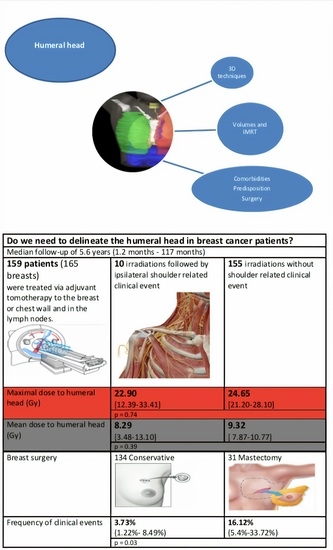
Do We Need to Delineate the Humeral Head in Breast Cancer Patients?
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Population
2.2. Terms of the Treatment
2.3. Data Collection
2.4. Statistical Analysis
3. Results
3.1. Population Description
3.2. Humeral Head Radiation Exposure
3.3. Clinical Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Offersen, B.V.; Boersma, L.J.; Kirkove, C.; Hol, S.; Aznar, M.; Sola, A.B.; Kirova, Y.M.; Pignol, J.-P.; Remouchamps, V.; Verhoeven, K.; et al. ESTRO consensus guideline on target volume delineation for elective radiation therapy of early stage breast cancer. Radiother. Oncol. 2015, 114, 3–10. [Google Scholar] [CrossRef]
- Arsene-Henry, A.; Fourquet, A.; Kirova, Y.M. Evolution of radiation techniques in the treatment of breast cancer (BC) patients: From 3D conformal radiotherapy (3DCRT) to intensity-modulated RT (IMRT) using Helical Tomotherapy (HT). Radiother. Oncol. 2017, 124, 333–334. [Google Scholar] [CrossRef]
- Arsene-Henry, A.; Foy, J.-P.; Robilliard, M.; Xu, H.-P.; Bazire, L.; Peurien, D.; Poortmans, P.; Fourquet, A.; Kirova, Y.M. The use of helical tomotherapy in the treatment of early stage breast cancer: Indications, tolerance, efficacy-a single center experience. Oncotarget 2018, 9, 23608–23619. [Google Scholar] [CrossRef]
- Zolcsák, Z.; Loap, P.; Fourquet, A.; Kirova, Y.M. Long-Term Follow-Up Results of Intensity Modulated Radiotherapy with Helicoïdal Tomotherapy in Non-Metastatic Breast Cancer Patients: Single Center Experience. Cancer Radiother. 2022; in press. [Google Scholar]
- Abbassi, L.M.; Arsène-Henry, A.; Amessis, M.; Kirova, Y.M. Radiation Dose to the Low Axilla in Patients Treated for Early-Stage Breast Cancer by Locoregional Intensity-Modulated Radiotherapy (IMRT). Cancer Radiother. 2021; in press. [Google Scholar] [CrossRef]
