Expression of Immunomodulatory Checkpoint Molecules in Drug-Resistant Neuroblastoma: An Exploratory Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines
2.2. Reagents
2.3. Cell Culture, Drug Treatment and Cytotoxicity Assay
2.4. In Vitro Invasion Assay
2.5. SDS-PAGE and Western Blot
2.6. Human Immuno-Oncology Checkpoint Protein Panel
2.7. Statistical Analysis
3. Results
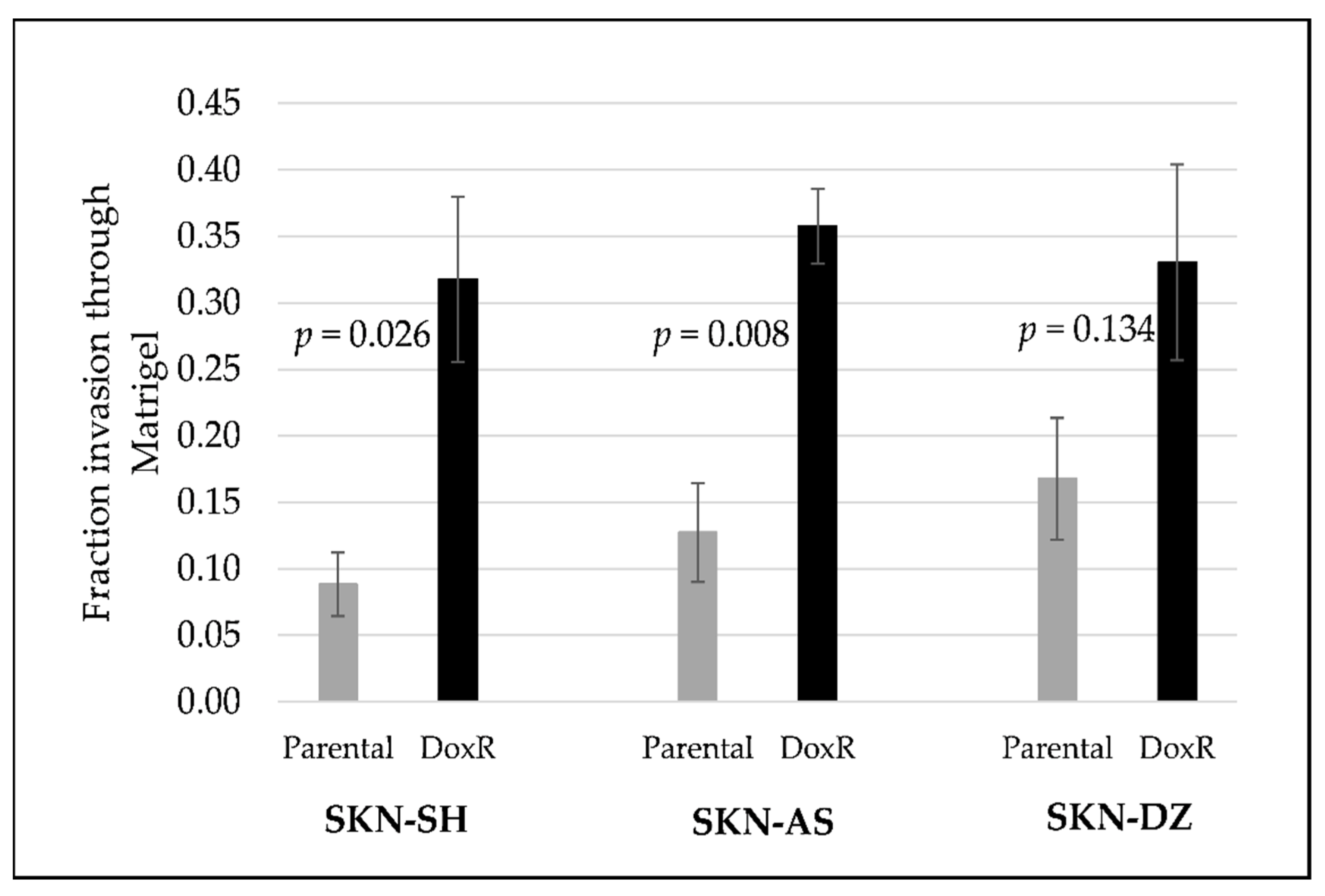
3.1. Doxorubicin-Resistant Cells Are More Invasive Than Their Parental Cells
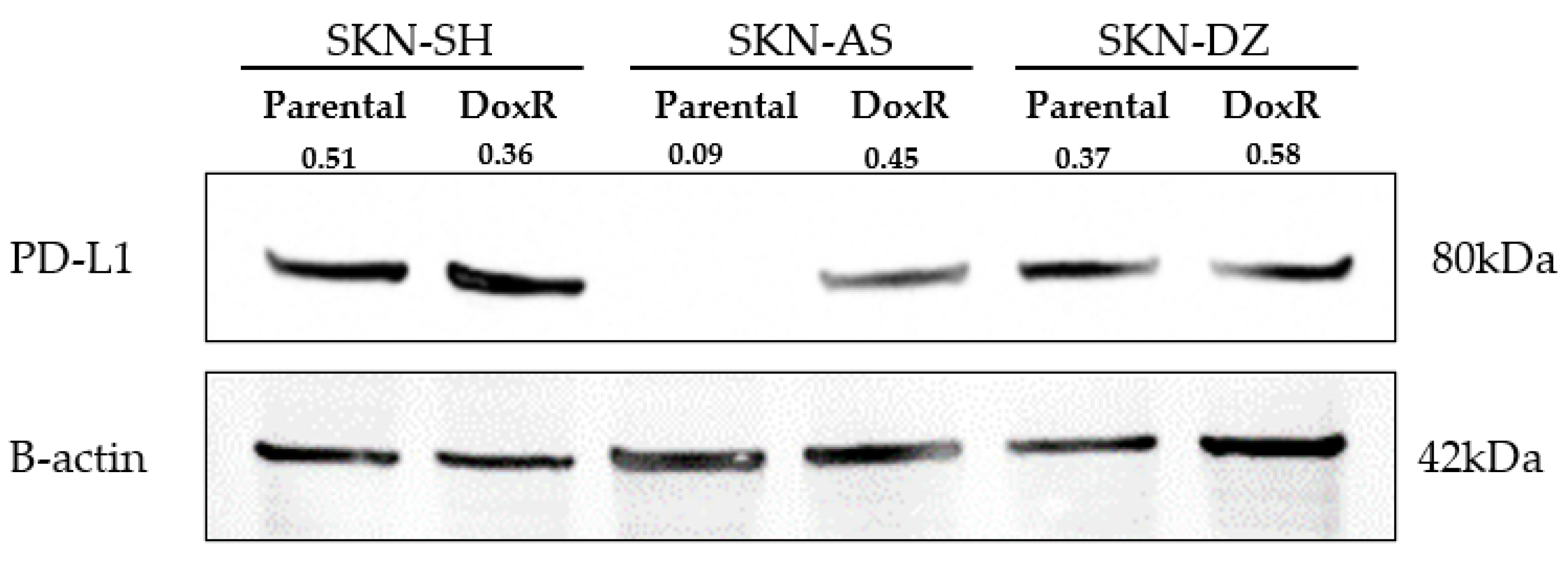
3.2. Neuroblastoma Expresses PD-L1 Regardless of Doxorubicin Resistance
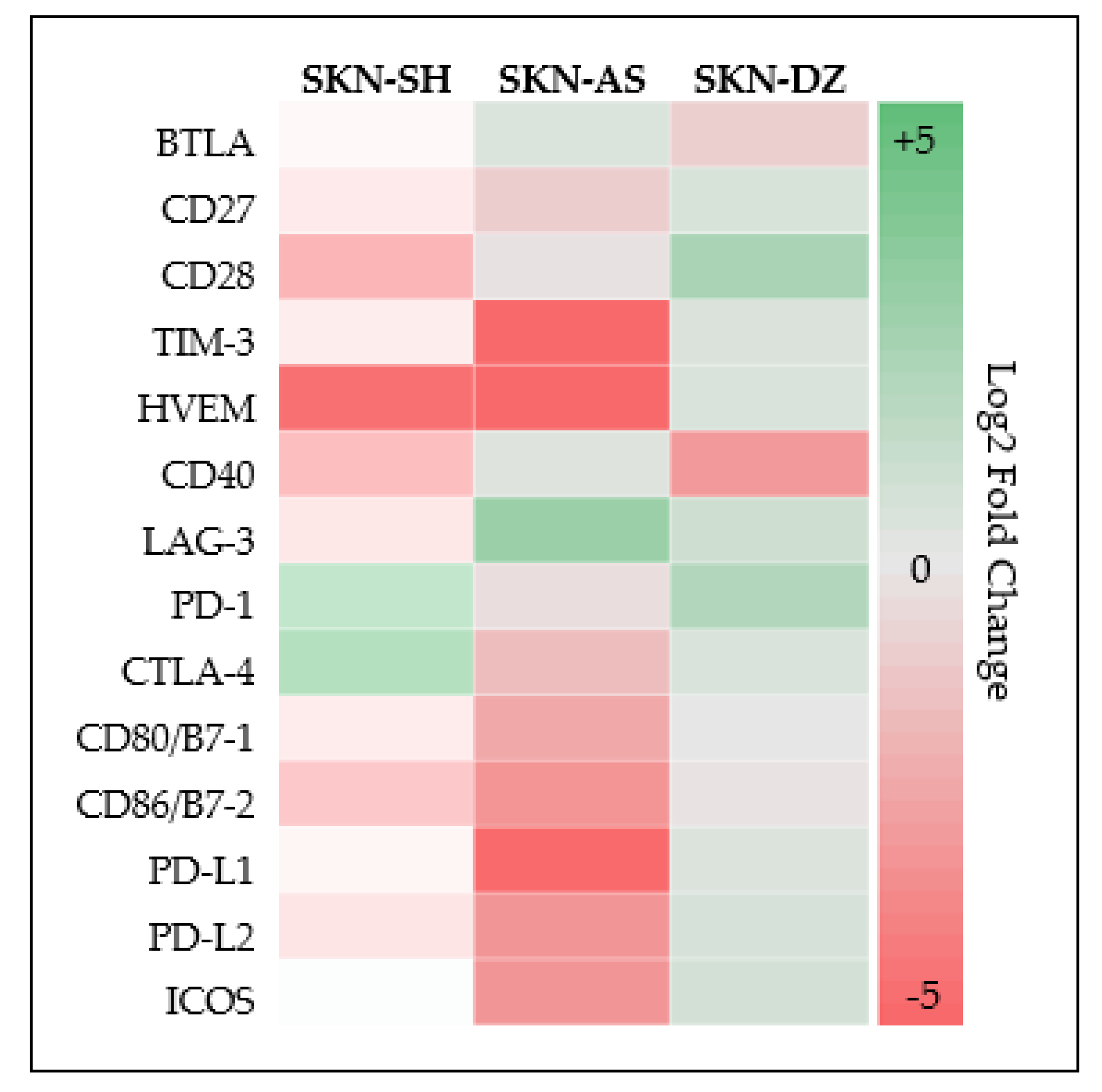
3.3. Neuroblastoma Expresses Multiple Additional Checkpoint Molecules Both Cellular and Soluble
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Park, J.R.; Eggert, A.; Caron, H. Neuroblastoma: Biology, Prognosis, and Treatment. Hematol. Oncol. Clin. N. Am. 2010, 24, 65–86. [Google Scholar] [CrossRef] [PubMed]
- Newman, E.A.; Abdessalam, S.; Aldrink, J.H.; Austin, M.; Heaton, T.E.; Bruny, J.; Ehrlich, P.; Dasgupta, R.; Baertschiger, R.M.; Lautz, T.B.; et al. Update on neuroblastoma. J. Pediatr. Surg. 2019, 54, 383–389. [Google Scholar] [CrossRef]
- Park, J.A.; Cheung, N.K.V. Limitations and opportunities for immune checkpoint inhibitors in pediatric malignancies. Cancer Treat. Rev. 2017, 58, 22–33. [Google Scholar] [CrossRef]
- Galluzzi, L.; Chan, T.; Kroemer, G.; Wolchock, J.; Lopez-Sota, A. The hallmarks of successful anticancer immunotherapy. Sci. Transl. Med. 2018, 10, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Tocheva, A.S.; Mor, A. Checkpoint Inhibitors: Applications for Autoimmunity. Curr. Allergy Asthma Rep. 2017, 17, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Riley, J.L. PD-1 signaling in primary T cells. Immunol. Rev. 2009, 229, 114–125. [Google Scholar] [CrossRef] [PubMed]
- Rozali, E.N.; Hato, S.V.; Robinson, B.W.; Lake, R.A.; Lesterhuis, W.J. Programmed death ligand 2 in cancer-induced immune suppression. Clin. Dev. Immunol. 2012, 2012, 656340. [Google Scholar] [CrossRef]
