The Evolution of Immune Checkpoint Inhibitors in Advanced Urothelial Carcinoma
Abstract
:Simple Summary
Abstract
1. Introduction
2. ICIs in Urothelial Carcinoma: Rationale for Clinical Efficacy
3. ICI after Failure of Platinum-Based Therapy: Better Than Chemotherapy
4. Determining the Optimal Place of ICIs: Moving towards the First-Line Setting
4.1. ICI in Maintenance Represents the New Standard Strategy
4.2. Combination of Chemotherapy and ICI Does Not Improve Survival in a First-Line Setting
4.3. Superiority of ICI over Platinum-Based Chemotherapy Has Not Been Demonstrated in A First-Line Metastatic Setting
4.4. Strategies in Cisplatin-Ineligible Patients: Monotherapy or Maintenance ICI
4.5. No Current Place for ICI–ICI Combinations in mUC
5. Optimizing Biomarker Profiles in UC
6. Future Strategies with ICIs in Urothelial Carcinoma Based on Immune Resistance Mechanisms
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bellmunt, J.; Powles, T.; Vogelzang, N.J. A review on the evolution of PD-1/PD-L1 immunotherapy for bladder cancer: The future is now. Cancer Treat. Rev. 2017, 54, 58–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boesteanu, A.C.; Katsikis, P.D. Memory T cells need CD28 costimulation to remember. Semin. Immunol. 2009, 21, 69–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jenkins, R.; Barbie, D.; Flaherty, K. Mechanisms of resistance to immune checkpoint inhibitors. Br. J. Cancer 2018, 118, 9–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patsoukis, N.; Sari, D.; Boussiotis, V.A. PD-1 inhibits T cell proliferation by upregulating p27 and p15 and suppressing Cdc25A. Cell Cycle 2012, 11, 4305–4309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schneider, H.; Valk, E.; Leung, R.; Rudd, C.E. CTLA-4 activation of phosphatidylinositol 3-kinase (PI 3-K) and protein kinase B (PKB/AKT) sustains T-cell anergy without cell death. PLoS ONE 2008, 3, e3842. [Google Scholar] [CrossRef] [Green Version]
- Nakanishi, J.; Wada, Y.; Matsumoto, K.; Azuma, M.; Kikuchi, K.; Ueda, S. Overexpression of B7-H1 (PD-L1) significantly associates with tumor grade and postoperative prognosis in human urothelial cancers. Cancer Immunol. Immunother. 2007, 56, 1173–1182. [Google Scholar] [CrossRef]
- Qin, S.; Xu, L.; Yi, M.; Yu, S.; Wu, K.; Luo, S. Novel immune checkpoint targets: Moving beyond PD-1 and CTLA-4. Mol. Cancer 2019, 18, 155. [Google Scholar] [CrossRef]
- Hamilton, G.; Rath, B. Avelumab: Combining immune checkpoint inhibition and antibody-dependent cytotoxicity. Expert Opin. Biol. Ther. 2017, 17, 515–523. [Google Scholar] [CrossRef]
- Yarchoan, M.; Johnson, B.A., 3rd; Lutz, E.R.; Laheru, D.A.; Jaffee, E.M. Targeting neoantigens to augment antitumour immunity. Nat. Rev. Cancer 2017, 17, 209–222. [Google Scholar] [CrossRef]
- Kandoth, C.; McLellan, M.D.; Vandin, F.; Ye, K.; Niu, B.; Lu, C.; Xie, M.; Zhang, Q.; McMichael, J.F.; Wyczalkowski, M.A.; et al. Mutational landscape and significance across 12 major cancer types. Nature 2013, 502, 333–339. [Google Scholar] [CrossRef] [Green Version]
- Hülsen, S.; Lippolis, E.; Ferrazzi, F.; Otto, W.; Distel, L.; Fietkau, R.; Denzinger, S.; Breyer, J.; Burger, M.; Bertz, S.; et al. High Stroma T-Cell Infiltration is Associated with Better Survival in Stage pT1 Bladder Cancer. Int. J. Mol. Sci. 2020, 21, 8407. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Shen, Y.; Wen, S.; Yamada, S.; Jungbluth, A.A.; Gnjatic, S.; Bajorin, D.F.; Reuter, V.E.; Herr, H.; Old, L.J.; et al. CD8 tumor-infiltrating lymphocytes are predictive of survival in muscle-invasive urothelial carcinoma. Proc. Natl. Acad. Sci. USA 2007, 104, 3967–3972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wen, Y.; Chen, Y.; Duan, X.; Zhu, W.; Cai, C.; Deng, T.; Zeng, G. The clinicopathological and prognostic value of PD-L1 in urothelial carcinoma: A meta-analysis. Clin. Exp. Med. 2019, 19, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Stein, J.P.; Lieskovsky, G.; Cote, R.; Groshen, S.; Feng, A.C.; Boyd, S.; Skinner, E.; Bochner, B.; Thangathurai, D.; Mikhail, M.; et al. Radical cystectomy in the treatment of invasive bladder cancer: Long-term results in 1,054 patients. J. Clin. Oncol. 2001, 19, 666–675. [Google Scholar] [CrossRef]