- Joseph, K.; Vos, L.J.; Gabos, Z.; Pervez, N.; Chafe, S.; Tankel, K.; Warkentin, H.; Ghosh, S.; Amanie, J.; Powell, K.; et al. Skin Toxicity in Early Breast Cancer Patients Treated with Field-In-Field Breast Intensity-Modulated Radiotherapy versus Helical Inverse Breast Intensity-Modulated Radiotherapy: Results of a Phase III Randomised Controlled Trial. Clin. Oncol. 2021, 33, 30–39. [Google Scholar] [CrossRef]
- Pignol, J.P.; Olivotto, I.; Rakovitch, E.; Gardner, S.; Sixel, K.; Beckham, W.; Vu, T.T.T.; Truong, P.; Ackerman, I.; Paszat, L. A multicenter randomized trial of breast intensity-modulated radiation therapy to reduce acute radiation dermatitis. J. Clin. Oncol. 2008, 26, 2085–2092. [Google Scholar] [CrossRef] [Green Version]
- Freedman, G.M.; Anderson, P.R.; Li, J.; Eisenberg, D.F.; Hanlon, A.L.; Wang, L.; Nicolaou, N. Intensity modulated radiation therapy (IMRT) decreases acute skin toxicity for women receiving radiation for breast cancer. Am. J. Clin. Oncol. 2006, 29, 66–70. [Google Scholar] [CrossRef]
- Krueger, E.A.; Fraass, B.A.; McShan, D.L.; Marsh, R.; Pierce, L.J. Potential gains for irradiation of chest wall and regional nodes with intensity modulated radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2003, 56, 1023–1037. [Google Scholar] [CrossRef]
- Popescu, C.C.; Olivotto, I.A.; Beckham, W.A.; Ansbacher, W.; Zavgorodni, S.; Shaffer, R.; Wai, E.S.; Otto, K. Volumetric modulated arc therapy improves dosimetry and reduces treatment time compared to conventional intensity-modulated radiotherapy for locoregional radiotherapy of left-sided breast cancer and internal mammary nodes. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, 287–295. [Google Scholar] [CrossRef]
