PBRM1 Immunohistochemical Expression Profile Correlates with Histomorphological Features and Endothelial Expression of Tumor Vasculature for Clear Cell Renal Cell Carcinoma
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Case Selection
2.2. Evaluation of Histomorphological Features
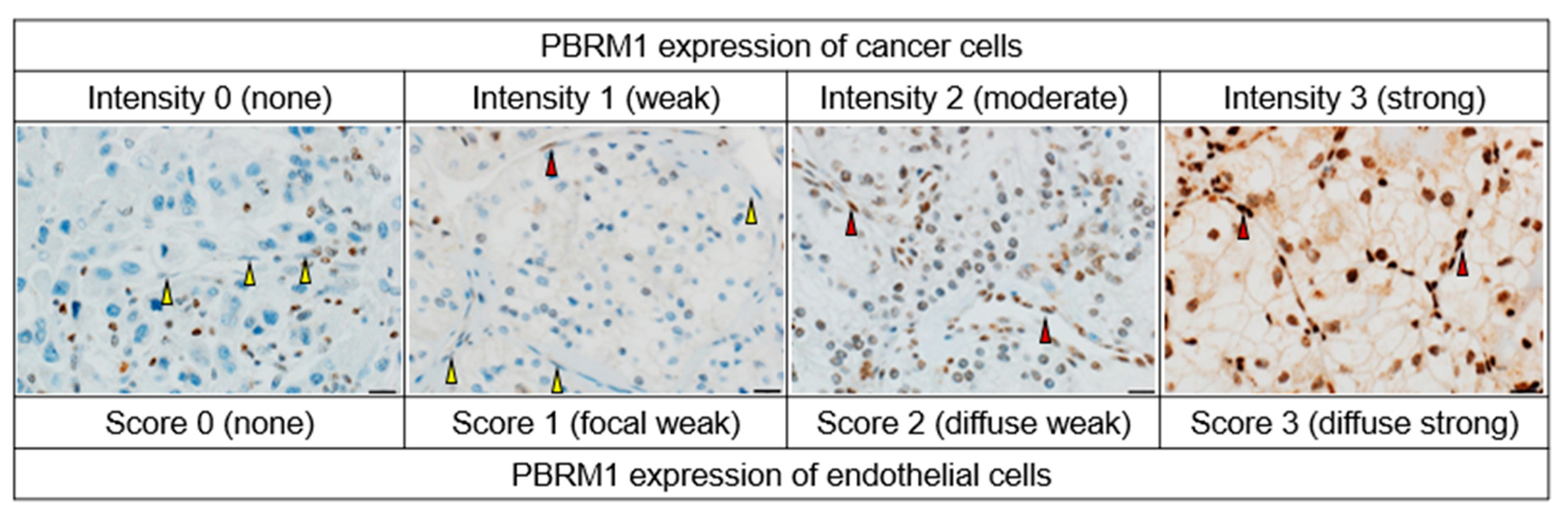
2.3. Tissue Microarray (TMA) Construction and Immunohistochemistry of PBRM1
2.4. Statistical Analysis
3. Results
3.1. Patients’ Characteristics and PBRM1 Expression in Cancer Cells
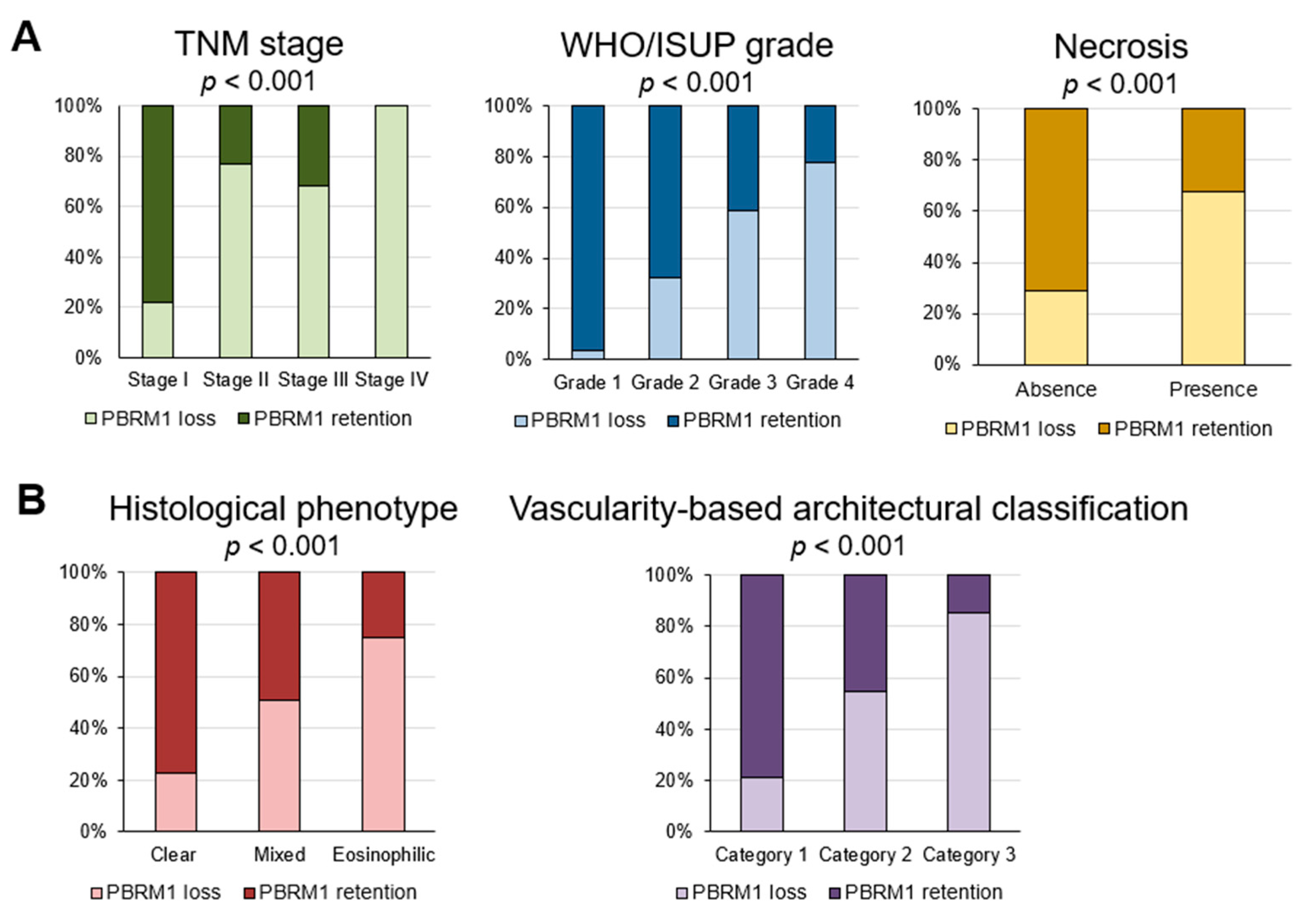
3.2. Association of PBRM1 Expression in Cancer Cells with Clinicopathological Factors
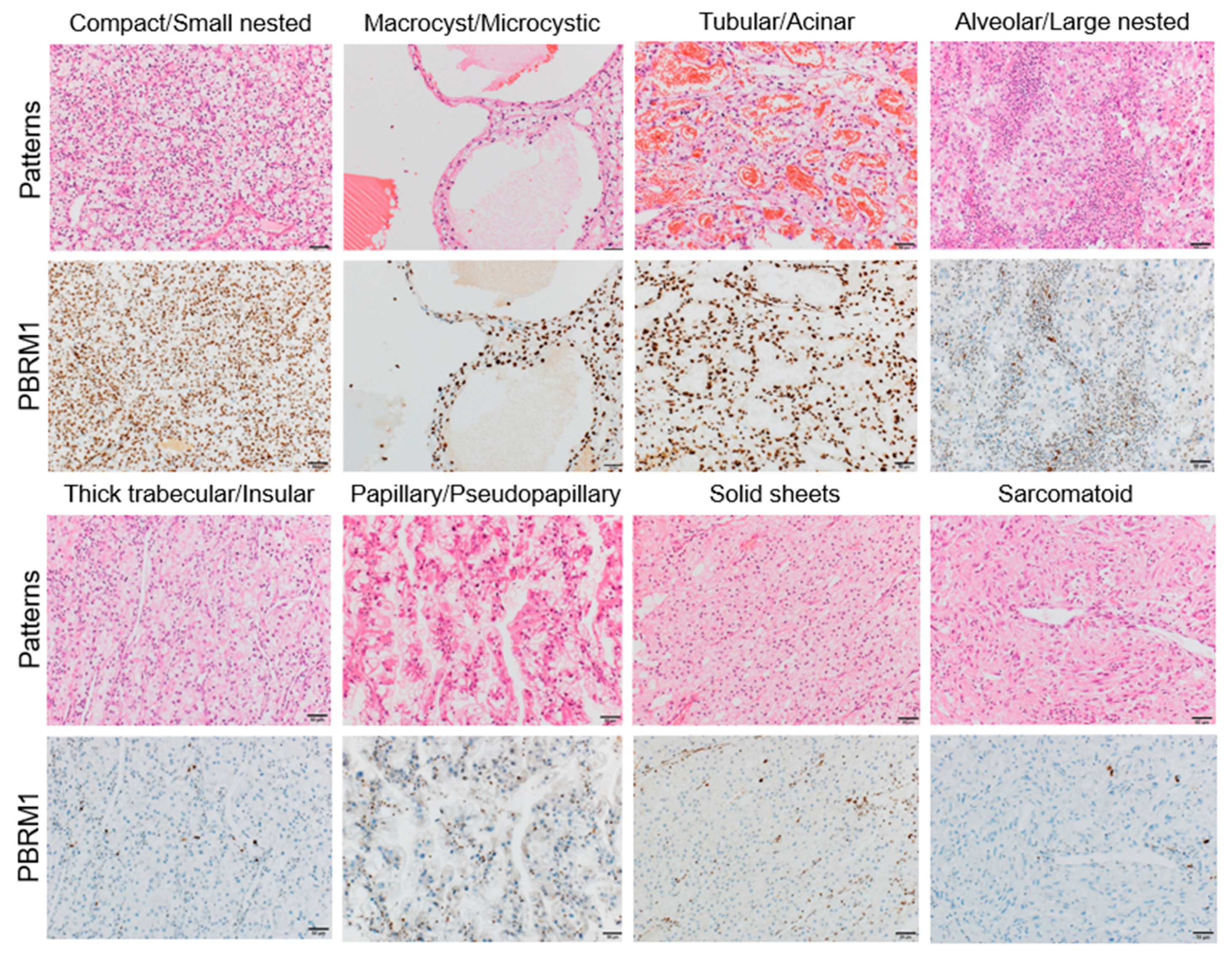
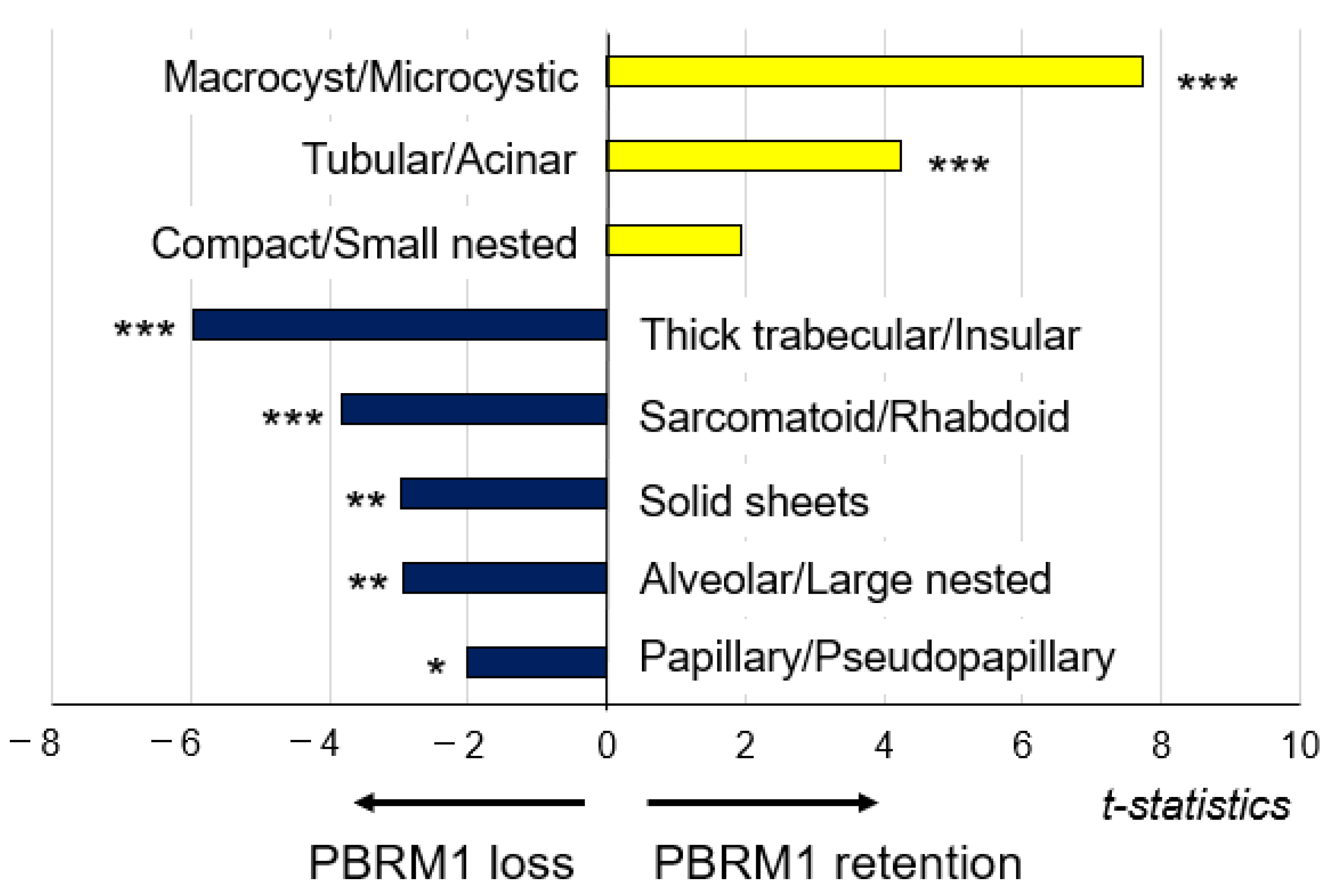
3.3. Association of PBRM1 Expression in Cancer Cells with Architectural Patterns
3.4. Association between Cancer Cells and Endothelial Cells
3.4.1. Correlation between PBRM1 Expression in Cancer Cells and Endothelial Cells
3.4.2. Prognostic Significance of PBRM1 Expression in Cancer Cells and Endothelial Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shuch, B.; Amin, A.; Armstrong, A.J.; Eble, J.N.; Ficarra, V.; Lopez-Beltran, A.; Martignoni, G.; Rini, B.I.; Kutikov, A. Understanding pathologic variants of renal cell carcinoma: Distilling therapeutic opportunities from biologic complexity. Eur. Urol. 2015, 67, 85–97. [Google Scholar] [CrossRef] [PubMed]
- George, D.J.; Kaelin, W.G., Jr. The von Hippel-Lindau protein, vascular endothelial growth factor, and kidney cancer. N. Engl. J. Med. 2003, 349, 419–421. [Google Scholar] [CrossRef] [PubMed]
- Rini, B.I. Vascular endothelial growth factor-targeted therapy in metastatic renal cell carcinoma. Cancer 2009, 115, 2306–2312. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Escudier, B.; McDermott, D.F.; George, S.; Hammers, H.J.; Srinivas, S.; Tykodi, S.S.; Sosman, J.A.; Procopio, G.; Plimack, E.R.; et al. