PET Imaging in Neuro-Oncology: An Update and Overview of a Rapidly Growing Area
Abstract
Simple Summary
Abstract
1. Introduction
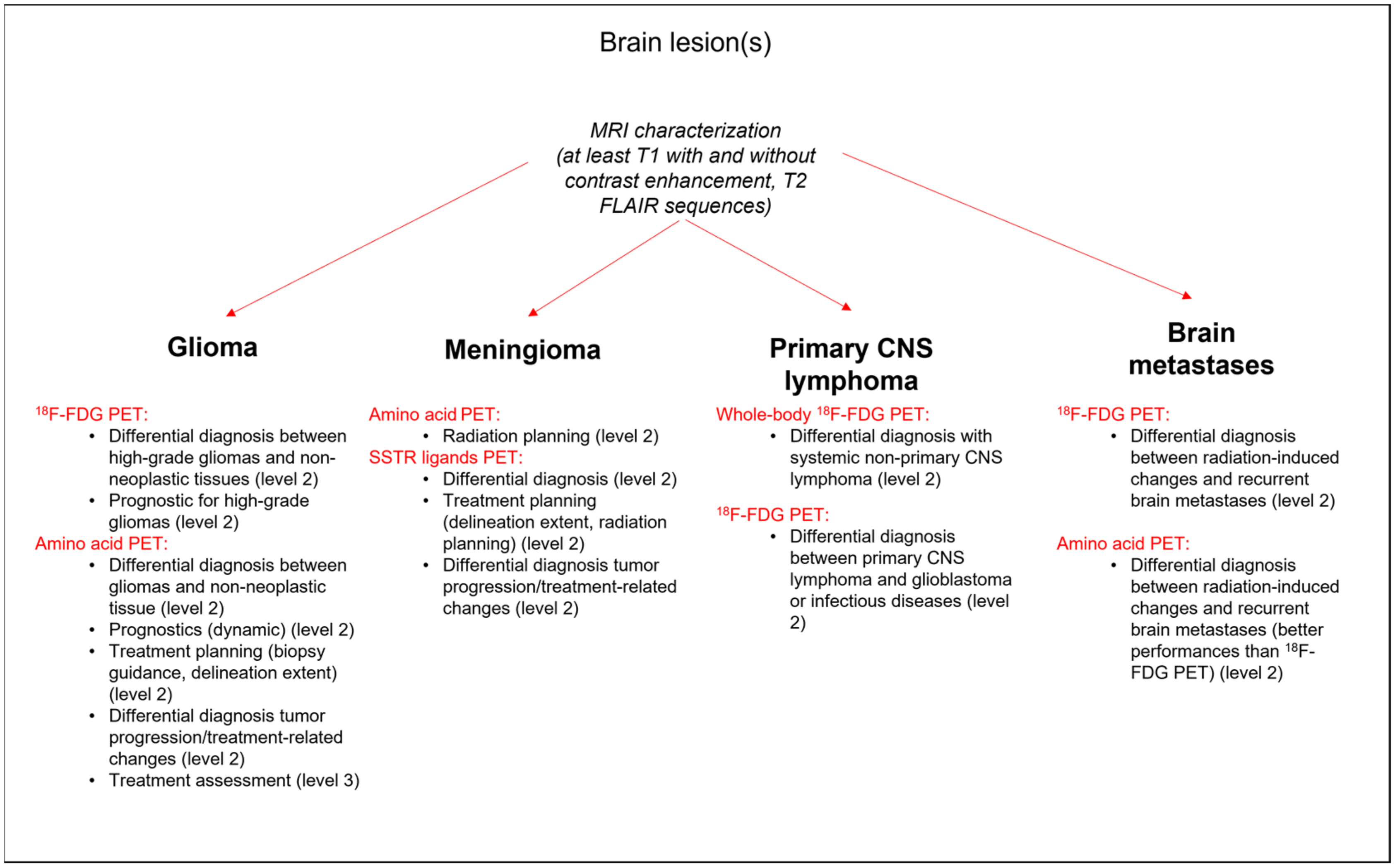
2. Current Uses of PET Imaging in Brain Tumors
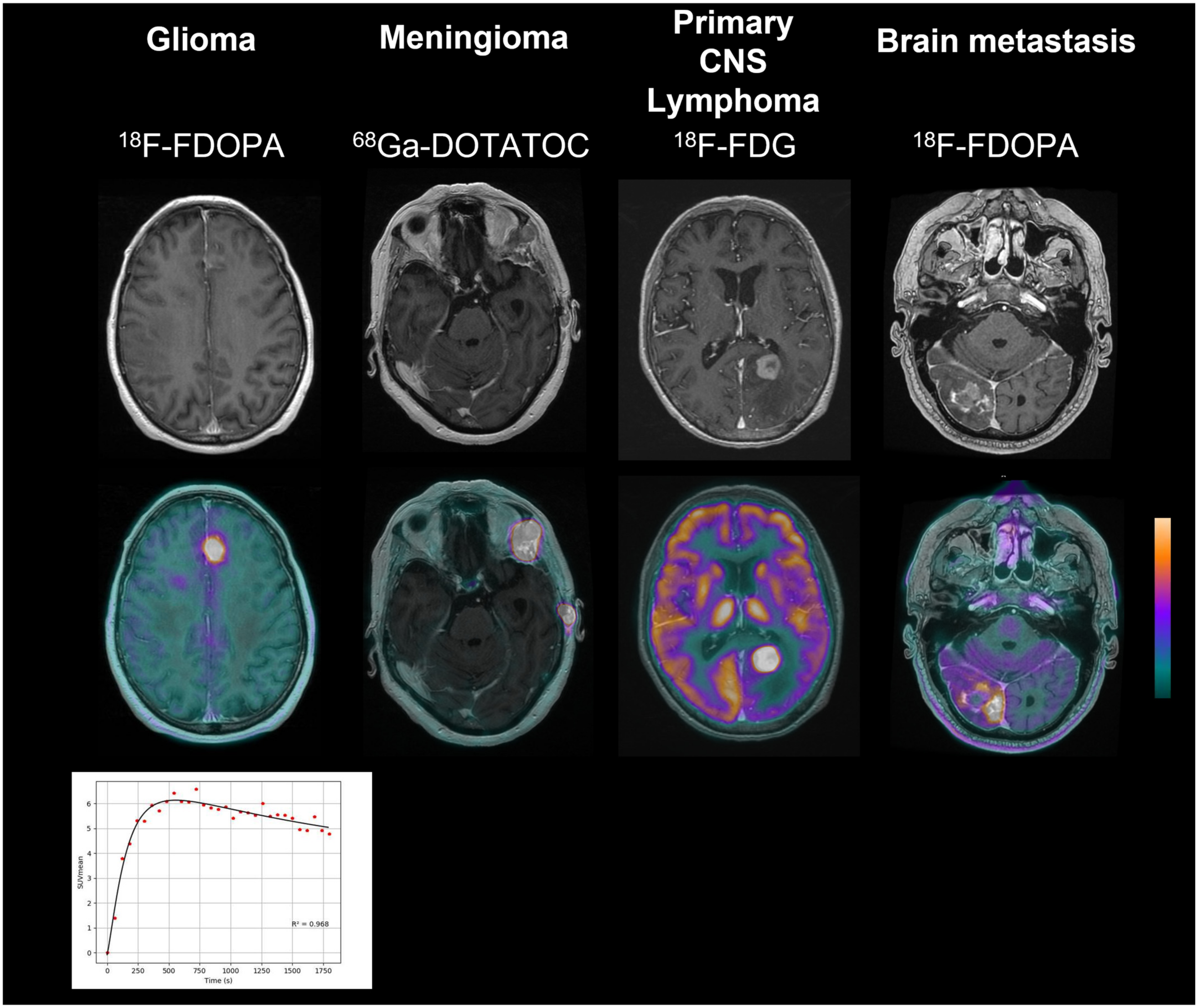
2.1. Glioma
2.1.1. Initial Characterization
2.1.2. Treatment Monitoring
2.1.3. Treatment Effectiveness
2.2. Meningioma
2.2.1. Initial Characterization
2.2.2. Treatment Monitoring
2.2.3. Treatment Effectiveness
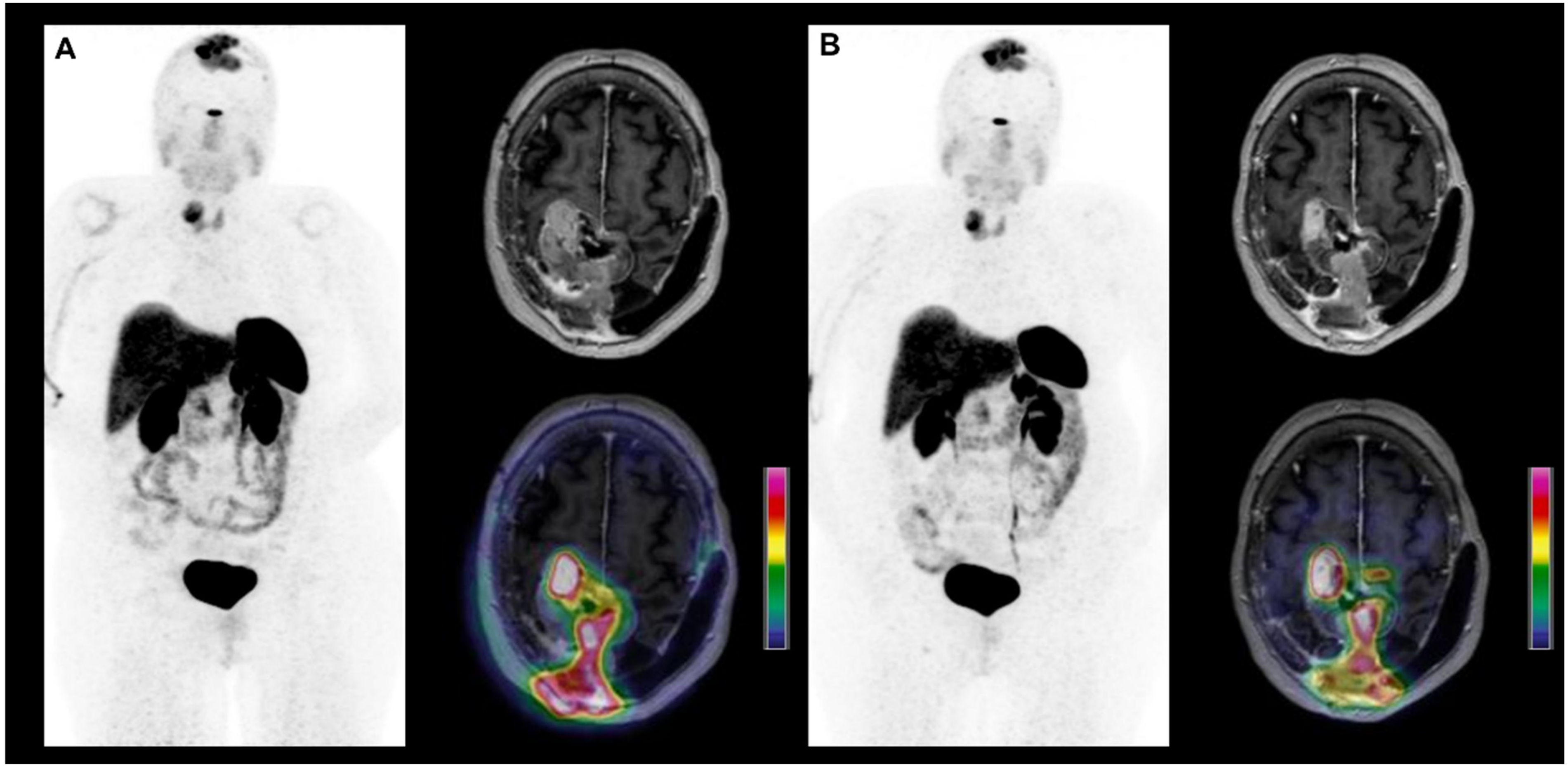
2.3. Primary CNS Lymphoma
2.3.1. Initial Characterization
2.3.2. Treatment Monitoring and Effectiveness
2.4. Brain Metastases
2.4.1. Treatment Monitoring
2.4.2. Initial Characterization
2.4.3. Treatment Effectiveness
3. Future Perspectives
3.1. Advances in Radiopharmaceuticals
3.1.1. PET Radiotracers for Tumor Perfusion
3.1.2. PET Radiotracers for Angiogenesis
3.1.3. PET Radiotracers for Neuroinflammation
3.1.4. Other PET Radiotracers
3.2. Advances in PET Systems and Image Analyses
3.2.1. PET Systems
3.2.2. Images Analyses
3.3. Translation to Peptide Receptor Radionuclide Therapy (PRRT)
3.3.1. PRRT in Meningiomas
3.3.2. PRRT in Gliomas
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Darlix, A.; Zouaoui, S.; Rigau, V.; Bessaoud, F.; Figarella-Branger, D.; Mathieu-Daudé, H.; Trétarre, B.; Bauchet, F.; Duffau, H.; Taillandier, L.; et al. Epidemiology for primary brain tumors: A nationwide population-based study. J. Neurooncol. 2017, 131, 525–546. [Google Scholar] [CrossRef] [PubMed]
- Ostrom, Q.T.; Cioffi, G.; Gittleman, H.; Patil, N.; Waite, K.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2012–2016. Neuro-Oncology 2019, 21, v1–v100. [Google Scholar] [CrossRef] [PubMed]
- Ostrom, Q.T.; Wright, C.H.; Barnholtz-Sloan, J.S. Brain metastases: Epidemiology. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2018; Volume 149, pp. 27–42. ISBN 978-0-12-811161-1. [Google Scholar]
- Verger, A.; Arbizu, J.; Law, I. Role of amino-acid PET in high-grade gliomas: Limitations and perspectives. Q. J. Nucl. Med. Mol. Imaging 2018, 62, 254–266. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Silverman, D.H.S. Advances in evaluation of primary brain tumors. Semin. Nucl. Med. 2008, 38, 240–250. [Google Scholar] [CrossRef] [PubMed]
- Colavolpe, C.; Metellus, P.; Mancini, J.; Barrie, M.; Béquet-Boucard, C.; Figarella-Branger, D.; Mundler, O.; Chinot, O.; Guedj, E. Independent prognostic value of pre-treatment 18-FDG-PET in high-grade gliomas. J. Neurooncol. 2012, 107, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Albert, N.L.; Weller, M.; Suchorska, B.; Galldiks, N.; Soffietti, R.; Kim, M.M.; La Fougère, C.; Pope, W.; Law, I.; Arbizu, J.; et al. Response Assessment in Neuro-Oncology working group and European Association for Neuro-Oncology recommendations for the clinical use of PET imaging in gliomas. Neuro-Oncology 2016, 18, 1199–1208. [Google Scholar] [CrossRef]
- Papin-Michault, C.; Bonnetaud, C.; Dufour, M.; Almairac, F.; Coutts, M.; Patouraux, S.; Virolle, T.; Darcourt, J.; Burel-Vandenbos, F. Study of LAT1 Expression in Brain Metastases: Towards a Better Understanding of the Results of Positron Emission Tomography Using Amino Acid Tracers. PLoS ONE 2016, 11, e0157139. [Google Scholar] [CrossRef]
- Habermeier, A.; Graf, J.; Sandhöfer, B.F.; Boissel, J.-P.; Roesch, F.; Closs, E.I. System L amino acid transporter LAT1 accumulates O-(2-fluoroethyl)-L-tyrosine (FET). Amino Acids 2015, 47, 335–344. [Google Scholar] [CrossRef]
- Okubo, S.; Zhen, H.-N.; Kawai, N.; Nishiyama, Y.; Haba, R.; Tamiya, T. Correlation of L-methyl-11C-methionine (MET) uptake with L-type amino acid transporter 1 in human gliomas. J. Neurooncol. 2010, 99, 217–225. [Google Scholar] [CrossRef]
- Vettermann, F.J.; Diekmann, C.; Weidner, L.; Unterrainer, M.; Suchorska, B.; Ruf, V.; Dorostkar, M.; Wenter, V.; Herms, J.; Tonn, J.-C.; et al. L-type amino acid transporter (LAT) 1 expression in 18F-FET-negative gliomas. EJNMMI Res. 2021, 11, 124. [Google Scholar] [CrossRef]
- Reubi, J.C.; Maurer, R.; Klijn, J.G.; Stefanko, S.Z.; Foekens, J.A.; Blaauw, G.; Blankenstein, M.A.; Lamberts, S.W. High incidence of somatostatin receptors in human meningiomas: Biochemical characterization. J. Clin. Endocrinol. Metab. 1986, 63, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Menke, J.R.; Raleigh, D.R.; Gown, A.M.; Thomas, S.; Perry, A.; Tihan, T. Somatostatin receptor 2a is a more sensitive diagnostic marker of meningioma than epithelial membrane antigen. Acta Neuropathol. 2015, 130, 441–443. [Google Scholar] [CrossRef] [PubMed]
- Rachinger, W.; Stoecklein, V.M.; Terpolilli, N.A.; Haug, A.R.; Ertl, L.; Pöschl, J.; Schüller, U.; Schichor, C.; Thon, N.; Tonn, J.-C. Increased 68Ga-DOTATATE Uptake in PET Imaging Discriminates Meningioma and Tumor-Free Tissue. J. Nucl. Med. 2015, 56, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Afshar-Oromieh, A.; Giesel, F.L.; Linhart, H.G.; Haberkorn, U.; Haufe, S.; Combs, S.E.; Podlesek, D.; Eisenhut, M.; Kratochwil, C. Detection of cranial meningiomas: Comparison of 68Ga-DOTATOC PET/CT and contrast-enhanced MRI. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 1409–1415. [Google Scholar] [CrossRef] [PubMed]
- Humbert, O.; Bourg, V.; Mondot, L.; Gal, J.; Bondiau, P.-Y.; Fontaine, D.; Saada-Bouzid, E.; Paquet, M.; Chardin, D.; Almairac, F.; et al. 18F-DOPA PET/CT in brain tumors: Impact on multidisciplinary brain tumor board decisions. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 558–568. [Google Scholar] [CrossRef]
- Kosaka, N.; Tsuchida, T.; Uematsu, H.; Kimura, H.; Okazawa, H.; Itoh, H. 18F-FDG PET of common enhancing malignant brain tumors. AJR Am. J. Roentgenol. 2008, 190, W365–W369. [Google Scholar] [CrossRef]
- Spence, A.M.; Muzi, M.; Mankoff, D.A.; O’Sullivan, S.F.; Link, J.M.; Lewellen, T.K.; Lewellen, B.; Pham, P.; Minoshima, S.; Swanson, K.; et al. 18F-FDG PET of gliomas at delayed intervals: Improved distinction between tumor and normal gray matter. J. Nucl. Med. 2004, 45, 1653–1659. [Google Scholar]