- Tierney, J.F.; Vogle, A.; Poirier, J.; Min, I.M.; Finnerty, B.; Zarnegar, R.; Pappas, S.G.; Scognamiglio, T.; Ghai, R.; Gattuso, P.; et al. Expression of programmed death ligand 1 and 2 in adrenocortical cancer tissues: An exploratory study. Surgery 2019, 165, 196–201. [Google Scholar] [CrossRef]
- Hamid, O.; Robert, C.; Daud, A.; Hodi, F.S.; Hwu, W.-J.; Kefford, R.; Wolchok, J.D.; Hersey, P.; Joseph, R.W.; Weber, J.S.; et al. Safety and Tumor Responses with Lambrolizumab (Anti–PD-1) in Melanoma. N. Engl. J. Med. 2013, 369, 134–144. [Google Scholar] [CrossRef] [Green Version]
- Ansell, S.M.; Lesokhin, A.M.; Borrello, I.; Halwani, A.; Scott, E.C.; Gutierrez, M.; Schuster, S.J.; Millenson, M.M.; Cattry, D.; Freeman, G.J.; et al. PD-1 Blockade with Nivolumab in Relapsed or Refractory Hodgkin’s Lymphoma. N. Engl. J. Med. 2015, 372, 311–319. [Google Scholar] [CrossRef] [Green Version]
- Garon, E.B.; Rizvi, N.A.; Hui, R.; Leighl, N.; Balmanoukian, A.S.; Eder, J.P.; Patnaik, A.; Aggarwal, C.; Gubens, M.; Horn, L.; et al. Pembrolizumab for the Treatment of Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 372, 2018–2028. [Google Scholar] [CrossRef] [PubMed]
- De Sousa Linhares, A.; Battin, C.; Jutz, S.; Leitner, J.; Hafner, C.; Tobias, J.; Wiedermann, U.; Kundi, M.; Zlabinger, G.J.; Grabmeier-Pfistershammer, K.; et al. Therapeutic PD-L1 antibodies are more effective than PD-1 antibodies in blocking PD-1/PD-L1 signaling. Sci. Rep. 2019, 9, 11427. [Google Scholar] [CrossRef]
- Rizvi, N.A.; Hellmann, M.D.; Brahmer, J.R.; Juergens, R.A.; Borghaei, H.; Gettinger, S.; Chow, L.Q.; Gerber, D.E.; Laurie, S.A.; Goldman, J.W.; et al. Nivolumab in combination with platinum-based doublet chemotherapy for first-line treatment of advanced non-small-cell lung cancer. J. Clin. Oncol. 2016, 34, 2969–2979. [Google Scholar] [CrossRef]
- Alfarra, H.; Weir, J.; Grieve, S.; Reiman, T. Targeting NK Cell Inhibitory Receptors for Precision Multiple Myeloma Immunotherapy. Front. Immunol. 2020, 11, 575609. [Google Scholar] [CrossRef]
