Using a 31-Gene Expression Profile Test to Stratify Patients with Stage I–II Cutaneous Melanoma According to Recurrence Risk: Update to a Prospective, Multicenter Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. 31-GEP Testing
2.3. Statistical Analysis
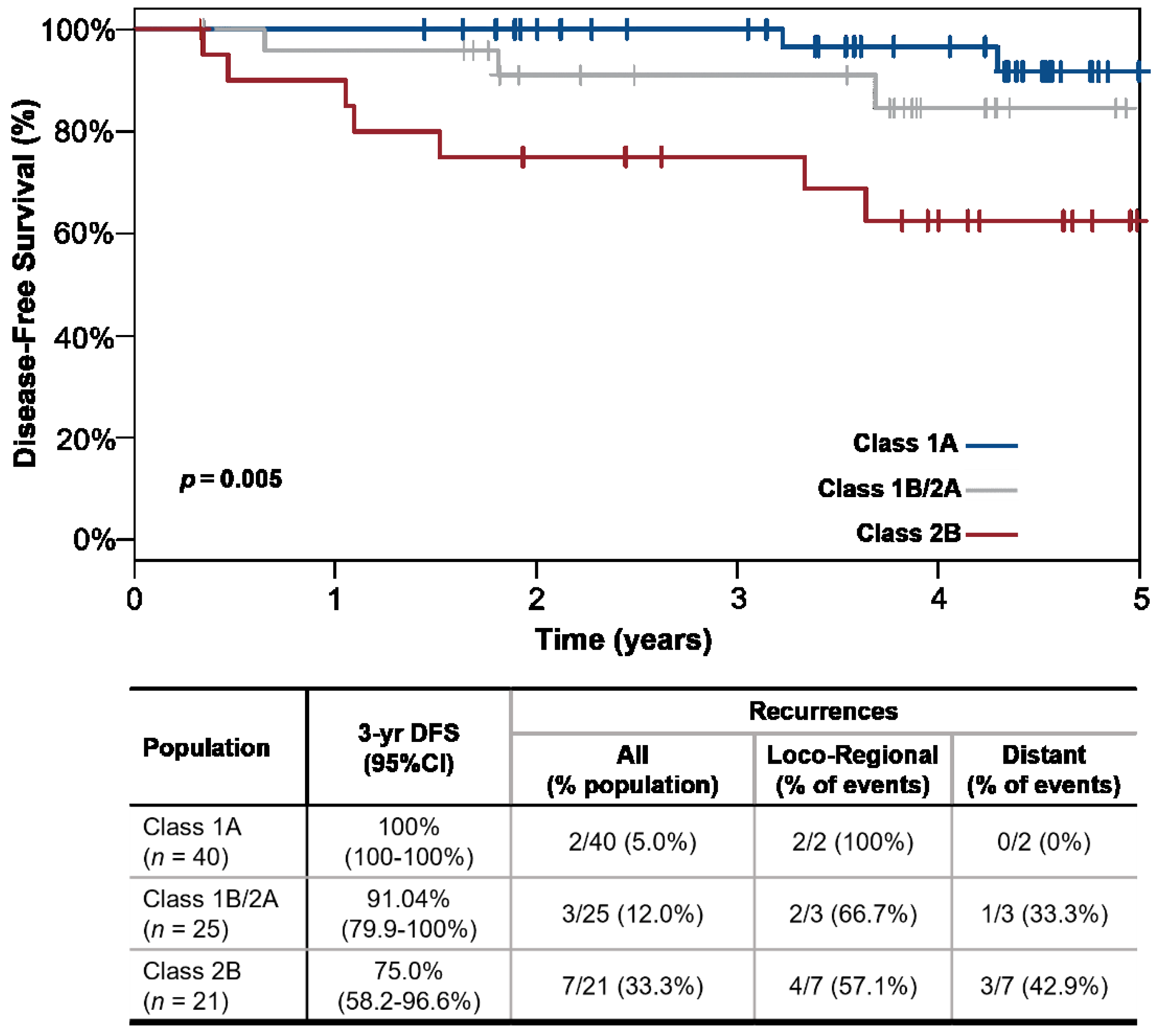
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Glazer, A.M.; Winkelmann, R.R.; Farberg, A.S.; Rigel, D.S. Analysis of Trends in US Melanoma Incidence and Mortality. JAMA Dermatol. 2017, 153, 225–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- American Cancer Society. Cancer Facts & Figures 2020; American Cancer Society: Atlanta, GA, USA, 2020; Volume 76. [Google Scholar]
- Curti, B.D.; Faries, M.B. Recent Advances in the Treatment of Melanoma. N. Engl. J. Med. 2021, 384, 2229–2240. [Google Scholar] [CrossRef] [PubMed]
- Herbert, A.; Koo, M.M.; Barclay, M.E.; Greenberg, D.C.; Abel, G.A.; Levell, N.J.; Lyratzopoulos, G. Stage-Specific Incidence Trends of Melanoma in an English Region, 1996–2015: Longitudinal Analyses of Population-Based Data. Melanoma Res. 2020, 30, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Kwatra, S.G.; Hines, H.; Semenov, Y.R.; Trotter, S.C.; Holland, E.; Leachman, S. A Dermatologist’s Guide to Implementation of Gene Expression Profiling in the Management of Melanoma. J. Clin. Aesthet. Dermatol. 2020, 13, S3–S14. [Google Scholar] [PubMed]
- Gershenwald, J.E.; Scolyer, R.A.; Hess, K.R.; Sondak, V.K.; Long, G.V.; Ross, M.I.; Lazar, A.J.; Faries, M.B.; Kirkwood, J.M.; McArthur, G.A.; et al. Melanoma Staging: Evidence-Based Changes in the American Joint Committee on Cancer Eighth Edition Cancer Staging Manual. CA Cancer J. Clin. 2017, 67, 472–492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rockberg, J.; Amelio, J.M.; Taylor, A.; Jörgensen, L.; Ragnhammar, P.; Hansson, J. Epidemiology of Cutaneous Melanoma in Sweden—Stage-Specific Survival and Rate of Recurrence. Int. J. Cancer 2016, 139, 2722–2729. [Google Scholar] [CrossRef] [PubMed]
- Podlipnik, S.; Carrera, C.; Boada, A.; Richarz, N.A.; López-Estebaranz, J.L.; Pinedo-Moraleda, F.; Elosua-González, M.; Martín-González, M.M.; Carrillo-Gijón, R.; Redondo, P.; et al. Early Outcome of a 31-Gene Expression Profile Test in 86 AJCC Stage IB-II Melanoma Patients. A Prospective Multicentre Cohort Study. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 857–862. [Google Scholar] [CrossRef] [PubMed]