- Grossman, H.B.; Natale, R.B.; Tangen, C.M.; Speights, V.O.; Vogelzang, N.J.; Trump, D.L.; deVere White, R.W.; Sarosdy, M.F.; Wood, D.P., Jr.; Raghavan, D.; et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N. Engl. J. Med. 2003, 349, 859–866. [Google Scholar] [CrossRef]
- von der Maase, H.; Sengelov, L.; Roberts, J.T.; Ricci, S.; Dogliotti, L.; Oliver, T.; Moore, M.J.; Zimmermann, A.; Arning, M. Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J. Clin. Oncol. 2005, 23, 4602–4608. [Google Scholar] [CrossRef]
- De Santis, M.; Bellmunt, J.; Mead, G.; Kerst, J.M.; Leahy, M.; Maroto, P.; Gil, T.; Marreaud, S.; Daugaard, G.; Skoneczna, I.; et al. Randomized phase II/III trial assessing gemcitabine/carboplatin and methotrexate/carboplatin/vinblastine in patients with advanced urothelial cancer who are unfit for cisplatin-based chemotherapy: EORTC study 30986. J. Clin. Oncol. 2012, 30, 191–199. [Google Scholar] [CrossRef]
- Bellmunt, J.; Théodore, C.; Demkov, T.; Komyakov, B.; Sengelov, L.; Daugaard, G.; Caty, A.; Carles, J.; Jagiello-Gruszfeld, A.; Karyakin, O.; et al. Phase III trial of vinflunine plus best supportive care compared with best supportive care alone after a platinum-containing regimen in patients with advanced transitional cell carcinoma of the urothelial tract. J. Clin. Oncol. 2009, 27, 4454–4461. [Google Scholar] [CrossRef]
- Rosenberg, J.E.; Hoffman-Censits, J.; Powles, T.; Van Der Heijden, M.S.; Balar, A.V.; Necchi, A.; Dawson, N.; O’Donnell, P.H.; Balmanoukian, A.; Loriot, Y.; et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: A single-arm, multicentre, phase 2 trial. Lancet 2016, 387, 1909–1920. [Google Scholar] [CrossRef] [Green Version]
- Powles, T.; Durán, I.; van der Heijden, M.S.; Loriot, Y.; Vogelzang, N.J.; De Giorgi, U.; Oudard, S.; Retz, M.M.; Castellano, D.; Bamias, A.; et al. Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): A multicentre, open-label, phase 3 randomised controlled trial. Lancet 2018, 391, 748–757. [Google Scholar] [CrossRef]
- Sharma, P.; Retz, M.; Siefker-Radtke, A.; Baron, A.; Necchi, A.; Bedke, J.; Plimack, E.R.; Vaena, D.; Grimm, M.-O.; Bracarda, S.; et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): A multicentre, single-arm, phase 2 trial. Lancet Oncol. 2017, 18, 312–322. [Google Scholar] [CrossRef]
- Bellmunt, J.; De Wit, R.; Vaughn, D.J.; Fradet, Y.; Lee, J.-L.; Fong, L.; Vogelzang, N.J.; Climent, M.A.; Petrylak, D.P.; Choueiri, T.K.; et al. Pembrolizumab as Second-Line Therapy for Advanced Urothelial Carcinoma. N. Engl. J. Med. 2017, 376, 1015–1026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellmunt, J.; Necchi, A.; De Wit, R.; Lee, J.L.; Fong, L.; Vogelzang, N.J.; Climent Durán, M.A.; Petrylak, D.P.; Choueiri, T.K.; Gerritsen, W.R.; et al. Pembrolizumab (pembro) versus investigator’s choice of paclitaxel, docetaxel, or vinflunine in recurrent, advanced urothelial cancer (UC): 5-year follow-up from the phase 3 KEYNOTE-045 trial. J. Clin. Oncol. 2021, 39, 4532. [Google Scholar] [CrossRef]
- Powles, T.; O’Donnell, P.H.; Massard, C.; Arkenau, H.-T.; Friedlander, T.W.; Hoimes, C.; Lee, J.L.; Ong, M.; Sridhar, S.S.; Vogelzang, N.J.; et al. Efficacy and Safety of Durvalumab in Locally Advanced or Metastatic Urothelial Carcinoma. JAMA Oncol. 2017, 3, e172411. [Google Scholar] [CrossRef]
