The Impact of a Precision-Based Exercise Intervention in Childhood Hematological Malignancies Evaluated by an Adapted Yo-Yo Intermittent Recovery Test
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
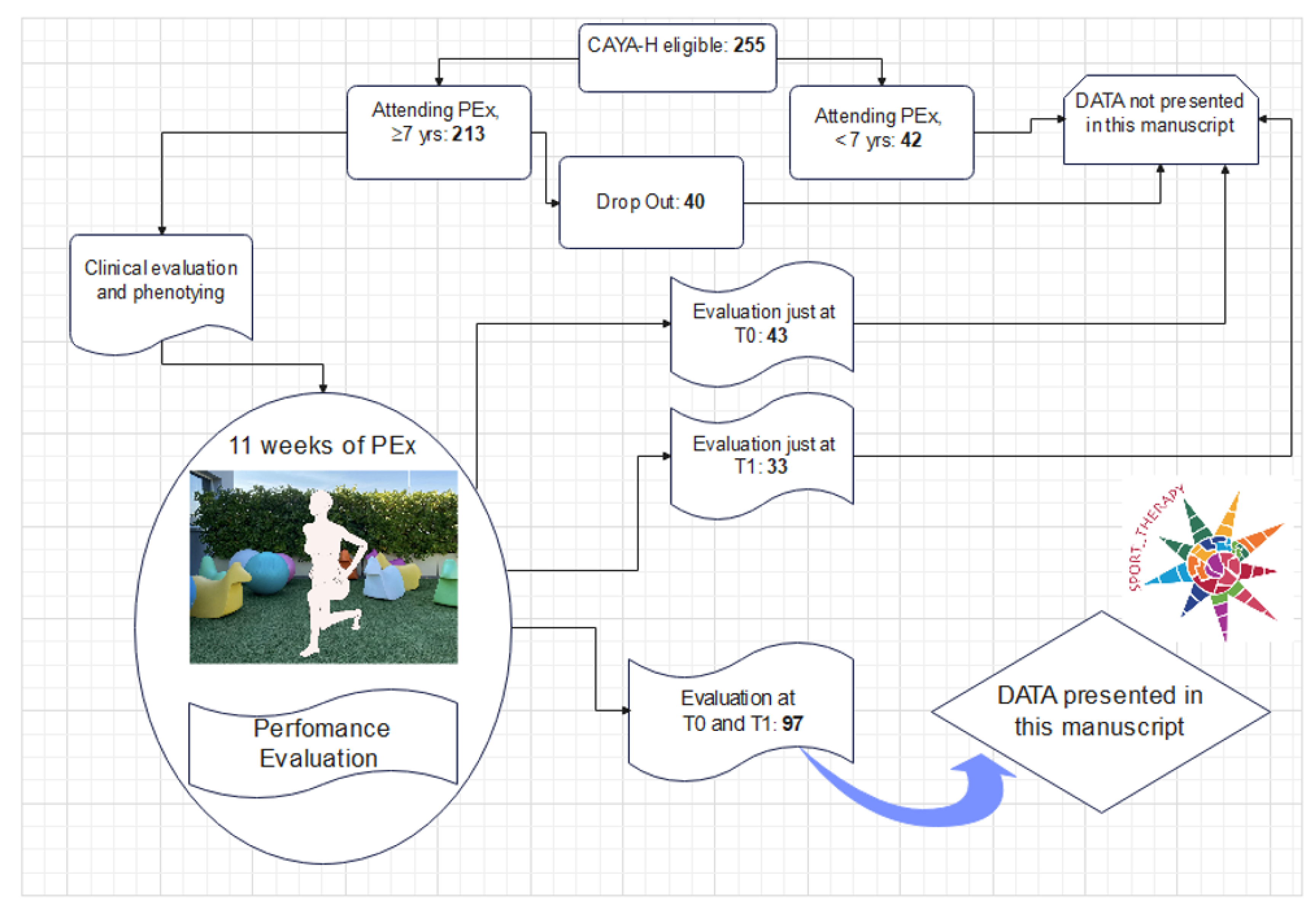
2.1. Participants and Intervention
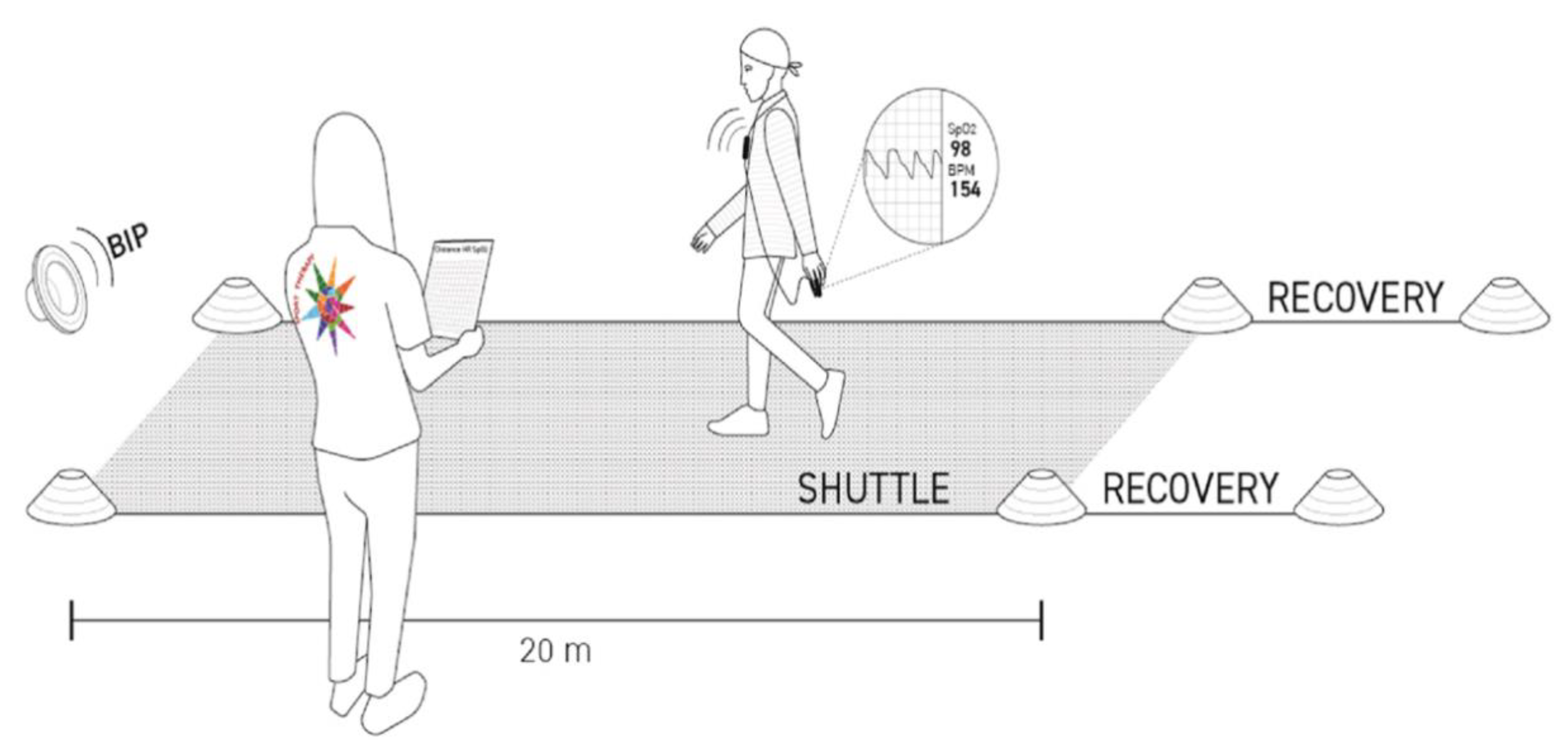
2.2. Assessment of the Exercise Tolerance
2.3. Assessment of Functional Capacity and Strength
2.4. Statistical Analysis
3. Results
| Clinical Characteristics | Num, (%) | Treatment Protocols |
|---|---|---|
| Patients, total | 97 | |
| Aged 7 < x < 11 years | 58, (60%) | |
| Aged 11 ≤ x < 22 years | 39, (40%) | |
| Level 4: Most Intensive Treatments | 25, (26%) | |
| Relapsed Disease—Excluding Hodgkin Lymphoma, first relapse | 8, (8%) | IntReALL SR or Personalized treatment |
| HSCT—All diseases | 12, (13%) | Personalized treatment |
| AML | 5, (5%) | AML 2013/01 |
| Level 3: Very Intensive Treatments, Total | 16, (17%) | |
| Relapse Protocols for Hodgkins | 3, (3%) | Personalized treatment |
| ALL (High Risk, Very High Risk, T-cell) | 10, (11%) | AIEOP BFM ALL 2009 |
| HL (Stages 3B or 4/High Risk) | 1, (1%) | EuroNet-PHL-C2 |
| NHL (Group C or Stage 4) | 2, (2%) | Euro LB-02/ NHL97 |
| Level 2: Moderately Intensive Treatments, Total | 51, (53%) | |
| ALL (Low, Standard, or Intermediate Risk; precursor B cell) | 36, (37%) | AIEOP BFM ALL 2009 |
| HL (Low/Intermediate risk: all stages except IIIB, IVB) | 13, (14%) | EuroNet-PHL-C2 |
| NHL (Stages 1, 2, 3 and Groups A, B) | 2, (2%) | Euro LB-02/ NHL97 |
| Level 0: Off Therapy, Total | ||
| Long-term HSCT, with GvHD and/or ON | 5, (4%) | Personalized treatment |
3.1. Reliability
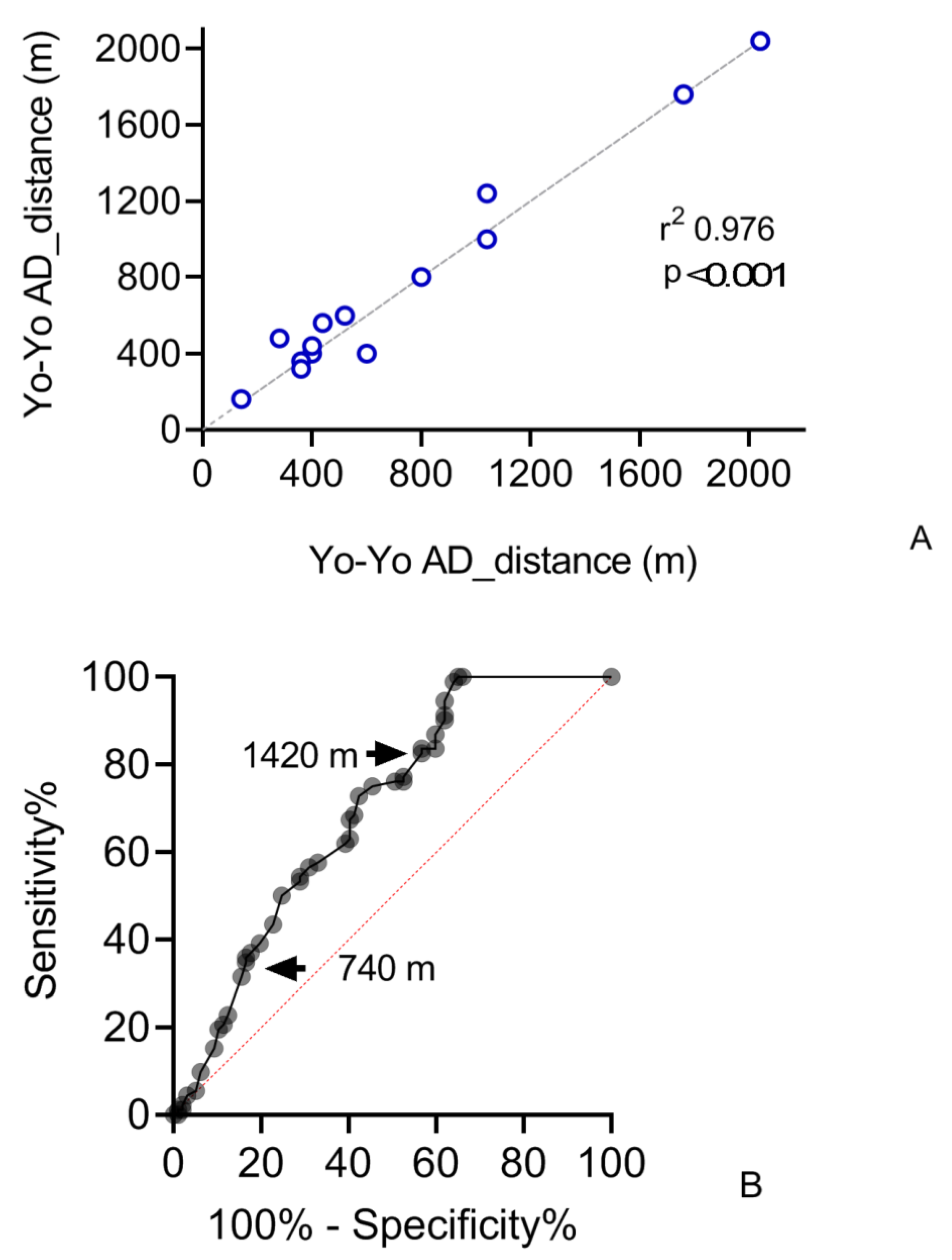
3.2. Sensitivity
3.3. Validity
4. Discussion
4.1. A Safe Test
4.2. A Reliable and Valid Test
4.3. Exercise Tolerance, Strength, and Metabolic Pathways
4.4. Patterns of Efficiency
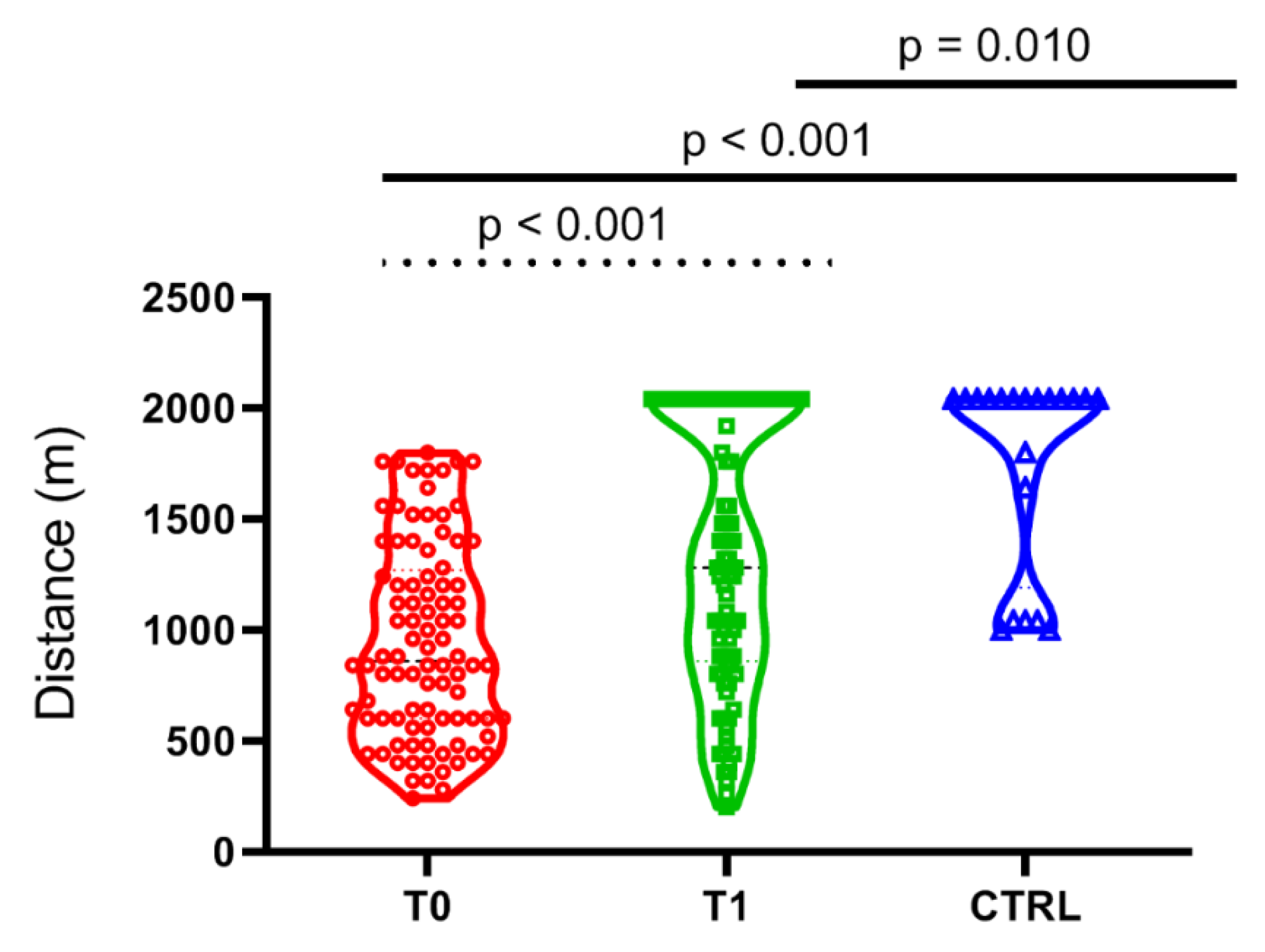
4.5. Effect of PEx
4.6. Role of Sex and Age in Performance
4.7. Limitation of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lanfranconi, F.; Zardo, W.; Moriggi, T.; Villa, E.; Radaelli, G.; Radaelli, S.; Paoletti, F.; Bottes, E.; Miraglia, T.; Pollastri, L.; et al. Precision-based exercise as a new therapeutic option for children and adolescents with haematological malignancies. Sci. Rep. 2020, 10, 12892. [Google Scholar] [CrossRef] [PubMed]