- Hodi, F.; O’Day, S.; McDermott, D.; Weber, R.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Ph, D.; Schadendorf, D.; et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Merchant, M.S.; Wright, M.; Baird, K.; Wexler, L.H.; Rodriguez-Galindo, C.; Bernstein, D.; Delbrook, C.; Lodish, M.; Bishop, R.; Wolchok, J.D.; et al. Phase i clinical trial of ipilimumab in pediatric patients with advanced solid tumors. Clin. Cancer Res. 2016, 22, 1364–1370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kramer, K.; Kushner, B.H.; Modak, S.; Pandit-Taskar, N.; Smith-Jones, P.; Zanzonico, P.; Humm, J.L.; Xu, H.; Wolden, S.L.; Souweidane, M.M.; et al. Compartmental intrathecal radioimmunotherapy: Results for treatment for metastatic CNS neuroblastoma. J. Neurooncol. 2010, 97, 409–418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, Y.; Cao, J.; Zhao, C.; Li, X.; Zhou, C.; Hirsch, F.R. TIM-3, a promising target for cancer immunotherapy. Onco. Targets. Ther. 2018, 11, 7005–7009. [Google Scholar] [CrossRef] [Green Version]
- Kabir, T.F.; Chauhan, A.; Anthony, L.; Hildebrandt, G.C. Immune Checkpoint Inhibitors in Pediatric Solid Tumors: Status in 2018. Ochsner J. 2018, 18, 370–376. [Google Scholar] [CrossRef] [Green Version]
- Pinto, N.; Park, J.R.; Murphy, E.; Yearley, J.; McClanahan, T.; Annamalai, L.; Hawkins, D.S.; Rudzinski, E.R. Patterns of PD-1, PD-L1, and PD-L2 expression in pediatric solid tumors. Pediatr. Blood Cancer 2017, 64, e26613. [Google Scholar] [CrossRef]
- Geoerger, B.; Kang, H.; Yalon-Oren, M.; Marshall, M.; Vezina, C.; Pappo, A.; Laetsch, T.; Petrilli, A.; Ebinger, M.; Toporski, J.; et al. Pembrolizumab in paediatric patients with advanced melanoma or a PD-L1-positive, advanced, relapsed, or refractory solid tumour or lymphoma (KEYNOTE-051): Interim analysis of an open-label, single-arm, phase 1-2 trial. Lancet Oncol. 2020, 21, 121–133. [Google Scholar] [CrossRef]
- Davis, K.L.; Fox, E.; Merchant, M.S.; Reid, J.M.; Kudgus, R.A.; Liu, X.; Minard, C.G.; Voss, S.; Berg, S.L.; Weigel, B.J.; et al. Nivolumab in children and young adults with relapsed or refractory solid tumours or lymphoma (ADVL1412): A multicentre, open-label, single-arm, phase 1–2 trial. Lancet Oncol. 2020, 21, 541–550. [Google Scholar] [CrossRef]