- Apolo, A.B.; Infante, J.R.; Balmanoukian, A.; Patel, M.R.; Wang, D.; Kelly, K.; Mega, A.E.; Britten, C.D.; Ravaud, A.; Mita, A.C.; et al. Avelumab, an anti-programmed death-ligand 1 antibody, in patients with refractory metastatic urothelial carcinoma: Results from a multicenter, Phase Ib study. J. Clin. Oncol. 2017, 35, 2117–2124. [Google Scholar] [CrossRef]
- Apolo, A.B.; Ellerton, J.A.; Infante, J.R.; Agrawal, M.; Gordon, M.S.; Aljumaily, R.; Gourdin, T.; Dirix, L.; Lee, K.W.; Taylor, M.H.; et al. Avelumab as second-line therapy for metastatic, platinum-treated urothelial carcinoma in the phase Ib JAVELIN Solid Tumor study: 2-year updated efficacy and safety analysis. J. Immunother. Cancer 2020, 8, e001246. [Google Scholar] [CrossRef]
- Powles, T.; Park, S.H.; Voog, E.; Caserta, C.; Valderrama, B.P.; Gurney, H.; Kalofonos, H.; Radulovic, S.; Demey, W.; Ullén, A.; et al. Avelumab Maintenance Therapy for Advanced or Metastatic Urothelial Carcinoma. N. Engl. J. Med. 2020, 383, 1218–1230. [Google Scholar] [CrossRef]
- Galsky, M.D.; Arija, J.Á.A.; Bamias, A.; Davis, I.D.; De Santis, M.; Kikuchi, E.; Garcia-Del-Muro, X.; De Giorgi, U.; Mencinger, M.; Izum, K.; et al. Atezolizumab with or without chemotherapy in metastatic urothelial cancer (IMvigor130): A multicentre, randomised, placebo-controlled phase 3 trial. Lancet 2020, 395, 1547–1557. [Google Scholar] [CrossRef]
- Powles, T.; Csőszi, T.; Özgüroğlu, M.; Matsubara, N.; Géczi, L.; Cheng, S.Y.-S.; Fradet, Y.; Oudard, S.; Vulsteke, C.; Barrera, R.M.; et al. Pembrolizumab alone or combined with chemotherapy versus chemotherapy as first-line therapy for advanced urothelial carcinoma (KEYNOTE-361): A randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 931–945. [Google Scholar] [CrossRef]
- Powles, T.; van der Heijden, M.S.; Castellano, D.; Galsky, M.D.; Loriot, Y.; Petrylak, D.P.; Ogawa, O.; Park, S.H.; Lee, J.-L.; De Giorgi, U.; et al. Durvalumab alone and durvalumab plus tremelimumab versus chemotherapy in previously untreated patients with unresectable, locally advanced or metastatic urothelial carcinoma (DANUBE): A randomised, open-label, multicentre, phase 3 trial. Lancet Oncol. 2020, 21, 1574–1588. [Google Scholar] [CrossRef]
- Balar, A.V.; Galsky, M.D.; Rosenberg, J.E.; Powles, T.; Petrylak, D.P.; Bellmunt, J.; Loriot, Y.; Necchi, A.; Hoffman-Censits, J.; Perez-Gracia, J.L.; et al. Atezolizumab as First-Line Treatment in Cisplatin-Ineligible Patients with Locally Advanced and Metastatic Urothelial Carcinoma: A Single-Arm, Multicentre, Phase 2 Trial. Lancet 2017, 389, 67–76. [Google Scholar] [CrossRef] [Green Version]
- Rosenberg, J.E.; Galsky, M.D.; Balar, A.V.; Loriot, Y.; Necchi, A.; Hoffman-Censits, J.; Srinivas, S.; Drakaki, A.; Javery, A.; Nelson, B.; et al. Atezolizumab monotherapy in cisplatin-ineligible patients with previously untreated metastatic urothelial carcinoma: 5-year response and survival analysis from the phase II IMvigor210 study (cohort 1). Ann. Oncol. 2021, 32, S678–S724. [Google Scholar] [CrossRef]
- Balar, A.V.; Castellano, D.; O’Donnell, P.H.; Grivas, P.; Vuky, J.; Powles, T.; Plimack, E.R.; Hahn, N.M.; de Wit, R.; Pang, L.; et al. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): A multicentre, single-arm, phase 2 study. Lancet Oncol. 2017, 18, 1483–1492. [Google Scholar] [CrossRef]
- Bellmunt, J.; De Wit, R.; Vaughn, D.J.; Fradet, Y.; Lee, J.L.; Fong, L.; Vogelzang, N.J.; Climent, M.A.; Petrylak, D.P.; Choueiri, T.K.; et al. Two-year follow-up from the phase 3 KEYNOTE-045 trial of pembrolizumab (pembro) vs investigator’s choice (paclitaxel, docetaxel, or vinflunine) in recurrent, advanced urothelial cancer (UC). J. Clin. Oncol. 2018, 36, 410. [Google Scholar] [CrossRef]
- Sharma, P.; Siefker-Radtke, A.; de Braud, F.; Basso, U.; Calvo, E.; Bono, P.; Morse, M.A.; Ascierto, P.A.; Lopez-Martin, J.; Brossart, P.; et al. Nivolumab Alone and With Ipilimumab in Previously Treated Metastatic Urothelial Carcinoma: CheckMate 032 Nivolumab 1 Mg/Kg Plus Ipilimumab 3 Mg/Kg Expansion Cohort Results. J. Clin. Oncol. 2019, 37, 1608–1616. [Google Scholar] [CrossRef] [Green Version]