- Morales, J.S.; Santana-Sosa, E.; Santos-Lozano, A.; Baño-Rodrigo, A.; Valenzuela, P.L.; Rincón-Castanedo, C.; Fernández-Moreno, D.; Vicent, M.G.; Pérez-Somarriba, M.; Fiuza-Luces, C.; et al. In hospital exercise benefits in childhood cancer: A prospective cohort study. Scand. J. Med. Sci. Sports 2020, 30, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Stössel, S.; Neu, M.A.; Wingerter, A.; Bloch, W.; Zimmer, P.; Paret, C.; Malki, K.E.; Baumann, F.T.; Russo, A.; Henninger, N.; et al. Benefits of Exercise Training for Children and Adolescents Undergoing Cancer Treatment: Results from the Randomized Controlled MUCKI Trial. Front. Pediatr. 2020, 8, 243. [Google Scholar] [CrossRef] [PubMed]
- Lanfranconi, F.; Pollastri, L.; Ferri, A.; Fraschini, D.; Masera, G.; Miserocchi, G. Near infrared spectroscopy (NIRS) as a new non-invasive tool to detect oxidative skeletal muscle impairment in children survived to acute lymphoblastic leukaemia. PLoS ONE 2014, 9, e99282. [Google Scholar] [CrossRef][Green Version]
- Ness, K.K.; Kaste, S.C.; Zhu, L.; Pui, C.; Jeha, S.; Nathan, P.C.; Inaba, H.; Wasilewski-Masker, K.; Shah, D.; Wells, R.J.; et al. Skeletal, neuromuscular and fitness impairments among children with newly diagnosed acute lymphoblastic leukemia. Leuk. Lymphoma 2015, 56, 1004–1011. [Google Scholar] [CrossRef]
- Deisenroth, A.; Söntgerath, R.; Schuster, A.J.; Von Busch, C.; Huber, G.; Eckert, K.; Kulozik, A.; Wiskemann, J. Muscle strength and quality of life in patients with childhood cancer at early phase of primary treatment. Pediatr. Hematol. Oncol. 2016, 33, 393–407. [Google Scholar] [CrossRef]
- Haupt, R.; Essiaf, S.; Dellacasa, C.; Ronckers, C.M.; Caruso, S.; Sugden, E.; Zadravec Zaletel, L.; Muraca, M.; Morsellino, V.; Kienesberger, A.; et al. The ‘Survivorship Passport’ for childhood cancer survivors. Eur. J. Cancer 2018, 102, 69–81. [Google Scholar] [CrossRef]
- Elnaggar, R.K. Within 5-year off-chemotherapy: How the cardio-respiratory response to exercise is related to energy expenditure, fatigue, and adiposity in children with acute lymphoblastic leukaemia? Eur. J. Cancer Care 2021, 1, e13418. [Google Scholar] [CrossRef]
- Mody, R.; Li, S.; Dover, D.C.; Salan, S.; Leisenring, W.; Oeffinger, K.C.; Yasui, Y.; Robison, L.L.; Neglia, J.P. Twenty-five-year follow-up among survivors of childhood acute lymphoblastic leukemia: A report from the Childhood Cancer Survivor Study. Blood 2008, 111, 5515–5523. [Google Scholar] [CrossRef]
