Bladder-Sparing Chemoradiotherapy Combined with Immune Checkpoint Inhibition for Locally Advanced Urothelial Bladder Cancer—A Review
Abstract
Simple Summary
Abstract
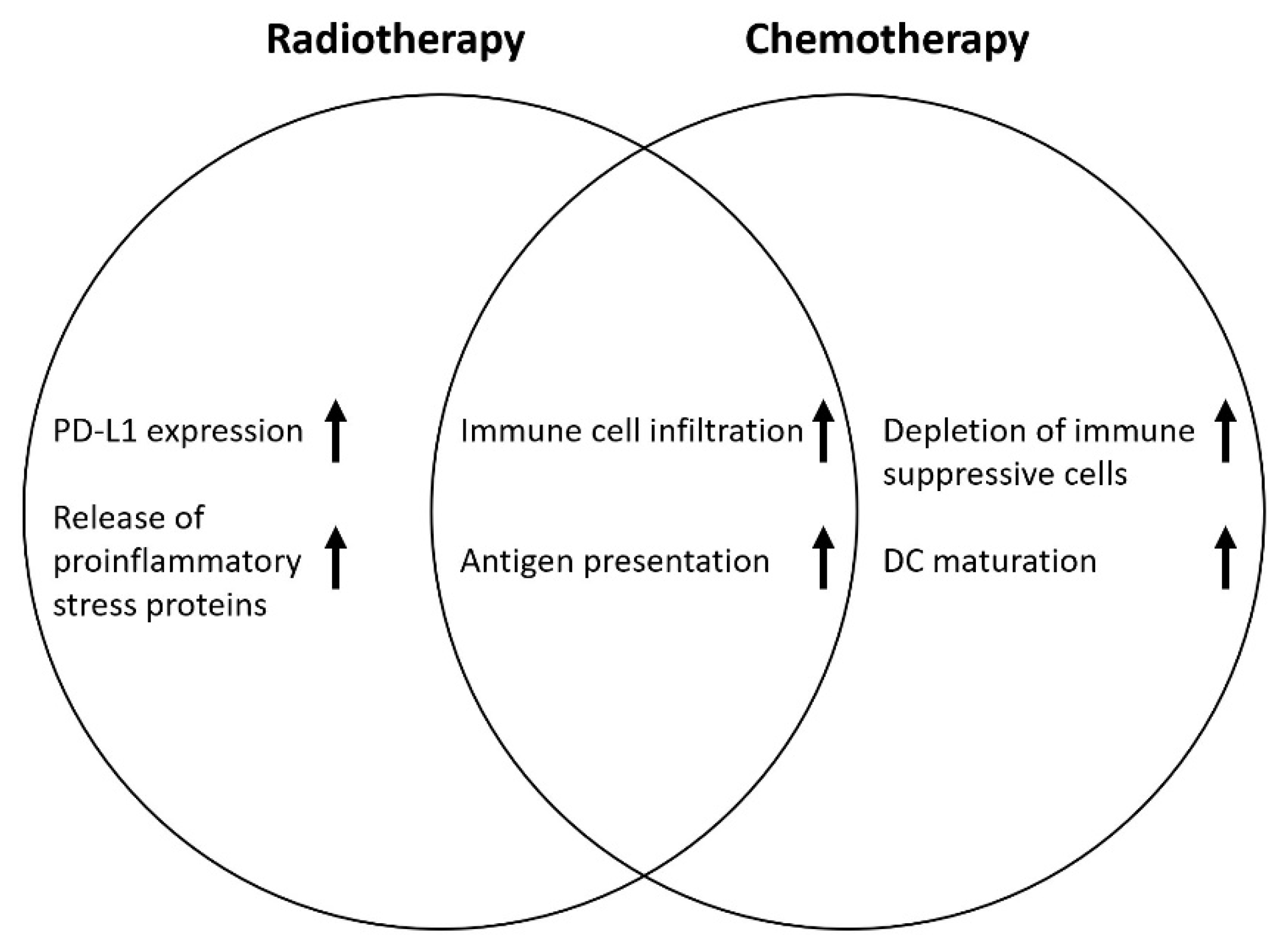
1. Introduction
2. Clinical Evidence
2.1. Overview of Studies
2.1.1. Study Design
2.1.2. Study Outcomes
2.1.3. Treatment Schedules
2.2. Reported Outcomes
2.2.1. Patient Characteristics and Outcomes
2.2.2. Treatment Toxicity
3. Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef] [PubMed]
- Howlader, N.; Noone, A.M.; Krapcho, M.; Miller, D.; Brest, A.; Yu, M.; Ruhl, J.; Tatalovich, Z.; Mariotto, A.; Lewis, D.R.; et al. SEER Cancer Statistics Review, 1975–2018; National Cancer Institute: Bethesda, MD, USA, April 2021; Based on November 2020 SEER Data Submission, Posted to the SEER Web Site. Available online: https://seer.cancer.gov/csr/1975_2018/ (accessed on 18 November 2021).
