From Interferon to Checkpoint Inhibition Therapy—A Systematic Review of New Immune-Modulating Agents in Bacillus Calmette–Guérin (BCG) Refractory Non-Muscle-Invasive Bladder Cancer (NMIBC)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Search
2.2. Study Selection
2.3. Cochrane Collaboration’s Tool for Assessing Risk of Bias
2.4. Data Extraction
2.5. Definition of Terms
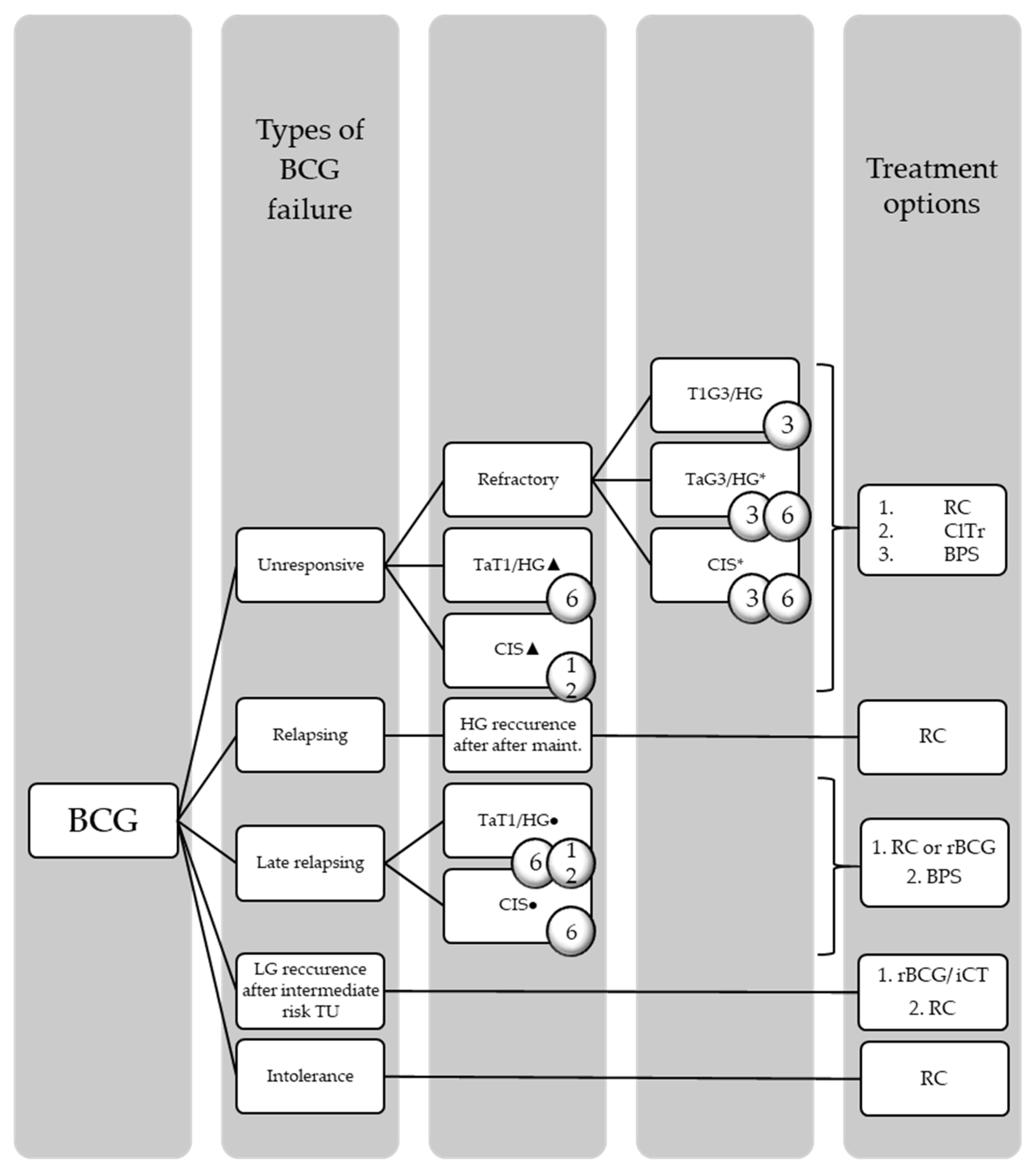
2.5.1. BCG-Refractory, -Unresponsive, and -Relapsing
2.5.2. Terms for Response to Therapy
3. Results
3.1. CPI
3.1.1. Atezolizumab (Tecentriq©)
3.1.2. Pembrolizumab (Keytruda©)
3.1.3. Durvalumab (Imfinzi©) in Combination with Vicineum™ (Oportuzumab monatox, OM)
3.2. Virus Vector-Mediated Immunotherapy/Immunostimulation and Vaccines
3.2.1. The Oncolytic Virus CG0070: Replication in Retinoblastoma (Rb)-Pathway Deficient UC Cells and Immune Induction via Granulocyte-Macrophage Colony-Stimulating Factor (GM-CSF)
3.2.2. Nadofaragene Firadenovec (rAd-IFNa/Syn3, Instiladrin®): Immune Enhancement via IFNα
3.2.3. Recombinant Pox-Viral Vector Vaccine PANVAC™ Plus BCG
3.3. Modified BCG
VPM1002BC
3.4. IL Agonists
IL-15RαFc Superagonist ALT-803 Instillation Plus BCG
4. Discussion
5. Conclusions
6. Limitations
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| (m)UC | (Metastatic) urothelial carcinoma |
| (N)MIBC | (Non-)muscle-invasive bladder cancer |
| AEs | Adverse events |
| ≥G3 AE | ≥Grade 3 adverse event |
| irAE | Immune-related adverse event |
| sAE | Serious adverse event |
| APC | Antigen-presenting cells |
| ASCO-GU | Genitourinary Cancers Symposium of the American Society of Clinical Oncology |
| AUA | American Urologic Association |
| BC | Bladder cancer |
| BCG | Bacille Calmette–Guérin |
| BPS | Bladder-preserving strategies |
| CEA | Carcinoembryonic antigen |
| CIS | Carcinoma in situ |
| ClTr | Clinical trials |
| CPI | Checkpoint inhibition |
| CPS | Combined positive score |
| CR | Complete response |
| CT | Center of the tumor |
| CUETO | Club Urologico Español deTratamiento Oncologico |
| DDM | Dodecyl maltoside |
| DoR | Duration of response |
| EAU | European Association of Urology |
| EBRT | External Beam Radiotherapy of the Bladder |
| ECOG | Eastern Cooperative Oncology Group |
| EFS | Event-free survival |
| EORTC | European Organisation for Research and Treatment of Cancer |
| FDA | United States Food and Drug Administration |
| FoxP3 | Forkhead box protein P3 |
| FU | Follow up |
| G1–3 | Grading 1–3 |
| LG | Low grade |
| HG | High grade |
| GM-CSF | Granulocyte-macrophage colony-stimulating factor |
| HG-RFS | High-grade recurrence-free survival |
| I | Immunoscore |
| i.v. | Intravenously |
| iCT | Intravesical chemotherapy |
| IFN | Interferon |
| IL | Interleukin |
| IM | Invasive margin |
| Maint. | Maintenance |
| MDSC | Myeloid-derived suppressor cells |
| MHC | Major histocompatibility complex |
| MMC | Mitomycin C |
| MUC | Antigens epithelial mucin 1 |
| n.a. | Not applicable |
| NA | Not available |
| NKT-cell | Natural killer T-cell |
| OM | Oportuzumab monatox |
| PD | Progressive disease |
| PD-(L)1 | Programmed cell death (ligand)-1 |
| PRISMA | Preferred Reporting Items for Systematic Review and Meta-Analysis statement |
| Rb | Retinoblastom |
| rBCG | Repeat BCG |
| RC | Radical cystectomy |
| RCT | Randomized controlled trial |
| RECIST | Response evaluation criteria in solid tumors |
| RR | Risk ratio |
| SD | Stable disease |
| TAM | Tumor-associated macrophage |
| TIL | Tumor-infiltrating lymphocyte |
| TLR | Toll-like receptor |
| TMB | Tumor mutational burden |
| TU | Tumor |
| TURBT | Transurethral resection of bladder tumor |
| UTI | Urinary tract infection |
| Vp | Viral particles |
| WHO | World Health Organization |
References
- Estimated Number of New Cases in 2020, Worldwide, Both Sexes, All Ages. Available online: https://gco.iarc.fr/today/online-analysis-table?v=2020&mode=cancer&mode_population=continents&population=900&populations=900&key=asr&sex=0&cancer=39&type=0&statistic=5&prevalence=0&population_group=0&ages_group%5B%5D=0&ages_group%5B%5D=17&group_cancer=1&include_nmsc=1&include_nmsc_other=1 (accessed on 21 May 2021).
