Anaplastic Thyroid Carcinoma: An Update
Simple Summary
Abstract
1. Introduction
2. Epidemiology and Clinical Presentation: A Rare Disease with a Rapid Onset and Poor Prognosis
3. Pathology and Biology: How Do We Understand the Aggressiveness of This Disease?
3.1. Pathology
3.2. Molecular Biology
3.3. Immune Infiltrate of ATC
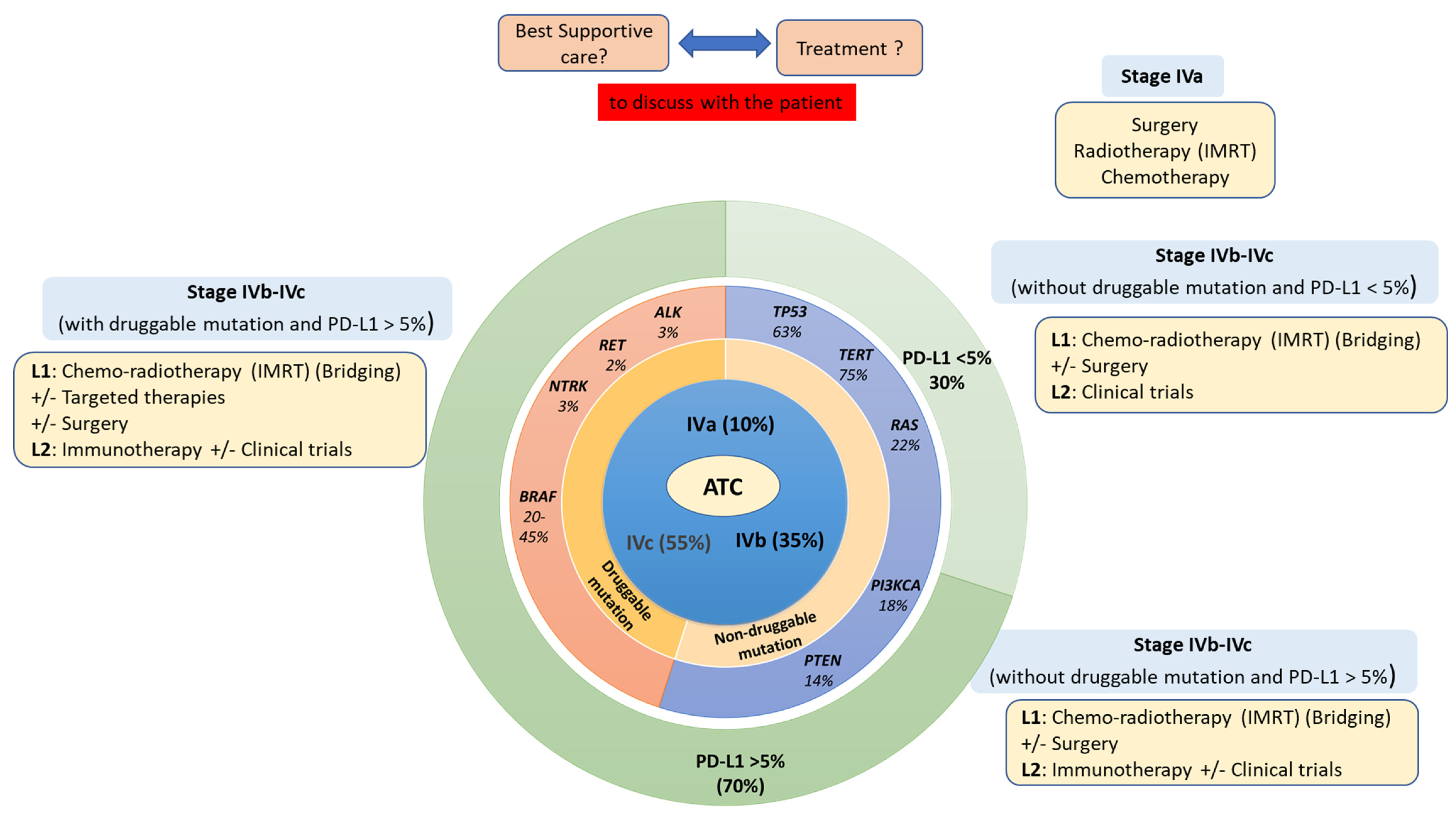
4. Treatment: To Treat Aggressively or to Palliate the Symptoms?
4.1. Multimodal Therapy or Palliative Care within a Fast-Dedicated Management Track
4.2. Radiation Therapy: Still the Mainstem of ATC Treatment
4.3. Systemic Therapies: Failure of Chemotherapies, Success of Targeted Therapies and the Promises of Immunotherapy
4.4. Reappraisal of Surgery in the Era of Targeted Therapies
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Molinaro, E.; Romei, C.; Biagini, A.; Sabini, E.; Agate, L.; Mazzeo, S.; Materazzi, G.; Sellari-Franceschini, S.; Ribechini, A.; Torregrossa, L.; et al. Anaplastic Thyroid Carcinoma: From Clinicopathology to Genetics and Advanced Therapies. Nat. Rev. Endocrinol. 2017, 13, 644–660. [Google Scholar] [CrossRef] [PubMed]
- Bible, K.C.; Kebebew, E.; Brierley, J.; Brito, J.P.; Cabanillas, M.E.; Clark, T.J.; Di Cristofano, A.; Foote, R.; Giordano, T.; Kasperbauer, J.; et al. 2021 American Thyroid Association Guidelines for Management of Patients with Anaplastic Thyroid Cancer. Thyroid 2021, 31, 337–386. [Google Scholar] [CrossRef] [PubMed]
- Filetti, S.; Durante, C.; Hartl, D.; Leboulleux, S.; Locati, L.D.; Newbold, K.; Papotti, M.G.; Berruti, A. Thyroid Cancer: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2019, 30, 1856–1883. [Google Scholar] [CrossRef] [PubMed]
- Hvilsom, G.B.; Londero, S.C.; Hahn, C.H.; Schytte, S.; Pedersen, H.B.; Christiansen, P.; Kiss, K.; Larsen, S.R.; Jespersen, M.L.; Lelkaitis, G.; et al. Anaplastic Thyroid Carcinoma in Denmark 1996-2012: A National Prospective Study of 219 Patients. Cancer Epidemiol. 2018, 53, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Wendler, J.; Kroiss, M.; Gast, K.; Kreissl, M.C.; Allelein, S.; Lichtenauer, U.; Blaser, R.; Spitzweg, C.; Fassnacht, M.; Schott, M.; et al. Clinical Presentation, Treatment and Outcome of Anaplastic Thyroid Carcinoma: Results of a Multicenter Study in Germany. Eur. J. Endocrinol. 2016, 175, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Sugitani, I.; Miyauchi, A.; Sugino, K.; Okamoto, T.; Yoshida, A.; Suzuki, S. Prognostic Factors and Treatment Outcomes for Anaplastic Thyroid Carcinoma: ATC Research Consortium of Japan Cohort Study of 677 Patients. World J. Surg. 2012, 36, 1247–1254. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Fuchs, T.; Dogan, S.; Landa, I.; Katabi, N.; Fagin, J.A.; Tuttle, R.M.; Sherman, E.; Gill, A.J.; Ghossein, R. Dissecting Anaplastic Thyroid Carcinoma: A Comprehensive Clinical, Histologic, Immunophenotypic, and Molecular Study of 360 Cases. Thyroid 2020, 30, 1505–1517. [Google Scholar] [CrossRef]
- Subbiah, V.; Kreitman, R.J.; Wainberg, Z.A.; Cho, J.Y.; Schellens, J.H.M.; Soria, J.C.; Wen, P.Y.; Zielinski, C.; Cabanillas, M.E.; Urbanowitz, G.; et al. Dabrafenib and Trametinib Treatment in Patients With Locally Advanced or Metastatic BRAF V600-Mutant Anaplastic Thyroid Cancer. J. Clin. Oncol. 2018, 36, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Subbiah, V.; Kreitman, R.J.; Wainberg, Z.A.; Cho, J.Y.; Schellens, J.H.M.; Soria, J.C.; Wen, P.Y.; Zielinski, C.C.; Cabanillas, M.E.; Boran, A.; et al. Dabrafenib plus Trametinib in Patients with BRAF V600E–Mutant Anaplastic Thyroid Cancer: Updated Analysis from the Phase II ROAR Basket Study. Ann. Oncol. 2022. [Google Scholar] [CrossRef]
- Capdevila, J.; Wirth, L.J.; Ernst, T.; Ponce Aix, S.; Lin, C.-C.; Ramlau, R.; Butler, M.O.; Delord, J.-P.; Gelderblom, H.; Ascierto, P.A.; et al. PD-1 Blockade in Anaplastic Thyroid Carcinoma. J. Clin. Oncol. 2020, 38, 2620–2627. [Google Scholar] [CrossRef]
- Dierks, C.; Seufert, J.; Aumann, K.; Ruf, J.; Klein, C.; Kiefer, S.; Rassner, M.; Boerries, M.; Zielke, A.; La Rosée, P.; et al. The Lenvatinib/Pembrolizumab Combination Is an Effective Treatment Option for Anaplastic and Poorly Differentiated Thyroid Carcinoma. Thyroid 2021, 31, 1076–1085. [Google Scholar] [CrossRef] [PubMed]
- Iyer, P.C.; Dadu, R.; Gule-Monroe, M.; Busaidy, N.L.; Ferrarotto, R.; Habra, M.A.; Zafereo, M.; Williams, M.D.; Gunn, G.B.; Grosu, H.; et al. Salvage Pembrolizumab Added to Kinase Inhibitor Therapy for the Treatment of Anaplastic Thyroid Carcinoma. J. Immunother. Cancer 2018, 6, 68. [Google Scholar] [CrossRef]
- Leboulleux, S.; Godbert, Y.; Penel, N.; Hescot, S.; De La Fouchardiere, C.; Blonski, M.; Lamartina, L.; Cousin, S.; Do Cao, C.; Hadoux, J.; et al. Benefits of Pembrolizumab in Progressive Radioactive Iodine Refractory Thyroid Cancer: Results of the AcSé Pembrolizumab Study from Unicancer. J. Clin. Oncol. 2021, 39, 6082. [Google Scholar] [CrossRef]
- Maniakas, A.; Dadu, R.; Busaidy, N.L.; Wang, J.R.; Ferrarotto, R.; Lu, C.; Williams, M.D.; Gunn, G.B.; Hofmann, M.-C.; Cote, G.; et al. Evaluation of Overall Survival in Patients With Anaplastic Thyroid Carcinoma, 2000–2019. JAMA Oncol. 2020, 6, 1397. [Google Scholar] [CrossRef] [PubMed]
- Casali, P.G.; Trama, A. Rationale of the Rare Cancer List: A Consensus Paper from the Joint Action on Rare Cancers (JARC) of the European Union (EU). ESMO Open 2020, 5, e000666. [Google Scholar] [CrossRef] [PubMed]
- Janz, T.A.; Neskey, D.M.; Nguyen, S.A.; Lentsch, E.J. Is the Incidence of Anaplastic Thyroid Cancer Increasing: A Population Based Epidemiology Study. World J. Otorhinolaryngol. Head Neck Surg. 2019, 5, 34–40. [Google Scholar] [CrossRef]
- Amphlett, B.; Lawson, Z.; Abdulrahman, G.O.; White, C.; Bailey, R.; Premawardhana, L.D.; Okosieme, O.E. Recent Trends in the Incidence, Geographical Distribution, and Survival from Thyroid Cancer in Wales, 1985–2010. Thyroid 2013, 23, 1470–1478. [Google Scholar] [CrossRef]
- de Ridder, M.; Nieveen van Dijkum, E.; Engelsman, A.; Kapiteijn, E.; Klümpen, H.-J.; Rasch, C.R.N. Anaplastic Thyroid Carcinoma: A Nationwide Cohort Study on Incidence, Treatment and Survival in the Netherlands over 3 Decades. Eur. J. Endocrinol. 2020, 183, 203–209. [Google Scholar] [CrossRef]
- Kebebew, E.; Greenspan, F.S.; Clark, O.H.; Woeber, K.A.; McMillan, A. Anaplastic Thyroid Carcinoma. Treatment Outcome and Prognostic Factors. Cancer 2005, 103, 1330–1335. [Google Scholar] [CrossRef]
