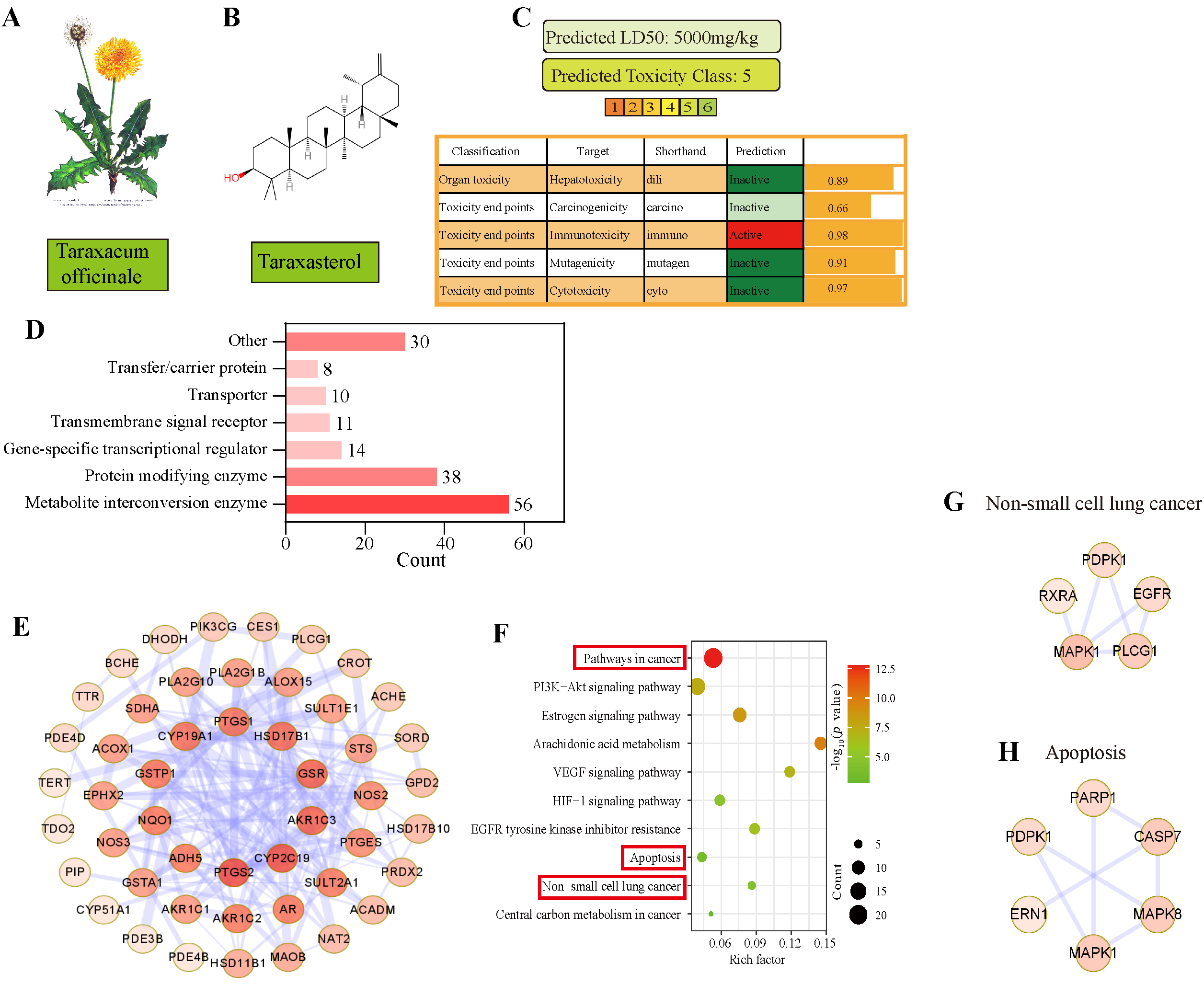
3.3. Apoptosis Was Predicted as the Main Pathway of TAX in NSCLC by Network Pharmacology
3.4. TAX Induces Early Apoptosis and Alters Mitochondrial Membrane Potential
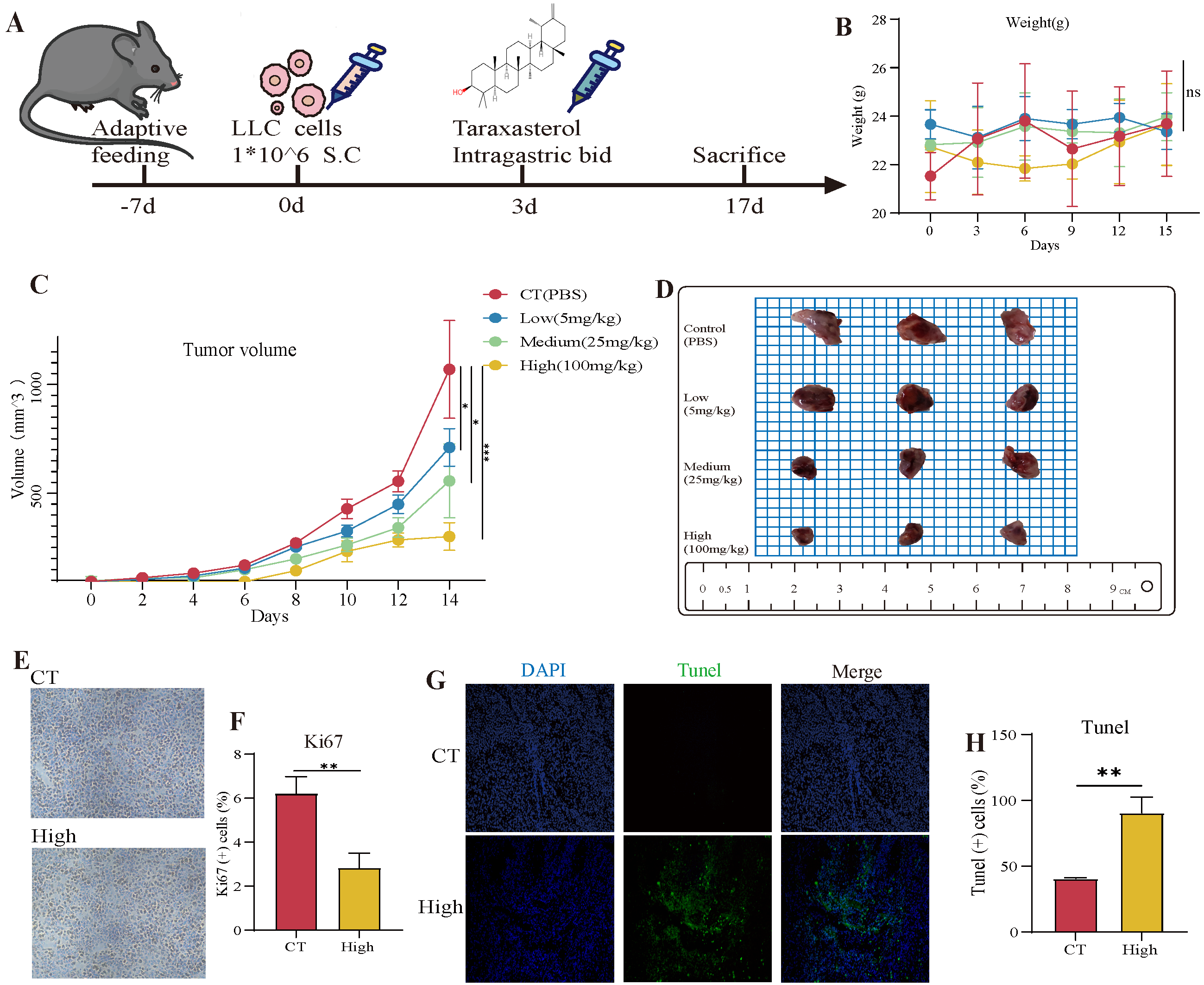
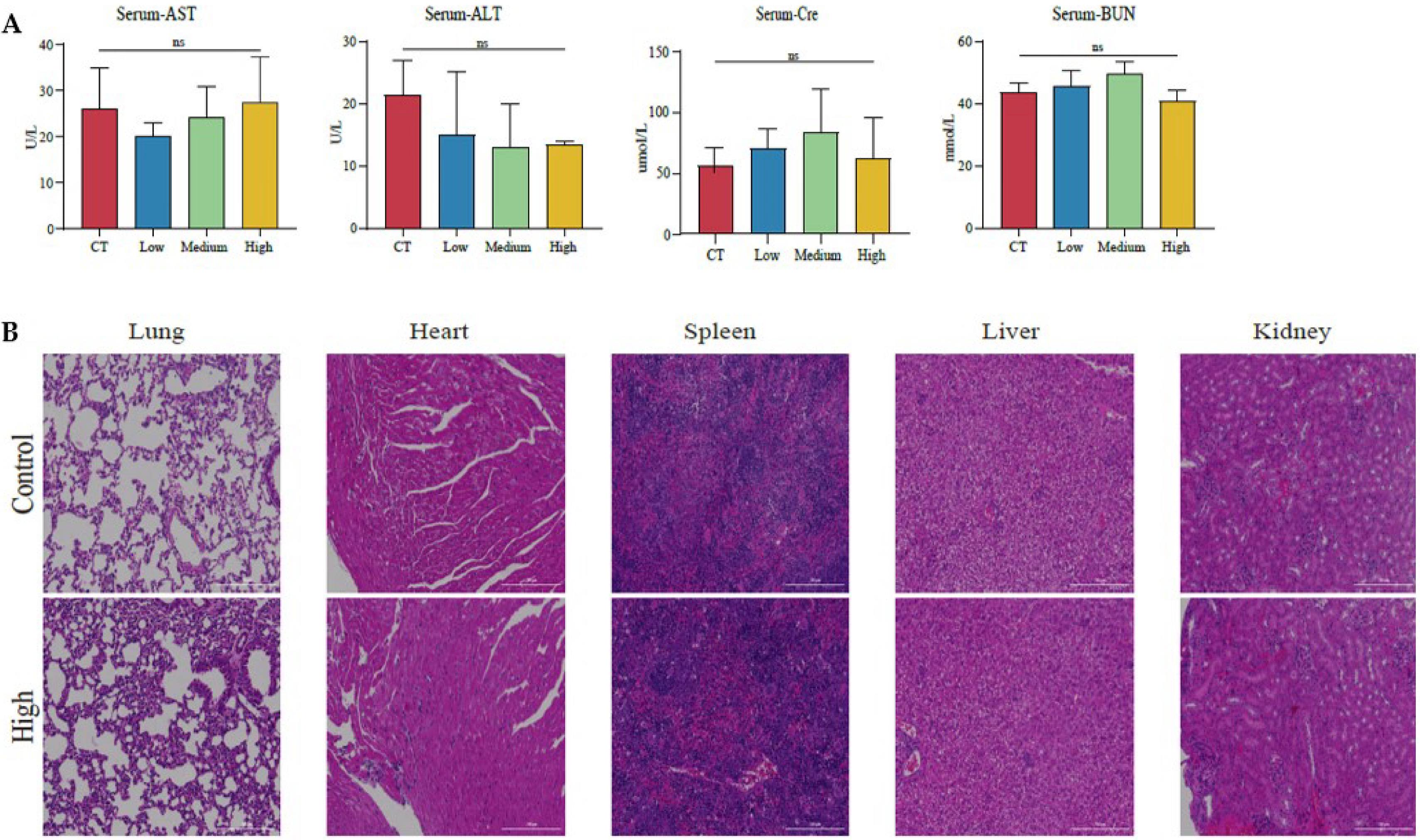
3.5. The Therapeutic Effects of TAX on NSCLC Growth in the Subcutaneous Tumor Model
3.6. TAX Inhibits EMT, Induces Tumor Cell Apoptosis, and Modulates the Internal Immune Microenvironment In Vivo
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA A Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Kocarnik, J.M.; Compton, K.; Dean, F.E.; Fu, W.; Gaw, B.L.; Harvey, J.D.; Henrikson, H.J.; Lu, D.; Pennini, A.; Xu, R.; et al. Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life Years for 29 Cancer Groups From 2010 to 2019: A Systematic Analysis for the Global Burden of Disease Study 2019. JAMA Oncol. 2022, 8, 420–444. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Herbst, R.S.; Boshoff, C. Toward personalized treatment approaches for non-small-cell lung cancer. Nat. Med. 2021, 27, 1345–1356. [Google Scholar] [CrossRef] [PubMed]
- Roberts, A.W.; Davids, M.S.; Pagel, J.M.; Kahl, B.S.; Puvvada, S.D.; Gerecitano, J.F.; Kipps, T.J.; Anderson, M.A.; Brown, J.R.; Gressick, L.; et al. Targeting BCL2 with Venetoclax in Relapsed Chronic Lymphocytic Leukemia. N. Engl. J. Med. 2016, 374, 311–322. [Google Scholar] [CrossRef]
- Mao, X.; Xu, J.; Wang, W.; Liang, C.; Hua, J.; Liu, J.; Zhang, B.; Meng, Q.; Yu, X.; Shi, S. Crosstalk between cancer-associated fibroblasts and immune cells in the tumor microenvironment: New findings and future perspectives. Mol. Cancer 2021, 20, 131. [Google Scholar] [CrossRef]