- Omuro, A.M.; Leite, C.C.; Mokhtari, K.; Delattre, J.-Y. Pitfalls in the diagnosis of brain tumours. Lancet Neurol. 2006, 5, 937–948. [Google Scholar] [CrossRef]
- De Witte, O.; Lefranc, F.; Levivier, M.; Salmon, I.; Brotchi, J.; Goldman, S. FDG-PET as a Prognostic Factor in High-Grade Astrocytoma. J. Neurooncol. 2000, 49, 157–163. [Google Scholar] [CrossRef]
- Colavolpe, C.; Chinot, O.; Metellus, P.; Mancini, J.; Barrie, M.; Bequet-Boucard, C.; Tabouret, E.; Mundler, O.; Figarella-Branger, D.; Guedj, E. FDG-PET Predicts Survival in Recurrent High-Grade Gliomas Treated with Bevacizumab and Irinotecan. Neuro-Oncology 2012, 14, 649–657. [Google Scholar] [CrossRef]
- Dunet, V.; Pomoni, A.; Hottinger, A.; Nicod-Lalonde, M.; Prior, J.O. Performance of 18F-FET versus 18F-FDG-PET for the diagnosis and grading of brain tumors: Systematic review and meta-analysis. Neuro-Oncology 2016, 18, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Zaragori, T.; Castello, A.; Guedj, E.; Girard, A.; Galldiks, N.; Albert, N.L.; Lopci, E.; Verger, A. Photopenic Defects in Gliomas With Amino-Acid PET and Relative Prognostic Value: A Multicentric 11C-Methionine and 18F-FDOPA PET Experience. Clin. Nucl. Med. 2021, 46, e36–e37. [Google Scholar] [CrossRef] [PubMed]
- Sala, Q.; Metellus, P.; Taieb, D.; Kaphan, E.; Figarella-Branger, D.; Guedj, E. 18F-DOPA, a clinically available PET tracer to study brain inflammation? Clin. Nucl. Med. 2014, 39, e283–e285. [Google Scholar] [CrossRef] [PubMed]
- Verger, A.; Stoffels, G.; Bauer, E.K.; Lohmann, P.; Blau, T.; Fink, G.R.; Neumaier, B.; Shah, N.J.; Langen, K.-J.; Galldiks, N. Static and dynamic (18)F-FET PET for the characterization of gliomas defined by IDH and 1p/19q status. Eur. J. Nucl. Med. Mol. Imaging 2017, 45, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Ginet, M.; Zaragori, T.; Marie, P.-Y.; Roch, V.; Gauchotte, G.; Rech, F.; Blonski, M.; Lamiral, Z.; Taillandier, L.; Imbert, L.; et al. Integration of dynamic parameters in the analysis of 18F-FDopa PET imaging improves the prediction of molecular features of gliomas. Eur. J. Nucl. Med. Mol. Imaging 2019, 47, 1381–1390. [Google Scholar] [CrossRef] [PubMed]
- Nomura, Y.; Asano, Y.; Shinoda, J.; Yano, H.; Ikegame, Y.; Kawasaki, T.; Nakayama, N.; Maruyama, T.; Muragaki, Y.; Iwama, T. Characteristics of time-activity curves obtained from dynamic 11C-methionine PET in common primary brain tumors. J. Neurooncol. 2018, 138, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Kaiser, L.; Holzgreve, A.; Ruf, V.C.; Suchorska, B.; Wenter, V.; Quach, S.; Herms, J.; Bartenstein, P.; Tonn, J.C.; et al. Albert Prediction of TERTp-mutation status in IDH-wildtype high-grade gliomas using pre-treatment dynamic 18F-FET PET radiomics. Eur. J. Nucl. Med. 2021, 48, 4415–4425. [Google Scholar] [CrossRef]
- Lohmann, P.; Herzog, H.; Rota Kops, E.; Stoffels, G.; Judov, N.; Filss, C.; Galldiks, N.; Tellmann, L.; Weiss, C.; Sabel, M.; et al. Dual-time-point O-(2-[(18)F]fluoroethyl)-L-tyrosine PET for grading of cerebral gliomas. Eur. Radiol. 2015, 25, 3017–3024. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro-Oncology 2021, 23, 1231–1251. [Google Scholar] [CrossRef]
- Pöpperl, G.; Kreth, F.W.; Mehrkens, J.H.; Herms, J.; Seelos, K.; Koch, W.; Gildehaus, F.J.; Kretzschmar, H.A.; Tonn, J.C.; Tatsch, K. FET PET for the evaluation of untreated gliomas: Correlation of FET uptake and uptake kinetics with tumour grading. Eur. J. Nucl. Med. Mol. Imaging 2007, 34, 1933–1942. [Google Scholar] [CrossRef]
- Kunz, M.; Albert, N.L.; Unterrainer, M.; La Fougere, C.; Egensperger, R.; Schüller, U.; Lutz, J.; Kreth, S.; Tonn, J.-C.; Kreth, F.-W.; et al. Dynamic 18F-FET PET is a powerful imaging biomarker in gadolinium-negative gliomas. Neuro-Oncology 2019, 21, 274–284. [Google Scholar] [CrossRef] [PubMed]
- Pafundi, D.H.; Laack, N.N.; Youland, R.S.; Parney, I.F.; Lowe, V.J.; Giannini, C.; Kemp, B.J.; Grams, M.P.; Morris, J.M.; Hoover, J.M.; et al. Biopsy validation of 18F-DOPA PET and biodistribution in gliomas for neurosurgical planning and radiotherapy target delineation: Results of a prospective pilot study. Neuro-Oncology 2013, 15, 1058–1067. [Google Scholar] [CrossRef] [PubMed]
- Ledezma, C.J.; Chen, W.; Sai, V.; Freitas, B.; Cloughesy, T.; Czernin, J.; Pope, W. 18F-FDOPA PET/MRI fusion in patients with primary/recurrent gliomas: Initial experience. Eur. J. Radiol. 2009, 71, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Nariai, T.; Momose, T.; Aoyagi, M.; Maehara, T.; Tomori, T.; Yoshino, Y.; Nagaoka, T.; Ishiwata, K.; Ishii, K.; et al. Glioma surgery using a multimodal navigation system with integrated metabolic images: Clinical article. J. Neurosurg. 2009, 110, 163–172. [Google Scholar] [CrossRef]
- Suchorska, B.; Jansen, N.L.; Linn, J.; Kretzschmar, H.; Janssen, H.; Eigenbrod, S.; Simon, M.; Pöpperl, G.; Kreth, F.W.; La Fougere, C.; et al. Biological tumor volume in 18FET-PET before radiochemotherapy correlates with survival in GBM. Neurology 2015, 84, 710–719. [Google Scholar] [CrossRef]
- Seidlitz, A.; Beuthien-Baumann, B.; Löck, S.; Jentsch, C.; Platzek, I.; Zöphel, K.; Linge, A.; Kotzerke, J.; Petr, J.; Van den Hoff, J.; et al. Final Results of the Prospective Biomarker Trial PETra: [11C]-MET-Accumulation in Postoperative PET/MRI Predicts Outcome after Radiochemotherapy in Glioblastoma. Clin. Cancer Res. 2021, 27, 1351–1360. [Google Scholar] [CrossRef]
- Kunz, M.; Thon, N.; Eigenbrod, S.; Hartmann, C.; Egensperger, R.; Herms, J.; Geisler, J.; La Fougere, C.; Lutz, J.; Linn, J.; et al. Hot spots in dynamic (18)FET-PET delineate malignant tumor parts within suspected WHO grade II gliomas. Neuro-Oncology 2011, 13, 307–316. [Google Scholar] [CrossRef]