- Onoda, N.; Sugitani, I.; Ito, K.; Suzuki, A.; Higashiyama, T.; Fukumori, T.; Suganuma, N.; Masudo, K.; Nakayama, H.; Uno, A.; et al. Evaluation of the 8th Edition TNM Classification for Anaplastic Thyroid Carcinoma. Cancers 2020, 12, 552. [Google Scholar] [CrossRef]
- Demeter, J.G.; De Jong, S.A.; Lawrence, A.M.; Paloyan, E. Anaplastic Thyroid Carcinoma: Risk Factors and Outcome. Surgery 1991, 110, 956–961, discussion 961–963. [Google Scholar] [PubMed]
- Loyd, R.; Osamura, R.; Klöppel, G.; Rosai, J. WHO Classification of Tumours of Endocrine Organs, 4th ed.; IARC Publications; WHO Press: Geneve, Switzerland, 2017; Volume 10, ISBN 978-92-832-4493-6. [Google Scholar]
- Ragazzi, M.; Ciarrocchi, A.; Sancisi, V.; Gandolfi, G.; Bisagni, A.; Piana, S. Update on Anaplastic Thyroid Carcinoma: Morphological, Molecular, and Genetic Features of the Most Aggressive Thyroid Cancer. Int. J. Endocrinol. 2014, 2014, 790834. [Google Scholar] [CrossRef] [PubMed]
- Lai, W.-A.; Hang, J.-F.; Liu, C.-Y.; Bai, Y.; Liu, Z.; Gu, H.; Hong, S.; Pyo, J.Y.; Jung, C.K.; Kakudo, K.; et al. PAX8 Expression in Anaplastic Thyroid Carcinoma Is Less than Those Reported in Early Studies: A Multi-Institutional Study of 182 Cases Using the Monoclonal Antibody MRQ-50. Virchows Arch. Int. J. Pathol. 2020, 476, 431–437. [Google Scholar] [CrossRef]
- Bishop, J.A.; Sharma, R.; Westra, W.H. PAX8 Immunostaining of Anaplastic Thyroid Carcinoma: A Reliable Means of Discerning Thyroid Origin for Undifferentiated Tumors of the Head and Neck. Hum. Pathol. 2011, 42, 1873–1877. [Google Scholar] [CrossRef] [PubMed]
- Aldinger, K.A.; Samaan, N.A.; Ibanez, M.; Hill, C.S. Anaplastic Carcinoma of the Thyroid: A Review of 84 Cases of Spindle and Giant Cell Carcinoma of the Thyroid. Cancer 1978, 41, 2267–2275. [Google Scholar] [CrossRef]
- Bonhomme, B.; Godbert, Y.; Perot, G.; Al Ghuzlan, A.; Bardet, S.; Belleannée, G.; Crinière, L.; Do Cao, C.; Fouilloux, G.; Guyetant, S.; et al. Molecular Pathology of Anaplastic Thyroid Carcinomas: A Retrospective Study of 144 Cases. Thyroid 2017, 27, 682–692. [Google Scholar] [CrossRef]
- Landa, I.; Ibrahimpasic, T.; Boucai, L.; Sinha, R.; Knauf, J.A.; Shah, R.H.; Dogan, S.; Ricarte-Filho, J.C.; Krishnamoorthy, G.P.; Xu, B.; et al. Genomic and Transcriptomic Hallmarks of Poorly Differentiated and Anaplastic Thyroid Cancers. J. Clin. Invest. 2016, 126, 1052–1066. [Google Scholar] [CrossRef]
- Pozdeyev, N.; Gay, L.M.; Sokol, E.S.; Hartmaier, R.; Deaver, K.E.; Davis, S.; French, J.D.; Borre, P.V.; LaBarbera, D.V.; Tan, A.-C.; et al. Genetic Analysis of 779 Advanced Differentiated and Anaplastic Thyroid Cancers. Clin. Cancer Res. 2018, 24, 3059–3068. [Google Scholar] [CrossRef]
- Romei, C.; Tacito, A.; Molinaro, E.; Piaggi, P.; Cappagli, V.; Pieruzzi, L.; Matrone, A.; Viola, D.; Agate, L.; Torregrossa, L.; et al. Clinical, Pathological and Genetic Features of Anaplastic and Poorly Differentiated Thyroid Cancer: A Single Institute Experience. Oncol. Lett. 2018, 15, 9174–9182. [Google Scholar] [CrossRef]
- Capdevila, J.; Mayor, R.; Mancuso, F.M.; Iglesias, C.; Caratù, G.; Matos, I.; Zafón, C.; Hernando, J.; Petit, A.; Nuciforo, P.; et al. Early Evolutionary Divergence between Papillary and Anaplastic Thyroid Cancers. Ann. Oncol. 2018, 29, 1454–1460. [Google Scholar] [CrossRef]
- Ngo, T.N.M.; Le, T.T.B.; Le, T.; Bychkov, A.; Oishi, N.; Jung, C.K.; Hassell, L.; Kakudo, K.; Vuong, H.G. Primary Versus Secondary Anaplastic Thyroid Carcinoma: Perspectives from Multi-Institutional and Population-Level Data. Endocr. Pathol. 2021, 32, 489–500. [Google Scholar] [CrossRef] [PubMed]
- Kunstman, J.W.; Juhlin, C.C.; Goh, G.; Brown, T.C.; Stenman, A.; Healy, J.M.; Rubinstein, J.C.; Choi, M.; Kiss, N.; Nelson-Williams, C.; et al. Characterization of the Mutational Landscape of Anaplastic Thyroid Cancer via Whole-Exome Sequencing. Hum. Mol. Genet. 2015, 24, 2318–2329. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.-K.; Song, Y.S.; Lee, E.K.; Hwang, J.; Kim, H.H.; Jung, G.; Kim, Y.A.; Kim, S.; Cho, S.W.; Won, J.-K.; et al. Integrative Analysis of Genomic and Transcriptomic Characteristics Associated with Progression of Aggressive Thyroid Cancer. Nat. Commun. 2019, 10, 2764. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.-H.; Wirth, L.J.; Farahani, A.A.; Nosé, V.; Faquin, W.C.; Dias-Santagata, D.; Sadow, P.M. Clinicopathologic Features of Kinase Fusion-Related Thyroid Carcinomas: An Integrative Analysis with Molecular Characterization. Mod. Pathol. 2020, 33, 2458–2472. [Google Scholar] [CrossRef]
- Prete, A.; Matrone, A.; Gambale, C.; Torregrossa, L.; Minaldi, E.; Romei, C.; Ciampi, R.; Molinaro, E.; Elisei, R. Poorly Differentiated and Anaplastic Thyroid Cancer: Insights into Genomics, Microenvironment and New Drugs. Cancers 2021, 13, 3200. [Google Scholar] [CrossRef]
- Yakushina, V.D.; Lerner, L.V.; Lavrov, A.V. Gene Fusions in Thyroid Cancer. Thyroid 2018, 28, 158–167. [Google Scholar] [CrossRef]
- Krishnamoorthy, G.P.; Davidson, N.R.; Leach, S.D.; Zhao, Z.; Lowe, S.W.; Lee, G.; Landa, I.; Nagarajah, J.; Saqcena, M.; Singh, K.; et al. EIF1AX and RAS Mutations Cooperate to Drive Thyroid Tumorigenesis through ATF4 and C-MYC. Cancer Discov. 2019, 9, 264–281. [Google Scholar] [CrossRef]
- Garcia-Rostan, G.; Camp, R.L.; Herrero, A.; Carcangiu, M.L.; Rimm, D.L.; Tallini, G. β-Catenin Dysregulation in Thyroid Neoplasms. Am. J. Pathol. 2001, 158, 987–996. [Google Scholar] [CrossRef]
- Lazzereschi, D.; Palmirotta, R.; Ranieri, A.; Ottini, L.; Verì, M.C.; Cama, A.; Cetta, F.; Nardi, F.; Colletta, G.; Mariani-Costantini, R. Microsatellite Instability in Thyroid Tumours and Tumour-like Lesions. Br. J. Cancer 1999, 79, 340–345. [Google Scholar] [CrossRef][Green Version]
- Ravi, N.; Yang, M.; Gretarsson, S.; Jansson, C.; Mylona, N.; Sydow, S.R.; Woodward, E.L.; Ekblad, L.; Wennerberg, J.; Paulsson, K. Identification of Targetable Lesions in Anaplastic Thyroid Cancer by Genome Profiling. Cancers 2019, 11, 402. [Google Scholar] [CrossRef]
- Rocha, M.L.; Schmid, K.W.; Czapiewski, P. The Prevalence of DNA Microsatellite Instability in Anaplastic Thyroid Carcinoma–Systematic Review and Discussion of Current Therapeutic Options. Contemp. Oncol. 2021, 25, 213–223. [Google Scholar] [CrossRef]