- Patel, M.R.; Ellerton, J.; Infante, J.R.; Agrawal, M.; Gordon, M.; Aljumaily, R.; Britten, C.D.; Dirix, L.; Lee, K.-W.; Taylor, M.; et al. Avelumab in Metastatic Urothelial Carcinoma after Platinum Failure (JAVELIN Solid Tumor): Pooled Results from Two Expansion Cohorts of an Open-Label, Phase 1 Trial. Lancet Oncol. 2018, 19, 51–64. [Google Scholar] [CrossRef]
- Sridhar, S.S.; Powles, T.; Loriot, Y.; Climent DuraÃÅn, M.A.; Gupta, S.; Tsuchiya, N.; Bamias, A.; Ardizzoni, A.; UlleÃÅn, A.; Huang, B.; et al. Avelumab First-Line (1L) Maintenance for Advanced Urothelial Carcinoma (UC) in the JAVELIN Bladder 100 Trial: Subgroup Analysis by Duration of Treatment-Free Interval (TFI) from End of Chemotherapy to Start of Maintenance. J. Clin. Oncol. 2021, 39, 4527. [Google Scholar] [CrossRef]
- Grivas, P.; Park, S.H.; Voog, E.; Caserta, C.; Perez Valderrama, B.; Gurney, H.; Kalofonos, H.; Radulovic, S.; Demey, W.; Ullén, A.; et al. Avelumab first-line (1L) maintenance + best supportive care (BSC) vs BSC alone with 1L chemotherapy (CTx) for advanced urothelial carcinoma (UC): Subgroup analyses from JAVELIN Bladder 100. Ann. Oncol. 2020, 31, S550. [Google Scholar] [CrossRef]
- Galsky, M.D.; Chen, G.J.; Oh, W.K.; Bellmunt, J.; Roth, B.J.; Petrioli, R.; Dogliotti, L.; Dreicer, R.; Sonpavde, G. Comparative effectiveness of cisplatin-based and carboplatin-based chemotherapy for treatment of advanced urothelial carcinoma. Ann. Oncol. 2012, 23, 406–410. [Google Scholar] [CrossRef]
- Galsky, M.D.; Wang, H.; Hahn, N.M.; Twardowski, P.; Pal, S.K.; Albany, C.; Fleming, M.T.; Starodub, A.; Hauke, R.J.; Yu, M.; et al. Phase 2 Trial of Gemcitabine, Cisplatin, plus Ipilimumab in Patients with Metastatic Urothelial Cancer and Impact of DNA Damage Response Gene Mutations on Outcomes. Eur. Urol. 2018, 73, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Powles, T.; Walker, J.; Williams, J.A.; Bellmunt, J. The evolving role of PD-L1 testing in patients with metastatic urothelial carcinoma. Cancer Treat. Rev. 2020, 82, 1019–1025. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klempner, S.J.; Fabrizio, D.; Bane, S.; Reinhart, M.; Peoples, T.; Ali, S.M.; Sokol, E.S.; Frampton, G.; Schrock, A.B.; Anhorn, R.; et al. Tumor Mutational Burden as a Predictive Biomarker for Response to Immune Checkpoint Inhibitors: A Review of Current Evidence. Oncologist 2020, 25, e147–e159. [Google Scholar] [CrossRef] [Green Version]
- Meeks, J.J.; Carneiro, B.A.; Pai, S.G.; Oberlin, D.T.; Rademaker, A.; Fedorchak, K.; Balasubramanian, S.; Elvin, J.; Beaubier, N.; Giles, F.J. Genomic characterization of high-risk non-muscle invasive bladder cancer. Oncotarget 2016, 7, 75176–75184. [Google Scholar] [CrossRef] [Green Version]
- Galsky, M.D.; Saci, A.; Szabo, P.M.; Han, G.C.; Grossfeld, G.; Collette, S.; Siefker-Radtke, A.; Necchi, A.; Sharma, P. Nivolumab in Patients with Advanced Platinum-resistant Urothelial Carcinoma: Efficacy, Safety, and Biomarker Analyses with Extended Follow-up from CheckMate 275. Clin. Cancer Res. 2020, 26, 5120–5128. [Google Scholar] [CrossRef] [PubMed]
- Powles, T.; Loriot, Y.; Ravaud, A.; Vogelzang, N.J.; Duran, I.; Retz, M.; De Giorgi, U.; Oudard, S.; Bamias, A.; Koeppen, H.; et al. Atezolizumab (atezo) vs. chemotherapy (chemo) in platinum-treated locally advanced or metastatic urothelial carcinoma (mUC): Immune biomarkers, tumor mutational burden (TMB), and clinical outcomes from the phase III IMvigor211 study. J. Clin. Oncol. 2018, 36, 409. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, N.; Wang, T.; Zhang, E.; Wang, X.; Zheng, J. Biomarkers of the Response to Immune Checkpoint Inhibitors in Metastatic Urothelial Carcinoma. Front. Immunol. 2020, 11, 1900. [Google Scholar] [CrossRef]