- Hudson, M.M.; Ness, K.K.; Gurney, J.G.; Mulrooney, D.A.; Chemaitilly, W.; Krull, K.R.; Green, D.M.; Armstrong, G.T.; Nottage, K.A.; Jones, K.E.; et al. Clinical Ascertainment of Health Outcomes among Adults Treated for Childhood Cancer: A Report from the St. Jude Lifetime Cohort Study. JAMA 2013, 309, 2371–2381. [Google Scholar] [CrossRef]
- Cox, C.L.; Rai, S.; Rosenthal, D.; Phipps, S.; Hudson, M. Subclinical late cardiac toxicity in childhood cancer survivors: Impact on self-reported health. Cancer 2008, 112, 1835–1844. [Google Scholar] [CrossRef] [PubMed]
- Jenney, M.E.; Faragher, E.B.; Jones, P.H.; Woodcock, A. Lung function and exercise capacity in survivors of childhood leukaemia. Med. Pediatr. Oncol. 1995, 24, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Bossi, G.; Cerveri, I.; Volpini, E.; Corsico, A.; Baio, A.; Corbella, F.; Klersy, C.; Arico, M. Long-term pulmonary sequelae after treatment of childhood Hodgkin’s disease. Ann. Oncol. 1997, 8 (Suppl. S1), 19–24. [Google Scholar] [CrossRef] [PubMed]
- Nysom, K.; Holm, K.; Hertz, H.; Hesse, B. Risk factors for reduced pulmonary function after malignant lymphoma in childhood. Med. Pediatr. Oncol. 1998, 30, 240–248. [Google Scholar] [CrossRef]
- Oguz, A.; Tayfun, T.; Citak, E.C.; Ceyda, K.; Turkan, T.; Oznur, B.; Huseyin, B. Long-term pulmonary function in survivors of childhood Hodgkin disease and non-Hodgkin lymphoma. Pediatr. Blood Cancer 2007, 49, 699–703. [Google Scholar] [CrossRef]
- Tseng-Tien, H.; Hudson, M.M.; Stokes, D.C.; Krasin, M.J.; Spunt, S.L.; Ness, K.K. Pulmonary Outcomes in Survivors of Childhood Cancer A Systematic Review. Chest 2011, 140, 881–901. [Google Scholar] [CrossRef]
- Akyay, A.; Olcay, L.; Sezer, N.; Sonmez, C.A. Muscle strength, motor performance, cardiac and muscle biomarkers in detection of muscle side effects during and after acute lymphoblastic leukemia treatment in children. J. Pediatr. Hematol. Oncol. 2014, 36, 594–598. [Google Scholar] [CrossRef]
- Harila-Saari, A.H.; Huuskonen, U.E.; Tolonen, U.; Vainionpaa, L.K.; Lanning, B.M. Motor nervous pathway function is impaired after treatment of childhood acute lymphoblastic leukemia: A study with motor evoked potentials. Med. Pediatr. Oncol. 2001, 36, 345–351. [Google Scholar] [CrossRef]