- Gerami, P.; Cook, R.W.; Wilkinson, J.; Russell, M.C.; Dhillon, N.; Amaria, R.N.; Gonzalez, R.; Lyle, S.; Johnson, C.E.; Oelschlager, K.M.; et al. Development of a Prognostic Genetic Signature to Predict the Metastatic Risk Associated with Cutaneous Melanoma. Clin. Cancer Res. 2015, 21, 175–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zager, J.S.; Gastman, B.R.; Leachman, S.; Gonzalez, R.C.; Fleming, M.D.; Ferris, L.K.; Ho, J.; Miller, A.R.; Cook, R.W.; Covington, K.R.; et al. Performance of a Prognostic 31-Gene Expression Profile in an Independent Cohort of 523 Cutaneous Melanoma Patients. BMC Cancer 2018, 18, 130. [Google Scholar] [CrossRef] [PubMed]
- Keller, J.; Schwartz, T.L.; Lizalek, J.M.; Chang, E.; Patel, A.D.; Hurley, M.Y.; Hsueh, E.C. Prospective Validation of the Prognostic 31-gene Expression Profiling Test in Primary Cutaneous Melanoma. Cancer Med. 2019, 8, 2205–2212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsueh, E.C.; DeBloom, J.R.; Lee, J.H.; Sussman, J.J.; Covington, K.R.; Caruso, H.G.; Quick, A.P.; Cook, R.W.; Slingluff, C.L.; McMasters, K.M. Long-Term Outcomes in a Multicenter, Prospective Cohort Evaluating the Prognostic 31-Gene Expression Profile for Cutaneous Melanoma. JCO Precis. Oncol. 2021, 5, 589–601. [Google Scholar] [CrossRef] [PubMed]
- Arnot, S.P.; Han, G.; Fortino, J.; Han, D.; Fowler, G.; Vetto, J.T. Utility of a 31-Gene Expression Profile for Predicting Outcomes in Patients with Primary Cutaneous Melanoma Referred for Sentinel Node Biopsy. Am. J. Surg. 2021, 221, 1195–1199. [Google Scholar] [CrossRef] [PubMed]
- Guy, G.P., Jr.; Thomas, C.C.; Thompson, T.; Watson, M.; Massetti, G.M.; Richardson, L.C. Centers for Disease Control and Prevention (CDC) Vital Signs: Melanoma Incidence and Mortality Trends and Projections—United States, 1982–2030. MMWR Morb. Mortal. Wkly. Rep. 2015, 64, 591–596. [Google Scholar] [PubMed]
- Gastman, B.R.; Gerami, P.; Kurley, S.J.; Cook, R.W.; Leachman, S.; Vetto, J.T. Identification of Patients at Risk of Metastasis Using a Prognostic 31-Gene Expression Profile in Subpopulations of Melanoma Patients with Favorable Outcomes by Standard Criteria. J. Am. Acad. Dermatol. 2019, 80, 149–157.e4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greenhaw, B.N.; Zitelli, J.A.; Brodland, D.G. Estimation of Prognosis in Invasive Cutaneous Melanoma: An Independent Study of the Accuracy of a Gene Expression Profile Test. Dermatol. Surg. 2018, 44, 1494–1500. [Google Scholar] [CrossRef] [PubMed]