- Galsky, M.D.; Banchereau, R.; Hamidi, H.R.; Leng, N.; Harris, W.; O’Donnell, P.H.; Kadel, E.E.; Yuen, K.C.Y.; Jin, D.; Koeppen, H.; et al. Tumor, Immune, and Stromal Characteristics Associated with Clinical Outcomes with Atezolizumab (Atezo) + Platinum-Based Chemotherapy (PBC) or Atezo Monotherapy (Mono) versus PBC in Metastatic Urothelial Cancer (MUC) from the Phase III IMvigor130 Study. JCO 2020, 38, 5011. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Research Network. Comprehensive molecular characterization of urothelial bladder carcinoma. Nature 2014, 507, 315–322. [Google Scholar] [CrossRef] [Green Version]
- Tang, C.; Ma, J.; Liu, X.; Liu, Z. Identification of Four Immune Subtypes in Bladder Cancer Based on Immune Gene Sets. Front. Oncol. 2020, 10, 544610. [Google Scholar] [CrossRef]
- Kim, J.; Kwiatkowski, D.; McConkey, D.J.; Meeks, J.J.; Freeman, S.S.; Bellmunt, J.; Getz, G.; Lerner, S.P. The Cancer Genome Atlas Expression Subtypes Stratify Response to Checkpoint Inhibition in Advanced Urothelial Cancer and Identify a Subset of Patients with High Survival Probability. Eur. Urol. 2019, 75, 961–964. [Google Scholar] [CrossRef] [PubMed]
- Kamoun, A.; de Reyniès, A.; Allory, Y.; Sjödahl, G.; Robertson, A.G.; Seiler, R.; Hoadley, K.A.; Groeneveld, C.S.; Al-Ahmadie, H.; Choi, W.; et al. Bladder Cancer Molecular Taxonomy Group. A Consensus Molecular Classification of Muscle-invasive Bladder Cancer. Eur. Urol. 2020, 77, 420–433. [Google Scholar] [CrossRef] [PubMed]
- Powles, T.; Sridhar, S.S.; Loriot, Y.; Bellmunt, J.; Mu, X.J.; Ching, K.A.; Pu, J.; Sternberg, C.N.; Petrylak, D.P.; Tambaro, R.; et al. Avelumab Maintenance in Advanced Urothelial Carcinoma: Biomarker Analysis of the Phase 3 JAVELIN Bladder 100 Trial. Nat. Med. 2021, 27, 2200–2211. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.M.; Lin, Y.; Dohadwala, M.; Gardner, B.; Luo, J.; Zhu, L.; Kronenberg, M.; Miller, P.W.; Portanova, J.; Lee, J.C.; et al. Specific inhibition of cyclooxygenase 2 restores antitumor reactivity by altering the balance of IL-10 and IL-12 synthesis. J. Immunol. 2000, 164, 361–370. [Google Scholar]
- Crispen, P.L.; Kusmartsev, S. Mechanisms of immune evasion in bladder cancer. Cancer Immunol. Immunother. 2020, 69, 3–14. [Google Scholar] [CrossRef] [Green Version]
- Rayahin, J.E.; Buhrman, J.S.; Zhang, Y.; Koh, T.J.; Gemeinhart, R.A. High and low molecular weight hyaluronic acid differentially influence macrophage activation. ACS Biomater. Sci. Eng. 2015, 1, 481–493. [Google Scholar] [CrossRef] [Green Version]
- Luke, J.J.; Tabernero, J.; Joshua, A.; Desai, J.; Varga, A.I.; Morenoet, V.; Gomez-Roca, C.A.; Markman, B.; De Braud, F.G.; Pravin Patelet, S.; et al. BMS-986205, an indoleamine 2, 3-dioxygenase 1 inhibitor (IDO1i), in combination with nivolumab (nivo): Updated safety across all tumor cohorts and efficacy in advanced bladder cancer (advBC). J. Clin. Oncol. 2019, 37 (Suppl. 7), abstr 358. [Google Scholar] [CrossRef]
- Tomczak, P.; Popovic, L.; Barthelemy, P.; Janicic, A.; Fernandez, E.S.; Borchiellini, D.; Aglietta, M.; Maroto, J.; Carnot, A.; O’Connell, B.; et al. Preliminary analysis of a phase II, multicenter, randomized, active-control study to evaluate the efficacy and safety of eganelisib (IPI 549) in combination with nivolumab compared to nivolumab monotherapy in patients with advanced urothelial carcinoma. J. Clin. Oncol. 2021, 39 (Suppl. 6), abstr 436. [Google Scholar] [CrossRef]
- Audenet, F.; Isharwal, S.; Cha, E.K.; Donoghue, M.T.A.; Drill, E.N.; Ostrovnaya, I.; Pietzak, E.J.; Sfakianos, J.P.; Bagrodia, A.; Murugan, P.; et al. Clonal relatedness and mutational differences between upper tract and bladder urothelial carcinoma. Clin. Cancer Res. 2019, 25, 967–976. [Google Scholar] [CrossRef] [Green Version]