- Lee, I.H.; Piert, M.; Gomez-Hassan, D.; Junck, L.; Rogers, L.; Hayman, J.; Ten Haken, R.K.; Lawrence, T.S.; Cao, Y.; Tsien, C. Association of 11C-methionine PET uptake with site of failure after concurrent temozolomide and radiation for primary glioblastoma multiforme. Int. J. Radiat. Oncol. Biol. Phys. 2009, 73, 479–485. [Google Scholar] [CrossRef]
- Law, I.; Albert, N.L.; Arbizu, J.; Boellaard, R.; Drzezga, A.; Galldiks, N.; La Fougère, C.; Langen, K.-J.; Lopci, E.; Lowe, V.; et al. Joint EANM/EANO/RANO practice guidelines/SNMMI procedure standards for imaging of gliomas using PET with radiolabelled amino acids and [18F]FDG: Version 1.0. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 540–557. [Google Scholar] [CrossRef]
- Brandsma, D.; Stalpers, L.; Taal, W.; Sminia, P.; Van den Bent, M.J. Clinical features, mechanisms, and management of pseudoprogression in malignant gliomas. Lancet Oncol. 2008, 9, 453–461. [Google Scholar] [CrossRef]
- Nihashi, T.; Dahabreh, I.J.; Terasawa, T. Diagnostic accuracy of PET for recurrent glioma diagnosis: A meta-analysis. AJNR Am. J. Neuroradiol. 2013, 34, 944–950. [Google Scholar] [CrossRef] [PubMed]
- Galldiks, N.; Dunkl, V.; Stoffels, G.; Hutterer, M.; Rapp, M.; Sabel, M.; Reifenberger, G.; Kebir, S.; Dorn, F.; Blau, T.; et al. Diagnosis of pseudoprogression in patients with glioblastoma using O-(2-[18F]fluoroethyl)-L-tyrosine PET. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 685–695. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, K.; Czernin, J.; Cloughesy, T.; Lai, A.; Pomykala, K.L.; Benz, M.R.; Buck, A.K.; Phelps, M.E.; Chen, W. Comparison of visual and semiquantitative analysis of 18F-FDOPA-PET/CT for recurrence detection in glioblastoma patients. Neuro-Oncology 2014, 16, 603–609. [Google Scholar] [CrossRef] [PubMed]
- Galldiks, N.; Stoffels, G.; Filss, C.; Rapp, M.; Blau, T.; Tscherpel, C.; Ceccon, G.; Dunkl, V.; Weinzierl, M.; Stoffel, M.; et al. The use of dynamic O-(2-18F-fluoroethyl)-l-tyrosine PET in the diagnosis of patients with progressive and recurrent glioma. Neuro-Oncology 2015, 17, 1293–1300. [Google Scholar] [CrossRef] [PubMed]
- Maurer, G.D.; Brucker, D.P.; Stoffels, G.; Filipski, K.; Filss, C.P.; Mottaghy, F.M.; Galldiks, N.; Steinbach, J.P.; Hattingen, E.; Langen, K.-J. 18F-FET PET Imaging in Differentiating Glioma Progression from Treatment-Related Changes: A Single-Center Experience. J. Nucl. Med. 2020, 61, 505–511. [Google Scholar] [CrossRef]
- Zaragori, T.; Ginet, M.; Marie, P.-Y.; Roch, V.; Grignon, R.; Gauchotte, G.; Rech, F.; Blonski, M.; Lamiral, Z.; Taillandier, L.; et al. Use of Static and Dynamic [18F]-FDopa PET Parameters for Detecting Patients with Glioma Recurrence or Progression. EJNMMI Res. 2020, 10, 56. [Google Scholar] [CrossRef]
- Yonezawa, S.; Miwa, K.; Shinoda, J.; Nomura, Y.; Asano, Y.; Nakayama, N.; Ohe, N.; Yano, H.; Iwama, T. Bevacizumab treatment leads to observable morphological and metabolic changes in brain radiation necrosis. J. Neurooncol. 2014, 119, 101–109. [Google Scholar] [CrossRef]
- Kawasaki, T.; Miwa, K.; Shinoda, J.; Asano, Y.; Takei, H.; Ikegame, Y.; Yokoyama, K.; Yano, H.; Iwama, T. Dissociation Between 11C-Methionine-Positron Emission Tomography and Gadolinium-Enhanced Magnetic Resonance Imaging in Longitudinal Features of Glioblastoma After Postoperative Radiotherapy. World Neurosurg. 2019, 125, 93–100. [Google Scholar] [CrossRef]
- Galldiks, N.; Langen, K.-J.; Holy, R.; Pinkawa, M.; Stoffels, G.; Nolte, K.W.; Kaiser, H.J.; Filss, C.P.; Fink, G.R.; Coenen, H.H.; et al. Assessment of Treatment Response in Patients with Glioblastoma Using O-(2-18F-Fluoroethyl)-L-Tyrosine PET in Comparison to MRI. J. Nucl. Med. 2012, 53, 1048–1057. [Google Scholar] [CrossRef]
- Ceccon, G.; Lohmann, P.; Werner, J.-M.; Tscherpel, C.; Dunkl, V.; Stoffels, G.; Rosen, J.; Rapp, M.; Sabel, M.; Herrlinger, U.; et al. Early Treatment Response Assessment Using 18F-FET PET Compared with Contrast-Enhanced MRI in Glioma Patients After Adjuvant Temozolomide Chemotherapy. J. Nucl. Med. 2021, 62, 918–925. [Google Scholar] [CrossRef]
- Galldiks, N.; Rapp, M.; Stoffels, G.; Fink, G.R.; Shah, N.J.; Coenen, H.H.; Sabel, M.; Langen, K.-J. Response assessment of bevacizumab in patients with recurrent malignant glioma using [18F]Fluoroethyl-L-tyrosine PET in comparison to MRI. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Schwarzenberg, J.; Czernin, J.; Cloughesy, T.F.; Ellingson, B.M.; Pope, W.B.; Grogan, T.; Elashoff, D.; Geist, C.; Silverman, D.H.S.; Phelps, M.E.; et al. Treatment response evaluation using 18F-FDOPA PET in patients with recurrent malignant glioma on bevacizumab therapy. Clin. Cancer Res. 2014, 20, 3550–3559. [Google Scholar] [CrossRef] [PubMed]
- Galldiks, N.; Niyazi, M.; Grosu, A.L.; Kocher, M.; Langen, K.-J.; Law, I.; Minniti, G.; Kim, M.M.; Tsien, C.; Dhermain, F.; et al. Contribution of PET imaging to radiotherapy planning and monitoring in glioma patients-a report of the PET/RANO group. Neuro-Oncology 2021, 23, 881–893. [Google Scholar] [CrossRef]
- Roelcke, U.; Wyss, M.T.; Nowosielski, M.; Rudà, R.; Roth, P.; Hofer, S.; Galldiks, N.; Crippa, F.; Weller, M.; Soffietti, R. Amino acid positron emission tomography to monitor chemotherapy response and predict seizure control and progression-free survival in WHO grade II gliomas. Neuro-Oncology 2016, 18, 744–751. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Suchorska, B.; Unterrainer, M.; Biczok, A.; Sosnova, M.; Forbrig, R.; Bartenstein, P.; Tonn, J.-C.; Albert, N.L.; Kreth, F.-W. 18F-FET-PET as a biomarker for therapy response in non-contrast enhancing glioma following chemotherapy. J. Neurooncol. 2018, 139, 721–730. [Google Scholar] [CrossRef] [PubMed]
- Galldiks, N.; Albert, N.L.; Sommerauer, M.; Grosu, A.L.; Ganswindt, U.; Law, I.; Preusser, M.; Le Rhun, E.; Vogelbaum, M.A.; Zadeh, G.; et al. PET imaging in patients with meningioma—report of the RANO/PET Group. Neuro-Oncology 2017, 19, 1576–1587. [Google Scholar] [CrossRef] [PubMed]
- Dutour, A.; Kumar, U.; Panetta, R.; Ouafik, L.; Fina, F.; Sasi, R.; Patel, Y.C. Expression of somatostatin receptor subtypes in human brain tumors. Int. J. Cancer 1998, 76, 620–627. [Google Scholar] [CrossRef]