- Altorki, N.K.; Markowitz, G.J.; Gao, D.; Port, J.L.; Saxena, A.; Stiles, B.; McGraw, T.; Mittal, V. The lung microenvironment: An important regulator of tumour growth and metastasis. Nat. Reviews. Cancer 2019, 19, 9–31. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wu, Y.; Wang, Z.; Chen, J.; Yang, Y.; Dong, G. Dandelion Extract Alleviated Lipopolysaccharide-Induced Oxidative Stress through the Nrf2 Pathway in Bovine Mammary Epithelial Cells. Toxins 2020, 12, 496. [Google Scholar] [CrossRef] [PubMed]
- Majewski, M.; Lis, B.; Juśkiewicz, J.; Ognik, K.; Borkowska-Sztachańska, M.; Jedrejek, D.; Stochmal, A.; Olas, B. Phenolic Fractions from Dandelion Leaves and Petals as Modulators of the Antioxidant Status and Lipid Profile in an In Vivo Study. Antioxidants 2020, 9, 131. [Google Scholar] [CrossRef] [PubMed]
- Hosseini Shekarabi, S.P.; Mostafavi, Z.S.; Mehrgan, M.S.; Islami, H.R. Dietary supplementation with dandelion (Taraxacum officinale) flower extract provides immunostimulation and resistance against Streptococcus iniae infection in rainbow trout (Oncorhynchusmykiss). Fish Shellfish. Immunol. 2021, 118, 180–187. [Google Scholar] [CrossRef]
- Ovadje, P.; Chochkeh, M.; Akbari-Asl, P.; Hamm, C.; Pandey, S. Selective induction of apoptosis and autophagy through treatment with dandelion root extract in human pancreatic cancer cells. Pancreas 2012, 41, 1039–1047. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Wang, M.; Shen, C. Bauerane Induces S-Phase Cell Cycle Arrest, Apoptosis, and Inhibition of Proliferation of A549 Human Lung Cancer Cells Through the Phosphoinositide 3-Kinase (PI3K)/AKT and Signal Transducer and Activator of Transcription 3 (STAT3) Signaling Pathway. Med. Sci.Monit. Int. Med. J. Exp. Clin. Res. 2020, 26, e919558. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.X.; Jiao, Y.N.; Hao, H.F.; Xue, D.; Bai, C.C.; Han, S.Y. Taraxacum mongolicum extract inhibited malignant phenotype of triple-negative breast cancer cells in tumor-associated macrophages microenvironment through suppressing IL-10 / STAT3 / PD-L1 signaling pathways. J. Ethnopharmacol. 2021, 274, 113978. [Google Scholar] [CrossRef]
- Liu, W.; Yu, Q.; Wang, F.; Li, Y.; Zhang, G.; Tao, S. Taraxasterol attenuates melanoma progression via inactivation of reactive oxygen species-mediated PI3K/Akt signaling pathway. Hum. Exp. Toxicol. 2022, 41, 9603271211069034. [Google Scholar] [CrossRef]
- Zhu, J.; Li, X.; Zhang, S.; Liu, J.; Yao, X.; Zhao, Q.; Kou, B.; Han, P.; Wang, X.; Bai, Y.; et al. Taraxasterol inhibits TGF-β1-induced epithelial-to-mesenchymal transition in papillary thyroid cancer cells through regulating the Wnt/β-catenin signaling. Hum. Exp. Toxicol. 2021, 40, S87–S95. [Google Scholar] [CrossRef]
- Tang, C.T.; Yang, J.; Liu, Z.D.; Chen, Y.; Zeng, C. Taraxasterol acetate targets RNF31 to inhibit RNF31/p53 axis-driven cell proliferation in colorectal cancer. Cell Death Discov. 2021, 7, 66. [Google Scholar] [CrossRef]
- Chen, P.; Wu, Q.; Feng, J.; Yan, L.; Sun, Y.; Liu, S.; Xiang, Y.; Zhang, M.; Pan, T.; Chen, X.; et al. Erianin, a novel dibenzyl compound in Dendrobium extract, inhibits lung cancer cell growth and migration via calcium/calmodulin-dependent ferroptosis. Signal Transduct. Target. Ther. 2020, 5, 51. [Google Scholar] [CrossRef]
- Kabakov, A.E.; Gabai, V.L. Cell Death and Survival Assays. Methods Mol. Biol. 2018, 1709, 107–127. [Google Scholar] [CrossRef]
- Chaoui, D.; Faussat, A.M.; Majdak, P.; Tang, R.; Perrot, J.Y.; Pasco, S.; Klein, C.; Marie, J.P.; Legrand, O. JC-1, a sensitive probe for a simultaneous detection of P-glycoprotein activity and apoptosis in leukemic cells. Cytom. Part B Clin. Cytom. 2006, 70, 189–196. [Google Scholar] [CrossRef]