- Jubb, A.M.; Oates, A.J.; Holden, S.; Koeppen, H. Predicting benefit from anti-angiogenic agents in malignancy. Nat. Rev. Cancer 2006, 6, 626–635. [Google Scholar] [CrossRef]
- Wilky, B.A.; Trucco, M.M.; Subhawong, T.K.; Florou, V.; Park, W.; Kwon, D.; Wieder, E.D.; Kolonias, D.; Rosenberg, A.E.; Kerr, D.A.; et al. Axitinib plus pembrolizumab in patients with advanced sarcomas including alveolar soft-part sarcoma: A single-centre, single-arm, phase 2 trial. Lancet Oncol. 2019, 20, 837–848. [Google Scholar] [CrossRef]
- Stessin, A.M.; Clausi, M.G.; Zhao, Z.; Lin, H.; Hou, W.; Jiang, Z.; Duong, T.Q.; Tsirka, S.E.; Ryu, S. Repolarized macrophages, induced by intermediate stereotactic dose radiotherapy and immune checkpoint blockade, contribute to long-term survival in glioma-bearing mice. J. Neurooncol. 2020, 147, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Sundahl, N.; Vandekerkhove, G.; Decaestecker, K.; Meireson, A.; De Visschere, P.; Fonteyne, V.; De Maeseneer, D.; Reynders, D.; Goetghebeur, E.; Van Dorpe, J.; et al. Randomized phase 1 trial of pembrolizumab with sequential versus concomitant stereotactic body radiotherapy in metastatic urothelial carcinoma. Eur. Urol. 2019, 75, 707–711. [Google Scholar] [CrossRef] [PubMed]
- Teo, M.Y.; Seier, K.; Ostrovnaya, I.; Regazzi, A.M.; Kania, B.E.; Moran, M.M.; Cipolla, C.K.; Bluth, M.J.; Chaim, J.; Al-Ahmadie, H.; et al. Alterations in DNA Damage Response and Repair Genes as Potential Marker of Clinical Benefit From PD-1/PD-L1 Blockade in Advanced Urothelia Cancers. J. Clin. Oncol. 2018, 36, 1685–1694. [Google Scholar] [CrossRef]
- Grivas, P.; Loriot, Y.; Morales-Barrera, R.; Teo, M.Y.; Zakharia, Y.; Feyerabend, S.; Vogelzang, N.J.; Grande, E.; Adra, N.; Alva, A.; et al. Efficacy and safety of rucaparib in previously treated, locally advanced or metastatic urothelial carcinoma from a phase 2, open-label trial (ATLAS). BMC Cancer 2021, 21, 593. [Google Scholar] [CrossRef]
- Rosenberg, J.E.; Park, S.H.; Dao, T.V.; Castellano, D.E.; Li, J.R.; Mukherjee, S.; Howells, K.; Dry, H.; Lanasa, M.C.; Stewart, R.; et al. BAYOU: A phase II, randomized, multicenter, double-blind, study of durvalumab (D) in combination with olaparib (O) for the first-line treatment of platinum-ineligible patients with unresectable, stage IV urothelial carcinoma (UC). J. Clin. Oncol. 2022, 40 (Suppl. 6), 437. [Google Scholar] [CrossRef]
- Challita-Eid, P.M.; Satpayev, D.; Yang, P.; An, Z.; Morrison, K.; Shostak, Y.; Raitano, A.; Nadell, R.; Liu, W.; Lortie, D.R.; et al. Enfortumab Vedotin Antibody-Drug Conjugate Targeting Nectin-4 Is a Highly Potent Therapeutic Agent in Multiple Preclinical Cancer Models. Cancer Res. 2016, 76, 3003–3013. [Google Scholar] [CrossRef] [Green Version]
- Tagawa, S.T.; Balar, A.V.; Petrylak, D.P.; Kalebasty, A.R.; Loriot, Y.; Fléchon, A.; Jain, R.K.; Agarwal, N.; Bupathi, M.; Barthelemy, P.; et al. TROPHY-U-01: A Phase II Open-Label Study of Sacituzumab Govitecan in Patients with Metastatic Urothelial Carcinoma Progressing After Platinum-Based Chemotherapy and Checkpoint Inhibitors. J. Clin. Oncol. 2021, 39, 2474–2485. [Google Scholar] [CrossRef]
- Powles, T.; Rosenberg, J.E.; Sonpavde, G.P.; Loriot, Y.; Durán, I.; Lee, J.-L.; Matsubara, N.; Vulsteke, C.; Castellano, D.; Wu, C.; et al. Enfortumab Vedotin in Previously Treated Advanced Urothelial Carcinoma. N. Engl. J. Med. 2021, 384, 1125–1135. [Google Scholar] [CrossRef]
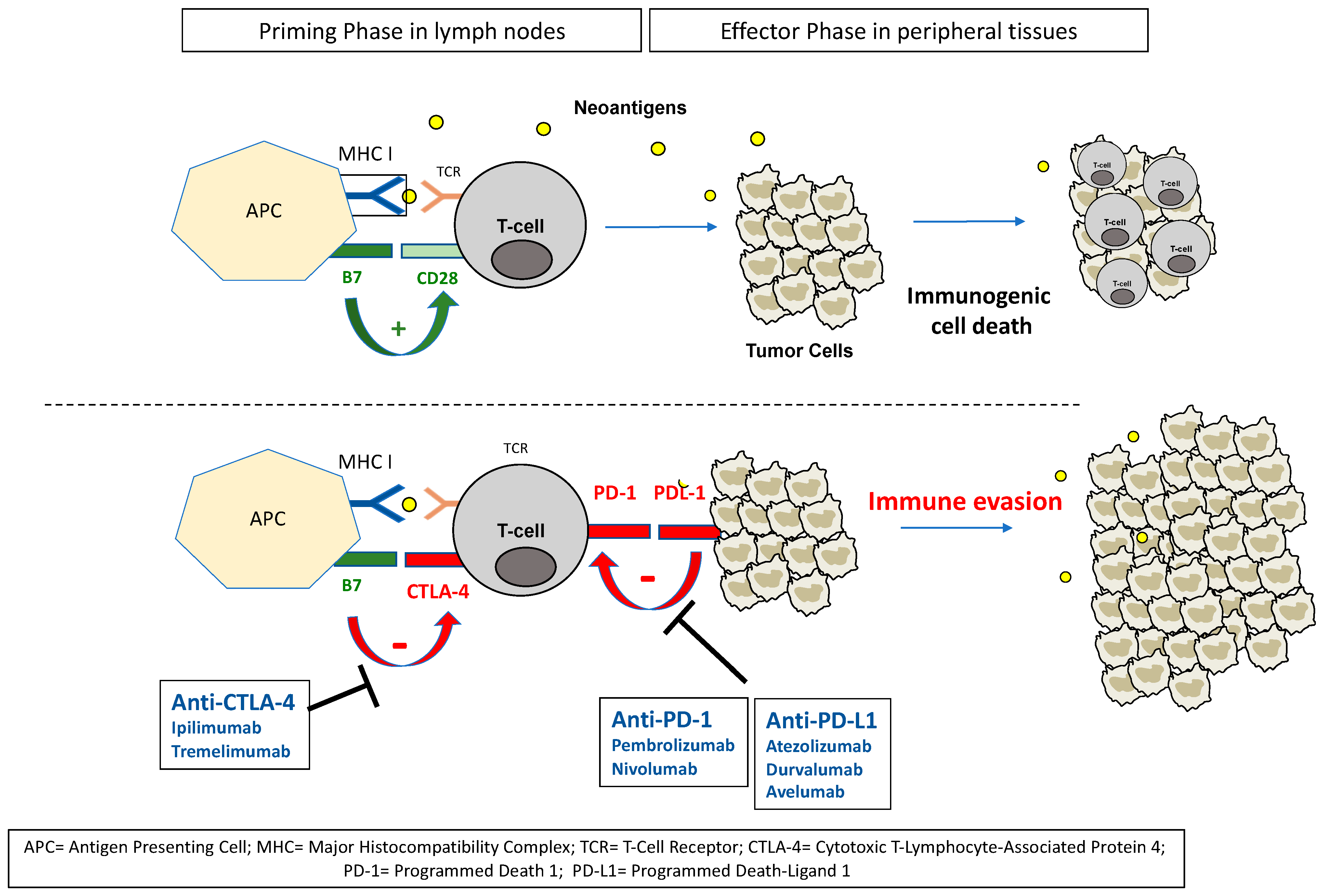
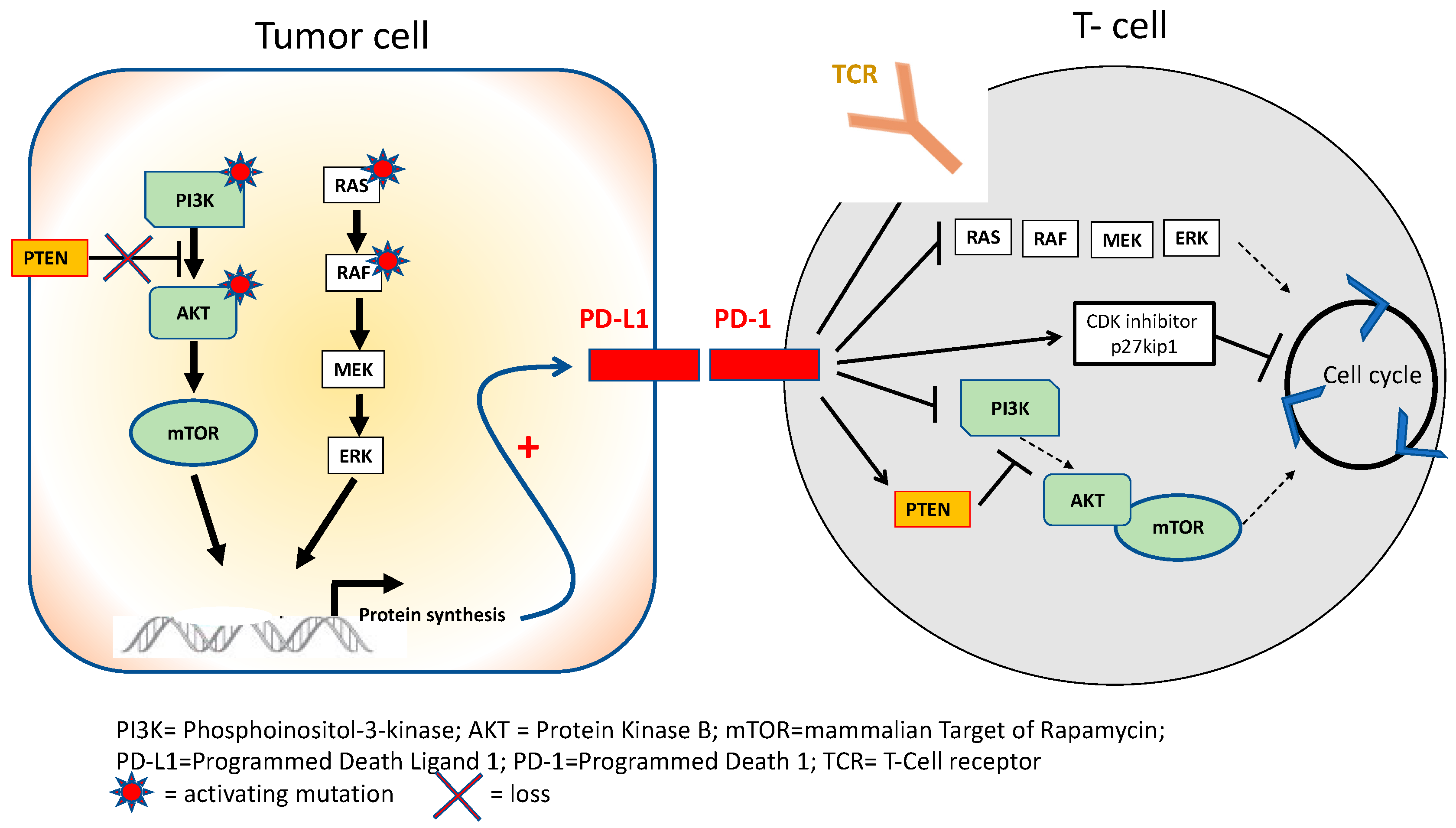
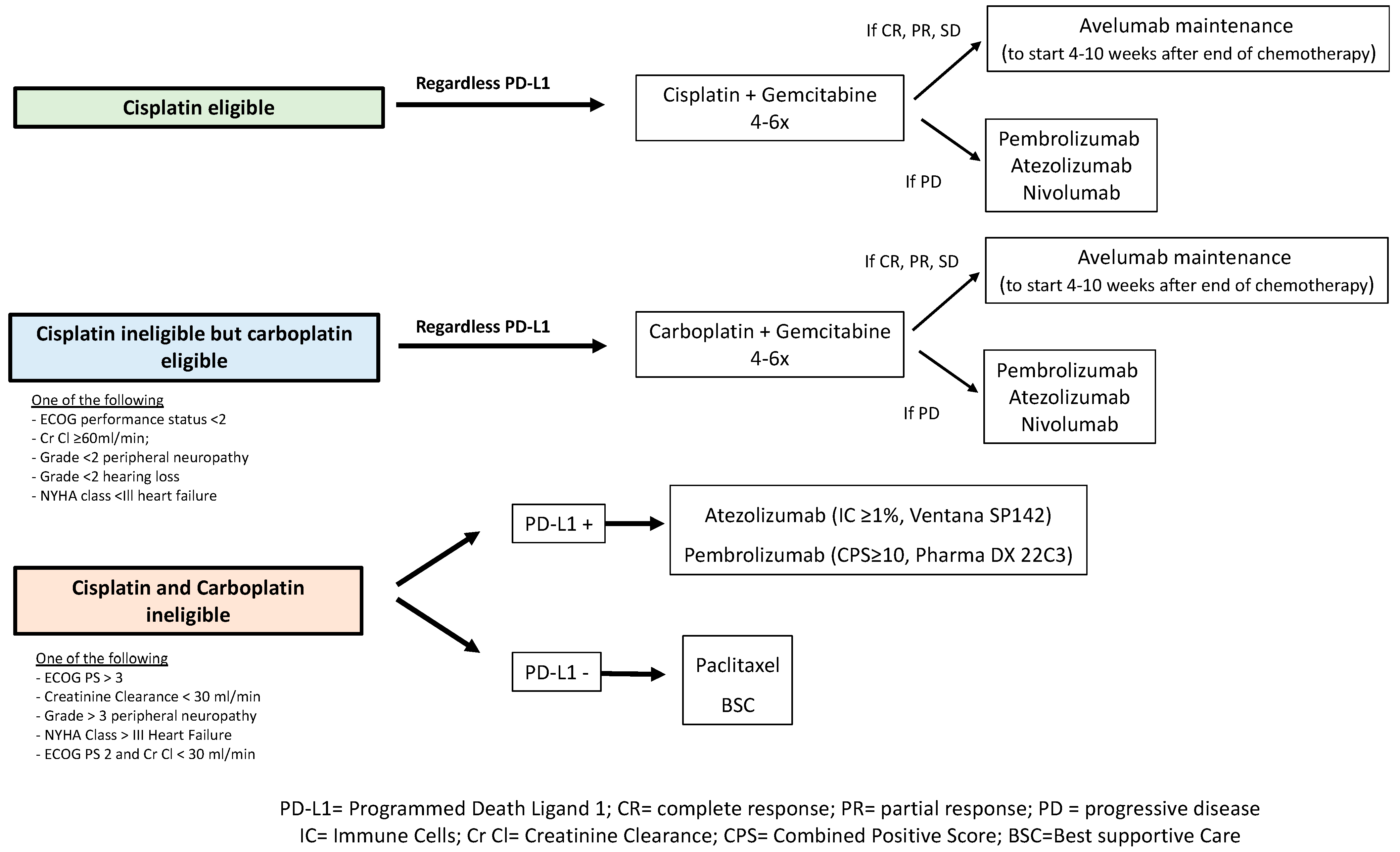

| Trial | Setting | Agent | N | ORR | OS (Months) | PD-L1 Positivity |
|---|---|---|---|---|---|---|
| Phase II Single-agent IMvigor210 Cohort 2 [20] | After failure of platinum-based therapy | Atezolizumab | 310 | All pts = 16%, CR = 7% IC2/3 PD-L1 = 28%, CR = 15% | All pts = 7.9; 1 y OS = 37% High PD-L1 = 11.9; 1 y OS = 50% | PD-L1 on tumor-infiltrating ICs: IC0 (<1%), IC1 (≥1% but <5%), and IC2/3 (≥5%) (Ventana SP142) |