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2015, 373, 1803–1813. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Tannir, N.M.; McDermott, D.F.; Arén Frontera, O.; Melichar, B.; Choueiri, T.K.; Plimack, E.R.; Barthélémy, P.; Porta, C.; George, S.; et al. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2018, 378, 1277–1290. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.L.; Siefker-Radtke, A.; McConkey, D.; McGregor, B. Renal Cell and Urothelial Carcinoma: Biomarkers for New Treatments. Am. Soc. Clin. Oncol. Educ. Book 2020, 40, 1–11. [Google Scholar] [CrossRef]
- Varela, I.; Tarpey, P.; Raine, K.; Huang, D.; Ong, C.K.; Stephens, P.; Davies, H.; Jones, D.; Lin, M.L.; Teague, J.; et al. Exome sequencing identifies frequent mutation of the SWI/SNF complex gene PBRM1 in renal carcinoma. Nature 2011, 469, 539–542. [Google Scholar] [CrossRef] [Green Version]
- The Cancer Genome Atlas Research Network. Comprehensive molecular characterization of clear cell renal cell carcinoma. Nature 2013, 499, 43–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Da Costa, W.H.; Rezende, M.; Carneiro, F.C.; Rocha, R.M.; da Cunha, I.W.; Carraro, D.M.; Guimaraes, G.C.; de Cassio Zequi, S. Polybromo-1 (PBRM1), a SWI/SNF complex subunit is a prognostic marker in clear cell renal cell carcinoma. BJU Int. 2014, 113, E157–E163. [Google Scholar] [CrossRef] [Green Version]
- Joseph, R.W.; Kapur, P.; Serie, D.J.; Parasramka, M.; Ho, T.H.; Cheville, J.C.; Frenkel, E.; Parker, A.S.; Brugarolas, J. Clear Cell Renal Cell Carcinoma Subtypes Identified by BAP1 and PBRM1 Expression. J. Urol. 2016, 195, 180–187. [Google Scholar] [CrossRef] [Green Version]
- Ohsugi, H.; Yoshida, T.; Ohe, C.; Ikeda, J.; Sugi, M.; Kinoshita, H.; Tsuta, K.; Matsuda, T. The SSPN Score, a Novel Scoring System Incorporating PBRM1 Expression, Predicts Postoperative Recurrence for Patients with Non-metastatic Clear Cell Renal Cell Carcinoma. Ann. Surg. Oncol. 2021, 28, 2359–2366. [Google Scholar] [CrossRef] [PubMed]