- Hirano, S. Western blot analysis. Methods Mol. Biol. 2012, 926, 87–97. [Google Scholar] [CrossRef]
- Jozefczuk, J.; Adjaye, J. Quantitative real-time PCR-based analysis of gene expression. Methods Enzymol. 2011, 500, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Young, Y.K.; Bolt, A.M.; Ahn, R.; Mann, K.K. Analyzing the Tumor Microenvironment by Flow Cytometry. Methods Mol. Biol. 2016, 1458, 95–110. [Google Scholar] [CrossRef]
- Cassetta, L.; Noy, R.; Swierczak, A.; Sugano, G.; Smith, H.; Wiechmann, L.; Pollard, J.W. Isolation of Mouse and Human Tumor-Associated Macrophages. Adv. Exp. Med. Biol. 2016, 899, 211–229. [Google Scholar] [CrossRef] [PubMed]
- Kos, K.; van Baalen, M.; Meijer, D.A.; de Visser, K.E. Flow cytometry-based isolation of tumor-associated regulatory T cells and assessment of their suppressive potential. Methods Enzymol. 2020, 632, 259–281. [Google Scholar] [CrossRef] [PubMed]
- De Craene, B.; Berx, G. Regulatory networks defining EMT during cancer initiation and progression. Nat. Rev. Cancer 2013, 13, 97–110. [Google Scholar] [CrossRef]
- Togashi, Y.; Shitara, K.; Nishikawa, H. Regulatory T cells in cancer immunosuppression—Implications for anticancer therapy. Nat. Rev. Clin. Oncol. 2019, 16, 356–371. [Google Scholar] [CrossRef] [PubMed]
- Aktas, E.; Kucuksezer, U.C.; Bilgic, S.; Erten, G.; Deniz, G. Relationship between CD107a expression and cytotoxic activity. Cell. Immunol. 2009, 254, 149–154. [Google Scholar] [CrossRef]
- Bykov, V.J.N.; Eriksson, S.E.; Bianchi, J.; Wiman, K.G. Targeting mutant p53 for efficient cancer therapy. Nat. Rev. Cancer 2018, 18, 89–102. [Google Scholar] [CrossRef]