- Aoki, T.; Hino, M.; Koh, K.; Hyushiki, M.; Kishimoto, H.; Arakawa, Y.; Hanada, R.; Jawashima, H.; Kurihara, J.; Shimojo, N.; et al. Low Frequency of Programmed Death Ligand 1 Expression in Pediatric Cancerso Title. Pediatr. Blood Cancer 2016, 63, 1461–1464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chowdhury, F.; Dunn, S.; Mitchell, S.; Mellows, T.; Ashton-Key, M.; Gray, J.C. PD-L1 and CD8+PD1+ lymphocytes exist as targets in the pediatric tumor microenvironment for immunomodulatory therapy. Oncoimmunology 2015, 4, e1029701. [Google Scholar] [CrossRef] [Green Version]
- Melaiu, O.; Mina, M.; Chierici, M.; Boldrini, R.; Jurman, G.; Romania, P.; D’Alicandro, V.; Benedetti, M.C.; Castellano, A.; Liu, T.; et al. PD-L1 is a therapeutic target of the bromodomain inhibitor JQ1 and, combined with HLA class I, a promising prognostic biomarker in neuroblastoma. Clin. Cancer Res. 2017, 23, 4462–4472. [Google Scholar] [CrossRef] [Green Version]
- Majzner, R.G.; Simon, J.S.; Grosso, J.F.; Martinez, D.; Pawel, B.R.; Santi, M.; Merchant, M.S.; Geoerger, B.; Hezam, I.; Marty, V.; et al. Assessment of programmed death-ligand 1 expression and tumor-associated immune cells in pediatric cancer tissues. Cancer 2017, 123, 3807–3815. [Google Scholar] [CrossRef] [Green Version]
- Zuo, S.; Sho, M.; Sawai, T.; Kanehiro, H.; Maeda, K.; Yoshida, M.; Tsukada, R.; Nomura, M.; Okuyama, H. Potential role of the PD-L1 expression and tumor-infiltrating lymphocytes on neuroblastoma. Pediatr. Surg. Int. 2020, 36, 137–143. [Google Scholar] [CrossRef]
- Mao, Y.; Eissler, N.; Le Blanc, K.; Johnsen, J.I.; Kogner, P.; Kiessling, R. Targeting suppressive myeloid cells potentiates checkpoint inhibitors to control spontaneous neuroblastoma. Clin. Cancer Res. 2016, 22, 3849–3859. [Google Scholar] [CrossRef] [Green Version]
- Eissler, N.; Mao, Y.; Brodin, D.; Reuterswärd, P.; Andersson Svahn, H.; Johnsen, J.I.; Kiessling, R.; Kogner, P. Regulation of myeloid cells by activated T cells determines the efficacy of PD-1 blockade. Oncoimmunology 2016, 5, e1235106. [Google Scholar] [CrossRef] [Green Version]
- Rigo, V.; Emionite, L.; Daga, A.; Astigiano, S.; Corrias, M.V.; Quintarelli, C.; Locatelli, F.; Ferrini, S.; Croce, M. Combined immunotherapy with anti-PDL-1/PD-1 and anti-CD4 antibodies cures syngeneic disseminated neuroblastoma. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Siebert, N.; Zumpe, M.; Jüttner, M.; Troschke-Meurer, S.; Lode, H.N. PD-1 blockade augments anti-neuroblastoma immune response induced by anti-GD2 antibody ch14.18/CHO. Oncoimmunology 2017, 6, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srinivasan, P.; Wu, X.; Basu, M.; Rossi, C.; Sandler, A.D. PD-L1 checkpoint inhibition and anti-CTLA-4 whole tumor cell vaccination counter adaptive immune resistance: A mouse neuroblastoma model that mimics human disease. PLoS Med. 2018, 15, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Shirinbak, S.; Chan, R.Y.; Shahani, S.; Muthugounder, S.; Kennedy, R.; Hung, L.T.; Fernandez, G.E.; Hadjidaniel, M.D.; Moghimi, B.; Sheard, M.A.; et al. Combined immune checkpoint blockade increases CD8+CD28+PD-1+ effector T cells and provides a therapeutic strategy for patients with neuroblastoma. Oncoimmunology 2021, 10, 1838140. [Google Scholar] [CrossRef] [PubMed]
- Skertich, N.J.; Chu, F.; Tarhoni, I.A.; Szajek, S.; Borgia, J.A.; Madonna, M.B. Expression of programmed death ligand 1 in drug-resistant osteosarcoma: An exploratory study. Surg. Open Sci. 2021, 6, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Bhola, N.E.; Njatcha, C.; Hu, L.; Lee, E.D.; Shiah, J.V.; Kim, M.O.; Johnson, D.E.; Grandis, J.R. PD-L1 is upregulated via BRD2 in head and neck squamous cell carcinoma models of acquired cetuximab resistance. Head Neck 2021, 43, 3364–3373. [Google Scholar] [CrossRef]
- Bishop, J.L.; Sio, A.; Angeles, A.; Roberts, M.E.; Azad, A.A.; Chi, K.N.; Zoubeidi, A. PD-L1 is highly expressed in Enzalutamide resistant prostate cancer. Oncotarget 2015, 6, 234–242. [Google Scholar] [CrossRef] [Green Version]
- American Type Culture Collection. Available online: https://www.atcc.org/ (accessed on 1 June 2021).
- Rebbaa, A.; Chou, P.M.; Mirkin, B.L. Factors Secreted by Human Neuroblastoma Mediate Doxorubicin Resistance by Activating STAT3 and Inhibiting Apoptosis. Mol. Med. 2001, 7, 393–400. [Google Scholar] [CrossRef] [Green Version]
- Boes, M.; Meyer-Wentrup, F. TLR3 triggering regulates PD-L1 (CD274) expression in human neuroblastoma cells. Cancer Lett. 2015, 361, 49–56. [Google Scholar] [CrossRef]