- Miao, D.; Margolis, C.A.; Gao, W.; Voss, M.H.; Li, W.; Martini, D.J.; Norton, C.; Bossé, D.; Wankowicz, S.M.; Cullen, D.; et al. Genomic correlates of response to immune checkpoint therapies in clear cell renal cell carcinoma. Science 2018, 359, 801–806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.D.; Kong, W.; Peterson, C.B.; McGrail, D.J.; Hoang, A.; Zhang, X.; Lam, T.; Pilie, P.G.; Zhu, H.; Beckermann, K.E.; et al. PBRM1 loss defines a nonimmunogenic tumor phenotype associated with checkpoint inhibitor resistance in renal carcinoma. Nat. Commun. 2020, 11, 2135. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Ohe, C.; Ikeda, J.; Atsumi, N.; Ohsugi, H.; Sugi, M.; Higasa, K.; Saito, R.; Tsuta, K.; Matsuda, T.; et al. Eosinophilic features in clear cell renal cell carcinoma correlate with outcomes of immune checkpoint and angiogenesis blockade. J. Immunother. Cancer 2021, e002922. [Google Scholar] [CrossRef] [PubMed]
- Ohe, C.; Yoshida, T.; Amin, M.B.; Atsumi, N.; Ikeda, J.; Saiga, K.; Noda, Y.; Yasukochi, Y.; Ohashi, R.; Ohsugi, H.; et al. Development and validation of a vascularity-based architectural classification for clear cell renal cell carcinoma: Correlation with conventional pathological prognostic factors, gene expression patterns, and clinical outcomes. Mod. Pathol. 2021, 1–9. [Google Scholar] [CrossRef]
- Verine, J.; Colin, D.; Nheb, M.; Prapotnich, D.; Ploussard, G.; Cathelineau, X.; Desgrandchamps, F.; Mongiat-Artus, P.; Feugeas, J.P. Architectural Patterns are a Relevant Morphologic Grading System for Clear Cell Renal Cell Carcinoma Prognosis Assessment: Comparisons with WHO/ISUP Grade and Integrated Staging Systems. Am. J. Surg. Pathol. 2018, 42, 423–441. [Google Scholar] [CrossRef]