- Kaste, S.C.; Jones-Walace, D.; Rose, S.R.; Boyett, J.M.; Lustig, R.H.; Rivera, G.K.; Pui, C.H.; Hudson, M.M. Bone mineral decrements in survivors of childhood acute lymphoblastic leukemia: Frequency of occurrence and risk factors for their development. Leukemia 2001, 15, 728–734. [Google Scholar] [CrossRef]
- Mostoufi-Moab, S.; Ward, L.M. Skeletal Morbidity in Children and Adolescents During and Following Cancer Therapy. Horm. Res. Paediatr. 2019, 91, 137–151. [Google Scholar] [CrossRef]
- Niinimäki, R.A.; Harila-Saari, A.H.; Jartti, A.E.; Seuri, R.M.; Riikonen, P.V.; Paakko, E.L.; Mottonen, M.I.; Lanning, M. High body mass index increases the risk for osteonecrosis in children with acute lymphoblastic leukemia. J. Clin. Oncol. 2007, 25, 1498–1504. [Google Scholar] [CrossRef] [PubMed]
- Marchese, V.G.; Connolly, B.H.; Able, C.; Booten, A.R.; Bowen, P.; Porter, B.M.; Rai, S.N.; Hancock, M.L.; Pui, C.H.; Howard, S.; et al. Relationships among severity of osteonecrosis, pain, range of motion, and functional mobility in children, adolescent, and young adults with acute lymphoblastic leukemia. Phys. Ther. 2008, 88, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Mattano, L.A., Jr.; Devidas, M.; Nachman, J.B.; Sather, H.; Hunger, S.; Steinherz, P.; Gaynon, P.; Seibel, N.; Children’s Oncology Group. Effect of alternate-week versus continuous dexamethasone scheduling on the risk of osteonecrosis in paediatric patients with acute lymphoblastic leukaemia: Results from the CCG-1961 randomised cohort trial. Lancet Oncol. 2012, 13, 906–915. [Google Scholar] [CrossRef]
- Girard, P.; Auquier, P.; Barlogis, V.; Contet, A.; Poiree, M.; Demeocq, F.; Berbis, J.; Herrmann, I.; Villes, V.; Sirvent, N.; et al. Symptomatic osteonecrosis in childhood leukaemia survivors: Prevalence, risk factors and impact of quality of life in adulthood. Haematologica 2013, 98, 1089–1097. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Global Disability Action Plan 2014–2021: Better Health for All People with Disability; WHO: Geneva, Switzerland, 2015. [Google Scholar]
- Chamorro-Viña, C.; Ruiz, J.R.; Santana-Sosa, E.; González Vicent, M.; Madero, L.; Pérez, M.; Fleck, S.J.; Pérez, A.; Ramírez, M.; Lucía, A. Exercise during hematopoietic stem cell transplant hospitalization in children. Med. Sci. Sports Exerc. 2010, 42, 1045–1053. [Google Scholar] [CrossRef]
- Perondi, M.B.; Gualano, B.; Artioli, G.G. Effects of a combined aerobic and strength training program in youth patients with acute lymphoblastic leukemia. J. Sports Sci. Med. 2012, 11, 387–392. [Google Scholar]