- Dondero, A.; Pastorino, F.; Della Chiesa, M.; Corrias, M.V.; Morandi, F.; Pistoia, V.; Olive, D.; Bellora, F.; Locatelli, F.; Castellano, A.; et al. PD-L1 expression in metastatic neuroblastoma as an additional mechanism for limiting immune surveillance. Oncoimmunology 2016, 5, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Toews, K.; Grunewald, L.; Schwiebert, S.; Klaus, A.; Winkler, A.; Ali, S.; Zirngibl, F.; Astrahantseff, K.; Wagner, D.L.; Henssen, A.G.; et al. Central memory phenotype drives success of checkpoint inhibition in combination with CAR T cells. Mol. Carcinog. 2020, 59, 724–735. [Google Scholar] [CrossRef]
- Vaddepally, R.K.; Kharel, P.; Pandey, R.; Garje, R.; Chandra, A.B. Review of indications of FDA-approved immune checkpoint inhibitors per NCCN guidelines with the level of evidence. Cancers 2020, 12, 738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Ni, L.; Dong, C. Immune checkpoint receptors in cancer: Redundant by design? Curr. Opin. Immunol. 2017, 45, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Marin-Acevedo, J.A.; Kimbrough, E.M.O.; Lou, Y. Next generation of immune checkpoint inhibitors and beyond. J. Hematol. Oncol. 2021, 14, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.L.; Lin, H.W.; Chien, C.L.; Lai, Y.L.; Sun, W.Z.; Chen, C.A.; Cheng, W.F. BTLA blockade enhances Cancer therapy by inhibiting IL-6/IL-10-induced CD19high B lymphocytes. J. Immunother. Cancer 2019, 7, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Huang, A.C.; Zhang, W.; Zhang, G.; Wu, M.; Xu, W.; Yu, Z.; Yang, J.; Wang, B.; Sun, H.; et al. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature 2018, 560, 382–386. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, J.; Ishikawa, T.; Doi, T.; Ota, T.; Yasuda, T.; Okayama, T.; Sakamoto, N.; Inoue, K.; Dohi, O.; Yoshida, N.; et al. Clinical significance of soluble forms of immune checkpoint molecules in advanced esophageal cancer. Med. Oncol. 2019, 36, 60. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, H.; Sun, X.; Wang, X.; Ren, T.; Huang, Y.; Zhang, R.; Zheng, B.; Guo, W. Exosomal PD-L1 and N-cadherin predict pulmonary metastasis progression for osteosarcoma patients. J. Nanobiotechnol. 2020, 18, 151. [Google Scholar] [CrossRef] [PubMed]