- Cai, Q.; Christie, A.; Rajaram, S.; Zhou, Q.; Araj, E.; Chintalapati, S.; Cadeddu, J.; Margulis, V.; Pedrosa, I.; Rakheja, D.; et al. Ontological analyses reveal clinically-significant clear cell renal cell carcinoma subtypes with convergent evolutionary trajectories into an aggressive type. EBioMedicine 2020, 51, 102526. [Google Scholar] [CrossRef] [Green Version]
- Peña-Llopis, S.; Vega-Rubín-de-Celis, S.; Liao, A.; Leng, N.; Pavía-Jiménez, A.; Wang, S.; Yamasaki, T.; Zhrebker, L.; Sivanand, S.; Spence, P.; et al. BAP1 loss defines a new class of renal cell carcinoma. Nat. Genet. 2012, 44, 751–759. [Google Scholar] [CrossRef]
- Heidegger, I.; Pircher, A.; Pichler, R. Targeting the Tumor Microenvironment in Renal Cell Cancer Biology and Therapy. Front. Oncol. 2019, 9, 490. [Google Scholar] [CrossRef] [Green Version]
- Moch, H.; Humphrey, P.A.; Ulbright, T.M.; Reuter, V.E. WHO Classification of Tumours of the Urinary System and Male Genital Organs, 4th ed.; IARC: Lyon, France, 2016. [Google Scholar]
- Brierley, J.D.; Gospodarowics, M.K.; Wittekind, C. Union for International Cancer Control. In TNM Classification of Malignant Tumours, 8th ed.; Wiley: New York, NY, USA, 2017. [Google Scholar]
- Delahunt, B.; Srigley, J.R.; Judge, M.J.; Amin, M.B.; Billis, A.; Camparo, P.; Evans, A.J.; Fleming, S.; Griffiths, D.F.; Lopez-Beltran, A.; et al. Data set for the reporting of carcinoma of renal tubular origin: Recommendations from the International Collaboration on Cancer Reporting (ICCR). Histopathology 2019, 74, 377–390. [Google Scholar] [CrossRef] [Green Version]
- Ikeda, J.; Ohe, C.; Yoshida, T.; Ohsugi, H.; Sugi, M.; Tsuta, K.; Kinoshita, H. PD-L1 Expression and Clinicopathological Factors in Renal Cell Carcinoma: A Comparison of Antibody Clone 73-10 with Clone 28-8. Anticancer Res. 2021, 41, 4577–4586. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Ohe, C.; Ikeda, J.; Atsumi, N.; Saito, R.; Taniguchi, H.; Ohsugi, H.; Sugi, M.; Tsuta, K.; Matsuda, T.; et al. Integration of NRP1, RGS5, and FOXM1 expression, and tumour necrosis, as a postoperative prognostic classifier based on molecular subtypes of clear cell renal cell carcinoma. J. Pathol. Clin. Res. 2021, 7, 590–603. [Google Scholar] [CrossRef] [PubMed]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kapur, P.; Christie, A.; Rajaram, S.; Brugarolas, J. What morphology can teach us about renal cell carcinoma clonal evolution. Kidney Cancer J. 2020, 18, 68–76. [Google Scholar] [CrossRef]
- Högner, A.; Krause, H.; Jandrig, B.; Kasim, M.; Fuller, T.F.; Schostak, M.; Erbersdobler, A.; Patzak, A.; Kilic, E. PBRM1 and VHL expression correlate in human clear cell renal cell carcinoma with differential association with patient’s overall survival. Urol. Oncol. 2018, 36, 94-e1. [Google Scholar] [CrossRef]
- Pawłowski, R.; Mühl, S.M.; Sulser, T.; Krek, W.; Moch, H.; Schraml, P. Loss of PBRM1 expression is associated with renal cell carcinoma progression. Int. J. Cancer 2013, 132, E11–E17. [Google Scholar] [CrossRef] [PubMed]
- Nam, S.J.; Lee, C.; Park, J.H.; Moon, K.C. Decreased PBRM1 expression predicts unfavorable prognosis in patients with clear cell renal cell carcinoma. Urol. Oncol. 2015, 33, e9–e16. [Google Scholar] [CrossRef]
- Wang, Z.; Peng, S.; Guo, L.; Xie, H.; Wang, A.; Shang, Z.; Niu, Y. Prognostic and clinicopathological value of PBRM1 expression in renal cell carcinoma. Clin. Chim. Acta 2018, 486, 9–17. [Google Scholar] [CrossRef]
- Bihr, S.; Ohashi, R.; Moore, A.L.; Rüschoff, J.H.; Beisel, C.; Hermanns, T.; Mischo, A.; Corrò, C.; Beyer, J.; Beerenwinkel, N.; et al. Expression and Mutation Patterns of PBRM1, BAP1 and SETD2 Mirror Specific Evolutionary Subtypes in Clear Cell Renal Cell Carcinoma. Neoplasia 2019, 21, 247–256. [Google Scholar] [CrossRef]