- Kabak, V.Y.; Duger, T.; Cetinkaya, D.U. Investigation of the Effects of an Exercise Program on Physical Functions and Activities of Daily Life in Pediatric Hematopoietic Stem Cell Transplantation. Pediatr. Blood Cancer 2016, 63, 1643–1648. [Google Scholar] [CrossRef]
- Fairman, C.; Zourdos, M.; Helms, E.; Focht, B. A scientific Rationale to improve resistance training prescription in Exercise Oncology. Sports Med. 2017, 47, 1457–1465. [Google Scholar] [CrossRef]
- Scott, J.M.; Nilsen, T.S.; Gupta, D.; Jones, L.W. Exercise Therapy and Cardiovascular Toxicity in Cancer. Circulation 2018, 137, 1176–1191. [Google Scholar] [CrossRef]
- Stuart, G.; Forsythe, L. Exercise prescription in young children with congenital heart disease: Time for a change in culture. Open Heart 2021, 8, e001669. [Google Scholar] [CrossRef]
- Sasso, J.P.; Eves, N.D.; Christensen, J.F.; Graeme, J.K.; Scott, J.; Lee, W. A framework for prescription in exercise-oncology research. J. Cachexia Sarcopenia Muscle 2015, 6, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Ross, W.L.; Le, A.; Zheng, D.J.; Mitchell, H.R.; Rotatori, J.; Li, F.; Ness, K.K.; Kadan-Lottick, N.S. Physical activity barriers, preferences, and beliefs in childhood cancer patients. Support. Care Cancer 2018, 26, 2177–2184. [Google Scholar] [CrossRef] [PubMed]
- San Juan, A.F.; Chamorro-Viña, C.; Maté-Muñoz, J.L.; Fernández del Vale, M.; Cardona, C.; Hernández, M.; Madero, L.; Pérez, M.; Ramírez, M.; Lucia, A. Functional capacity of children with leukemia. Int. J. Sports Med. 2008, 29, 163–167. [Google Scholar] [CrossRef] [PubMed]
- San Juan, A.F.; Fleck, S.J.; Chamorro-Vina, C.; Maté-Muñoz, J.L.; Moral, S.; Pérez, M.; Cardona, C.; Del Valle, M.F.; Hernández, M.; Ramírez, M.; et al. Effects of an intrahospital exercise program intervention for children with leukemia. Med. Sci. Sports Exerc. 2007, 39, 13–21. [Google Scholar] [CrossRef]
- Davis, N.L.; Tolfrey, K.; Jenney, M.; Elson, R.; Stewart, C.; Moss, A.D.; Cornish, J.M.; Stevens, M.; Crowne, E.C. Combined resistance and aerobic exercise intervention improves fitness, insulin resistance and quality of life in survivors of childhood haemopoietic stem cell transplantation with total body irradiation. Pediatr. Blood Cancer 2020, 67, e28687. [Google Scholar] [CrossRef]