- Lauche, O.; Kirova, Y.M.; Fenoglietto, P.; Costa, E.; Lemanski, C.; Bourgier, C.; Riou, O.; Tiberi, D.; Campana, F.; Fourquet, A.; et al. Helical tomotherapy and volumetric modulated arc therapy: New therapeutic arms in the breast cancer radiotherapy. World J. Radiol. 2016, 28, 735–742. [Google Scholar] [CrossRef]
- Osman, S.O.; Hol, S.; Poortmans, P.M.; Essers, M. Volumetric modulated arc therapy and breath-hold in image-guided locoregional left-sided breast irradiation. Radiother. Oncol. 2014, 112, 17–22. [Google Scholar] [CrossRef]
- Vrieling, C.; Collette, L.; Fourquet, A.; Hoogenraad, W.J.; Horiot, J.H.; Jager, J.J.; Pierart, M.; Poortmans, P.M.; Struikmans, H.; Maat, B.; et al. The influence of patient, tumor and treatment factors on the cosmetic results after breast-conserving therapy in the EORTC ‘boost vs. no boost’ trial. Radiother. Oncol. 2000, 55, 219–232. [Google Scholar] [CrossRef]
- Byun, H.K.; Chang, J.S.; Im, S.H.; Kirova, Y.M.; Arsene-Henry, A.; Choi, S.H.; Cho, Y.U.; Park, H.S.; Kim, J.Y.; Suh, C.-O.; et al. Risk of Lymphedema Following Contemporary Treatment for Breast Cancer: An Analysis of 7617 Consecutive Patients from a Multidisciplinary Perspective. Ann. Surg. 2019, 274, 170–178. [Google Scholar] [CrossRef]
- Uhl, M.; Sterzing, F.; Habl, G.; Schubert, K.; Holger, H.; Debus, J.; Herfarth, K. Breast cancer and funnel chest. Comparing helical tomotherapy and three-dimensional conformal radiotherapy with regard to the shape of pectus excavatum. Strahlenther. Onkol. 2012, 188, 127–135. [Google Scholar] [CrossRef]
- Coon, A.B.; Dickler, A.; Kirk, M.C.; Liao, Y.; Shah, A.P.; Strauss, J.B.; Chen, S.; Turian, J.; Griem, K.L. Tomotherapy and multifield intensity-modulated radiotherapy planning reduce cardiac doses in left-sided breast cancer patients with unfavorable cardiac anatomy. Int. J. Radiat. Oncol. Biol. Phys. 2010, 78, 104–110. [Google Scholar] [CrossRef]