| Phase III Randomized IMvigor211 [21] | After failure of platinum-based therapy | Atezolizumab | 467 | All pts = 14%, CR = 4% High PD-L1 = 23% | All pts = 8.6 (p = 0.038); 1 y OS = 40% High PD-L1 = 11.1 (p = 0.41) | PD-L1 on tumor-infiltrating ICs: IC0 (<1%), IC1 (≥1% but <5%), and IC2/3 (≥5%) (Ventana SP142) |
| Vinflunine or paclitaxel or docetaxel | 464 | All pts = 15%, CR = 4% High PD-L1 = 22% | All pts = 8.0; 1 y OS = 33% High PD-L1 = 10.6 | |||
| Phase II Single-agent Checkmate 275 [22] | After failure of platinum-based therapy | Nivolumab | 265 | All pts = 19.6% High PD-L1 = 28.4% Low PD-L1 = 16.1% | All pts = 8.7; 1 y OS = 41% High PD-L1 = 11.3; Low PD-L1 = 5.9 | PD-L1 on TCs: high (≥5%), medium (≥1%) and low (<1%) (Pharma Dx 28-8) |
| Phase III Randomized KEYNOTE-045 [23,24] | After failure of platinum-based therapy | Pembrolizumab | 270 | All pts = 21.1%, CR = 7% | All pts = 10.1 High PD-L1 = 8 | CPS = percentage of PD-L1 in ICs and TCs related to numbers of TCs. Positive if ≥10 (Pharma Dx 22C3) |
| Vinflunine or Paclitaxel or Docetaxel | 272 | All pts = 11.4%, CR = 3.3% | All pts = 7.4 High PD-L1 = 5.2 | |||
| Phase I/II Single agent [25,26] | After failure of platinum-based therapy | Durvalumab | 182 | All pts = 17% High PD-L1 = 26.3% Low PD-L1 = 4.1% | All pts = 14.1; 1 y OS = 50% | PD-L1 positivity if ≥25% of TCs or ≥25% of ICs if >1% of the tumor area contained ICs or 100% of ICs if ≤1% of the tumor area contained ICs (Ventana SP263) |
| Phase Ib Single agent (Javelin) [27] | After failure of platinum-based therapy | Avelumab | 242 | All pts = 16.1% High PD-L1 = 25% Low PD-L1 = 14.7% | All patients = 7.4; 1 y OS = 54.9% | PD-L1 on TCs: Positive if ≥5% (Ventana SP263) |
| Phase III Randomized JAVELIN Bladder 100 [28] | Maintenance setting after response or stable disease on first-line platinum-based therapy | Avelumab | 350 | All pts = 9.7%, CR = 6% High PD-L1 = 13.8%, CR = 9.5% | All pts = 21.4 (p = 0.0005); 1 y OS = 71.3% High PD-L1 = NR; 1 y OS = 79.1% | PD-<L1 positivity if ≥25% of TCs or ≥25% of ICs if >1% of the tumor area contained ICs or 100% of ICs if ≤1% of the tumor area contained ICs (Ventana SP263) |
| BSC | 350 | All pts = 1.4%, CR = 0.9% High PD-L1 = 1.2%, CR = 0.6% | All pts = 14.3; 1 y OS = 58.5% High PD-L1 = 17.1; 1 y OS = 60.4% | |||
| Phase III Randomized IMvigor130 [29] | 1st-line mUC | Atezolizumab + plt/gem | 451 | All pts = 48%, CR = 13% | All pts = 16 (p = 0.027 but did not cross boundary p-value 0.007) | PD-L1 on tumor-infiltrating ICs: IC0 (<1%), IC1 (≥1% but <5%), and IC2/3 (≥5%) (Ventana SP142) |
| Atezolizumab | 362 | All pts = 23%, CR 6% | All pts = 15.7 High PD-L1 = 27.5 | |||
| Placebo + plt/gem | 400 | All pts = 44%, CR = 7% | All pts = 13.4 | |||
| Phase III Randomized KEYNOTE-361 [30] | 1st-line mUC | Pembrolizumab + plt/gem | 351 | All pts = 54.7%, CR = 15%, High PD-L1 = 57.2%, CR = 16% | All pts = 17 (p = 0.0407 but did not cross boundary p-value 0.014 1 y OS = 62% | CPS = percentage of PD-L1 in ICs and TCs related to numbers of TCs. Positive if ≥10 (Pharma Dx 22C3) |
| Pembrolizumab | 307 | All pts = 30.3%, CR = 11% High PD-L1 = 32.5%, CR = 13% | All pts = 15.6 High PD-L1 = 16.1 | |||
| Plt/gem | 352 | All pts = 44.9%, CR = 12% High PD-L1 = 46.2%, CR = 17% | All pts = 14.3 1 y OS = 56% | |||
| Phase III Randomized DANUBE [31] | 1st-line mUC | Durvalumab + Tremelimumab | 342 | All pts = 36%, CR = 8% High PD-L1 = 47%, CR = 12% | All pts = 15.1 (p = 0.075) High PD-L1 = 17.9 | PD-<L1 positivity if ≥25% of TCs Or ≥25% of ICs if >1% of the tumor area contained ICs Or 100% of ICs if ≤1% of the tumor area contained ICs (Ventana SP263) |
| Durvalumab | 346 | All pts = 26%, CR = 8% High PD-L1 = 28%, CR = 10% | All pts = 13.2 High PD-L1 = 14.4 | |||