- Van Osch, F.H.; Jochems, S.H.; Van Schooten, F.-J.; Bryan, R.T.; Zeegers, M.P. Quantified relations between exposure to tobacco smoking and bladder cancer risk: A meta-analysis of 89 observational studies. Int. J. Epidemiol. 2016, 45, 857–870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brierley, J.D.; Gospodarowicz, M.K.; Wittekind, C. TNM Classification of Malignant Tumours, 8th ed.; Wiley-Blackwell: Oxford, UK; Hoboken, NJ, USA, 2017. [Google Scholar]
- Sylvester, R.J.; van der Meijden, A.P.; Oosterlinck, W.; Witjes, J.A.; Bouffioux, C.; Denis, L.; Newling, D.W.; Kurth, K. Predicting Recurrence and Progression in Individual Patients with Stage Ta T1 Bladder Cancer Using EORTC Risk Tables: A Combined Analysis of 2596 Patients from Seven EORTC Trials. Eur. Urol. 2006, 49, 466–477. [Google Scholar] [CrossRef] [PubMed]
- Compérat, E.; Larré, S.; Rouprêt, M.; Neuzillet, Y.; Pignot, G.; Quintens, H.; Houede, N.; Roy, C.; Durand, X.; Varinot, J.; et al. Clinicopathological characteristics of urothelial bladder cancer in patients less than 40 years old. Virchows Arch. 2015, 466, 589–594. [Google Scholar] [CrossRef]
- Sylvester, R.J.; Rodríguez, O.; Hernández, V.; Turturica, D.; Bauerová, L.; Bruins, H.M.; Bründl, J.; van der Kwast, T.H.; Brisuda, A.; Rubio-Briones, J.; et al. European Association of Urology (EAU) Prognostic Factor Risk Groups for Non–muscle-invasive Bladder Cancer (NMIBC) Incorporating the WHO 2004/2016 and WHO 1973 Classification Systems for Grade: An Update from the EAU NMIBC Guidelines Panel. Eur. Urol. 2021, 79, 480–488. [Google Scholar] [CrossRef]
- EAU Guidelines. Edn. Presented at the EAU Annual Congress Milan 2021; EAU Guidelines Office: Arnhem, The Netherlands, 2021. [Google Scholar]
- Guallar-Garrido, S.; Julián, E. Bacillus Calmette-Guérin (BCG) Therapy for Bladder Cancer: An Update. ImmunoTargets Ther. 2020, 9, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morales, A. Treatment of carcinoma in situ of the bladder with BCG. Cancer Immunol. Immunother. 1980, 9, 69–72. [Google Scholar] [CrossRef]
- Morales, A.; Eidinger, D.; Bruce, A. Intracavitary Bacillus Calmette-guerin in the Treatment of Superficial Bladder Tumors. J. Urol. 1976, 116, 180–182. [Google Scholar] [CrossRef]
- Lamm, D.L.; Blumenstein, B.A.; Crissman, J.D.; Montie, J.E.; Gottesman, J.E.; Lowe, B.A.; Sarosdy, M.F.; Bohl, R.D.; Grossman, H.B.; Beck, T.M.; et al. Maintenance bacillus Calmette-Guerin immunotherapy for recurrent TA, T1 and carci-noma in situ transitional cell carcinoma of the bladder: A randomized Southwest Oncology Group Study. J. Urol. 2000, 163, 1124–1129. [Google Scholar] [CrossRef]
- Malmström, P.-U.; Sylvester, R.J.; Crawford, D.E.; Friedrich, M.; Krege, S.; Rintala, E.; Solsona, E.; Di Stasi, S.M.; Witjes, J.A. An Individual Patient Data Meta-Analysis of the Long-Term Outcome of Randomised Studies Comparing Intravesical Mitomycin C versus Bacillus Calmette-Guérin for Non–Muscle-Invasive Bladder Cancer. Eur. Urol. 2009, 56, 247–256. [Google Scholar] [CrossRef] [Green Version]
- Sylvester, R.J.; Van Der Meijden, A.P.M.; Lamm, D.L. Intravesical bacillus Calmette-Guerin reduces the risk of progression in patients with superficial bladder cancer: A meta-analysis of the published results of randomized clinical trials. J. Urol. 2002, 168, 1964–1970. [Google Scholar] [CrossRef]
- Oddens, J.; Brausi, M.; Sylvester, R.; Bono, A.; van de Beek, C.; van Andel, G.; Gontero, P.; Hoeltl, W.; Turkeri, L.; Marreaud, S.; et al. Final Results of an EORTC-GU Cancers Group Randomized Study of Maintenance Bacillus Calmette-Guérin in Intermediate- and High-risk Ta, T1 Papillary Carcinoma of the Urinary Bladder: One-third Dose Versus Full Dose and 1 Year Versus 3 Years of Maintenance. Eur. Urol. 2013, 63, 462–472. [Google Scholar] [CrossRef] [PubMed]
- Okafor, C.N.; Rewane, A.; Momodu, I.I. Bacillus Calmette Guerin; StatPearls: Treasure Island, FL, USA, 2021. [Google Scholar]