- Arsene Henry, A.; Amessis, M.; Kirova, Y.M. Helical Tomotherapy for patients with pectus excavatum treated for early-stage breast cancer. Breast J. 2019, 26, 1483–1484. [Google Scholar] [CrossRef]
- Chira, C.; Kirova, Y.M.; Liem, X.; Campana, F.; Peurien, D.; Amessis, M.; Fournier-Bidoz, N.; Pierga, J.-Y.; Dendale, R.; Bey, P.; et al. Helical tomotherapy for inoperable breast cancer: A new promising tool. Biomed Res. Int. 2013, 2013, 264306. [Google Scholar] [CrossRef]
- Joste, M.; Arsene-Henry, A.; Reyal, F.; Amessis, M.; Fourquet, A.; Kirova, Y.M. Helical tomotherapy for patients presented with implant breast reconstruction in case of adjuvant breast cancer radiotherapy: A single center experience. Breast J. 2020, 26, 1436–1438. [Google Scholar] [CrossRef]
- Dogan, N.; Cuttino, L.; Lloyd, R.; Bump, E.A.; Arthur, D.W. Optimized dose coverage of regional lymph nodes in breast cancer: The role of intensity-modulated radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2007, 68, 1238–1250. [Google Scholar] [CrossRef]
- Lin, P.P.; Schupak, K.D.; Boland, P.J.; Brennan, M.F.; Healey, J.H. Pathologic femoral fracture after periosteal excision and radiation for the treatment of soft tissue sarcoma. Cancer 1998, 82, 2356–2365. [Google Scholar] [CrossRef]
- Holt, G.E.; Griffin, A.M.; Pintilie, M.; Wunder, J.S.; Catton, C.; O’Sullivan, B.; Bell, R.S. Fractures following radiotherapy and limb-salvage surgery for lower extremity soft-tissue sarcomas. A comparison of high-dose and low-dose radiotherapy. J. Bone Jt. Surg. Am. 2005, 87, 315–319. [Google Scholar] [CrossRef]