- Suhaili, S.H.; Karimian, H.; Stellato, M.; Lee, T.H.; Aguilar, M.I. Mitochondrial outer membrane permeabilization: A focus on the role of mitochondrial membrane structural organization. Biophys. Rev. 2017, 9, 443–457. [Google Scholar] [CrossRef]
- Carneiro, B.A.; El-Deiry, W.S. Targeting apoptosis in cancer therapy. Nat. Rev. Clin. Oncol. 2020, 17, 395–417. [Google Scholar] [CrossRef]
- Ray-Coquard, I.; Pautier, P.; Pignata, S.; Pérol, D.; González-Martín, A.; Berger, R.; Fujiwara, K.; Vergote, I.; Colombo, N.; Mäenpää, J.; et al. Olaparib plus Bevacizumab as First-Line Maintenance in Ovarian Cancer. N. Engl. J. Med. 2019, 381, 2416–2428. [Google Scholar] [CrossRef] [PubMed]
- Leone, R.D.; Powell, J.D. Metabolism of immune cells in cancer. Nat. Rev. Cancer 2020, 20, 516–531. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Zhang, Y.; Zheng, L.; Zheng, C.; Song, J.; Zhang, Q.; Kang, B.; Liu, Z.; Jin, L.; Xing, R.; et al. Global characterization of T cells in non-small-cell lung cancer by single-cell sequencing. Nat. Med. 2018, 24, 978–985. [Google Scholar] [CrossRef]
- Martínez-Reyes, I.; Chandel, N.S. Cancer metabolism: Looking forward. Nat. Rev. Cancer 2021, 21, 669–680. [Google Scholar] [CrossRef] [PubMed]
- Hiam-Galvez, K.J.; Allen, B.M.; Spitzer, M.H. Systemic immunity in cancer. Nat. Rev. Cancer 2021, 21, 345–359. [Google Scholar] [CrossRef]
- Bruni, D.; Angell, H.K.; Galon, J. The immune contexture and Immunoscore in cancer prognosis and therapeutic efficacy. Nat. Rev. Cancer 2020, 20, 662–680. [Google Scholar] [CrossRef]
- Shimasaki, N.; Jain, A.; Campana, D. NK cells for cancer immunotherapy. Nat. Rev. Drug Discov. 2020, 19, 200–218. [Google Scholar] [CrossRef]
- Alter, G.; Malenfant, J.M.; Altfeld, M. CD107a as a functional marker for the identification of natural killer cell activity. J. Immunol. Methods 2004, 294, 15–22. [Google Scholar] [CrossRef]