- Stein, J.P.; Skinner, D.G. Radical cystectomy for invasive bladder cancer: Long-term results of a standard procedure. World J. Urol. 2006, 24, 296–304. [Google Scholar] [CrossRef]
- EAU Guidelines: Muscle-invasive and Metastatic Bladder Cancer [Internet]. 2015. Available online: https://uroweb.org/guideline/bladder-cancer-muscle-invasive-and-metastatic/ (accessed on 18 November 2021).
- Hautmann, R.E.; Abol-Enein, H.; Hafez, K.; Haro, I.; Mansson, W.; Mills, R.D.; Montie, J.D.; Sagalowsky, A.I.; Stein, J.P.; Stenzl, A.; et al. Urinary Diversion. Urology 2007, 69, 17–49. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.; Joshi, M.; Meijer, R.P.; Glantz, M.; Holder, S.; Harvey, H.A.; Kaag, M.; Van De Putte, E.E.F.; Horenblas, S.; Drabick, J.J. Neoadjuvant Chemotherapy for Muscle-Invasive Bladder Cancer: A Systematic Review and Two-Step Meta-Analysis. Oncol. 2016, 21, 708–715. [Google Scholar] [CrossRef]
- Vale, C. Neoadjuvant Chemotherapy in Invasive Bladder Cancer: Update of a Systematic Review and Meta-Analysis of Individual Patient Data: Advanced Bladder Cancer (ABC) Meta-analysis Collaboration. Eur. Urol. 2005, 48, 202–206. [Google Scholar] [CrossRef]
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved Survival with Ipilimumab in Patients with Metastatic Melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar] [CrossRef]
- Twomey, J.D.; Zhang, B. Cancer Immunotherapy Update: FDA-Approved Checkpoint Inhibitors and Companion Diagnostics. AAPS J. 2021, 23, 39. [Google Scholar] [CrossRef]
- Balar, A.V.; Castellano, D.; O’Donnell, P.H.; Grivas, P.; Vuky, J.; Powles, T.; Plimack, E.R.; Hahn, N.M.; de Wit, R.; Pang, L.; et al. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): A multicentre, single-arm, phase 2 study. Lancet Oncol. 2017, 18, 1483–1492. [Google Scholar] [CrossRef]
- Balar, A.V.; Galsky, M.D.; Rosenberg, J.E.; Powles, T.; Petrylak, D.P.; Bellmunt, J.; Loriot, Y.; Necchi, A.; Hoffman-Censits, J.; Perez-Gracia, J.L.; et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: A single-arm, multicentre, phase 2 trial. Lancet 2017, 389, 67–76. [Google Scholar] [CrossRef]
- Powles, T.; Park, S.H.; Voog, E.; Caserta, C.; Valderrama, B.P.; Gurney, H.; Kalofonos, H.; Radulović, S.; Demey, W.; Ullén, A.; et al. Avelumab Maintenance Therapy for Advanced or Metastatic Urothelial Carcinoma. N. Engl. J. Med. 2020, 383, 1218–1230. [Google Scholar] [CrossRef] [PubMed]
- Necchi, A.; Anichini, A.; Raggi, D.; Briganti, A.; Massa, S.; Lucianò, R.; Colecchia, M.; Giannatempo, P.; Mortarini, R.; Bianchi, M.; et al. Pembrolizumab as Neoadjuvant Therapy Before Radical Cystectomy in Patients With Muscle-Invasive Urothelial Bladder Carcinoma (PURE-01): An Open-Label, Single-Arm, Phase II Study. J. Clin. Oncol. 2018, 36, 3353–3360. [Google Scholar] [CrossRef] [PubMed]
- Powles, T.; Kockx, M.; Rodriguez-Vida, A.; Duran, I.; Crabb, S.J.; Van Der Heijden, M.S.; Szabados, B.; Pous, A.F.; Gravis, G.; Herranz, U.A.; et al. Clinical efficacy and biomarker analysis of neoadjuvant atezolizumab in operable urothelial carcinoma in the ABACUS trial. Nat. Med. 2019, 25, 1706–1714. [Google Scholar] [CrossRef]
- van Dijk, N.; Gil-Jimenez, A.; Silina, K.; Hendricksen, K.; Smit, L.A.; de Feijter, J.M.; van Montfoort, M.L.; van Rooijen, C.; Peters, D.; Broeks, A.; et al. Preoperative ipilimumab plus nivolumab in locoregionally advanced urothelial cancer: The NABUCCO trial. Nat. Med. 2020, 26, 1839–1844. [Google Scholar] [CrossRef]