- Dickie, C.I.; Parent, A.L.; Griffin, A.M.; Fung, S.; Chung, P.W.; Catton, C.N.; Ferguson, P.C.; Wunder, J.S.; Bell, R.S.; Sharpe, M.B.; et al. Bone fractures following external beam radiotherapy and limb-preservation surgery for lower extremity soft tissue sarcoma: Relationship to irradiated bone length, volume, tumor location and dose. Int. J. Radiat. Oncol. Biol. Phys. 2009, 75, 1119–1124. [Google Scholar] [CrossRef]
- Soares, C.B.G.; Araújo, I.D.; Pádua, B.J.; Vilela, J.C.S.; Souza, R.H.R.; Teixeira, L.E.M. Pathological fracture after radiotherapy: Systematic review of literature. Rev. Assoc. Med. Bras. 2019, 65, 902–908. [Google Scholar] [CrossRef]
- Bazan, J.G.; DiCostanzo, D.; Hock, K.; Jhawar, S.; Kuhn, K.; Lindsey, K.; Tedrick, K.; Healy, E.; Beyer, S.; White, J.R. Analysis of Radiation Dose to the Shoulder by Treatment Technique and Correlation With Patient Reported Outcomes in Patients Receiving Regional Nodal Irradiation. Front. Oncol. 2021, 11, 617926. [Google Scholar] [CrossRef]
- Johansen, S.; Fosså, K.; Nesvold, I.L.; Malinen, E.; Fosså, S.D. Arm and shoulder morbidity following surgery and radiotherapy for breast cancer. Acta Oncol. 2014, 53, 521–529. [Google Scholar] [CrossRef]
- Lipps, D.B.; Sachdev, S.; Strauss, J.B. Quantifying radiation dose delivered to individual shoulder muscles during breast radiotherapy. Radiother. Oncol. 2017, 122, 431–436. [Google Scholar] [CrossRef] [Green Version]
- Gupta, A.; Lynn Henry, N.; Lopinzi, C.L. Management of Aromatase Inhibitor–Induced Musculoskeletal Symptoms. JCO Oncol. Pract. 2020, 11, 733–739. [Google Scholar] [CrossRef]
- Buzdar, A.U. Clinical features of joint symptoms observed in the Arimidex, Tamoxifen, Alone or in Combination (ATAC) trial. J. Clin. Oncol. 2006, 24, 551–555. [Google Scholar] [CrossRef]