| Clinicopathologic Feature | Class 1A (n = 40) | Class 1B/2A (n = 25) | Class 2B (n = 21) | Combined (n = 86) | p-Value † |
|---|---|---|---|---|---|
| Age (years) | |||||
| Median (Range) | 58 (26–79) | 66 (23–82) | 65 (32–86) | 60 (23–86) | p = 0.133 |
| Sex | |||||
| Female | 24/40 (60%) | 14/25 (56%) | 8/21 (38.1%) | 46/86 (53.49%) | p = 0.253 |
| Male | 16/40 (40%) | 11/25 (44%) | 13/21 (61.9%) | 40/86 (46.51%) | |
| AJCC 7th Ed. Stage | |||||
| IB | 32/40 (80%) | 9/25 (36%) | 3/21 (14.29%) | 44/86 (51.16%) | p < 0.001 |
| IIA | 5/40 (12.5%) | 10/25 (40%) | 3/21 (14.29%) | 18/86 (20.93%) | |
| IIB | 3/40 (7.5%) | 3/25 (12%) | 9/21 (42.86%) | 15/86 (17.44%) | |
| IIC | 0/40 (0%) | 3/25 (12%) | 6/21 (28.57%) | 9/86 (10.47%) | |
| AJCC 8th Ed. Stage | p < 0.001 | ||||
| IA | 17/40 (43%) | 2/25 (8%) | 0/21 (0%) | 19/86 (22%) | |
| IB | 16/40 (40%) | 7/25 (28%) | 3/21 (14%) | 26/86 (30%) | |
| IIA | 4/40 (10%) | 10/25 (40%) | 2/21 (10%) | 16/86 (19%) | |
| IIB | 3/40 (8%) | 4/25 (16%) | 11/21 (52%) | 18/86 (21%) | |
| IIC | 0/40 (0%) | 2/25 (8%) | 5/21 (24%) | 7/86 (8%) | |
| Breslow Thickness (mm) | |||||
| Median (Range) | 1.3 (0.4–7.0) | 2.0 (0.8–10) | 3.5 (1.1–15.0) | 1.7 (0.4–15.0) | p < 0.001 |
| Mitoses (1/mm2) | |||||
| Median (Range) | 1 (0–6) | 3 (0–12) | 8 (0–32) | 2 (0–32) | p < 0.001 |
| Ulceration | |||||
| Absent | 39/40 (97.5%) | 15/25 (60%) | 6/21 (28.57%) | 60/86 (69.77%) | p < 0.001 |
| Present | 1/40 (2.5%) | 10/25 (40%) | 15/21 (71.43%) | 26/86 (30.23%) | |
| Disease Relapse | |||||
| No | 38/40 (95%) | 22/25 (88%) | 14/21 (66.67%) | 74/86 (86.05%) | p = 0.009 |
| Yes | 2/40 (5%) | 3/25 (12%) | 7/21 (33.33%) | 12/86 (13.95%) | |
| Follow-up (years) | |||||
| Median follow up with no relapse, years, median (range) | 4.2 (1.4–5.3) | 3.8 (0.3–4.9) | 4.1 (0.3–5.0) | 3.9 (0.3–5.3) | p = 0.237 |
| Time to relapse, years, median (range) | 3.8 (3.2–4.3) | 1.8 (0.6–3.7) | 1.1 (0.3–3.6) | 1.7 (0.3–4.3) | p = 0.283 |
| Feature | Univariate HR (95% CI) | p-Value |
|---|---|---|
| 31-GEP Class 1A 31-GEP Class 1B/2A | reference 2.8 (0.5–17.2) | - 0.253 |
| 31-GEP Class 2B | 8.4 (1.7–40.7) | 0.008 |