- Kim, S.H.; Park, W.S.; Park, E.Y.; Park, B.; Joo, J.; Joung, J.Y.; Seo, H.K.; Lee, K.H.; Chung, J. The prognostic value of BAP1, PBRM1, pS6, PTEN, TGase2, PD-L1, CA9, PSMA, and Ki-67 tissue markers in localized renal cell carcinoma: A retrospective study of tissue microarrays using immunohistochemistry. PLoS ONE 2017, 12, e0179610. [Google Scholar] [CrossRef] [Green Version]
- Gerlinger, M.; Rowan, A.J.; Horswell, S.; Math, M.; Larkin, J.; Endesfelder, D.; Gronroos, E.; Martinez, P.; Matthews, N.; Stewart, A.; et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N. Engl. J. Med. 2012, 366, 883–892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, W.; Wang, Z.; Cai, B.; Yuan, Y.; Kong, W.; Zhang, J.; Chen, Y.; Liu, Q.; Huang, Y.; Huang, J.; et al. Expression of PBRM1 as a prognostic predictor in metastatic renal cell carcinoma patients treated with tyrosine kinase inhibitor. Int. J. Clin. Oncol. 2020, 25, 338–346. [Google Scholar] [CrossRef] [PubMed]
- Braun, D.A.; Ishii, Y.; Walsh, A.M.; Van Allen, E.M.; Wu, C.J.; Shukla, S.A.; Choueiri, T.K. Clinical Validation of PBRM1 Alterations as a Marker of Immune Checkpoint Inhibitor Response in Renal Cell Carcinoma. JAMA Oncol. 2019, 5, 1631–1633. [Google Scholar] [CrossRef] [PubMed]
- Dizman, N.; Lyou, Y.; Salgia, N.; Bergerot, P.G.; Hsu, J.; Enriquez, D.; Izatt, T.; Trent, J.M.; Byron, S.; Pal, S. Correlates of clinical benefit from immunotherapy and targeted therapy in metastatic renal cell carcinoma: Comprehensive genomic and transcriptomic analysis. J. Immunother. Cancer 2020, e000953. [Google Scholar] [CrossRef] [PubMed]
- McDermott, D.F.; Huseni, M.A.; Atkins, M.B.; Motzer, R.J.; Rini, B.I.; Escudier, B.; Fong, L.; Joseph, R.W.; Pal, S.K.; Reeves, J.A.; et al. Clinical activity and molecular correlates of response to atezolizumab alone or in combination with bevacizumab versus sunitinib in renal cell carcinoma. Nat. Med. 2018, 24, 749–757. [Google Scholar] [CrossRef] [PubMed]