- Gao, J.; Navai, N.; Alhalabi, O.; Siefker-Radtke, A.; Campbell, M.T.; Tidwell, R.S.; Guo, C.C.; Kamat, A.M.; Matin, S.F.; Araujo, J.C.; et al. Neoadjuvant PD-L1 plus CTLA-4 blockade in patients with cisplatin-ineligible operable high-risk urothelial carcinoma. Nat. Med. 2020, 26, 1845–1851. [Google Scholar] [CrossRef]
- Lutkenhaus, L.J.; Van Os, R.M.; Bel, A.; Hulshof, M.C.C.M. Clinical results of conformal versus intensity-modulated radiotherapy using a focal simultaneous boost for muscle-invasive bladder cancer in elderly or medically unfit patients. Radiat. Oncol. 2016, 11, 45. [Google Scholar] [CrossRef]
- James, N.D.; Hussain, S.; Hall, E.; Jenkins, P.; Tremlett, J.; Rawlings, C.; Crundwell, M.; Sizer, B.; Sreenivasan, T.; Hendron, C.; et al. Radiotherapy with or without Chemotherapy in Muscle-Invasive Bladder Cancer. N. Engl. J. Med. 2012, 366, 1477–1488. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, G.S.; Hermanns, T.; Wei, Y.; Bhindi, B.; Satkunasivam, R.; Athanasopoulos, P.; Bostrom, P.J.; Kuk, C.; Li, K.; Templeton, A.J.; et al. Propensity Score Analysis of Radical Cystectomy Versus Bladder-Sparing Trimodal Therapy in the Setting of a Multidisciplinary Bladder Cancer Clinic. J. Clin. Oncol. 2017, 35, 2299–2305. [Google Scholar] [CrossRef]
- Fahmy, O.; Khairul-Asri, M.G.; Schubert, T.; Renninger, M.; Malek, R.; Kübler, H.; Stenzl, A.; Gakis, G. A systematic review and meta-analysis on the oncological long-term outcomes after trimodality therapy and radical cystectomy with or without neoadjuvant chemotherapy for muscle-invasive bladder cancer. Urol. Oncol. Semin. Orig. Investig. 2017, 36, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Schmid, T. Radiation-induced stress proteins—The role of heat shock proteins (HSP) in anti- tumor responses. Curr. Med. Chem. 2012, 19, 1765–1770. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-T.; Chen, W.-C.; Chang, Y.-H.; Lin, W.-Y.; Chen, M.-F. The role of PD-L1 in the radiation response and clinical outcome for bladder cancer. Sci. Rep. 2016, 6, 19740. [Google Scholar] [CrossRef]
- Sharabi, A.B.; Nirschl, C.J.; Kochel, C.M.; Nirschl, T.R.; Francisca, B.J.; Velarde, E.; Deweese, T.L.; Drake, C.G. Stereotactic Radiation Therapy Augments Antigen-Specific PD-1–Mediated Antitumor Immune Responses via Cross-Presentation of Tumor Antigen. Cancer Immunol. Res. 2014, 3, 345–355. [Google Scholar] [CrossRef]
- Heinhuis, K.; Ros, W.; Kok, M.; Steeghs, N.; Beijnen, J.; Schellens, J. Enhancing antitumor response by combining immune checkpoint inhibitors with chemotherapy in solid tumors. Ann. Oncol. 2019, 30, 219–235. [Google Scholar] [CrossRef]
- Ende, T.V.D.; Boorn, H.G.V.D.; Hoonhout, N.M.; van Etten-Jamaludin, F.S.; Meijer, S.L.; Derks, S.; de Gruijl, T.D.; Bijlsma, M.F.; van Oijen, M.G.; van Laarhoven, H.W. Priming the tumor immune microenvironment with chemo(radio)therapy: A systematic review across tumor types. Biochim. Et Biophys. Acta Bioenerg. 2020, 1874, 188386. [Google Scholar] [CrossRef]
- Antonia, S.J.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Kurata, T.; Chiappori, A.; Lee, K.H.; De Wit, M.; et al. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N. Engl. J. Med. 2018, 379, 2342–2350. [Google Scholar] [CrossRef]
- Systematic review of neoadjuvant therapy by immune checkpoint inhibitors before radical cystectomy: Where do we stand? Minerva Urol. E Nefrol. 2020, 72. [CrossRef]
- Rouanne, M.; Bajorin, D.F.; Hannan, R.; Galsky, M.D.; Williams, S.B.; Necchi, A.; Sharma, P.; Powles, T. Rationale and Outcomes for Neoadjuvant Immunotherapy in Urothelial Carcinoma of the Bladder. Eur. Urol. Oncol. 2020, 3, 728–738. [Google Scholar] [CrossRef]