- Bangsbo, J.; Iaia, M.; Krustrup, P. The Intermittent Recovery Test a Useful Tool for Evaluation of Physical Performance in Intermittent Sports. Sports Med. 2008, 38, 37–51. [Google Scholar] [CrossRef] [PubMed]
- Haupt, R.; Spinetta, J.J.; Ban, I.; Barr, R.; Beck, J.D.; Byrne, J.; Calaminus, G.; Coenen, E.; Chesler, M.; D’Angio, J.G.; et al. Long term survivors of childhood cancer: Cure and care. The Erice statement. Eur. J. Cancer 2007, 43, 1778–1780. [Google Scholar] [CrossRef]
- Kazak, A.E.; Hocking, M.C.; Ittenbach, R.F.; Meadows, A.T.; Hobbie, W.; DeRosa, W.B.; Leahey, A.; Kersun, L.; Reilly, A.A. Revision of the Intensity of Treatment Rating Scale: Classifying the Intensity of Pediatric Cancer Treatment. Pediatr. Blood Cancer 2012, 59, 96–99. [Google Scholar] [CrossRef]
- Prahm, K.P.; Witting, N.; Vissing, J. Decreased variability of the 6-min walk test by heart rate correction in patients with neuromuscular disease. PLoS ONE 2014, 9, e114273. [Google Scholar] [CrossRef][Green Version]
- Zaino, C.A.; Marchese, V.G.; Westcoot, S.L. Timed up and down stairs test: Preliminary reliability and validity of a new measure of functional mobility. Pediatr. Phys. Ther. 2004, 16, 90–98. [Google Scholar] [CrossRef]
- Geiger, R.; Strasak, A.; Treml, B.; Gasser, K.; Kleinsasser, A.; Fischer, V.; Geiger, H.; Loeckinger, J.I. Six-minut walking test in children and adolescents. J. Pediatr. 2007, 150, 395–399.e2. [Google Scholar] [CrossRef] [PubMed]
- Fiuza-Luces, C.; Padilla, J.R.; Soares-Miranda, L.; Santana-Sosa, E.; Quiroga, J.V.; Santos-Lozano, A.; Pareja-Galeano, H.; Sanchis-Gomar, F.; Lorenzo-González, R.; Verde, Z.; et al. Exercise Intervention in Pediatric Patients with Solid Tumors: The Physical Activity in Pediatric Cancer Trial. Med. Sci. Sports Exerc. 2017, 49, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Currell, K.; Jeukendrup, A.E. Validity, Reliability and Sensitivity of Measures of Sporting Performance. Sports Med. 2008, 38, 297–316. [Google Scholar] [CrossRef]
- D’Arrigo, G.; Provenzano, F.; Torino, C.; Zaccali, C.; Tripepi, G. Diagnostic tests and ROC curve analysis. G. Ital. Nefrol. 2011, 28, 642–647. [Google Scholar] [PubMed]
- Jones, L.W. Precision Oncology Framework for Investigation of Exercise as Treatment for Cancer. J. Clin. Oncol. 2015, 33, 4134–4137. [Google Scholar] [CrossRef]