- Welgemoed, C.; Coughlan, S.; McNaught, P.; Gujral, D.; Riddle, P. A dosimetric study to improve the quality of nodal radiotherapy in breast cancer. BJR Open 2021, 2, 20210013. [Google Scholar] [CrossRef]
- Surmann, K.; van der Leer, J.; Branje, T.; van der Sangen, M.; van Lieshout, M.; Hurkmans, C.W. Elective breast radiotherapy including level I and II lymph nodes: A planning study with the humeral head as planning risk volume. Radiat. Oncol. 2017, 12, 22. [Google Scholar] [CrossRef] [Green Version]
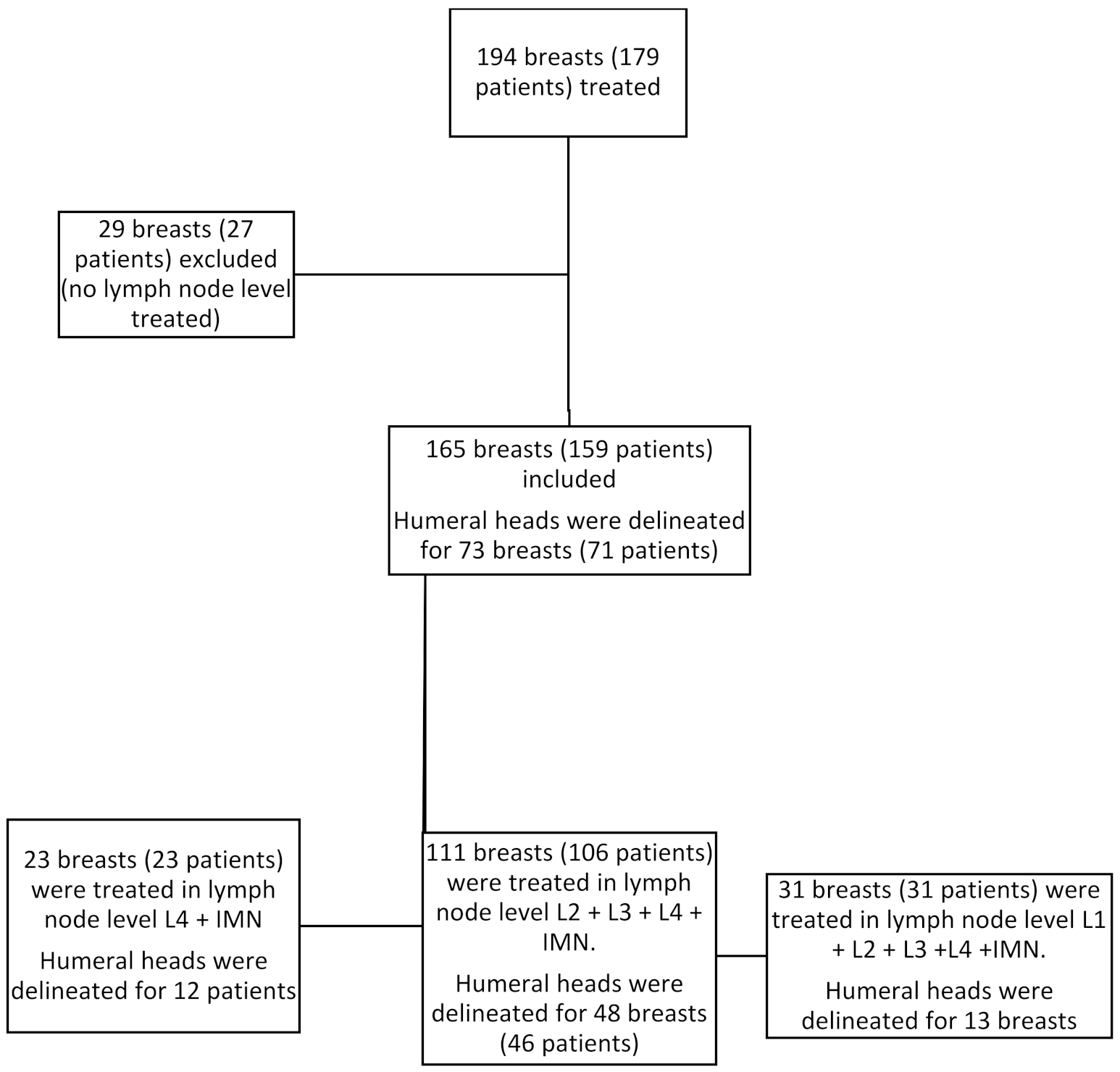
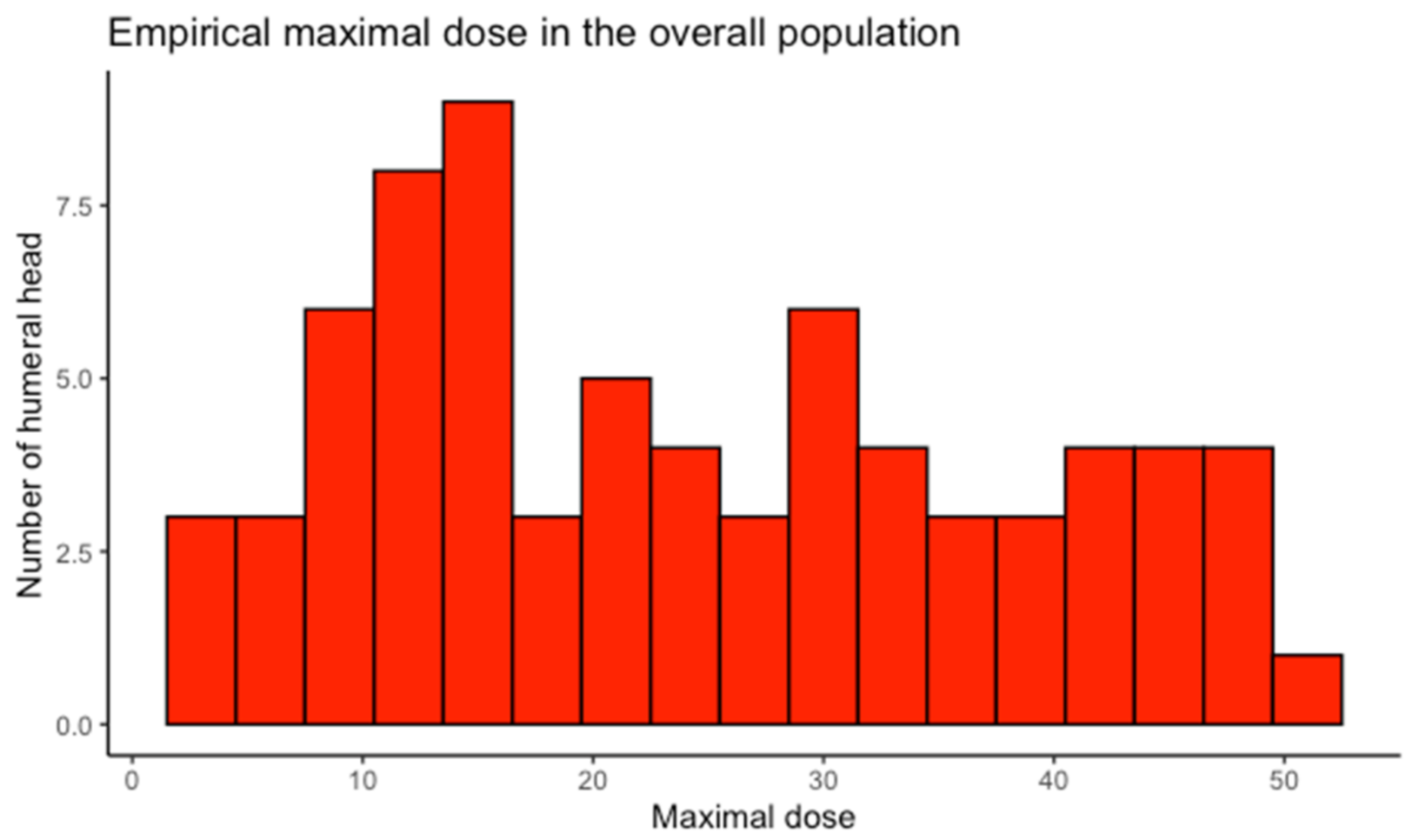
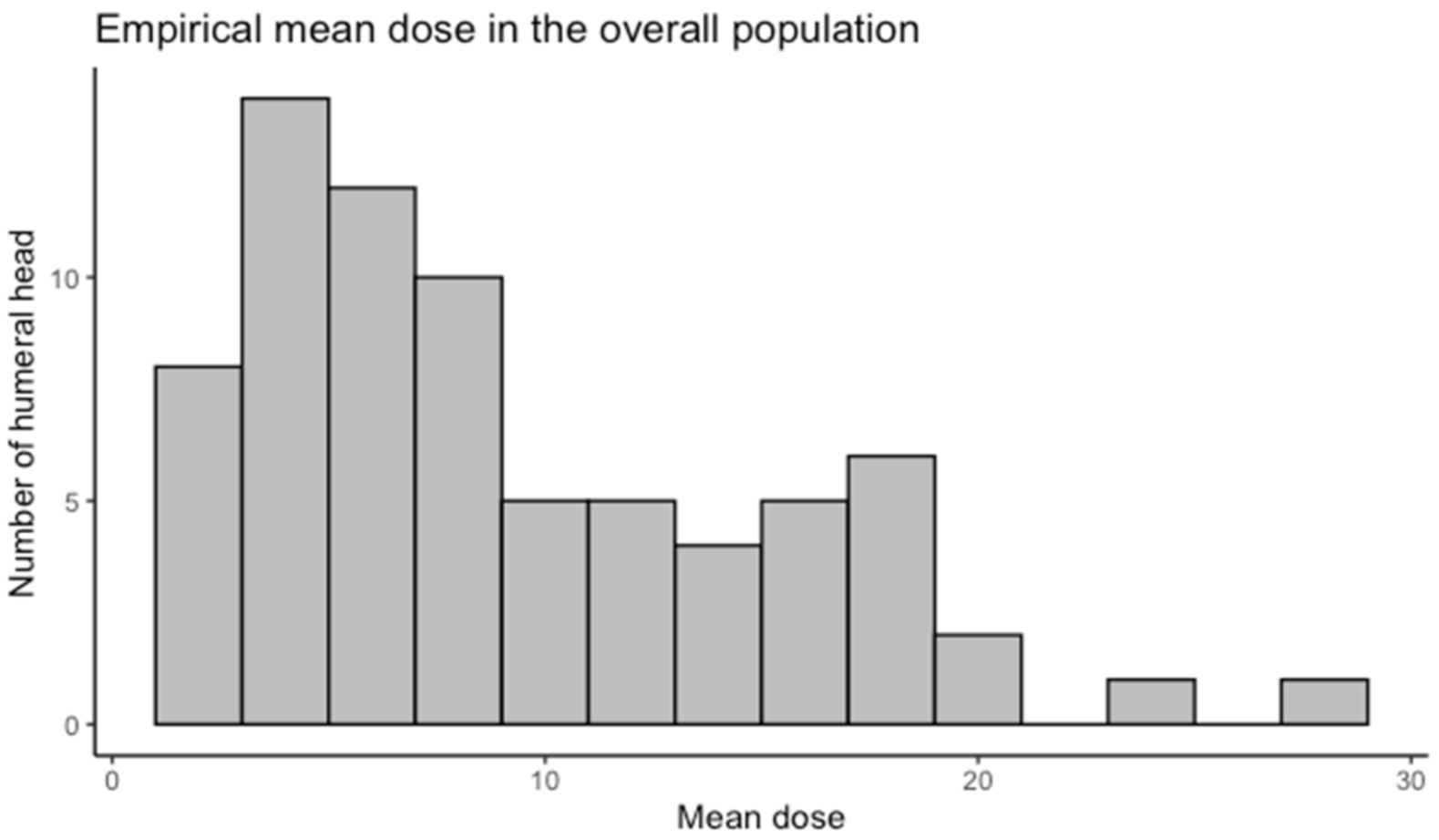
| Dosimetric Study | Maximal Dose (Gy): Mean (95%CI) | Mean Dose (Gy): Mean (95%CI) | p-Value for Difference in Maximal Dose |
|---|---|---|---|
| All breasts (n = 73) | 24.41 (21.20–27.61) | 9.18 (7.81–10.54) | |
| Lymph node level treated | |||
| L4 + IMN (n = 12) | 10.99 (7.49–14.49) | 5.08 (3.72–6.44) | reference |
| L2 + L3 + L4 + IMN (n = 48) | 24.72 (21.09–28.35) | 9.40 (7.74–11.06) | <0.001 |
| L1 + L2 + L3 + L4 + IMN (n = 13) | 35.64 (27.62–43.66) | 12.15 (7.99–16.30) | <0.0001 |
| Side treated | |||
| Right (n = 33) | 28.24 (23.47–33.01) | 10.44 (8.20–12.68) | reference |
| Left (n = 40) | 21.25 (16.98–25.51) | 8.14 (6.44–9.84) | 0.03 |
| Clinical event | |||
| No (n = 63) | 24.65 (21.20–28.10) | 9.32 (7.87–10.77) | reference |
| Yes (n = 10) | 22.90 (12.39–33.41) | 8.29 (3.48–13.10) | 0.74 |
| Breast surgery | |||
| Conservative (n = 62) | 24.20 (20.65–27.75) | 9.24 (7.73–10.76) | reference |
| Mastectomy (n = 11) | 25.61 (16.91–34.31) | 8.82 (5.14–12.50) | 0.67 |
| Age | 0.62 | ||
| BMI | 0.19 | ||
| Height | 0.25 |
| Clinical Events | Number of Events | Median Time to Onset of Symptoms in Month (Min–Max) |
|---|---|---|
| All | 10 | 48 (6–99) |