- Wong, K.S.; Lorch, J.H.; Alexander, E.K.; Nehs, M.A.; Nowak, J.A.; Hornick, J.L.; Barletta, J.A. Clinicopathologic Features of Mismatch Repair-Deficient Anaplastic Thyroid Carcinomas. Thyroid 2019, 29, 666–673. [Google Scholar] [CrossRef] [PubMed]
- Caillou, B.; Talbot, M.; Weyemi, U.; Pioche-Durieu, C.; Al Ghuzlan, A.; Bidart, J.M.; Chouaib, S.; Schlumberger, M.; Dupuy, C. Tumor-Associated Macrophages (TAMs) Form an Interconnected Cellular Supportive Network in Anaplastic Thyroid Carcinoma. PLoS ONE 2011, 6, e22567. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.Y.; Cho, S.W.; Kim, Y.A.; Kim, D.; Oh, B.-C.; Park, D.J.; Park, Y.J. Cancers with Higher Density of Tumor-Associated Macrophages Were Associated with Poor Survival Rates. J. Pathol. Transl. Med. 2015, 49, 318–324. [Google Scholar] [CrossRef]
- Kim, H.; Park, Y.W.; Oh, Y.-H.; Sim, J.; Ro, J.Y.; Pyo, J.Y. Anaplastic Transformation of Papillary Thyroid Carcinoma Only Seen in Pleural Metastasis: A Case Report with Review of the Literature. Head Neck Pathol. 2016, 11, 162–167. [Google Scholar] [CrossRef]
- Ryder, M.; Ghossein, R.A.; Ricarte-Filho, J.C.M.; Knauf, J.A.; Fagin, J.A. Increased Density of Tumor-Associated Macrophages Is Associated with Decreased Survival in Advanced Thyroid Cancer. Endocr. Relat. Cancer 2008, 15, 1069–1074. [Google Scholar] [CrossRef]
- Lv, J.; Feng, Z.-P.; Chen, F.-K.; Liu, C.; Jia, L.; Liu, P.-J.; Yang, C.-Z.; Hou, F.; Deng, Z.-Y. M2-like Tumor-Associated Macrophages-Secreted Wnt1 and Wnt3a Promotes Dedifferentiation and Metastasis via Activating β-Catenin Pathway in Thyroid Cancer. Mol. Carcinog. 2021, 60, 25–37. [Google Scholar] [CrossRef]
- Schürch, C.M.; Roelli, M.A.; Forster, S.; Wasmer, M.-H.; Brühl, F.; Maire, R.S.; Di Pancrazio, S.; Ruepp, M.-D.; Giger, R.; Perren, A.; et al. Targeting CD47 in Anaplastic Thyroid Carcinoma Enhances Tumor Phagocytosis by Macrophages and Is a Promising Therapeutic Strategy. Thyroid 2019, 29, 979–992. [Google Scholar] [CrossRef]
- Giannini, R.; Moretti, S.; Ugolini, C.; Macerola, E.; Menicali, E.; Nucci, N.; Morelli, S.; Colella, R.; Mandarano, M.; Sidoni, A.; et al. Immune Profiling of Thyroid Carcinomas Suggests the Existence of Two Major Phenotypes: An ATC-like and a PDTC-Like. J. Clin. Endocrinol. Metab. 2019, 104, 3557–3575. [Google Scholar] [CrossRef]
- Adam, P.; Kircher, S.; Sbiera, I.; Koehler, V.F.; Berg, E.; Knösel, T.; Sandner, B.; Fenske, W.K.; Bläker, H.; Smaxwil, C.; et al. FGF-Receptors and PD-L1 in Anaplastic and Poorly Differentiated Thyroid Cancer: Evaluation of the Preclinical Rationale. Front. Endocrinol. 2021, 12, 712107. [Google Scholar] [CrossRef]
- Bastman, J.J.; Serracino, H.S.; Zhu, Y.; Koenig, M.R.; Mateescu, V.; Sams, S.B.; Davies, K.D.; Raeburn, C.D.; McIntyre, R.C.; Haugen, B.R.; et al. Tumor-Infiltrating T Cells and the PD-1 Checkpoint Pathway in Advanced Differentiated and Anaplastic Thyroid Cancer. J. Clin. Endocrinol. Metab. 2016, 101, 2863–2873. [Google Scholar] [CrossRef] [PubMed]
- Chintakuntlawar, A.V.; Rumilla, K.M.; Smith, C.Y.; Jenkins, S.M.; Foote, R.L.; Kasperbauer, J.L.; Morris, J.C.; Ryder, M.; Alsidawi, S.; Hilger, C.; et al. Expression of PD-1 and PD-L1 in Anaplastic Thyroid Cancer Patients Treated With Multimodal Therapy: Results From a Retrospective Study. J. Clin. Endocrinol. Metab. 2017, 102, 1943–1950. [Google Scholar] [CrossRef]
- Noguchi, T.; Ward, J.P.; Gubin, M.M.; Arthur, C.D.; Lee, S.H.; Hundal, J.; Selby, M.J.; Graziano, R.F.; Mardis, E.R.; Korman, A.J.; et al. Temporally Distinct PD-L1 Expression by Tumor and Host Cells Contributes to Immune Escape. Cancer Immunol. Res. 2017, 5, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Zhao, Q.; Gao, Z.; Lao, X.-M.; Lin, W.-M.; Chen, D.-P.; Mu, M.; Huang, C.-X.; Liu, Z.-Y.; Li, B.; et al. The Local Immune Landscape Determines Tumor PD-L1 Heterogeneity and Sensitivity to Therapy. J. Clin. Investig. 2019, 129, 3347–3360. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, Y.S.; Ordonez, N.G.; Schultz, P.N.; Hickey, R.C.; Goepfert, H.; Samaan, N.A. Anaplastic Carcinoma of the Thyroid. A Clinicopathologic Study of 121 Cases. Cancer 1990, 66, 321–330. [Google Scholar] [CrossRef]
- Haigh, P.I. Anaplastic Thyroid Carcinoma. Curr. Treat. Options Oncol. 2000, 1, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Smallridge, R.C.; Copland, J.A. Anaplastic Thyroid Carcinoma: Pathogenesis and Emerging Therapies. Clin. Oncol. 2010, 22, 486–497. [Google Scholar] [CrossRef]
- Akaishi, J.; Sugino, K.; Kitagawa, W.; Nagahama, M.; Kameyama, K.; Shimizu, K.; Ito, K.; Ito, K. Prognostic Factors and Treatment Outcomes of 100 Cases of Anaplastic Thyroid Carcinoma. Thyroid 2011, 21, 1183–1189. [Google Scholar] [CrossRef]
- Sherman, E.J.; Lim, S.H.; Ho, A.L.; Ghossein, R.A.; Fury, M.G.; Shaha, A.R.; Rivera, M.; Lin, O.; Wolden, S.; Lee, N.Y.; et al. Concurrent Doxorubicin and Radiotherapy for Anaplastic Thyroid Cancer: A Critical Re-Evaluation Including Uniform Pathologic Review. Radiother. Oncol. 2011, 101, 425–430. [Google Scholar] [CrossRef]
- Ito, K.-I.; Hanamura, T.; Murayama, K.; Okada, T.; Watanabe, T.; Harada, M.; Ito, T.; Koyama, H.; Kanai, T.; Maeno, K.; et al. Multimodality Therapeutic Outcomes in Anaplastic Thyroid Carcinoma: Improved Survival in Subgroups of Patients with Localized Primary Tumors. Head Neck 2012, 34, 230–237. [Google Scholar] [CrossRef]
- Cabanillas, M.E.; Williams, M.D.; Gunn, G.B.; Weitzman, S.P.; Burke, L.; Busaidy, N.L.; Ying, A.K.; Yiin, Y.H.; William, W.N.; Lu, C.; et al. Facilitating Anaplastic Thyroid Cancer Specialized Treatment: A Model for Improving Access to Multidisciplinary Care for Patients with Anaplastic Thyroid Cancer. Head Neck 2017, 39, 1291–1295. [Google Scholar] [CrossRef] [PubMed]
- De Crevoisier, R.; Baudin, E.; Bachelot, A.; Leboulleux, S.; Travagli, J.-P.; Caillou, B.; Schlumberger, M. Combined Treatment of Anaplastic Thyroid Carcinoma with Surgery, Chemotherapy, and Hyperfractionated Accelerated External Radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2004, 60, 1137–1143. [Google Scholar] [CrossRef] [PubMed]