- Graef, J.; Furth, C.; Kluge, A.K.; Acker, G.; Kord, M.; Zimmermann, Z.; Amthauer, H.; Makowski, M.; Loebel, F.; Vajkoczy, P.; et al. 68Ga-DOTATOC-PET/MRI—A Secure One-Stop Shop Imaging Tool for Robotic Radiosurgery Treatment Planning in Patients with Optic Nerve Sheath Meningioma. Cancers 2021, 13, 3305. [Google Scholar] [CrossRef]
- Grosu, A.-L.; Weber, W.A.; Astner, S.T.; Adam, M.; Krause, B.J.; Schwaiger, M.; Molls, M.; Nieder, C. 11C-methionine PET improves the target volume delineation of meningiomas treated with stereotactic fractionated radiotherapy. Int. J. Radiat. Oncol. *Biol. *Phys. 2006, 66, 339–344. [Google Scholar] [CrossRef]
- Astner, S.T.; Dobrei-Ciuchendea, M.; Essler, M.; Bundschuh, R.A.; Sai, H.; Schwaiger, M.; Molls, M.; Weber, W.A.; Grosu, A.-L. Effect of 11C-Methionine-Positron Emission Tomography on Gross Tumor Volume Delineation in Stereotactic Radiotherapy of Skull Base Meningiomas. Int. J. Radiat. Oncol. *Biol. *Phys. 2008, 72, 1161–1167. [Google Scholar] [CrossRef]
- Kowalski, E.S.; Khairnar, R.; Gryaznov, A.A.; Kesari, V.; Koroulakis, A.; Raghavan, P.; Chen, W.; Woodworth, G.; Mishra, M. 68Ga-DOTATATE PET-CT as a tool for radiation planning and evaluating treatment responses in the clinical management of meningiomas. Radiat. Oncol. 2021, 16, 151. [Google Scholar] [CrossRef] [PubMed]
- Milker-Zabel, S.; Zabel-du Bois, A.; Henze, M.; Huber, P.; Schulz-Ertner, D.; Hoess, A.; Haberkorn, U.; Debus, J. Improved target volume definition for fractionated stereotactic radiotherapy in patients with intracranial meningiomas by correlation of CT, MRI, and [68Ga]-DOTATOC-PET. Int. J. Radiat. Oncol. *Biol. *Phys. 2006, 65, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Mahase, S.S.; Roth O’Brien, D.A.; No, D.; Roytman, M.; Skafida, M.E.; Lin, E.; Karakatsanis, N.A.; Osborne, J.R.; Brandmaier, A.; Pannullo, S.C.; et al. [68Ga]-DOTATATE PET/MRI as an adjunct imaging modality for radiation treatment planning of meningiomas. Neuro-Oncol. Adv. 2021, 3, vdab012. [Google Scholar] [CrossRef] [PubMed]
- Floeth, F.W.; Pauleit, D.; Sabel, M.; Stoffels, G.; Reifenberger, G.; Riemenschneider, M.J.; Jansen, P.; Coenen, H.H.; Steiger, H.-J.; Langen, K.-J. Prognostic value of O-(2-18F-fluoroethyl)-L-tyrosine PET and MRI in low-grade glioma. J. Nucl. Med. 2007, 48, 519–527. [Google Scholar] [CrossRef]
- Dittmar, J.O.; Kratochwil, C.; Dittmar, A.; Welzel, T.; Habermehl, D.; Rieken, S.; Giesel, F.L.; Haberkorn, U.; Debus, J.; Combs, S.E. First intraindividual comparison of contrast-enhanced MRI, FET- and DOTATOC- PET in patients with intracranial meningiomas. Radiat. Oncol. 2017, 12, 169. [Google Scholar] [CrossRef]
- Seystahl, K.; Stoecklein, V.; Schüller, U.; Rushing, E.; Nicolas, G.; Schäfer, N.; Ilhan, H.; Pangalu, A.; Weller, M.; Tonn, J.-C.; et al. Somatostatin-receptor-targeted radionuclide therapy for progressive meningioma: Benefit linked to 68Ga-DOTATATE/-TOC uptake. Neuro-Oncology 2016, 18, 1538–1547. [Google Scholar] [CrossRef]
- Sommerauer, M.; Burkhardt, J.-K.; Frontzek, K.; Rushing, E.; Buck, A.; Krayenbuehl, N.; Weller, M.; Schaefer, N.; Kuhn, F.P. 68Gallium-DOTATATE PET in meningioma: A reliable predictor of tumor growth rate? Neuro-Oncology 2016, 18, 1021–1027. [Google Scholar] [CrossRef]
- Graillon, T.; Romano, D.; Defilles, C.; Saveanu, A.; Mohamed, A.; Figarella-Branger, D.; Roche, P.-H.; Fuentes, S.; Chinot, O.; Dufour, H.; et al. Octreotide therapy in meningiomas: In vitro study, clinical correlation, and literature review. J. Neurosurg. 2017, 127, 660–669. [Google Scholar] [CrossRef]
- Kunz, W.G.; Jungblut, L.M.; Kazmierczak, P.M.; Vettermann, F.J.; Bollenbacher, A.; Tonn, J.C.; Schichor, C.; Rominger, A.; Albert, N.L.; Bartenstein, P.; et al. Improved Detection of Transosseous Meningiomas Using 68Ga-DOTATATE PET/CT Compared with Contrast-Enhanced MRI. J. Nucl. Med. 2017, 58, 1580–1587. [Google Scholar] [CrossRef]
- Houillier, C.; Soussain, C.; Ghesquières, H.; Soubeyran, P.; Chinot, O.; Taillandier, L.; Lamy, T.; Choquet, S.; Ahle, G.; Damaj, G.; et al. Management and outcome of primary CNS lymphoma in the modern era: An LOC network study. Neurology 2020, 94, e1027–e1039. [Google Scholar] [CrossRef]
- Barajas, R.F.; Politi, L.S.; Anzalone, N.; Schöder, H.; Fox, C.P.; Boxerman, J.L.; Kaufmann, T.J.; Quarles, C.C.; Ellingson, B.M.; Auer, D.; et al. Consensus recommendations for MRI and PET imaging of primary central nervous system lymphoma: Guideline statement from the International Primary CNS Lymphoma Collaborative Group (IPCG). Neuro-Oncology 2021, 23, 1056–1071. [Google Scholar] [CrossRef] [PubMed]
- Makino, K.; Hirai, T.; Nakamura, H.; Murakami, R.; Kitajima, M.; Shigematsu, Y.; Nakashima, R.; Shiraishi, S.; Uetani, H.; Iwashita, K.; et al. Does adding FDG-PET to MRI improve the differentiation between primary cerebral lymphoma and glioblastoma? Observer performance study. Ann. Nucl. Med. 2011, 25, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Hatakeyama, J.; Ono, T.; Takahashi, M.; Oda, M.; Shimizu, H. Differentiating between Primary Central Nervous System Lymphoma and Glioblastoma: The Diagnostic Value of Combining 18F-fluorodeoxyglucose Positron Emission Tomography with Arterial Spin Labeling. Neurol. Med. Chir. 2021, 61, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Gupta, T.; Manjali, J.J.; Kannan, S.; Purandare, N.; Rangarajan, V. Diagnostic Performance of Pretreatment 18F-Fluorodeoxyglucose Positron Emission Tomography With or Without Computed Tomography in Patients With Primary Central Nervous System Lymphoma: Updated Systematic Review and Diagnostic Test Accuracy Meta-analyses. Clin. Lymphoma Myeloma Leuk. 2021, 21, 497–507. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Sun, J.; Bai, H.X.; Tao, Y.; Tang, X.; States, L.J.; Zhang, Z.; Zhou, J.; Farwell, M.D.; Zhang, P.; et al. Diagnostic accuracy of SPECT, PET, and MRS for primary central nervous system lymphoma in HIV patients: A systematic review and meta-analysis. Medicine 2017, 96, e6676. [Google Scholar] [CrossRef]