| Stage I–IIA | reference | - |
| Stage IIB–IIC (V7) | 8.8 (2.4–32.6) | 0.001 |
| Stage IIB–IIC (V8) | 8.5 (2.3–31.4) | 0.001 |
| Metric Class 1A vs. Class 2B | %, (95% CI) | Likelihood Ratios |
|---|---|---|
| Sensitivity | 77.8% (40.2–96.1%) | Positive: 2.9 (1.6–5.1) |
| Specificity | 73.1% (58.7–84.0%) | |
| PPV | 33.3% (15.8–56.9%) | Negative: 0.3 (0.1–1.0) |
| NPV | 95.0% (81.8–99.1%) | |
| Metric Class 1 vs. Class 2 | %, (95% CI) | Likelihood Ratios |
| Sensitivity | 75.0% (42.8–93.3%) | Positive: 2.3 (1.5–3.7) |
| Specificity | 67.6% (55.6–77.7%) | |
| PPV | 27.3% (13.9–45.8%) | Negative: 0.4 (0.1–1.0) |
| NPV | 94.3% (83.4–98.5%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Podlipnik, S.; Boada, A.; López-Estebaranz, J.L.; Martín-González, M.M.; Redondo, P.; Martin, B.; Quick, A.P.; Bailey, C.N.; Kurley, S.J.; Cook, R.W.; et al. Using a 31-Gene Expression Profile Test to Stratify Patients with Stage I–II Cutaneous Melanoma According to Recurrence Risk: Update to a Prospective, Multicenter Study. Cancers 2022, 14, 1060. https://doi.org/10.3390/cancers14041060
Podlipnik S, Boada A, López-Estebaranz JL, Martín-González MM, Redondo P, Martin B, Quick AP, Bailey CN, Kurley SJ, Cook RW, et al. Using a 31-Gene Expression Profile Test to Stratify Patients with Stage I–II Cutaneous Melanoma According to Recurrence Risk: Update to a Prospective, Multicenter Study. Cancers. 2022; 14(4):1060. https://doi.org/10.3390/cancers14041060
Chicago/Turabian StylePodlipnik, Sebastian, Aram Boada, Jose L. López-Estebaranz, Manuel M. Martín-González, Pedro Redondo, Brian Martin, Ann P. Quick, Christine N. Bailey, Sarah J. Kurley, Robert W. Cook, and et al. 2022. "Using a 31-Gene Expression Profile Test to Stratify Patients with Stage I–II Cutaneous Melanoma According to Recurrence Risk: Update to a Prospective, Multicenter Study" Cancers 14, no. 4: 1060. https://doi.org/10.3390/cancers14041060
APA StylePodlipnik, S., Boada, A., López-Estebaranz, J. L., Martín-González, M. M., Redondo, P., Martin, B., Quick, A. P., Bailey, C. N., Kurley, S. J., Cook, R. W., & Puig, S. (2022). Using a 31-Gene Expression Profile Test to Stratify Patients with Stage I–II Cutaneous Melanoma According to Recurrence Risk: Update to a Prospective, Multicenter Study. Cancers, 14(4), 1060. https://doi.org/10.3390/cancers14041060