- Levy, A.; Leboulleux, S.; Lepoutre-Lussey, C.; Baudin, E.; Ghuzlan, A.A.; Hartl, D.; Deutsch, E.; Deandreis, D.; Lumbroso, J.; Tao, Y.; et al. (18)F-Fluorodeoxyglucose Positron Emission Tomography to Assess Response after Radiation Therapy in Anaplastic Thyroid Cancer. Oral Oncol. 2015, 51, 370–375. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.N.; Zafereo, M.; Dadu, R.; Busaidy, N.L.; Hess, K.; Cote, G.J.; Williams, M.D.; William, W.N.; Sandulache, V.; Gross, N.; et al. Patterns of Treatment Failure in Anaplastic Thyroid Carcinoma. Thyroid 2017, 27, 672–681. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.-S.; Nam, K.-H.; Chung, W.Y.; Park, C.S. Anaplastic Thyroid Carcinoma: A Therapeutic Dilemma. Yonsei Med. J. 2005, 46, 759–764. [Google Scholar] [CrossRef] [PubMed]
- Haigh, P.I.; Ituarte, P.H.; Wu, H.S.; Treseler, P.A.; Posner, M.D.; Quivey, J.M.; Duh, Q.Y.; Clark, O.H. Completely Resected Anaplastic Thyroid Carcinoma Combined with Adjuvant Chemotherapy and Irradiation Is Associated with Prolonged Survival. Cancer 2001, 91, 2335–2342. [Google Scholar] [CrossRef]
- Chen, J.; Tward, J.D.; Shrieve, D.C.; Hitchcock, Y.J. Surgery and Radiotherapy Improves Survival in Patients with Anaplastic Thyroid Carcinoma: Analysis of the Surveillance, Epidemiology, and End Results 1983-2002. Am. J. Clin. Oncol. 2008, 31, 460–464. [Google Scholar] [CrossRef]
- Haymart, M.R.; Banerjee, M.; Yin, H.; Worden, F.; Griggs, J.J. Marginal Treatment Benefit in Anaplastic Thyroid Cancer. Cancer 2013, 119, 3133–3139. [Google Scholar] [CrossRef]
- Huang, N.-S.; Shi, X.; Lei, B.-W.; Wei, W.-J.; Lu, Z.-W.; Yu, P.-C.; Wang, Y.; Ji, Q.-H.; Wang, Y.-L. An Update of the Appropriate Treatment Strategies in Anaplastic Thyroid Cancer: A Population-Based Study of 735 Patients. Int. J. Endocrinol. 2019, 2019, 8428547. [Google Scholar] [CrossRef]
- Kwon, J.; Kim, B.H.; Jung, H.-W.; Besic, N.; Sugitani, I.; Wu, H.-G. The Prognostic Impacts of Postoperative Radiotherapy in the Patients with Resected Anaplastic Thyroid Carcinoma: A Systematic Review and Meta-Analysis. Eur. J. Cancer 2016, 59, 34–45. [Google Scholar] [CrossRef]
- Liu, T.-R.; Xiao, Z.-W.; Xu, H.-N.; Long, Z.; Wei, F.-Q.; Zhuang, S.-M.; Sun, X.-M.; Xie, L.-E.; Mu, J.-S.; Yang, A.-K.; et al. Treatment and Prognosis of Anaplastic Thyroid Carcinoma: A Clinical Study of 50 Cases. PLoS ONE 2016, 11, e0164840. [Google Scholar] [CrossRef]
- Pezzi, T.A.; Mohamed, A.S.R.; Sheu, T.; Blanchard, P.; Sandulache, V.C.; Lai, S.Y.; Cabanillas, M.E.; Williams, M.D.; Pezzi, C.M.; Lu, C.; et al. Radiation Therapy Dose Is Associated with Improved Survival for Unresected Anaplastic Thyroid Carcinoma: Outcomes from the National Cancer Data Base. Cancer 2017, 123, 1653–1661. [Google Scholar] [CrossRef] [PubMed]
- Prasongsook, N.; Kumar, A.; Chintakuntlawar, A.V.; Foote, R.L.; Kasperbauer, J.; Molina, J.; Garces, Y.; Ma, D.; Wittich, M.A.N.; Rubin, J.; et al. Survival in Response to Multimodal Therapy in Anaplastic Thyroid Cancer. J. Clin. Endocrinol. Metab. 2017, 102, 4506–4514. [Google Scholar] [CrossRef] [PubMed]
- Smallridge, R.C. Approach to the Patient with Anaplastic Thyroid Carcinoma. J. Clin. Endocrinol. Metab. 2012, 97, 2566–2572. [Google Scholar] [CrossRef]
- Song, T.; Chen, L.; Zhang, H.; Lu, Y.; Yu, K.; Zhan, W.; Fang, M. Multimodal Treatment Based on Thyroidectomy Improves Survival in Patients with Metastatic Anaplastic Thyroid Carcinoma: A SEER Analysis from 1998 to 2015. Gland Surg. 2020, 9, 1205–1213. [Google Scholar] [CrossRef]
- Baek, S.-K.; Lee, M.-C.; Hah, J.H.; Ahn, S.-H.; Son, Y.-I.; Rho, Y.-S.; Chung, P.-S.; Lee, Y.-S.; Koo, B.S.; Jung, K.-Y.; et al. Role of Surgery in the Management of Anaplastic Thyroid Carcinoma: Korean Nationwide Multicenter Study of 329 Patients with Anaplastic Thyroid Carcinoma, 2000 to 2012. Head Neck 2017, 39, 133–139. [Google Scholar] [CrossRef]
- Brierley, J.; Sherman, E. The Role of External Beam Radiation and Targeted Therapy in Thyroid Cancer. Semin. Radiat. Oncol. 2012, 22, 254–262. [Google Scholar] [CrossRef]
- Bhatia, A.; Rao, A.; Ang, K.-K.; Garden, A.S.; Morrison, W.H.; Rosenthal, D.I.; Evans, D.B.; Clayman, G.; Sherman, S.I.; Schwartz, D.L. Anaplastic Thyroid Cancer: Clinical Outcomes with Conformal Radiotherapy. Head Neck 2010, 32, 829–836. [Google Scholar] [CrossRef]
- Nachalon, Y.; Stern-Shavit, S.; Bachar, G.; Shvero, J.; Limon, D.; Popovtzer, A. Aggressive Palliation and Survival in Anaplastic Thyroid Carcinoma. JAMA Otolaryngol. Neck Surg. 2015, 141, 1128. [Google Scholar] [CrossRef]
- Sun, X.S.; Sun, S.R.; Guevara, N.; Marcy, P.Y.; Peyrottes, I.; Lassalle, S.; Lacout, A.; Sadoul, J.L.; Santini, J.; Benisvy, D.; et al. Indications of External Beam Radiation Therapy in Non-Anaplastic Thyroid Cancer and Impact of Innovative Radiation Techniques. Crit. Rev. Oncol. Hematol. 2013, 86, 52–68. [Google Scholar] [CrossRef]
- Tennvall, J.; Lundell, G.; Wahlberg, P.; Bergenfelz, A.; Grimelius, L.; Akerman, M.; Hjelm Skog, A.-L.; Wallin, G. Anaplastic Thyroid Carcinoma: Three Protocols Combining Doxorubicin, Hyperfractionated Radiotherapy and Surgery. Br. J. Cancer 2002, 86, 1848–1853. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tsang, R.; Asa, S.; Dickson, B.; Arenovich, T.; Brierley, J. Clinical Outcome of Anaplastic Thyroid Carcinoma Treated with Radiotherapy of Once- and Twice-Daily Fractionation Regimens. Cancer 2006, 107, 1786–1792. [Google Scholar] [CrossRef] [PubMed]
- Lacas, B.; Bourhis, J.; Overgaard, J.; Zhang, Q.; Grégoire, V.; Nankivell, M.; Zackrisson, B.; Szutkowski, Z.; Suwiński, R.; Poulsen, M.; et al. Role of Radiotherapy Fractionation in Head and Neck Cancers (MARCH): An Updated Meta-Analysis. Lancet Oncol. 2017, 18, 1221–1237. [Google Scholar] [CrossRef]
- Takahashi, N.; Matsushita, H.; Umezawa, R.; Yamamoto, T.; Ishikawa, Y.; Katagiri, Y.; Tasaka, S.; Takeda, K.; Fukui, K.; Kadoya, N.; et al. Hypofractionated Radiotherapy for Anaplastic Thyroid Carcinoma: 15 Years of Experience in a Single Institution. Eur. Thyroid J. 2019, 8, 24–30. [Google Scholar] [CrossRef]
- Jacobsen, A.-B.; Grøholt, K.K.; Lorntzsen, B.; Osnes, T.A.; Falk, R.S.; Sigstad, E. Anaplastic Thyroid Cancer and Hyperfractionated Accelerated Radiotherapy (HART) with and without Surgery. Eur. Arch. Oto-Rhino-Laryngol. 2017, 274, 4203–4209. [Google Scholar] [CrossRef]
- Dumke, A.-K.; Pelz, T.; Vordermark, D. Long-Term Results of Radiotherapy in Anaplastic Thyroid Cancer. Radiat. Oncol. Lond. Engl. 2014, 9, 90. [Google Scholar] [CrossRef]
- Houlihan, O.A.; Moore, R.; Jamaluddin, M.F.; Sharifah, A.; Redmond, H.P.; O’Reilly, S.; Feeley, L.; Sheahan, P.; Rock, K. Anaplastic Thyroid Cancer: Outcomes of Trimodal Therapy. Rep. Pract. Oncol. Radiother. 2021, 26, 416–422. [Google Scholar] [CrossRef]