- Lamm, D.L.; Van Der Meijden, A.P.; Morales, A.; Brosman, S.A.; Catalona, W.J.; Herr, H.W.; Soloway, M.S.; Steg, A.; Debruyne, F.M. Incidence and treatment of complications of bacillus calmette-guerin intravesical therapy in superficial bladder cancer. J. Urol. 1992, 147, 596–600. [Google Scholar] [CrossRef]
- Van der Meijden, A.P.; Sylvester, R.J.; Oosterlinck, W.; Hoeltl, W.; Bono, A.V. Maintenance Bacillus Calmette-Guerin for Ta T1 Bladder Tumors Is Not Associated with Increased Toxicity: Results from a European Organisation for Research and Treatment of Cancer Genito-Urinary Group Phase III Trial. Eur. Urol. 2003, 44, 429–434. [Google Scholar] [CrossRef]
- Takeda, T.; Kikuchi, E.; Yuge, K.; Matsumoto, K.; Miyajima, A.; Nakagawa, K.; Oya, M. Discontinuance of Bacille Calmette-Guérin Instillation Therapy for Nonmuscle-invasive Bladder Cancer Has Negative Effect on Tumor Recurrence. Urology 2009, 73, 1318–1322. [Google Scholar] [CrossRef]
- Mayor, N.; Fankhauser, C.; Sangar, V.; Mostafid, H. Management of NMIBC during BCG shortage and COVID -19. Trends Urol. Men’s Health 2021, 12, 7–11. [Google Scholar] [CrossRef]
- Figueroa, A.J.; Stein, J.P.; Dickinson, M.; Skinner, E.C.; Thangathurai, D.; Mikhail, M.S.; Skinner, D.G.; Boyd, S.D.; Lieskovsky, G.; Donald, G. Skinner Radical cystectomy for elderly patients with bladder carcinoma: An updated experience with 404 patients. Cancer 1998, 83, 141–147. [Google Scholar] [CrossRef]
- Young, M.J.; Elmussareh, M.; Weston, P.; Dooldeniya, M. Radical cystectomy in the elderly—Is this a safe treatment option? Arab J. Urol. 2017, 15, 360–365. [Google Scholar] [CrossRef] [Green Version]
- Steinberg, G.; Bahnson, R.; Brosman, S.; Middleton, R.; Wajsman, Z.; Wehle, M. The valrubicin study group efficacy and safety of valrubicin for the treatment of bacillus calmette-guerin refractory carcinoma in situ of the bladder. J. Urol. 2000, 163, 761–767. [Google Scholar] [CrossRef]
- Kamat, A.M.; Lerner, S.P.; O’Donnell, M.; Georgieva, M.V.; Yang, M.; Inman, B.A.; Kassouf, W.; Boorjian, S.A.; Tyson, M.D.; Kulkarni, G.S.; et al. Evidence-based Assessment of Current and Emerging Bladder-sparing Therapies for Non–muscle-invasive Bladder Cancer After Bacillus Calmette-Guerin Therapy: A Systematic Review and Meta-analysis. Eur. Urol. Oncol. 2020, 3, 318–340. [Google Scholar] [CrossRef] [Green Version]
- Powles, T.; Durán, I.; Van Der Heijden, M.S.; Loriot, Y.; Vogelzang, N.J.; De Giorgi, U.; Oudard, S.; Retz, M.M.; Castellano, D.; Bamias, A.; et al. Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): A multicentre, open-label, phase 3 randomised controlled trial. Lancet 2018, 391, 748–757. [Google Scholar] [CrossRef]
- Starkman, J.S.; Smith, C.P.; Staskin, D.R. Surgical options for drug-refractory overactive bladder patients. Rev. Urol. 2010, 12, e97–e110. [Google Scholar] [PubMed]
- Balar, A.V.; Galsky, M.D.; Rosenberg, J.E.; Powles, T.; Petrylak, D.P.; Bellmunt, J.; Loriot, Y.; Necchi, A.; Hoffman-Censits, J.; Perez-Gracia, J.L.; et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: A single-arm, multicentre, phase 2 trial. Lancet 2017, 389, 67–76. [Google Scholar] [CrossRef] [Green Version]
- Bellmunt, J.; De Wit, R.; Vaughn, D.J.; Fradet, Y.; Lee, J.-L.; Fong, L.; Vogelzang, N.J.; Climent, M.A.; Petrylak, D.P.; Choueiri, T.K.; et al. Pembrolizumab as Second-Line Therapy for Advanced Urothelial Carcinoma. N. Engl. J. Med. 2017, 376, 1015–1026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grivas, P.; Plimack, E.R.; Balar, A.V.; Castellano, D.; O’Donnell, P.H.; Bellmunt, J.; Powles, T.; Hahn, N.M.; de Wit, R.; Bajorin, D.F.; et al. Pembrolizumab as First-line Therapy in Cisplatin-ineligible Advanced Urothelial Cancer (KEYNOTE-052): Outcomes in Older Patients by Age and Performance Status. Eur. Urol. Oncol. 2020, 3, 351–359. [Google Scholar] [CrossRef]
- Powles, T.; Park, S.H.; Voog, E.; Caserta, C.; Valderrama, B.P.; Gurney, H.; Kalofonos, H.; Radulović, S.; Demey, W.; Ullén, A.; et al. Avelumab Maintenance Therapy for Advanced or Metastatic Urothelial Carcinoma. N. Engl. J. Med. 2020, 383, 1218–1230. [Google Scholar] [CrossRef]
- PRISMA. Available online: http://www.prisma-statement.org (accessed on 23 April 2020).