- Bejarano, L.; Jordāo, M.J.C.; Joyce, J.A. Therapeutic Targeting of the Tumor Microenvironment. Cancer Discov. 2021, 11, 933–959. [Google Scholar] [CrossRef]






| Species | Gene | Primer Sequence (5′-3′) |
|---|---|---|
| Human | β-actin-F | TCGTGCGTGACATTAAGGAG |
| β-actin-R | ATGCCAGGGTACATGGTGGT | |
| Human | PARP1-F | CCAAGCCAGTTCAGGACCTCAT |
| PARP1-R | GGATCTGCCTTTTGCTCAGCTTC | |
| Human | Bax-F | CGTGAAGGACGCACGTTC |
| Bax-R | AGTGCTTGGAGATCGCACAG | |
| Human | Bcl-2-F | AGGCGGCAGTTAAAGCTCAT |
| Bcl-2-R | CGCATCTGCGGCAAGAG | |
| Human | Caspase-3-F | TCACCATGGCTCAGAAGCAC |
| Caspase-3-R | CAACTTCCTAAAATGGTTTGAGATGTGTT | |
| Human | Caspase-9-F | GAGGACGAGCCCTTCTGATG |
| Caspase-9-R | ATGACAAGAGGTGTTTGTTTCTGCT | |
| Human | N-Cadherin-F | CCTCCAGAGTTTACTGCCATGAC |
| N-Cadherin-R | GTAGGATCTCCGCCACTGATTC | |
| Human | E-Cadherin-F | GCCTCCTGAAAAGAGAGTGGAAG |
| E-Cadherin-R | TGGCAGTGTCTCTCCAAATCCG | |
| Human | Vimentin-F | AGGCAAAGCAGGAGTCCACTGA |
| Vimentin-R | ATCTGGCGTTCCAGGGACTCAT | |
| Human | Snail-1-F | TGCCCTCAAGATGCACATCCGA |
| Snail-1-R | GGGACAGGAGAAGGGCTTCTC | |
| Human | Snail-2-F | ATCTGCGGCAAGGCGTTTTCCA |
| Snail-2-R | GAGCCCTCAGATTTGACCTGTC | |
| Human | MMP3-F | CACTCACAGACCTGACTCGGTT |
| MMP3-R | AAGCAGGATCACAGTTGGCTGG | |
| Human | MMP9-F | GCCACTACTGTGCCTTTGAGTC |
| MMP9-R | CCCTCAGAGAATCGCCAGTACT | |
| Human | SLC7a11-F | TCCTGCTTTGGCTCCATGAACG |
| SLC7a11-R | AGAGGAGTGTGCTTGCGGACAT | |
| Human | GPX4-F | ACAAGAACGGCTGCGTGGTGAA |
| GPX4-R | GCCACACACTTGTGGAGCTAGA | |
| Human | LC3II-F | GAGAAGCAGCTTCCTGTTCTGG |
| LC3II-R | GTGTCCGTTCACCAACAGGAAG | |
| Human | P62-F | TGTGTAGCGTCTGCGAGGGAAA |
| P62-R | AGTGTCCGTGTTTCACCTTCCG | |