| Proximal humerus fracture | 1 | 39 |
| Shoulder pain | 6 | 54 (21–99) |
| Functional limitation | 3 | 45 (6–72) |
| Clinical Study | Number of Clinical Events | Frequency of Clinical Event * | p-Value |
|---|---|---|---|
| Total (n = 165) | 10 | 6.06% (3.20–10.92%) | |
| Lymph node level treated | |||
| L4 + IMN (n = 23) | 0 | 0% (0–14.8%) | reference |
| L2 + L3 + L4 + IMN (n = 111) | 7 | 6.31% (2.87–12.66%) | 0.60 |
| L1 + L2 + L3 + L4 + IMN (n = 31) | 3 | 9.68 % (2.56–25.69%) | 0.27 |
| Side treated | |||
| Right (n =86) | 4 | 4.65% (1.28–11.48%) | reference |
| Left (n = 79) | 6 | 7.59% (2.83–15.8%) | 0.53 |
| Breast surgery | |||
| Conservative (n = 134) | 5 | 3.73% (1.22–8.49%) | reference |
| Mastectomy (n = 31) | 5 | 16.12% (5.4–33.72%) | 0.03 |
| ALND | |||
| Yes (n = 129) | 10 | 7.75% (3.78–13.79%) | reference |
| No (n = 36) | 0 | 0% (0–9.73%) | 0.22 |
| Diabetes | |||
| Yes (n = 9) | 1 | 11.11% (2.81–48.25%) | reference |
| No (n = 153) | 9 | 5.88% (2.72–10.87%) | 0.46 |
| Age | 0.21 | ||
| BMI | 0.09 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belaidi, L.; Loap, P.; Kirova, Y. Do We Need to Delineate the Humeral Head in Breast Cancer Patients? Cancers 2022, 14, 496. https://doi.org/10.3390/cancers14030496
Belaidi L, Loap P, Kirova Y. Do We Need to Delineate the Humeral Head in Breast Cancer Patients? Cancers. 2022; 14(3):496. https://doi.org/10.3390/cancers14030496
Chicago/Turabian StyleBelaidi, Lahcene, Pierre Loap, and Youlia Kirova. 2022. "Do We Need to Delineate the Humeral Head in Breast Cancer Patients?" Cancers 14, no. 3: 496. https://doi.org/10.3390/cancers14030496
APA StyleBelaidi, L., Loap, P., & Kirova, Y. (2022). Do We Need to Delineate the Humeral Head in Breast Cancer Patients? Cancers, 14(3), 496. https://doi.org/10.3390/cancers14030496