- He, X.; Li, D.; Hu, C.; Wang, Z.; Ying, H.; Wu, Y. Outcome after Intensity Modulated Radiotherapy for Anaplastic Thyroid Carcinoma. BMC Cancer 2014, 14, 235. [Google Scholar] [CrossRef]
- Jiménez-Fonseca, P.; Gómez Saez, J.M.; Santamaria Sandi, J.; Capdevila, J.; Navarro Gonzalez, E.; Zafon Llopis, C.; Ramón Y Cajal Asensio, T.; Riesco-Eizaguirre, G.; Grande, E.; Galofré, J.C. Spanish Consensus for the Management of Patients with Anaplastic Cell Thyroid Carcinoma. Clin. Transl. Oncol. 2017, 19, 12–20. [Google Scholar] [CrossRef]
- Mangoni, M.; Gobitti, C.; Autorino, R.; Cerizza, L.; Furlan, C.; Mazzarotto, R.; Monari, F.; Simontacchi, G.; Vianello, F.; Basso, M.; et al. External Beam Radiotherapy in Thyroid Carcinoma: Clinical Review and Recommendations of the AIRO “Radioterapia Metabolica” Group. Tumori 2017, 103, 114–123. [Google Scholar] [CrossRef]
- Nutting, C.M.; Convery, D.J.; Cosgrove, V.P.; Rowbottom, C.; Vini, L.; Harmer, C.; Dearnaley, D.P.; Webb, S. Improvements in Target Coverage and Reduced Spinal Cord Irradiation Using Intensity-Modulated Radiotherapy (IMRT) in Patients with Carcinoma of the Thyroid Gland. Radiother. Oncol. 2001, 60, 173–180. [Google Scholar] [CrossRef]
- Posner, M.D.; Quivey, J.M.; Akazawa, P.F.; Xia, P.; Akazawa, C.; Verhey, L.J. Dose Optimization for the Treatment of Anaplastic Thyroid Carcinoma: A Comparison of Treatment Planning Techniques. Int. J. Radiat. Oncol. Biol. Phys. 2000, 48, 475–483. [Google Scholar] [CrossRef]
- Park, J.W.; Choi, S.H.; Yoon, H.I.; Lee, J.; Kim, T.H.; Kim, J.W.; Lee, I.J. Treatment Outcomes of Radiotherapy for Anaplastic Thyroid Cancer. Radiat. Oncol. J. 2018, 36, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Swaak-Kragten, A.T.; de Wilt, J.H.W.; Schmitz, P.I.M.; Bontenbal, M.; Levendag, P.C. Multimodality Treatment for Anaplastic Thyroid Carcinoma--Treatment Outcome in 75 Patients. Radiother. Oncol. 2009, 92, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Vulpe, H.; Kwan, J.Y.Y.; McNiven, A.; Brierley, J.D.; Tsang, R.; Chan, B.; Goldstein, D.P.; Le, L.W.; Hope, A.; Giuliani, M. Patterns of Failure in Anaplastic and Differentiated Thyroid Carcinoma Treated with Intensity-Modulated Radiotherapy. Curr. Oncol. 2017, 24, e226–e232. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.W.; Foote, R.L.; Garces, Y.I.; Ma, D.J.; Neben-Wittich, M.; Routman, D.M.; Patel, S.H.; Ko, S.J.; McGee, L.A.; Bible, K.C.; et al. Outcomes and Patterns of Recurrence for Anaplastic Thyroid Cancer Treated With Comprehensive Chemoradiotherapy. Pract. Radiat. Oncol. 2021. [Google Scholar] [CrossRef]
- Kim, T.H.; Chung, K.-W.; Lee, Y.J.; Park, C.S.; Lee, E.K.; Kim, T.S.; Kim, S.K.; Jung, Y.S.; Ryu, J.S.; Kim, S.S.; et al. The Effect of External Beam Radiotherapy Volume on Locoregional Control in Patients with Locoregionally Advanced or Recurrent Nonanaplastic Thyroid Cancer. Radiat. Oncol. 2010, 5, 69. [Google Scholar] [CrossRef]
- Besic, N.; Auersperg, M.; Us-Krasovec, M.; Golouh, R.; Frkovic-Grazio, S.; Vodnik, A. Effect of Primary Treatment on Survival in Anaplastic Thyroid Carcinoma. Eur. J. Surg. Oncol. 2001, 27, 260–264. [Google Scholar] [CrossRef]
- Arora, S.; Christos, P.; Pham, A.; Desai, P.; Wernicke, A.G.; Nori, D.; Chao, K.S.C.; Parashar, B. Comparing Outcomes in Poorly-Differentiated versus Anaplastic Thyroid Cancers Treated with Radiation: A Surveillance, Epidemiology, and End Results Analysis. J. Cancer Res. Ther. 2014, 10, 526–530. [Google Scholar] [CrossRef]
- Zhou, W.; Yue, Y.; Zhang, X. Radiotherapy Plus Chemotherapy Leads to Prolonged Survival in Patients With Anaplastic Thyroid Cancer Compared With Radiotherapy Alone Regardless of Surgical Resection and Distant Metastasis: A Retrospective Population Study. Front. Endocrinol. 2021, 12, 748023. [Google Scholar] [CrossRef]
- Sherman, E.J.; Harris, J.; Bible, K.C.; Xia, P.; Ghossein, R.A.; Chung, C.H.; Riaz, N.; Gunn, B.; Foote, R.L.; Yom, S.; et al. 1914MO Randomized Phase II Study of Radiation Therapy and Paclitaxel with Pazopanib or Placebo: NRG-RTOG 0912. Ann. Oncol. 2020, 31, S1085. [Google Scholar] [CrossRef]
- Jonker, P.K.C.; Turchini, J.; Kruijff, S.; Lin, J.F.; Gill, A.J.; Eade, T.; Aniss, A.; Clifton-Bligh, R.; Learoyd, D.; Robinson, B.; et al. Multimodality Treatment Improves Locoregional Control, Progression-Free and Overall Survival in Patients with Anaplastic Thyroid Cancer: A Retrospective Cohort Study Comparing Oncological Outcomes and Morbidity between Multimodality Treatment and Limited Treatment. Ann. Surg. Oncol. 2021, 28, 7520–7530. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Smith, J.L.; Carlino, M.S.; Burmeister, B.; Pinkham, M.B.; Fogarty, G.B.; Christie, D.R.H.; Estall, V.; Shackleton, M.; Clements, A.; et al. Phase I/II Trial of Concurrent Extracranial Palliative Radiation Therapy with Dabrafenib and Trametinib in Metastatic BRAF V600E/K Mutation-Positive Cutaneous Melanoma. Clin. Transl. Radiat. Oncol. 2021, 30, 95–99. [Google Scholar] [CrossRef]
- Werner, B.; Abele, J.; Alveryd, A.; Björklund, A.; Franzén, S.; Granberg, P.O.; Landberg, T.; Lundell, G.; Löwhagen, T.; Sundblad, R. Multimodal Therapy in Anaplastic Giant Cell Thyroid Carcinoma. World J. Surg. 1984, 8, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Shimaoka, K.; Schoenfeld, D.A.; DeWys, W.D.; Creech, R.H.; DeConti, R. A Randomized Trial of Doxorubicin versus Doxorubicin plus Cisplatin in Patients with Advanced Thyroid Carcinoma. Cancer 1985, 56, 2155–2160. [Google Scholar] [CrossRef]
- Tallroth, E.; Wallin, G.; Lundell, G.; Löwhagen, T.; Einhorn, J. Multimodality Treatment in Anaplastic Giant Cell Thyroid Carcinoma. Cancer 1987, 60, 1428–1431. [Google Scholar] [CrossRef]
- Kim, J.H.; Leeper, R.D. Treatment of Locally Advanced Thyroid Carcinoma with Combination Doxorubicin and Radiation Therapy. Cancer 1987, 60, 2372–2375. [Google Scholar] [CrossRef]
- Schlumberger, M.; Parmentier, C.; Delisle, M.J.; Couette, J.E.; Droz, J.P.; Sarrazin, D. Combination Therapy for Anaplastic Giant Cell Thyroid Carcinoma. Cancer 1991, 67, 564–566. [Google Scholar] [CrossRef]
- Wong, C.S.; Van Dyk, J.; Simpson, W.J. Myelopathy Following Hyperfractionated Accelerated Radiotherapy for Anaplastic Thyroid Carcinoma. Radiother. Oncol. 1991, 20, 3–9. [Google Scholar] [CrossRef]