- Krebs, S.; Mauguen, A.; Yildirim, O.; Hatzoglou, V.; Francis, J.H.; Schaff, L.R.; Mellinghoff, I.K.; Schöder, H.; Grommes, C. Prognostic value of [18F]FDG PET/CT in patients with CNS lymphoma receiving ibrutinib-based therapies. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 3940–3950. [Google Scholar] [CrossRef]
- Palmedo, H.; Urbach, H.; Bender, H.; Schlegel, U.; Schmidt-Wolf, I.G.H.; Matthies, A.; Linnebank, M.; Joe, A.; Bucerius, J.; Biersack, H.-J.; et al. FDG-PET in immunocompetent patients with primary central nervous system lymphoma: Correlation with MRI and clinical follow-up. Eur. J. Nucl. Med. Mol. Imaging 2006, 33, 164–168. [Google Scholar] [CrossRef]
- Jo, J.-C.; Yoon, D.H.; Kim, S.; Lee, K.; Kang, E.H.; Park, J.S.; Ryu, J.-S.; Huh, J.; Park, C.-S.; Kim, J.H.; et al. Interim 18F-FGD PET/CT may not predict the outcome in primary central nervous system lymphoma patients treated with sequential treatment with methotrexate and cytarabine. Ann. Hematol. 2017, 96, 1509–1515. [Google Scholar] [CrossRef]
- Birsen, R.; Blanc, E.; Willems, L.; Burroni, B.; Legoff, M.; Le Ray, E.; Pilorge, S.; Salah, S.; Quentin, A.; Deau, B.; et al. Prognostic value of early 18F-FDG PET scanning evaluation in immunocompetent primary CNS lymphoma patients. Oncotarget 2018, 9, 16822–16831. [Google Scholar] [CrossRef]
- Ahn, S.-Y.; Kwon, S.Y.; Jung, S.-H.; Ahn, J.-S.; Yoo, S.W.; Min, J.-J.; Bom, H.-S.; Ki, S.Y.; Kim, H.-J.; Lee, J.-J.; et al. Prognostic Significance of Interim 11C-Methionine PET/CT in Primary Central Nervous System Lymphoma. Clin. Nucl. Med. 2018, 43, e259–e264. [Google Scholar] [CrossRef]
- Galldiks, N.; Langen, K.-J.; Albert, N.L.; Chamberlain, M.; Soffietti, R.; Kim, M.M.; Law, I.; Le Rhun, E.; Chang, S.; Schwarting, J.; et al. PET imaging in patients with brain metastasis—report of the RANO/PET group. Neuro-Oncology 2019, 21, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Minniti, G.; Clarke, E.; Lanzetta, G.; Osti, M.F.; Trasimeni, G.; Bozzao, A.; Romano, A.; Enrici, R.M. Stereotactic radiosurgery for brain metastases: Analysis of outcome and risk of brain radionecrosis. Radiat. Oncol. 2011, 6, 48. [Google Scholar] [CrossRef] [PubMed]
- Chao, S.T.; Suh, J.H.; Raja, S.; Lee, S.Y.; Barnett, G. The sensitivity and specificity of FDG PET in distinguishing recurrent brain tumor from radionecrosis in patients treated with stereotactic radiosurgery. Int. J. Cancer 2001, 96, 191–197. [Google Scholar] [CrossRef]
- Tomura, N.; Kokubun, M.; Saginoya, T.; Mizuno, Y.; Kikuchi, Y. Differentiation between Treatment-Induced Necrosis and Recurrent Tumors in Patients with Metastatic Brain Tumors: Comparison among 11C-Methionine-PET, FDG-PET, MR Permeability Imaging, and MRI-ADC—Preliminary Results. AJNR Am. J. Neuroradiol. 2017, 38, 1520–1527. [Google Scholar] [CrossRef] [PubMed]
- Horky, L.L.; Hsiao, E.M.; Weiss, S.E.; Drappatz, J.; Gerbaudo, V.H. Dual phase FDG-PET imaging of brain metastases provides superior assessment of recurrence versus post-treatment necrosis. J. Neurooncol. 2011, 103, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Cicone, F.; Minniti, G.; Romano, A.; Papa, A.; Scaringi, C.; Tavanti, F.; Bozzao, A.; Maurizi Enrici, R.; Scopinaro, F. Accuracy of F-DOPA PET and perfusion-MRI for differentiating radionecrotic from progressive brain metastases after radiosurgery. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Minamimoto, R.; Saginoya, T.; Kondo, C.; Tomura, N.; Ito, K.; Matsuo, Y.; Matsunaga, S.; Shuto, T.; Akabane, A.; Miyata, Y.; et al. Differentiation of Brain Tumor Recurrence from Post-Radiotherapy Necrosis with 11C-Methionine PET: Visual Assessment versus Quantitative Assessment. PLoS ONE 2015, 10, e0132515. [Google Scholar] [CrossRef] [PubMed]
- Lizarraga, K.J.; Allen-Auerbach, M.; Czernin, J.; DeSalles, A.A.F.; Yong, W.H.; Phelps, M.E.; Chen, W. (18)F-FDOPA PET for differentiating recurrent or progressive brain metastatic tumors from late or delayed radiation injury after radiation treatment. J. Nucl. Med. 2014, 55, 30–36. [Google Scholar] [CrossRef]
- Ceccon, G.; Lohmann, P.; Stoffels, G.; Judov, N.; Filss, C.P.; Rapp, M.; Bauer, E.; Hamisch, C.; Ruge, M.I.; Kocher, M.; et al. Dynamic O-(2-18F-fluoroethyl)-L-tyrosine positron emission tomography differentiates brain metastasis recurrence from radiation injury after radiotherapy. Neuro-Oncology 2016, 19, 281–288. [Google Scholar] [CrossRef]
- Cicone, F.; Carideo, L.; Scaringi, C.; Romano, A.; Mamede, M.; Papa, A.; Tofani, A.; Cascini, G.L.; Bozzao, A.; Scopinaro, F.; et al. Long-term metabolic evolution of brain metastases with suspected radiation necrosis following stereotactic radiosurgery: Longitudinal assessment by F-DOPA PET. Neuro-Oncology 2021, 23, 1024–1034. [Google Scholar] [CrossRef]
- Heinzel, A.; Müller, D.; Yekta-Michael, S.S.; Ceccon, G.; Langen, K.-J.; Mottaghy, F.M.; Wiesmann, M.; Kocher, M.; Hattingen, E.; Galldiks, N. O-(2-18F-fluoroethyl)-L-tyrosine PET for evaluation of brain metastasis recurrence after radiotherapy: An effectiveness and cost-effectiveness analysis. Neuro-Oncology 2017, 19, 1271–1278. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Jin, G.; Su, D. Comparison of Gadolinium-enhanced MRI and 18FDG PET/PET-CT for the diagnosis of brain metastases in lung cancer patients: A meta-analysis of 5 prospective studies. Oncotarget 2017, 8, 35743–35749. [Google Scholar] [CrossRef] [PubMed]
- Unterrainer, M.; Galldiks, N.; Suchorska, B.; Kowalew, L.-C.; Wenter, V.; Schmid-Tannwald, C.; Niyazi, M.; Bartenstein, P.; Langen, K.-J.; Albert, N.L. 18F-FET PET Uptake Characteristics in Patients with Newly Diagnosed and Untreated Brain Metastasis. J. Nucl. Med. 2017, 58, 584–589. [Google Scholar] [CrossRef] [PubMed]