- Packiam, V.T.; Lamm, D.L.; Barocas, D.A.; Trainer, A.; Fand, B.; Davis, R.L.; Clark, W.; Kroeger, M.; Dumbadze, I.; Chamie, K.; et al. An open label, single-arm, phase II multicenter study of the safety and efficacy of CG0070 oncolytic vector regimen in patients with BCG-unresponsive non–muscle-invasive bladder cancer: Interim results. Urol. Oncol. Semin. Orig. Investig. 2018, 36, 440–447. [Google Scholar] [CrossRef]
- Balar, A.V.; Kamat, A.M.; Kulkarni, G.S.; Uchio, E.M.; Boormans, J.L.; Roumiguié, M.; Krieger, L.E.M.; Singer, E.A.; Bajorin, D.F.; Grivas, P.; et al. Pembrolizumab monotherapy for the treatment of high-risk non-muscle-invasive bladder cancer unre-sponsive to BCG (KEYNOTE-057): An open-label, single-arm, multicentre, phase 2 study. Lancet Oncol. 2021, 22, 919–930. [Google Scholar] [CrossRef]
- Boorjian, S.A.; Alemozaffar, M.; Konety, B.R.; Shore, N.D.; Gomella, L.G.; Kamat, A.M.; Bivalacqua, T.J.; Montgomery, J.S.; Lerner, S.P.; Busby, J.E.; et al. Intravesical nadofaragene firadenovec gene therapy for BCG-unresponsive non-muscle-invasive bladder cancer: A single-arm, open-label, repeat-dose clinical trial. Lancet Oncol. 2020, 22, 107–117. [Google Scholar] [CrossRef]
- Rentsch, C.A.; Bosshard, P.; Mayor, G.; Rieken, M.; Püschel, H.; Wirth, G.; Cathomas, R.; Parzmair, G.P.; Grode, L.; Eisele, B.; et al. Results of the phase I open label clinical trial SAKK 06/14 assessing safety of intravesical instillation of VPM1002BC, a recombinant mycobacterium Bacillus Calmette Guérin (BCG), in patients with non-muscle invasive bladder cancer and previous failure of conventional BCG therapy. OncoImmunology 2020, 9, 1748981. [Google Scholar] [CrossRef] [Green Version]
- The Cochrane Collaboration’s Tool for Assessing Risk of Bias in Randomised Trials. Available online: https://www.bmj.com/content/343/bmj.d5928 (accessed on 8 November 2021).
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2008, 45, 228–247. [Google Scholar] [CrossRef]
- Black, P.C.; Tangen, C.; Singh, P.; McConkey, D.J.; Lucia, S.; Lowrance, W.T.; Koshkin, V.S.; Stratton, K.L.; Bivalacqua, T.; Kassouf, W.; et al. Phase II trial of atezolizumab in BCG-unresponsive non-muscle invasive bladder cancer: SWOG S1605 (NCT #02844816). J. Clin. Oncol. 2021, 39, 4541. [Google Scholar] [CrossRef]
- ASCO 2021: Phase II Trial of Atezolizumab in BCG-Unresponsive Non-Muscle Invasive Bladder Cancer: SWOG S1605. Available online: https://www.urotoday.com/conference-highlights/asco-2021/asco-2021-bladder-cancer/129954-asco-2021-phase-ii-trial-of-atezolizumab-in-bcg-unresponsive-non-muscle-invasive-bladder-cancer-swog-s1605.html (accessed on 8 November 2021).
- AUA 2021: Interim Analysis of a Phase I Single-Arm Study of the Combination of Durvalumab and Vicineum in Subjects with High-Grade NMIBC Previously Treated with BCG. Available online: https://www.urotoday.com/conference-highlights/aua-2021-program/aua-2021-bladder-cancer/131953-aua-2021-interim-analy-sis-of-a-phase-i-single-arm-study-of-the-combination-of-durvalumab-and-vicineum-in-subjects-with-high-grade-nmibc-previously-treated-with-bcg.html (accessed on 7 October 2021).
- Burke, J.M.; Lamm, D.L.; Meng, M.V.; Nemunaitis, J.J.; Stephenson, J.J.; Arseneau, J.C.; Aimi, J.; Lerner, S.; Yeung, A.W.; Kazarian, T.; et al. A First in Human Phase 1 Study of CG0070, a GM-CSF Expressing Oncolytic Adenovirus, for the Treatment of Nonmuscle Invasive Bladder Cancer. J. Urol. 2012, 188, 2391–2397. [Google Scholar] [CrossRef] [PubMed]
- CG Oncology Announces Clinical Trial Collaboration with Bristol Myers Squibb to Evaluate Oncolytic Immunotherapy CG0070 in Combination with OPDIVO® (nivolumab) in Metastatic Urothelial Cancer. Available online: https://www.cgoncology.com/news/press-releases/090921 (accessed on 8 November 2021).
- Dickstein, R.; Wu, N.; Cowan, B.; Dunshee, C.; Franks, M.; Wolk, F.; Belkoff, L.; Castelucci, S.; Holzbeierlein, J.; Kulkarni, G.; et al. LBA27 PHASE 3 study of vicinium in BCG-unresponsive non-muscle invasive bladder cancer: Initial results. J. Urol. 2018, 199, e1167. [Google Scholar] [CrossRef] [Green Version]
- Brunner, A.; Prelog, M.; Verdorfer, I.; Tzankov, A.; Mikuz, G.; Ensinger, C. EpCAM is predominantly expressed in high grade and advanced stage urothelial carcinoma of the bladder. J. Clin. Pathol. 2007, 61, 307–310. [Google Scholar] [CrossRef] [PubMed]
- FDA Denies Vicineum Approval for Non-Muscle Invasive Bladder Cancer. Available online: https://www.managedhealthcareexecutive.com/view/approval-of-vicineum-for-bladder-cancer-denied (accessed on 8 November 2021).