- Auersperg, M.; Us-Krasovec, M.; Petric, G.; Pogacnik, A.; Besic, N. Results of Combined Modality Treatment in Poorly Differentiated and Anaplastic Thyroid Carcinoma. Wien. Klin. Wochenschr. 1990, 102, 267–270. [Google Scholar]
- Derbel, O.; Limem, S.; Ségura-Ferlay, C.; Lifante, J.-C.; Carrie, C.; Peix, J.-L.; Borson-Chazot, F.; Bournaud, C.; Droz, J.-P.; de la Fouchardière, C. Results of Combined Treatment of Anaplastic Thyroid Carcinoma (ATC). BMC Cancer 2011, 11, 469. [Google Scholar] [CrossRef]
- Lim, S.M.; Shin, S.-J.; Chung, W.Y.; Park, C.S.; Nam, K.-H.; Kang, S.-W.; Keum, K.C.; Kim, J.H.; Cho, J.Y.; Hong, Y.K.; et al. Treatment Outcome of Patients with Anaplastic Thyroid Cancer: A Single Center Experience. Yonsei Med. J. 2012, 53, 352–357. [Google Scholar] [CrossRef] [PubMed]
- Sosa, J.A.; Balkissoon, J.; Lu, S.; Langecker, P.; Elisei, R.; Jarzab, B.; Bal, C.S.; Marur, S.; Gramza, A.; Ondrey, F. Thyroidectomy Followed by Fosbretabulin (CA4P) Combination Regimen Appears to Suggest Improvement in Patient Survival in Anaplastic Thyroid Cancer. Surgery 2012, 152, 1078–1087. [Google Scholar] [CrossRef] [PubMed]
- Higashiyama, T.; Ito, Y.; Hirokawa, M.; Fukushima, M.; Uruno, T.; Miya, A.; Matsuzuka, F.; Miyauchi, A. Induction Chemotherapy with Weekly Paclitaxel Administration for Anaplastic Thyroid Carcinoma. Thyroid 2010, 20, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Seto, A.; Sugitani, I.; Toda, K.; Kawabata, K.; Takahashi, S.; Saotome, T. Chemotherapy for Anaplastic Thyroid Cancer Using Docetaxel and Cisplatin: Report of Eight Cases. Surg. Today 2015, 45, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Glaser, S.M.; Mandish, S.F.; Gill, B.S.; Balasubramani, G.K.; Clump, D.A.; Beriwal, S. Anaplastic Thyroid Cancer: Prognostic Factors, Patterns of Care, and Overall Survival. Head Neck 2016, 38 (Suppl. 1), E2083–E2090. [Google Scholar] [CrossRef]
- Lin, B.; Ma, H.; Ma, M.; Zhang, Z.; Sun, Z.; Hsieh, I.-Y.; Okenwa, O.; Guan, H.; Li, J.; Lv, W. The Incidence and Survival Analysis for Anaplastic Thyroid Cancer: A SEER Database Analysis. Am. J. Transl. Res. 2019, 11, 5888–5896. [Google Scholar] [PubMed]
- Cabanillas, M.E.; Zafereo, M.; Gunn, G.B.; Ferrarotto, R. Anaplastic Thyroid Carcinoma: Treatment in the Age of Molecular Targeted Therapy. J. Oncol. Pract. 2016, 12, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Smallridge, R.C.; Ain, K.B.; Asa, S.L.; Bible, K.C.; Brierley, J.D.; Burman, K.D.; Kebebew, E.; Lee, N.Y.; Nikiforov, Y.E.; Rosenthal, M.S.; et al. American Thyroid Association Guidelines for Management of Patients with Anaplastic Thyroid Cancer. Thyroid 2012, 22, 1104–1139. [Google Scholar] [CrossRef]
- Ryder, M.; Gild, M.; Hohl, T.M.; Pamer, E.; Knauf, J.; Ghossein, R.; Joyce, J.A.; Fagin, J.A. Genetic and Pharmacological Targeting of CSF-1/CSF-1R Inhibits Tumor-Associated Macrophages and Impairs BRAF-Induced Thyroid Cancer Progression. PLoS ONE 2013, 8, e54302. [Google Scholar] [CrossRef]
- Keam, B.; Kreitman, R.J.; Wainberg, Z.A.; Cabanillas, M.E.; Cho, D.C.; Italiano, A.; Stein, A.; Cho, J.Y.; Schellens, J.H.M.; Wen, P.Y.; et al. Updated Efficacy and Safety Data of Dabrafenib (D) and Trametinib (T) in Patients (Pts) with BRAF V600E–Mutated Anaplastic Thyroid Cancer (ATC). Ann. Oncol. 2018, 29, viii645–viii646. [Google Scholar] [CrossRef]
- Cabanillas, M.E.; Dadu, R.; Iyer, P.; Wanland, K.B.; Busaidy, N.L.; Ying, A.; Gule-Monroe, M.; Wang, J.R.; Zafereo, M.; Hofmann, M.-C. Acquired Secondary RAS Mutation in BRAFV600E-Mutated Thyroid Cancer Patients Treated with BRAF Inhibitors. Thyroid 2020, 30, 1288–1296. [Google Scholar] [CrossRef] [PubMed]
- Knauf, J.A.; Luckett, K.A.; Chen, K.-Y.; Voza, F.; Socci, N.D.; Ghossein, R.; Fagin, J.A. Hgf/Met Activation Mediates Resistance to BRAF Inhibition in Murine Anaplastic Thyroid Cancers. J. Clin. Invest. 2018, 128, 4086–4097. [Google Scholar] [CrossRef] [PubMed]
- Ofir Dovrat, T.; Sokol, E.; Frampton, G.; Shachar, E.; Pelles, S.; Geva, R.; Wolf, I. Unusually Long-Term Responses to Vemurafenib in BRAF V600E Mutated Colon and Thyroid Cancers Followed by the Development of Rare RAS Activating Mutations. Cancer Biol. Ther. 2018, 19, 871–874. [Google Scholar] [CrossRef] [PubMed]
- Cabanillas, M.E.; Drilon, A.; Farago, A.F.; Brose, M.S.; McDermott, R.; Sohal, D.; Oh, D.-Y.; Almubarak, M.; Bauman, J.; Chu, E.; et al. 1916P Larotrectinib Treatment of Advanced TRK Fusion Thyroid Cancer. Ann. Oncol. 2020, 31, S1086. [Google Scholar] [CrossRef]
- Dias-Santagata, D.; Lennerz, J.K.; Sadow, P.M.; Frazier, R.P.; Govinda Raju, S.; Henry, D.; Chung, T.; Kherani, J.; Rothenberg, S.M.; Wirth, L.J. Response to RET-Specific Therapy in RET Fusion-Positive Anaplastic Thyroid Carcinoma. Thyroid 2020, 30, 1384–1389. [Google Scholar] [CrossRef]
- Godbert, Y.; Henriques de Figueiredo, B.; Bonichon, F.; Chibon, F.; Hostein, I.; Pérot, G.; Dupin, C.; Daubech, A.; Belleannée, G.; Gros, A.; et al. Remarkable Response to Crizotinib in Woman With Anaplastic Lymphoma Kinase–Rearranged Anaplastic Thyroid Carcinoma. J. Clin. Oncol. 2015, 33, e84–e87. [Google Scholar] [CrossRef]
- Schneider, T.C.; de Wit, D.; Links, T.P.; van Erp, N.P.; van der Hoeven, J.J.M.; Gelderblom, H.; Roozen, I.C.F.M.; Bos, M.; Corver, W.E.; van Wezel, T.; et al. Everolimus in Patients With Advanced Follicular-Derived Thyroid Cancer: Results of a Phase II Clinical Trial. J. Clin. Endocrinol. Metab. 2017, 102, 698–707. [Google Scholar] [CrossRef]
- Lim, S.M.; Chang, H.; Yoon, M.J.; Hong, Y.K.; Kim, H.; Chung, W.Y.; Park, C.S.; Nam, K.H.; Kang, S.W.; Kim, M.K.; et al. A Multicenter, Phase II Trial of Everolimus in Locally Advanced or Metastatic Thyroid Cancer of All Histologic Subtypes. Ann. Oncol. 2013, 24, 3089–3094. [Google Scholar] [CrossRef]
- Sherman, E.J.; Dunn, L.A.; Ho, A.L.; Baxi, S.S.; Ghossein, R.A.; Fury, M.G.; Haque, S.; Sima, C.S.; Cullen, G.; Fagin, J.A.; et al. Phase 2 Study Evaluating the Combination of Sorafenib and Temsirolimus in the Treatment of Radioactive Iodine-Refractory Thyroid Cancer. Cancer 2017, 123, 4114–4121. [Google Scholar] [CrossRef]
- Takahashi, S.; Kiyota, N.; Yamazaki, T.; Chayahara, N.; Nakano, K.; Inagaki, L.; Toda, K.; Enokida, T.; Minami, H.; Imamura, Y.; et al. A Phase II Study of the Safety and Efficacy of Lenvatinib in Patients with Advanced Thyroid Cancer. Future Oncol. 2019, 15, 717–726. [Google Scholar] [CrossRef] [PubMed]