- Piva, F.; Giulietti, M.; Occhipinti, G.; Santoni, M.; Massari, F.; Sotte, V.; Iacovelli, R.; Burattini, L.; Santini, D.; Montironi, R.; et al. Computational analysis of the mutations in BAP1, PBRM1 and SETD2 genes reveals the impaired molecular processes in renal cell carcinoma. Oncotarget 2015, 6, 32161–32168. [Google Scholar] [CrossRef]
- D’Aniello, C.; Berretta, M.; Cavaliere, C.; Rossetti, S.; Facchini, B.A.; Iovane, G.; Mollo, G.; Capasso, M.; Pepa, C.D.; Pesce, L.; et al. Biomarkers of Prognosis and Efficacy of Anti-angiogenic Therapy in Metastatic Clear Cell Renal Cancer. Front. Oncol. 2019, 9, 1400. [Google Scholar] [CrossRef] [Green Version]
- Sato, M.; Kojima, M.; Nagatsuma, A.K.; Nakamura, Y.; Saito, N.; Ochiai, A. Optimal fixation for total preanalytic phase evaluation in pathology laboratories. A comprehensive study including immunohistochemistry, DNA, and RNA assays. Pathol. Int. 2014, 64, 209–216. [Google Scholar] [CrossRef]


| Variables | PBRM1 Retention | PBRM1 Loss |
|---|---|---|
| Gender, n (%) | ||
| Female | 81 (71.7) | 32 (28.3) |
| Male | 196 (62.8) | 116 (37.2) |
| TNM stage, n (%) | ||
| I | 242 (78.1) | 68 (21.9) |
| II | 3 (23.1) | 10 (76.9) |
| III | 32 (32.0) | 68 (68.0) |
| IV | 0 (0.0) | 2 (100.0) |
| WHO/ISUP grade, n (%) | ||
| 1 | 58 (96.7) | 2 (3.3) |
| 2 | 155 (68.0) | 73 (32.0) |
| 3 | 58 (52.7) | 52 (47.3) |
| 4 | 6 (22.2) | 21 (77.8) |
| Necrosis, n (%) | ||
| Absent | 256 (71.1) | 104 (28.9) |
| Present | 21 (32.3) | 44 (67.7) |
| Histological phenotype, n (%) | ||
| Clear | 201 (77.3) | 59 (22.7) |
| Mixed | 71 (49.0) | 74 (51.0) |
| Eosinophilic | 5 (25.0) | 15 (75.0) |
| Vascularity-based architectural classification, n (%) | ||
| Category 1 | 218 (79.0) | 58 (21.0) |
| Category 2 | 55 (45.1) | 67 (54.9) |
| Category 3 | 4 (14.8) | 23 (85.2) |
| Recurrence, n (%) | 11 (19.3) | 46 (80.7) |
| Cancer-specific mortality, n (%) | 2 (13.3) | 13 (86.7) |
| Architectural Patterns, n (%) | PBRM1 Retention | PBRM1 Loss |
|---|---|---|
| Compact/Small nested | 127 (71.8) | 50 (28.2) |