- Thompson, P.D.; Baggish, A.L.; Franklin, B.; Jaworski, C.; Riebe, D. American College of Sports Medicine Expert Consensus Statement to Update Recommendations for Screening, Staffing, and Emergency Policies to Prevent Cardiovascular Events at Health Fitness Facilities. Curr. Sports Med. Rep. 2020, 19, 223–231. [Google Scholar] [CrossRef]
- Taipale, R.S.; Mikkola, J.; Vesterinen, V.; Nummela, A.; Häkkinen, K. Neuromuscolar adaptations during combined strength and endurance training in endurance runners: Maximal versus explosive strength training or a mix of both. Eur. J. Appl. Physiol. 2013, 113, 325–335. [Google Scholar] [CrossRef]

| Type | Cardiorespiratory | Resistance | Neuromotor | Flexibility |
|---|---|---|---|---|
| Elaborated meaning | Regular, purposeful exercise that involves major muscle groups and is continuous and rhythmic in nature (e.g., walking on an elastic trampoline) | Major muscle groups with a variety of exercise equipment and/or body weight (e.g., leg extension machine). Different muscle groups are used every day | Exercise involving balance, agility, coordination, and gait, proprioceptive training, and multifaceted activities (e.g., balance on proprioceptive surfaces) | A series of flexibility exercises for each of the major muscle-tendon units |
| Frequency | ≥3 day × week−1 | ≥3 day × week−1 | ≥3 day × week−1 | ≥3 day × week−1 |
| Pattern | One continuous session of supervised exercise per day with active recoveries for each 2 min of activity | Rest intervals of 1 min between each set of repetitions | / | At the end of each training session. One repetition |
| Progression | Increased exercise volume each 4 weeks | Increased exercise volume each 4 weeks | / | / |
| Most intensive treatment, exercise training protocol | ||||
| Intensity | Light–moderate, 40–60% of HRR | Light, 40–50% of the 1 RM | Indeterminable | Stretch to the point of feeling tightness or slight discomfort |
| Time | 30 min × week−1 | 30 min × week−1 | 15 min × week−1 | 15 min × week−1 |
| Volume/repetitions | ≥120 MET min × week−1 | 8–12 repetitions, 2–3 sets | / | 30 s |
| Very intensive treatment, exercise training protocol | ||||
| Intensity | Moderate–vigorous, 60–80% of HRR | Moderate–hard, 60–70% of the 1 RM | Indeterminable | Stretch to the point of feeling tightness or slight discomfort |
| Time | 60 min × week−1 | 60 min × week−1 | 30 min × week−1 | 30 min × week−1 |
| Volume/repetitions | ≥240 MET min × week−1 | 8–12 repetitions, 3–4 sets | / | 30 s |