- Meyer, H.S.; Liesche-Starnecker, F.; Mustafa, M.; Yakushev, I.; Wiestler, B.; Meyer, B.; Gempt, J. [18F]FET PET Uptake Indicates High Tumor and Low Necrosis Content in Brain Metastasis. Cancers 2021, 13, 355. [Google Scholar] [CrossRef] [PubMed]
- Purandare, N.C.; Puranik, A.; Shah, S.; Agrawal, A.; Gupta, T.; Moiyadi, A.; Shetty, P.; Shridhar, E.; Jalali, R.; Rangarajan, V. Common malignant brain tumors: Can 18F-FDG PET/CT aid in differentiation? Nucl. Med. Commun. 2017, 38, 1109–1116. [Google Scholar] [CrossRef] [PubMed]
- Galldiks, N.; Abdulla, D.S.Y.; Scheffler, M.; Wolpert, F.; Werner, J.-M.; Hüllner, M.; Stoffels, G.; Schweinsberg, V.; Schlaak, M.; Kreuzberg, N.; et al. Treatment Monitoring of Immunotherapy and Targeted Therapy Using 18F-FET PET in Patients with Melanoma and Lung Cancer Brain Metastases: Initial Experiences. J. Nucl. Med. 2021, 62, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Laudicella, R.; Quartuccio, N.; Argiroffi, G.; Alongi, P.; Baratto, L.; Califaretti, E.; Frantellizzi, V.; De Vincentis, G.; Del Sole, A.; Evangelista, L.; et al. Unconventional non-amino acidic PET radiotracers for molecular imaging in gliomas. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 3925–3939. [Google Scholar] [CrossRef]
- McKenzie, C.G.; Lenzi, G.L.; Jones, T.; Moss, S. Radioactive oxygen 15O studies in cerebral neoplasms. J. R. Soc. Med. 1978, 71, 417–425. [Google Scholar] [CrossRef]
- Xiangsong, Z.; Changhong, L.; Weian, C.; Dong, Z. PET Imaging of cerebral astrocytoma with 13N-ammonia. J. Neurooncol. 2006, 78, 145–151. [Google Scholar] [CrossRef]
- Verger, A.; Imbert, L.; Zaragori, T. Dynamic amino-acid PET in neuro-oncology: A prognostic tool becomes essential. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 4129–4132. [Google Scholar] [CrossRef]
- Cai, W.; Chen, X. Multimodality Molecular Imaging of Tumor Angiogenesis. J. Nucl. Med. 2008, 49, 113S–128S. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, N.; Gao, S.; Hu, X.; Zhao, W.; Tao, R.; Chen, Z.; Zheng, J.; Sun, X.; Xu, L.; et al. Can an 18F-ALF-NOTA-PRGD2 PET/CT Scan Predict Treatment Sensitivity to Concurrent Chemoradiotherapy in Patients with Newly Diagnosed Glioblastoma? J. Nucl. Med. 2016, 57, 524–529. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhang, J.; Ji, N.; Zhao, X.; Zheng, K.; Qiao, Z.; Li, F.; Lang, L.; Iagaru, A.; Niu, G.; et al. Combined 68Ga-NOTA-PRGD2 and 18F-FDG PET/CT Can Discriminate Uncommon Meningioma Mimicking High-Grade Glioma. Clin. Nucl. Med. 2018, 43, 648–654. [Google Scholar] [CrossRef]
- Jansen, M.H.; Veldhuijzen van Zanten, S.E.M.; Van Vuurden, D.G.; Huisman, M.C.; Vugts, D.J.; Hoekstra, O.S.; Van Dongen, G.A.; Kaspers, G.-J.L. Molecular Drug Imaging: 89Zr-Bevacizumab PET in Children with Diffuse Intrinsic Pontine Glioma. J. Nucl. Med. 2017, 58, 711–716. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Lisok, A.; Dahmane, E.; McCoy, M.; Shelake, S.; Chatterjee, S.; Allaj, V.; Sysa-Shah, P.; Wharram, B.; Lesniak, W.G.; et al. Peptide-based PET quantifies target engagement of PD-L1 therapeutics. J. Clin. Investig. 2019, 129, 616–630. [Google Scholar] [CrossRef]
- Dupont, A.-C.; Largeau, B.; Santiago Ribeiro, M.; Guilloteau, D.; Tronel, C.; Arlicot, N. Translocator Protein-18 kDa (TSPO) Positron Emission Tomography (PET) Imaging and Its Clinical Impact in Neurodegenerative Diseases. Int. J. Mol. Sci. 2017, 18, 785. [Google Scholar] [CrossRef]
- Zanotti-Fregonara, P.; Pascual, B.; Rostomily, R.C.; Rizzo, G.; Veronese, M.; Masdeu, J.C.; Turkheimer, F. Anatomy of 18F-GE180, a failed radioligand for the TSPO protein. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 2233–2236. [Google Scholar] [CrossRef]
- Janczar, K.; Su, Z.; Raccagni, I.; Anfosso, A.; Kelly, C.; Durrenberger, P.F.; Gerhard, A.; Roncaroli, F. The 18-kDa mitochondrial translocator protein in gliomas: From the bench to bedside. Biochem. Soc. Trans. 2015, 43, 579–585. [Google Scholar] [CrossRef]
- Albert, N.L.; Unterrainer, M.; Fleischmann, D.F.; Lindner, S.; Vettermann, F.; Brunegraf, A.; Vomacka, L.; Brendel, M.; Wenter, V.; Wetzel, C.; et al. TSPO PET for glioma imaging using the novel ligand 18F-GE-180: First results in patients with glioblastoma. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 2230–2238. [Google Scholar] [CrossRef]
- Junck, L.; Olson, J.M.M.; Ciliax, B.J.; Koeppe, R.A.; Watkins, G.L.; Jewett, D.M.; McKeever, P.E.; Wieland, D.M.; Kilbourn, M.R.; Starosta-Rubinstein, S.; et al. PET Imaging of human gliomas with ligands for the peripheral benzodiazepine binding site. Ann. Neurol. 1989, 26, 752–758. [Google Scholar] [CrossRef]
- Zinnhardt, B.; Roncaroli, F.; Foray, C.; Agushi, E.; Osrah, B.; Hugon, G.; Jacobs, A.H.; Winkeler, A. Imaging of the glioma microenvironment by TSPO PET. Eur. J. Nucl. Med. Mol. Imaging 2021, 49, 174–185. [Google Scholar] [CrossRef] [PubMed]
- Unterrainer, M.; Fleischmann, D.F.; Vettermann, F.; Ruf, V.; Kaiser, L.; Nelwan, D.; Lindner, S.; Brendel, M.; Wenter, V.; Stöcklein, S.; et al. TSPO PET, tumour grading and molecular genetics in histologically verified glioma: A correlative 18F-GE-180 PET study. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 1368–1380. [Google Scholar] [CrossRef] [PubMed]
- Vettermann, F.J.; Harris, S.; Schmitt, J.; Unterrainer, M.; Lindner, S.; Rauchmann, B.-S.; Palleis, C.; Weidinger, E.; Beyer, L.; Eckenweber, F.; et al. Impact of TSPO Receptor Polymorphism on [18F]GE-180 Binding in Healthy Brain and Pseudo-Reference Regions of Neurooncological and Neurodegenerative Disorders. Life 2021, 11, 484. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, M.; Wang, L.; Wang, S.; Kang, F.; Li, G.; Jacobson, O.; Niu, G.; Yang, W.; Wang, J.; et al. Prospective Study of 68Ga-NOTA-NFB: Radiation Dosimetry in Healthy Volunteers and First Application in Glioma Patients. Theranostics 2015, 5, 882–889. [Google Scholar] [CrossRef]