- Romero, Y.; Wise, R.; Zolkiewska, A. Proteolytic processing of PD-L1 by ADAM proteases in breast cancer cells. Cancer Immunol. Immunother. 2020, 69, 43–55. [Google Scholar] [CrossRef]
- Wei, W.; Xu, B.; Wang, Y.; Wu, C.; Jiang, J.; Wu, C. Prognostic significance of circulating soluble programmed death ligand-1 in patients with solid tumors. Medicine 2018, 97, 1–6. [Google Scholar] [CrossRef]
- Chiarucci, C.; Cannito, S.; Daffinà, M.G.; Amato, G.; Giacobini, G.; Cutaia, O.; Lofiego, M.F.; Fazio, C.; Giannarelli, D.; Danielli, R.; et al. Circulating levels of PD-L1 in mesothelioma patients from the NIBIT-MESO-1 study: Correlation with survival. Cancers 2020, 12, 361. [Google Scholar] [CrossRef] [Green Version]
- Li, R.; Ladisch, S. Abrogation of shedding of immunosuppressive neuroblastoma gangliosides. Cancer Res. 1996, 56, 4602–4605. [Google Scholar] [PubMed]
- Balis, F.M.; Busch, C.M.; Desai, A.V.; Hibbitts, E.; Naranjo, A.; Bagatell, R.; Irwin, M.; Fox, E. The ganglioside GD2 as a circulating tumor biomarker for neuroblastoma. Pediatr. Blood Cancer 2020, 67, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Li, C.W.; Lim, S.O.; Xia, W.; Lee, H.H.; Chan, L.C.; Kuo, C.W.; Khoo, K.H.; Chang, S.S.; Cha, J.H.; Kim, T.; et al. Glycosylation and stabilization of programmed death ligand-1 suppresses T-cell activity. Nat. Commun. 2016, 7, 12632. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Cell Line | SKN-SH | SKN-AS | SKN-DZ | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Target | Parenteral | DoxR | p-Value | Parenteral | DoxR | p-Value | Parenteral | DoxR | p-Value |
| BTLA | 36.1 | 31.3 | 1 | 1136.0 | 32.1 | 0.2 | 99.3 | 52.9 | 0.667 |
| CD27 | 4.9 | 3.0 | 0.4 | 1.9 | 2.6 | 0.4 | 2.8 | 4.3 | 0.4 |
| CD28 | 11.4 | 2.1 | 0.1 | 6.2 | 3.1 | 0.8 | 3.3 | 17.4 | 0.5 |
| TIM-3 | 3.5 | 2.3 | 0.1 | 2.5 | 2.2 | 0.7 | 2.2 | 3.2 | 0.4 |
| HVEM | 0.3 | 0.0 | 0.1 | 97.8 | 0.1 | 0.5 | 0.1 | 0.2 | 0.1 |
| CD40 | 15.8 | 3.6 | 0.7 | 1820.0 | 23.1 | 0.1 | 6.5 | 0.8 | 0.4 |
| GITR | 4.4 | 4.0 | 0.1 | 2.4 | 3.1 | 0.1 | 2.7 | 4.0 | 0.1 |
| LAG-3 | 107.1 | 62.0 | 0.1 | 34.0 | 63.7 | 0.4 | 45.8 | 90.5 | 0.7 |
| TLR-2 | <LLoQ | <LLoQ | <LLoQ | <LLoQ | <LLoQ | <LLoQ | |||
| GITRL | <LLoQ | <LLoQ | <LLoQ | <LLoQ | <LLoQ | <LLoQ | |||
| PD-1 | 0.8 | <LLoQ | 2.8 | <LLoQ | 0.1 | <LLoQ | <LLoQ | ||