- Raggi, D.; Moschini, M.; Necchi, A. Neoadjuvant Immunotherapy: The Next Gold Standard before Radical Surgery for Urothelial Cancer. Eur. Urol. Open Sci. 2021, 30, 34–36. [Google Scholar] [CrossRef] [PubMed]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef]
- Marcq, G.; Souhami, L.; Cury, F.L.; Salimi, A.; Aprikian, A.; Tanguay, S.; Vanhuyse, M.; Rajan, R.; Brimo, F.; Mansure, J.J.; et al. Phase 1 Trial of Atezolizumab Plus Trimodal Therapy in Patients With Localized Muscle-Invasive Bladder Cancer. Int. J. Radiat. Oncol. 2021, 110, 738–741. [Google Scholar] [CrossRef] [PubMed]
- de Ruiter, B.-M.; van Hattum, J.W.; De Reijke, T.M.; Oddens, J.R.; Hulshof, M.C.; Bins, A.D. Safety evaluation of combined PD-1+CTLA4 inhibition concurrently to chemoradiotherapy (CRT) in localized muscle invasive bladder carcinoma (MIBC). J. Clin. Oncol. 2021, 39, 4531. [Google Scholar] [CrossRef]
- Weickhardt, A.J.; Foroudi, F.; Lawrentschuk, N.; Galleta, L.; Seegum, A.; Herschtal, A.; Link, E.; McJannett, M.M.; Liow, E.C.H.; Grimison, P.S.; et al. Pembrolizumab with chemoradiotherapy as treatment for muscle invasive bladder cancer: A planned interim analysis of safety and efficacy of the PCR-MIB phase II clinical trial (ANZUP 1502). J. Clin. Oncol. 2020, 38, 485. [Google Scholar] [CrossRef]
- Balar, A.V.; James, N.D.; Shariat, S.F.; Shore, N.D.; Van Der Heijden, M.S.; Weickhardt, A.J.; Fang, X.; Godwin, J.L.; Kapadia, E.; Michalski, J.M. Phase III study of pembrolizumab (pembro) plus chemoradiotherapy (CRT) versus CRT alone for patients (pts) with muscle-invasive bladder cancer (MIBC): KEYNOTE-992. J. Clin. Oncol. 2020, 38, TPS5093. [Google Scholar] [CrossRef]
- A Study of Chemotherapy and Radiation Therapy Compared to Chemotherapy and Radiation Therapy Plus MEDI4736 (Durvalumab) Immunotherapy for Bladder Cancer Which Has Spread to the Lymph Nodes (The INSPIRE Study) [Internet]. Available online: https://clinicaltrials.gov/ct2/show/NCT04216290 (accessed on 18 November 2021).
- Gupta, S.; Maughan, B.L.; Dechet, C.B.; Lowrance, W.T.; Neil, B.O.; Kokeny, K.E.; Lloyd, S.; Tward, J.D.; Boucher, K.M.; Agarwal, N. NEXT: A phase II, open-label study of nivolumab adjuvant to chemoradiation in patients (pts) with localized muscle invasive bladder cancer. J. Clin. Oncol. 2020, 38, TPS605. [Google Scholar] [CrossRef]
- Nivolumab Plus Chemoradiotherapy in Patients With Muscle-invasive Bladder Cancer (MIBC) Not Undergoing Cystectomy [Internet]. Available online: https://clinicaltrials.gov/ct2/show/NCT03993249 (accessed on 18 November 2021).
- Trimodality Therapy With/Out Durvalumab to Treat Patients with Muscle-Invasive Bladder Cancer [Internet]. Available online: https://clinicaltrials.gov/ct2/show/NCT03768570 (accessed on 18 November 2021).
- Balar, A.V.; Milowsky, M.I.; O’Donnell, P.H.; Alva, A.S.; Kollmeier, M.; Rose, T.L.; Pitroda, S.; Kaffenberger, S.D.; Rosenberg, J.E.; Francese, K.; et al. Pembrolizumab (pembro) in combination with gemcitabine (Gem) and concurrent hypofractionated radiation therapy (RT) as bladder sparing treatment for muscle-invasive urothelial cancer of the bladder (MIBC): A multicenter phase 2 trial. J. Clin. Oncol. 2021, 39, 4504. [Google Scholar] [CrossRef]
- Atezolizumab after Chemo-radiotherapy for MIBC Patients Not Eligible for Radical Cystectomy—Full Text View—Clinical-Trials.gov [Internet]. Available online: https://clinicaltrials.gov/ct2/show/NCT03697850 (accessed on 18 November 2021).
- Pembrolizumab Monotherapy Following Tri-modality Treatment for Selected Patients with Muscle-invasive Bladder Cancer [Internet]. Available online: https://clinicaltrials.gov/ct2/show/NCT05072600 (accessed on 18 November 2021).