- Sparano, C.; Godbert, Y.; Attard, M.; Do Cao, C.; Zerdoud, S.; Roudaut, N.; Joly, C.; Berdelou, A.; Hadoux, J.; Lamartina, L.; et al. Limited Efficacy of Lenvatinib in Heavily Pretreated Anaplastic Thyroid Cancer: A French Overview. Endocr. Relat. Cancer 2021, 28, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Wirth, L.J.; Brose, M.S.; Sherman, E.J.; Licitra, L.; Schlumberger, M.; Sherman, S.I.; Bible, K.C.; Robinson, B.; Rodien, P.; Godbert, Y.; et al. Open-Label, Single-Arm, Multicenter, Phase II Trial of Lenvatinib for the Treatment of Patients With Anaplastic Thyroid Cancer. J. Clin. Oncol. 2021, 39, 2359–2366. [Google Scholar] [CrossRef] [PubMed]
- Cabanillas, M.E.; Dadu, R.; Ferrarotto, R.; Liu, S.; Fellman, B.M.; Gross, N.D.; Gule-Monroe, M.; Lu, C.; Grosu, H.; Williams, M.D.; et al. Atezolizumab Combinations with Targeted Therapy for Anaplastic Thyroid Carcinoma (ATC). J. Clin. Oncol. 2020, 38, 6514. [Google Scholar] [CrossRef]
- McIver, B.; Hay, I.D.; Giuffrida, D.F.; Dvorak, C.E.; Grant, C.S.; Thompson, G.B.; van Heerden, J.A.; Goellner, J.R. Anaplastic Thyroid Carcinoma: A 50-Year Experience at a Single Institution. Surgery 2001, 130, 1028–1034. [Google Scholar] [CrossRef]
- Hu, S.; Helman, S.N.; Hanly, E.; Likhterov, I. The Role of Surgery in Anaplastic Thyroid Cancer: A Systematic Review. Am. J. Otolaryngol. 2017, 38, 337–350. [Google Scholar] [CrossRef] [PubMed]
- Green, L.D.; Mack, L.; Pasieka, J.L. Anaplastic Thyroid Cancer and Primary Thyroid Lymphoma: A Review of These Rare Thyroid Malignancies. J. Surg. Oncol. 2006, 94, 725–736. [Google Scholar] [CrossRef]
- Are, C.; Shaha, A.R. Anaplastic Thyroid Carcinoma: Biology, Pathogenesis, Prognostic Factors, and Treatment Approaches. Ann. Surg. Oncol. 2006, 13, 453–464. [Google Scholar] [CrossRef]
- Brignardello, E.; Palestini, N.; Felicetti, F.; Castiglione, A.; Piovesan, A.; Gallo, M.; Freddi, M.; Ricardi, U.; Gasparri, G.; Ciccone, G.; et al. Early Surgery and Survival of Patients with Anaplastic Thyroid Carcinoma: Analysis of a Case Series Referred to a Single Institution between 1999 and 2012. Thyroid 2014, 24, 1600–1606. [Google Scholar] [CrossRef]
- Onoda, N.; Sugino, K.; Higashiyama, T.; Kammori, M.; Toda, K.; Ito, K.-I.; Yoshida, A.; Suganuma, N.; Nakashima, N.; Suzuki, S.; et al. The Safety and Efficacy of Weekly Paclitaxel Administration for Anaplastic Thyroid Cancer Patients: A Nationwide Prospective Study. Thyroid 2016, 26, 1293–1299. [Google Scholar] [CrossRef]
- Wang, J.R.; Zafereo, M.E.; Dadu, R.; Ferrarotto, R.; Busaidy, N.L.; Lu, C.; Ahmed, S.; Gule-Monroe, M.K.; Williams, M.D.; Sturgis, E.M.; et al. Complete Surgical Resection Following Neoadjuvant Dabrafenib Plus Trametinib in BRAFV600E-Mutated Anaplastic Thyroid Carcinoma. Thyroid 2019, 29, 1036–1043. [Google Scholar] [CrossRef] [PubMed]
- Cabanillas, M.E.; Ferrarotto, R.; Garden, A.S.; Ahmed, S.; Busaidy, N.L.; Dadu, R.; Williams, M.D.; Skinner, H.; Gunn, G.B.; Grosu, H.; et al. Neoadjuvant BRAF- and Immune-Directed Therapy for Anaplastic Thyroid Carcinoma. Thyroid 2018, 28, 945–951. [Google Scholar] [CrossRef] [PubMed]
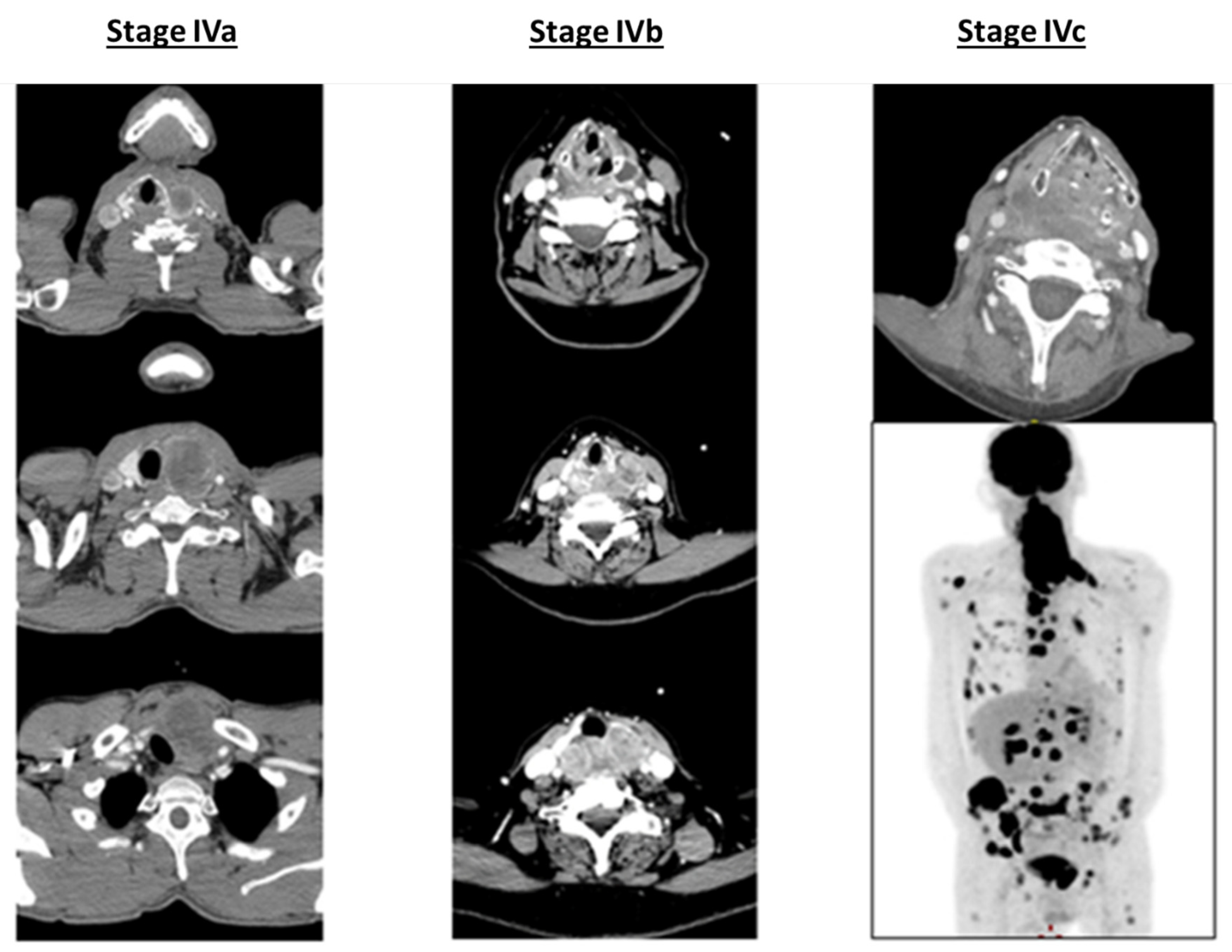


| Authors, References | Study | Number of Patients (Total and According to the Stage | Surgery | Radiotherapy (Dose) | Chemotherapy Protocol (n = Number of Patients) | Outcomes (ORR (n and %), Median OS (Months) and PFS (Months) Local and Distant Control at 1 Year (%)) |
|---|---|---|---|---|---|---|
| [105] | Retrospective | n = 19 metastatic: n = 9 | 12 | 30 | Bleomycin + Cyclophosphamide + 5-FU | OS: 7–12 months ORR, PFS, local control: ND Distant control: 16% |
| [106] | Prospective, randomized, | n = 39 | ND | ND | Doxorubicin (n = 21) Doxorubicin + Cisplatin (n = 18) | ORR: 1/21 (4.8%); OS: ND; PFS: ND ORR: 6/18 (22.2%); OS: ND; PFS: ND |
| [107] | Retrospective | n = 34 | 15 | 30 | Bleomycin + Cyclophosphamide + 5-FU | ORR: 22/34 (64%); OS: 4 months; PFS: ND |
| [108] | Prospective, no randomization, non-controlled | n = 19 (limited to the neck) | 10 | 19 (57.6 Gy) | Doxorubicin per week (n = 19) | OS: 12 months Local control: 68% Distant control: 21% |
| [109] | Prospective randomized but non-controlled | n = 20 metastatic: n = 6 metastatic: n = 3 | 12 −9 −3 | 20 | Doxorubicin + Cisplatin (n = 12) Mitoxantrone (n = 8) | OS: 2–6 months; PFS: 6.5 months Local control: 9/12 (75%); Distant control: 4/12 (33.3%); Local control: 3/8 (37.5%); Distant control: 0/12 (0%); PFS: 4 months |
| [110] | Prospective, no randomization, non-controlled | n = 32 Stage IVb: n = 23 Stage IVc: n = 9 | 0/32 | 32 (30–45 Gy) | Doxorubicin (n = 14/32) | OS: 6 months; PFS: ND Local control: 7/32 (21.9%); Distant control: ND |
| [111] | Retrospective | n = 89 | 10 | ND | Vinblastine or Cisplatin or Doxorubicin or Novantrone | ND |
| [56] | Retrospective | n = 121 Stage IVc: n = 64 | 106 | 58 | n = 64 | OS: 6 months (mean OS: 7.2 +/− 10 months) ORR, PFS, local or distant control: ND |
| [21] | Retrospective | n = 17 | 17 | 12 | ND | OS: 12 months |
| [82] | Prospective study, randomized but non-controlled | n = 55 Stage IVc: n = 17 | 40 | 55 (46 Gy) | Doxorubicin (n = 55) | OS: 2–4.5 months ORR local: 60% ORR distant: 22% PFS: ND |
| [63] | Prospective, no randomization, non-controlled | n = 30 Stage IVa: n = 4 Stage IVb: n = 20 Stage IVc: n = 6 | 7 | 30 (40 Gy) | Doxorubicin + Cisplatin | OS: 10 months Local control: 47%; Distant control: 37% |
| [60] | Retrospective | n = 37 | 19 | 37 (57.6 Gy) | Doxorubicin (n = 37) | OS: 6 months Median Loco-regional-PFS: 10.1 months Local control: 45% |
| [112] | Retrospective | n = 44 Local: n = 12 Regional: n = 12 Distant: n = 20 | 44 | 39 (46–50 Gy) | Doxorubicin + Cisplatin (n = 33) Doxorubicin + Carboplatin (n = 3) Doxorubicin (n = 1) Paclitaxel (n = 1) | OS: 8.5 months ORR: 22/44 (50%) PFS: 6.5 months |
| [113] | Retrospective | n = 13 Stage IVc: n = 6 | 8 | 5 (45–65 Gy) | Doxorubicin (n = 5) | OS: 3.8 months ORR: ND PFS: 2.8 months |
| [6] | Retrospective | n = 547 Stage IVa: n = 69 Stage IVb: n = 242 Stage IVc: n = 233 | n = 301 | 319 | n = 255 Etoposide + Cisplatin (EP) Etoposide + Cisplatin + Doxorubicin 5FU + Cisplatin + Doxorubicin Paclitaxel | Stage-dependent OS: Stage IVa: 7.8 months Stage IVb: 4.8 months Stage IVc: 2.7 months PFS/ORR: ND |
| [114] | Phase 3 | n = 80 Stage IVa: n = 1 Stage IVb: n = 6 Stage IVc: n = 72 ND: n = 1 | 44/80 | n = 28 | Fosbretabulin + Carboplatin + Paclitaxel Control: Carboplatin + Paclitaxel | OS: 8.2 months if surgery versus 4.0 months on the control arm OS: 4.0 months if no surgery and 4.6 months on the control arm |