| CTLA-4 | 0.4 | <LLoQ | 2.0 | <LLoQ | 0.7 | <LLoQ | <LLoQ | ||
| CD80/B7-1 | 2.2 | 1.4 | 0.4 | 3.5 | 1.1 | 0.1 | 1.4 | 1.5 | 0.7 |
| CD86/B7-2 | 2.9 | 0.8 | 0.1 | 4.4 | 0.8 | 0.1 | 1.1 | 1.0 | 1 |
| PD-L1 | 4.5 | 3.6 | 0.7 | 29.5 | 3.1 | 0.1 | 2.8 | 3.8 | 0.7 |
| PD-L2 | 8.4 | 4.7 | 0.1 | 246.8 | 8.0 | 0.1 | 5.8 | 9.0 | 0.2 |
| ICOS | 15.0 | 16.4 | 0.7 | 153.9 | 16.4 | 0.1 | 10.9 | 19.1 | 0.2 |
| Cell Line | SKN-SH | SKN-AS | SKN-DZ | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Target | Parenteral | DoxR | p-Value | Parenteral | DoxR | p-Value | Parenteral | DoxR | p-Value |
| CD40 | 3.205 | 4.65 | 0.667 | 16.97 | 1.34 | 0.121 | 0.285 | 1.83 | 0.121 |
| LAG-3 | 24.755 | 32.62 | 1 | 17.13 | 32.28 | 0.439 | 11.12 | 71.33 | 0.121 |
| CD80/B7-1 | <LLoQ | 0.75 | N/A | <LLoQ | <LLoQ | N/A | <LLoQ | <LLoQ | N/A |
| CD80/B7-2 | <LLoQ | 0.04 | N/A | <LLoQ | <LLoQ | N/A | <LLoQ | <LLoQ | N/A |
| PD-L1 | <LLoQ | 0.57 | N/A | <LLoQ | <LLoQ | N/A | <LLoQ | <LLoQ | N/A |
| PD-L2 | 3.19 | 2.42 | 0.121 | 40.38 | 2.095 | 0.121 | 1.06 | 3.65 | 0.121 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skertich, N.J.; Chu, F.; Tarhoni, I.A.M.; Szajek, S.; Borgia, J.A.; Madonna, M.B. Expression of Immunomodulatory Checkpoint Molecules in Drug-Resistant Neuroblastoma: An Exploratory Study. Cancers 2022, 14, 751. https://doi.org/10.3390/cancers14030751
Skertich NJ, Chu F, Tarhoni IAM, Szajek S, Borgia JA, Madonna MB. Expression of Immunomodulatory Checkpoint Molecules in Drug-Resistant Neuroblastoma: An Exploratory Study. Cancers. 2022; 14(3):751. https://doi.org/10.3390/cancers14030751
Chicago/Turabian StyleSkertich, Nicholas J., Fei Chu, Imad A. M. Tarhoni, Stephen Szajek, Jeffrey A. Borgia, and Mary Beth Madonna. 2022. "Expression of Immunomodulatory Checkpoint Molecules in Drug-Resistant Neuroblastoma: An Exploratory Study" Cancers 14, no. 3: 751. https://doi.org/10.3390/cancers14030751
APA StyleSkertich, N. J., Chu, F., Tarhoni, I. A. M., Szajek, S., Borgia, J. A., & Madonna, M. B. (2022). Expression of Immunomodulatory Checkpoint Molecules in Drug-Resistant Neuroblastoma: An Exploratory Study. Cancers, 14(3), 751. https://doi.org/10.3390/cancers14030751