- Singh, P.; Efstathiou, J.A.; Tangen, C.; Jhavar, S.G.; Hahn, N.M.; Costello, B.A.; Delacroix, S.E.; Tripathi, A.; Sachdev, S.; Gills, J.; et al. INTACT (S/N1806) phase III randomized trial of concurrent chemoradiotherapy with or without atezolizumab in localized muscle-invasive bladder cancer: Safety update on first 73 patients. J. Clin. Oncol. 2021, 39, 428. [Google Scholar] [CrossRef]
- Bajorin, D.F.; Witjes, J.A.; Gschwend, J.E.; Schenker, M.; Valderrama, B.P.; Tomita, Y.; Bamias, A.; Lebret, T.; Shariat, S.F.; Park, S.H.; et al. Adjuvant Nivolumab versus Placebo in Muscle-Invasive Urothelial Carcinoma. N. Engl. J. Med. 2021, 384, 2102–2114. [Google Scholar] [CrossRef]
- van den Ende, T.; de Clercq, N.C.; van Berge Henegouwen, M.I.; Gisbertz, S.S.; Geijsen, E.D.; Verhoeven, R.H.A.; Meijer, S.L.; Schokker, S.; Dings, M.P.; Bergman, J.J.; et al. Neoadjuvant Chemoradiotherapy Combined with Atezolizumab for Resectable Esophageal Adenocarcinoma: A Single-arm Phase II Feasi-bility Trial (PERFECT). Clin. Cancer Res. 2021, 27, 3351–3359. Available online: https://pubmed.ncbi.nlm.nih.gov/33504550/ (accessed on 15 November 2021). [CrossRef]
- Jabbour, S.K.; Lee, K.H.; Frost, N.; Breder, V.; Kowalski, D.M.; Pollock, T.; Levchenko, E.; Reguart, N.; Martinez-Marti, A.; Houghton, B.; et al. Pembrolizumab Plus Concurrent Chemoradiation Therapy in Patients With Unresectable, Locally Advanced, Stage III Non–Small Cell Lung Cancer. JAMA Oncol. 2021, 7, 1351. [Google Scholar] [CrossRef]
- Ahn, J.S.; Ahn, Y.C.; Kim, J.-H.; Lee, C.G.; Cho, E.K.; Lee, K.C.; Chen, M.; Kim, D.-W.; Kim, H.-K.; Min, Y.J.; et al. Multinational Randomized Phase III Trial With or Without Consolidation Chemotherapy Using Docetaxel and Cisplatin After Concurrent Chemoradiation in Inoperable Stage III Non–Small-Cell Lung Cancer: KCSG-LU05-04. J. Clin. Oncol. 2015, 33, 2660–2666. [Google Scholar] [CrossRef] [PubMed]
- Choy, H.; Schwartzberg, L.S.; Dakhil, S.R.; Garon, E.B.; Gerber, D.E.; Choksi, J.K.; Govindan, R.; Peng, G.; Koustenis, A.; Treat, J.; et al. Phase 2 Study of Pemetrexed Plus Carboplatin, or Pemetrexed Plus Cisplatin with Concurrent Radiation Therapy Followed by Pemetrexed Consolidation in Patients with Favorable-Prognosis Inoperable Stage IIIA/B Non–Small-Cell Lung Cancer. J. Thorac. Oncol. 2013, 8, 1308–1316. [Google Scholar] [CrossRef] [PubMed]
- Senan, S.; Brade, A.; Wang, L.-H.; Vansteenkiste, J.; Dakhil, S.; Biesma, B.; Aguillo, M.M.; Aerts, J.; Govindan, R.; Rubio-Viqueira, B.; et al. PROCLAIM: Randomized Phase III Trial of Pemetrexed-Cisplatin or Etoposide-Cisplatin Plus Thoracic Radiation Therapy Followed by Consolidation Chemotherapy in Locally Advanced Nonsquamous Non–Small-Cell Lung Cancer. J. Clin. Oncol. 2016, 34, 953–962. [Google Scholar] [CrossRef]
- Choudhury, A.; Porta, N.; Hall, E.; Song, Y.P.; Owen, R.; MacKay, R.; West, C.M.L.; Lewis, R.; Hussain, S.A.; James, N.D.; et al. Hypofractionated radiotherapy in locally advanced bladder cancer: An individual patient data meta-analysis of the BC2001 and BCON trials. Lancet Oncol. 2021, 22, 246–255. [Google Scholar] [CrossRef]