- Lapa, C.; Lückerath, K.; Kleinlein, I.; Monoranu, C.M.; Linsenmann, T.; Kessler, A.F.; Rudelius, M.; Kropf, S.; Buck, A.K.; Ernestus, R.-I.; et al. 68Ga-Pentixafor-PET/CT for Imaging of Chemokine Receptor 4 Expression in Glioblastoma. Theranostics 2016, 6, 428–434. [Google Scholar] [CrossRef]
- Kumar, A.; ArunRaj, S.T.; Bhullar, K.; Haresh, K.P.; Gupta, S.; Ballal, S.; Yadav, M.; Singh, M.; Damle, N.A.; Garg, A.; et al. Ga-68 PSMA PET/CT in recurrent high-grade gliomas: Evaluating PSMA expression in vivo. Neuroradiology 2021, 1–11. [Google Scholar] [CrossRef]
- Marner, L.; Henriksen, O.M.; Lundemann, M.; Larsen, V.A.; Law, I. Clinical PET/MRI in neurooncology: Opportunities and challenges from a single-institution perspective. Clin. Transl. Imaging 2017, 5, 135–149. [Google Scholar] [CrossRef]
- Wang, K.; Qiao, Z.; Zhao, X.; Li, X.; Wang, X.; Wu, T.; Chen, Z.; Fan, D.; Chen, Q.; Ai, L. Individualized discrimination of tumor recurrence from radiation necrosis in glioma patients using an integrated radiomics-based model. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 1400–1411. [Google Scholar] [CrossRef]
- Liao, C.-Y.; Jen, J.-H.; Chen, Y.-W.; Li, C.-Y.; Wang, L.-W.; Liu, R.-S.; Huang, W.-S.; Lu, C.-F. Comparison of Conventional and Radiomic Features between 18F-FBPA PET/CT and PET/MR. Biomolecules 2021, 11, 1659. [Google Scholar] [CrossRef]
- Verger, A.; Filss, C.P.; Lohmann, P.; Stoffels, G.; Sabel, M.; Wittsack, H.J.; Kops, E.R.; Galldiks, N.; Fink, G.R.; Shah, N.J.; et al. Comparison of (18)F-FET PET and Perfusion-Weighted MRI for Glioma Grading: A Hybrid PET/MR Study. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 2257–2265. [Google Scholar] [CrossRef]
- Pellerin, A.; Khalifé, M.; Sanson, M.; Rozenblum-Beddok, L.; Bertaux, M.; Soret, M.; Galanaud, D.; Dormont, D.; Kas, A.; Pyatigorskaya, N. Simultaneously Acquired PET and ASL Imaging Biomarkers May Be Helpful in Differentiating Progression from Pseudo-Progression in Treated Gliomas. Eur. Radiol. 2021, 31, 7395–7405. [Google Scholar] [CrossRef] [PubMed]
- Tatekawa, H.; Hagiwara, A.; Yao, J.; Oughourlian, T.C.; Ueda, I.; Uetani, H.; Raymond, C.; Lai, A.; Cloughesy, T.F.; Nghiemphu, P.L.; et al. Voxelwise and Patientwise Correlation of 18F-FDOPA PET, Relative Cerebral Blood Volume, and Apparent Diffusion Coefficient in Treatment-Naïve Diffuse Gliomas with Different Molecular Subtypes. J. Nucl. Med. 2021, 62, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Lohmeier, J.; Bohner, G.; Siebert, E.; Brenner, W.; Hamm, B.; Makowski, M.R. Quantitative biparametric analysis of hybrid 18F-FET PET/MR-neuroimaging for differentiation between treatment response and recurrent glioma. Sci. Rep. 2019, 9, 14603. [Google Scholar] [CrossRef] [PubMed]
- Werner, J.-M.; Stoffels, G.; Lichtenstein, T.; Borggrefe, J.; Lohmann, P.; Ceccon, G.; Shah, N.J.; Fink, G.R.; Langen, K.-J.; Kabbasch, C.; et al. Differentiation of treatment-related changes from tumour progression: A direct comparison between dynamic FET PET and ADC values obtained from DWI MRI. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 1889–1901. [Google Scholar] [CrossRef]
- D’Souza, M.M.; Sharma, R.; Jaimini, A.; Panwar, P.; Saw, S.; Kaur, P.; Mondal, A.; Mishra, A.; Tripathi, R.P. 11C-MET PET/CT and advanced MRI in the evaluation of tumor recurrence in high-grade gliomas. Clin. Nucl. Med. 2014, 39, 791–798. [Google Scholar] [CrossRef]
- Mauler, J.; Maudsley, A.A.; Langen, K.-J.; Nikoubashman, O.; Stoffels, G.; Sheriff, S.; Lohmann, P.; Filss, C.; Galldiks, N.; Kops, E.R.; et al. Spatial Relationship of Glioma Volume Derived from 18F-FET PET and Volumetric MR Spectroscopy Imaging: A Hybrid PET/MRI Study. J. Nucl. Med. 2018, 59, 603–609. [Google Scholar] [CrossRef]
- Bumes, E.; Wirtz, F.-P.; Fellner, C.; Grosse, J.; Hellwig, D.; Oefner, P.J.; Häckl, M.; Linker, R.; Proescholdt, M.; Schmidt, N.O.; et al. Non-Invasive Prediction of IDH Mutation in Patients with Glioma WHO II/III/IV Based on F-18-FET PET-Guided In Vivo 1H-Magnetic Resonance Spectroscopy and Machine Learning. Cancers 2020, 12, 3406. [Google Scholar] [CrossRef]
- Stopa, B.M.; Juhász, C.; Mittal, S. Comparison of Amino Acid PET to Advanced and Emerging MRI Techniques for Neurooncology Imaging: A Systematic Review of the Recent Studies. Mol. Imaging 2021, 2021, 1–19. [Google Scholar] [CrossRef]
- Salvadori, J.; Imbert, L.; Perrin, M.; Karcher, G.; Lamiral, Z.; Marie, P.-Y.; Verger, A. Head-to-head comparison of image quality between brain 18F-FDG images recorded with a fully digital versus a last-generation analog PET camera. EJNMMI Res. 2019, 9, 61. [Google Scholar] [CrossRef]
- Salvadori, J.; Odille, F.; Karcher, G.; Marie, P.-Y.; Imbert, L. Fully digital PET is unaffected by any deterioration in TOF resolution and TOF image quality in the wide range of routine PET count rates. EJNMMI Phys. 2021, 8, 1. [Google Scholar] [CrossRef]
- Lohmann, P.; Elahmadawy, M.A.; Gutsche, R.; Werner, J.-M.; Bauer, E.K.; Ceccon, G.; Kocher, M.; Lerche, C.W.; Rapp, M.; Fink, G.R.; et al. FET PET Radiomics for Differentiating Pseudoprogression from Early Tumor Progression in Glioma Patients Post-Chemoradiation. Cancers 2020, 12, 3835. [Google Scholar] [CrossRef] [PubMed]
- Pyka, T.; Gempt, J.; Hiob, D.; Ringel, F.; Schlegel, J.; Bette, S.; Wester, H.-J.; Meyer, B.; Förster, S. Textural Analysis of Pre-Therapeutic [18F]-FET-PET and Its Correlation with Tumor Grade and Patient Survival in High-Grade Gliomas. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Haubold, J.; Demircioglu, A.; Gratz, M.; Glas, M.; Wrede, K.; Sure, U.; Antoch, G.; Keyvani, K.; Nittka, M.; Kannengiesser, S.; et al. Non-Invasive Tumor Decoding and Phenotyping of Cerebral Gliomas Utilizing Multiparametric 18F-FET PET-MRI and MR Fingerprinting. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 1435–1445. [Google Scholar] [CrossRef] [PubMed]
- Lohmann, P.; Lerche, C.; Bauer, E.K.; Steger, J.; Stoffels, G.; Blau, T.; Dunkl, V.; Kocher, M.; Viswanathan, S.; Filss, C.P.; et al. Predicting IDH Genotype in Gliomas Using FET PET Radiomics. Sci. Rep. 2018, 8, 13328. [Google Scholar] [CrossRef] [PubMed]