| [115] | Prospective, controlled, non-randomized | n = 13 Stage IVb, n = 9 Stage IVc, n = 4 | 4 | ND | Paclitaxel | OS and PFS: ND ORR Stage IVb: 33% ORR Stage IVc: 25% |
| [64] | Retrospective | n = 92 Stage IVa: n = 6 Stage IVb, n = 22 Stage IVc, n = 61 ND: n = 3 | 35 | 56 (55 Gy) | 59 Doxorubicin + Cisplatin (n = 56) Carboplatin + Paclitaxel (n = 3) | OS: 7 months; PFS: 5 months Local control: 75%; Distant control: 63% |
| [116] | Retrospective | n = 8 Stage IVb: n = 2 Stage IVc: n = 4 ND: n = 2 | 6 | 5 (40–60 Gy) | Docetaxel + Cisplatin | OS: 30.4 months ORR: 3/8 (37.5%) PFS: 5.5 months |
| [73] | Retrospective | n = 1288 Stage IVc: n = 608 | 0 | 613 | 471 (treatment not available) | OS: 2.27 months |
| [5] | Retrospective | n = 100 Stage IVa: n = 9 Stage IVb: n = 32 Stage IVc: n = 54 ND: n = 5 | 83 | 81 (57.6 Gy) | Doxorubicin weekly (n = 25) Paclitaxel weekly (n = 9) Paclitaxel + Pemetrexed (n = 8) Doxorubicin + Cisplatin (n = 8) Carboplatin + Paclitaxel (n = 14) Tyrosine kinase inhibitors (n = 10) Other (n = 10) | Median OS: 5.7 months Stage-dependent OS (months and % at 1 year): Stage IVa: 26 months (66%) Stage IVb: 11 months (39%) Stage IVc: 3 months (13%) ORR and PFS: ND |
| [74] | Retrospective | n = 30 Stage IVa: n = 2 Stage IVb: n = 22 Stage IVc: n = 6 ND: n = 5 | 27 | 30 (66 Gy) | Doxorubicin + Docetaxel (n = 19) Carboplatin + Paclitaxel (n = 5) Doxorubicin only (n = 4) Cisplatin only (n = 2) | Median OS: 21 months Median PFS: 8.3 months ORR: 19/30 (63.3%) Local control: 93% Distant control: 22% |
| [65] | Retrospective | n = 44 Stage IVa: n = 10 Stage IVb: n = 17 Stage IVc: n = 27 | 23 | 29 | platinum or taxane based agents (n = 46) | OS: 11.9 months (total cohort) and 22.1 months in patients treated with chemotherapy and EBRT TTF: 3.8 months |
| Clinical Trials Gov. Identifier | Treatments/Interventions (Settings) | Phase | Status |
|---|---|---|---|
| NCT03565536 | Sorafenib (Neoadjuvant treatment of ATC) | Phase 2 | Unknown |
| NCT03085056 | Trametinib + Paclitaxel (Advanced ATC) | Early Phase 1 | Recruiting |
| NCT02688608 | Pembrolizumab (Advanced ATC) | Phase 2 | Unknown |
| NCT02244463 | MLN0128 (Advanced ATC) | Phase 2 | Active, not recruiting |
| NCT04739566 | Dabrafenib + Trametinib (Neoadjuvant Strategy in ATC with BRAF mutation) | Phase 2 | Recruiting |
| NCT03122496 | Durvalumab + Tremelimumab + Stereotactic Body Radiotherapy (Advanced ATC) | Phase 1 | Active, not recruiting |
| NCT01236547 | IMRT + Paclitaxel with or without Pazopanib Hydrochloride (Advanced ATC) | Phase 2 | Active, not recruiting |
| NCT05102292 | HLX208 (Advanced ATC with BRAFV600 mutation) | Phase 1b/2 | Recruiting |
| NCT02152137 | Efatutazone + Paclitaxel (Advanced ATC) | Phase 2 | Active, not recruiting |
| NCT04552769 | Abemaciclib (CDK4 + CDK6 inhibitor) (Advanced ATC) | Phase 2 | Recruiting |
| NCT04675710 | Pembrolizumab + Dabrafenib + Trametinib (Neoadjuvant BRAF-Mutated ATC) | Phase 2 | Recruiting |
| NCT04238624 | Cemiplimab + Dabrafenib + Trametinib (Advanced ATC) | Phase 2 | Recruiting |
| NCT04420754 | AIC100 Chimeric Antigen Receptor T-cells (Relapsed/Refractory Thyroid Cancer) | Phase 1 | Recruiting |
| NCT03975231 | Dabrafenib + Trametinib + IMRT in (Advanced BRAF Mutated ATC) | Phase 1 | Recruiting |
| NCT03449108 | LN-145/LN-145-S1 (Autologous Centrally Manufactured Tumor Infiltrating Lymphocytes) (Advanced ATC) | Phase 2 | Recruiting |
| NCT04592484 | CDK-002 (exoSTING) (Advanced/Metastatic, Recurrent, Injectable ATC) | Phase 1 | Recruiting |
| NCT03181100 | Cohort I (BRAF mutation): Vemurafenib + Cobimetinib + Atezolizumab. Cohort II (RAS, NF1 or NF2 mutations): Cobimetinib + Atezolizumab Cohort III (non BRAF or RAS mutation): Bevacizumab + Atezolizumab Cohort IV: Nab-paclitaxel + Atezolizumab | Phase 2 | Recruiting |
| NCT03246958 | Nivolumab + Ipilimumab (Advanced ATC) | Phase 2 | Active non-recruiting |
| NCT04400474 | Cabozantinib + Atezolizumab (Advanced ATC) | Phase 2 | Recruiting |
| NCT04579757 | Surufatinib + Tislelizumab (Advanced ATC) | Phase 1/2 | Recruiting |
| NCT04759911 | Selpercatinib (Neoadjuvant ATC with RET alterations) | Phase 2 | Recruiting |
| Treatment | Protocols and Dose | |
|---|---|---|
| Chemotherapy | Every 3 or 4 weeks Doxorubicin (60 mg/m2) + Cisplatin (120 mg/m2) every 4 weeks Paclitaxel (175 mg/m2) + Carboplatin (AUC 5) every 3 weeks Docetaxel (60 mg/m2) + Doxorubicin (60 mg/m2) every 3–4 weeks Paclitaxel (135–200 mg/m2) every 3–4 weeks Doxorubicin (60–75 mg/m2) every 3 weeks Every week Paclitaxel 50–100 mg/m2 + Carboplatin AUC2 Docetaxel (20 mg/m2) + Doxorubicin (20 mg/m2) Paclitaxel (30–60 mg/m2) Docetaxel (20 mg/m2) | |
| BRAF and MEK inhibitors | Dabrafenib 150 mg twice daily + Trametinib 2 mg once daily | |
| RET inhibitor | Selpercatinib 160 mg twice daily, reduced to 120 mg twice daily in patients weighing less than 50 kg | |
| Praseltinib 400 mg per day | ||
| NTRK inhibitor | Larotrectinib 100 mg twice daily Entrectinib 600 mg once daily | |
| ALK inhibitor | Crizotinib 250 mg twice daily | |
| Larotrectinib 100 mg twice daily | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jannin, A.; Escande, A.; Al Ghuzlan, A.; Blanchard, P.; Hartl, D.; Chevalier, B.; Deschamps, F.; Lamartina, L.; Lacroix, L.; Dupuy, C.; et al. Anaplastic Thyroid Carcinoma: An Update. Cancers 2022, 14, 1061. https://doi.org/10.3390/cancers14041061
Jannin A, Escande A, Al Ghuzlan A, Blanchard P, Hartl D, Chevalier B, Deschamps F, Lamartina L, Lacroix L, Dupuy C, et al. Anaplastic Thyroid Carcinoma: An Update. Cancers. 2022; 14(4):1061. https://doi.org/10.3390/cancers14041061
Chicago/Turabian StyleJannin, Arnaud, Alexandre Escande, Abir Al Ghuzlan, Pierre Blanchard, Dana Hartl, Benjamin Chevalier, Frédéric Deschamps, Livia Lamartina, Ludovic Lacroix, Corinne Dupuy, and et al. 2022. "Anaplastic Thyroid Carcinoma: An Update" Cancers 14, no. 4: 1061. https://doi.org/10.3390/cancers14041061
APA StyleJannin, A., Escande, A., Al Ghuzlan, A., Blanchard, P., Hartl, D., Chevalier, B., Deschamps, F., Lamartina, L., Lacroix, L., Dupuy, C., Baudin, E., Do Cao, C., & Hadoux, J. (2022). Anaplastic Thyroid Carcinoma: An Update. Cancers, 14(4), 1061. https://doi.org/10.3390/cancers14041061