- Arafat, W.; Darwish, A.; Naoum, G.E.; Sameh, W.; El Husseiny, G.; Abd-El-Gawad, F.; Samir, M. Comparison between standard and reduced volume radiotherapy in bladder preservation trimodality protocol for muscle-invasive bladder cancer patients. Ecancermedicalscience 2016, 10. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tunio, M.A.; Hashmi, A.; Qayyum, A.; Mohsin, R.; Zaeem, A. Whole-Pelvis or Bladder-Only Chemoradiation for Lymph Node–Negative Invasive Bladder Cancer: Single-Institution Experience. Int. J. Radiat. Oncol. 2012, 82, e457–e462. [Google Scholar] [CrossRef] [PubMed]
- Powles, T.; Csőszi, T.; Özgüroğlu, M.; Matsubara, N.; Géczi, L.; Cheng, S.Y.-S.; Fradet, Y.; Oudard, S.; Vulsteke, C.; Barrera, R.M.; et al. Pembrolizumab alone or combined with chemotherapy versus chemotherapy as first-line therapy for advanced urothelial carcinoma (KEYNOTE-361): A randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 931–945. [Google Scholar] [CrossRef]
- Galsky, M.D.; Arija, J.Á.A.; Bamias, A.; Davis, I.D.; De Santis, M.; Kikuchi, E.; Garcia-Del-Muro, X.; De Giorgi, U.; Mencinger, M.; Izumi, K.; et al. Atezolizumab with or without chemotherapy in metastatic urothelial cancer (IMvigor130): A multicentre, randomised, placebo-controlled phase 3 trial. Lancet 2020, 395, 1547–1557. [Google Scholar] [CrossRef]
- Voskuilen, C.S.; van de Kamp, M.W.; Schuring, N.; Mertens, L.S.; Noordzij, A.; Pos, F.; van Rhijn, B.W.; van der Heijden, M.S.; Schaake, E.E. Radiation with concurrent radiosensitizing capecitabine tablets and single-dose mitomycin-C for muscle-invasive bladder cancer: A convenient alternative to 5-fluorouracil. Radiother. Oncol. 2020, 150, 275–280. [Google Scholar] [CrossRef]
- Seymour, L.; Bogaerts, J.; Perrone, A.; Ford, R.; Schwartz, L.H.; Mandrekar, S.; Lin, N.U.; Litière, S.; Dancey, J.; Chen, A.; et al. iRECIST: Guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 2017, 18, e143–e152. [Google Scholar] [CrossRef]
- López-Beltrán, A.; Luque, R.J.; Mazzucchelli, R.; Scarpelli, M.; Montironi, R. Changes produced in the urothelium by traditional and newer therapeutic procedures for bladder cancer. J. Clin. Pathol. 2002, 55, 641–647. [Google Scholar] [CrossRef]
- Schulz, G.B.; Todorova, R.; Braunschweig, T.; Rodler, S.; Volz, Y.; Eismann, L.; Pfitzinger, P.; Jokisch, F.; Buchner, A.; Stief, C.; et al. PD-L1 expression in bladder cancer: Which scoring algorithm in what tissue? Urol. Oncol. Semin. Orig. Investig. 2021, 39, 734.e1–734.e10. [Google Scholar] [CrossRef]
- Necchi, A.; Marandino, L.; Raggi, D.; Bandini, M.; Gallina, A.; Moschini, M.; Briganti, A.; Montorsi, F. Is it Time to Consider Eliminating Surgery from the Treatment of Locally Advanced Bladder Cancer? Eur. Urol. 2020, 79, 713–716. [Google Scholar] [CrossRef] [PubMed]