- Yamashita, M.; Rosser, C.J.; Zhou, J.-H.; Zhang, X.-Q.; Connor, R.J.; Engler, H.; Maneval, D.C.; Karashima, T.; Czerniak, B.A.; Dinney, C.P.N.; et al. Syn3 provides high levels of intravesical adenoviral-mediated gene transfer for gene therapy of genetically altered urothelium and superficial bladder cancer. Cancer Gene Ther. 2002, 9, 687–691. [Google Scholar] [CrossRef] [Green Version]
- Dinney, C.P.; Fisher, M.B.; Navai, N.; O’Donnell, M.; Cutler, D.L.; Abraham, A.; Young, S.; Hutchins, B.; Caceres, M.; Kishnani, N.; et al. Phase I Trial of Intravesical Recombinant Adenovirus Mediated Interferon-α2b Formulated in Syn3 for Bacillus Calmette-Guérin Failures in Nonmuscle Invasive Bladder Cancer. J. Urol. 2013, 190, 850–856. [Google Scholar] [CrossRef]
- Castellano, D.; de Velasco, G.; Martin Soberón, M.C.; Carretero-González, A.; Dueñas, M.; Paramio, J.; Guerrero, F.; de la Rosa, F.; Sanz, J.L.; Guerrero, F. Atezolizumab + intravesical BCG (Bacillus Calmette-Guerin) in high-risk non-muscle invasive bladder cancer (NMIBC) patients: Institutional clinical and translational study (BladderGATE). J. Clin. Oncol. 2020, 38. [Google Scholar] [CrossRef]
- Winerdal, M.E.; Marits, P.; Winerdal, M.; Hasan, M.; Rosenblatt, R.; Tolf, A.; Selling, K.; Sherif, A.; Winqvist, O. FOXP3 and survival in urinary bladder cancer. Br. J. Urol. 2011, 108, 1672–1678. [Google Scholar] [CrossRef]
- Dysthe, M.; Parihar, R. Myeloid-Derived Suppressor Cells in the Tumor Microenvironment. Tumor Microenviron. 2020, 1224, 117–140. [Google Scholar] [CrossRef]
- Zeng, D.; Yu, Y.-F.; Ou, Q.-Y.; Li, X.-Y.; Zhong, R.-Z.; Xie, C.-M.; Hu, Q.-G. Prognostic and predictive value of tumor-infiltrating lymphocytes for clinical therapeutic research in patients with non-small cell lung cancer. Oncotarget 2016, 7, 13765–13781. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.H.; Huh, J.W.; Kim, H.R. Prognostic Significance of Tumor-Infiltrating Lymphocytes for Patients with Colorectal Cancer. Arch. Surg. 2012, 147, 366–372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krpina, K.; Babarović, E.; Jonjić, N. Correlation of tumor-infiltrating lymphocytes with bladder cancer recurrence in patients with solitary low-grade urothelial carcinoma. Virchows Arch. 2015, 467, 443–448. [Google Scholar] [CrossRef]
- Zhang, Q.; Hao, C.; Cheng, G.; Wang, L.; Wang, X.; Li, C.; Qiu, J.; Ding, K. High CD4⁺ T cell density is associated with poor prognosis in patients with non-muscle-invasive bladder cancer. Int. J. Clin. Exp. Pathol. 2015, 8, 11510–11516. [Google Scholar] [PubMed]
- Yu, D.-S.; Wu, C.-L.; Ping, S.-Y.; Keng, C.; Shen, K.-H. Bacille Calmette-Guerin can induce cellular apoptosis of urothelial cancer directly through toll-like receptor 7 activation. Kaohsiung J. Med. Sci. 2015, 31, 391–397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- See, W.A.; Zhang, G.; Chen, F.; Cao, Y.; Langenstroer, P.; Sandlow, J. Bacille-Calmette Guèrin induces caspase-independent cell death in urothelial carcinoma cells together with release of the necrosis-associated chemokine high molecular group box protein 1. BJU Int. 2009, 103, 1714–1720. [Google Scholar] [CrossRef]
- Shah, G.; Zielonka, J.; Chen, F.; Zhang, G.; Cao, Y.; Kalyanaraman, B.; See, W. H2O2Generation by bacillus Calmette-Guérin Induces the Cellular Oxidative Stress Response Required for bacillus Calmette-Guérin Direct Effects on Urothelial Carcinoma Biology. J. Urol. 2014, 192, 1238–1248. [Google Scholar] [CrossRef] [Green Version]
- Bevers, R.F.M.; De Boer, E.C.; Kurth, K.H.; Schamhart, D.H.J. BCG-induced interleukin-6 upregulation and BCG internalization in well and poorly differ-entiated human bladder cancer cell lines. Eur. Cytokine Netw. 1998, 9, 181–186. [Google Scholar]
- Han, J.; Gu, X.; Li, Y.; Wu, Q. Mechanisms of BCG in the treatment of bladder cancer-current understanding and the prospect. Biomed. Pharmacother. 2020, 129, 110393. [Google Scholar] [CrossRef]
- Mitropoulos, D.N. Novel insights into the mechanism of action of intravesical immunomodulators. Vivo 2005, 19, 611–621. [Google Scholar]
- Bakhru, P.; Sirisaengtaksin, N.; Soudani, E.; Mukherjee, S.; Khan, A.; Jagannath, C. BCG vaccine mediated reduction in the MHC-II expression of macrophages and dendritic cells is reversed by activation of Toll-like receptors 7 and 9. Cell. Immunol. 2013, 287, 53–61. [Google Scholar] [CrossRef] [Green Version]
- Farinacci, M.; Weber, S.; Kaufmann, S.H. The recombinant tuberculosis vaccine rBCG ΔureC::hly+ induces apoptotic vesicles for improved priming of CD4+ and CD8+ T cells. Vaccine 2012, 30, 7608–7614. [Google Scholar] [CrossRef] [PubMed]
- Saiga, H.; Nieuwenhuizen, N.; Gengenbacher, M.; Koehler, A.-B.; Schuerer, S.; Moura-Alves, P.; Wagner, I.; Mollenkopf, H.-J.; Dorhoi, A.; Kaufmann, S.H. The Recombinant BCG ΔureC::hlyVaccine Targets the AIM2 Inflammasome to Induce Autophagy and Inflammation. J. Infect. Dis. 2014, 211, 1831–1841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andersen, C.S.; Dietrich, J.; Agger, E.M.; Lycke, N.Y.; Lövgren, K.; Andersen, P. The Combined CTA1-DD/ISCOMs Vector Is an Effective Intranasal Adjuvant for Boosting Prior Mycobacterium bovis BCG Immunity to Mycobacterium tuberculosis. Infect. Immun. 2007, 75, 408–416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grode, L.; Seiler, P.; Baumann, S.; Hess, J.; Brinkmann, V.; Eddine, A.N.; Mann, P.; Goosmann, C.; Bandermann, S.; Smith, D.; et al. Increased vaccine efficacy against tuberculosis of recombinant Mycobacterium bovis bacille Calmette-Guerin mutants that secrete listeriolysin. J. Clin. Investig. 2005, 115, 2472–2479. [Google Scholar] [CrossRef] [Green Version]
- FDA Approves Pembrolizumab for BCG-Unresponsive, High-Risk Non-Muscle Invasive Bladder Cancer. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-pembrolizumab-bcg-unresponsive-high-risk-non-muscle-invasive-bladder-cancer (accessed on 4 November 2021).
- Sesen Bio Withdraws EU Application for Cancer Drug FDA Just Turned Down. Available online: https://www.fdanews.com/articles/204156-sesen-bio-withdraws-eu-application-for-cancer-drug-fda-just-turned-down?v=preview (accessed on 11 August 2021).