| Macrocyst/Microcystic | 35 (97.2) | 1 (2.8) |
| Tubular/Acinar | 56 (88.9) | 7 (11.1) |
| Alveolar/Large nested | 27 (57.4) | 20 (42.6) |
| Thick trabecular/Insular | 18 (32.7) | 37 (67.3) |
| Papillary/Pseudopapillary | 10 (50.0) | 10 (50.0) |
| Solid sheets | 1 (11.1) | 8 (88.9) |
| Sarcomatoid/Rhabdoid | 3 (16.7) | 15 (83.3) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saiga, K.; Ohe, C.; Yoshida, T.; Ohsugi, H.; Ikeda, J.; Atsumi, N.; Noda, Y.; Yasukochi, Y.; Higasa, K.; Taniguchi, H.; et al. PBRM1 Immunohistochemical Expression Profile Correlates with Histomorphological Features and Endothelial Expression of Tumor Vasculature for Clear Cell Renal Cell Carcinoma. Cancers 2022, 14, 1062. https://doi.org/10.3390/cancers14041062
Saiga K, Ohe C, Yoshida T, Ohsugi H, Ikeda J, Atsumi N, Noda Y, Yasukochi Y, Higasa K, Taniguchi H, et al. PBRM1 Immunohistochemical Expression Profile Correlates with Histomorphological Features and Endothelial Expression of Tumor Vasculature for Clear Cell Renal Cell Carcinoma. Cancers. 2022; 14(4):1062. https://doi.org/10.3390/cancers14041062
Chicago/Turabian StyleSaiga, Kazuho, Chisato Ohe, Takashi Yoshida, Haruyuki Ohsugi, Junichi Ikeda, Naho Atsumi, Yuri Noda, Yoshiki Yasukochi, Koichiro Higasa, Hisanori Taniguchi, and et al. 2022. "PBRM1 Immunohistochemical Expression Profile Correlates with Histomorphological Features and Endothelial Expression of Tumor Vasculature for Clear Cell Renal Cell Carcinoma" Cancers 14, no. 4: 1062. https://doi.org/10.3390/cancers14041062
APA StyleSaiga, K., Ohe, C., Yoshida, T., Ohsugi, H., Ikeda, J., Atsumi, N., Noda, Y., Yasukochi, Y., Higasa, K., Taniguchi, H., Kinoshita, H., & Tsuta, K. (2022). PBRM1 Immunohistochemical Expression Profile Correlates with Histomorphological Features and Endothelial Expression of Tumor Vasculature for Clear Cell Renal Cell Carcinoma. Cancers, 14(4), 1062. https://doi.org/10.3390/cancers14041062