| Mouse | PARP1-F | CTCTCCCAGAACAAGGACGAAG |
| PARP1-R | CCGCTTTCACTTCCTCCATCTTC | |
| Mouse | Bax-F | AGGATGCGTCCACCAAGAAGCT |
| Bax-R | TCCGTGTCCACGTCAGCAATCA | |
| Mouse | Bcl-2-F | CCTGTGGATGACTGAGTACCTG |
| Bcl-2-R | AGCCAGGAGAAATCAAACAGAGG | |
| Mouse | Caspase-3-F | GGAGTCTGACTGGAAAGCCGAA |
| Caspase-3-R | CTTCTGGCAAGCCATCTCCTCA | |
| Mouse | Caspase-9-F | GCTGTGTCAAGTTTGCCTACCC |
| Caspase-9-R | CCAGAATGCCATCCAAGGTCTC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, J.; Shuai, B.; Shou, Z.; Guo, W.; Zhou, C.; Ouyang, X.; Zhou, H.; Li, J.; Cui, J.; Jiang, F.; et al. Taraxasterol Inhibits Tumor Growth by Inducing Apoptosis and Modulating the Tumor Microenvironment in Non-Small Cell Lung Cancer. Cancers 2022, 14, 4645. https://doi.org/10.3390/cancers14194645
Lu J, Shuai B, Shou Z, Guo W, Zhou C, Ouyang X, Zhou H, Li J, Cui J, Jiang F, et al. Taraxasterol Inhibits Tumor Growth by Inducing Apoptosis and Modulating the Tumor Microenvironment in Non-Small Cell Lung Cancer. Cancers. 2022; 14(19):4645. https://doi.org/10.3390/cancers14194645
Chicago/Turabian StyleLu, Junjie, Bo Shuai, Zhexing Shou, Weina Guo, Cong Zhou, Xiaohu Ouyang, Haifeng Zhou, Junyi Li, Jing Cui, Feng Jiang, and et al. 2022. "Taraxasterol Inhibits Tumor Growth by Inducing Apoptosis and Modulating the Tumor Microenvironment in Non-Small Cell Lung Cancer" Cancers 14, no. 19: 4645. https://doi.org/10.3390/cancers14194645
APA StyleLu, J., Shuai, B., Shou, Z., Guo, W., Zhou, C., Ouyang, X., Zhou, H., Li, J., Cui, J., Jiang, F., Jin, K. Y., Sarapultsev, A., Li, F., Zhang, G., Luo, S., & Hu, D. (2022). Taraxasterol Inhibits Tumor Growth by Inducing Apoptosis and Modulating the Tumor Microenvironment in Non-Small Cell Lung Cancer. Cancers, 14(19), 4645. https://doi.org/10.3390/cancers14194645