- Shore, N.D.; Boorjian, S.A.; Canter, D.J.; Ogan, K.; Karsh, L.I.; Downs, T.M.; Gomella, L.G.; Kamat, A.M.; Lotan, Y.; Svatek, R.S.; et al. Intravesical rAd–IFNα/Syn3 for Patients with High-Grade, Bacillus Calmette-Guerin–Refractory or Relapsed Non–Muscle-Invasive Bladder Cancer: A Phase II Randomized Study. J. Clin. Oncol. 2017, 35, 3410–3416. [Google Scholar] [CrossRef] [PubMed]
- Madan, R.A.; Arlen, P.M.; Gulley, J.L. PANVAC™-VF: Poxviral-based vaccine therapy targeting CEA and MUC1 in carcinoma. Expert Opin. Biol. Ther. 2007, 7, 543–554. [Google Scholar] [CrossRef]
- Petrulio, C.A.; Kaufman, H.L. Development of the PANVAC™-VF vaccine for pancreatic cancer. Expert Rev. Vaccines 2006, 5, 9–19. [Google Scholar] [CrossRef]
- Heery, C.R.; Ibrahim, N.K.; Arlen, P.M.; Mohebtash, M.; Murray, J.L.; Koenig, K.; Madan, R.A.; McMahon, S.; Marté, J.L.; Steinberg, S.M.; et al. Docetaxel Alone or in Combination With a Therapeutic Cancer Vaccine (PANVAC) in Patients With Metastatic Breast Cancer. JAMA Oncol. 2015, 1, 1087–1095. [Google Scholar] [CrossRef]
- Saoud, R.; Telfer, S.; Maruf, M.; Singer, E.; Weiss, R.; Jang, T.; Elsamra, S.; Marino, M.; Valera, V.; Rodriguez, B.W.; et al. MP16-14 clinical outcomes of a randomized, prospective, phase ii study to determine the efficacy of bacillus calmette-guerin (BCG) given in combination with panvac versus BCG given alone in adults with high grade BCG-refractory non-muscle invasive bladder cancer. J. Urol. 2021, 206, e302. [Google Scholar] [CrossRef]
- AUA 2021: Clinical Outcomes of a Randomized, Prospective, Phase II Study to Determine the Efficacy of BCG Given in Combination with PANVAC™ vs BCG Alone in Adults with High Grade BCG-Refractory NMIBC. Available online: https://www.urotoday.com/conference-highlights/aua-2021-program/aua-2021-bladder-cancer/131964-aua-2021-clinical-outcomes-of-a-randomized-prospective-phase-ii-study-to-determine-the-efficacy-of-bacillus-calmette-guerin-bcg-given-in-combination-with-panvac-versus-bcg-given-alone-in-adults-with-high-grade-bcg-refractory-non-muscle-invasive-bladde.html (accessed on 21 October 2021).
- Study of Bacillus Calmette-Guerin (BCG) Combined with PANVAC Versus BCG Alone in Adults With High Grade Non-Muscle Invasive Bladder Cancer Who Failed At Least 1 Course of BCG. Available online: https://clinicaltrials.gov/ct2/show/NCT02015104 (accessed on 7 October 2021).
- Disease Recurrence Not Significantly Delayed with Panvac Plus BCG in Advanced NMIBC. Available online: https://www.targetedonc.com/view/disease-recurrence-not-significantly-delayed-with-panvac-plus-bcg-in-advanced-nmibc (accessed on 7 October 2021).
- Novel IL-15 superagonist N-803 Hits High Complete Response Rate in BCG-Unresponsive NMIBC. Available online: https://www.urologytimes.com/view/novel-il-15-superagonist-n-803-hits-high-complete-response-rate-in-bcg-unresponsive-nmibc (accessed on 1 November 2021).
- Chamie, K.; Chang, S.; Gonzalgo, M.L.; Kramolowsky, E.V.; Sexton, W.J.; Reddy, S.K.; Bhar, P.; Garner, C.; Soon-Shiong, P. Phase II/III clinical results of IL-15RαFc superagonist N-803 with BCG in BCG-unresponsive non-muscle invasive bladder cancer (NMIBC) carcinoma in situ (CIS) patients. J. Clin. Oncol. 2021, 39, 510. [Google Scholar] [CrossRef]
- Joseph, M.; Enting, D. Immune Responses in Bladder Cancer-Role of Immune Cell Populations, Prognostic Factors and Therapeutic Implications. Front. Oncol. 2019, 9, 1270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luckheeram, R.V.; Zhou, R.; Verma, A.D.; Xia, B. CD4 + T Cells: Differentiation and Functions. Clin. Dev. Immunol. 2012, 2012, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mora-Bau, G.; Platt, A.M.; Van Rooijen, N.; Randolph, G.J.; Albert, M.L.; Ingersoll, M.A. Macrophages Subvert Adaptive Immunity to Urinary Tract Infection. PLoS Pathog. 2015, 11, e1005044. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.J.; Nguyen, P.H.D.; Wasser, M.; Kumar, P.; Lee, Y.H.; Nasir, N.J.M.; Chua, C.; Lai, L.; Hazirah, S.N.; Loh, J.J.H.; et al. Immunological Hallmarks for Clinical Response to BCG in Bladder Cancer. Front. Immunol. 2021, 11, 91. [Google Scholar] [CrossRef] [PubMed]
- Kates, M.; Matoso, A.; Choi, W.; Baras, A.S.; Daniels, M.J.; Lombardo, K.; Brant, A.; Mikkilineni, N.; McConkey, D.J.; Kamat, A.M.; et al. Adaptive Immune Resistance to Intravesical BCG in Non–Muscle Invasive Bladder Cancer: Implications for Prospective BCG-Unresponsive Trials. Clin. Cancer Res. 2019, 26, 882–891. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-H.; Cao, Y.-W.; Yang, X.-C.; Niu, H.-T.; Sun, L.-J.; Wang, X.-S.; Liu, J. Effect of TLR4 and B7-H1 on Immune Escape of Urothelial Bladder Cancer and its Clinical Significance. Asian Pac. J. Cancer Prev. 2014, 15, 1321–1326. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Liu, J.; Yang, X.; Liu, Y.; Liu, Y.; Li, Y.; Sun, L.; Yang, X.; Niu, H. Bacillus Calmette–Guérin and anti-PD-L1 combination therapy boosts immune response against bladder cancer. OncoTargets Ther. 2018, 11, 2891–2899. [Google Scholar] [CrossRef] [Green Version]