- Siefker-Radtke, A.O.; Steinberg, G.D.; Bedke, J.; Nishiyama, H.; Fang, X.; Kataria, R.; Moreno, B.H.; Hoimes, C.J. Phase III study of perioperative pembrolizumab (pembro) plus neoadjuvant chemotherapy (chemo) versus placebo plus neoadjuvant chemo in cisplatin-eligible patients (pts) with muscle-invasive bladder cancer (MIBC): KEYNOTE-866. J. Clin. Oncol. 2020, 38, TPS599. [Google Scholar] [CrossRef]
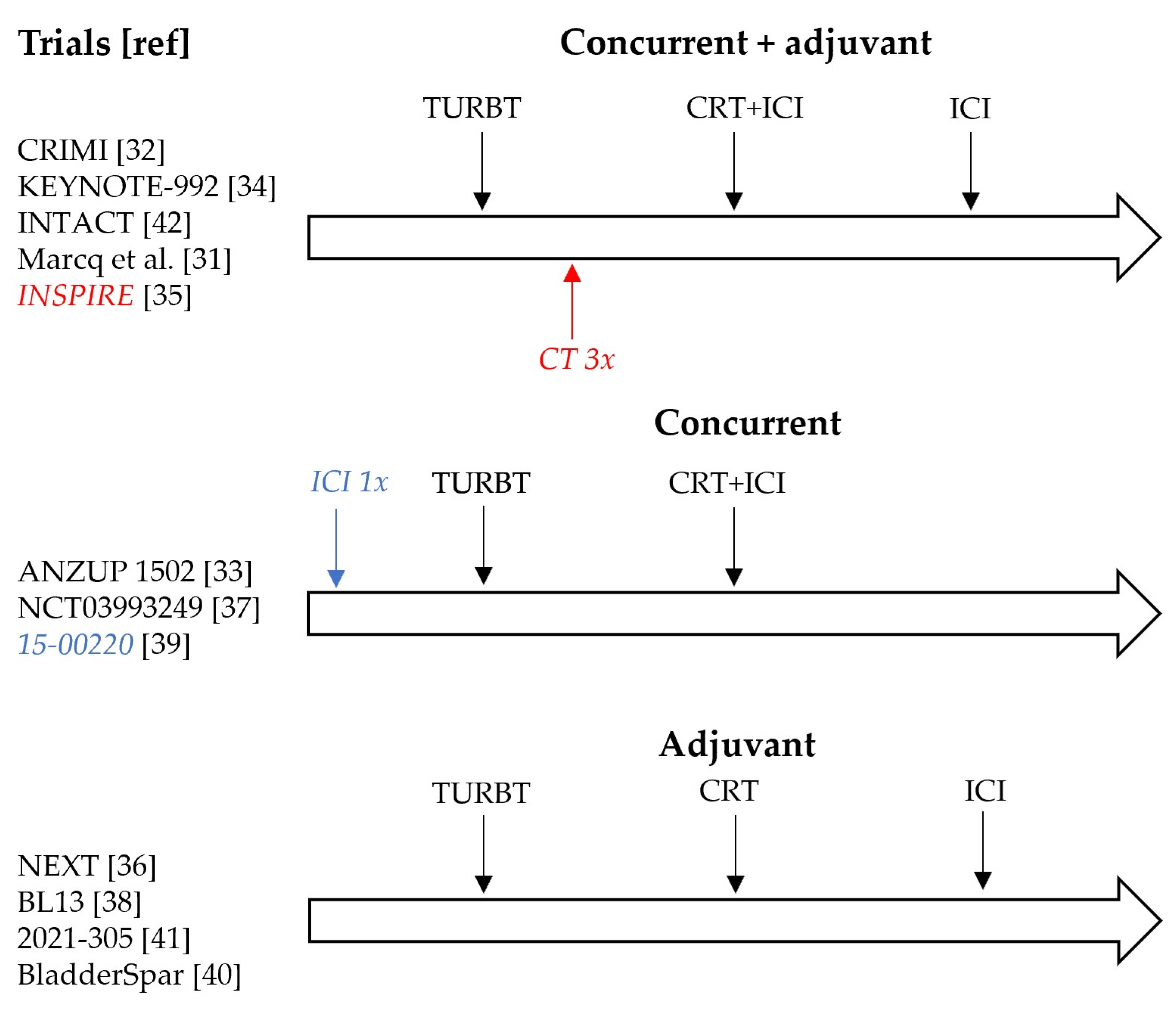
| NCT (Title), Ref | Phase | N | ICI (CP) | Chemotherapy | Radiotherapy | Concurrent/Adjuvant | Primary Endpoint | Secondary Endpoints |
|---|---|---|---|---|---|---|---|---|
| NCT03620435 (Marcq et al.) [31] | I | 8 | Atezolizumab (PD-L1) | Gem | 20 × 2.5 Gy and 20 × 2 pelvic nodes IMRT | Concurrent + adjuvant 9 m | Safety/toxicity | RR, OS, QoL |
| NCT03844256 (CRIMI) [32] | Ib/II | 30–50 | Nivolumab (PD-1) +/− Ipilimumab (CTLA-4) | MMC + Cape | 20 × 2 Gy whole bladder, tumor boost 20 × 0.75 Gy | Concurrent + 1 y adjuvant nivolumab | Safety/toxicity | DFS, OS |
| NCT03171025 (NEXT) [36] | II | 28 | Nivolumab (PD-1) | Radiosensitizing NOS | NR | Adjuvant 1 y | Failure free survival | BI-EFS, AE Cystectomy rate QoL |
| NCT03993249 [37] | II | 78 | Nivolumab (PD-1) | SoC | SoC | Concurrent | Locoregional control rate | AE, RFS, OS, QoL |
| NCT02662062 (ANZUP 1502) [33] | II | 30 | Pembrolizumab (PD-1) | Cis | 32 × 2 Gy | Concurrent | Safety/toxicity | RR, MFS, Cystectomy rate |
| NCT02621151 (15-00220) [39] | II | 54 | Pembrolizumab (PD-1) | Gem | 20 × 2.6 Gy | Concurrent | BI-DFS | AE, RR, MFS, OS |
| NCT04216290 (INSPIRE) [35] | II | 114 | Durvalumab (PD-L1) | Gem or Gem + Carbo or Gem + Cis or MVAC | 6.5–8 weeks NOS | Concurrent + 9 m adjuvant | Complete response rate | BI-EFS, Cystectomy rate, PFS, MFS, CSS, OS |
| NCT03768570 (BL13) [38] | II | 190 | Durvalumab (PD-L1) | Cis or 5-FU + MMC or Gem | Bladder: 32 × 2 Gy or 20 × 2.5 Gy. Pelvis: 45–46 Gy + 17–20 Gy bladder | Adjuvant 1 y | DFS | BI-EFS, locoregional control, MFS |
| NCT03697850 (BladderSpar) [40] | II | 77 | Atezolizumab (PD-L1) | SoC | ≥60 Gy | Adjuvant 1 y | DFS | Local control, AE, DFS, OS, QoL |
| NCT04241185 (Keynote-992) [34] | III | 636 | Pembrolizumab (PD-1) | Cis or 5-FU + MMC or Gem | 32 × 2 Gy +/− Nodes or 20 × 2.75 Gy | Concurrent + 1 y adjuvant | BI-EFS | AE, OS, MFS. NMIBC recurrence, QoL |
| NCT05072600 (2021-305) [41] | III | 54 | Pembrolizumab (PD-1) | SoC | SOC | Adjuvant 1 y | PFS | NR |
| NCT03775265 (INTACT) [42] | III | 475 | Atezolizumab (PD-L1) | Cis or 5-FU + MMC or Gem | Bladder or Pelvis | Concurrent + 6 m adjuvant | BI-EFS | AE, OS, QoL |
| NCT (Title), Ref | N (Total) | Age y (Median) | Completed Full Therapy | Gr ≥ 3 AEs | Complete Response | Additional Outcome |
|---|---|---|---|---|---|---|
| NCT03620435 (Marcq et al.) [31] | 8 | 68 | 63% | 62.5% (5/8) | NR | NA |
| NCT03844256 (CRIMI) [32] | Nivo: 10 Nivo/ipi 10 (50) | Nivo: 68 Nivo/ipi: 70 | nivo: 100% nivo/ipi: 50% | Nivo: 10% (1/10) Nivo/ipi: 30% (3/10) | NR | Nivo (1 year): DFS 100% OS 100% |
| NCT02662062 (ANZUP 1502) [33] | 10 (30) | NR | 80% (1 CT, 1 ICI) | 40% (4/10) | 9/10 (at 24 weeks) | 24-week MFS 90% |