| Moderately intensive treatment, exercise training protocol | ||||
| Intensity | Vigorous, 70–90% of HRR | Hard, 70–80% of the 1 RM | Indeterminable | Stretch to the point of feeling tightness or slight discomfort |
| Time | 60–70 min × week−1 | 60–70 min × week−1 | 30–35 min × week−1 | 30–35 min × week−1 |
| Volume/repetitions | ≥240 MET min × week−1 | 8–12 repetitions, 3–4 sets | / | 30 s |
| 6MWT vs. Yo-Yo AD | ||
| T0 | T1 | |
| Equation | Y = 4.046 × X − 1.147 | Y = 4.583 × X − 1.278 |
| p value | <0.001 | <0.001 |
| R square | 0.420 | 0.554 |
| TUDS vs. Yo-Yo AD | ||
| T0 | T1 | |
| Equation | Y = −162.8 × X + 2.210 | Y = −249.5 × X + 2.970 |
| p value | <0.001 | <0.001 |
| R square | 0.215 | 0.377 |
| LEG EXTENSION vs. Yo-Yo AD | ||
| T0 | T1 | |
| Equation | Y = 18.31 × X + 381.3 | Y = 22.86 × X + 367.8 |
| p value | <0.001 | <0.001 |
| R square | 0.417 | 0.335 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zardo, W.; Villa, E.; Corti, E.; Moriggi, T.; Radaelli, G.; Ferri, A.; Marzorati, M.; Eirale, C.; Vago, P.; Biondi, A.; et al. The Impact of a Precision-Based Exercise Intervention in Childhood Hematological Malignancies Evaluated by an Adapted Yo-Yo Intermittent Recovery Test. Cancers 2022, 14, 1187. https://doi.org/10.3390/cancers14051187
Zardo W, Villa E, Corti E, Moriggi T, Radaelli G, Ferri A, Marzorati M, Eirale C, Vago P, Biondi A, et al. The Impact of a Precision-Based Exercise Intervention in Childhood Hematological Malignancies Evaluated by an Adapted Yo-Yo Intermittent Recovery Test. Cancers. 2022; 14(5):1187. https://doi.org/10.3390/cancers14051187
Chicago/Turabian StyleZardo, William, Emanuele Villa, Eleonora Corti, Tommaso Moriggi, Giorgia Radaelli, Alessandra Ferri, Mauro Marzorati, Cristiano Eirale, Paola Vago, Andrea Biondi, and et al. 2022. "The Impact of a Precision-Based Exercise Intervention in Childhood Hematological Malignancies Evaluated by an Adapted Yo-Yo Intermittent Recovery Test" Cancers 14, no. 5: 1187. https://doi.org/10.3390/cancers14051187
APA StyleZardo, W., Villa, E., Corti, E., Moriggi, T., Radaelli, G., Ferri, A., Marzorati, M., Eirale, C., Vago, P., Biondi, A., Jankovic, M., Balduzzi, A., & Lanfranconi, F. (2022). The Impact of a Precision-Based Exercise Intervention in Childhood Hematological Malignancies Evaluated by an Adapted Yo-Yo Intermittent Recovery Test. Cancers, 14(5), 1187. https://doi.org/10.3390/cancers14051187