| plt/gem | 344 | All pts = 49%, CR = 6% High PD-L1 = 48%, CR = 7%, | All pts = 12.1 High PD-L1 = 12.1 | |||
| Phase II Single-agent IMvigor210 Cohort 1 [20,32,33] | 1st-line mUC (cisplatin ineligible) | Atezolizumab | 119 | All pts = 24%, CR = 7% High PD-L1 = 24% | All pts = 15.9 High PD-L1 = 12.3 Low PD-L1 = 19.1 | PD-L1 on tumor-infiltrating ICs: IC0 (<1%), IC1 (≥1% but <5%), and IC2/3 (≥5%) (Ventana SP142) |
| Phase II Single-agent KEYNOTE-052 [34,35] | 1st-line mUC (cisplatin ineligible) | Pembrolizumab | 370 | All pts = 29%, CR = 7% | All pts = 11.3 High PD-L1 = 18.5 | CPS = percentage of PD-L1 in ICs and TCs related to numbers of TCs. Positive if ≥10 (Pharma Dx 22C3) |
| Phase I–II CheckMate 032 [36] | After failure of platinum-based therapy | N 1 mg/kg + I 3 mg/kg | 61 | All pts = 23%, CR = 2% | All pts = 10.2 | PD-L1 on TCs: high (≥5%), medium (≥1%) and low (<1%) (Pharma Dx 28-8) |
| N 3 mg/kg + I 1 mg/kg | 54 | All pts = 19%, CR = 0% | All pts = 7.3 |
| Trial | Cancer | Associated Agent | ICI |
|---|---|---|---|
| Phase I/II (NCT02658890) [54] | Second- or later line mUC | Inhibitor of IDO (Linrodostat mesylate) | Nivolumab |
| Phase II NCT03915405 | Second- or later line mUC | Inhibitor of IDO (KHK2455) | Avelumab |
| Phase II MARIO-275 (NCT03980041) [55] | Second- or later line mUC ICI-naïve | PI3K inhibitor Eganelisib | Nivolumab |
| Phase Ib/II trial (NCT03473743) | First-line treatment in mUC (cisplatin ineligible) | FGFR inhibitor Erdafitinib | Cetrelimab (anti-PD-1) |
| Phase Ib/II trial FORT-2 (NCT03473756) | First-line treatment in mUC (cisplatin ineligible) | FGFR inhibitor Rogaratinib | Atezolizumab |
| Phase Ib/II trial FIERCE-22 (NCT03123055) | Second- or later line mUC | FGFR3 inhibitor Vofatamab | Pembrolizumab |
| Phase I NCT02496208 | Second- or later line Advanced Genito-urinary cancers | Antiangiogenic tyrosine kinase inhibitor Cabozantinib | Nivolumab +/− Ipilimumab |
| Phase I/II NCT03170960 | First- or later line Advanced metastatic cancer | Antiangiogenic tyrosine kinase inhibitor Cabozantinib | Atezolizumab |
| Phase II (NCT03601455) | Locally advanced or metastatic UC; Ineligible for chemotherapy or refusing chemotherapy | External Beam RT (5 fractions beginning on day 8 of cycle 1 of ICI) | Durvalumab +RT vs. Durvalumab + tremelimumab + RT |
| Phase II (NCT03115801) | Previously treated mUC with ≥2 metastatic sites ICI-naïve patient | RT on 1 lesion (30 Gy in 3 fractions of 10 Gy) | ICI (nivolumab, atezolizumab, pembrolizumab) vs. ICI + RT |
| Phase II (NCT03693014) | Previously treated metastatic cancer of any histology with limited progression on ICI | SBRT | Continuing ICI |
| Phase II NCT03486197 | mUC ≥2 metastatic sites | Neutron-based RT 3 × 5 fractions over 2 weeks | Pembrolizumab |
| Phase II TALASUR (NCT04678362) | mUC in maintenance setting | Talazoparib | Avelumab in maintenance in platinum-sensitive mUC patients |
| Randomized Phase III NCT04223856 | Previously untreated mUC | Enfortumab Vedotin | Pembrolizumab |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Houssiau, H.; Seront, E. The Evolution of Immune Checkpoint Inhibitors in Advanced Urothelial Carcinoma. Cancers 2022, 14, 1640. https://doi.org/10.3390/cancers14071640
Houssiau H, Seront E. The Evolution of Immune Checkpoint Inhibitors in Advanced Urothelial Carcinoma. Cancers. 2022; 14(7):1640. https://doi.org/10.3390/cancers14071640
Chicago/Turabian StyleHoussiau, Hélène, and Emmanuel Seront. 2022. "The Evolution of Immune Checkpoint Inhibitors in Advanced Urothelial Carcinoma" Cancers 14, no. 7: 1640. https://doi.org/10.3390/cancers14071640
APA StyleHoussiau, H., & Seront, E. (2022). The Evolution of Immune Checkpoint Inhibitors in Advanced Urothelial Carcinoma. Cancers, 14(7), 1640. https://doi.org/10.3390/cancers14071640