| NCT02621151 (15-00220) [39] | SC: 6 EC: 48 (54) | SC 67 EC 74 | 88% (1 CRT, 2 CT, 4 ICI) | 31% (17/54) | ±87% (at 12 weeks) | 1-year eBI-DFS 77% (95% CI: 0.60–0.87). 1-year MFS 85% (95% CI 0.71–0.93) |
| NCT03775265 (INTACT) [42] | 73 (475) | NR | NR | iCRT 62% (23/37) CRT 30% (11/35) | NR | NA |
| NCT (Title), Ref | Immune AEs Gr ≤ 2 | Immune AEs Gr ≥ 3 | Urinary AEs Gr ≤ 2 | Urinary AEs Gr ≥ 3 | Hematological AEs Gr ≤ 2 | Hematological AEs Gr ≥ 3 |
|---|---|---|---|---|---|---|
| 03620435 (Marcq et al.) [31] | 25% (2/8) | 63% (5/8 Colitis, lymphopenia) | 63% (5/8 dysuria) | 0% | -Anemia 25% (2/8) -Lymphopenia 25% (2/8) -Neutropenia 25% (2/8) | -Neutropenia 13% (1/8) -Lymphopenia 13% (1/8) |
| 03844256 (CRIMI) [32] | Nivo: 10% (1/10 hepatitis) Nivo3/ipi1: 20% (2/10 colitis, pancreatitis) | Nivo: 0% Nivo3/ipi1: 10% (1/10 colitis) | Nivo: 10% (1/10 AKI) Nivo3/Ipi1: 40% (4/10 UTI, AKI, Urgency) | Nivo: 0% Nivo3/Ipi1: 10% (1/10 UTI) | Nivo: 0% Nivo3/Ipi1: 20% (2/10 anemia, thrombocytopenia) | Nivo: 0% Nivo3/Ipi1: 20% (2/10 anemia, thrombocytopenia) |
| 02662062 (ANZUP 1502) [33] | 10% (1/10 Nephritis) | 0% | NR | 0% | NR | NR |
| 02621151 (15-00220) [39] | NR | 7% (4/54 GI) | NR | 12% (6/54 UTI, obstruction) | NR | 4% (2/54 neutropenia, thrombocytopenia) |
| 03775265 (INTACT) [42] | NR | 0% | iCRT: 9% (3/37 UTI) CRT: 9% (3/36 AKI 1, UTI 2) | iCRT: 25% (9/37 AKI 2, UTI 7) CRT: 3% (1/36 UTI) | iCRT: -Anemia 41% (15/37) -Lymphopenia 3% (1/37) -Neutropenia: 20% (7/37) -Leukopenia 22% (8/37) CRT: -Anemia 33% (12/36) -Lymphopenia 0% -Neutropenia 11% (4/36) - Leukopenia 14% (5/36) | iCRT: -Anemia 4% (11/37) -Lymphopenia 16% (6//37) -Neutropenia: 8% (3/37) -Leukopenia 19% (7/37) CRT: -Anemia 3% (1/36) -Lymphopenia 17% (6/36) -Neutropenia 8% (3/36) -Leukopenia 8% (3/36) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van Hattum, J.W.; de Ruiter, B.-M.; Oddens, J.R.; Hulshof, M.C.C.M.; de Reijke, T.M.; Bins, A.D. Bladder-Sparing Chemoradiotherapy Combined with Immune Checkpoint Inhibition for Locally Advanced Urothelial Bladder Cancer—A Review. Cancers 2022, 14, 38. https://doi.org/10.3390/cancers14010038
van Hattum JW, de Ruiter B-M, Oddens JR, Hulshof MCCM, de Reijke TM, Bins AD. Bladder-Sparing Chemoradiotherapy Combined with Immune Checkpoint Inhibition for Locally Advanced Urothelial Bladder Cancer—A Review. Cancers. 2022; 14(1):38. https://doi.org/10.3390/cancers14010038
Chicago/Turabian Stylevan Hattum, Jons W., Ben-Max de Ruiter, Jorg R. Oddens, Maarten C. C. M. Hulshof, Theo M. de Reijke, and Adriaan D. Bins. 2022. "Bladder-Sparing Chemoradiotherapy Combined with Immune Checkpoint Inhibition for Locally Advanced Urothelial Bladder Cancer—A Review" Cancers 14, no. 1: 38. https://doi.org/10.3390/cancers14010038
APA Stylevan Hattum, J. W., de Ruiter, B.-M., Oddens, J. R., Hulshof, M. C. C. M., de Reijke, T. M., & Bins, A. D. (2022). Bladder-Sparing Chemoradiotherapy Combined with Immune Checkpoint Inhibition for Locally Advanced Urothelial Bladder Cancer—A Review. Cancers, 14(1), 38. https://doi.org/10.3390/cancers14010038