- Frederick, J.; Guerrero, L.; Evans, T.; Barreto, J.; Kulangara, K. Abstract 749: Pathology training for combined positive score algorithm for the assessment of PD-L1 in human cancer tissues. In Proceedings of the AACR Annual Meeting 2020, Philadelphia, PA, USA, 27–28 April 2020; Volume 80, p. 749. [Google Scholar] [CrossRef]
- Golden, E.B.; Frances, D.; Pellicciotta, I.; Demaria, S.; Barcellos-Hoff, M.H.; Formenti, S.C. Radiation fosters dose-dependent and chemotherapy-induced immunogenic cell death. OncoImmunology 2014, 3, e28518. [Google Scholar] [CrossRef] [Green Version]
- Boustani, J.; Lecoester, B.; Baude, J.; Latour, C.; Adotevi, O.; Mirjolet, C.; Truc, G. Anti-PD-1/Anti-PD-L1 Drugs and Radiation Therapy: Combinations and Optimization Strategies. Cancers 2021, 13, 4893. [Google Scholar] [CrossRef]
- Chen, H.-N.; Liang, K.-H.; Lai, J.-K.; Lan, C.-H.; Liao, M.-Y.; Hung, S.-H.; Chuang, Y.-T.; Chen, K.-C.; Tsuei, W.W.-F.; Wu, H.-C. EpCAM Signaling Promotes Tumor Progression and Protein Stability of PD-L1 through the EGFR Pathway. Cancer Res. 2020, 80, 5035–5050. [Google Scholar] [CrossRef]
- MacDonald, G.C.; Kowalski, M.; Entwistle, J.; Cizeau, J.; Niforos, D.; Loewen, S.; Chapman, W. A Phase I study of an intravesically administered immunotoxin targeting EpCAM for the treatment of nonmuscle-invasive bladder cancer in BCG-refractory and BCG-intolerant patients. Drug Des. Dev. Ther. 2010, 4, 313–320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shepherd, A.R.; Shepherd, E.; Brook, N.R. Intravesical Bacillus Calmette-Guérin with interferon-alpha versus intravesical Bacillus Calmette-Guérin for treating non-muscle-invasive bladder cancer. Cochrane Database Syst. Rev. 2017, 2017, CD012112. [Google Scholar] [CrossRef] [PubMed]
- Kamat, A.M.; Lamm, D.L. Immunotherapy for bladder cancer. Curr. Urol. Rep. 2001, 2, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Lamm, D.; Brausi, M.; O’Donnell, M.A.; Witjes, J.A. Interferon alfa in the treatment paradigm for non–muscle-invasive bladder cancer. Urol. Oncol. Semin. Orig. Investig. 2013, 32, 35.e21–35.e30. [Google Scholar] [CrossRef] [PubMed]
- Connor, R.J.; Anderson, J.M.; Machemer, T.; Maneval, D.C.; Engler, H. Sustained intravesical interferon protein exposure is achieved using an adenoviral-mediated gene delivery system: A study in rats evaluating dosing regimens. Urology 2005, 66, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Dranoff, G. GM-CSF-based cancer vaccines. Immunol. Rev. 2002, 188, 147–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, Z.; Tan, W.; Zhang, L.; Liang, Z.; Xu, C.; Su, H.; Lu, J.; Gao, J. A novel immunotherapy for superficial bladder cancer by intravesical immobilization of GM-CSF. J. Cell. Mol. Med. 2009, 14, 1836–1844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyake, M.; Hori, S.; Ohnishi, S.; Toritsuka, M.; Fujii, T.; Shimizu, T.; Owari, T.; Morizawa, Y.; Gotoh, D.; Itami, Y.; et al. Supplementary granulocyte macrophage colony-stimulating factor to chemotherapy and programmed death-ligand 1 blockade decreases local recurrence after surgery in bladder cancer. Cancer Sci. 2019, 110, 3315–3327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rong, Q.-X.; Wang, F.; Guo, Z.-X.; Hu, Y.; An, S.-N.; Luo, M.; Zhang, H.; Wu, S.-C.; Huang, H.-Q.; Fu, L.-W. GM-CSF mediates immune evasion via upregulation of PD-L1 expression in extranodal natural killer/T cell lymphoma. Mol. Cancer 2021, 20, 1–17. [Google Scholar] [CrossRef]
- Takeuchi, H.; Tanaka, M.; Tanaka, A.; Tsunemi, A.; Yamamoto, H. Predominance of M2-polarized macrophages in bladder cancer affects angiogenesis, tumor grade and invasiveness. Oncol. Lett. 2016, 11, 3403–3408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ajili, F.; Kourda, N.; Darouiche, A.; Chebil, M.; Boubaker, S. Prognostic Value of Tumor-associated Macrophages Count in Human Non-muscle-invasive Bladder Cancer Treated by BCG Immunotherapy. Ultrastruct. Pathol. 2013, 37, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.K.; Mantovani, A. Macrophage plasticity and interaction with lymphocyte subsets: Cancer as a paradigm. Nat. Immunol. 2010, 11, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ni, S.; Chen, Q.; Ma, L.; Jiao, Z.; Wang, C.; Jia, G. Bladder cancer cells induce immunosuppression of T cells by supporting PD-L1 expression in tumour macrophages partially through interleukin 10. Cell Biol. Int. 2017, 41, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Kaempfer, R.; Gerez, L.; Farbstein, H.; Madar, L.; Hirschman, O.; Nussinovich, R.; Shapiro, A. Prediction of response to treatment in superficial bladder carcinoma through pattern of inter-leukin-2 gene expression. J. Clin. Oncol. 2016, 14, 1778–1786. [Google Scholar] [CrossRef]
- Sonpavde, G.; Rosser, C.J.; Pan, C.X.; Parikh, R.A.; Nix, J.; Gingrich, J.R.; Wong, H.C.; Hernandez, L.; Huang, B.-Y. Phase I trial of ALT-801, a first-in-class T-cell receptor (TCR)-interleukin (IL)-2 fusion molecule, plus gemcitabine (G) for Bacillus Calmette Guerin (BCG)-resistant non-muscle-invasive bladder cancer (NMIBC). J. Clin. Oncol. 2015, 33. [Google Scholar] [CrossRef]