- Zaragori, T.; Oster, J.; Roch, V.; Hossu, G.; Chawki, M.B.; Grignon, R.; Pouget, C.; Gauchotte, G.; Rech, F.; Blonski, M.; et al. 18F-FDOPA PET for the Non-Invasive Prediction of Glioma Molecular Parameters: A Radiomics Study. J. Nucl. Med. 2021, 63, 147–157. [Google Scholar] [CrossRef]
- Kong, Z.; Lin, Y.; Jiang, C.; Li, L.; Liu, Z.; Wang, Y.; Dai, C.; Liu, D.; Qin, X.; Wang, Y.; et al. 18F-FDG-PET-Based Radiomics Signature Predicts MGMT Promoter Methylation Status in Primary Diffuse Glioma. Cancer Imaging 2019, 19, 58. [Google Scholar] [CrossRef]
- Meißner, A.-K.; Gutsche, R.; Galldiks, N.; Kocher, M.; Jünger, S.T.; Eich, M.-L.; Montesinos-Rongen, M.; Brunn, A.; Deckert, M.; Wendl, C.; et al. Radiomics for the Non-Invasive Prediction of the BRAF Mutation Status in Patients with Melanoma Brain Metastases. Neuro-Oncology 2021, noab294. [Google Scholar] [CrossRef]
- Carles, M.; Popp, I.; Starke, M.M.; Mix, M.; Urbach, H.; Schimek-Jasch, T.; Eckert, F.; Niyazi, M.; Baltas, D.; Grosu, A.L. FET-PET radiomics in recurrent glioblastoma: Prognostic value for outcome after re-irradiation? Radiat. Oncol. 2021, 16, 46. [Google Scholar] [CrossRef]
- Lohmann, P.; Kocher, M.; Ceccon, G.; Bauer, E.K.; Stoffels, G.; Viswanathan, S.; Ruge, M.I.; Neumaier, B.; Shah, N.J.; Fink, G.R.; et al. Combined FET PET/MRI radiomics differentiates radiation injury from recurrent brain metastasis. NeuroImage Clin. 2018, 20, 537–542. [Google Scholar] [CrossRef]
- Ahrari, S.; Zaragori, T.; Rozenblum, L.; Oster, J.; Imbert, L.; Kas, A.; Verger, A. Relevance of Dynamic 18F-DOPA PET Radiomics for Differentiation of High-Grade Glioma Progression from Treatment-Related Changes. Biomedicines 2021, 9, 1924. [Google Scholar] [CrossRef]
- Martens, C.; Debeir, O.; Decaestecker, C.; Metens, T.; Lebrun, L.; Leurquin-Sterk, G.; Trotta, N.; Goldman, S.; Van Simaeys, G. Voxelwise Principal Component Analysis of Dynamic [S-Methyl-11C]Methionine PET Data in Glioma Patients. Cancers 2021, 13, 2342. [Google Scholar] [CrossRef] [PubMed]
- Kaley, T.; Barani, I.; Chamberlain, M.; McDermott, M.; Panageas, K.; Raizer, J.; Rogers, L.; Schiff, D.; Vogelbaum, M.; Weber, D.; et al. Historical benchmarks for medical therapy trials in surgery- and radiation-refractory meningioma: A RANO review. Neuro-Oncology 2014, 16, 829–840. [Google Scholar] [CrossRef] [PubMed]
- Guedj, E.; Graillon, T.; Chinot, O.; Taieb, D. Treatment of aggressive recurrent meningiomas: Spinning towards peptide receptor radionuclide therapy. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 537–538. [Google Scholar] [CrossRef] [PubMed]
- Mirian, C.; Duun-Henriksen, A.K.; Maier, A.; Pedersen, M.M.; Jensen, L.R.; Bashir, A.; Graillon, T.; Hrachova, M.; Bota, D.; Van Essen, M.; et al. Somatostatin Receptor-Targeted Radiopeptide Therapy in Treatment-Refractory Meningioma: Individual Patient Data Meta-analysis. J. Nucl. Med. 2021, 62, 507–513. [Google Scholar] [CrossRef]
- Schumacher, T.; Hofer, S.; Eichhorn, K.; Wasner, M.; Zimmerer, S.; Freitag, P.; Probst, A.; Gratzl, O.; Reubi, J.-C.; Maecke, H.; et al. Local injection of the 90Y-labelled peptidic vector DOTATOC to control gliomas of WHO grades II and III: An extended pilot study. Eur. J. Nucl. Med. 2002, 29, 486–493. [Google Scholar] [CrossRef]
- Heute, D.; Kostron, H.; Von Guggenberg, E.; Ingorokva, S.; Gabriel, M.; Dobrozemsky, G.; Stockhammer, G.; Virgolini, I.J. Response of Recurrent High-Grade Glioma to Treatment with 90Y-DOTATOC. J. Nucl. Med. 2010, 51, 397–400. [Google Scholar] [CrossRef]
- Lange, F.; Kaemmerer, D.; Behnke-Mursch, J.; Brück, W.; Schulz, S.; Lupp, A. Differential somatostatin, CXCR4 chemokine and endothelin A receptor expression in WHO grade I–IV astrocytic brain tumors. J. Cancer Res. Clin. Oncol. 2018, 144, 1227–1237. [Google Scholar] [CrossRef]
- Kneifel, S.; Bernhardt, P.; Uusijärvi, H.; Good, S.; Plasswilm, L.; Buitrago-Téllez, C.; Müller-Brand, J.; Mäcke, H.; Merlo, A. Individual voxelwise dosimetry of targeted 90Y-labelled substance P radiotherapy for malignant gliomas. Eur. J. Nucl. Med. Mol. Imaging 2007, 34, 1388–1395. [Google Scholar] [CrossRef]
- Krolicki, L.; Bruchertseifer, F.; Kunikowska, J.; Koziara, H.; Królicki, B.; Jakuciński, M.; Pawlak, D.; Apostolidis, C.; Mirzadeh, S.; Rola, R.; et al. Prolonged survival in secondary glioblastoma following local injection of targeted alpha therapy with 213Bi-substance P analogue. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1636–1644. [Google Scholar] [CrossRef]
- Kumar, A.; Ballal, S.; Yadav, M.P.; ArunRaj, S.T.; Haresh, K.P.; Gupta, S.; Damle, N.A.; Garg, A.; Tripathi, M.; Bal, C. 177Lu-/68Ga-PSMA Theranostics in Recurrent Glioblastoma Multiforme: Proof of Concept. Clin. Nucl. Med. 2020, 45, e512–e513. [Google Scholar] [CrossRef]
- Kirchner, M.A.; Holzgreve, A.; Brendel, M.; Orth, M.; Ruf, V.C.; Steiger, K.; Pötter, D.; Gold, L.; Unterrainer, M.; Mittlmeier, L.M.; et al. PSMA PET Imaging in Glioblastoma: A Preclinical Evaluation and Theranostic Outlook. Front. Oncol. 2021, 11, 774017. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verger, A.; Kas, A.; Darcourt, J.; Guedj, E. PET Imaging in Neuro-Oncology: An Update and Overview of a Rapidly Growing Area. Cancers 2022, 14, 1103. https://doi.org/10.3390/cancers14051103
Verger A, Kas A, Darcourt J, Guedj E. PET Imaging in Neuro-Oncology: An Update and Overview of a Rapidly Growing Area. Cancers. 2022; 14(5):1103. https://doi.org/10.3390/cancers14051103
Chicago/Turabian StyleVerger, Antoine, Aurélie Kas, Jacques Darcourt, and Eric Guedj. 2022. "PET Imaging in Neuro-Oncology: An Update and Overview of a Rapidly Growing Area" Cancers 14, no. 5: 1103. https://doi.org/10.3390/cancers14051103
APA StyleVerger, A., Kas, A., Darcourt, J., & Guedj, E. (2022). PET Imaging in Neuro-Oncology: An Update and Overview of a Rapidly Growing Area. Cancers, 14(5), 1103. https://doi.org/10.3390/cancers14051103