| Medication | Atezolizumab [39,40] | Durvalumab + OM [41] | Pembrolizumab [42] | CG0070 [43] | Nadofaragene Firadenovec [44] | PANVAC + BCG vs. BCG Alone [45] | ALT-803 + BCG [46] | |||
|---|---|---|---|---|---|---|---|---|---|---|
| PANVAC + BCG | BCG Alone | |||||||||
| Study phase | 2 | 1 | 2 | 2 | 3 | 1b/2 | 2/3 | |||
| Number of patients (n) | 128 1 | 12 | 101 * | 67 | 157 | 15 | 15 | 80 | ||
| Median age in years (range) | NA | 69.5 (57–82) | 73 (63–79) | 72 (64–80) | 71 (66–77) | NA | 72.5 | |||
| Sex (male), % | NA | 91.7 | 84.2 | 80.6 | 82.2 | NA | 86 | |||
| ECOG | 0, % ≥1, % | NA | NA | 73.3 | NA | 89.2 | NA | 82 | ||
| NA | NA | 26.7 | NA | 10.8 | NA | 18 | ||||
| Median number of previous BCG instillations (range) | NA | NA | 12.0 (9.0–16.5) | NA | NA | NA | 16.2 | |||
| Initial T-stage | CIS | 74 | NA | 63.4 | 46.3 | 51.6 | 6 | 69 | ||
| Ta/HG | NA | 50.0 | NA | 16.4 | 22.3 | 14 | 0 | |||
| Ta/HG + CIS | NA | NA | 24.9 | 14.9 | 13.4 | 2 | 21 | |||
| T1 | NA | 16.7 | NA | 11.9 | 9.6 | 7 | 0 | |||
| T1 + CIS | NA | NA | 11.9 | 8.9 | 3.2 | 1 | 9 | |||
| TaT1/HG | 55 | NA | NA | 28.4 | NA | NA | 0 | |||
| TaT1/HG + CIS | 0 | 33.3 | 36.8 | 23.8 | 16.6 | 3 | 30 | |||
| Initial T-Stage | FU in Months | Atezolizumab [39,40] | Durvalumab + OM [41] | Pembrolizumab [42] | CG0070 [43] | Nadofaragene Firadenovec [44] |
|---|---|---|---|---|---|---|
| CIS | 3 | NA | NA | 45.0 | NA | 53.4 |
| 6 | 27.0 | NA | NA | 58.3 | 40.8 | |
| 12 | NA | NA | NA | NA | 24.3 | |
| Ta + CIS | 3 | NA | NA | 29.2 | NA | NA |
| 6 | NA | NA | NA | 37.5 | NA | |
| 12 | NA | NA | NA | NA | NA | |
| T1 + CIS | 3 | NA | NA | 41.7 | NA | NA |
| 6 | NA | NA | NA | 25.0 | NA | |
| 12 | NA | NA | NA | NA | NA | |
| Complete cohort | 3 | NA | 41.6 | 40.6 | NA | 59.6 |
| 6 | NA | 33.3 | NA | NA | 47.7 | |
| 12 | NA | 16.7 | NA | NA | 30.5 |
| Medication | Atezolizumab [39,40] | Durvalumab + OM [41] | Pembrolizumab [42] | CG0070 [43] | Nadofaragene Firadenovec [44] |
|---|---|---|---|---|---|
| Number of patients (n) | 166 | 12 | 101 | 67 | 157 |
| Treatment-related AE, % | 85.5 | 100.0 | 66.3 | 56.7 | 70.1 |
| Treatment-related ≥ G3 AE, % | 16.9 | 8.0 | 12.9 | NA | 3.8 |
| Trial Name | PREVERT | ADAPT-BLADDER | Check-Mate 9UT | MK-3475-676/ KEYNOTE-676 | CORE-001 | QUILT-3.032 | ALT-801 in Patients with BCG NMIBC |
|---|---|---|---|---|---|---|---|
| NCT number | NCT03950362 | NCT03317158 | NCT03519256 | NCT03711032 | NCT04387461 | NCT03022825 | NCT01625260 |
| Immune mediating agent | Avelumab 10 mg/kg i.v. Q3W for 8 cycles | Durvalumab 1120 mg i.v. Q3W for 8 cycles | Nivolumab i.v. | Pembrolizumab 200 mg i.v. Q3W or 400 mg i.v. Q6W for 2 years | CG0070 Inst. + Pembrolizumab 200 mg i.v. Q3W | ALT-803 Inst. | ALT-801 i.v. |
| Molecular mechanism of action | Anti-PD-L1 antibody | Anti-PD-L1 antibody | Anti-PD-1 antibody | Anti-PD-1 antibody | Oncolytic virus + Anti-PD-1 antibody | IL-15RαFc superagonist | T-cell receptor -interleukin-2 fusion molecule |
| In combination with | EBRT-B with 60–66 Gray in 30–33 fractions | Mono or + BGC Inst. or + EBRT-B | Mono or + BGC Inst. or + Linrodostat (BMS-986205) * or + both | BCG Inst. | - | BGC Inst. | Gemcitabine i.v. |
| Treatment arms | 1 | 3 | 4 | 2 ♦ | 1 | 1 | 1 |
| Study phase | 2 | 1/2 | 2 | 3 | 2 | 2/3 | 1b/2 |
| Histology | CIS or TaT1/HG or both | CIS or TaT1/HG or both | CIS+/−TaT1/HG | CIS or TaT1/HG or both | CIS+/−pTa/T1 HG | CIS+/−TaT1/HG | CIS or TaT1/HG or both |
| Trial status | NYR | R | ANR | R | R | R | ANR |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deininger, S.; Törzsök, P.; Mitterberger, M.; Pallauf, M.; Oswald, D.; Deininger, C.; Lusuardi, L. From Interferon to Checkpoint Inhibition Therapy—A Systematic Review of New Immune-Modulating Agents in Bacillus Calmette–Guérin (BCG) Refractory Non-Muscle-Invasive Bladder Cancer (NMIBC). Cancers 2022, 14, 694. https://doi.org/10.3390/cancers14030694
Deininger S, Törzsök P, Mitterberger M, Pallauf M, Oswald D, Deininger C, Lusuardi L. From Interferon to Checkpoint Inhibition Therapy—A Systematic Review of New Immune-Modulating Agents in Bacillus Calmette–Guérin (BCG) Refractory Non-Muscle-Invasive Bladder Cancer (NMIBC). Cancers. 2022; 14(3):694. https://doi.org/10.3390/cancers14030694
Chicago/Turabian StyleDeininger, Susanne, Peter Törzsök, Michael Mitterberger, Maximilian Pallauf, David Oswald, Christian Deininger, and Lukas Lusuardi. 2022. "From Interferon to Checkpoint Inhibition Therapy—A Systematic Review of New Immune-Modulating Agents in Bacillus Calmette–Guérin (BCG) Refractory Non-Muscle-Invasive Bladder Cancer (NMIBC)" Cancers 14, no. 3: 694. https://doi.org/10.3390/cancers14030694
APA StyleDeininger, S., Törzsök, P., Mitterberger, M., Pallauf, M., Oswald, D., Deininger, C., & Lusuardi, L. (2022). From Interferon to Checkpoint Inhibition Therapy—A Systematic Review of New Immune-Modulating Agents in Bacillus Calmette–Guérin (BCG) Refractory Non-Muscle-Invasive Bladder Cancer (NMIBC). Cancers, 14(3), 694. https://doi.org/10.3390/cancers14030694







